Effects of Fertilization on Stoichiometric Characteristics, Rhizosphere Microorganisms and Metabolites Under Substrate Cultivation for Pepper
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Materials
2.2. Experimental Design
2.3. Determination of Physiological Indices of Plants and Cultivation Substrates
2.3.1. Determination of Plant Morphology and Biomass of Peppers
2.3.2. Determination of Plant Photosynthetic Capacity
2.4. Analysis of Plant and Soil Elemental Content
2.5. Determination of Pepper Fruit Quality
2.6. Analysis of Microbial Diversity in Cultivation Substrates
2.7. Non-Targeted Metabolome Analysis of Cultivation Substrates
2.8. Data Analysis
3. Results
3.1. Effects of Fertilization Amount and Frequency on the Substrate Element Content
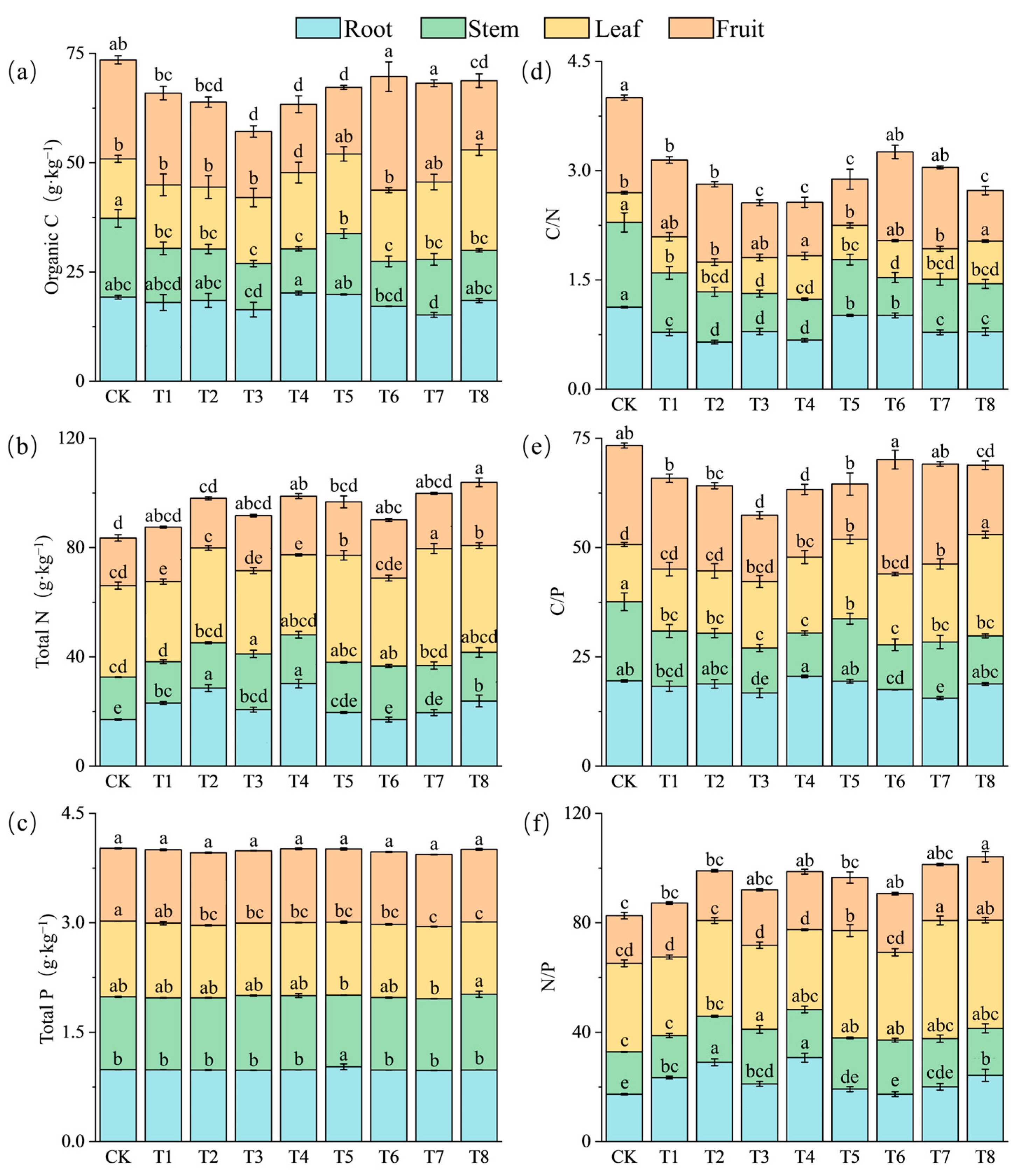
3.2. Effects of Fertilization Amount and Frequency on Element Content of Pepper Plants
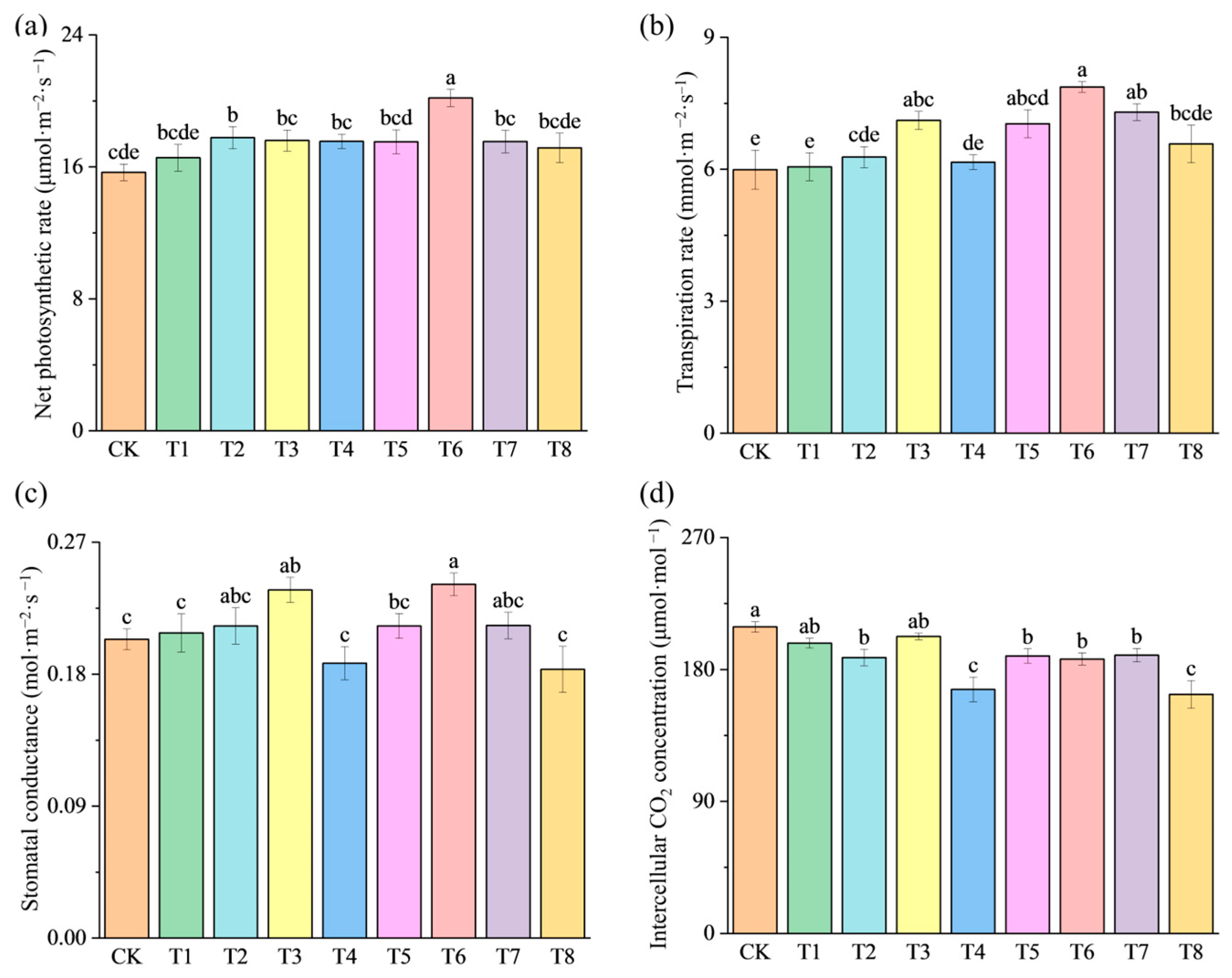
3.3. Effects of Fertilization Amount and Frequency on Photosynthesis of Pepper Plants
3.4. Effects of Fertilization Amount and Frequency on Pepper Quality
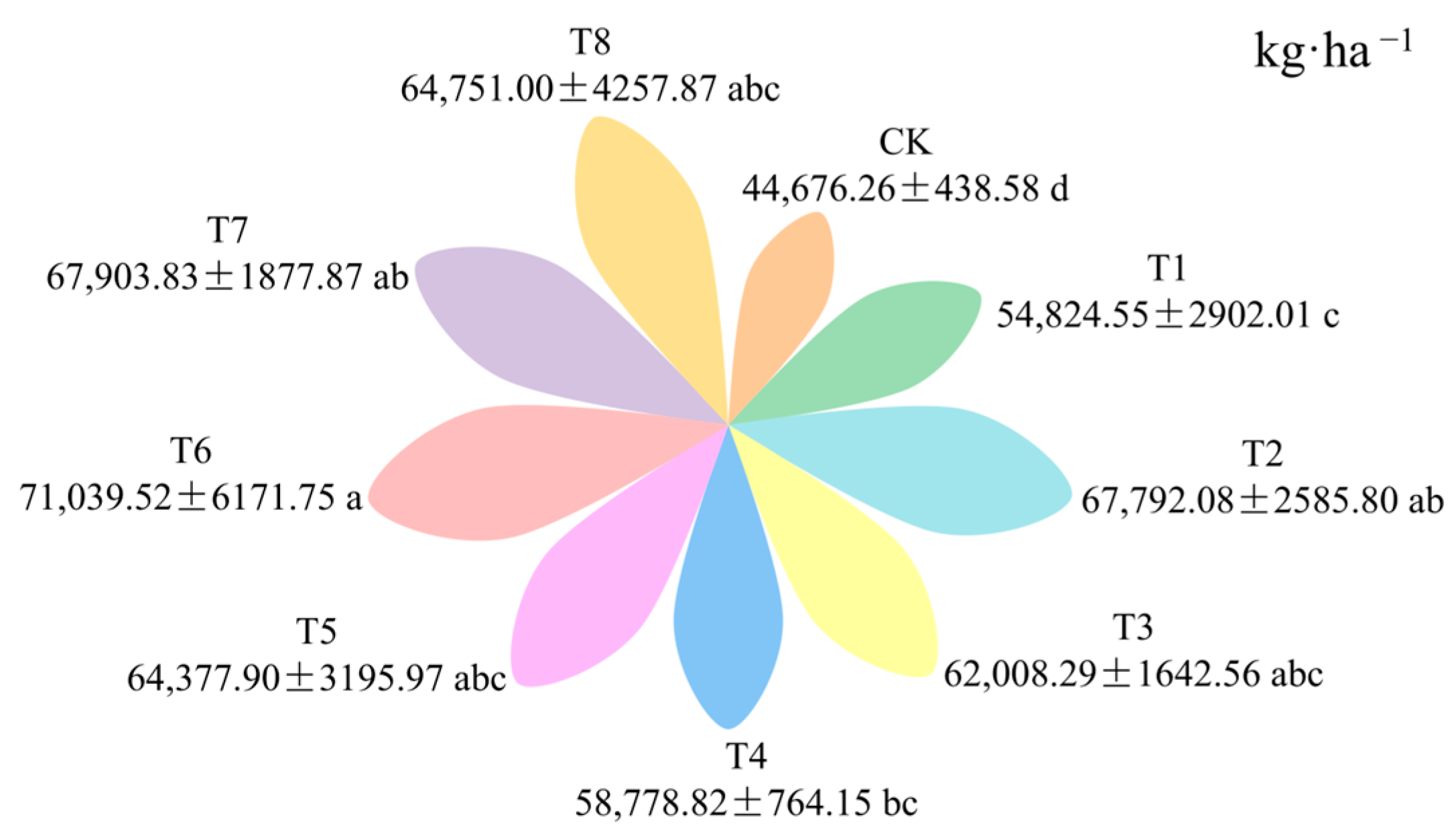
3.5. Effects of Fertilization Amount and Frequency on Pepper Yield
3.6. Comprehensive Evaluation of the Effects of Fertilization Amount and Frequency on Pepper
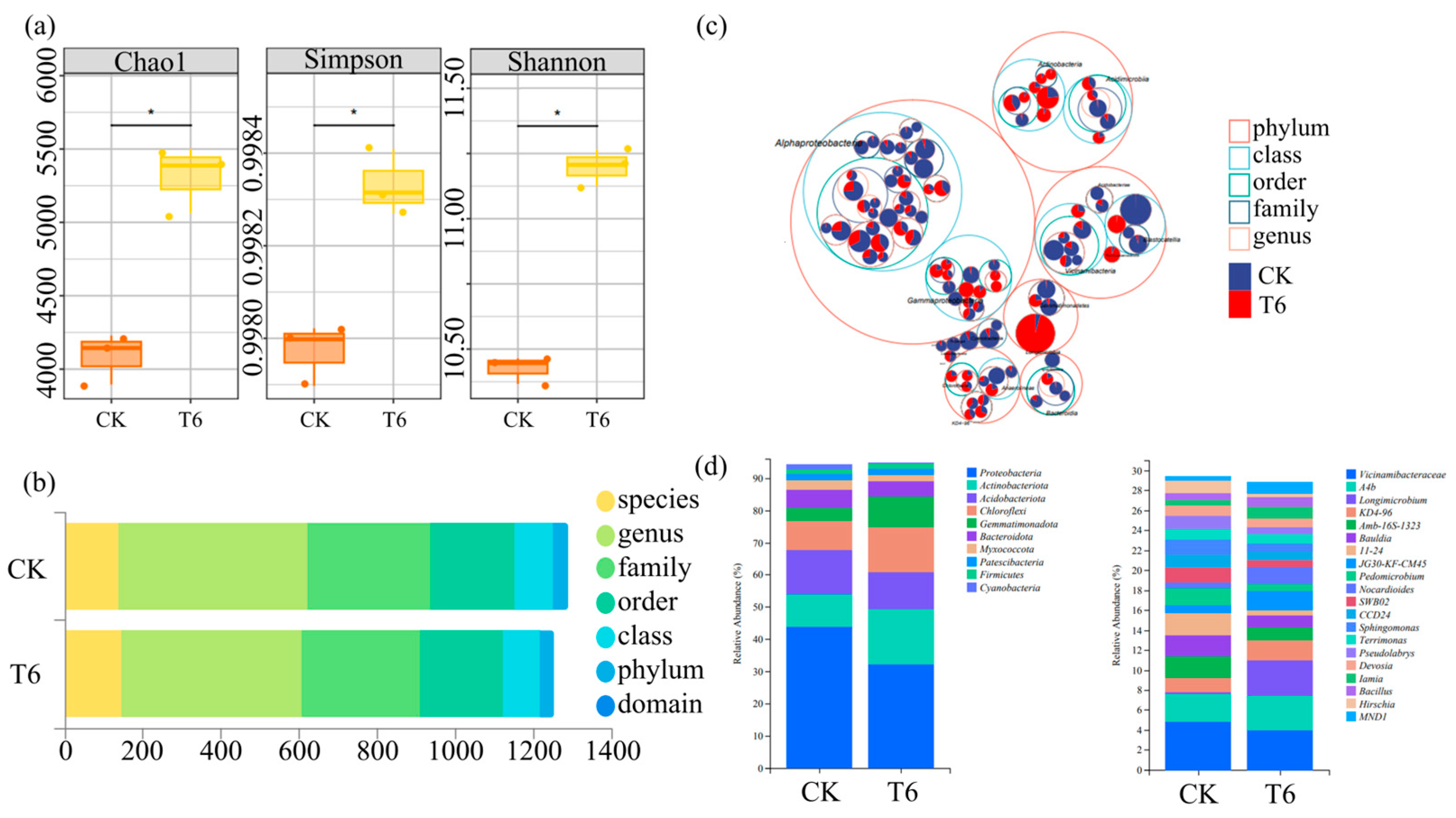
3.7. Effects of Fertilization Amount and Frequency on Rhizosphere Microorganisms
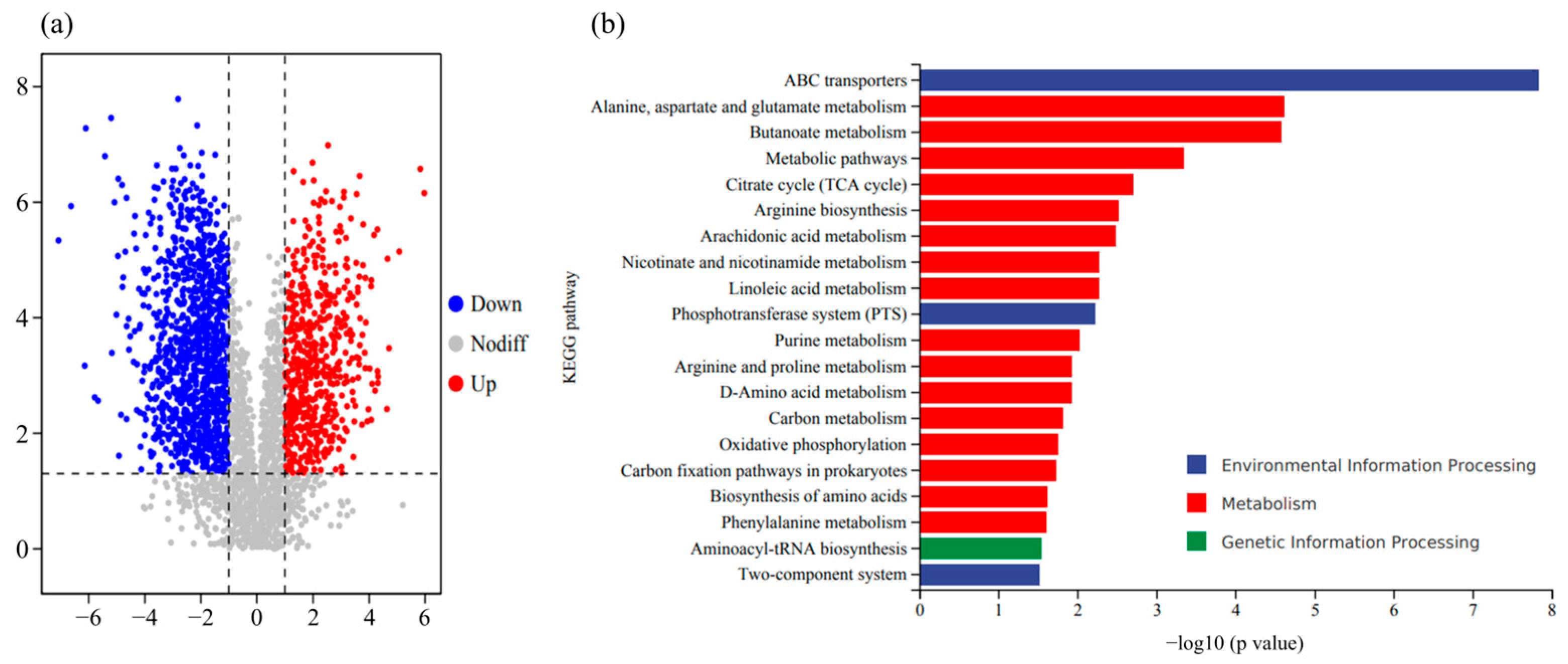
3.8. Effects of Fertilization Amount and Frequency on Pepper Metabolites
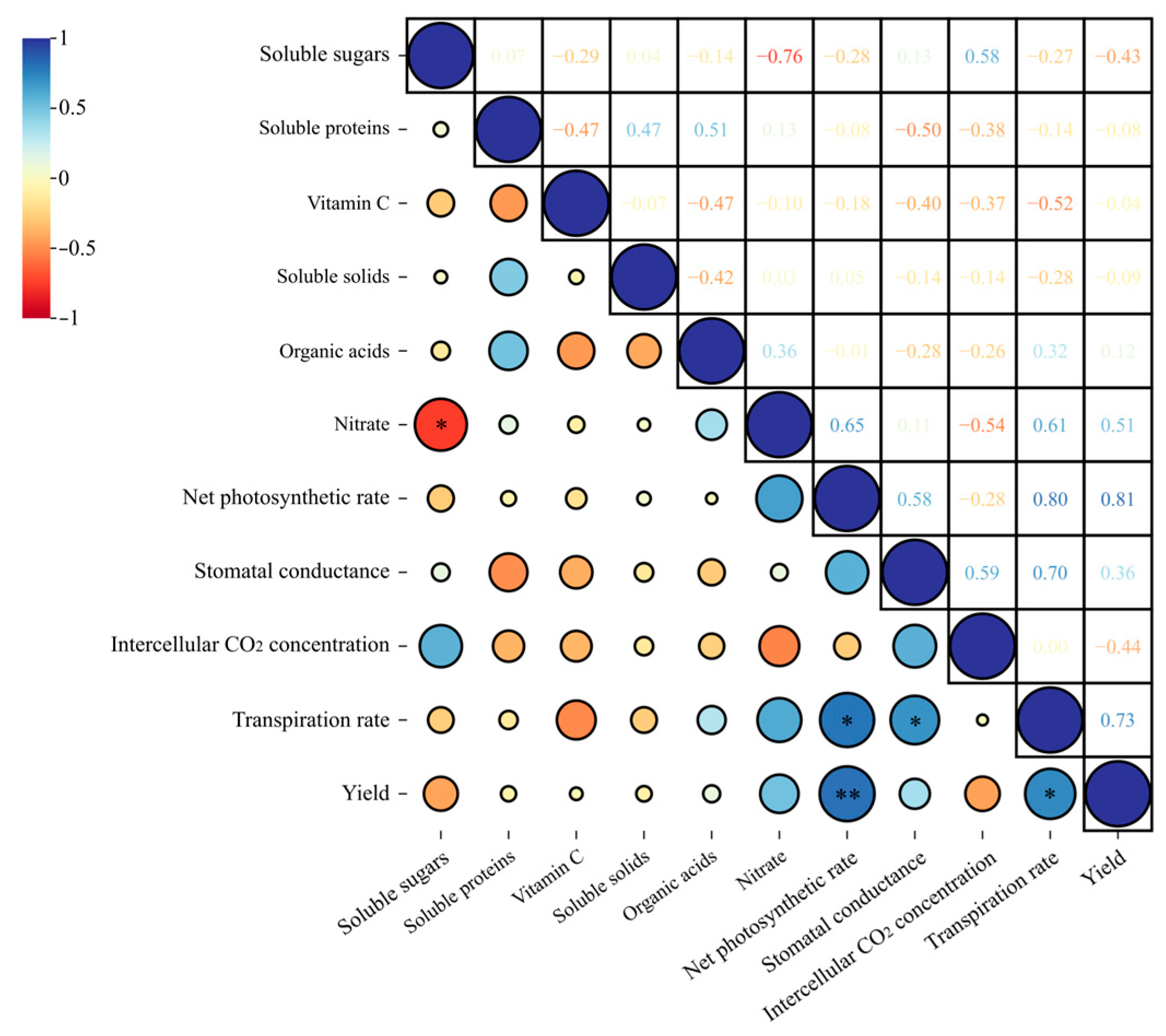
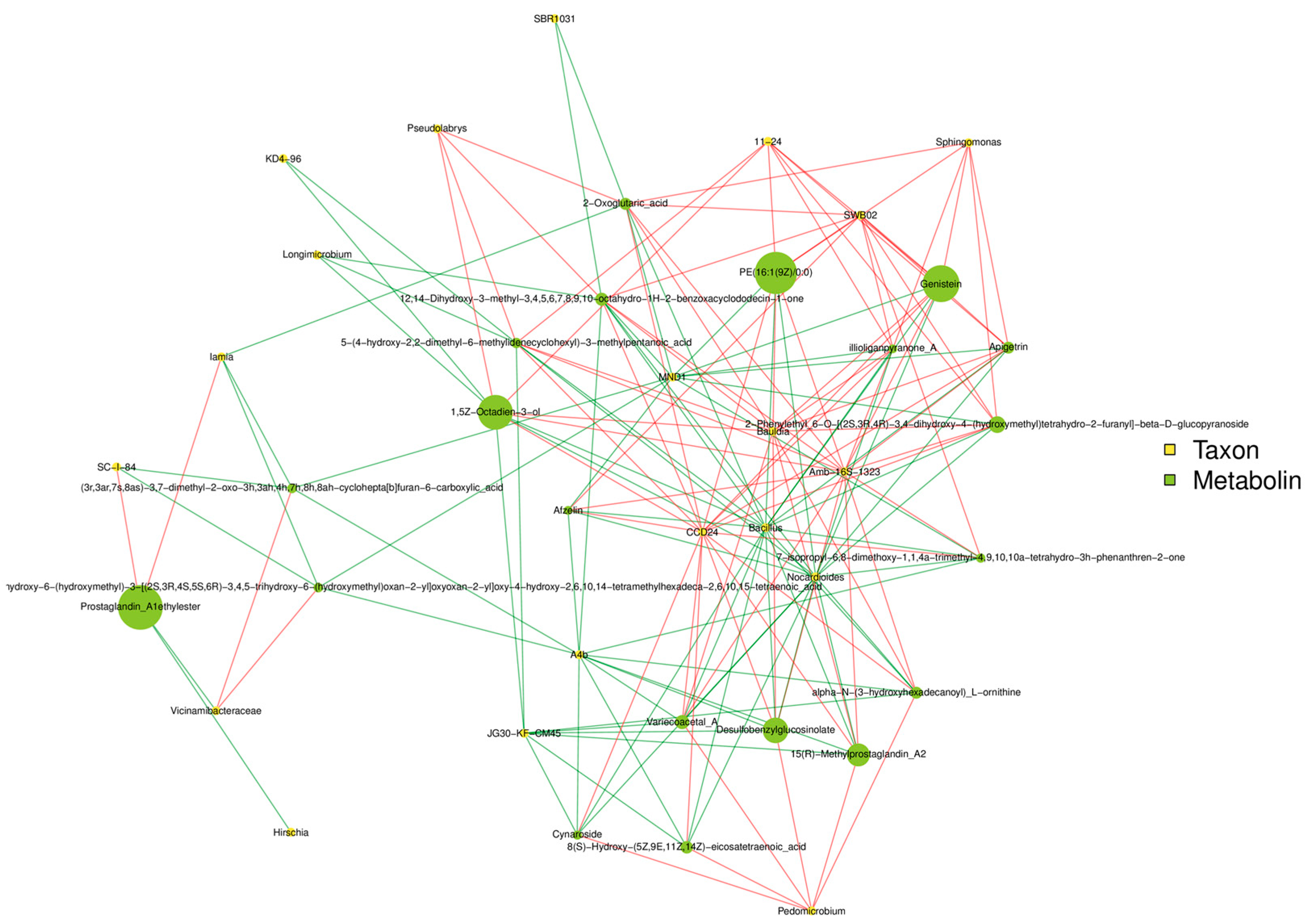
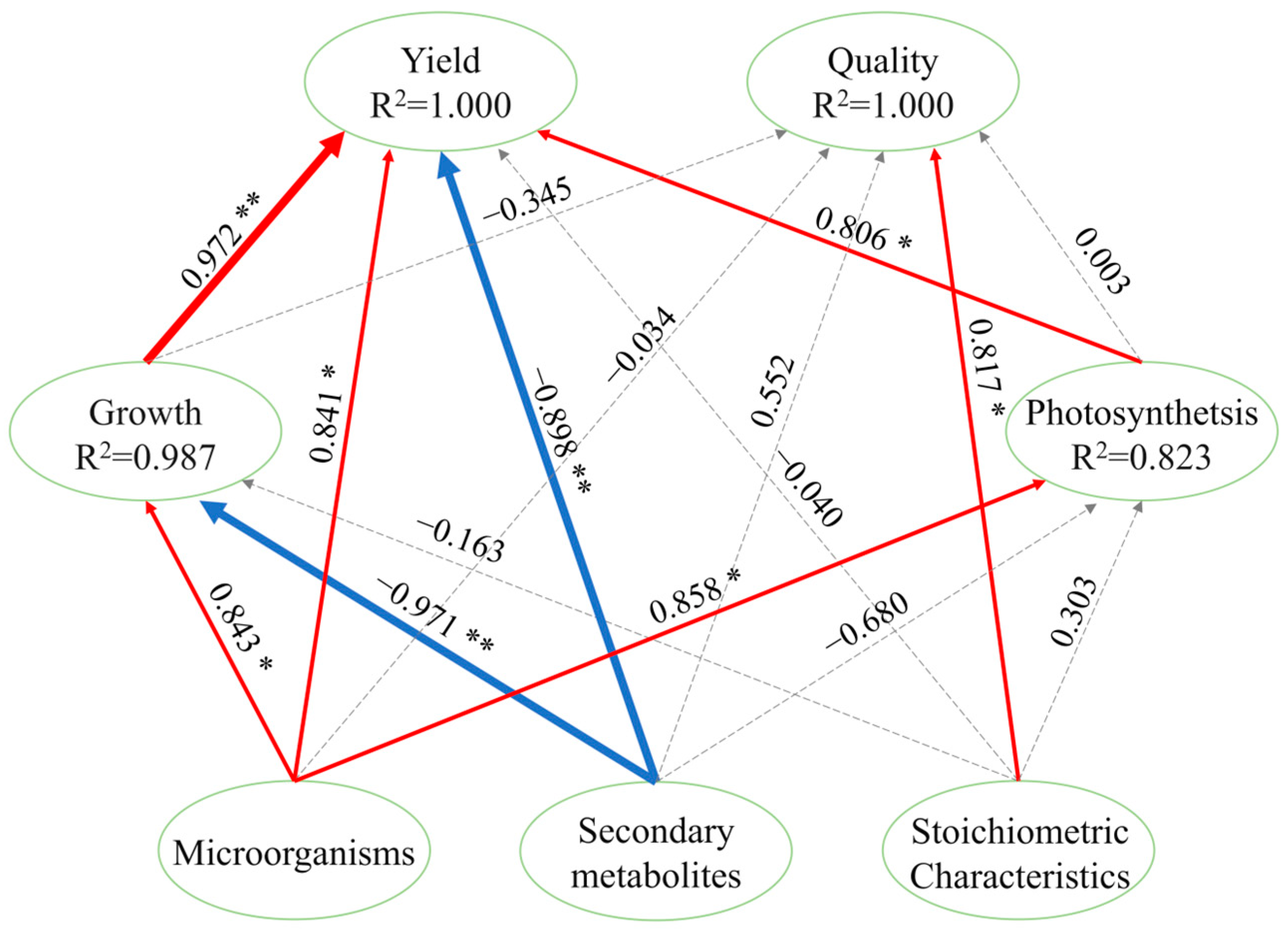
3.9. Combined Analysis of Pepper Yield and Quality Considering Growth, Stoichiometric Characteristics, Microorganisms, and Metabolites
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Wang, Y.Y.; Piao, F.Z.; Di, Y.C.; Xu, J.X.; Wang, Z.C.; Wang, T.; Li, P.J.; Hu, C.Y.; Du, N.S.; Zhang, T.; et al. Serratia plymuthica HK9-3 enhances tomato resistance against Phytophthora capsici by modulating antioxidant defense systems and rhizosphere micro-ecological condition. Physiol. Plant. 2024, 176, e14323. [Google Scholar] [CrossRef] [PubMed]
- Ren, Y.; Yu, P.B.; Wang, Y.; Hou, W.X.; Yang, X.; Fan, J.L.; Wu, X.H.; Lv, X.L.; Zhang, N.; Zhao, L.; et al. Development of a rapid approach for detecting sharp eyespot resistance in seedling-stage wheat and its application in Chinese wheat cultivars. Plant Dis. 2020, 104, 1662–1667. [Google Scholar] [CrossRef] [PubMed]
- Feng, Z.H.; Wang, L.Y.; Yang, Z.Q.; Zhang, Y.Y.; Li, X.; Song, L.; He, L.; Duan, J.Z.; Feng, W. Hyperspectral monitoring of powdery mildew disease severity in wheat based on machine learning. Front. Plant Sci. 2022, 13, 828454. [Google Scholar] [CrossRef]
- Lai, Y.; Ma, J.H.; Zhang, X.B.; Xuan, X.B.; Zhu, F.Y.; Ding, S.; Shang, F.D.; Chen, Y.Y.; Zhao, B.; Chen, L.; et al. High-quality chromosome-level genome assembly and multi-omics analysis of rosemary (Salvia rosmarinus) reveals new insights into the environmental and genome adaptation. Plant Biotechnol. J. 2024, 22, 1833–1847. [Google Scholar] [CrossRef]
- Lakhiar, I.A.; Gao, J.M.; Syed, T.N.; Chandio, F.A.; Buttar, N.A. Modern plant cultivation technologies in agriculture under controlled environment: A review on aeroponics. J. Plant Interact. 2018, 13, 338–352. [Google Scholar] [CrossRef]
- Wang, R.N.; Qian, J.J.; Fang, Z.M.; Tang, J.H. Transcriptomic and physiological analyses of rice seedlings under different nitrogen supplies provide insight into the regulation involved in axillary bud outgrowth. BMC Plant Biol. 2020, 20, 197. [Google Scholar] [CrossRef]
- Verruma-Bernardi, M.R.; Pimenta, D.M.; Levrero, G.R.; Forti, V.A.; Medeiros, S.D.; Ceccato-Antonini, S.R.; Covre, E.A.; Ferreira, M.D.; Moret, R.; Bernardi, A.C.; et al. Yield and quality of curly kale grown using organic fertilizers. Hortic. Bras. 2021, 39, 112–121. [Google Scholar] [CrossRef]
- Liu, W.X.; Wang, J.R.; Wang, C.Y.; Ma, G.; Wei, Q.R.; Lu, H.F.; Xie, Y.X.; Ma, D.Y.; Kang, G.Z. Root growth, water and nitrogen use efficiencies in winter wheat under different irrigation and nitrogen regimes in North China Plain. Front. Plant Sci. 2018, 9, 1798. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.X.; Ma, G.; Wang, C.Y.; Wang, J.R.; Lu, H.F.; Li, S.S.; Fang, W.; Xie, Y.X.; Ma, D.Y.; Kang, G.Z. Irrigation and nitrogen regimes promote the use of soil water and nitrate nitrogen from deep soil layers by regulating root growth in wheat. Front. Plant Sci. 2018, 9, 32. [Google Scholar] [CrossRef]
- Pahuja, S.; Bheri, M.; Bisht, D.; Pandey, G.K. Calcium signalling components underlying NPK homeostasis: Potential avenues for exploration. Biochem. J. 2023, 48, 1015–1034. [Google Scholar] [CrossRef]
- Nosheen, S.; Ajmal, I.; Song, Y.D. Microbes as biofertilizers, a potential approach for sustainable crop production. Sustainability 2021, 13, 1868. [Google Scholar] [CrossRef]
- Zhou, Z.F.; Shi, X.J.; Zheng, Y.; Qin, Z.X.; Xie, D.T.; Li, Z.L.; Guo, T. Abundance and community structure of ammonia-oxidizing bacteria and archaea in purple soil under long-term fertilization. Eur. J. Soil Biol. 2014, 60, 24–33. [Google Scholar] [CrossRef]
- Stringlis, I.A.; Yu, K.; Feussner, K.; Jonge, R.D.; Bentum, S.V.; Verk, M.C.V.; Berendsen, R.L.; Bakker, P.A.H.M.; Feussner, I.; Pieterse, C.M.J. MYB72-dependent coumarin exudation shapes root microbiome assembly to promote plant health. Proc. Natl. Acad. Sci. USA 2018, 115, 5213–5222. [Google Scholar] [CrossRef]
- Thakur, M.P.; Geisen, S. Trophic regulations of the soil microbiome. Trends Microbiol. 2019, 27, 771–780. [Google Scholar] [CrossRef]
- Leff, J.W.; Jones, S.E.; Prober, S.M.; Barberán, A.; Borer, E.T.; Firn, J.L.; Harpole, W.S.; Hobbie, S.E.; Hofmockel, K.S.; Knops, J.M.H.; et al. Consistent responses of soil microbial communities to elevated nutrient inputs in grasslands across the globe. Proc. Natl. Acad. Sci. USA 2015, 112, 10967–10972. [Google Scholar] [CrossRef]
- Saxena, D.; Stewart, C.N.; Altosaar, I.; Shu, Q.Y.; Stotzky, G. Larvicidal Cry proteins from Bacillus thuringiensis are released in root exudates of transgenic B. thuringiensis corn, potato, and rice but not of B. thuringiensis canola, cotton, and tobacco. Plant Physiol. Biochem. 2004, 42, 383–387. [Google Scholar] [CrossRef] [PubMed]
- Ansari, M.M.; Bisht, N.; Singh, T.; Chauhan, P.S. Symphony of survival: Insights into cross-talk mechanisms in plants, bacteria, and fungi for strengthening plant immune responses. Microbiol. Res. 2024, 285, 127762. [Google Scholar] [CrossRef] [PubMed]
- Yang, T.; Siddique, K.; Liu, K. Cropping systems in agriculture and their impact on soil health-A review. Glob. Ecol. Conserv. 2020, 23, e01118. [Google Scholar] [CrossRef]
- Xu, L.T.; Jin, S.; Lyu, X.C.; Yan, S.S.; Wang, C.; Cao, L.; Yan, C.; Ma, C.M. Combined metagenomics and metabolomic analysis of microbial community structure and metabolic function in continuous soybean cropping soils of Songnen Plain, China. Chem. Biol. Technol. Agric. 2024, 11, 46. [Google Scholar] [CrossRef]
- Eichler-Löbermann, B.; Köhne, S.; Köppen, D. Effect of organic, inorganic, and combined organic and inorganic P fertilization on plant P uptake and soil P pools. J. Plant Nutr. Soil Sci. 2007, 170, 623–628. [Google Scholar] [CrossRef]
- You, C.; Qin, A.; Wang, Y.M.; Lan, W.Y.; Li, Y.H.; Yu, B.H.; Peng, Y.J.; Xu, J.R.; Dong, J.Y. Plant triterpenoids regulate endophyte community to promote medicinal plant Schisandra sphenanthera growth and metabolites accumulation. J. Fungi 2022, 7, 788. [Google Scholar] [CrossRef] [PubMed]
- Lucas, J.; Koester, I.; Wichels, A.; Niggemann, J.; Dittmar, T.; Callies, U.; Wiltshire, K.H.; Gerdts, G. Short-term dynamics of north sea bacterioplankton-dissolved organic matter coherence on molecular level. Front. Microbiol. 2016, 7, 321. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.J.; Sun, D.L.; Chen, L.J.; An, Y.X. Integrative analysis of the microbiome and metabolome in understanding the causes of sugarcane bitterness. Sci. Rep. 2021, 11, 6024. [Google Scholar] [CrossRef]
- Stipcovich, T.; Barbero, G.F.; Ferreiro-González, M.; Palma, M.; Barroso, C.G. Fast analysis of capsaicinoids in Naga Jolokia extracts (Capsicum chinense) by high-performance liquid chromatography using fused core columns. Food Chem. 2018, 239, 217–224. [Google Scholar] [CrossRef] [PubMed]
- Li, H.S. Principles and Techniques of Plant Physiological and Biochemical Experiments; Higher Education Press: Beijing, China, 2000. (In Chinese) [Google Scholar]
- Bao, S.D. Analysis of Soil Agrochemistry, 3rd ed.; China Agriculture Press: Beijing, China, 2000. (In Chinese) [Google Scholar]
- Gao, J.F. Plant Physiology Experimental Guidance; Higher Education Press: Beijing, China, 2006. (In Chinese) [Google Scholar]
- Chao, A. Nonparametric Estimation of the Number of Classes in a Population. Scand. J. Stat. 1984, 11, 265–270. [Google Scholar]
- Shannon, C.E. A mathematical theory of communication. Bell Syst. Tech. J. 1948, 27, 379–423. [Google Scholar] [CrossRef]
- Simpson, E.H. Measurement of Diversity. Nature 1949, 163, 688. [Google Scholar] [CrossRef]
- Chen, P.Y. A novel coordinated TOPSIS based on coefficient of variation. Mathematics 2019, 7, 614. [Google Scholar] [CrossRef]
- Yang, Q.; Li, J.L.; Xu, W.X.; Wang, J.J.; Jiang, Y.M.; Ali, W.Q.; Liu, W.J. Substitution of inorganic fertilizer with organic fertilizer influences soil carbon and nitrogen content and enzyme activity under rubber plantation. Forests 2024, 15, 756. [Google Scholar] [CrossRef]
- Brown, R.W.; Chadwick, D.R.; Bending, G.D.; Collins, C.D.; Whelton, H.L.; Daulton, E.; Covington, J.A.; Bull, I.D.; Jones, D.L. Nutrient (C, N and P) enrichment induces significant changes in the soil metabolite profile and microbial carbon partitioning. Soil Biol. Biochem. 2022, 172, 108779. [Google Scholar] [CrossRef]
- Lian, X.L.; Liu, S.; Sikandar, A.; Kang, Z.L.; Feng, Y.X.; Jiang, L.Y.W.; Wang, Y.Y. The influence of 6-Benzylaminopurine (BAP) on yield responses and photosynthetic physiological indices of soybean. Kuwait J. Sci. 2023, 50, 345–352. [Google Scholar] [CrossRef]
- Sade, N.; Gebretsadik, M.; Seligmann, R.; Schwartz, A.; Wallach, R.; Moshelion, M. The role of tobacco aquaporin1 in improving water use efficiency, hydraulic conductivity, and yield production under salt stress. Plant Physiol. 2010, 152, 245–254. [Google Scholar] [CrossRef]
- Weksler, S.; Rozenstein, O.; Haish, N.; Moshelion, M.; Wallach, R.; Ben-Dor, E. Detection of potassium deficiency and momentary transpiration rate estimation at early growth stages using proximal hyperspectral imaging and extreme gradient boosting. Sensors 2021, 21, 958. [Google Scholar] [CrossRef] [PubMed]
- Sheikh, F.R.; Jose-Santhi, J.; Kalia, D.; Singh, K.; Singh, R.K. Sugars as the regulators of dormancy and sprouting in geophytes. Ind. Crops Prod. 2022, 189, 115817. [Google Scholar] [CrossRef]
- Glockow, T.; Kaster, A.; Rabe, K.S.; Niemeyer, C.M. Sustainable agriculture: Leveraging microorganisms for a circular economy. Appl. Microbiol. Biotechnol. 2024, 108, 452. [Google Scholar] [CrossRef] [PubMed]
- Krzmarzick, M.J.; Crary, B.B.; Harding, J.J.; Oyerinde, O.O.; Leri, A.C.; Myneni, S.C.B.; Novak, P.J. Natural niche for organohalide-respiring Chloroflexi. Appl. Environ. Microbiol. 2012, 78, 393–401. [Google Scholar] [CrossRef]
- Pan, Y.Q.; Kang, P.; Qu, X.; Zhang, H.X.; Li, X.R. Response of the soil bacterial community to seasonal variations and land reclamation in a desert grassland. Ecol. Indic. 2024, 165, 112227. [Google Scholar] [CrossRef]
- Mujakić, I.; Piwosz, K.; Koblížek, M. Phylum gemmatimonadota and its role in the environment. Microorganisms 2022, 10, 151. [Google Scholar] [CrossRef]
- Sánchez-López, A.M.; Bahaji, A.; Diego, N.D.; Baslam, M.; Li, J.; Muñoz, F.J.; Almagro, G.; García-Gómez, P.; Ameztoy, P.; Ricarte-Bermejo, A.; et al. Arabidopsis responds to Alternaria alternata volatiles by triggering plastid phosphoglucose isomerase-independent mechanisms. Plant Physiol. 2016, 172, 1989–2001. [Google Scholar] [CrossRef]
- Gámez-Arcas, S.; Baroja-Fernández, E.; García-Gómez, P.; José Muñoz, F.; Almagro, G.; Bahaji, A.; Sánchez-López, A.M.; Pozueta-Romero, J. Action mechanisms of small microbial volatile compounds in plants. J. Exp. Bot. 2022, 73, 498–510. [Google Scholar] [CrossRef]
- Wu, Y.J.; Ma, L.Y.; Liu, Q.Z.; Sikder, M.M.; Vestergård, M.; Zhou, K.; Wang, Q.; Yang, X.; Feng, Y. Pseudomonas fluorescens promote photosynthesis, carbon fixation and cadmium phytoremediation of hyperaccumulator Sedum alfredii. Sci. Total Environ. 2020, 726, 138554. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.Q.; Kong, D.X.; Fu, Y.; Sussman, M.R.; Wu, H. The effect of developmental and environmental factors on secondary metabolites in medicinal plants. Plant Physiol. Biochem. 2020, 148, 80–89. [Google Scholar] [CrossRef] [PubMed]
- Chaiwanon, J.; Wang, W.F.; Zhu, J.Y.; Oh, E.; Wang, Z.Y. Information integration and communication in plant growth regulation. Cell 2016, 164, 1257–1268. [Google Scholar] [CrossRef]
- Wang, S.; Bian, T.; Wu, T.; Zhang, Y.D.; Awais, M.; Fu, H.D.; Sun, Z.P. Co-analysis of cucumber rhizosphere metabolites and microbial PLFAs under excessive fertilization in solar greenhouse. Frotiers Microbiol. 2022, 13, 1004836. [Google Scholar] [CrossRef]
- Fernandez, M.; Paulucci, N.S.; Reynos, E.; Morales, G.M.; Agostini, E.; González, P.S. Morphological and structural response of Bacillus sp. SFC 500-1E after Cr(VI) and phenol treatment. J. Basic Microbiol. 2020, 60, 679–690. [Google Scholar] [CrossRef] [PubMed]
- Mahgoub, S.A.; Qattan, S.Y.A.; Salem, S.S.; Abdelbasit, H.M.; Raafat, M.; Ashkan, M.F.; Al-Quwaie, D.A.; Motwali, E.A.; Alqahtani, F.S.; El-Fattah, H.A. Characterization and biodegradation of phenol by Pseudomonas aeruginosa and Klebsiella variicola strains isolated from sewage sludge and their effect on soybean seeds germination. Molecules 2023, 28, 1203. [Google Scholar] [CrossRef]
- Fang, K.; Kou, Y.P.; Tang, N.; Liu, J.; Zhang, X.Y.; He, H.L.; Xia, R.X.; Zhao, W.Q.; Li, D.D.; Liu, Q. Differential responses of soil bacteria, fungi and protists to root exudates and temperature. Microbiol. Res. 2024, 286, 127829. [Google Scholar] [CrossRef]

| Treatment | Fertilization Frequency | Fertilization Amount/kg·ha−1 | ||
|---|---|---|---|---|
| Amount of Fertilizer Applied Each Time During Vegetative Growth Period (28 March to 7 May 2024) | Amount of Fertilizer Applied Each Time During Fruiting Period (8 May to 6 July 2024) | Total Amount of Fertilizer over the Growing Period | ||
| CK | Not fertilized | 0 | 0 | 0 |
| T1 | Once every 5 days | 18.93 (8 times in total) | 18.93 (12 times in total) | 378.5 |
| T2 | Once every 5 days | 18.93 (8 times in total) | 37.85 (12 times in total) | 605.6 |
| T3 | Once every 5 days | 37.85 (8 times in total) | 18.93 (12 times in total) | 529.9 |
| T4 | Once every 5 days | 37.85 (8 times in total) | 37.85 (12 times in total) | 757.0 |
| T5 | Once every 10 days | 37.85 (4 times in total) | 37.85 (6 times in total) | 378.5 |
| T6 | Once every 10 days | 37.85 (4 times in total) | 75.70 (6 times in total) | 605.6 |
| T7 | Once every 10 days | 75.70 (4 times in total) | 37.85 (6 times in total) | 529.9 |
| T8 | Once every 10 days | 75.70 (4 times in total) | 75.70 (6 times in total) | 757.0 |
| Treatment | Organic C/g·kg−1 | Total N/g·kg−1 | Total P/g·kg−1 | Available N/mg·kg−1 | Available P/mg·kg−1 |
|---|---|---|---|---|---|
| CK | 16.14 ± 0.96 a | 8.42 ± 0.11 a | 0.99 ± 0.00 c | 350.00 ± 0.00 a | 7.21 ± 0.58 ab |
| T1 | 13.01 ± 0.20 bc | 8.05 ± 0.77 a | 1.01 ± 0.01 abc | 350.00 ± 0.00 a | 8.02 ± 0.06 a |
| T2 | 13.68 ± 0.10 abc | 8.27 ± 0.31 a | 1.00 ± 0.01 abc | 233.33 ± 23.33 bc | 7.33 ± 0.69 ab |
| T3 | 14.32 ± 0.19 ab | 8.65 ± 0.30 a | 1.00 ± 0.00 bc | 303.33 ± 46.67 bc | 7.40 ± 0.66 ab |
| T4 | 11.54 ± 0.96 bcd | 8.12 ± 0.41 a | 1.00 ± 0.01 bc | 210.00 ± 0.00 c | 6.89 ± 0.49 ab |
| T5 | 11.08 ± 2.41 cde | 8.09 ± 0.45 a | 1.04 ± 0.01 a | 233.33 ± 46.67 bc | 5.93 ± 0.59 b |
| T6 | 10.80 ± 1.19 cde | 8.20 ± 0.24 a | 1.01 ± 0.03 abc | 233.33 ± 23.33 bc | 7.40 ± 0.48 ab |
| T7 | 8.40 ± 0.27 e | 7.93 ± 0.12 a | 1.02 ± 0.01 abc | 326.67 ± 46.67 a | 6.64 ± 0.66 ab |
| T8 | 9.53 ± 0.30 de | 8.50 ± 0.16 a | 1.03 ± 0.01 ab | 373.33 ± 23.33 a | 6.51 ± 0.73 ab |
| Treatment | Soluble Sugars/% | Soluble Proteins/mg·g−1 | Soluble Solids/% | Vitamin C/mg·100g−1 | Organic Acids/% | Nitrate/mg·kg−1 |
|---|---|---|---|---|---|---|
| CK | 59.25 ± 12.32 a | 28.20 ± 0.14 abc | 2.43 ± 0.09 bc | 111.67 ± 18.78 bcd | 0.12 ± 0.01 ab | 9.76 ± 0.01 b |
| T1 | 41.26 ± 0.46 bc | 28.03 ± 0.15 bc | 2.93 ± 0.32 b | 144.22 ± 6.90 abc | 0.11 ± 0.01 bc | 10.41 ± 0.24 ab |
| T2 | 53.17 ± 1.36 ab | 27.80 ± 0.28 cd | 3.03 ± 0.18 b | 155.72 ± 15.91 ab | 0.10 ± 0.00 c | 9.75 ± 0.11 b |
| T3 | 43.42 ± 1.57 bc | 27.09 ± 0.13 d | 2.37 ± 0.49 bc | 128.76 ± 10.42 abcd | 0.10 ± 0.00 c | 10.34 ± 0.25 ab |
| T4 | 43.08 ± 2.20 bc | 29.02 ± 0.13 a | 4.40 ± 0.47 a | 129.60 ± 27.90 abcd | 0.11 ± 0.01 bc | 10.71 ± 0.13 ab |
| T5 | 48.70 ± 3.53 abc | 28.84 ± 0.23 ab | 2.17 ± 0.35 bc | 86.10 ± 14.15 d | 0.13 ± 0.00 a | 10.23 ± 0.08 ab |
| T6 | 45.12 ± 6.52 abc | 27.99 ± 0.51 bcd | 2.53 ± 0.38 b | 107.97 ± 7.49 bcd | 0.12 ± 0.01 bc | 11.18 ± 0.79 a |
| T7 | 43.64 ± 1.80 bc | 28.51 ± 0.24 abc | 3.07 ± 0.20 b | 94.72 ± 28.32 cd | 0.13 ± 0.00 a | 10.68 ± 0.13 ab |
| T8 | 37.86 ± 2.69 c | 27.90 ± 0.57 cd | 1.47 ± 0.48 c | 164.48 ± 7.81 a | 0.13 ± 0.00 a | 10.78 ± 0.41 ab |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Di, Q.; Ji, E.; Du, Q.; Gu, G.; Li, J.; Li, M.; Wang, H.; Wang, P.; Xiao, H. Effects of Fertilization on Stoichiometric Characteristics, Rhizosphere Microorganisms and Metabolites Under Substrate Cultivation for Pepper. Horticulturae 2025, 11, 764. https://doi.org/10.3390/horticulturae11070764
Di Q, Ji E, Du Q, Gu G, Li J, Li M, Wang H, Wang P, Xiao H. Effects of Fertilization on Stoichiometric Characteristics, Rhizosphere Microorganisms and Metabolites Under Substrate Cultivation for Pepper. Horticulturae. 2025; 11(7):764. https://doi.org/10.3390/horticulturae11070764
Chicago/Turabian StyleDi, Qianqian, Enling Ji, Qingjie Du, Guilan Gu, Juanqi Li, Meng Li, Hu Wang, Panqiao Wang, and Huaijuan Xiao. 2025. "Effects of Fertilization on Stoichiometric Characteristics, Rhizosphere Microorganisms and Metabolites Under Substrate Cultivation for Pepper" Horticulturae 11, no. 7: 764. https://doi.org/10.3390/horticulturae11070764
APA StyleDi, Q., Ji, E., Du, Q., Gu, G., Li, J., Li, M., Wang, H., Wang, P., & Xiao, H. (2025). Effects of Fertilization on Stoichiometric Characteristics, Rhizosphere Microorganisms and Metabolites Under Substrate Cultivation for Pepper. Horticulturae, 11(7), 764. https://doi.org/10.3390/horticulturae11070764





