Exogenous Methyl Jasmonate Effects of Sugar, Acid, and Calcium Accumulation During Fruit Development in Prunus humilis Bunge
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Materials
2.2. Experimental Design
2.3. Sample Collection
2.4. Experimental Index Measurement
2.4.1. Determination of Sugar Components in Fruits
2.4.2. Determination of Organic Acid Components in Fruits
2.4.3. Fruit Flavor Evaluation Parameters
2.4.4. Determination of Calcium Components in Fruits
2.5. Statistical Analysis
3. Results
3.1. Effect of Exogenous Methyl Jasmonate on Sugar Component Content in P. humilis Fruits
3.2. Effect of Exogenous Methyl Jasmonate on Organic Acid Component Content in P. humilis Fruits
3.3. Effect of Exogenous Methyl Jasmonate on Flavor Evaluation Parameters in P. humilis Fruits
3.4. Effect of Exogenous Methyl Jasmonate on Calcium Component Content in P. humilis Fruits
3.5. Correlation Between Sugar, Acid, and Calcium Quality Parameters During the Development and Maturation of P. humilis Fruits
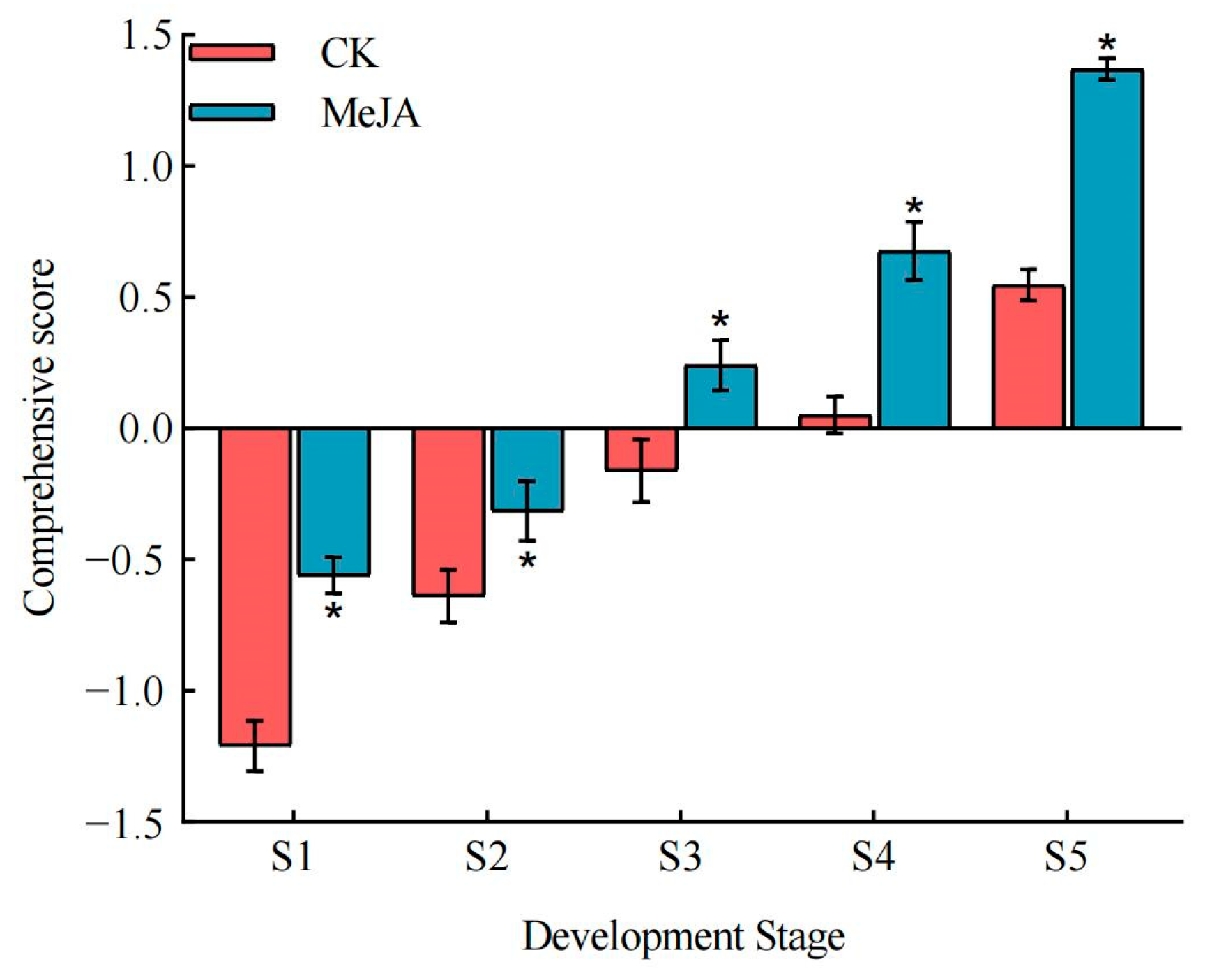
3.6. Principal Component Analysis of Sugar, Acid, and Calcium Quality in P. humilis Fruits
4. Discussion
4.1. The Effect of Exogenous Methyl Jasmonate on the Sugar and Acid Quality of P. humilis Fruits
4.2. The Effect of Exogenous Methyl Jasmonate on the Calcium Quality of P. humilis Fruits
4.3. Effect of Exogenous Methyl Jasmonate on the Comprehensive Sugar, Acid, and Calcium Quality of P. humilis Fruits
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Mo, C.; Li, W.D.; He, Y.X.; Zhang, Z.S.; Jin, J.S. Variability in the sugar and organic acid composition of the fruit of 57 genotypes of Chinese dwarf cherry [Cerasus humilis (Bge.) Sok.]. J. Pomol. Hortic. Sci. 2015, 90, 419–426. [Google Scholar] [CrossRef]
- Li, W.D.; Li, O.; Zhang, A.R.; Li, L.; Hao, J.H.; Jin, J.S.; Yin, S.J. Genotypic diversity of phenolic compounds and antioxidant capacity of Chinese dwarf cherry (Cerasus humilis (Bge.) Sok.) in China. Sci. Hortic. 2014, 175, 208–213. [Google Scholar] [CrossRef]
- Cao, H.Q.; Liu, S.Y.; Wang, G.Y.; Liao, G.Z.; Bai, J. Nutritional composition and development of Prunus humilis Bunge. Farm Prod. Process 2015, 24, 70–72. [Google Scholar] [CrossRef]
- Zhang, M.L.; Deng, Q.C.; Yang, H.X.; Zhang, Z.Y. Study on nutrition components from fresh fruit and pip of Inner Mongolia Cerasus humilis (Bge.) Sok. Amino Acids Biot. Resour. 2007, 29, 18–20. [Google Scholar] [CrossRef]
- Zhang, S.K.; Xi, L.Q.; Zhang, Q.Y.; Shi, J.Y.; Wu, P. Research progress on the development and utilization of Cerasus humilis (Bge.). Sok. Sci. Technol. Food Ind. 2020, 41, 361–367. [Google Scholar] [CrossRef]
- Wang, P.F.; Xue, X.F.; Mu, X.P.; Zhang, J.C.; Cao, Q.; Du, J.J. Analysis of organic acid accumulation characteristics and organic acid-metabolizing enzyme activities of Chinese dwarf cherry (Cerasus humilis Bunge) fruit. Sci. Agric. Sin. 2013, 46, 4101–4109. [Google Scholar] [CrossRef]
- Ye, L.Q.; Sun, M.; Zhang, Z.S.; Liu, H.J.; Gu, J.R.; Li, W.D. Analysis on the changes and correlations of sugar and organic acid contents in Chinese Cerasus humilis [Cerasus humilis (Bge.) Sok.] during different development stages. Sci. Technol. Food Ind. 2017, 38, 98–102. [Google Scholar] [CrossRef]
- Yang, Y.; Yan, S.T.; Fu, H.B.; Wang, P.F.; Du, J.J.; Mu, X.P. Effects of bagging and shading on the content of sugar, acid and flavonoids in fruit of Cerasus humilis. Acta Bot. Breali-Occident. Snica 2024, 44, 1201–1207. [Google Scholar] [CrossRef]
- Zhang, L.; Zhang, J.; Guo, J. Relationships between organic acid metabolism and the accumulation of sugars and calcium in fruits of Cerasus humilis during different development stages. Plants 2024, 13, 3053. [Google Scholar] [CrossRef] [PubMed]
- Siddeeg, A.; Zeng, X.A.; Ammar, A.F.; Han, Z. Sugar profile, volatile compounds, composition and antioxidant activity of Sukkari date palm fruit. J. Food Sci. Technol. 2019, 56, 754–762. [Google Scholar] [CrossRef]
- Zhou, J.T.; Yang, S.W.; Ma, Y.; Liu, Z.S.; Tu, H.X.; Wang, H.; Zhang, J.; Chen, Q.; He, W.; Li, M.Y.; et al. Soluble sugar and organic acid composition and flavor evaluation of Chinese cherry fruits. Food Chem X 2023, 20, 100953. [Google Scholar] [CrossRef]
- Zhang, L.; Zhang, W.; Guo, J.L. Analysis of changes and correlations between calciums and organic acids in fruits of Cerasus humilis during different development stages. J. Fruit Sci. 2024, 41, 494–504. [Google Scholar] [CrossRef]
- Qin, Y.Y.; Luo, Y.C.; Deng, Y.Z.; Wang, Y.R.; Wu, F.; Zhang, L.J.; Shan, B.; Cen, S.M. Changes in sugar and acid components contents and comprehensive quality evaluation during fruit development of Orah with different rootstocks. J. South. Agric. 2024, 55, 2201–2214. [Google Scholar] [CrossRef]
- Liu, S.; Liu, Y.C.; Liu, N.; Zhang, Q.P.; Xu, M.; Zhang, Y.J.; Liu, W.S. Sugar and organic acid components in fruits of plum cultivar resources of genus Prunus. Sci. Agric. Sin. 2016, 49, 3188–3198. [Google Scholar] [CrossRef]
- Ma, J.J.; Zhang, L.B.; Liu, Y.Y.; Xu, X.F.; Yu, F.M. Variation of different forms of calcium contents and their correlation during the growth stage of Prunus humilis tissues. Acta Hortic. Sin. 2008, 35, 631–636. [Google Scholar] [CrossRef]
- Wang, S.Y.; Shi, X.C.; Liu, F.Q.; Laborda, P. Effects of exogenous methyl jasmonate on quality and preservation of postharvest fruits: A review. Food Chem. 2021, 353, 129482. [Google Scholar] [CrossRef]
- Ju, Y.L.; Liu, B.C.; Xu, X.L.; Wu, J.R.; Sun, W.; Fang, Y.L. Targeted metabolomic and transcript level analysis reveals the effects of exogenous strigolactone and methyl jasmonate on grape quality. Sci. Hortic. 2022, 299, 111009. [Google Scholar] [CrossRef]
- Ameye, M.; Allmann, S.; Verwaeren, J.; Smagghe, G.; Haesaert, G.; Schuurink, R.C.; Audenaert, K. Green leaf volatile production by plants: A meta-analysis. New Phytol. 2018, 220, 666–683. [Google Scholar] [CrossRef] [PubMed]
- Ho, T.-T.; Murthy, H.N.; Park, S.-Y. Methyl Jasmonate induced oxidative stress and accumulation of secondary metabolites in plant cell and organ cultures. Int. J. Mol. Sci. 2020, 21, 716. [Google Scholar] [CrossRef]
- Ye, N.; Zhang, Z.; Lv, Z.Z.; Du, J.M.; Zhang, S.X.; Li, W. Effects of the plant growth regulator methyl jasmonate on fruit quality and lipoxygenase metabolic pathway of cabernet gernischt. Food Sci. 2024, 45, 248–256. [Google Scholar] [CrossRef]
- Sudheeran, P.K.; Love, C.; Feygenberg, O.; Maurer, D.; Ovadia, R.; Oren-Shamir, M.; Alkan, N. Induction of red skin and improvement of fruit quality in ‘kent’, ‘shelly’ and ‘maya’ mangoes by preharvest spraying of prohydrojasmon at the orchard. Postharvest Biol. Tec. 2019, 149, 18–26. [Google Scholar] [CrossRef]
- Ding, X.; Wang, B.; Gong, Y.; Yan, X.; Chen, X.; Zhong, Y.; Zhao, Z. Exogenous methyl jasmonate (MeJA) improves ‘Ruixue’ apple fruit quality by regulating cell wall metabolism. Foods 2024, 13, 1594. [Google Scholar] [CrossRef]
- Balbontín, C.; Gutiérrez, C.; Wolff, M.; Figueroa, C.R. Effect of abscisic acid and methyl jasmonate preharvest applications on fruit quality and cracking tolerance of sweet cherry. Chil. J. Agric. Res. 2018, 78, 438–446. [Google Scholar] [CrossRef]
- Sheng, W.S.; Jiang, J.S.; Wang, X.B.; Li, S.L.; Wan, D. Determination of fructose glucose and sucrose content in Honey. J. Anhui Agri. Sci. 2010, 38, 17182–17183. [Google Scholar] [CrossRef]
- Ji, X.H.; Zhang, R.; Mao, Z.Q.; Kuang, L.G.; Lu, M.F.; Wang, Y.; Zhang, Y.M.; Chen, X.S. The analysis of characteristic variations of the seedlings of Xin jiang wild myrobalan plum and excavation of the excellent germplasm resources. Acta Hortic. Sin. 2012, 39, 1551–1558. [Google Scholar] [CrossRef]
- Wang, H.L.; Xie, P.; Ma, Y.H.; Zhao, K.; Wang, H.; Zhao, X.Z.; Zheng, Q.M. (2024). Differences in sugar and acid compositions and quality evaluation of fengtang Plum fruit from different producing areas in Guizhou. Guizhou Agric. Sci. 2024, 52, 84–93. [Google Scholar] [CrossRef]
- Huang, X.M.; Wang, H.C.; Zhong, W.L.; Yuan, W.Q.; Lu, J.M.; Li, J.G. Spraying calcium is not an effective way to increase structural calcium in litchi pericarp. Sci. Hortic. 2008, 117, 39–44. [Google Scholar] [CrossRef]
- Chen, C.; Hu, Q.Q.; Yang, K.B.; Wang, K.P.; Yu, F.Y.; Dai, P.F. Research progress on the regulation of plant growth and development by methyl jasmonate. Jiangsu Agric. Sci 2023, 51, 1–11. [Google Scholar] [CrossRef]
- Xiong, B.; Wang, Y.; Zhang, Y.; Ma, M.; Gao, Y.; Zhou, Z.; Wang, B.; Wang, T.; Lv, X.; Wang, X.; et al. Alleviation of drought stress and the physiological mechanisms in Citrus cultivar (Huangguogan) treated with methyl jasmonate. Biosci Biotechnol Biochem. 2020, 84, 1958–1965. [Google Scholar] [CrossRef]
- Rehman, M.; Singh, Z.; Khurshid, T. Methyl jasmonate alleviates chilling injury and regulates fruit quality in ‘Midknight’ Valencia orange. Postharvest Biol. Tec. 2018, 141, 58–62. [Google Scholar] [CrossRef]
- Karimi, R.; Gavili-Kilaneh, K.; Khadivi, A. Methyl jasmonate promotes salinity adaptation responses in two grapevine (Vitis vinifera L.) cultivars differing in salt tolerance. Food Chem. 2022, 375, 131667. [Google Scholar] [CrossRef]
- Schaller, A.; Stintzi, A. Enzymes in jasmonate biosynthesis–structure, function, regulation. Phytochemistry 2009, 70, 1532–1538. [Google Scholar] [CrossRef]
- Zheng, L.J.; Nie, J.Y.; Yan, Z. Advances in research on sugars, organic acids and their effects on taste of fruits. J. Fruit Sci. 2015, 32, 304–312. [Google Scholar] [CrossRef]
- Liang, J.; Guo, Y.; Liu, Y.L.; Li, M.M.; Zhao, Z.Y. Analysis of contents and constitutents of sugar and organic acid in different apple cultivars. J. Northwest Sci-Tech Univ. Agric. For. (Nat. Sci. Ed.) 2011, 39, 163–170. [Google Scholar] [CrossRef]
- Guo, K.X.; Sun, Z.J.; Li, Q.Y.; Han, L.; Zhang, Y.; Wang, C.H.; Tian, Y.K.; Zheng, X.D. Analysis of components and content of sugar and acid in fruits of different Pyrus communis L. varieties. J. Qingdao Agric. Univ. (Nat. Sci.) 2024, 41, 79–86+115. [Google Scholar] [CrossRef]
- Chen, M.X.; Chen, X.S.; Ci, Z.J.; Shi, Z.A. Changes of sugar and acid constituents in apricot during fruit development. Acta Hortic. Sin. 2006, 33, 805–808. [Google Scholar] [CrossRef]
- Asghari, M.; Merrikhi, M.; Kavoosi, B. Methyl jasmonate foliar spray substantially enhances the productivity, quality and phytochemical contents of pomegranate fruit. J. Plant Growth Regul. 2020, 39, 1153–1161. [Google Scholar] [CrossRef]
- Kucuker, E.; Ozturk, B.; Celik, S.M.; Aksit, H. Pre-harvest spray application of methyl jasmonate plays an important role in fruit ripening, fruit quality and bioactive compounds of Japanese plums. Sci. Hortic. 2014, 176, 162–169. [Google Scholar] [CrossRef]
- Wang, S.Y.; Zheng, W. Preharvest application of methyl jasmonate increases fruit quality and antioxidant capacity in raspberries. Int. J. Food Sci. Technol. 2005, 40, 187–195. [Google Scholar] [CrossRef]
- Yang, G.K.; Wu, Y.L.; Gao, Y.; Zhang, X.J.; Hao, Y.Y. Effects of exogenous methyl jasmonate on the quality of apple. J. Fruit Resour. Guoshu 2021, 2, 15–22. [Google Scholar] [CrossRef]
- Ozturk, B.; Yıldız, K.; Kucuker, E. Effect of pre-harvest methyl jasmonate treatments on ethylene production, water-soluble phenolic compounds and fruit quality of Japanese plums. J. Sci. Food Agric. 2015, 95, 583–591. [Google Scholar] [CrossRef]
- Lv, J.Y.; Sun, C.X.; Qiu, Y.X.; Ge, Y.H.; Chen, J.X. Exogenous methyl jasmonate (MeJA) mediated carbohydrate metabolism in apple fruit during ripening. Plant Physiol. Bioc. 2024, 218, 109329. [Google Scholar] [CrossRef]
- Li, Q.L.; Cao, D.T.; Wei, Z.F.; Wang, Z.Q.; Liu, J.W.; Yang, W.J. Effect of different concentrations of methyl-jasmonate on fruit quality of Chunmi peach. Acta Agric. Zhejiangensis 2018, 30, 985–991. [Google Scholar] [CrossRef]
- Yang, W.Y.; Xie, H.J.; Tao, L.; Huan, Y.M.; Chen, S.B.; Lin, L.J.; Liao, M.A. Comparison of organic acid metabolism characteristics between ‘golden delicious’ apple and its excellent variation material ‘SGP-1’ during fruit development. Acta Bot. Boreali-Occident. Sin. 2022, 42, 0829–0839. [Google Scholar] [CrossRef]
- Xu, W.Q.; He, Y.H.; Wang, W.; Yi, C.Y.; Wei, J. Effects of rootstocks on componrnts and content changes of organic acids in ‘Hosui’ pear fruits. South China Fruits 2017, 46, 16–19. [Google Scholar] [CrossRef]
- Xu, Z.Y.; Yan, J.; Cai, Z.X.; Sun, M.; Su, Z.W.; Shen, Z.J.; Ma, R.J.; Yu, M.L. Correlation between soluble sugar, organic acid and phenolic substances with tasted flavor in peach fruit. Jiangsu J. Agric. Sci. 2022, 38, 190–199. [Google Scholar] [CrossRef]
- Min, H.; Li, C.Z.; Fu, X.J.; Zhu, J.H.; Xing, S.P.; Xiong, H.W. Changes of nutrient and functional components in Nanfeng tangerine green fruits at different development stages. J. Anhui Agric. Sci. 2022, 50, 172–175. [Google Scholar] [CrossRef]
- Hepler, P.K. Calcium: A central regulator of plant growth and development. Plant Cell 2005, 17, 2142–2155. [Google Scholar] [CrossRef]
- Hua, Q.Y.; Wei, T.; Han, W.; Wu, G.H.; Liu, Q. Progress in the study of composition, volatile flavor compounds and bioactivity of Chinese dwarf cherry. Food Mach. 2024, 40, 186–192. [Google Scholar] [CrossRef]
- Ma, J.J.; Zhang, L.B.; Yu, F.M.; Xu, X.F. Contents and distribution of different forms of calcium in Prunus humilis. Acta Hortic. Sin. 2007, 34, 755–759. [Google Scholar] [CrossRef]
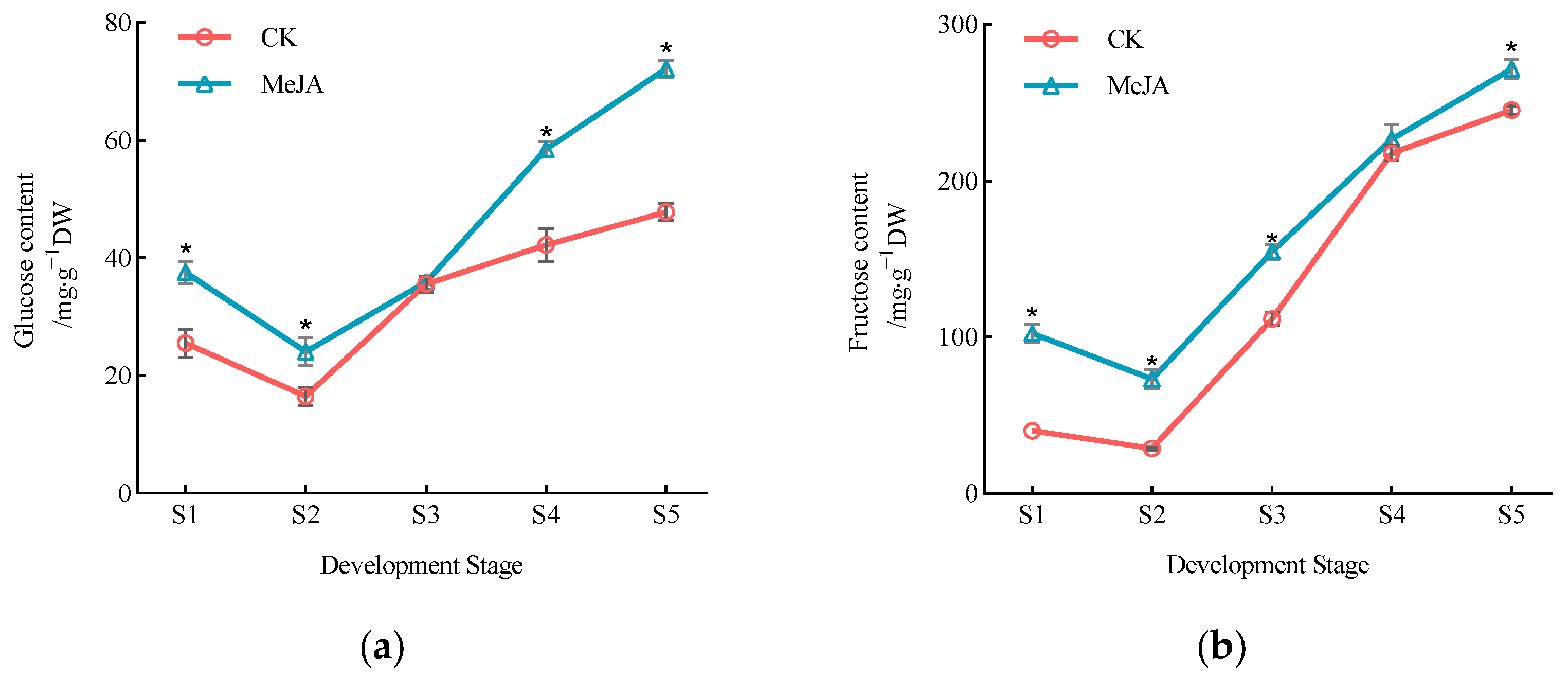

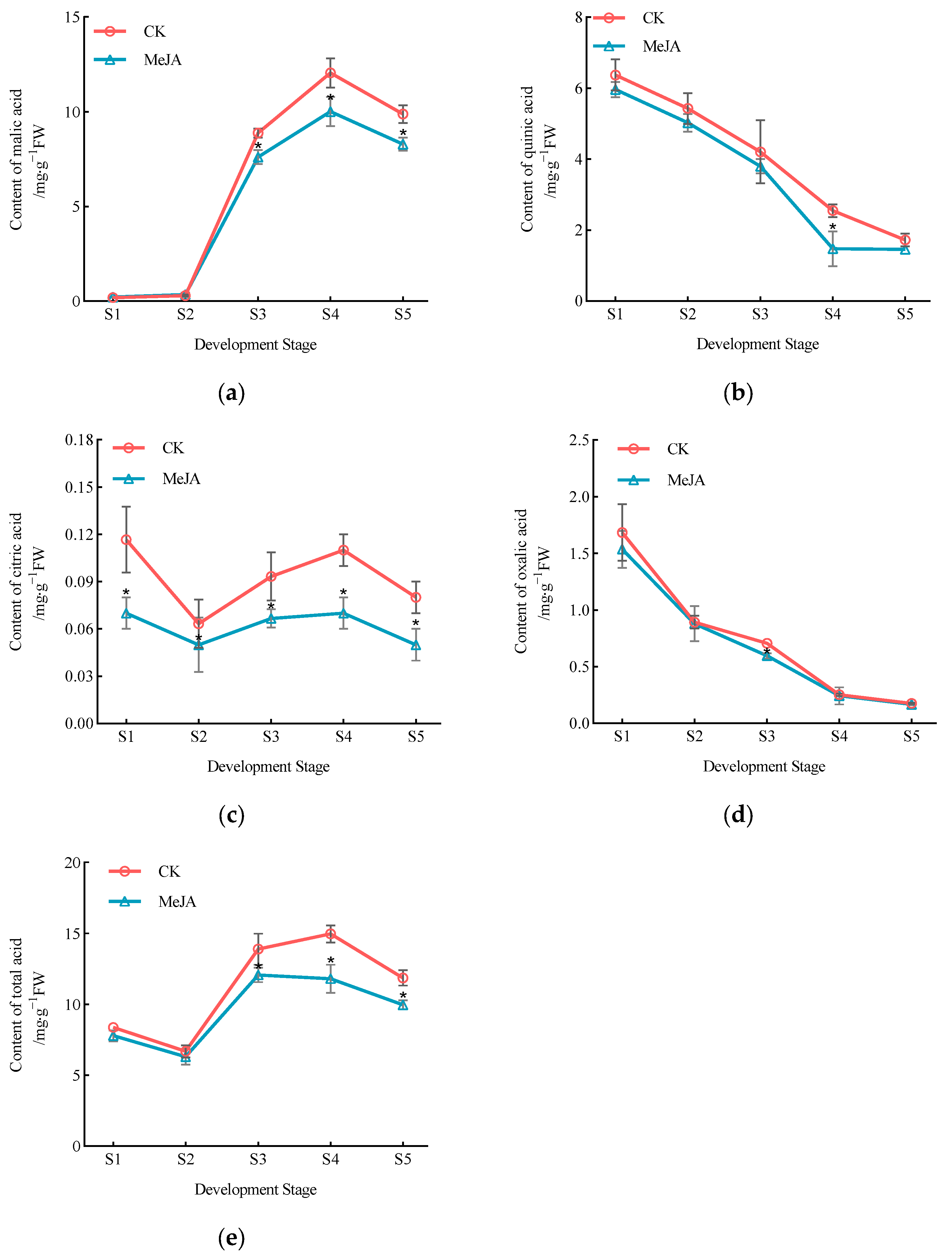
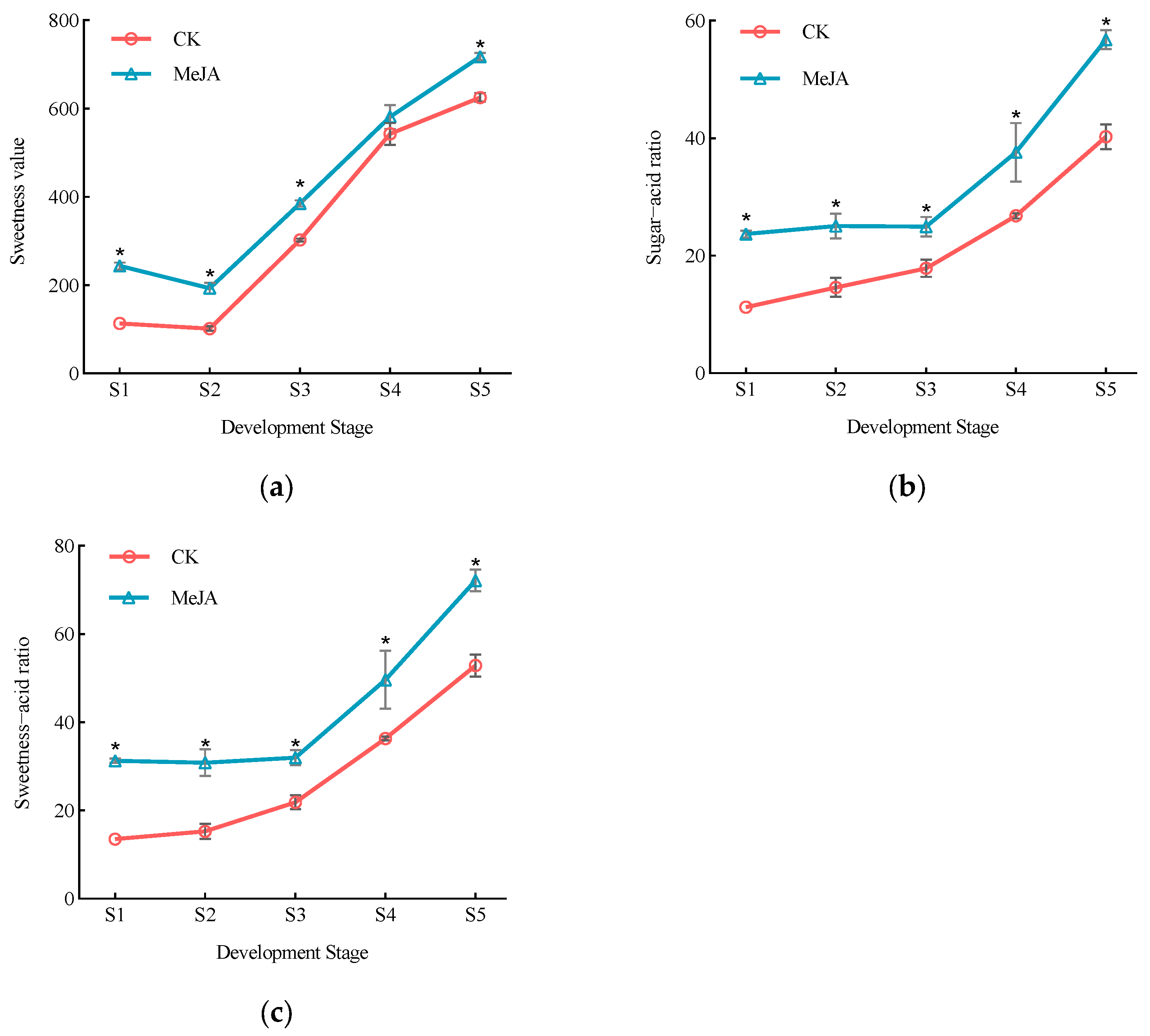
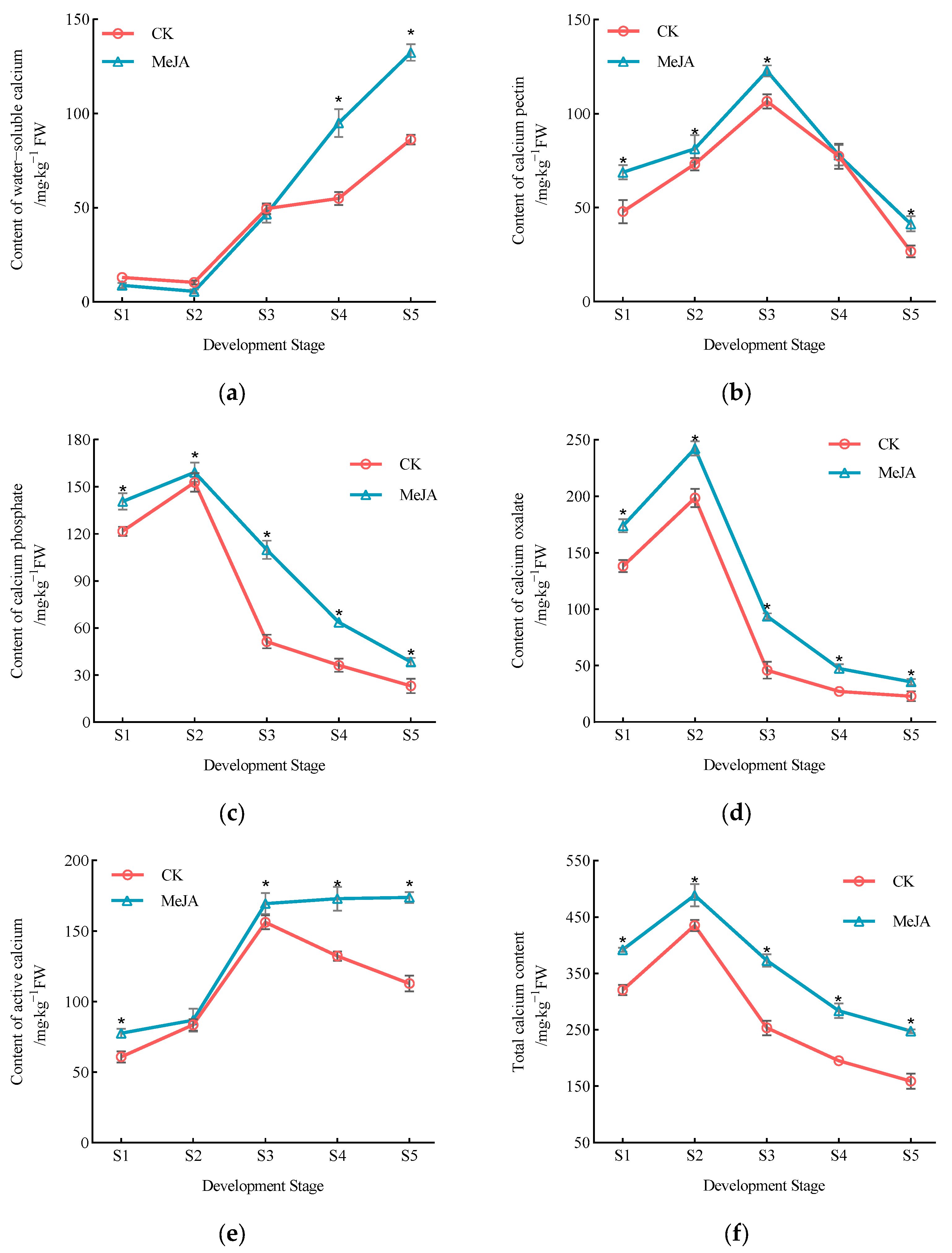
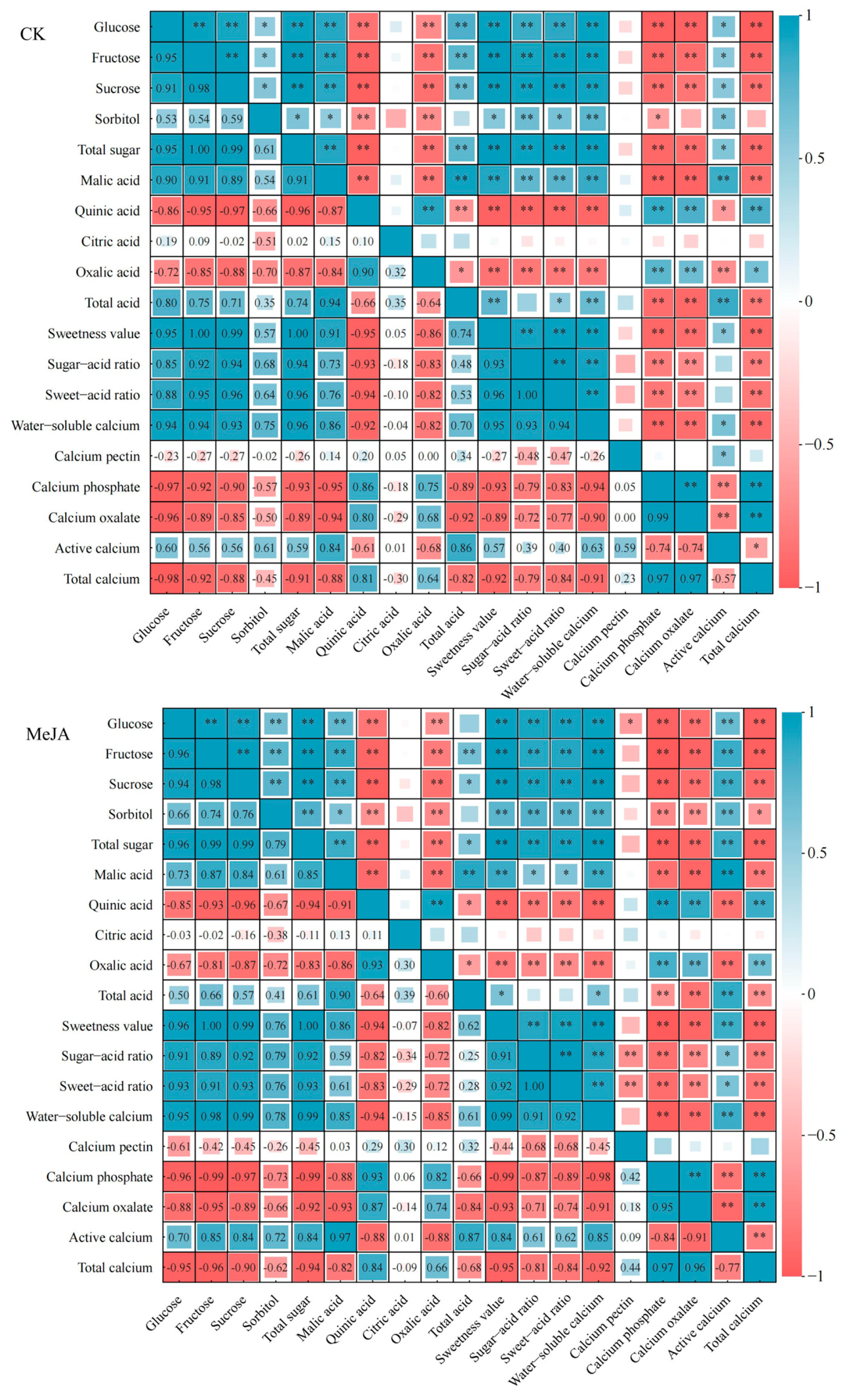
| Fruit Development Stage | Treatment | Proportion of Sugar Components/% | |||
|---|---|---|---|---|---|
| Glucose | Fructose | Sucrose | Sorbitol | ||
| S1 | CK | 27.07 ± 1.77 | 42.68 ± 1.06 | 22.38 ± 1.15 | 7.87 ± 1.01 |
| MeJA | 20.33 ± 0.56 * | 55.58 ± 1.98 * | 16.51 ± 2.54 * | 7.57 ± 0.51 | |
| S2 | CK | 16.86 ± 0.72 | 29.55 ± 0.71 | 30.71 ± 1.50 | 22.88 ± 1.49 |
| MeJA | 15.34 ± 1.93 | 46.65 ± 2.31 * | 24.20 ± 0.91 * | 13.81 ± 1.28 * | |
| S3 | CK | 14.38 ± 0.56 | 45.16 ± 1.65 | 27.49 ± 1.65 | 12.97 ± 0.41 |
| MeJA | 11.94 ± 0.27 * | 51.51 ± 2.14 * | 24.29 ± 1.77 | 12.26 ± 0.38 | |
| S4 | CK | 10.55 ± 0.93 | 54.39 ± 1.65 | 30.68 ± 2.62 | 4.38 ± 0.26 |
| MeJA | 13.28 ± 0.29 * | 51.41 ± 0.57 * | 29.87 ± 0.97 | 5.44 ± 0.42 * | |
| S5 | CK | 10.03 ± 0.41 | 51.48 ± 1.14 | 30.42 ± 0.89 | 8.08 ± 0.33 |
| MeJA | 12.76 ± 0.37 * | 48.03 ± 0.97 * | 29.47 ± 0.55 | 9.75 ± 0.03 * | |
| Fruit Development Stage | Treatment | Proportion of Acid Components/% | |||
|---|---|---|---|---|---|
| Malic Acid | Quinic Acid | Citric Acid | Oxalic Acid | ||
| S1 | CK | 2.35 ± 0.76 | 72.93 ± 3.17 | 1.40 ± 0.29 | 20.17 ± 3.43 |
| MeJA | 2.83 ± 0.33 | 76.58 ± 1.39 | 0.90 ± 0.09 * | 19.70 ± 1.23 | |
| S2 | CK | 4.53 ± 1.00 | 81.15 ± 1.39 | 0.95 ± 0.23 | 13.90 ± 0.49 |
| MeJA | 5.37 ± 1.44 | 79.95 ± 3.01 | 0.78 ± 0.22 | 13.37 ± 1.35 | |
| S3 | CK | 64.14 ± 4.00 | 32.39 ± 4.24 | 0.67 ± 0.06 | 5.10 ± 0.31 |
| MeJA | 63.02 ± 1.19 | 31.49 ± 1.17 | 0.55 ± 0.03 * | 4.94 ± 0.03 | |
| S4 | CK | 80.49 ± 1.89 | 15.08 ± 1.84 | 0.74 ± 0.08 | 1.69 ± 0.07 |
| MeJA | 84.92 ± 2.72 | 12.40 ± 3.50 | 0.60 ± 0.10 | 2.08 ± 0.69 | |
| S5 | CK | 83.30 ± 1.38 | 15.57 ± 1.26 | 0.68 ± 0.09 | 1.49 ± 0.07 |
| MeJA | 83.19 ± 0.92 | 14.64 ± 0.94 | 0.50 ± 0.11 | 1.67 ± 0.02 * | |
| Fruit Development Stage | Treatment | Proportion of Calcium Components/% | ||||
|---|---|---|---|---|---|---|
| Water-Soluble Calcium | Calcium Pectin | Active Calcium | Calcium Phosphate | Calcium Oxalate | ||
| S1 | CK | 4.04 ± 0.78 | 14.91 ± 1.63 | 18.96 ± 0.88 | 37.94 ± 0.51 | 43.10 ± 1.22 |
| MeJA | 2.23 ± 0.33 * | 17.55 ± 1.07 | 19.78 ± 0.95 | 35.86 ± 1.30 | 44.35 ± 1.17 | |
| S2 | CK | 2.38 ± 0.27 | 16.84 ± 1.10 | 19.22 ± 1.30 | 35.13 ± 0.88 | 45.65 ± 0.88 |
| MeJA | 1.13 ± 0.19 * | 16.62 ± 0.79 | 17.75 ± 0.90 | 32.60 ± 0.30 * | 49.65 ± 0.91 * | |
| S3 | CK | 19.55 ± 0.56 | 42.07 ± 1.67 | 61.61 ± 1.45 | 20.29 ± 1.30 | 18.10 ± 2.26 |
| MeJA | 12.50 ± 0.89 * | 32.91 ± 0.21 * | 45.41 ± 0.73 * | 29.45 ± 0.71 * | 25.15 ± 1.42 * | |
| S4 | CK | 28.10 ± 1.86 | 39.56 ± 3.41 | 67.66 ± 1.87 | 18.55 ± 2.09 | 13.79 ± 0.42 |
| MeJA | 33.47 ± 2.28 * | 27.42 ± 1.11 * | 60.89 ± 1.20 * | 22.44 ± 0.56 * | 16.67 ± 0.79 * | |
| S5 | CK | 54.38 ± 2.87 | 16.82 ± 1.26 | 71.19 ± 3.04 | 14.49 ± 1.55 | 14.32 ± 1.85 |
| MeJA | 53.44 ± 2.42 * | 16.69 ± 1.41 | 70.13 ± 1.76 | 15.52 ± 0.85 | 14.35 ± 0.95 | |
| Quality Index | PC1 | PC2 | PC3 |
|---|---|---|---|
| Glucose | 0.856 | 0.337 | −0.131 |
| Fructose | 0.839 | 0.498 | −0.065 |
| Sucrose | 0.852 | 0.493 | −0.084 |
| Sorbitol | 0.836 | 0.042 | 0.187 |
| Total sugar | 0.879 | 0.462 | −0.059 |
| Malic acid | 0.55 | 0.779 | 0.267 |
| Quinic acid | −0.843 | −0.454 | −0.044 |
| Citric acid | −0.577 | 0.721 | −0.197 |
| Oxalic acid | −0.782 | −0.38 | −0.242 |
| Total acid | 0.202 | 0.900 | 0.354 |
| Sweetness value | 0.859 | 0.485 | −0.068 |
| Sugar–acid ratio | 0.968 | 0.074 | −0.198 |
| Sweet–acid ratio | 0.956 | 0.125 | −0.202 |
| Water–soluble calcium | 0.875 | 0.412 | −0.053 |
| Calcium pectin | −0.259 | −0.042 | 0.945 |
| Calcium phosphate | −0.559 | −0.801 | 0.073 |
| Calcium oxalate | −0.503 | −0.843 | −0.036 |
| Active calcium | 0.688 | 0.377 | 0.573 |
| Total calcium | −0.371 | −0.875 | 0.245 |
| Eigenvalue | 13.371 | 2.691 | 1.703 |
| Contribution rate/% | 70.373 | 14.161 | 8.964 |
| Cumulative contribution rate/% | 70.373 | 84.534 | 93.498 |
| Treatment | Fruit Development Stage | F1 | F2 | F3 | Comprehensive Score |
|---|---|---|---|---|---|
| CK | S1 | −1.602 | 0.275 | −1.352 | −1.210 |
| S2 | −0.698 | −1.114 | 0.119 | −0.638 | |
| S3 | −0.587 | 0.979 | 1.252 | −0.162 | |
| S4 | −0.271 | 1.658 | 0.090 | 0.052 | |
| S5 | 0.774 | 0.823 | −1.270 | 0.547 | |
| MeJA | S1 | −0.547 | −0.823 | −0.657 | −0.560 |
| S2 | −0.140 | −1.658 | 0.201 | −0.315 | |
| S3 | 0.139 | −0.166 | 1.861 | 0.241 | |
| S4 | 0.841 | 0.368 | 0.374 | 0.677 | |
| S5 | 2.093 | −0.342 | −0.618 | 1.369 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, L.; Liang, Z.; Guo, J. Exogenous Methyl Jasmonate Effects of Sugar, Acid, and Calcium Accumulation During Fruit Development in Prunus humilis Bunge. Horticulturae 2025, 11, 1008. https://doi.org/10.3390/horticulturae11091008
Zhang L, Liang Z, Guo J. Exogenous Methyl Jasmonate Effects of Sugar, Acid, and Calcium Accumulation During Fruit Development in Prunus humilis Bunge. Horticulturae. 2025; 11(9):1008. https://doi.org/10.3390/horticulturae11091008
Chicago/Turabian StyleZhang, Li, Zhaoyang Liang, and Jinli Guo. 2025. "Exogenous Methyl Jasmonate Effects of Sugar, Acid, and Calcium Accumulation During Fruit Development in Prunus humilis Bunge" Horticulturae 11, no. 9: 1008. https://doi.org/10.3390/horticulturae11091008
APA StyleZhang, L., Liang, Z., & Guo, J. (2025). Exogenous Methyl Jasmonate Effects of Sugar, Acid, and Calcium Accumulation During Fruit Development in Prunus humilis Bunge. Horticulturae, 11(9), 1008. https://doi.org/10.3390/horticulturae11091008





