Characteristics and Transcriptome Analysis of Anther Abortion in Male Sterile Celery (Apium graveolens L.)
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material
2.2. Phenotypic Characters of Floral Organs and Anther Histology of Celery
2.3. Total RNA Library Construction, Transcriptome Sequencing, and Assembly
2.4. Differentially Expressed Genes (DEGs) Analysis
2.5. Identification and Analysis of AgGSL Gene Family Members
2.6. qRT-PCR Validation of DEGs
3. Results
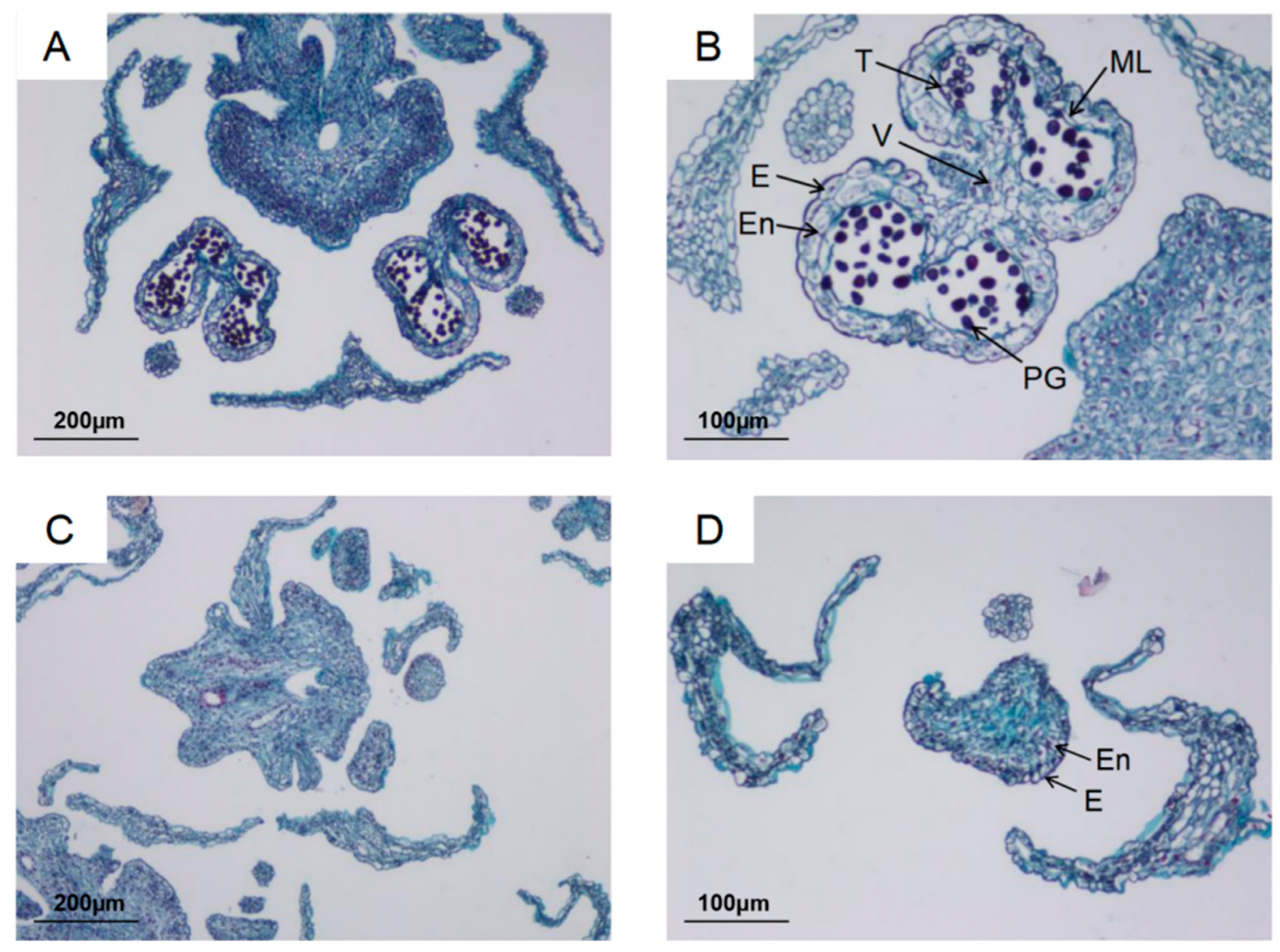
3.1. Analysis of Differences in Phenotypic Characters and Histological Analysis Between Fertile and Sterile Flowers
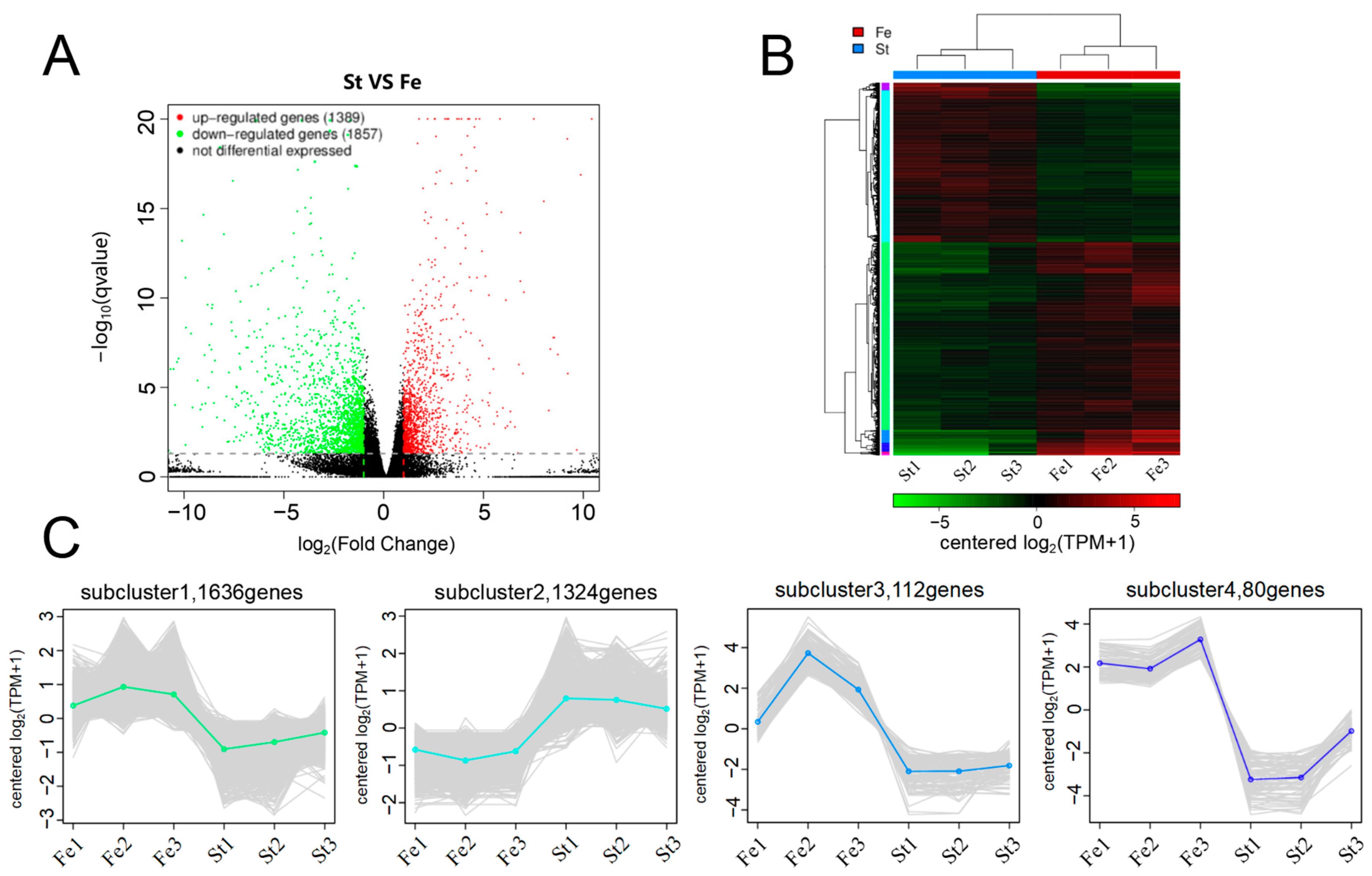
3.2. Sequencing Quality and Gene Expression Analysis
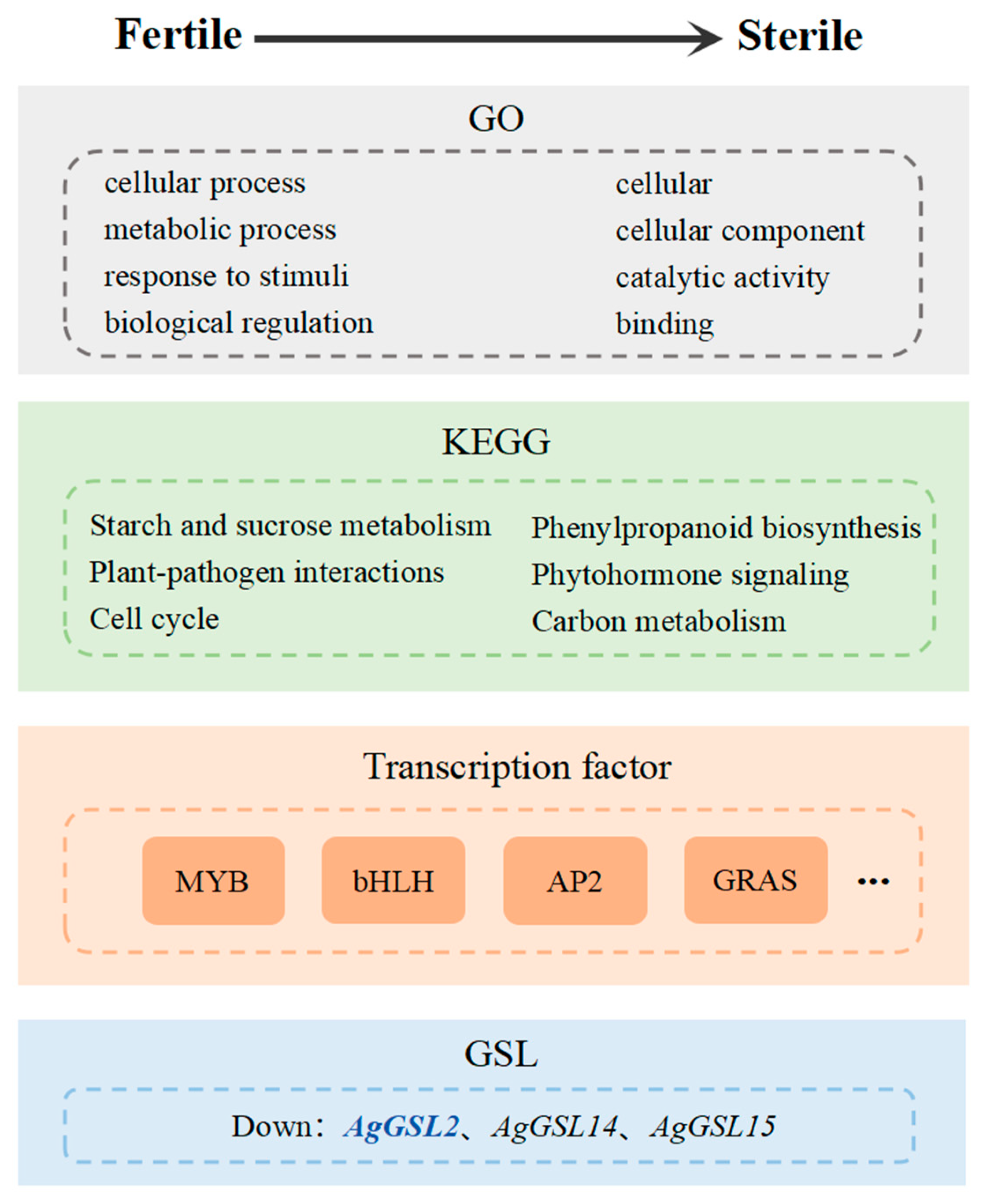
3.3. GO Functional Annotation and KEGG Pathway Enrichment Analysis of DEGs
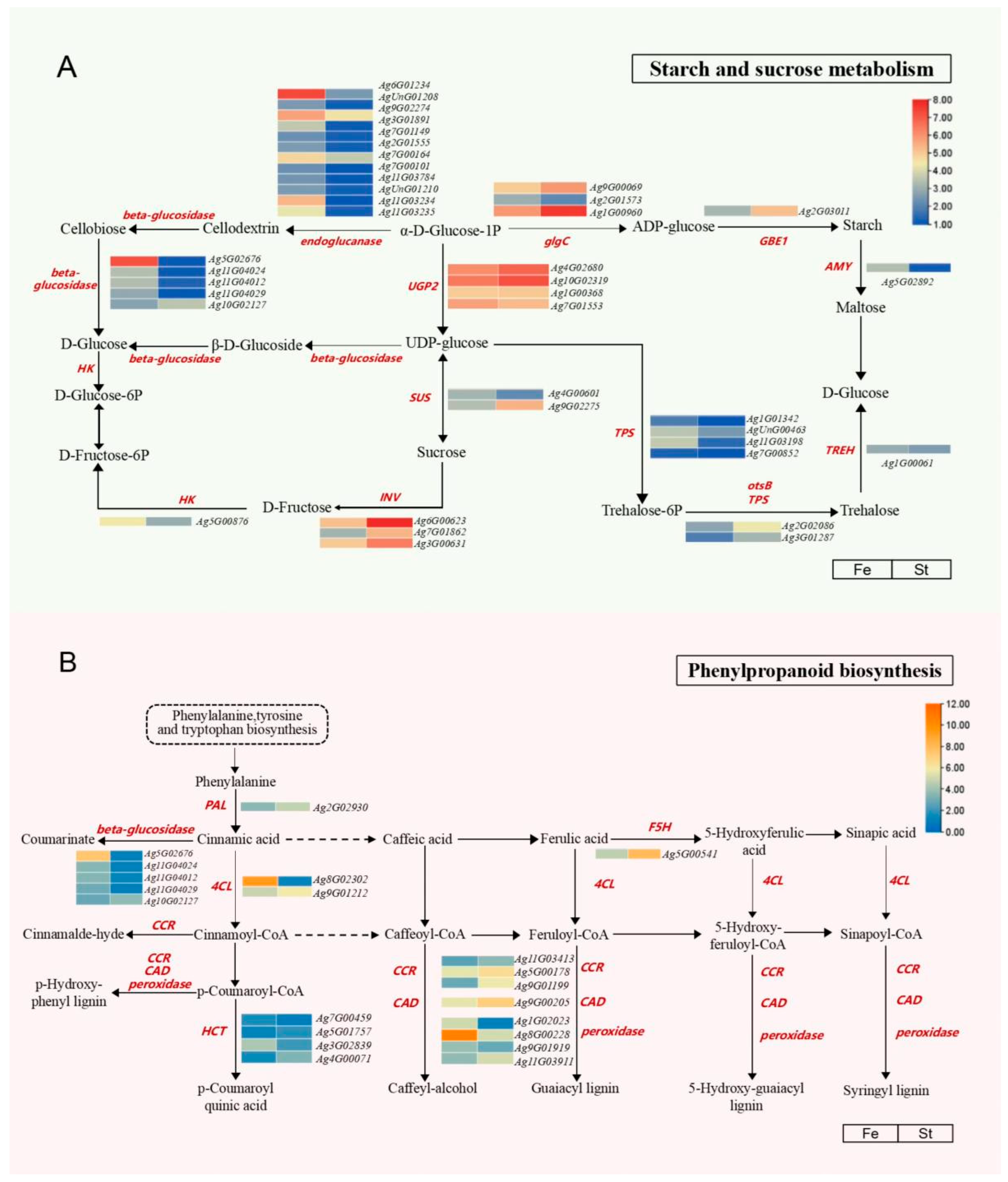
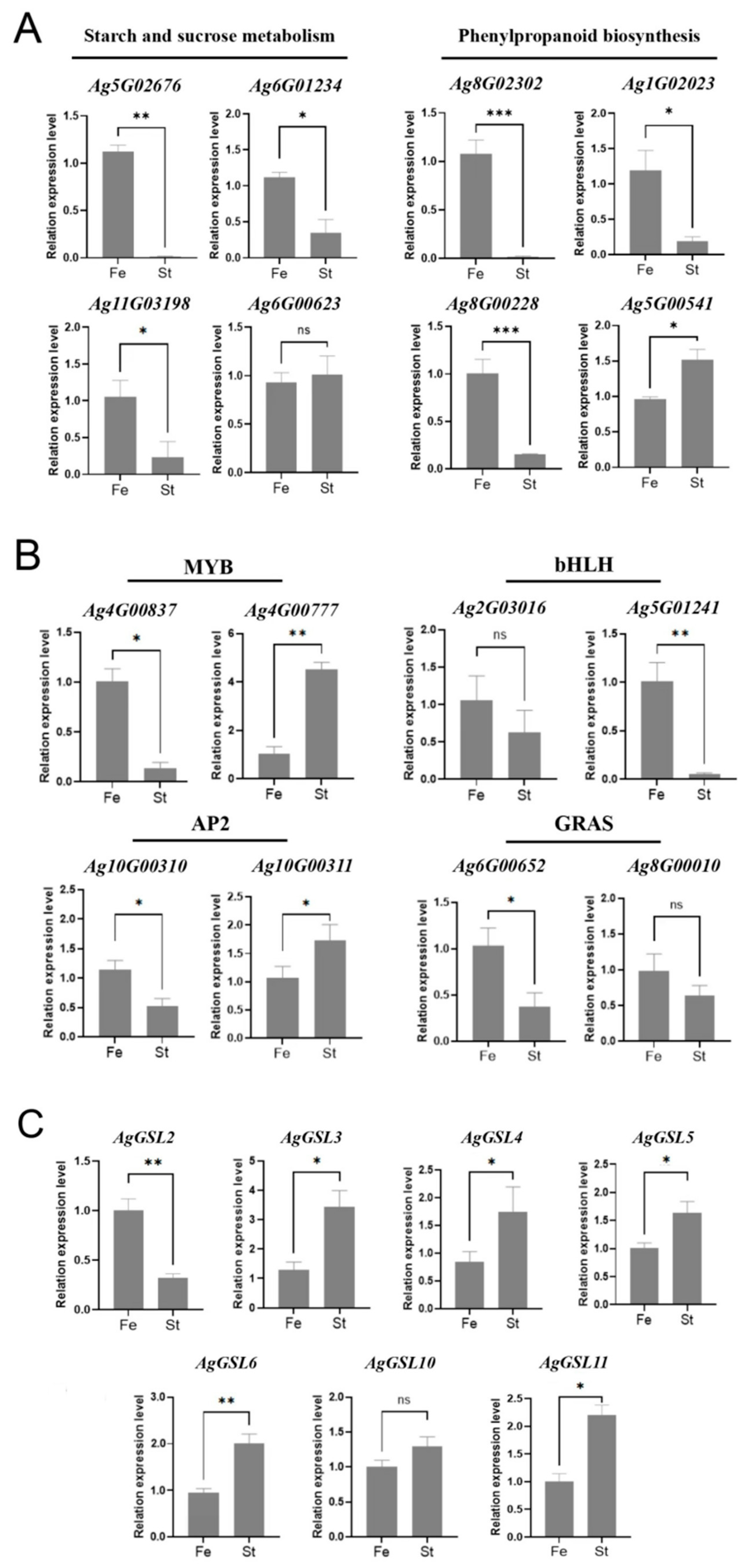
3.4. Analysis of Starch and Sucrose Metabolism and Phenylpropanoid Biosynthesis Pathways and Gene Expression
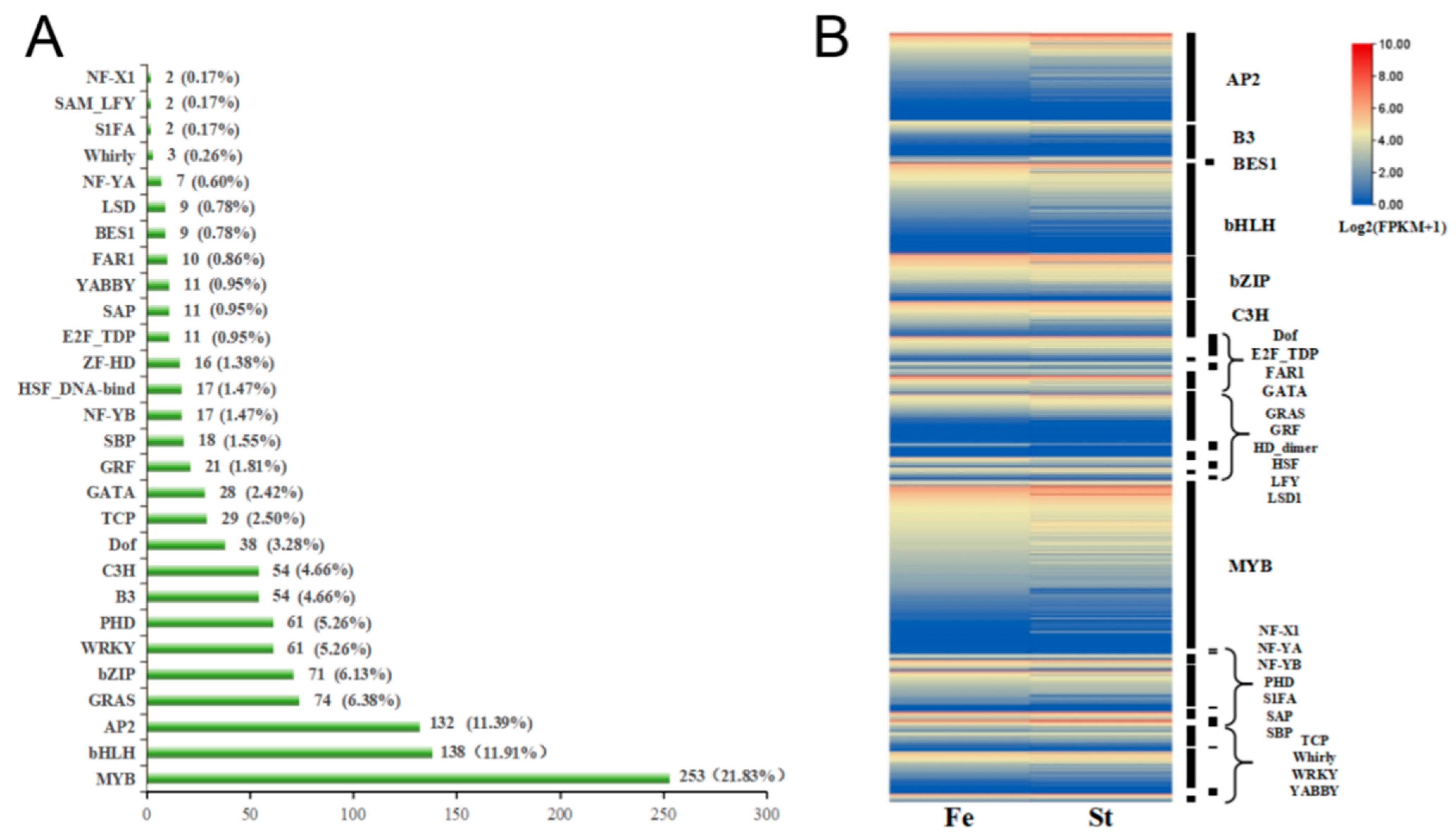
3.5. Transcription Factor Analysis of DEGs in Celery Anthers
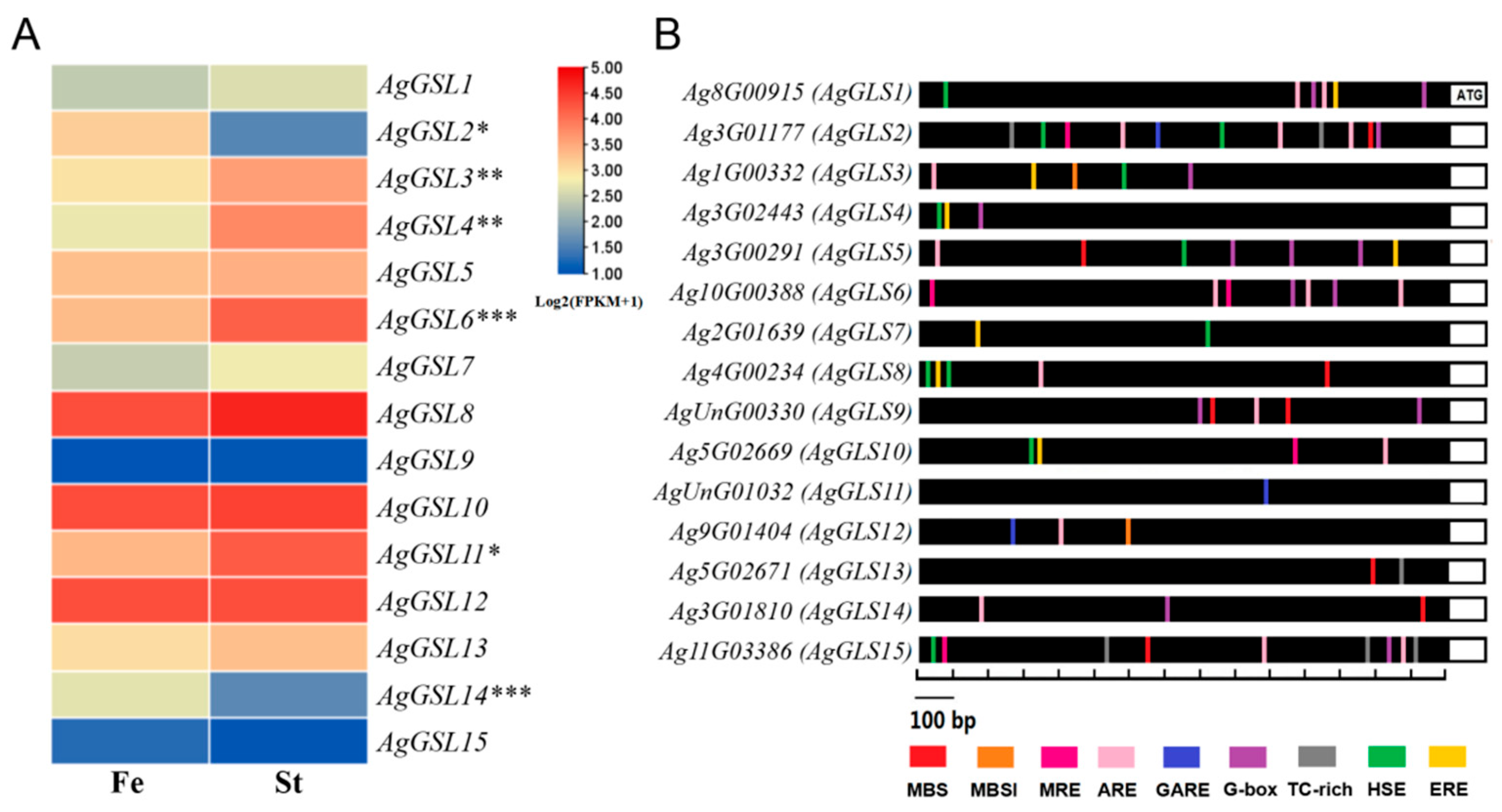
3.6. Identification and Analysis of the AgGSL Family in Celery
3.7. qRT-PCR Analysis of DEGs
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Smith, A.R.; Zhao, D. Sterility caused by floral organ degeneration and abiotic stresses in Arabidopsis and cereal grains. Front. Plant Sci. 2016, 7, 1503. [Google Scholar] [CrossRef]
- Tester, M.; Langridge, P. Breeding technologies to increase crop production in a changing world. Science 2010, 327, 818–822. [Google Scholar] [CrossRef]
- Cheng, Z.; Song, W.; Zhang, X. Genic male and female sterility in vegetable crops. Hortic. Res. 2022, 10, uhac232. [Google Scholar] [CrossRef]
- Zhou, Q.; Yuan, R.; Zhang, W.; Gu, J.; Liu, L.; Zhang, H.; Wang, Z.; Yang, J. Grain yield, nitrogen use efficiency and physiological performance of indica/japonica hybrid rice in response to various nitrogen rates. J. Integr. Agric. 2023, 22, 63–79. [Google Scholar] [CrossRef]
- Liu, J.; Wang, S.; Wang, H.; Luo, B.; Cai, Y.; Li, X.; Zhang, Y.; Wang, X. Rapid generation of tomato male-sterile lines with a marker use for hybrid seed production by CRISPR/Cas9 system. Mol. Breed. 2021, 41, 25. [Google Scholar] [CrossRef]
- Xue, M.; Li, J.; Liao, R.; Xu, J.; Zhou, M.; Yao, R.; Liu, Z.; Feng, H.; Huang, S. Fine mapping and expression characteristics analysis of male-sterile gene BrRNR1 in Chinese cabbage (Brassica rapa L. ssp. pekinensis). BMC Plant Biol. 2025, 25, 49. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; An, D.; Cao, Y.; Yu, H.; Zhu, Y.; Mei, Y.; Zhang, B.; Wang, L. Development and application of KASP markers associated with Restorer-of-fertility gene in Capsicum annuum L. Physiol. Mol. Biol. Plants 2021, 27, 2757–2765. [Google Scholar] [CrossRef]
- Duan, N.; Bai, J.; Xie, K.; Wang, J.; Wang, X. De novo and comparative transcriptome analysis of genetic male sterile and fertile lines in radish (Raphanus sativus). J. Hortic. Sci. Biotech. 2020, 95, 32–43. [Google Scholar] [CrossRef]
- He, Y.; Wang, J.; Hu, J.; Zheng, J.; Guo, Z.; Yu, Q.; Shi, Q.; Zhang, Y. Knockdown of SlEMS1 causes male sterility in tomato. Hortic. Plant J. 2025, 11, 939–942. [Google Scholar] [CrossRef]
- Dong, J.; Hu, F.; Guan, W.; Yuan, F.; Lai, Z.; Zhong, J.; Liu, J.; Wu, Z.; Cheng, J.; Hu, K. A 163-bp insertion in the Capana10g000198 encoding a MYB transcription factor causes male sterility in pepper (Capsicum annuum L.). Plant J. 2023, 113, 521–535. [Google Scholar] [CrossRef]
- Li, C.; Zhao, Z.; Liu, Y.; Liang, B.; Guan, S.; Lan, H.; Wang, J.; Lu, Y.; Cao, M. Comparative transcriptome analysis of isonuclear-alloplasmic lines unmask key transcription factor genes and metabolic pathways involved in sterility of maize CMS-C. PeerJ 2017, 5, e3408. [Google Scholar] [CrossRef]
- Shan, S.; Tang, P.; Wang, R.; Ren, Y.; Wu, B.; Yan, N.; Zhang, G.; Niu, N.; Song, Y. The characteristic analysis of TaTDF1 reveals its function related to male sterility in wheat (Triticum aestivum L.). BMC Plant Biol. 2024, 24, 746. [Google Scholar] [CrossRef]
- Bian, S.; Tian, T.; Ding, Y.; Yan, N.; Wang, C.; Fang, N.; Liu, Y.; Zhang, Z.; Zhang, H. bHLH Transcription factor NtMYC2a regulates carbohydrate metabolism during the pollen development of tobacco (Nicotiana tabacum L. cv. TN90). Plants 2022, 11, 17. [Google Scholar] [CrossRef]
- Krogan, N.T.; Hogan, K.; Long, J.A. APETALA2 negatively regulates multiple floral organ identity genes in Arabidopsis by recruiting the co-repressor TOPLESS and the histone deacetylase HDA19. Development 2012, 139, 4180–4190. [Google Scholar] [CrossRef] [PubMed]
- Ge, S.; Ding, F.; Daniel, B.; Wu, C.; Ran, M.; Ma, C.; Xue, Y.; Zhao, D.; Liu, Y.; Zhu, Z.; et al. Carbohydrate metabolism and cytology of S-type cytoplasmic male sterility in wheat. Front. Plant Sci. 2023, 14, 1255670. [Google Scholar] [CrossRef] [PubMed]
- Duan, Y.; Yang, L.; Han, R.; Gu, L.; Guo, J.; Sun, H.; Gong, H. Tomato sucrose synthase SUS3 is involved in flower and seed development. Plant Physiol. Biochem. 2025, 222, 109715. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Niu, F.; Yuan, S.; Feng, S.; Li, Y.; Lu, F.; Zhang, T.; Bai, J.; Zhao, C.; Zhang, L. Comparative transcriptome analysis reveals key insights into fertility conversion in the thermo-sensitive cytoplasmic male sterile wheat. Int. J. Mol. Sci. 2022, 23, 14354. [Google Scholar] [CrossRef]
- Guseman, J.M.; Lee, J.S.; Bogenschutz, N.L.; Peterson, K.M.; Virata, R.E.; Xie, B.; Kanaoka, M.M.; Hong, Z.; Torii, K.U. Dysregulation of cell-to-cell connectivity and stomatal patterning by loss-of-function mutation in Arabidopsis chorus (glucan synthase-like 8). Development 2010, 137, 1731–1741. [Google Scholar] [CrossRef]
- De Storme, N.; De Schrijver, J.; Van Criekinge, W.; Wewer, V.; Dörmann, P.; Geelen, D. GLUCAN SYNTHASE-LIKE8 and STEROL METHYLTRANSFERASE2 are required for ploidy consistency of the sexual reproduction system in Arabidopsis. Plant Cell 2013, 25, 387–403. [Google Scholar] [CrossRef] [PubMed]
- Töller, A.; Brownfield, L.; Neu, C.; Twell, D.; Schulze-Lefert, P. Dual function of Arabidopsis glucan synthase-like genes GSL8 and GSL10 in male gametophyte development and plant growth. Plant J. 2008, 54, 911–923. [Google Scholar] [CrossRef]
- Shi, X.; Sun, X.; Zhang, Z.; Feng, D.; Zhang, Q.; Han, L.; Wu, J.; Lu, T. GLUCAN SYNTHASE-LIKE 5 (GSL5) plays an essential role in male fertility by regulating callose metabolism during microsporogenesis in rice. Plant Cell Physiol. 2015, 56, 497–509. [Google Scholar] [CrossRef]
- Liu, Z.; Liu, Y.; Sun, Y.; Yang, A.; Li, F. Comparative transcriptome analysis reveals the potential mechanism of abortion in tobacco sua-cytoplasmic male sterility. Int. J. Mol. Sci. 2020, 21, 2445. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Xiong, X.; Wang, Q.; Zhu, L.; Wang, L.; He, Y.; Zeng, H. Integrated analysis of small RNA, transcriptome, and degradome sequencing reveals the MiR156, MiR5488 and MiR399 are involved in the regulation of male sterility in PTGMS rice. Int. J. Mol. Sci. 2021, 22, 2260. [Google Scholar] [CrossRef]
- Nie, Z.; Chen, J.; Song, Y.; Fu, H.; Wang, H.; Niu, Q.; Zhu, W. Comparative transcriptome analysis of the anthers from the cytoplasmic male-sterile pepper line HZ1A and its maintainer line HZ1B. Horticulturae 2021, 7, 580. [Google Scholar] [CrossRef]
- Wang, X.; Luo, Q.; Li, T.; Meng, P.; Pu, Y.; Liu, J.; Zhang, J.; Liu, H.; Tan, G.; Xiong, A. Origin, evolution, breeding, and omics of Apiaceae: A family of vegetables and medicinal plants. Hortic. Res. 2022, 9, uhac076. [Google Scholar] [CrossRef]
- Quiros, C.F.; Rugama, A.; Dong, Y.Y.; Orton, T.J. Cytological and genetic-studies of a male sterile celery. Euphytica 1986, 35, 867–875. [Google Scholar] [CrossRef]
- Jin, L.; Gao, G.; Zhu, X.; Lu, Z. Key points of hybrid seed production technology of male sterile dual-purpose lines in celery. Pei Fang Yuan I 2011, 8, 62. (In Chinese) [Google Scholar]
- Cheng, Q.; Wang, P.; Li, T.; Liu, J.; Zhang, Y.; Wang, Y.; Sun, L.; Shen, H. Complete mitochondrial genome sequence and identification of a candidate gene responsible for cytoplasmic male sterility in celery (Apium graveolens L.). Int. J. Mol. Sci. 2021, 22, 8584. [Google Scholar] [CrossRef]
- Tan, G.; Li, M.; Luo, Q.; Zhao, Q.; Zhong, X.; Meng, P.; Xiong, A. Creation of a male sterility line and identification of its candidate mitochondrial male sterility gene in celery. J. Plant Genet. Resour. 2022, 23, 1807–1815. (In Chinese) [Google Scholar]
- Li, M.; Tan, S.; Tan, G.; Luo, Y.; Sun, B.; Zhang, Y.; Chen, Q.; Wang, Y.; Zhang, F.; Zhang, Y.; et al. Transcriptome analysis reveals important transcription factor families and reproductive biological processes of flower development in celery (Apium graveolens L.). Agronomy 2020, 10, 653. [Google Scholar] [CrossRef]
- Li, H. Celery variety-Jinnan Shiqin. Sci. Technol. Tianjin Agric. For. 2004, 4, 21. (In Chinese) [Google Scholar]
- Jia, X.; Wang, G.; Xiong, F.; Yu, X.; Xu, Z.; Wang, F.; Xiong, A. De novo assembly, transcriptome characterization, lignin accumulation, and anatomic characteristics: Novel insights into lignin biosynthesis during celery leaf development. Sci. Rep. 2015, 5, 8259. [Google Scholar] [CrossRef]
- Love, M.I.; Huber, W.; Anders, S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014, 15, 550. [Google Scholar] [CrossRef] [PubMed]
- Jin, J.; He, K.; Tang, X.; Li, Z.; Lv, L.; Zhao, Y.; Luo, J.; Gao, G. An Arabidopsis transcriptional regulatory map reveals distinct functional and evolutionary features of novel transcription factors. Mol. Boil. Evol. 2015, 32, 1767–1773. [Google Scholar] [CrossRef]
- Li, M.; Feng, K.; Hou, X.; Jiang, Q.; Xu, Z.; Wang, G.; Liu, J.; Wang, F.; Xiong, A. The genome sequence of celery (Apium graveolens L.), an important leaf vegetable crop rich in apigenin in the Apiaceae family. Hortic Res. 2020, 7, 9. [Google Scholar] [CrossRef]
- Pei, Q.; Li, N.; Bai, Y.; Wu, T.; Yang, Q.; Yu, T.; Wang, Z.; Liu, Z.; Li, Q.; Lin, H.; et al. Comparative analysis of the TCP gene family in celery, coriander and carrot (family Apiaceae). Veg. Res. 2021, 1, 1–12. [Google Scholar] [CrossRef]
- Thiele, K.; Wanner, G.; Kindzierski, V.; Jürgens, G.; Mayer, U.; Pachl, F.; Assaad, F.F. The timely deposition of callose is essential for cytokinesis in Arabidopsis. Plant J. 2009, 58, 13–26. [Google Scholar] [CrossRef]
- Pu, Y.; Hou, L.; Guo, Y.; Ullah, I.; Yang, Y.; Yue, Y. Genome-wide analysis of the callose enzyme families of fertile and sterile flower buds of the Chinese cabbage (Brassica rapa L. ssp. pekinensis). FEBS Open Bio. 2019, 9, 1432–1449. [Google Scholar] [CrossRef]
- Sun, Y.; Fu, M.; Ang, Y.; Zhu, L.; Wei, L.; He, Y.; Zeng, H. Combined analysis of transcriptome and metabolome reveals that sugar, lipid, and phenylpropane metabolism are essential for male fertility in temperature-induced male sterile rice. Front. Plant Sci. 2022, 13, 945105. [Google Scholar] [CrossRef]
- Li, J.; Zhang, J.; Li, H.; Niu, H.; Xu, Q.; Jiao, Z.; An, J.; Jiang, Y.; Li, Q.; Niu, J. The major factors causing the microspore abortion of genic male sterile mutant NWMS1 in wheat (Triticum aestivum L.). Int. J. Mol. Sci. 2019, 20, 6252. [Google Scholar] [CrossRef] [PubMed]
- Mo, Z.; Ke, Y.; Huang, Y.; Duan, L.; Wang, P.; Luo, W.; Que, Y.; Pi, K.; Zeng, S.; Liu, R. Integrated transcriptome and proteome analysis provides insights into the mechanism of cytoplasmic male sterility (CMS) in tobacco (Nicotiana tabacum L.). J. Proteom. 2023, 275, 104825. [Google Scholar] [CrossRef] [PubMed]
- Dong, N.; Lin, H. Contribution of phenylpropanoid metabolism to plant development and plant-environment interactions. J. Integr. Plant Biol. 2021, 63, 180–209. [Google Scholar] [CrossRef]
- Yang, X.; Ye, J.; Zhang, L.; Song, X. Blocked synthesis of sporopollenin and jasmonic acid leads to pollen wall defects and anther indehiscence in genic male sterile wheat line 4110S at high temperatures. Funct. Integr. Genom. 2020, 20, 383–396. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Wang, J.; Liu, Z.; Yang, X.; Chen, X.; Zhang, L.; Song, X. The R2R3 MYB gene TaMYB305 positively regulates anther and pollen development in thermo-sensitive male-sterility wheat with Aegilops kotschyi cytoplasm. Planta 2024, 259, 64. [Google Scholar] [CrossRef]
- Ortolan, F.; Trenz, T.S.; Delaix, C.L.; Lazzarotto, F.; Margis-Pinheiro, M. bHLH-regulated routes in anther development in rice and Arabidopsis. Genet. Mol. Biol. 2024, 46, e20230171. [Google Scholar] [CrossRef]
- Zhao, L.; Xu, S.; Chai, T.; Wang, T. OsAP2-1, an AP2-like gene from Oryza sativa, is required for flower development and male fertility. Sex. Plant Reprod. 2006, 19, 197–206. [Google Scholar] [CrossRef]
- Zentella, R.; Zhang, Z.; Park, M.; Thomas, S.G.; Endo, A.; Murase, K.; Fleet, C.M.; Jikumaru, Y.; Nambara, E.; Kamiya, Y.; et al. Global analysis of della direct targets in early gibberellin signaling in Arabidopsis. Plant Cell 2007, 19, 3037–3057. [Google Scholar] [CrossRef]
- Virág, E.; Nagy, Á.; Tóth, B.B.; Kutasy, B.; Pallos, J.P.; Szigeti, Z.M.; Máthé, C.; Kardos, G.; Hegedűs, G. Master regulatory transcription factors in β-Aminobutyric acid-induced resistance (BABA-IR): A perspective on phytohormone biosynthesis and signaling in Arabidopsis thaliana and Hordeum vulgare. Int. J. Mol. Sci. 2024, 25, 9179. [Google Scholar] [CrossRef]
- Hong, Z.; Delauney, A.J.; Verma, D.P. A cell plate-specific callose synthase and its interaction with phragmoplastin. Plant Cell 2001, 13, 755–768. [Google Scholar] [CrossRef] [PubMed]
- Dong, X.; Hong, Z.; Sivaramakrishnan, M.; Mahfouz, M.; Verma, D.P. Callose synthase (CalS5) is required for exine formation during microgametogenesis and for pollen viability in Arabidopsis. Plant J. 2005, 42, 315–328. [Google Scholar] [CrossRef]
- Shin, D.H.; Choi, M.; Kim, K.; Bang, G.; Cho, M.; Choi, S.B.; Choi, G.; Park, Y.I. HY5 regulates anthocyanin biosynthesis by inducing the transcriptional activation of the MYB75/PAP1 transcription factor in Arabidopsis. FEBS Lett. 2013, 587, 1543–1547. [Google Scholar] [CrossRef] [PubMed]
- Himani, S.; Sonia, S.; Sneh, N.; Rekha, M.; Indu, S.; Ravish, C. Computational analysis of cis-acting regulatory elements in 5′ regulatory regions of sucrose transporter gene families in wheat and Arabidopsis. Res. J. Biotechnol. 2014, 9, 75–81. [Google Scholar]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gong, Y.; Yang, Z.; Li, H.; Lu, K.; Wang, C.; Xiong, A.; Zheng, Y.; Tan, G.; Li, M. Characteristics and Transcriptome Analysis of Anther Abortion in Male Sterile Celery (Apium graveolens L.). Horticulturae 2025, 11, 901. https://doi.org/10.3390/horticulturae11080901
Gong Y, Yang Z, Li H, Lu K, Wang C, Xiong A, Zheng Y, Tan G, Li M. Characteristics and Transcriptome Analysis of Anther Abortion in Male Sterile Celery (Apium graveolens L.). Horticulturae. 2025; 11(8):901. https://doi.org/10.3390/horticulturae11080901
Chicago/Turabian StyleGong, Yao, Zhenyue Yang, Huan Li, Kexiao Lu, Chenyang Wang, Aisheng Xiong, Yangxia Zheng, Guofei Tan, and Mengyao Li. 2025. "Characteristics and Transcriptome Analysis of Anther Abortion in Male Sterile Celery (Apium graveolens L.)" Horticulturae 11, no. 8: 901. https://doi.org/10.3390/horticulturae11080901
APA StyleGong, Y., Yang, Z., Li, H., Lu, K., Wang, C., Xiong, A., Zheng, Y., Tan, G., & Li, M. (2025). Characteristics and Transcriptome Analysis of Anther Abortion in Male Sterile Celery (Apium graveolens L.). Horticulturae, 11(8), 901. https://doi.org/10.3390/horticulturae11080901







