The Role of SQUAMOSA-PROMOTER BINDING PROTEIN-like (SPL) Transcription Factors in Plant Growth and Environmental Stress Response: A Comprehensive Review of Recent Advances
Abstract
1. Introduction
2. Structural Composition of SPL TFs
3. Regulatory Functions of SPL TFs
3.1. Seed Development
3.2. Root Biology
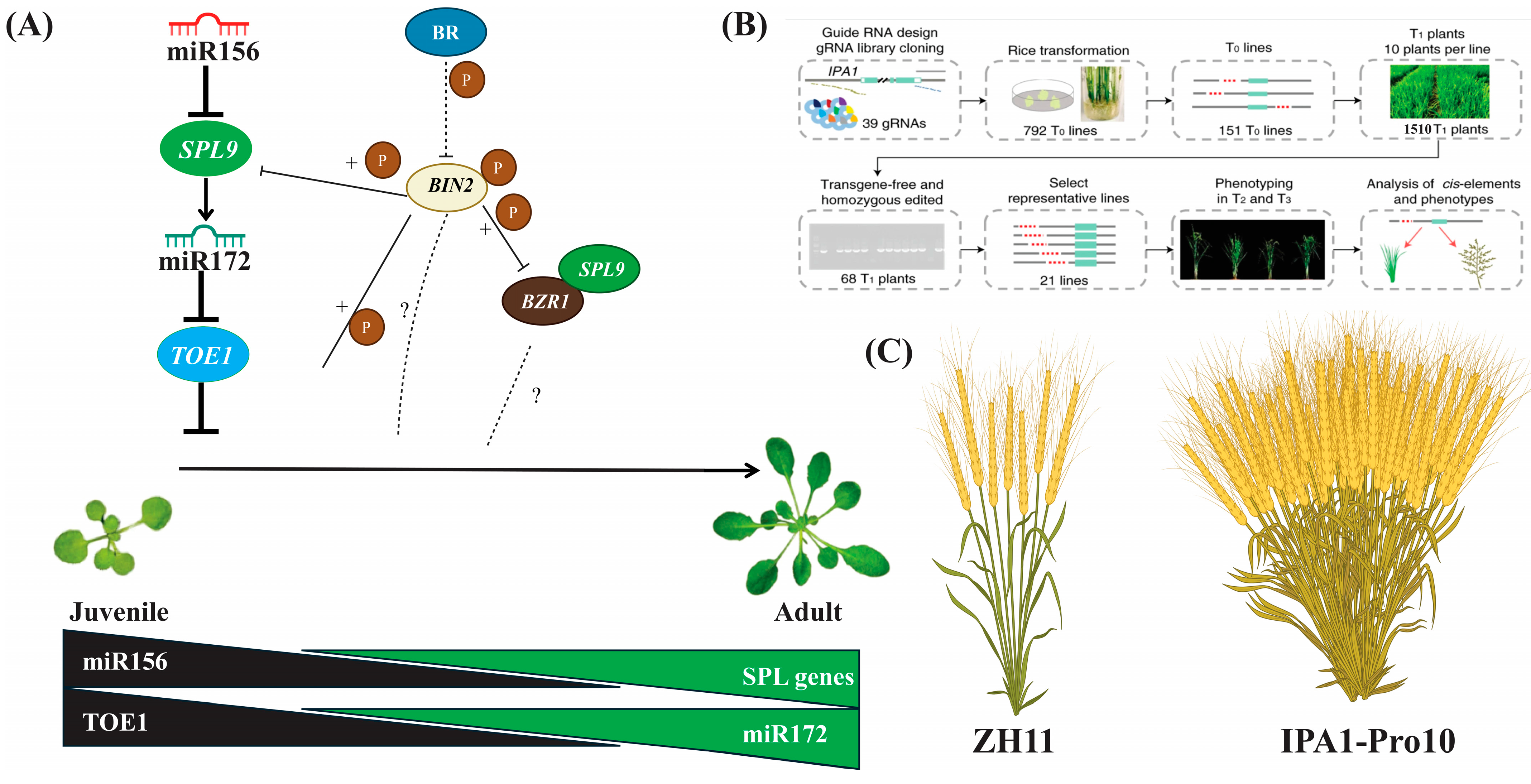
3.3. Vegetative Growth
3.4. Reproductive Biology
3.4.1. Flower Induction
3.4.2. Phase Transition
3.4.3. Tillering
3.4.4. Grains and Yield
3.4.5. Fruit Development
4. Postharvest Biology
5. Stress Response
5.1. Abiotic Stress
5.1.1. Cold Stress
5.1.2. Drought Stress
5.1.3. Flooding Stress
5.1.4. Heat Stress
5.1.5. Heavy Metal
5.2. Biotic Stresses
6. Conclusions and Future Perspectives
- (1)
- Since SPL genes are largely involved in hormonal metabolism, the functional role of these genes in regulating root development can be further evaluated.
- (2)
- The comprehensive role of these genes in the fruit development of horticultural crops is poorly understood. For instance, a high expression of SPL16-like in natural parthenocarpic cucumber lines has been observed. Its functional characterization could be vital in producing parthenocarpic fruits.
- (3)
- The questions of whether the targeted overexpression or silencing of SPL genes can optimize plant architecture to enhance agricultural productivity, and how stress-induced epigenetic modifications or post-translational regulatory mechanisms might be strategically leveraged to develop resilient, high-yielding crop varieties, remain unanswered.
- (4)
- SPL genes regulate rice responses to fungi, bacteria, and insects by modulating the JA and SA signaling pathways. However, no reports on their regulation in horticultural crops are available. In light of this research gap, the role of SPL genes could be studied in cucumbers under powdery mildew conditions or in tomato plants subjected to B. cinerea.
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| SPL | SQUAMOSA-PROMOTER BINDING PROTEIN-like |
| NLS | Nuclear localization signal |
| ROS | Reactive Oxygen Species |
| AP2 | Apetala2 |
| ERF | Ethylene Response Factor |
| HYL1 | Hyponastic Leaves 1 |
| TaTB1 | Teosinte Branched1 |
| SFT | Single Flower Truss |
References
- Sharif, R.; Zhu, Y.; Huang, Y.; Sohail, H.; Li, S.; Chen, X.; Qi, X. microRNA regulates cytokinin induced parthenocarpy in Cucumber (Cucumis sativus L.). Plant Physiol. Biochem. 2024, 212, 108681. [Google Scholar] [CrossRef] [PubMed]
- Sharif, R.; Su, L.; Chen, X.; Qi, X. Hormonal interactions underlying parthenocarpic fruit formation in horticultural crops. Hortic Res. 2022, 9, uhab024. [Google Scholar] [CrossRef]
- Liu, W.; Yang, Y.; Hu, Y.; Peng, X.; He, L.; Ma, T.; Zhu, S.; Xiang, L.; Chen, N. Overexpression of SQUAMOSA promoter binding protein-like 4a (NtSPL4a) alleviates Cd toxicity in Nicotiana tabacum. Plant Physiol. Biochem. 2024, 210, 108656. [Google Scholar] [CrossRef]
- Mouri, I.J.; Islam, M.S. A comprehensive in silico genome-wide identification and characterization of SQUAMOSA promoter binding protein (SBP) gene family in Musa acuminata. J. Genet. Eng. Biotechnol. 2025, 23, 100461. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Guo, H.; Liu, Z.; Wang, C.; Wang, Y. The homeodomain (PHD) protein, PdbPHD3, confers salt tolerance by regulating squamosa promoter binding protein PdbSBP3 in Populus davidiana × P. bolleana. Plant Physiol. Biochem. 2024, 216, 109128. [Google Scholar] [CrossRef] [PubMed]
- Preston, J.C.; Hileman, L. Functional Evolution in the Plant SQUAMOSA-PROMOTER BINDING PROTEIN-LIKE (SPL) Gene Family. Front. Plant Sci. 2013, 4, 80. [Google Scholar] [CrossRef]
- Wang, H.; Wang, H. The miR156/SPL Module, a Regulatory Hub and Versatile Toolbox, Gears up Crops for Enhanced Agronomic Traits. Mol. Plant 2015, 8, 677–688. [Google Scholar] [CrossRef]
- Li, Y.; Deng, Y.; Qin, D.; An, X. Study of the SPL gene family and miR156-SPL module in Populus tomentosa: Potential roles in juvenile-to-adult phase transition and reproductive phase. Int. J. Biol. Macromol. 2025, 296, 139547. [Google Scholar] [CrossRef]
- Ma, L.; Liu, X.; Liu, W.; Wen, H.; Zhang, Y.; Pang, Y.; Wang, X. Characterization of Squamosa-Promoter Binding Protein-Box Family Genes Reveals the Critical Role of MsSPL20 in Alfalfa Flowering Time Regulation. Front. Plant Sci. 2021, 12, 775690. [Google Scholar] [CrossRef]
- Leprince, O.; Pellizzaro, A.; Berriri, S.; Buitink, J. Late seed maturation: Drying without dying. J. Exp. Bot. 2016, 68, 827–841. [Google Scholar] [CrossRef]
- Yin, H.; Hong, G.; Li, L.; Zhang, X.; Kong, Y.; Sun, Z.; Li, J.; Chen, J.; He, Y. miR156/SPL9 Regulates Reactive Oxygen Species Accumulation and Immune Response in Arabidopsis thaliana. Phytopathology 2019, 109, 632–642. [Google Scholar] [CrossRef] [PubMed]
- Parreira, J.R.; Cappuccio, M.; Balestrazzi, A.; Fevereiro, P.; Araújo, S.d.S. MicroRNAs expression dynamics reveal post-transcriptional mechanisms regulating seed development in Phaseolus vulgaris L. Hortic. Res. 2021, 8, 18. [Google Scholar] [CrossRef] [PubMed]
- Dong, H.; Yan, S.; Jing, Y.; Yang, R.; Zhang, Y.; Zhou, Y.; Zhu, Y.; Sun, J. MIR156-Targeted SPL9 Is Phosphorylated by SnRK2s and Interacts With ABI5 to Enhance ABA Responses in Arabidopsis. Front. Plant Sci. 2021, 12, 708573. [Google Scholar] [CrossRef] [PubMed]
- Pan, J.; Sohail, H.; Sharif, R.; Hu, Q.; Song, J.; Qi, X.; Chen, X.; Xu, X. Cucumber JASMONATE ZIM-DOMAIN 8 interaction with transcription factor MYB6 impairs waterlogging-triggered adventitious rooting. Plant Physiol. 2024, 197, kiae351. [Google Scholar] [CrossRef]
- Pan, J.; Song, J.; Sohail, H.; Sharif, R.; Yan, W.; Hu, Q.; Qi, X.; Yang, X.; Xu, X.; Chen, X. RNA-seq-based comparative transcriptome analysis reveals the role of CsPrx73 in waterlogging triggered adventitious root formation in cucumber. Hortic. Res. 2024, 11, uhae062. [Google Scholar] [CrossRef]
- Shao, Y.; Zhou, H.-Z.; Wu, Y.; Zhang, H.; Lin, J.; Jiang, X.; He, Q.; Zhu, J.; Li, Y.; Yu, H.; et al. OsSPL3, an SBP-Domain Protein, Regulates Crown Root Development in Rice. Plant Cell 2019, 31, 1257–1275. [Google Scholar] [CrossRef]
- Ye, B.-B.; Shang, G.-D.; Pan, Y.; Xu, Z.-G.; Zhou, C.-M.; Mao, Y.-B.; Bao, N.; Sun, L.; Xu, T.; Wang, J.-W. AP2/ERF Transcription Factors Integrate Age and Wound Signals for Root Regeneration[OPEN]. Plant Cell 2019, 32, 226–241. [Google Scholar] [CrossRef]
- Wang, B.; Guo, X.; Qi, X.; Feng, F.; Zhu, X.; Hu, Y.; Li, J.; Zhao, Q.; Sun, H. OsSPL14 is involved in nitrogen-deficiency-induced root elongation in rice. Environ. Exp. Bot. 2022, 198, 104852. [Google Scholar] [CrossRef]
- Lian, L.; Xu, H.; Zhang, H.; He, W.; Cai, Q.; Lin, Y.; Wei, L.; Pan, L.; Xie, X.; Zheng, Y.; et al. Overexpression of OsSPL14 results in transcriptome and physiology changes in indica rice ‘MH86’. Plant Growth Regul. 2020, 90, 265–278. [Google Scholar] [CrossRef]
- Liu, K.; Cao, J.; Yu, K.; Liu, X.; Gao, Y.; Chen, Q.; Zhang, W.; Peng, H.; Du, J.; Xin, M.; et al. Wheat TaSPL8 Modulates Leaf Angle Through Auxin and Brassinosteroid Signaling. Plant Physiol. 2019, 181, 179–194. [Google Scholar] [CrossRef]
- Singer, S.D.; Burton Hughes, K.; Subedi, U.; Dhariwal, G.K.; Kader, K.; Acharya, S.; Chen, G.; Hannoufa, A. The CRISPR/Cas9-Mediated Modulation of SQUAMOSA PROMOTER-BINDING PROTEIN-LIKE 8 in Alfalfa Leads to Distinct Phenotypic Outcomes. Front. Plant Sci. 2021, 12, 774146. [Google Scholar] [CrossRef]
- Ren, W.; Wu, F.; Bai, J.; Li, X.; Yang, X.; Xue, W.; Liu, H.; He, Y. BcpLH organizes a specific subset of microRNAs to form a leafy head in Chinese cabbage (Brassica rapa ssp. pekinensis). Hortic. Res. 2020, 7, 1. [Google Scholar] [CrossRef]
- Jiao, Y.; Wang, Y.; Xue, D.; Wang, J.; Yan, M.; Liu, G.; Dong, G.; Zeng, D.; Lu, Z.; Zhu, X.; et al. Regulation of OsSPL14 by OsmiR156 defines ideal plant architecture in rice. Nat. Genet. 2010, 42, 541–544. [Google Scholar] [CrossRef]
- Cao, J.; Liu, K.; Song, W.; Zhang, J.; Yao, Y.; Xin, M.; Hu, Z.; Peng, H.; Ni, Z.; Sun, Q.; et al. Pleiotropic function of the SQUAMOSA PROMOTER-BINDING PROTEIN-LIKE gene TaSPL14 in wheat plant architecture. Planta 2021, 253, 44. [Google Scholar] [CrossRef]
- Zhou, J.; He, R.; Liu, X.; Zhang, B.; Chen, G.; Wang, D.; Zhu, Y. Manipulation of plant height in garden asparagus (Asparagus officinalis L.) through CRISPR/Cas9-mediated aspSPL14 allele editing. Hortic. Res. 2023, 10, uhad096. [Google Scholar] [CrossRef]
- Yang, R.; Wu, Z.; Bai, C.; Sun, Z.; Wang, M.; Huo, Y.; Zhang, H.; Wang, Y.; Zhou, H.; Dai, S.; et al. Overexpression of PvWOX3a in switchgrass promotes stem development and increases plant height. Hortic. Res. 2021, 8, 252. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Yu, R.; Tang, Y.; Meng, J.; Tao, J. Identification and Functional Studies on the Role of PlSPL14 in Herbaceous Peony Stem Development. Int. J. Mol. Sci. 2024, 25, 8443. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; He, G.; Li, Y.; Yang, Z.; Liu, T.; Xie, X.; Kong, X.; Sun, J. PIL transcription factors directly interact with SPLs and repress tillering/branching in plants. New Phytol. 2022, 233, 1414–1425. [Google Scholar] [CrossRef] [PubMed]
- Sharif, R.; Su, L.; Chen, X.; Qi, X. Involvement of auxin in growth and stress response of cucumber. Veg. Res. 2022, 2, 1–9. [Google Scholar] [CrossRef]
- Liu, N.; Tu, L.; Wang, L.; Hu, H.; Xu, J.; Zhang, X. MicroRNA 157-targeted SPL genes regulate floral organ size and ovule production in cotton. BMC Plant Biol. 2017, 17, 7. [Google Scholar] [CrossRef]
- Li, L.; Shi, F.; Wang, Y.; Yu, X.; Zhi, J.; Guan, Y.; Zhao, H.; Chang, J.; Chen, M.; Yang, G.; et al. TaSPL13 regulates inflorescence architecture and development in transgenic wheat (Triticum aestivum L.). Plant Sci. Int. J. Exp. Plant Biol. 2020, 296, 110516. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Li, Y.; Zhang, Y.; Xia, L.; Hu, W.; Huang, X.; Li, K.; He, X.; Luo, C. Overexpression of MiSPL3a and MiSPL3b confers early flowering and stress tolerance in Arabidopsis thaliana. Int. J. Biol. Macromol. 2024, 262, 129913. [Google Scholar] [CrossRef] [PubMed]
- Ye, X.; Deng, Q.; Xu, S.; Huang, Y.; Wei, D.; Wang, Z.; Zhang, H.; Wang, H.; Tang, Q. CsSPL13A directly binds and positively regulates CsFT and CsBAM to accelerate flowering in cucumber. Plant Physiol. Biochem. 2024, 207, 108395. [Google Scholar] [CrossRef]
- Wang, L.; Yu, P.; Lyu, J.; Hu, Y.; Han, C.; Bai, M.Y.; Fan, M. BZR1 Physically Interacts with SPL9 to Regulate the Vegetative Phase Change and Cell Elongation in Arabidopsis. Int. J. Mol. Sci. 2021, 22, 10415. [Google Scholar] [CrossRef] [PubMed]
- Zhou, B.; Luo, Q.; Shen, Y.; Wei, L.; Song, X.; Liao, H.; Ni, L.; Shen, T.; Du, X.; Han, J.; et al. Coordinated regulation of vegetative phase change by brassinosteroids and the age pathway in Arabidopsis. Nat. Commun. 2023, 14, 2608. [Google Scholar] [CrossRef]
- Riaz, A.; Alqudah, A.M.; Kanwal, F.; Pillen, K.; Ye, L.-z.; Dai, F.; Zhang, G.-p. Advances in studies on the physiological and molecular regulation of barley tillering. J. Integr. Agric. 2023, 22, 1–13. [Google Scholar] [CrossRef]
- Jia, L.; Dai, Y.; Peng, Z.; Cui, Z.; Zhang, X.; Li, Y.; Tian, W.; He, G.; Li, Y.; Sang, X. The auxin transporter OsAUX1 regulates tillering in rice (Oryza sativa). J. Integr. Agric. 2024, 23, 1454–1467. [Google Scholar] [CrossRef]
- Song, X.; Meng, X.; Guo, H.; Cheng, Q.; Jing, Y.; Chen, M.; Liu, G.; Wang, B.; Wang, Y.; Li, J.; et al. Targeting a gene regulatory element enhances rice grain yield by decoupling panicle number and size. Nat. Biotechnol. 2022, 40, 1403–1411. [Google Scholar] [CrossRef]
- Li, L.; Shi, F.; Wang, G.; Guan, Y.; Zhang, Y.; Chen, M.; Chang, J.; Yang, G.; He, G.; Wang, Y.; et al. Conservation and Divergence of SQUAMOSA-PROMOTER BINDING PROTEIN-LIKE (SPL) Gene Family between Wheat and Rice. Int. J. Mol. Sci. 2022, 23, 2099. [Google Scholar] [CrossRef]
- Hu, J.; Huang, L.; Chen, G.; Liu, H.; Zhang, Y.; Zhang, R.; Zhang, S.; Liu, J.; Hu, Q.; Hu, F.; et al. The Elite Alleles of OsSPL4 Regulate Grain Size and Increase Grain Yield in Rice. Rice 2021, 14, 90. [Google Scholar] [CrossRef]
- Zhang, X.-F.; Yang, C.-Y.; Lin, H.-X.; Wang, J.-W.; Xue, H.-W. Rice SPL12 coevolved with GW5 to determine grain shape. Sci. Bull. 2021, 66, 2353–2357. [Google Scholar] [CrossRef] [PubMed]
- Si, L.; Chen, J.; Huang, X.; Gong, H.; Luo, J.; Hou, Q.; Zhou, T.; Lu, T.; Zhu, J.; Shangguan, Y.; et al. OsSPL13 controls grain size in cultivated rice. Nat. Genet. 2016, 48, 447–456. [Google Scholar] [CrossRef] [PubMed]
- Zeng, X.; Fan, K.; Shi, Y.; Chen, R.; Liu, W.; Wang, X.; Ye, G.; Lin, W.; Li, Z. OsSPL11 positively regulates grain size by activating the expression of GW5L in rice. Plant Cell Rep. 2024, 43, 228. [Google Scholar] [CrossRef]
- Gupta, A.; Hua, L.; Zhang, Z.; Yang, B.; Li, W. CRISPR-induced miRNA156-recognition element mutations in TaSPL13 improve multiple agronomic traits in wheat. Plant Biotechnol. J. 2023, 21, 536–548. [Google Scholar] [CrossRef]
- Cui, L.; Zheng, F.; Wang, J.; Zhang, C.; Xiao, F.; Ye, J.; Li, C.; Ye, Z.; Zhang, J. miR156a-targeted SBP-Box transcription factor SlSPL13 regulates inflorescence morphogenesis by directly activating SFT in tomato. Plant Biotechnol. J. 2020, 18, 1670–1682. [Google Scholar] [CrossRef]
- Xu, S.; Wang, Y.; Yang, S.; Fan, S.; Shi, K.; Wang, F.; An, M.; Qi, Y.; Wang, M.; Feng, M.; et al. The WUSCHEL-related homeobox transcription factor CsWOX3 negatively regulates fruit spine morphogenesis in cucumber (Cucumis sativus L.). Hortic. Res. 2024, 11, uhae163. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Mou, W.; Xia, R.; Li, L.; Zawora, C.; Ying, T.; Mao, L.; Liu, Z.; Luo, Z. Integrated analysis of high-throughput sequencing data shows abscisic acid-responsive genes and miRNAs in strawberry receptacle fruit ripening. Hortic. Res. 2019, 6, 26. [Google Scholar] [CrossRef]
- Gao, Y.; Zhu, N.; Zhu, X.; Wu, M.; Jiang, C.-Z.; Grierson, D.; Luo, Y.; Shen, W.; Zhong, S.; Fu, D.-Q.; et al. Diversity and redundancy of the ripening regulatory networks revealed by the fruitENCODE and the new CRISPR/Cas9 CNR and NOR mutants. Hortic. Res. 2019, 6, 39. [Google Scholar] [CrossRef]
- Zhou, L.; Sun, Z.; Hu, T.; Chen, D.; Chen, X.; Zhang, Q.; Cao, J.; Zhu, B.; Fu, D.; Zhu, H.; et al. Increasing flavonoid contents of tomato fruits through disruption of the SlSPL-CNR, a suppressor of SlMYB12 transcription activity. Plant Biotechnol. J. 2024, 22, 290–292. [Google Scholar] [CrossRef]
- Chen, D.; Huang, H.; Zhang, Q.; Hu, T.; Sun, Z.; Wang, T.; Chen, X.; Nie, R.; Zhou, L.; Fu, D.; et al. The tomato SBP-box protein SlCNR negatively regulates genes involved in fruit cutin deposition. Plant Physiol. 2025, 197, kiaf099. [Google Scholar] [CrossRef]
- Wei, W.; Yang, Y.-y.; Wu, C.-j.; Kuang, J.-f.; Chen, J.-y.; Shan, W. MaSPL16 positively regulates fruit ripening in bananas via the direct transcriptional induction of MaNAC029. Hortic. Adv. 2023, 1, 10. [Google Scholar] [CrossRef]
- Yan, W.; Sharif, R.; Sohail, H.; Zhu, Y.; Chen, X.; Xu, X. Surviving a Double-Edged Sword: Response of Horticultural Crops to Multiple Abiotic Stressors. Int. J. Mol. Sci. 2024, 25, 5199. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, S.; Chen, Y.; Shah, A.Z.; Wang, H.; Xi, C.; Zhu, H.; Ge, L. The Homeodomain-Leucine Zipper Genes Family Regulates the Jinggangmycin Mediated Immune Response of Oryza sativa to Nilaparvata lugens, and Laodelphax striatellus. Bioengineering 2022, 9, 398. [Google Scholar] [CrossRef]
- Jia, M.; Meng, X.; Song, X.; Zhang, D.; Kou, L.; Zhang, J.; Jing, Y.; Liu, G.; Liu, H.; Huang, X.; et al. Chilling-induced phosphorylation of IPA1 by OsSAPK6 activates chilling tolerance responses in rice. Cell Discov. 2022, 8, 71. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Shi, M.; Yu, J.; Guo, C. SPL9 mediates freezing tolerance by directly regulating the expression of CBF2 in Arabidopsis thaliana. BMC Plant Biol. 2022, 22, 59. [Google Scholar] [CrossRef]
- Kong, X.; Peng, K.; Shan, Y.; Yun, Z.; Dalmay, T.; Duan, X.; Jiang, Y.; Qu, H.; Zhu, H. Transcriptional regulation of miR528-PPO module by miR156 targeted SPLs orchestrates chilling response in banana. Mol. Hortic. 2025, 5, 2. [Google Scholar] [CrossRef] [PubMed]
- Zareen, S.; Ali, A.; Yun, D.-J. Significance of ABA Biosynthesis in Plant Adaptation to Drought Stress. J. Plant Biol. 2024, 67, 175–184. [Google Scholar] [CrossRef]
- Zhao, Y.; He, J.; Liu, M.; Miao, J.; Ma, C.; Feng, Y.; Qian, J.; Li, H.; Bi, H.; Liu, W. The SPL transcription factor TaSPL6 negatively regulates drought stress response in wheat. Plant Physiol. Biochem. 2024, 206, 108264. [Google Scholar] [CrossRef]
- Li, Y.; Han, S.; Sun, X.; Khan, N.U.; Zhong, Q.; Zhang, Z.; Zhang, H.; Ming, F.; Li, Z.; Li, J. Variations in OsSPL10 confer drought tolerance by directly regulating OsNAC2 expression and ROS production in rice. J. Integr. Plant Biol. 2023, 65, 918–933. [Google Scholar] [CrossRef]
- Feng, C.; Zhang, X.; Du, B.; Xiao, Y.; Wang, Y.; Sun, Y.; Zhou, X.; Wang, C.; Liu, Y.; Li, T.-H. MicroRNA156ab regulates apple plant growth and drought tolerance by targeting transcription factor MsSPL13. Plant Physiol. 2023. [Google Scholar] [CrossRef]
- Hussain, S.; Chang, J.; Li, J.; Chen, L.; Ahmad, S.; Song, Z.; Zhang, B.; Chen, X. Multifunctional Role of Cytokinin in Horticultural Crops. Int. J. Mol. Sci. 2025, 26, 1037. [Google Scholar] [CrossRef] [PubMed]
- Qi, X.; Li, Q.; Ma, X.; Qian, C.; Wang, H.; Ren, N.; Shen, C.; Huang, S.; Xu, X.; Xu, Q. Waterlogging-induced adventitious root formation in cucumber is regulated by ethylene and auxin through reactive oxygen species signalling. Plant Cell Environ. 2019, 42, 1458–1470. [Google Scholar] [CrossRef]
- Feyissa, B.A.; Amyot, L.; Nasrollahi, V.; Papadopoulos, Y.; Kohalmi, S.E.; Hannoufa, A. Involvement of the miR156/SPL module in flooding response in Medicago sativa. Sci. Rep. 2021, 11, 3243. [Google Scholar] [CrossRef]
- Raza, A.; Charagh, S.; Zahid, Z.; Mubarik, M.S.; Javed, R.; Siddiqui, M.H.; Hasanuzzaman, M. Jasmonic acid: A key frontier in conferring abiotic stress tolerance in plants. Plant Cell Rep. 2021, 40, 1513–1541. [Google Scholar] [CrossRef] [PubMed]
- Sharif, R.; Raza, A.; Chen, P.; Li, Y.; El-Ballat, E.M.; Rauf, A.; Hano, C.; El-Esawi, M.A. HD-ZIP Gene Family: Potential Roles in Improving Plant Growth and Regulating Stress-Responsive Mechanisms in Plants. Genes 2021, 12, 1256. [Google Scholar] [CrossRef] [PubMed]
- Raza, A.; Tabassum, J.; Kudapa, H.; Varshney, R.K. Can omics deliver temperature resilient ready-to-grow crops? Crit. Rev. Biotechnol. 2021, 41, 1209–1232. [Google Scholar] [CrossRef]
- Chao, L.-M.; Liu, Y.-Q.; Chen, D.-Y.; Xue, X.-Y.; Mao, Y.-B.; Chen, X.-Y. Arabidopsis transcription factors SPL1 and SPL12 confer plant thermotolerance at reproductive stage. Mol. Plant 2017, 10, 735–748. [Google Scholar] [CrossRef]
- Liu, W.; Ji, X.; Cao, H.; Huo, C.; He, L.; Peng, X.; Yang, Y.; Yang, F.; Xiong, S. Comparative Transcriptome Analysis Reveals the Effect of miR156a Overexpression on Mineral Nutrient Homeostasis in Nicotiana tabacum. Plants 2023, 12, 1739. [Google Scholar] [CrossRef]
- Yue, E.; Rong, F.; Liu, Z.; Ruan, S.; Lu, T.; Qian, H. Cadmium induced a non-coding RNA microRNA535 mediates Cd accumulation in rice. J. Environ. Sci. 2023, 130, 149–162. [Google Scholar] [CrossRef]
- Ahmad, S.; Ali, S.; Shah, A.Z.; Khan, A.; Faria, S. Chalcone synthase (CHS) family genes regulate the growth and response of cucumber (Cucumis sativus L.) to Botrytis cinerea and abiotic stresses. Plant Stress 2023, 8, 100159. [Google Scholar] [CrossRef]
- Sun, T.; Zhou, Q.; Zhou, Z.; Song, Y.; Li, Y.; Wang, H.B.; Liu, B. SQUINT Positively Regulates Resistance to the Pathogen Botrytis cinerea via miR156-SPL9 Module in Arabidopsis. Plant Cell Physiol. 2022, 63, 1414–1432. [Google Scholar] [CrossRef] [PubMed]
- Zhong, Z.; Zhong, L.; Zhu, X.; Jiang, Y.; Zheng, Y.; Lan, T.; Cui, H. Transcription factor OsSPL10 interacts with OsJAmyb to regulate blast resistance in rice. Crop J. 2024, 12, 301–307. [Google Scholar] [CrossRef]
- Zhang, L.L.; Li, Y.; Zheng, Y.P.; Wang, H.; Yang, X.; Chen, J.F.; Zhou, S.X.; Wang, L.F.; Li, X.P.; Ma, X.C.; et al. Expressing a Target Mimic of miR156fhl-3p Enhances Rice Blast Disease Resistance Without Yield Penalty by Improving SPL14 Expression. Front. Genet. 2020, 11, 327. [Google Scholar] [CrossRef] [PubMed]
- Gull, S.; Arfan, M.; Uddin, S.; Qasim, M.; Babar, S.; Rajput, N.; Ullah, H.; Ain, Q.U.; Alharthi, B.; Ahmad, S.; et al. Identification and characterization of cysteine-rich polycomb-like protein (CPP) gene family in rice (Oryza sativa L.) in response to phytohormones and Xanthomonas oryzae pv. oryzae stress. Plant Stress 2024, 14, 100677. [Google Scholar] [CrossRef]
- Hui, S.; Ke, Y.; Chen, D.; Wang, L.; Li, Q.; Yuan, M. Rice microRNA156/529-SQUAMOSA PROMOTER BINDING PROTEIN-LIKE7/14/17 modules regulate defenses against bacteria. Plant Physiol. 2023, 192, 2537–2553. [Google Scholar] [CrossRef]
- Mao, Y.-B.; Liu, Y.-Q.; Chen, D.-Y.; Chen, F.-Y.; Fang, X.; Hong, G.-J.; Wang, L.-J.; Wang, J.-W.; Chen, X.-Y. Jasmonate response decay and defense metabolite accumulation contributes to age-regulated dynamics of plant insect resistance. Nat. Commun. 2017, 8, 13925. [Google Scholar] [CrossRef]
- Lu, L.; Sun, Z.; Wang, R.; Du, Y.; Zhang, Z.; Lan, T.; Song, Y.; Zeng, R. Integration of transcriptome and metabolome analyses reveals the role of OsSPL10 in rice defense against brown planthopper. Plant Cell Rep. 2023, 42, 2023–2038. [Google Scholar] [CrossRef]
- Sun, B.; Zhong, Y.; Tao, Z.; Zhu, L.; Miao, X.; Shi, Z.; Li, H. OsMYB1 antagonizes OsSPL14 to mediate rice resistance to brown planthopper and Xanthomonas oryzae pv. oryzae. Plant Cell Rep. 2024, 44, 13. [Google Scholar] [CrossRef]



| Gene | Species | Role | References |
|---|---|---|---|
| AtSPL9 | Arabidopsis | Inhibition of pod germination. | [13] |
| OsSPL13 | Rice | Mutation caused a low number crown roots. | [16] |
| AsSPL14 | Asparagus | Mutation leads to a semi-dwarf phenotype. | [25] |
| CsSPL13A | Cucumber | Overexpression of CsSPL13A induced male flowers. | [33] |
| SPL9 | Arabidopsis | Positive regulator of brassinosteroid-mediated phase transition. | [35] |
| TaSPL13 | Wheat | Mutation in the MRE enhanced wheat yield. | [44] |
| CsSPL15 | Cucumber | Silencing leads to an increase in fruit spine density. | [46] |
| SlSPL-CNR | Tomato | Knocking out the produce tomato with a thick cutin layer. | [50] |
| SPL9 | Rice | Overexpression enhanced the freezing tolerance by activating CBF. | [55] |
| MsSPL13 | Apple | Overexpression significantly reduced apple tolerance to drought. | [60] |
| SPL13 | Alfalfa | RNAi-silencing enhanced flooding tolerance by increasing ABA accumulation. | [63] |
| SPL1 | Arabidopsis | Overexpression boosted the heat tolerance via the ABA pathway. | [67] |
| NtSPL4a | Tobacco | Reduced cadmium toxicity and uptake. | [3] |
| SPL9 | Arabidopsis | Knock-out mutant exhibited increased resistance to B. cinerea. | [71] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bu, R.; Qiu, Z.; Dong, J.; Chen, L.; Zhou, Y.; Wang, H.; Hu, L. The Role of SQUAMOSA-PROMOTER BINDING PROTEIN-like (SPL) Transcription Factors in Plant Growth and Environmental Stress Response: A Comprehensive Review of Recent Advances. Horticulturae 2025, 11, 584. https://doi.org/10.3390/horticulturae11060584
Bu R, Qiu Z, Dong J, Chen L, Zhou Y, Wang H, Hu L. The Role of SQUAMOSA-PROMOTER BINDING PROTEIN-like (SPL) Transcription Factors in Plant Growth and Environmental Stress Response: A Comprehensive Review of Recent Advances. Horticulturae. 2025; 11(6):584. https://doi.org/10.3390/horticulturae11060584
Chicago/Turabian StyleBu, Runhua, Zongqing Qiu, Jing Dong, Liqin Chen, Yu Zhou, Huilin Wang, and Liangliang Hu. 2025. "The Role of SQUAMOSA-PROMOTER BINDING PROTEIN-like (SPL) Transcription Factors in Plant Growth and Environmental Stress Response: A Comprehensive Review of Recent Advances" Horticulturae 11, no. 6: 584. https://doi.org/10.3390/horticulturae11060584
APA StyleBu, R., Qiu, Z., Dong, J., Chen, L., Zhou, Y., Wang, H., & Hu, L. (2025). The Role of SQUAMOSA-PROMOTER BINDING PROTEIN-like (SPL) Transcription Factors in Plant Growth and Environmental Stress Response: A Comprehensive Review of Recent Advances. Horticulturae, 11(6), 584. https://doi.org/10.3390/horticulturae11060584






