Effects of Container Substrate Composition on the Growth and Performance of Garberia heterophylla (W. Bartram) Merr. & F. Harper: A Native Xeric Species
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material and Substrate Sourcing
2.2. Physical, Chemical and Elemental Properties
2.3. Plant Growth and Performance Under Greenhouse Conditions
2.4. Experimental Design and Statistical Analysis
3. Results
3.1. Analysis of Substrate Properties
3.2. Growth, SPAD, and Dry Mass
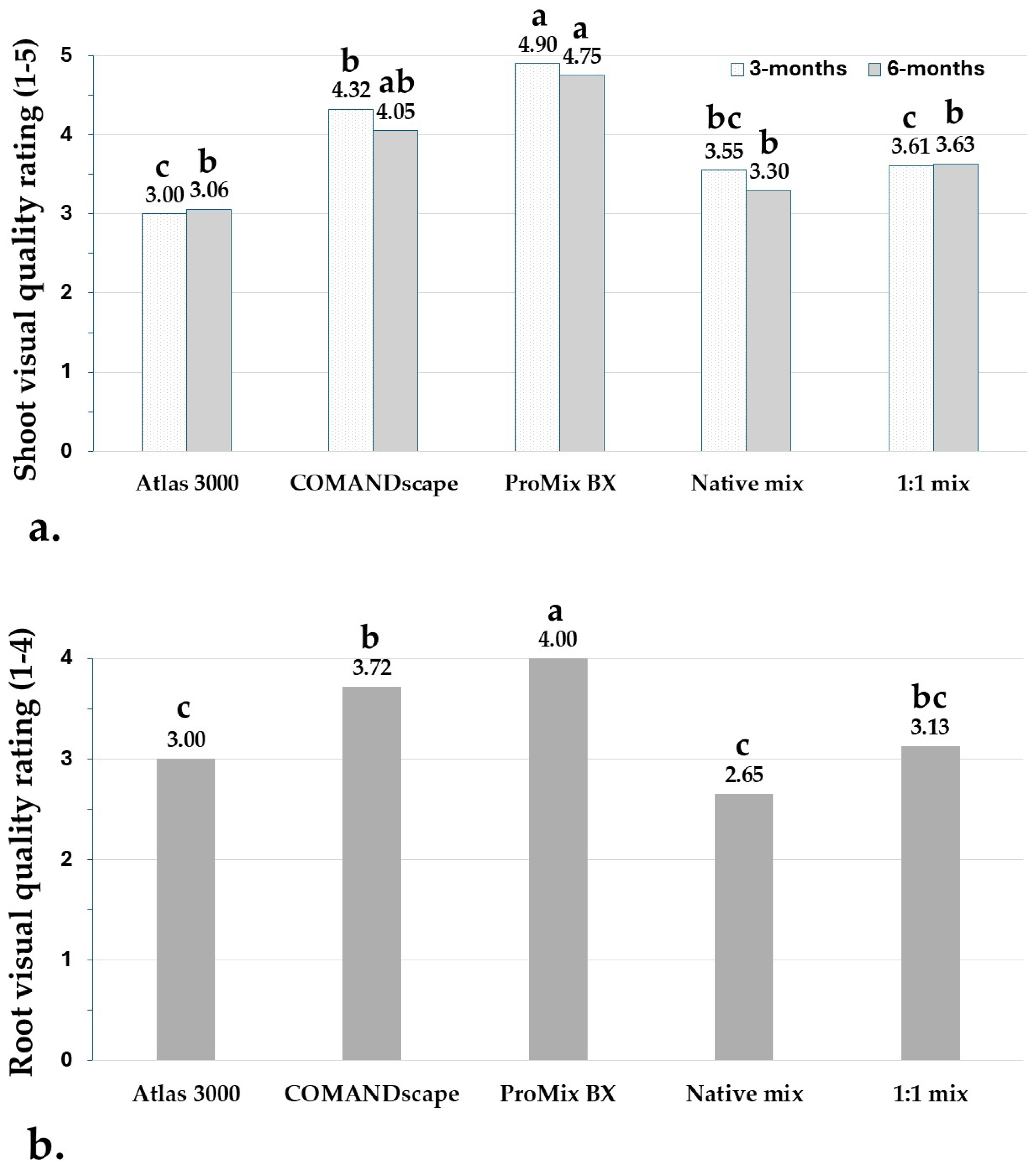
3.3. Root and Shoot Visual Quality
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rihn, A.L.; Knuth, M.J.; Peterson, B.J.; Torres, A.P.; Campbell, J.H.; Boyer, C.R.; Palma, M.A.; Khachatryan, H. Investigating drivers of native plant production in the United States green industry. Sustainability 2022, 14, 6774. [Google Scholar] [CrossRef]
- Nabors, A.; Hung, K.L.J.; Corkidi, L.; Bethke, J.A. California native perennials attract greater native pollinator abundance and diversity than nonnative, commercially available ornamentals in Southern California. Environ. Entomol. 2022, 51, 836–847. [Google Scholar] [CrossRef]
- Silva, J.J.; Wilson, S.B.; Knox, G.W.; Mallinger, R.E. Evaluation of plant growth and flowering performance of Florida native and non-native ornamentals under varying irrigation. HortTechnology 2024, 34, 629–643. [Google Scholar] [CrossRef]
- Threlfall, C.G.; Mata, L.; Mackie, J.A.; Hahs, A.K.; Stork, N.E.; Williams, N.S.G.; Livesley, S.J.; Beggs, J. Increasing biodiversity in urban green spaces through simple vegetation interventions. J. Appl. Ecol. 2017, 54, 1874–1883. [Google Scholar] [CrossRef]
- Rihn, A.L.; Torres, A.; Behe, B.K.; Barton, S. Unwrapping the native plant black box: Consumer perceptions and segments for target market strategies. HortTechnology 2024, 34, 361–371. [Google Scholar] [CrossRef]
- Henry, A.L.; Robinson, R.; Sinnott, K.; Brunson, M.; Ernst, A.; Tarsa, E.; Kettenring, K.M. Got plants? Availability of and challenges to production of native plants for wetland restoration. Res. Ecol. 2024, 32, e14120. [Google Scholar] [CrossRef]
- Wilson, S.B.; Perez, H.; Thetford, M. Propagation, Production, and Landscape Evaluation of Native Wildflowers in West, Central and South Florida; Condensed Progress Report; Florida Wildflower Foundation: Maitland, FL, USA, 2010; Available online: https://www.flawildflowers.org/wp-content/resources/pdfs/Research/Wildflower%20year-end%20progress%20report-Wilson%20et%20al.pdf (accessed on 15 May 2025).
- Lavallee, K.; Soti, P.G.; Rodrigo, H.; Kariyat, R.; Racelis, A. Pre-sowing treatments improve germinability of South Texas native plant seeds. Plants 2021, 10, 2545. [Google Scholar] [CrossRef]
- Smith, A.M.; Wilson, S.B.; Thetford, M.; Nolan, K.L.; Adams, C.R. Performance of nine Florida native wildflower species grown in varying container substrates. Nativ. Plants J. 2014, 15, 75–86. [Google Scholar] [CrossRef]
- Zinnen, J.; Barak, R.S.; Matthews, J.W. Influence of ecological characteristics and phylogeny on native plant species’ commercial availability. Ecol. Appl. 2024, 35, e3070. [Google Scholar] [CrossRef]
- LeBude, A.V.; White, S.A.; Fulcher, A.F.; Frank, S.; Klingeman, W.E., III; Chong, J.; Chappell, M.R.; Windham, A.; Braman, K.; Hale, F.; et al. Assessing the integrated pest management practices of southeastern US ornamental nursery operations. Pest Manag. Sci. 2012, 68, 1278–1288. [Google Scholar] [CrossRef]
- Godts, M. (Green Isle Gardens, Groveland, FL, USA). Personal communication. 2024. [Google Scholar]
- Beaulieu, J.; Belayneh, B.; Lea-Cox, J.D.; Swett, C.L. Improving container nursery crop sustainability: Effects of conservation-driven adaptations in soilless substrate and water use on plant growth and soil-borne disease development. HortScience 2022, 57, 674–683. [Google Scholar] [CrossRef]
- European Union Parliament; European Council. Nature Restoration Law; European Union Parliament/European Council: Brussels, Belgium, 2024. [Google Scholar]
- Fields, J.; Owen, J., Jr.; Lamm, A.; Altland, J.E.; Jackson, B.; Zheng, Y.; Oki, L.; Fontenot, K.; Samtani, J.; Campbell, B. Soilless substrate science: A North American needs assessment to steer soilless substrate research into the future. Acta Hortic. 2021, 1317, 313–318. [Google Scholar] [CrossRef]
- Choi, S.; Xu, L.; Kim, H.J. Influence of physical properties of peat-based potting mixes substituted with parboiled rice hulls on plant growth under two irrigation regimes. Hortic. Environ. Biotechnol. 2019, 60, 895–911. [Google Scholar] [CrossRef]
- Chilosi, G.; Esposito, A.; Castellani, F.; Stanzione, V.; Aleandri, M.; Dell’Unto, D.; Tomassini, A.; Vannini, A.; Altieri, R. Characterization and use of olive mill waste compost as peat surrogate in substrate for cultivation of Photinia potted plants: Assessment of growth performance and in vitro suppressiveness. Waste Biomass Valorization 2018, 9, 919–928. [Google Scholar] [CrossRef]
- Asp, H.; Bergstrand, K.J.; Casperson, S.; Hultberg, M. Anaerobic digestate as peat substitute and fertilizer in pot production of basil. Biol. Agric. Hortic. 2022, 38, 247–257. [Google Scholar] [CrossRef]
- Nocentini, M.; Mastrolonardo, G.; Michelozzi, M.; Cencetti, G.; Lenzi, A.; Panettieri, M.; Knicker, H.; Certini, G. Effects of biochar and compost addition in potting substrates on growth and volatile compounds profile in basil (Ocimum basilicum L.). J. Sci. Food Agric. 2024, 104, 1609–1620. [Google Scholar] [CrossRef]
- Danielson, H.E.; Wilson, S.B.; Schoellhorn, R.K.; Stoffella, P.S. Container and field-evaluation of Gaillardia pulchella production in compost-based media. Comb. Proc.-Int. Plant Propagators Soc. 2004, 54, 637–642. [Google Scholar]
- Wilson, S.B.; Stoffella, P.J. Using compost for container production of ornamental wetland and flatwood species native to Florida. Nativ. Plants J. 2006, 7, 293–300. [Google Scholar] [CrossRef]
- Wilson, S.B.; Mecca, L.K.; Stoffella, P.J.; Graetz, D.A. Using compost for container production of ornamental hammock species native to Florida. Nativ. Plants J. 2005, 5, 186–194. [Google Scholar] [CrossRef]
- Ostos, J.C.; López-Garrido, R.; Murillo, J.M.; López, R. Substitution of peat for municipal solid waste-and sewage sludge-based composts in nursery growing media: Effects on growth and nutrition of the native shrub Pistacia lentiscus L. Bioresour. Technol. 2008, 99, 1793–1800. [Google Scholar] [CrossRef]
- Wilson, S.B.; Mecca, L.K.; Danielson, H.E.; Graetz, D.A.; Stoffella, P.J. Container and field evaluation of three native shrubs grown in compost-based media. Compost Sci. Util. 2006, 14, 178–183. [Google Scholar] [CrossRef]
- Di Lonardo, S.; Cacini, S.; Becucci, L.; Lenzi, A.; Orsenigo, S.; Zubani, L.; Rossi, G.; Zaccheo, P.; Massa, D. Testing new peat-free substrate mixtures for the cultivation of perennial herbaceous species: A case study on Leucanthemum vulgare Lam. Sci. Hortic. 2021, 289, 110472. [Google Scholar] [CrossRef]
- Manrique-Vega, S.M.; Alvarado-Sanabria, O. Compost increases soil fertility and promotes the growth of five tropical species used in urban forestry. Floresta Ambiente 2023, 30, e20230018. [Google Scholar] [CrossRef]
- LaPierre, G.D.J.; Check, C.; Wilson, S.B. Performance of three Florida native grasses grown in varying container substrates. Nativ. Plants J. 2025, 25, 192–199. [Google Scholar] [CrossRef]
- Do, T.C.V.; Scherer, H.W. Compost as growing media component for salt-sensitive plants. Plant Soil Environ. 2013, 59, 214–220. [Google Scholar] [CrossRef]
- Witcher, A.L.; Pickens, J.M.; Blythe, E.K. Container color and compost substrate affect root zone temperature and growth of “Green Giant” arborvitae. Agronomy 2020, 10, 484. [Google Scholar] [CrossRef]
- Stanton, K.M.; Mickelbart, M.V. Growth and foliar nutrition of Spiraea alba Du Roi and Spiraea tomentosa L. in response to root zone pH. Sci. Hortic. 2014, 165, 23–28. [Google Scholar] [CrossRef]
- Florida Association of Native Nurseries (FANN). Native Plant and Service Directory. 2025. Available online: https://www.fann.org/plants/detail/garberia-heterophylla (accessed on 20 March 2025).
- Wunderlin, R.P.; Hansen, B.F.; Franck, A.R.; Essig, F.B. Atlas of Florida Plants. Available online: https://florida.plantatlas.usf.edu (accessed on 20 March 2025).
- Wilde, H.D.; Gandhi, K.J.; Colson, G. State of the science and challenges of breeding landscape plants with ecological function. Hortic. Res. 2015, 2, 14069. [Google Scholar] [CrossRef]
- Carapezza, G.C.; Wilson, S.B.; McMillan, M.; vanSanten, E. Seed germination of garberia (Garberia heterophylla (W. Bartram) Merr. & F. Harper), a pollinator plant with ornamental appeal. Seeds 2025, 4, 23. [Google Scholar] [CrossRef]
- [USEPA] United States Environmental Protection Agency. Test Methods for Evaluating Solid Waste; SW-846 (Methods 3050); United States Environmental Protection Agency: Washington, DC, USA, 1998.
- Bragg, N.C.; Chambers, B.J. Interpretation and advisory applications of compost air-filled porosity (AFP) measurements. Acta Hortic. 1988, 221, 35–44. [Google Scholar] [CrossRef]
- Niedziela, C.E.; Nelson, P.V. A rapid method for determining physical properties of undisturbed substrate. HortScience 1992, 27, 1279–1280. [Google Scholar] [CrossRef]
- Mikell, L.; Wilson, S.B.; Marbel, C.S.; Vendrame, W.; van Santen, E. Sexual and asexual reproduction of Wild lime (Zanthoxylum fagara L. Sarg), a native Florida plant with ornamental and ecological value. J. Environ. Hortic. 2024, 42, 131–139. [Google Scholar] [CrossRef]
- Mylavarapu, R.; Yeager, T. UF/IFAS Extension Nutrient Management Series: Container Media Nutrient Test Interpretation; University of Florida Institute of Food and Agricultural Sciences: Gainesville, FL, USA, 2015; Available online: https://edis.ifas.ufl.edu/publication/SS316 (accessed on 17 May 2025).
- Jackson, K.; Thounaojam, T.; Meetei, T.T. Influence of soil pH on nutrient availability: A review. J. Emerg. Technol. Innov. Res. 2018, 5, 707–713. Available online: http://www.jetir.org/papers/JETIRDZ06090.pdf (accessed on 10 May 2025).
- Niemiera, A.X.; Wright, R.D. Effect of liming rate on nitrification in a pine bark medium. J. Am. Soc. Hortic. Sci. 1986, 111, 713–715. Available online: https://api.semanticscholar.org/CorpusID:101068913 (accessed on 10 May 2025). [CrossRef]
- Zhang, W.; Zwiazek, J.J. Effects of root medium pH on root water transport and apoplastic pH in red-osier dogwood (Cornus sericea) and paper birch (Betula papyrifera) seedlings. J. Plant Biol. 2016, 18, 1001–1007. [Google Scholar] [CrossRef]
- Li, Y.C.; Zhang, M. Effects of urea and nitric acid on water and medium quality and on response of anthurium. HortTechnology 2002, 12, 131–134. [Google Scholar] [CrossRef]
- Nemali, K.; van Iersel, M.W. Light intensity and fertilizer concentration: I. estimating optimal fertilizer concentrations from water-use efficiency of wax begonia. HortScience 2004, 36, 1287–1292. [Google Scholar] [CrossRef]
- Aydinsakir, K.; Dinc, N.; Buyuktas, D.; Karaguzel, U.O.; Ozkan, C.F.; Vuran, F.A. Effects of salinity levels and substrates on yield and flower quality of soilless cultivated rose grown in an unheated greenhouse. Fresenius Environ. Bull. 2018, 27, 1424–1436. [Google Scholar] [CrossRef]
- Coca, L.I.R.; González, M.T.G.; Unday, Z.G.; Hernández, J.J.; Jáuregui, M.M.R.; Cancio, Y.F. Effects of sodium salinity on rice (Oryza sativa L.) cultivation: A review. Sustainability 2023, 15, 1804. [Google Scholar] [CrossRef]
- Galviz, Y.C.; Valerio, R. Leaf morphoanatomical traits of Jacquinia armillaris Jacq. (Theophrastoideae-Primulaceae) in two xeric shrublands from Venezuela. Neotrop. Biodivers. 2021, 7, 364–375. [Google Scholar] [CrossRef]
- Iqbal, N.; Umar, S.; Khan, N.A.; Khan, M.I.R. A new perspective of phytohormones in salinity tolerance: Regulation of proline metabolism. Environ. Exp. Bot. 2014, 100, 34–42. [Google Scholar] [CrossRef]
- Picchioni, G.A.; Ruiz, J.; Goss, R.M.; Mexal, J.G. Nursery crop growth response to municipal biosolids: Species salt and xeric adaptation a factor? Compost Sci. Util. 2014, 22, 138–152. [Google Scholar] [CrossRef]
- Bustamante, M.A.; Gomis, M.P.; Pérez-Murcia, M.D.; Gangi, D.; Ceglie, F.G.; Paredes, C.; Pérez-Espinosa, A.; Bernal, M.P.; Moral, R. Use of livestock waste composts as nursery growing media: Effect of a washing pre-treatment. Sci. Hortic. 2021, 281, 109954. [Google Scholar] [CrossRef]
- Jayasinghe, G.Y.; Arachchi-Liyana, I.D.; Tokashiki, Y. Evaluation of containerized substrates developed from cattle manure compost and synthetic aggregates for ornamental plant production as a peat alternative. Resour. Conserv. Recycl. 2010, 54, 1412–1418. [Google Scholar] [CrossRef]
- Bayabil, H.K.; Tilahun, F.T.; Li, Y.; Campoverde, E.V. Moisture Retention and Chemical Properties of Nursery Potting Substrates; University of Florida Institute of Food and Agricultural Sciences: Gainesville, FL, USA, 2024; AE562; Available online: https://edis.ifas.ufl.edu/publication/AE562 (accessed on 17 May 2025).
- Gondek, M.; Weindorf, D.C.; Thiel, C.; Kleinheinz, G. Soluble salts in compost and their effects on soil and plants: A review. Compost Sci. Util. 2020, 28, 59–75. [Google Scholar] [CrossRef]
- Yeager, T.H. Southern Nurserymen’s Association. Best Management Practices Guide for Producing Container Grown Plants; Southern Nurserymen’s Association: Marietta, GA, USA, 1997. [Google Scholar]
- Carmona, E.; Moreno, M.T.; Avilés, M.; Ordovás, J. Composting of wine industry wastes and their use as a substrate for growing soilless ornamental plants. Span. J. Agric. Res. 2012, 10, 482–491. [Google Scholar] [CrossRef]
- Bunt, A.C. Media and Mixes for Container Grown Plants; Unwin Hyman Ltd.: London, UK, 1988. [Google Scholar]
- Kaderabek, L.E.; Jackson, B.E.; Fonteno, W.C. Changes in the physical, chemical, and hydrological properties of pine bark over twelve months of aging. Comb. Proc.-Int. Plant Propagators Soc. 2016, 66, 313–317. [Google Scholar]
- Altland, J.E.; Krause, C. Change in physical properties of pine bark and switchgrass substrates over time. J. Environ. Hortic. 2012, 30, 113–117. [Google Scholar] [CrossRef]
- Yao, J.J.; Barés, J.; Dupuy, L.X.; Kold, E. Physical obstacles in the substrate cause maize root growth trajectories to switch from vertical to oblique. J. Exp. Bot. 2024, 76, 546–561. [Google Scholar] [CrossRef]
- Fu, Y.F.; Yang, X.Y.; Zhang, Z.W.; Yuan, S. Synergistic effects of nitrogen metabolites on auxin regulating plant growth and development. Front. Plant Sci. 2022, 13, 1098787. [Google Scholar] [CrossRef]
- Zhang, Y.H.; Liu, B.H.; Kong, F.J.; Chen, L.Y. Nutrient-mediated modulation of flowering time. Front. Plant Sci. 2023, 14, 1101611. [Google Scholar] [CrossRef] [PubMed]
- Fields, J.S.; Owen, J.S.; Altland, J.E.; van Iersel, M.W.; Jackson, B.E. Soilless substrate can be hydrology can be engineered to influence plant water status for an ornamental containerized crop grown within optimal water potentials. J. Am. Soc. Hortic. Sci. 2018, 143, 268–281. [Google Scholar] [CrossRef]
- Worrall, R.J.; Lamont, G.P.; O’Connell, M.A.; Nicholls, P.J. The growth response of container-grown woody ornamentals to controlled-release fertilizers. Sci. Hortic. 1987, 32, 275–286. [Google Scholar] [CrossRef]
- Kantz, R.S.; Hoehn, T.S.; Haddad, K.; Rogers, T.; Atkeson, T.; Estevez, E. Natural systems. In Water Resources Atlas of Florida; Fernald, E.A., Purdum, E.D., Eds.; Florida State University: Tallahassee, FL, USA, 1998; pp. 82–113. [Google Scholar]
- Baruzzi, C.; Hong, J.; Zamora, C.; Stein, C.; Crandall, R.M. The pyrogenic bunchgrass Aristida beyrichiana is negatively affected by soil biota when planted outside of its home soil. Plant Soil 2022, 479, 621–630. [Google Scholar] [CrossRef]
- Wang, X.P.; Fang, J.Y.; Zhu, B. Forest biomass and root-shoot allocation in northeast China. For. Ecol. Manag. 2008, 255, 4007–4020. [Google Scholar] [CrossRef]
- Reich, P.B.; Luo, Y.; Bradford, J.B.; Poorter, H.; Perry, C.H.; Oleksyn, J. Temperature drives global patterns in forest biomass distribution in leaves, stems, and roots. Proc. Natl. Acad. Sci. USA 2014, 111, 1721–13726. [Google Scholar] [CrossRef]
- Qi, Y.; Wei, W.; Chen, C.; Chen, L. Plant root-shoot biomass allocation over diverse biomes: A global synthesis. Glob. Ecol. Conserv. 2019, 18, e00606. [Google Scholar] [CrossRef]
- Wei, X.; Knuth, M.; Khachatryan, H. The role of consumers’ knowledge of native and pollinator-friendly plants and their prioritization of plant characteristics in purchase decisions. HortScience 2024, 59, 941–948. [Google Scholar] [CrossRef]
- Garbez, M.; Symoneaux, R.; Belin, É.; Caraglio, Y.; Chéné, Y.; Donès, N.; Durand, J.B.; Hunault, G.; Relion, D.; Sigogne, M.; et al. Ornamental plants architectural characteristics in relation to visual sensory attributes: A new approach on the rose bush for objective evaluation of the visual quality. Eur. J. Hortic. Sci. 2018, 83, 187–201. [Google Scholar] [CrossRef]
- Wilson, S.B.; Stoffella, P.J.; Graetz, D.A. Compost amended media and irrigation system influence containerized perennial Salvia. J. Am. Soc. Hortic. Sci. 2003, 128, 260–268. [Google Scholar] [CrossRef]
- Massa, D.; Prisa, D.; Lazzereschi, S.; Cacini, S.; Burchi, G. Heterogenous response of two bedding plants to peat substitution by two green composts. Hort. Sci. 2018, 45, 164–172. [Google Scholar] [CrossRef]
- Suzigan, N.M.; Cardoso, G.D.; Barbosa, N.A.; da Costa, G.C.P.; Gomes, G.; Moura, S.D.; de Oliveira, M.E.R.; Campos, L.F.C.; de Melo, H.C.; Gonçalves, J.D. Organic compost in promoting growth and photosynthetic performance of Pereskia aculeata Mill. (Cactaceae) saplings. Cienc. Rural 2025, 55, e20240038. [Google Scholar] [CrossRef]
- Bignami, C.; Melegari, F.; Zaccardelli, M.; Pane, C.; Ronga, D. Composted solid digestate and vineyard winter prunings partially replace peat in growing substrates for micropropagated highbush blueberry in the nursery. Agronomy 2022, 12, 337. [Google Scholar] [CrossRef]
- Cabrera, M.; Wilson, S.B.; Rostan, V.; Wilson, P.C. Influence of thiamethoxam application method, timing and rate on contamination of floral resources in lantana. Comb. Proc. Int. Plant Propagators Soc. 2024, 74, 375–386. Available online: https://ipps.org/uploads/docs/6d_sr_cabrera.pdf (accessed on 12 March 2025).
- Jo, S.; Chae, Y.; Lee, S.; Koo, Y.; Kim, H.; Kang, B.T.; Yun, T. Gastrointestinal complications following ingestion of potting soil in a dog. Vet. Sci. 2025, 12, 355. [Google Scholar] [CrossRef] [PubMed]
- Murphy, A.M.; Gilliam, C.H.; Fain, G.B.; Torbert, H.A.; Gallagher, T.V.; Sibley, J.L.; Marble, S.C.; Witcher, A.L. Extending pine bark supplies with whole tree and clean chip residual substrates. J. Environ. Hortic. 2010, 28, 217–223. [Google Scholar] [CrossRef]
- Lubell, J.D. Evaluating landscape performance of six native shrubs as alternatives to invasive exotics. HortTechnology 2013, 23, 119–125. [Google Scholar] [CrossRef]


| Substrate | Cost (USD 1) | Peat | Pine | Perlite | Vermiculite | Saw Dust | Coarse Sand | Wood Chips | Compost |
|---|---|---|---|---|---|---|---|---|---|
| Atlas 3000 | USD 10.00 | 40 | 50 | - | - | - | 10 | - | - |
| COMANDscape | USD 11.50 | - | - | - | - | - | - | - | 100 2 |
| Native mix | USD 3.00 | 6 | 47 | - | - | 10 | 10 | 27 | - |
| ProMix BX | USD 16.76 | 80 | - | 10 | 10 | - | - | - | - |
| 1:1 compost mix 3 | USD 7.25 | 20 | 25 | - | - | - | 5 | - | 50 |
| Substrate | pH | EC (dS/m) | Organic Matter (%) | NO3N (mg/L) | P (mg/L) | K (mg/L) | Ca (mg/L) | Mg (mg/L) |
|---|---|---|---|---|---|---|---|---|
| Atlas 3000 | 7.6 b | 2.1 c | 40.5 c | 2.4 c | 10.0 d | 244.2 c | 135.1 c | 32.9 c |
| COMANDscape | 6.6 d | 5.1 a | 73.2 b | 321.1 a | 36.3 b | 552.7 a | 285.6 a | 110.6 a |
| Native mix | 7.8 a | 0.4 e | 82.6 a | 0.1 c | 4.0 e | 49.8 e | 63.8 e | 5.8 d |
| ProMix BX | 5.3 e | 1.4 d | 73.4 b | 0.1 c | 47.3 a | 157.8 d | 88.4 d | 104.9 a |
| 1:1 compost mix 1 | 7.2 c | 3.9 b | 61.9 b | 179.8 b | 24.5 c | 432.0 b | 243.1 b | 76.1 b |
| Substrate | Water-Holding Capacity (% v/v) | Air-Filled Porosity (% v/v) | Total Porosity (% v/v) | Bulk Density (g∙cm−3) | Particle Density (g∙cm−3) |
|---|---|---|---|---|---|
| Atlas 3000 | 54.46 b | 10.86 ab | 65.31 ab | 0.42 a | 1.23 a |
| COMANDscape | 47.54 c | 16.40 a | 63.94 b | 0.16 d | 0.66 bc |
| Native mix | 46.32 c | 11.37 ab | 57.68 b | 0.34 b | 0.81 b |
| ProMix BX | 64.15 a | 7.83 b | 71.98 a | 0.24 c | 0.56 c |
| 1:1 compost mix 1 | 51.76 bc | 8.19 b | 59.95 b | 0.33 b | 0.81 b |
| Month | Atlas 3000 | COMAND-Scape | Native Mix | ProMix BX | 1:1 Mix 1 |
|---|---|---|---|---|---|
| 3 Months | |||||
| Height (cm) | 28.97 b | 31.56 b | 30.85 b | 33.84 a | 26.99 b |
| Width 2 (cm) | 17.39 b | 19.04 b | 17.74 b | 24.35 a | 17.42 b |
| SPAD 3 | 48.50 b | 58.38 a | 56.12 a | 58.87 a | 52.79 b |
| 6 Months | |||||
| Height (cm) | 31.82 a | 33.80 a | 30.71 a | 35.95 a | 30.64 a |
| Width (cm) | 22.23 a | 20.69 a | 21.66 a | 25.62 a | 18.98 a |
| SPAD | 49.89 b | 56.12 a | 50.43 b | 63.45 a | 54.14 b |
| Shoot DM (g) | 9.56 b | 13.84 b | 10.97 b | 22.66 a | 10.31 b |
| Root DM (g) | 1.89 b | 2.90 b | 2.51 b | 6.00 a | 2.29 b |
| Shoot: root ratio | 5.37 a | 5.02 ab | 5.02 ab | 3.97 b | 5.13 ab |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Carapezza, G.; Wilson, S.B.; McMillan, M.; Thetford, M. Effects of Container Substrate Composition on the Growth and Performance of Garberia heterophylla (W. Bartram) Merr. & F. Harper: A Native Xeric Species. Horticulturae 2025, 11, 982. https://doi.org/10.3390/horticulturae11080982
Carapezza G, Wilson SB, McMillan M, Thetford M. Effects of Container Substrate Composition on the Growth and Performance of Garberia heterophylla (W. Bartram) Merr. & F. Harper: A Native Xeric Species. Horticulturae. 2025; 11(8):982. https://doi.org/10.3390/horticulturae11080982
Chicago/Turabian StyleCarapezza, Grace, Sandra B. Wilson, Mica McMillan, and Mack Thetford. 2025. "Effects of Container Substrate Composition on the Growth and Performance of Garberia heterophylla (W. Bartram) Merr. & F. Harper: A Native Xeric Species" Horticulturae 11, no. 8: 982. https://doi.org/10.3390/horticulturae11080982
APA StyleCarapezza, G., Wilson, S. B., McMillan, M., & Thetford, M. (2025). Effects of Container Substrate Composition on the Growth and Performance of Garberia heterophylla (W. Bartram) Merr. & F. Harper: A Native Xeric Species. Horticulturae, 11(8), 982. https://doi.org/10.3390/horticulturae11080982








