Abstract
Ripe rot (caused by Colletotrichum species) severely compromises the yield and quality of grapes. Biocontrol approaches, such as antagonistic bacteria, represent an effective strategy to prevent and control grape ripe rot. In this study, 325 strains of Bacillus species were isolated from the rhizosphere soil of healthy grapevine plants. Among them, two strains, LJBA01 and LJBV047, exhibit strong antagonistic effects against C. viniferum, suggesting their potential role as biocontrol agents against grape ripe rot. Treatment with the fermentation broth of these strains significantly reduced disease incidence and lesion diameter in infected grape berries. Whole genome sequencing, combined with morphological characterization and 16S rRNA gene sequence, confirmed LJBA01 as Bacillus amyloliquefaciens and LJBV047 as Bacillus velezensis. Furthermore, GC-MS analysis of volatile compounds in fermentation broth from four strains (LJBA01, LJBV047, LJBS06, and LJBS17) identified 29 potential antimicrobial components. Among these, 2-nonanone and 2-decanol demonstrated highly significant inhibitory effects on C. viniferum. Overall, our research confirmed the potential value of two Bacillus strains as biocontrol bacteria against grape ripe rot.
1. Introduction
Grapevine is among the most economically significant cultivated plants globally, primarily for grape production and wine [1], which can be negatively affected by various environmental factors, including a range of diseases. Among them, grape ripe rot, caused by the fungus Colletotrichum, typically occurs during the later stages of plant growth and progressively worsens as the berries ripen [2]. In addition, stems, rachises, young leaves, and new shoots are also susceptible to this disease [3], posing a major threat to the growth and development of grapevines in humid and warm regions [4,5]. In recent years, grape ripe rot has spread and occurred in many regions around the world, such as Germany and France in Europe and China, India, Japan, South Korea, etc., in Asia [6]. The widespread prevalence of this disease has caused significant losses to grape cultivation in these nations.
Over 100 species are currently recognized within the Colletotrichum genus [7]. Studies indicate that several Colletotrichum species cause grape ripe rot, such as C. gloeosporioides, C. nymphaeae, C. fructicola, and C. viniferum [8]. The pathogenesis of Colletotrichum diseases is varied. Some species (e.g., C. circinans and C. capsici) employ a subcuticular, intramural infection strategy after penetrating the host surface, involving massive secretion of cell wall-degrading enzymes [9]. A few species (mostly C. gloeosporioides and C. acutatum) are either mutualistic endophytes or quiescent endophytes that resort to necrotrophy after a spell of latency [10].
Current strategies for preventing and managing grape ripe rot are primarily chemical-based, including fungicides such as carbendazim, copper oxychloride, and thiophanate methyl [11]. However, resistance to these fungicides has been reported, compelling the search for other safe and sustainable methods of ripe rot management. Biological control, which employs diverse groups of antagonistic microorganisms [11,12,13], is more environmentally friendly for managing fungal diseases. In grapes, Bacillus species have been found to be an environmentally friendly strategy for phytopathogenic fungi management, and they are known to be very resistant to heat treatment [14,15,16]. Specially, the antagonistic potential of Bacillus species is mainly attributed to their ability to produce numerous antimicrobial compounds (e.g., lipopeptides, lytic enzymes), as well as volatile organic compounds (VOCs), including 2-nonanone, acetophenone, 2-ethylhexanol, and acetoin (3-hydro-2-butanone) [17,18,19,20,21]. However, it is necessary to systematically analyze the biocontrol effect of Bacillus species and determine the effects of their compounds against plant pathogens.
Grapevines and vineyard soils are rich in microbial diversity; thus, they are potential sources of antagonists and can be effectively utilized for biological control [11]. Therefore, we collected the rhizosphere soil of healthy grapevine plants and identified 325 Bacillus strains through 16S rRNA sequencing. Using dual-culture plate assays and in vitro grape berry inoculation experiments, two strains (LJBA01 and LJBV047) with the most biocontrol efficacy against grape ripe rot were selected. Whole genome sequencing of these strains was performed for functional gene prediction, and GC-MS analysis of volatile compounds in their fermentation broth identified potential antimicrobial components, providing important clues for further exploration of their biocontrol mechanisms.
2. Materials and Methods
2.1. Plant Materials and Strains
Fresh grape berries were harvested from the Centennial Seedless cultivar (Vitis vinifera L.). Biocontrol strains were isolated from 30 rhizosphere soil samples of healthy grapevines in vineyards and germplasm repositories across Jilin (National Grape Germplasm Repository at Zuojia Mountain and Jilin Academy of Agricultural Sciences), Shanxi (Xi’an Jinyang Ecological Grape Planting Co., Ltd. in Xi’an, China), Shandong (Dazeshan Grape Agricultural Demonstration Park in Qingdao, China and Shandong Academy of Agricultural Sciences), Shanghai (Grapevine Germplasm Nursery of Shanghai Jiao Tong University), Zhejiang (Hangzhou Experiment Station), Fujian (Zhanghu Town in Nanping, China and Guangxingzhou Leisure Farm Co., Ltd. in Sanming, China), and Guangxi provinces (Guangxi Academy of Agricultural Sciences, Nanning, China) in China.
The Colletotrichum species ‘82A11’ and ‘85a1’ (C. viniferum) were kindly provided by Prof. Wei Zhang from the Beijing Academy of Agriculture and Forestry Sciences, while ‘LJCg1’ and ‘LJCg2’ (C. gloeosporioides) were previously isolated and preserved by our research group.
2.2. Medium, Fermentation Broth, and Fermentation Filtrate Preparation
For potato dextrose agar (PDA), 120 g of potatoes are diced into cubes and boiled in 500 mL hot water for 15 min. Then, 12 g of glucose and 12 g of agar powder were added to the filtered filtrate and made up to 600 mL with water. They were sealed and sterilized in an autoclave.
Single colonies of Bacillus strains were selected and inoculated into NB liquid medium, followed by shaking at 30 °C and 200 rpm for 12 h. Then, 1 mL of the bacterial suspension was transferred to 30 mL of NB liquid medium and cultured at 30 °C, 200 rpm for 24 h, followed by dilution with NB liquid medium to 1 × 108 CFU/mL to obtain the fermentation broth. The fermentation broth was centrifuged at 8000 rpm for 10 min at 30 °C, and the supernatant was filtered through a 0.45 mm sterile filter to obtain the fermentation filtrate.
2.3. Isolation and Identification of Bacillus Strains
Each soil sample was fully soaked and shaken in 0.85% NaCl [22]. The samples were allowed to stand to obtain the supernatant. The supernatant was treated at 80 °C for 30 min; then, 100 μL of supernatant was diluted to 10−1, 10−2, and 10−3 with sterile water, and 100 μL of the diluted solution was evenly spread on LB solid medium. After 24 h of incubation at 30 °C in the dark, milky-white single colonies were selected and streaked onto fresh LB solid medium. After 1 day, colonies were transferred to 1.5 mL centrifuge tubes, mixed with 50% glycerol, and stored at −80 °C. For activation, 600 μL of the stored suspension was pipetted into a 1.5 mL centrifuge tube, combined with 600 μL of liquid LB, and shaken at 200 rpm and 30 °C for 72 h.
The 16S rRNA genes were amplified using primers 27F (5′ AGAGTTTGATCMTGGCTCAG 3′)/1492R (5′ TACGGYTACCTTGTTACGACTT 3′) [23]. Sequencing was performed on an Illumina Nova 6000 platform at GENEWIZ (Suzhou) Biotechnology Co., Ltd. in Suzhou, China. The 16S rRNA gene sequences were used to search for homologous sequences on the NCBI website (https://www.ncbi.nlm.nih.gov/, accessed on 2 April 2023). According to the preliminary analysis, 325 Bacillus strains were isolated and classified, including 56 B. subtilis (LJBS01–LJBS56), 19 B. amyloliquefaciens (LJBA01–LJBA19), 25 B. mojavensis (LJBM01–LJBM25), and 225 B. velezensis (LJBV001–LJBV225).
2.4. Evaluation of Control Efficacy Against Grape Ripe Rot
Healthy, disease-free mature grape berries with uniform growth status were selected, washed with sterile water, and immersed in a 10% hypochlorite solution for 5 min. Then, they were placed in Petri dishes (10 grape berries per dish), with three biological replicates. A sterile needle was used to create a wound (1 mm in diameter and 3 mm deep) at the center of each berry, and 10 μL of Bacillus fermentation broth was dripped onto the wound site. A total of 10 μL of LB liquid or sterile water was used as a control. The samples were left in the dark for 12 h and then dried. A total of 10 μL of C. viniferum ‘82A11’ spore suspension (1 × 105/mL) was inoculated onto the wounds of both treatment groups with Bacillus fermentation broth and control groups with LB liquid/sterile water, and then the inoculated berries were dried in a sterile environment. The Petri dishes were sealed, wrapped in aluminum foil, and incubated at 25 °C in the dark for 5 days. Disease incidence and lesion diameter were then photographed and analyzed.
2.5. Determination of Inhibitory Spectrum Against Ripe Rot Pathogen
Dip the Bacillus fermentation broth with a sterile pipette tip and streak a 5.2 cm line in the center of the PDA medium. Colletotrichum spp. (C. viniferum ‘82A11’ and ‘85a1’, C. gloeosporioides ‘LJCg1’ and ‘LJCg2’) mycelial plugs were inoculated at positions 2.6 cm from both ends of the streak. PDA medium without any treatment before the inoculation of Colletotrichum spp. was used as the control. All plates were sealed and incubated in the dark at 30 °C for 5 days. The colony radius of the pathogen and inhibition rates were analyzed. The inhibition rate was calculated as follows:
Inhibition rate (%) = [(radius of control colony − radius of treatment colony)/radius of control colony] × 100. Each treatment was performed with three plates, and the experiment was repeated three times.
2.6. Genome Sequencing, Phylogenetic Tree Construction, and Gene Annotation of B. amyloliquefaciens LJBA01 and B. velezensis LJBV047
The genomic DNA was extracted using the Cetyltrimethyl Ammonium Bromide (CTAB) method with minor modification, and then the DNA concentration, quality, and integrity were determined using a Qubit Flurometer (Invitrogen, Shanghai, China) and a NanoDrop Spectrophotometer (Thermo Fisher Scientific, Waltham, MA, USA). Sequencing libraries were generated using the TruSeq DNA Sample Preparation Kit (Illumina, San Diego, CA, USA) and the Template Prep Kit (Pacific Biosciences, Menlo Park, CA, USA). Genome sequencing was then performed by Personal Biotechnology Company (Shanghai, China) using the Nanopore PromethION48 platform/(Pacific Biosciences, Menlo Park, CA, USA) and the Illumina Novaseq platform (Illumina, San Diego, CA, USA).
Data assembly was proceeding after adapter contamination removal and data filtering using AdapterRemoval (v2.1.3). Flye (v2.9.1) and Unicycler (v0.5.0) software were used to assemble the data obtained by nanopore platform sequencing. Subsequently, all assembled results were integrated to generate a complete sequence. Finally, the genome sequence was acquired after the rectification using pilon software.
To construct phylogenetic trees of B. amyloliquefaciens LJBA01 and B. velezensis LJBV047 based on genomic sequences, GTDB-Tk (v2.4.1) was employed to identify conserved marker genes and generate a concatenated multiple protein sequence alignment. The analysis was performed using the classify workflow with reference to the GTDB database (https://gtdb.ecogenomic.org/, accessed on 27 June 2025). The resulting aligned sequences were subsequently trimmed using Trim Galore (v0.4.5). Maximum likelihood phylogenetic analysis was constructed using IQ-TREE (v2.0.3) with the optimal model selected automatically via the ‘-m MFP’ command. The robustness for individual branches was estimated by bootstrapping with 1000 replications.
GeneMarkS software was utilized to predict genes within the complete genome sequences. The PHI (Pathogen-Host Interactions) database was employed to deduce functions of proteins predicted from gene sequences using BLAST (v2.13.0) with the e-value as 1 × 10−6 through PHIB-BLAST (http://phi-blast.phi-base.org/, accessed on 20 April 2024) and to detect potential virulence-related proteins.
2.7. Effects of Secretions from Bacillus Strains LJBA01, LJBV47, LJBS06, and LJBS17 on C. viniferum ‘82A11’
2.7.1. Inhibition of C. viniferum ‘82A11’ by Fermentation Filtrate
A mixture of Bacillus fermentation filtrate and PDA (1:9, v:v) was prepared, homogenized, and poured into plates. An 8 mm diameter mycelial plug of C. viniferum ‘82A11’ (mycelium facing up) was inoculated at the center of the PDA plate. The plates were incubated at 30 °C in the dark, using the PDA medium with 10% sterile water as the control. After 7 days, the colony diameter of Colletotrichum spp. was measured.
2.7.2. Inhibition of C. viniferum ‘82A11’ by Volatile Antimicrobial Substances
A total of 100 μL of Bacillus fermentation broth was evenly spread on an LB solid medium. An 8 mm mycelial plug of C. viniferum ‘82A11’ (mycelium facing up) was placed at the center of a PDA plate. The LB medium plate was inverted over the PDA plate, sealed with parafilm, and incubated at 30 °C in the dark. A control group with 100 μL sterile water was included. After 7 days, the colony diameter of Colletotrichum spp. was measured.
2.8. Determination of Volatile Components in Fermentation Broth
The volatile organic compounds (VOCs) from strains LJBA01, LJBV47, LJBS06, and LJBS17 were analyzed using gas chromatography–mass spectrometry (GC-MS). Samples were equilibrated at 50 °C for 15 min to release volatiles, followed by extraction using a solid-phase microextraction (SPME) fiber for 30 min. After adsorption, the VOCs were thermally desorbed in the GC inlet at 260 °C and separated through a DB-Wax chromatographic column under a helium flow rate of 1 mL/min. The column temperature program was set as follows: initial hold at 40 °C for 5 min, followed by a ramp of 5 °C/min to 220 °C, and then 20 °C/min to 250 °C with a final hold for 2.5 min. The separated compounds were then identified by mass spectrometry in electron ionization (EI) mode, with a transfer line temperature of 260 °C, an ion source temperature of 230 °C, and a mass scan range of 29–400 m/z. Data processing was performed using MassHunter soſtware (Agilent Technologies). VOCs were initially identified by comparing MS peaks with those in the National Institute of Standards and Technology (NIST) 2017 MS Library.
2.9. Evaluation of Antimicrobial Effects of Volatile Components in the Fermentation Broth Against C. viniferum ‘82A11’
To assess the antimicrobial activity of selected differential volatile compounds, filter paper discs were placed in Petri dishes and loaded with 10, 20, 30, 40, or 50 μL of pure volatile compounds (2-nonanone and 2-decanol). The corresponding concentrations (pure compound concentration/sealed chamber volume) of 2-nonanone were 0.405, 0.810, 1.215, 1.620, and 2.025 mM, respectively; the corresponding concentrations of 2-decanol were 0.36, 0.72, 1.08, 1.44, and 1.80 mM, respectively. An 8 mm mycelial plug of C. viniferum ‘82A11’ was placed at the center of the PDA plate. Using the inverted plate method, two Petri dishes were sealed together with parafilm. Sterile water-treated plates served as the control. All plates were incubated at 30 °C in a constant-temperature chamber, and the colony diameter of C. viniferum ‘82A11’ was measured every 24 h over a 7-day period.
2.10. Statistical Analysis
Statistical analysis was performed using SPSS statistics software (version 22.0). One-way analysis of variance (ANOVA) followed by a Tukey’s post hoc test was used to analyze the results for statistical analysis. Data that did not meet the criteria for normality and homoscedasticity, determined, respectively, by the Shapiro–Wilk and Levene’s tests, were compared using the nonparametric Kruskal–Wallis H test. p < 0.05 was considered to be significantly different. Data were expressed as the means of replicates with standard errors.
3. Results and Analysis
3.1. Screening of Antagonistic Bacteria from Rhizosphere Soil
A total of 10 g of rhizosphere soil samples were collected from healthy grapevine plants across multiple vineyards in many places from the northern to southern parts of China to ensure the diversity of the samples. In order to selectively isolate Bacillus strains, we utilized their heat-resistant property and employed heat treatment on the supernatant of soaked soil samples. Single colonies were cultivated and subjected to 16S rRNA sequencing, with subsequent BLAST alignments performed in the NCBI database. Based on the sequencing results, 325 strains of Bacillus were isolated and categorized into four species: 56 B. subtilis (LJBS01-LJBS56), 19 B. amyloliquefaciens (LJBA01-LJBA19), 25 B. mojavensis (LJBM01-LJBM25), and 225 B. velezensis (LJBV001-LJBV225).
The antagonistic effects of these Bacillus strains against C. viniferum ‘82A11’ were evaluated using dual-culture plate assays. Overall, a significant proportion of the LJBV strains exhibited strong antagonistic activity, with partial LJBA strains also demonstrating notable inhibition. In contrast, LJBS and LJBM strains showed weaker antifungal efficacy (Figure S1). LJBS06 and LJBS17 with poor antagonistic effects were selected as negative controls, and the top 20 strains with the best antagonistic effects were further confirmed. Compared with CK (4.20 cm), LJBS06 (2.84 cm), and LJBS17 (3.19 cm), strain LJBV047 exhibited the strongest inhibition effect with a Colletotrichum colony growth radius of 1.81 cm, followed by LJBA01 (1.91 cm) (Figure 1, Table S1).
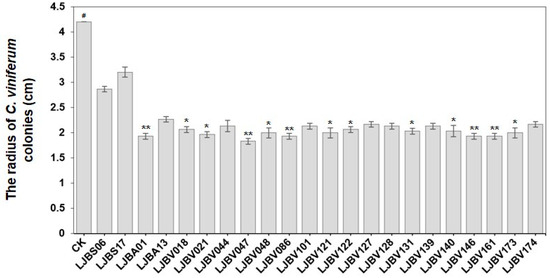
Figure 1.
Inhibitory effects of the top 20 Bacillus strains on C. viniferum ‘82A11’. The inhibitory effects of the top 20 antagonistic Bacillus strains against C. viniferum ‘82A11’ were verified using the dual-culture assay. The radius of C. viniferum ‘82A11’ colonies was measured to quantify growth suppression. Significance stars were from the Kruskal–Wallis H test (#, control; * p < 0.05; ** p < 0.001). All treatments were measured with three biological replicates.
Hyphae from C. viniferum ‘82A11’ under dual-culture with LJBA01 and LJBV047 were observed. The results revealed that the hyphae of C. viniferum ‘82A11’ subjected to these Bacillus spp. exhibited distinct morphological anomalies, including breakage, swelling, and distortion, compared to normal hyphae. Moreover, the hyphal diameter under antagonistic conditions showed a significant increase (Figure S2A–C).
3.2. Bacillus Strains LJBA01 and LJBV047 Exhibit Broad-Spectrum Antagonistic Activity Against Colletotrichum spp.
We further performed resistance evaluation against C. viniferum ‘82A11’ on Vitis vinifera ‘Centennial Seedless’ grape berries. Compared to controls (LB and H2O), treatment with the fermentation broth of LJBA01 and LJBV047 significantly reduced ripe rot incidence, accompanied by reductions in lesion diameter. In contrast, the inhibition effect of LJBS06 and LJBS17 was not obvious (Figure 2A,B).
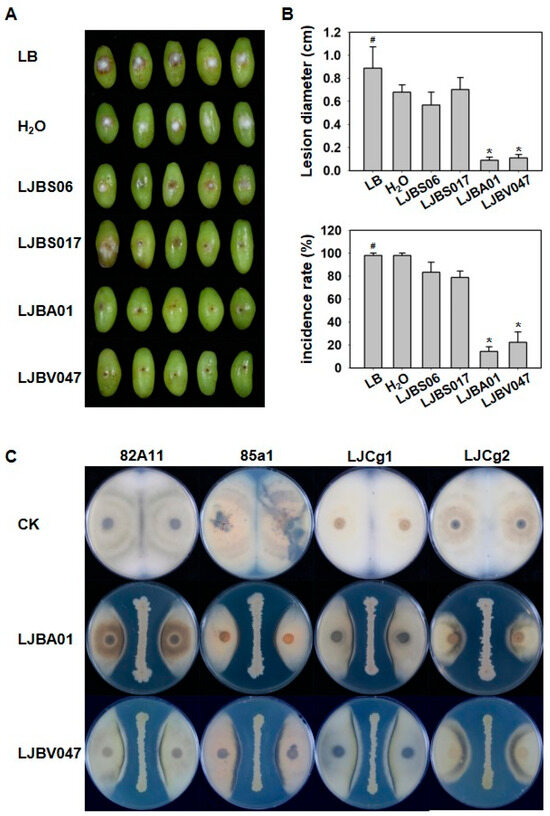
Figure 2.
Bacillus strains LJBA01 and LJBV047 exhibit significant inhibitory effects on ripe rot. (A,B) LJBA01 and LJBV047 significantly reduced ripe rot incidence on Vitis vinifera ‘Centennial Seedless’ grape berries. Using LB and sterile water as controls, phenotypic evaluation of ripe rot resistance of Bacillus strains was conducted on Vitis vinifera ‘Centennial Seedless’ grape berries (A). Disease incidence and lesion diameter were statistically analyzed (B). Significance stars were from the Kruskal–Wallis H test (#, control; * p < 0.05). Each treatment included three biological replicates, with 10 grape berries per replicate. (C). Determination of the antimicrobial spectrum of LJBA01 and LJBV047. LJBA01 or LJBV047 were subjected to dual-culture assays with four distinct Colletotrichum strains (‘82A11’, ‘85a1’, ‘LJCg1’, and ‘LJCg2’). Phenotypes were observed to assess their inhibitory effects.
To verify the broad-spectrum resistance of these two Bacillus strains to Colletotrichum, LJBA01 and LJBV047 were then subjected to dual-culture assays with four Colletotrichum strains: C. viniferum ‘82A11’ and ‘85a1’, and C. gloeosporioides ‘LJCg1’ and ‘LJCg2’. The results indicated that both LJBA01 and LJBV047 significantly inhibited the growth of each Colletotrichum strain. Specifically, the inhibition rate of LJBA01 against C. viniferum ‘82A11’ reached 60.77%, and that of LJBV047 reached 52.95%. The inhibitory effect of the two biocontrol bacteria on C. gloeosporioides ‘LJCg2’ was the most obvious, with inhibition rates of 74.10% (LJBA01) and 61.67% (LJBV047) (Figure 2C, Tables S2 and S3). These results demonstrate that the screened LJBA01 and LJBV047 strains exhibit a broad-spectrum antagonistic effect against Colletotrichum spp., qualifying them as promising biocontrol agents for further investigation.
3.3. Sequencing and Characterization of Bacillus Strains LJBA01 and LJBV047
For Next-Generation Sequencing, the Q20 for LJBA01 and LJBV047 reached 98.31% and 98.44%, respectively, while the Q30 reached 95.35% and 95.68%, respectively. Further filtering of the raw data yielded high-quality sequences, with a total of 8,249,862 reads for LJBA01 and 6,699,224 reads for LJBV047. For the third-generation single-molecular sequencing, the sequencing data was 1,292,387,287 bp for LJBA01 and 2,194,598,898 bp for LJBV047, respectively. The N50 read length was 13,776 bp and 13,266 bp, respectively.
Genomic maps were constructed through whole genome sequencing analysis integrated with gene prediction and non-coding RNA annotation. The LJBA01 genome consists of a single circular chromosome (3.97 Mbp, 46.49% GC content), without plasmids. A total of 3853 ORFs, 27 rRNA, 87 tRNA, and 88 ncRNA were predicted (Figure 3A). In contrast, LJBV047 contains a circular chromosome (3.95 Mbp, 46.52% GC content) and two plasmids: plasmid 1, 20,615 bp (35.23% GC), and plasmid 2, 8564 bp (39.86% GC). A total of 3925 ORFs were predicted in the LJBV047 genome, including 3883 on the chromosome, 29 on plasmid 1, and 13 on plasmid 2. Except for one ncRNA from plasmid 2, the other RNA was all from the chromosome, including twenty-seven rRNA, eighty-six tRNA, and eighty-nine ncRNA (Figure 4A).
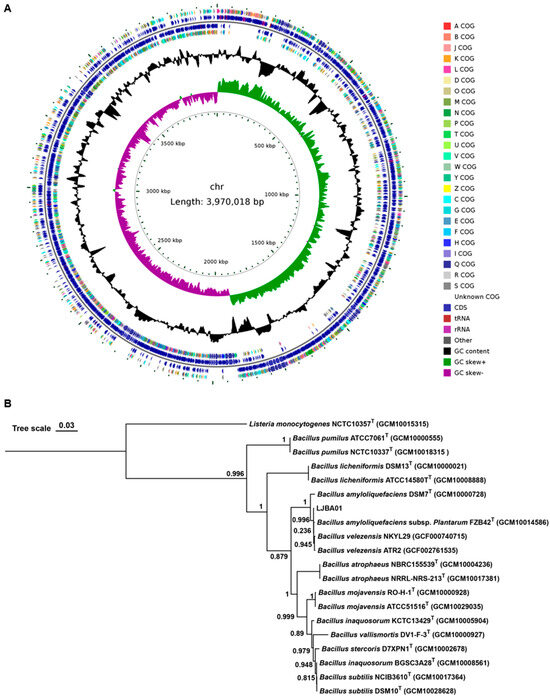
Figure 3.
LJBA01 was identified as Bacillus amyloliquefaciens. (A) Genomic circle diagram of LJBA01. The LJBA01 genome consists of a single circular chromosome. From the innermost to the outermost, the 1st circle represents the scale marks of the genome, the 2nd circle represents the GC skew (green positive skew; purple, negative skew), and the 3rd circle represents the G + C content. The 4th and 7th circles indicate reverse and forward COG annotated coding sequences, respectively. The 5th and 6th circles represent reverse and forward CDSs, respectively. Very short features were enlarged to enhance visibility. Clustered genes, such as several rRNA genes, may appear as one line due to space limitations. (B) A phylogenetic tree of LJBA01 was constructed based on genomic sequences. The type strain Listeria monocytogenes NCTC10357T (GCM10015315) was used as the outgroup. The phylogenetic tree was constructed using the Maximum Likelihood (ML) method. The robustness for individual branches was estimated by bootstrapping with 1000 replications. ‘T’ indicates type strains. The scale bar indicates 0.03 nucleotide substitutions per site.
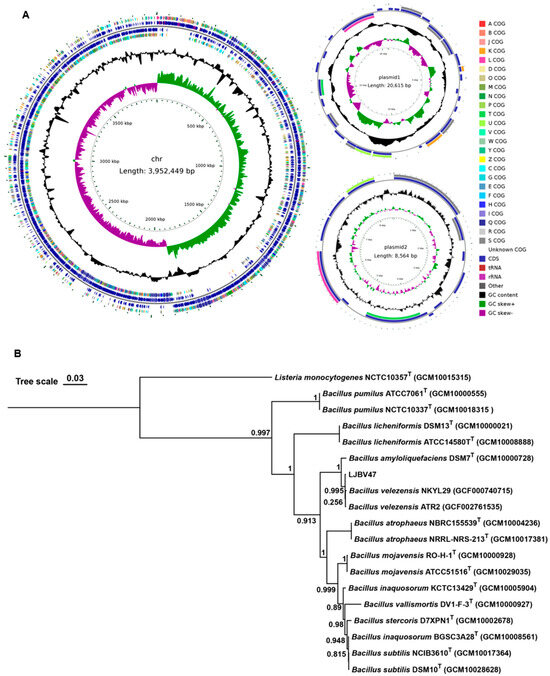
Figure 4.
LJBV047 was identified as Bacillus velezensis. (A) Genomic circle diagram of LJBA047. LJBV047 contains a circular chromosome and two plasmids. From the innermost to the outermost of the chromosome, the 1st circle represents the scale marks of the genome, the 2nd circle represents the GC skew (green, positive skew; purple, negative skew), and the 3rd circle represents the G + C content. The 4th and 7th circles indicate reverse and forward COG annotated coding sequences, respectively. The 5th and 6th circles represent reverse and forward CDSs, respectively. Very short features were enlarged to enhance visibility. Clustered genes, such as several rRNA genes, may appear as one line due to space limitations. (B) A phylogenetic tree of LJBV047 was constructed based on genomic sequences. The type strain Listeria monocytogenes NCTC10357T (GCM10015315) was used as the outgroup. The phylogenetic tree was constructed using the Maximum Likelihood (ML) method. The robustness for individual branches was estimated by bootstrapping with 1000 replications. ‘T’ indicates type strains. The scale bar indicates 0.03 nucleotide substitutions per site.
Strain LJBA01 exhibits opaque yellowish-white colonies with irregular edges and a Gram-positive reaction (Figure S2D). The 16S rRNA sequencing results indicate that LJBA01 has 100% similarity to Bacillus amyloliquefaciens BV2007 (MT613661.1). Phylogenetic analysis based on genomic sequences revealed that LJBA01 has high homology to B. amyloliquefaciens DSM7T (GCM10000728) and B. amyloliquefaciens subsp. Plantarum FZB42T (GCM10014586) (Figure 3B). Moreover, genomic sequence comparison against the NCBI Nucleotide Sequence Database (NT) demonstrated high similarity to the chromosome sequence of B. amyloliquefaciens TPS17 (CP085282.1). Together with these results, LJBA01 was identified as Bacillus amyloliquefaciens.
In contrast, strain LJBV047 also exhibits opaque milky-white colonies with irregular edges, displaying Gram-positive characteristics (Figure S2E). Blast analysis of 16S rRNA sequences revealed 100% similarity between LJBV047 and Bacillus velezensis WGB11 (KY962336.1). Phylogenetic analysis based on genomic sequences further demonstrated that LJBV047 has high homology to B. velezensis NKYL29 (GCF000740715) (Figure 4B). Both the chromosome and plasmid 1 of LJBV047 exhibit high similarity to the chromosomal sequence of Bacillus sp. LJBV19 (CP072563.1), a strain previously identified as B. velezensis by our research group [24], and plasmid 2 shows significant homology to the plasmid of B. velezensis 504 (CP092440.1). These collective findings confirm LJBV047 as Bacillus velezensis.
To further investigate the antimicrobial mechanisms of biocontrol strains LJBA01 and LJBV047, functional annotation of their genes was performed using the PHI database (commonly employed for predicting antifungal compounds and target genes). Both B. amyloliquefaciens LJBA01 and B. velezensis LJBV047 were annotated with 825 genes, with the largest proportion of annotated genes categorized under ‘Reduced Virulence’ (Figure S3), which is consistent with their potential biocontrol functions.
3.4. The Metabolites of LJBA01 and LJBV047 Suppress the Growth of C. viniferum ‘82A11’
Bacillus spp. often endows plants with antibacterial metabolites (e.g., antibiotics) that protect plants from pathogenic attacks, including the production of polyketides and lipopeptides [25,26]. The results showed that B. subtilis LJBS06 and LJBS17 exhibited weaker antagonistic effects against C. viniferum ‘82A11’ (Figure 1) and less suppression of ripe rot incidence on grape berries (Figure 2A,B) compared to B. amyloliquefaciens LJBA01 and B. velezensis LJBV047. Consequently, we thus evaluated the effects of metabolites from B. amyloliquefaciens LJBA01 and B. velezensis LJBV047 (both fermentation filtrate and volatile compounds) on the growth of C. viniferum ‘82A11’, using B. subtilis LJBS06 and LJBS17 as controls.
Colletotrichum viniferum ‘82A11’ was inoculated on the PDA medium containing 10% fermentation filtrate, and then the growth diameter of colonies was calculated 6 days after inoculation. Compared to the controls, the fermentation filtrate of LJBA01 and LJBV047 demonstrated superior inhibitory rates of 24.4% and 25.1%, respectively, significantly outperforming LJBS06 and LJBS17 (Figure 5).
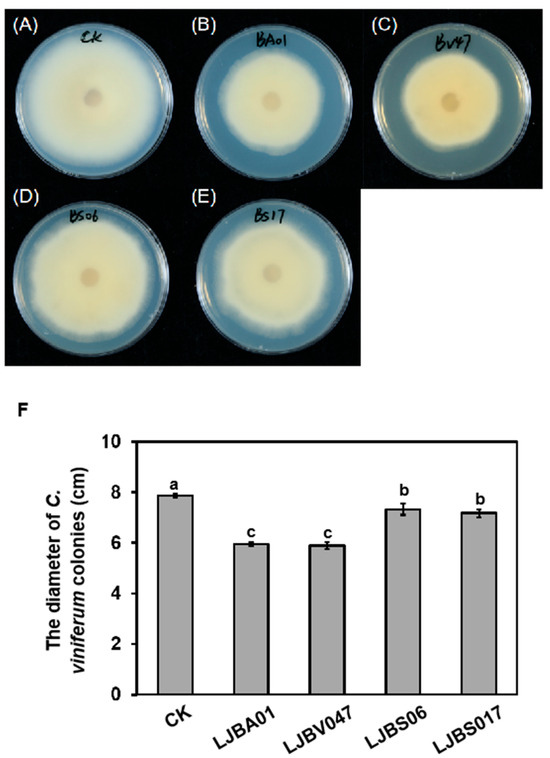
Figure 5.
The fermentation filtrates of LJBA01 and LJBV047 inhibit the growth of C. viniferum ‘82A11’. C. viniferum ‘82A11’ was inoculated on PDA medium containing 10% (v/v) fermentation filtrates from LJBA01 or LJBV047. Phenotypic observations (A–E) and measurements of colony diameter (F) were recorded after 6 days. One-way ANOVA was used for multiple comparisons (p < 0.05). Different letters indicate statistically significant differences. Each treatment had three biological replicates.
LB plates coated with fermentation broth were inverted over PDA plates inoculated with C. viniferum ‘82A11’ and incubated for 6 days. LJBA01 and LJBV047 again exhibited stronger suppression, with inhibition rates of 52.6% and 57.3%, respectively. Notably, the diameter of C. viniferum ‘82A11’colonies with the treatment of LJBV047 volatile compounds decreased to 3.38 cm (Figure 6). These results demonstrated that both fermentation broth and volatile metabolites of LJBA01 and LJBV047 critically impair the growth of C. viniferum.
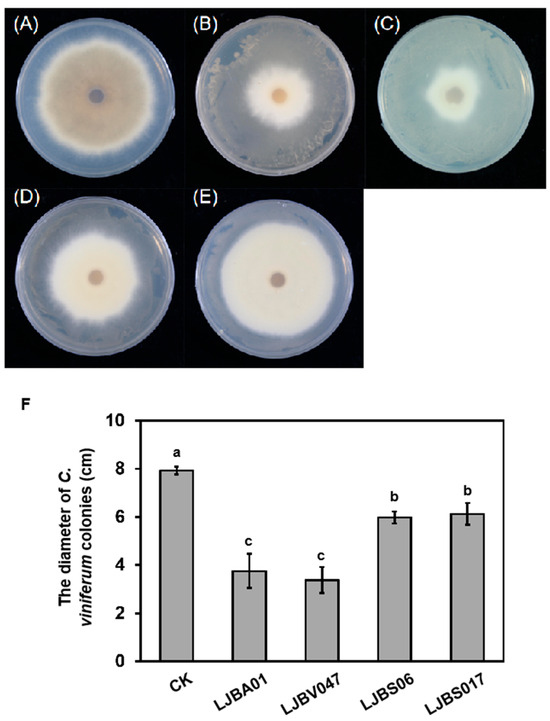
Figure 6.
Inhibition of Colletotrichum growth by volatile compounds produced by LJBA01 and LJBV047. The fermentation broth of LJBA01 or LJBV047 was spread uniformly on LB agar plates, which were then inverted over PDA plates inoculated with C. viniferum ‘82A11’. Phenotypic observations (A–E) and measurements of colony diameter (F) were performed after 6 days. One-way ANOVA was used for multiple comparisons (p < 0.05). Different letters denote statistically significant differences. Each treatment had three biological replicates.
3.5. B. amyloliquefaciens LJBA01 and B. velezensis LJBV047 Inhibit the Growth of C. viniferum Through the Production of 2-Nonanone and 2-Decanol
Similarly, using B. subtilis LJBS06 and LJBS17 as controls, the volatile components in the fermentation broth of B. amyloliquefaciens LJBA01 and B. velezensis LJBV047 were analyzed via GC-MS (gas chromatography–mass spectrometry) to investigate the mechanism for their superior efficacy. The metabolism profiles of LJBA01 and LJBV047 exhibited significant similarity but were markedly distinct from those of LJBS06 and LJBS17 (Figure S4).
The ratio of individual component peak area to total peak area was calculated, and components with peak area ratios in LJBA01 and LJBV047 exceeding those in LJBS06 and LJBS17 by more than tenfold were identified. A total of 29 components were filtered out, primarily including alcohols, ketones, esters, and alkanes (Table S4). This metabolism profiling underscores the strain-specific metabolic basis for their biocontrol efficacy.
Due to the strongest inhibitory effect of B. velezensis LJBV047 volatile compounds on C. viniferum ‘82A11’ colonies (Figure 6), two substances with relatively high peak areas in LJBV047 fermentation broth—2-nonanone and 2-decanol—were selected, and their pure forms were purchased for antimicrobial assays. The results demonstrated that both 2-nonanone and 2-decanol significantly inhibited C. viniferum growth, with efficacy escalating alongside increasing concentrations within a specific range (Figure 7). At 1.620 mM of 2-nonanone, the hyphae growth of C. viniferum was completely halted (Figure 7A), likely due to direct lethality to the hyphae. For 2-decanol, limited hyphal growth occurred only at 0.36 mM, and growth ceased entirely at 0.72 mM (Figure 7B). These findings confirm the dose-dependent antifungal activity of these compounds against C. viniferum.
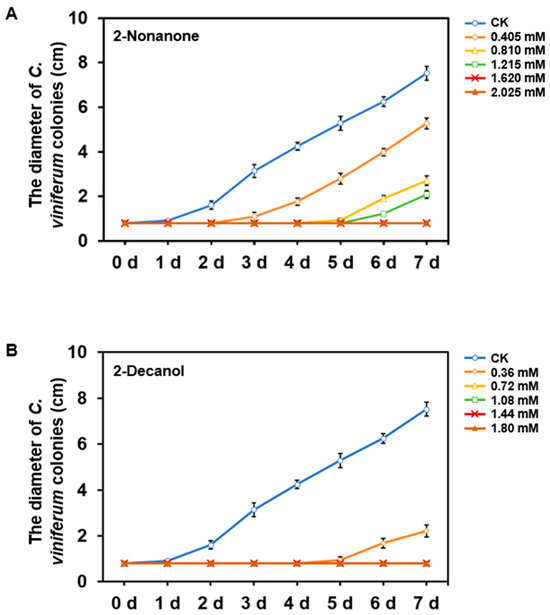
Figure 7.
Inhibition of Colletotrichum growth by volatile compounds. Effects of different concentrations of pure 2-nonanone (A) and 2-decanol (B) on the growth of C. viniferum ‘82A11’. Colony diameters of C. viniferum ‘82A11’ were measured to assess inhibitory effects. Each treatment had three biological replicates.
4. Discussion
Green and sustainable agriculture has emerged as the predominant direction for the future development of agriculture. Bacillus species, as biocontrol microorganisms, have received extensive attention in promoting plant growth and controlling diseases. Currently, there are relevant reports on the application of Bacillus strains in controlling ripe rot of mango (Mangifera indica L.), Camellia oleifera, Chili pepper (Capsicum annuum Linn.), and tomato (Lycopersicon esculentum Mill.) [27,28,29]. However, studies on their efficacy against grape ripe rot (Colletotrichum spp.) remain limited.
In this study, 325 Bacillus strains were isolated from the rhizosphere soil of healthy grapevines (Figure S1). Through dual-culture assays and resistance evaluation on grape berries, two strains with the strongest antagonistic effects against ripe rot pathogens were screened out, namely, LJBA01 and LJBV047 (Figure 1 and Figure 2A). Further antifungal spectrum analysis revealed that both LJBA01 and LJBV047 had significant inhibitory effects on the most common ripe rot-causing fungi, C. gloeosporioides and C. viniferum (Figure 2B). Through morphological characterization, 16S rRNA sequencing, and whole genome sequence analysis, LJBA01 was identified as B. amyloliquefaciens, and LJBV047 was classified as B. velezensis (Figure 3, Figure 4 and Figure S2).
Secondary metabolites are low-molecular-weight compounds produced by microorganisms, which are recognized for their diverse biological activities. Notably, many Bacillus species, including B. licheniformis, B. subtilis, and B. amyloliquefaciens, can synthesize secondary metabolites with biocontrol functionality, such as non-ribosomal synthesized peptides (e.g., bacilysin), lipopeptides (e.g., surfactin, fengycin), polyketides (e.g., difficidin), bacteriocins, and siderophores [30,31,32]. In this study, we demonstrated that the fermentation filtrate and broth of B. amyloliquefaciens LJBA01 and B. velezensis LJBV047 significantly suppress the growth of Colletotrichum spp. (Figure 5 and Figure 6), presumably attributed to bioactive metabolites.
Among the volatile components of the fermentation broth of LJBA01 and LJBV047, 2-nonanone and 2-decanol have significant antibacterial effects (Figure 7). 2-nonanone, a bacterial bioactive compound, has been reported for antifungal effects [17,33,34,35]. For instance, the anthracnose fungus showed mycelia growth inhibitions in the presence of 2-nonanone produced by B. pumilus and B. thuringiensis [33]. 2-nonanone demonstrated an inhibitory action on protein refolding in Escherichia coli [36] and may target proteins involved in the biogenesis and functioning of the cyanobacteria cell wall [37]. Moreover, it also suppresses the expression of genes involved in quorum-sensing systems in bacterial cells [38]. The antifungal mechanism of 2-nonanone against C. viniferum has not been extensively reported. We speculate that 2-nonanone may potentially inhibit C. viniferum growth through similar mechanisms—either by targeting different proteins or disrupting cell wall integrity.
The antifungal effect of 2-decanol has not been reported previously, although it has been shown to inhibit plant root-knot nematodes when produced by Paenibacillus polymyxa [39]. In the present study, we demonstrated that 2-decanol produced by LJBA01 and LJBV047 exhibits stronger inhibition activity against C. viniferum, suggesting its potential applications in developing novel biopesticides or preventing postharvest fruit diseases in mature grapes. Future experiments with the application of transcriptomic and proteomic methods may help to clarify more details of the molecular mechanisms of their biological action.
Here, two relatively abundant substances in the VOCs of B. velezensis LJBV047—2-nonanone and 2-decanol—were selected and verified for their antimicrobial effects. The functions of the remaining 27 VOCs (out of 29) were under further elucidation to identify more antimicrobial agents for agricultural applications.
5. Conclusions
Ripe rot, caused by fungal pathogens such as Colletotrichum species, is one of the most critical diseases affecting grape production. Current management in vineyards relies heavily on chemical pesticides, which can easily cause environmental pollution. Biocontrol agents, particularly microbial antagonists, have emerged as a sustainable alternative. This study identified two potent biocontrol strains, LJBA01 and LJBV047, demonstrating excellent inhibitory effects on grape ripe rot. Further analysis identified LJBA01 as Bacillus amyloliquefaciens, and LJBV047 was confirmed as Bacillus velezensis.
Functional annotation of their genomes revealed numerous genes linked to ‘Reduced Virulence’ in fungal pathogens. Volatile compound profiling via GC-MS identified 29 differentially expressed metabolites across four strains (LJBA01, LJBV047, LJBS06, LJBS17). Among these, 2-nonanone and 2-decanol exhibited significant antifungal activity against Colletotrichum spp., highlighting their potential for novel pesticide development and postharvest disease management. This research provides critical theoretical support for advancing microbial-based biocontrol strategies in sustainable viticulture.
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/horticulturae11070802/s1, Figure S1: Preliminary screening of Bacillus strains against C. viniferum ‘82A11’. The antagonistic effects of 325 Bacillus strains against C. viniferum ‘82A11’ were evaluated using the dual-culture plate assay. The colony diameter of C. viniferum ‘82A11’ was measured to assess inhibition efficacy. Figure S2: Characterization and identification of LJBA01 and LJBV047. A–C. Effects of LJBA01 and LJBV047 on the hyphae of C. viniferum ‘82A11’. Compared to normally grown hyphae (A), the hyphae of C. viniferum ‘82A11’ under confrontation with B. amyloliquefaciens LJBA01 or B. velezensis LJBV047 exhibited significant fragmentation, swelling, or distortion (B, LJBA01; C, LJBV047). Bars, 20 mm. D–E. Colonies of LJBA01 (D) and LJBV047 (E) cultured on PDA medium (left, bars, 5 mm) and their Gram-staining results (right, bars, 10 μm). Figure S3: PHI annotation results of LJBA01 (A) and LJBV047 (B). The number of genes matched to each term (x-axis) is shown on the y-axis. Figure S4: Total ion chromatograms of GC-MS for the volatile components of fermentation broth of each Bacillus strain. The volatile components of the fermentation broth from LJBA01 (A), LJBV047 (B), LJBS06 (C), and LJBS17 (D) were analyzed using gas chromatography–mass spectrometry (GC-MS). The x-axis indicates the retention time (min), and the y-axis represents the ion abundance. Table S1: The inhibitory rate of the top 20 Bacillus strains with the best antagonistic effects on C. viniferum ‘82A11’. Inhibition rate (%) = [(radius of control colony − radius of treatment colony)/radius of control colony] × 100. Table S2: The inhibitory effect of LJBA01 on Colletotrichum species. Inhibition rate (%) = [(radius of control colony − radius of treatment colony)/radius of control colony] × 100. Table S3: The inhibitory effect of LJBV047 on Colletotrichum species. Inhibition rate (%) = [(radius of control colony − radius of treatment colony)/radius of control colony] × 100. Table S4: Differential metabolites screened based on the GC-MS results. The peak area ratio (ratio of individual component peak area to total peak area) was calculated. Components with peak area ratios exceeding tenfold in LJBA01 and LJBV047 compared to LJBS06 and LJBS17 were screened, yielding a total of 29 identified components.
Author Contributions
X.-Q.D. and Y.-L.Y. designed the experiments and analyzed the data. Y.-L.Y. performed the experiments. X.-Q.D. and J.L. contributed to manuscript discussions and drafting. J.L. wrote the final manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This study was supported by the Ningxia Hui Autonomous Region key research and development program (grant number 2023BCF01026).
Data Availability Statement
The complete genome sequence of B. amyloliquefaciens LJBA01 and B. velezensis LJBV047 have been deposited in the NCBI genome database. The data that support the findings of this study are available from the corresponding author, J.L., upon reasonable request. All authors approved the submitted version.
Conflicts of Interest
The authors declare no competing interests.
References
- Zapparata, A. Grape Ripe Rot Disease Caused by Colletotrichum spp.: Current Status, Concerns and Future Perspectives. J. Plant Pathol. 2024, 106, 487–497. [Google Scholar] [CrossRef]
- Ye, B.; Zhang, J.; Chen, X.; Xiao, W.; Wu, J.; Yu, H.; Zhang, C. Genetic Diversity of Colletotrichum spp. Causing Grape Anthracnose in Zhejiang, China. Agronomy 2023, 13, 952. [Google Scholar] [CrossRef]
- Liu, Z.; Du, S.; Ren, Y.; Liu, Y. Biocontrol Ability of Killer Yeasts (Saccharomyces cerevisiae) Isolated from Wine against Colletotrichum gloeosporioides on Grape. J. Basic Microbiol. 2018, 58, 60–67. [Google Scholar] [CrossRef] [PubMed]
- Vasanthaiah, H.K.N.; Basha, S.M.; Katam, R. Differential Expression of Chitinase and Stilbene Synthase Genes in Florida Hybrid Bunch Grapes to Elsinoë ampelina Infection. Plant Growth Regul. 2010, 61, 127–134. [Google Scholar] [CrossRef]
- Li, Z.; Zhang, S.; Han, R.; Zhang, H.; Li, K.; Wang, X. Infection Process and Host Responses to Elsinoë ampelina, the Causal Organism of Grapevine Anthracnose. Eur. J. Plant Pathol. 2019, 155, 571–582. [Google Scholar] [CrossRef]
- Kono, A.; Sato, A.; Ban, Y.; Mitani, N. Resistance of Vitis Germplasm to Elsinoë ampelina (de Bary) Shear Evaluated by Lesion Number and Diameter. HortScience 2013, 48, 1433–1439. [Google Scholar] [CrossRef]
- Cannon, P.F.; Damm, U.; Johnston, P.R.; Weir, B.S. Colletotrichum—Current Status and Future Directions. Stud. Mycol. 2012, 73, 181–213. [Google Scholar] [CrossRef]
- Steiner, D.R.M.; Modesto, L.R.; Dias, A.H.; Zappelini, J.; Petters-Vandresen, D.A.L.; Castellar, C.; De Mio, L.L.M.; Nodari, R.O. Colletotrichum nymphaeae and Colletotrichum theobromicola Isolated from Anthracnose Symptoms Cause Grape Ripe Rot. Plant Pathol. 2025, 74, 813–824. [Google Scholar] [CrossRef]
- Latunde-Dada, A.O. Colletotrichum: Tales of Forcible Entry, Stealth, Transient Confinement and Breakout. Mol. Plant Pathol. 2001, 2, 187–198. [Google Scholar] [CrossRef]
- Latunde-Dada, A.O.; O’Connell, R.J.; Nash, C.; Lucas, J.A. Stomatal Penetration of Cowpea (Vigna unguiculata) Leaves by A Colletotrichum Species Causing Latent Anthracnose. Plant Pathol. 1999, 48, 777–785. [Google Scholar] [CrossRef]
- Sawant, I.S.; Wadkar, P.N.; Rajguru, Y.R.; Mhaske, N.H.; Salunkhe, V.P.; Sawant, S.D.; Upadhyay, A. Biocontrol Potential of Two Novel Grapevine Associated Bacillus Strains for Management of Anthracnose Disease Caused by Colletotrichum gloeosporioides. Biocontrol Sci. Technol. 2016, 26, 964–979. [Google Scholar] [CrossRef]
- Bastías, D.A.; Balestrini, R.; Pollmann, S.; Gundel, P.E. Environmental Interference of Plant−microbe Interactions. Plant Cell Environ. 2022, 45, 3387–3398. [Google Scholar] [CrossRef] [PubMed]
- O’Brien, P.A. Biological Control of Plant Diseases. Australas. Plant Pathol. 2017, 46, 293–304. [Google Scholar] [CrossRef]
- Mochizuki, M.; Yamamoto, S.; Aoki, Y.; Suzuki, S. Isolation and Characterisation of Bacillus amyloliquefaciens S13-3 as A Biological Control Agent for Anthracnose Caused by Colletotrichum gloeosporioides. Biocontrol Sci. Technol. 2012, 22, 697–709. [Google Scholar] [CrossRef]
- Russi, A.; Granada, C.E.; Schwambach, J. Suppression of Colletotrichum spp. on Grape Berries, Vine Leaves, and Plants using Bacillus velezensis S26 Endospores. Sci. Hortic. 2024, 326, 112696. [Google Scholar] [CrossRef]
- Aouadhi, C.; Al-zaban, M.I.; Alsaloom, A.N.; Maaroufi, A. Review: Inactivation of Very Heat-Resistant Spores of Bacilus sporothermodurans by High Pressure Treatment Combined with Others Treatments. High Press. Res. 2022, 42, 236–244. [Google Scholar] [CrossRef]
- Li, X.Y.; Mao, Z.C.; Wu, Y.X.; Ho, H.H.; He, Y.Q. Comprehensive Volatile Organic Compounds Profiling of Bacillus Species with Biocontrol Properties by Head Space Solid Phase Microextraction with Gas Chromatography-Mass Spectrometry. Biocontrol Sci. Technol. 2015, 25, 132–143. [Google Scholar] [CrossRef]
- Bakker, C.E.; Barghouth, Z.; Ramlawi, S.; Avis, T.J. Biochemistry and Differential Mechanistic Activity of Antimicrobial Lipopeptides from Plant Pathogen Antagonists from the Genus Bacillus. Can. J. Plant Pathol. 2025, 47, 98–110. [Google Scholar] [CrossRef]
- Dobrzyński, J.; Jakubowska, Z.; Kulkova, I.; Kowalczyk, P.; Kramkowski, K. Biocontrol of Fungal Phytopathogens by Bacillus pumilus. Front. Microbiol. 2023, 14, 1194606. [Google Scholar] [CrossRef]
- Hirozawa, M.T.; Ono, M.A.; Suguiura, I.M.d.S.; Bordini, J.G.; Ono, E.Y.S. Lactic Acid Bacteria and Bacillus spp. as Fungal Biological Control Agents. J. Appl. Microbiol. 2023, 134, lxac083. [Google Scholar] [CrossRef]
- Xue, J.; Sun, L.; Xu, H.; Gu, Y.; Lei, P. Bacillus atrophaeus NX-12 Utilizes Exosmotic Glycerol from Fusarium oxysporum f. sp. cucumerinum for Fengycin Production. J. Agric. Food Chem. 2023, 71, 10565–10574. [Google Scholar] [CrossRef] [PubMed]
- Jumpathong, W.; Intra, B.; Euanorasetr, J.; Wanapaisan, P. Biosurfactant-Producing Bacillus velezensis PW192 as an Anti-Fungal Biocontrol Agent against Colletotrichum gloeosporioides and Colletotrichum musae. Microorganisms 2022, 10, 1017. [Google Scholar] [CrossRef]
- López, A.C.; Alippi, A.M. Feasibility of Using RFLP of PCR-Amplified 16S rRNA Gene(s) for Rapid Differentiation of Isolates of Aerobic Spore-Forming Bacteria from Honey. J. Microbiol. Meth. 2019, 165, 105690. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Yang, B.; Peng, H.; Lu, J.; Fu, P. Genome Sequence and Comparative Analysis of Fungal Antagonistic Strain Bacillus velezensis LJBV19. Folia Microbiol. 2022, 68, 73–86. [Google Scholar] [CrossRef]
- Wang, J.; Peng, Y.; Xie, S.; Yu, X.; Bian, C.; Wu, H.; Wang, Y.; Ding, T. Biocontrol and Molecular Characterization of Bacillus velezensis D against Tobacco Bacterial Wilt. Phytopathol. Res. 2023, 5, 50. [Google Scholar] [CrossRef]
- Villegas-Escobar, V.; González-Jaramillo, L.M.; Ramírez, M.; Moncada, R.N.; Sierra-Zapata, L.; Orduz, S.; Romero-Tabarez, M. Lipopeptides from Bacillus sp. EA-CB0959: Active Metabolites Responsible for in vitro and in vivo Control of Ralstonia solanacearum. Biol. Control 2018, 125, 20–28. [Google Scholar] [CrossRef]
- Liang, Y.S.; Fu, J.Y.; Chao, S.H.; Tzean, Y.; Hsiao, C.Y.; Yang, Y.Y.; Chen, Y.K.; Lin, Y.H. Postharvest Application of Bacillus amyloliquefaciens PMB04 Fermentation Broth Reduces Anthracnose Occurrence in Mango Fruit. Agriculture 2022, 12, 1646. [Google Scholar] [CrossRef]
- Chen, Z.; Cao, H.; Jin, J.; Li, Z.; Zhang, S.; Chen, J. Enhanced Antifungal Efficacy of Validamycin A Co-Administered with Bacillus velezensis TCS001 against Camellia anthracnose. Plants 2024, 13, 2743. [Google Scholar] [CrossRef]
- Srikhong, P.; Lertmongkonthum, K.; Sowanpreecha, R.; Rerngsamran, P. Bacillus sp. Strain M10 as A Potential Biocontrol Agent Protecting Chili Pepper and Tomato Fruits from Anthracnose Disease Caused by Colletotrichum capsici. BioControl 2018, 63, 833–842. [Google Scholar] [CrossRef]
- Rokni-Zadeh, H.; Mangas-Losada, A.; De Mot, R. PCR Detection of Novel Non-ribosomal Peptide Synthetase Genes in Lipopeptide-producing Pseudomonas. Microb. Ecol. 2011, 62, 941–947. [Google Scholar] [CrossRef]
- Gao, Y.; Guo, R.; Li, S. Draft Genome Sequence of Bacillus velezensis B6, A Rhizobacterium That Can Control Plant Diseases. Genome Ann. 2018, 6, e00182-18. [Google Scholar] [CrossRef]
- Lyng, M.; Jørgensen, J.P.B.; Schostag, M.D.; Jarmusch, S.A.; Aguilar, D.K.C.; Lozano-Andrade, C.N.; Kovács, Á.T. Competition for Iron Shapes Metabolic Antagonism between Bacillus subtilis and Pseudomonas marginalis. ISME J. 2024, 18, wrad001. [Google Scholar] [CrossRef]
- Zheng, M.; Shi, J.; Shi, J.; Wang, Q.; Li, Y. Antimicrobial Effects of Volatiles Produced by Two Antagonistic Bacillus Strains on the Anthracnose Pathogen in Postharvest Mangos. Biol. Control 2013, 65, 200–206. [Google Scholar] [CrossRef]
- Calvo, H.; Mendiara, I.; Arias, E.; Gracia, A.P.; Blanco, D.; Venturini, M.E. Antifungal Activity of the Volatile Organic Compounds Produced by Bacillus velezensis Strains Against Postharvest Fungal Pathogens. Postharvest Biol. Technol. 2020, 166, 111208. [Google Scholar] [CrossRef]
- Garrido, A.; Atencio, L.A.; Bethancourt, R.; Bethancourt, A.; Guzmán, H.; Gutiérrez, M.; Durant-Archibold, A.A. Antibacterial Activity of Volatile Organic Compounds Produced by the Octocoral-Associated Bacteria Bacillus sp. BO53 and Pseudoalteromonas sp. GA327. Antibiotics 2020, 9, 923. [Google Scholar] [CrossRef] [PubMed]
- Melkina, O.E.; Khmel, I.A.; Plyuta, V.A.; Koksharova, O.A.; Zavilgelsky, G.B. Ketones 2-Heptanone, 2-Nonanone, and 2-Undecanone Inhibit DnaK-Dependent Refolding of Heat-Inactivated Bacterial Luciferases in Escherichia coli Cells Lacking Small Chaperon IbpB. Appl. Microbiol. Biotechnol. 2017, 101, 5765–5771. [Google Scholar] [CrossRef]
- Koksharova, O.A.; Popova, A.A.; Plyuta, V.A.; Khmel, I.A. Four New Genes of Cyanobacterium Synechococcus elongatus PCC 7942 Are Responsible for Sensitivity to 2-Nonanone. Microorganisms 2020, 8, 1234. [Google Scholar] [CrossRef] [PubMed]
- Plyuta, V.A.; Popova, A.A.; Koksharova, O.A.; Kuznetsov, A.E.; Khmel, I.A. The Ability of Natural Ketones to Interact with Bacterial Quorum Sensing Systems. Mol. Genet. Microbiol. 2014, 29, 167–171. [Google Scholar] [CrossRef]
- Cheng, W.; Yang, J.; Nie, Q.; Huang, D.; Yu, C.; Zheng, L.; Cai, M.; Thomashow, L.S.; Weller, D.M.; Yu, Z.; et al. Volatile Organic Compounds from Paenibacillus polymyxa KM2501-1 Control Meloidogyne incognita by Multiple Strategies. Sci. Rep. 2017, 7, 16213. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).