Skeleton Photoperiod Enhances Photosynthetic Yield in Celery via Circadian-Regulated Metabolic Coordination
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Materials and Growth Conditions
2.2. Photoperiodic Treatments
2.3. Determination of Photosynthetic Characteristic Index
2.4. Extraction of Total RNA and Preparation of cDNA
2.5. Real-Time Fluorescence Quantitative (RT-qPCR) Analysis
2.6. Measurement of Chl and N Contents in Leaves
2.7. Statistical Analyses
2.8. Model Description and Construction
2.9. Numerical Simulation
3. Results
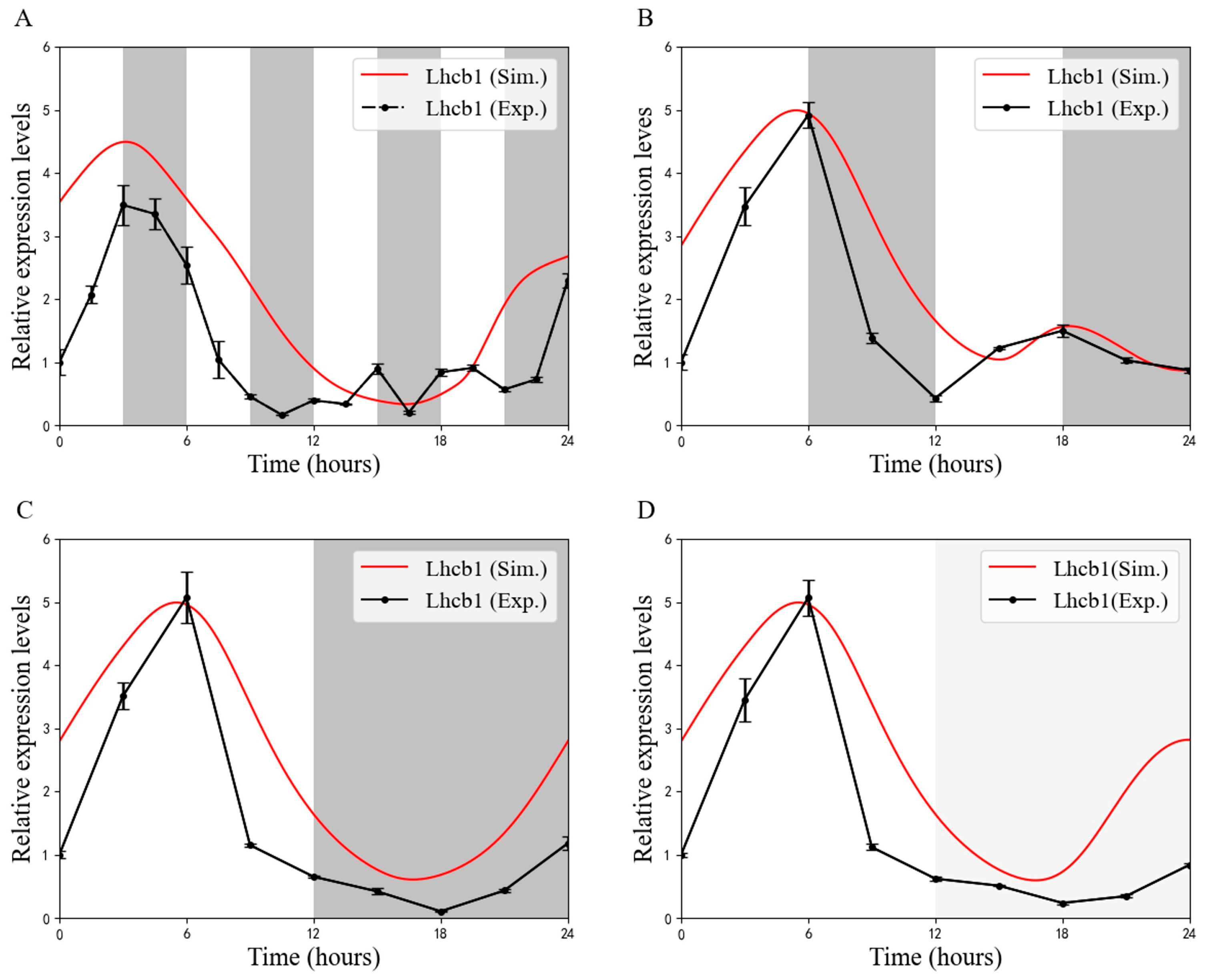
3.1. Circadian Expression of Lhcb1 During the Skeleton Photoperiod
3.2. Photosynthetic Parameters Have Different Expression Patterns in the Skeleton Photoperiod
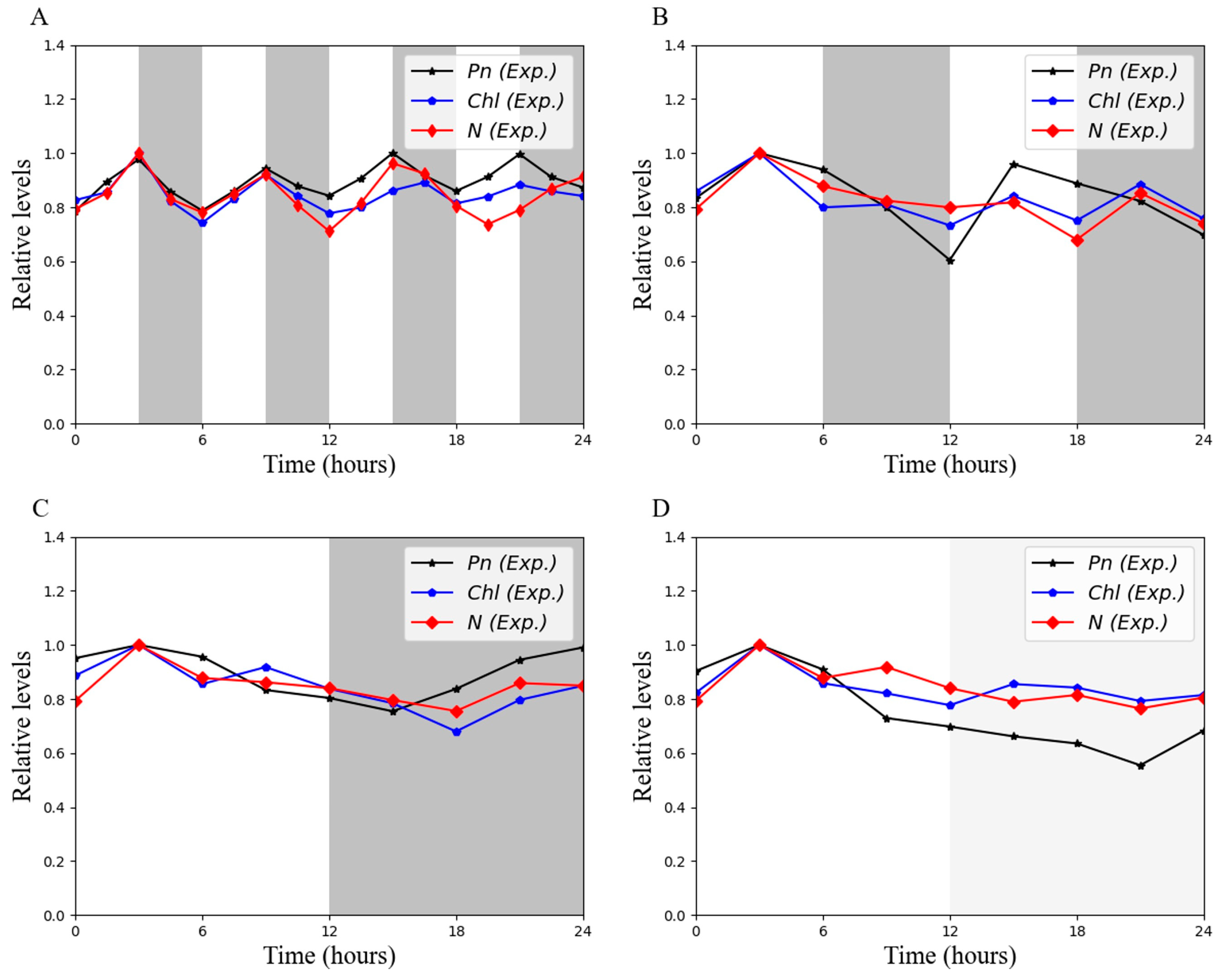
3.3. Skeletal Photoperiod Can Increase Intracellular Chl and N Contents
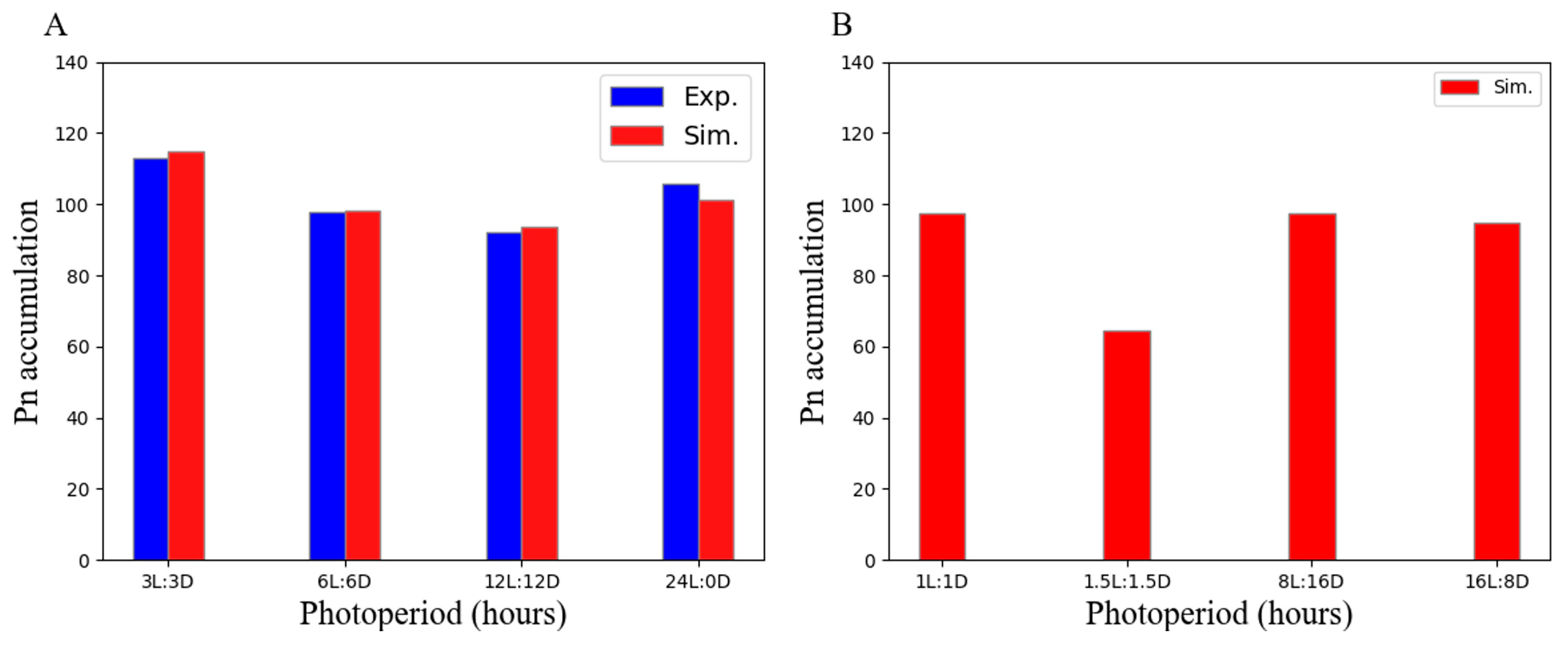
3.4. Skeletal Photoperiod Enhances Pn Accumulation
3.5. Skeleton Photoperiod Can Sustain High Photosynthetic Yields
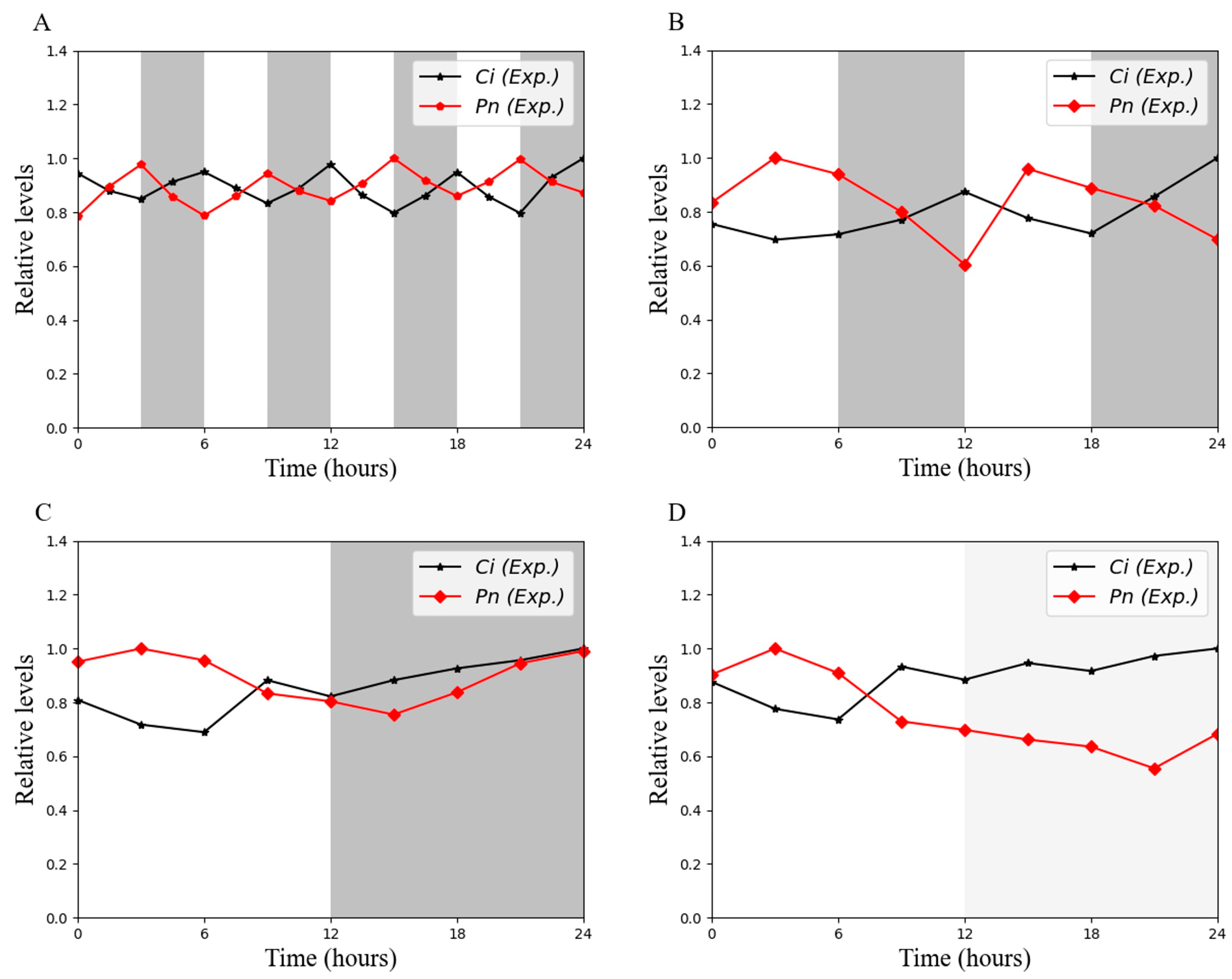
3.6. Effect of Ci on Photosynthesis Under Skeleton Photoperiod
3.7. Chl and N Collectively Influence the Rate of Photosynthesis
3.8. Circadian Rhythms in Photosynthetic Yield
4. Model
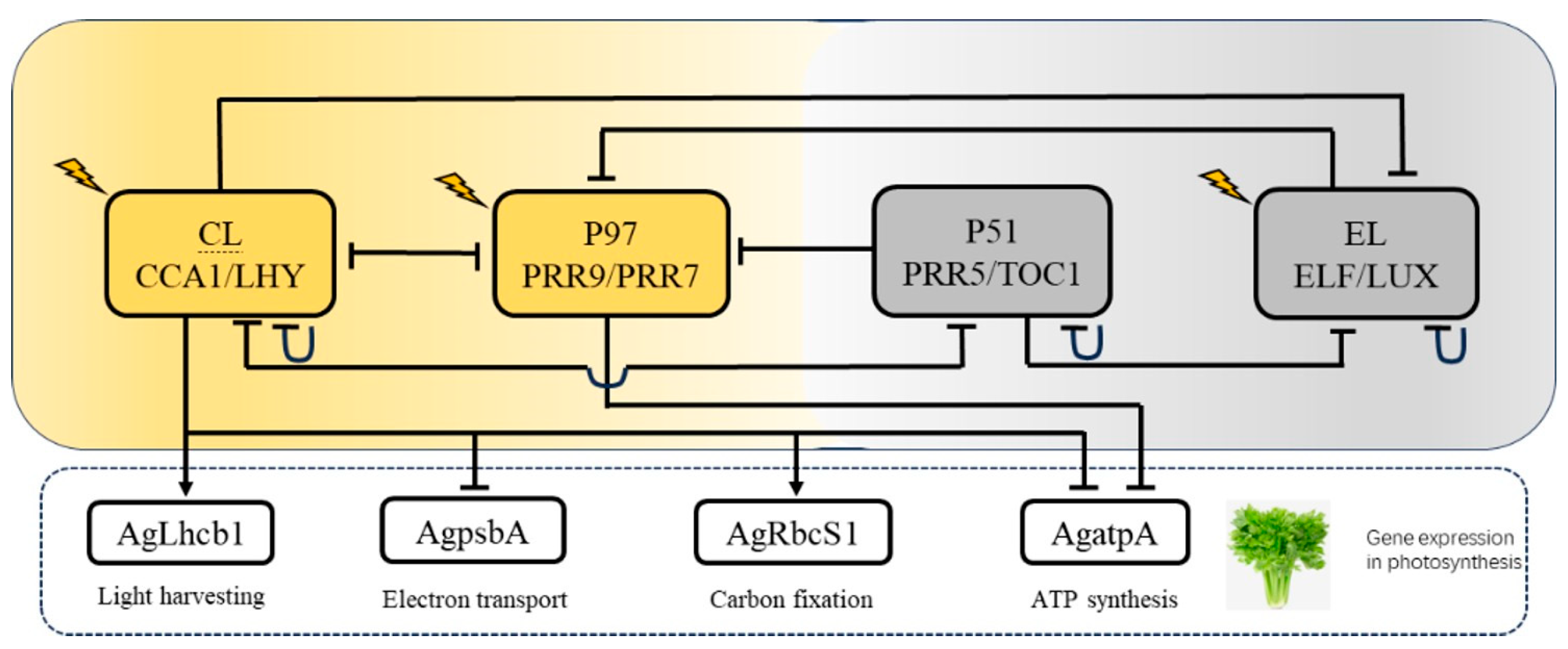
4.1. Biological Implications of Model Components
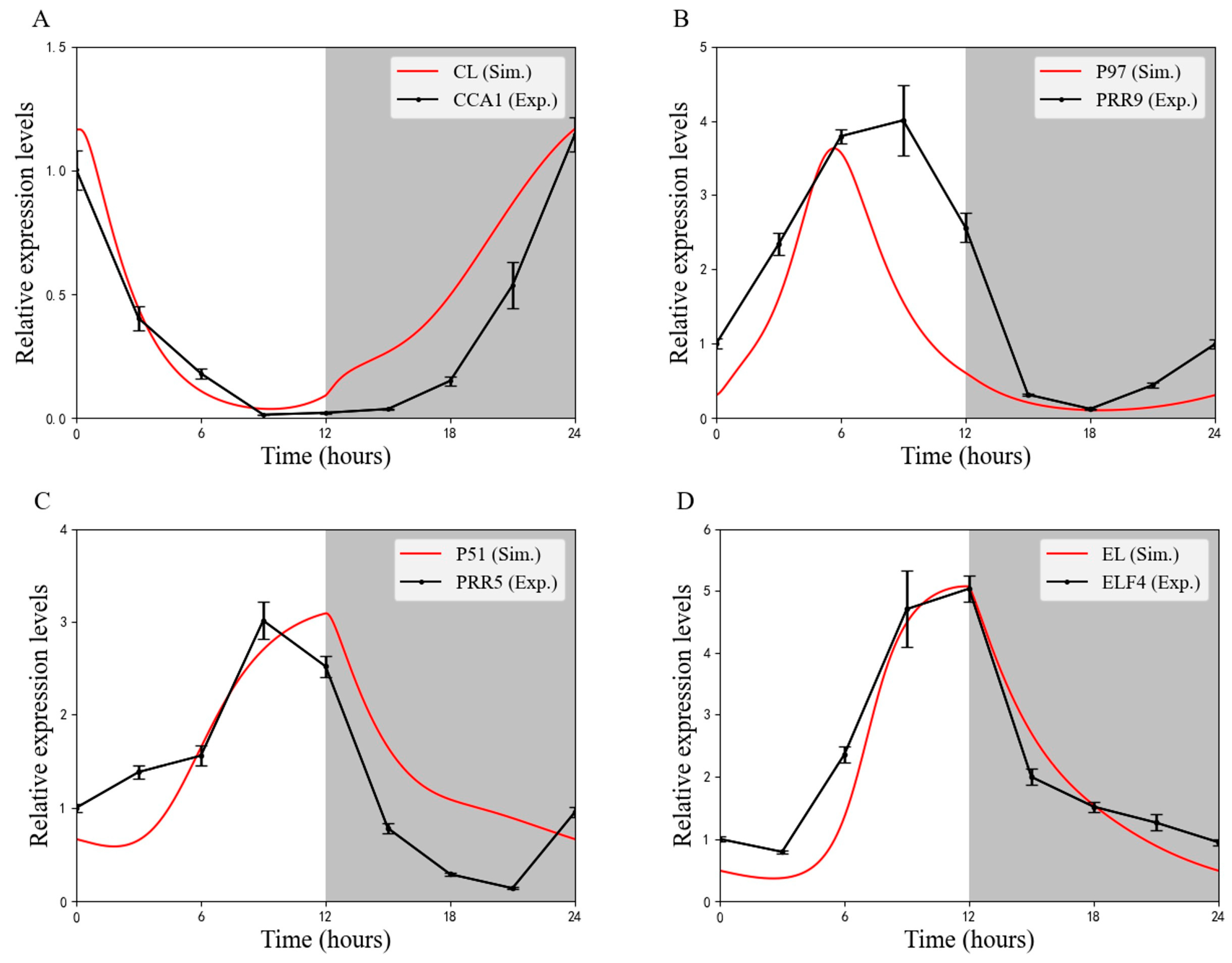
4.2. Parameter Estimation of the Model
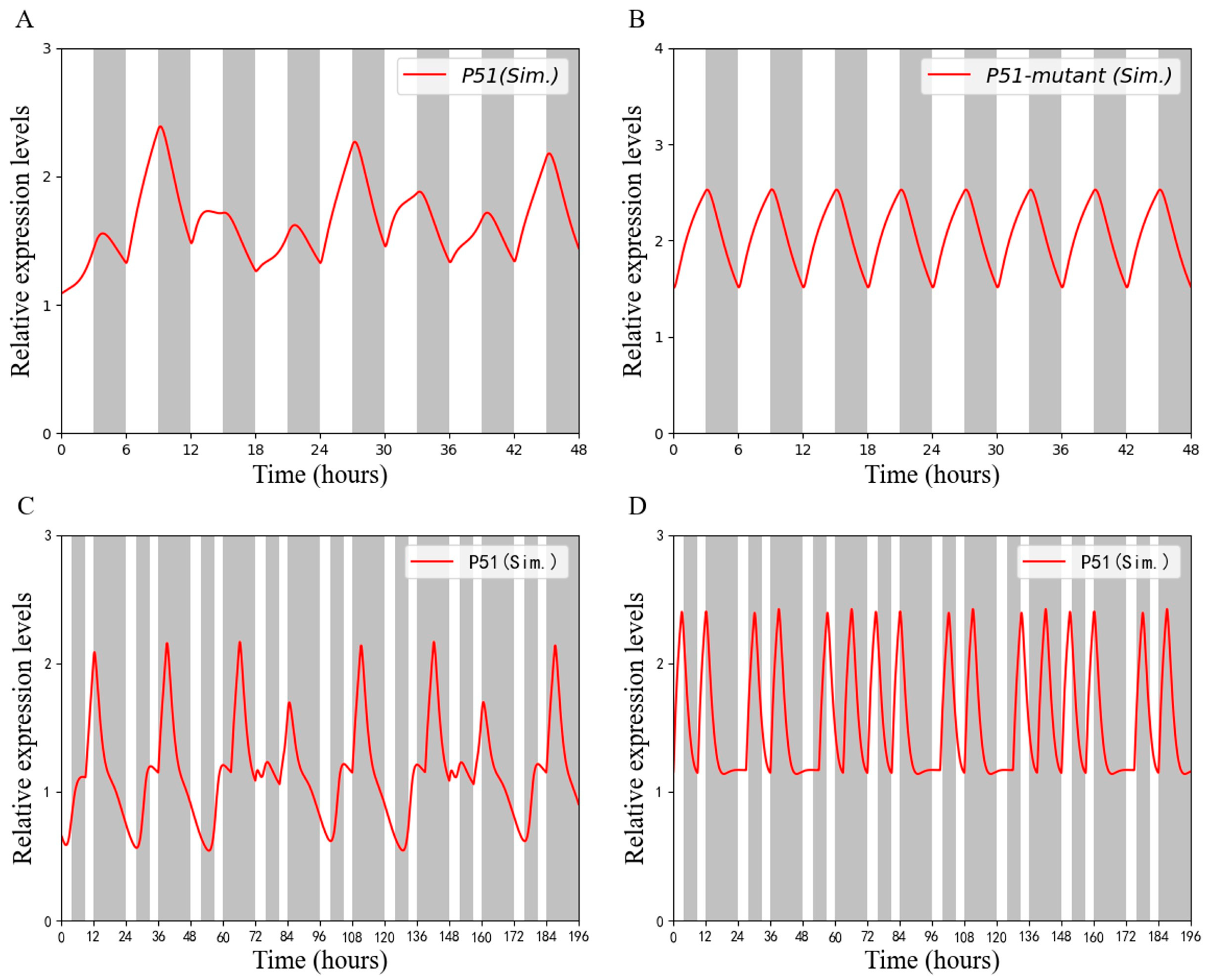
4.3. Robustness Analysis of the Model
4.4. Parameter Sensitivity Analysis
4.5. Rhythmic Expression of Clock Genes in Skeleton Photoperiod
5. Discussion
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Li, M.-Y.; Feng, K.; Hou, X.-L.; Jiang, Q.; Xu, Z.-S.; Wang, G.-L.; Liu, J.-X.; Wang, F.; Xiong, A.-S. The Genome Sequence of Celery (Apium graveolens L.), an Important Leaf Vegetable Crop Rich in Apigenin in the Apiaceae Family. Hortic. Res. 2020, 7, 9. [Google Scholar] [CrossRef] [PubMed]
- Singh, M.; Nara, U.; Kaur, K.; Rani, N.; Jaswal, C. Genetic, Genomic and Biochemical Insights of Celery (Apium graveolens L.) in the Era of Molecular Breeding. J. Appl. Res. Med. Aromat. Plants 2022, 31, 100420. [Google Scholar] [CrossRef]
- Duan, A.-Q.; Feng, K.; Wang, G.; Liu, J.-X.; Xu, Z.-S.; Xiong, A.-S. Elevated Gibberellin Enhances Lignin Accumulation in Celery (Apium graveolens L.) Leaves. Protoplasma 2019, 256, 777–788. [Google Scholar] [CrossRef]
- Funakoshi-Tago, M.; Nakamura, K.; Tago, K.; Mashino, T.; Kasahara, T. Anti-Inflammatory Activity of Structurally Related Flavonoids, Apigenin, Luteolin and Fisetin. Int. Immunopharmacol. 2011, 11, 1150–1159. [Google Scholar] [CrossRef]
- Huang, C.-S.; Lii, C.-K.; Lin, A.-H.; Yeh, Y.-W.; Yao, H.-T.; Li, C.-C.; Wang, T.-S.; Chen, H.-W. Protection by Chrysin, Apigenin, and Luteolin against Oxidative Stress Is Mediated by the Nrf2-Dependent up-Regulation of Heme Oxygenase 1 and Glutamate Cysteine Ligase in Rat Primary Hepatocytes. Arch. Toxicol. 2013, 87, 167–178. [Google Scholar] [CrossRef]
- Huang, T.; Shui, Y.; Wu, Y.; Hou, X.; You, X. Red Light Resets the Expression Pattern, Phase, and Period of the Circadian Clock in Plants: A Computational Approach. Biology 2022, 11, 1479. [Google Scholar] [CrossRef]
- Li, T.; Fang, K.; Tie, Y.; Lu, Y.; Lei, Y.; Li, W.; Zheng, T.; Yao, X. NAC Transcription Factor ATAF1 Negatively Modulates the PIF-regulated Hypocotyl Elongation under a Short-day Photoperiod. Plant Cell Environ. 2024, 47, 3253–3265. [Google Scholar] [CrossRef]
- Zheng, Z.; Zhou, W.; Yang, Q.; Jiang, J.; Miao, M. Effect of Furrow Straw Mulching and Straw Decomposer Application on Celery (Apium graveolens L.) Production and Soil Improvement. Agronomy 2023, 13, 2774. [Google Scholar] [CrossRef]
- Proietti, S.; Moscatello, S.; Riccio, F.; Downey, P.; Battistelli, A. Continuous Lighting Promotes Plant Growth, Light Conversion Efficiency, and Nutritional Quality of Eruca vesicaria (L.) Cav. in Controlled Environment With Minor Effects Due to Light Quality. Front. Plant Sci. 2021, 12, 730119. [Google Scholar] [CrossRef]
- Osnato, M. Photoperiod Control of Plant Growth: Flowering Time Genes Beyond Flowering. Front. Plant Sci. 2022, 12, 805635. [Google Scholar] [CrossRef]
- Roeber, V.M.; Schmülling, T.; Cortleven, A. The Photoperiod: Handling and Causing Stress in Plants. Front. Plant Sci. 2022, 12, 781988. [Google Scholar] [CrossRef] [PubMed]
- Roeber, V.M.; Bajaj, I.; Rohde, M.; Schmülling, T.; Cortleven, A. Light Acts as a Stressor and Influences Abiotic and Biotic Stress Responses in Plants. Plant Cell Environ. 2021, 44, 645–664. [Google Scholar] [CrossRef] [PubMed]
- Gendron, J.M.; Leung, C.C.; Liu, W. Energy as a Seasonal Signal for Growth and Reproduction. Curr. Opin. Plant Biol. 2021, 63, 102092. [Google Scholar] [CrossRef]
- Wu, S.; Li, R.; Bu, C.; Zhu, C.; Miao, C.; Zhang, Y.; Cui, J.; Jiang, Y.; Ding, X. Photoperiodic Effect on Growth, Photosynthesis, Mineral Elements, and Metabolome of Tomato Seedlings in a Plant Factory. Plants 2024, 13, 3119. [Google Scholar] [CrossRef]
- Bhatta, M.; Sandro, P.; Smith, M.R.; Delaney, O.; Voss-Fels, K.P.; Gutierrez, L.; Hickey, L.T. Need for Speed: Manipulating Plant Growth to Accelerate Breeding Cycles. Curr. Opin. Plant Biol. 2021, 60, 101986. [Google Scholar] [CrossRef]
- Wang, Q.; Liu, W.; Leung, C.C.; Tarté, D.A.; Gendron, J.M. Plants Distinguish Different Photoperiods to Independently Control Seasonal Flowering and Growth. Science 2024, 383, eadg9196. [Google Scholar] [CrossRef] [PubMed]
- Abuelsoud, W.; Cortleven, A.; Schmülling, T. Photoperiod Stress Induces an Oxidative Burst-like Response and Is Associated with Increased Apoplastic Peroxidase and Decreased Catalase Activities. J. Plant Physiol. 2020, 253, 153252. [Google Scholar] [CrossRef]
- Cortleven, A.; Roeber, V.M.; Frank, M.; Bertels, J.; Lortzing, V.; Beemster, G.T.S.; Schmülling, T. Photoperiod Stress in Arabidopsis Thaliana Induces a Transcriptional Response Resembling That of Pathogen Infection. Front. Plant Sci. 2022, 13, 838284. [Google Scholar] [CrossRef]
- Chu, Q.; Qin, Y.; Li, C.; Cheng, S.; Su, L.; He, Z.; Zhou, X.; Shao, D.; Guo, X. Effects of Different Photoperiods on the Growth and Nutritional Characteristics of Two Celery Cultivars in Plant Factory. Agronomy 2023, 13, 3039. [Google Scholar] [CrossRef]
- Xu, Y.; Ibrahim, I.M.; Harvey, P.J. The Influence of Photoperiod and Light Intensity on the Growth and Photosynthesis of Dunaliella Salina (Chlorophyta) CCAP 19/30. Plant Physiol. Biochem. 2016, 106, 305–315. [Google Scholar] [CrossRef]
- Huang, T.; Liu, H.; Tao, J.-P.; Zhang, J.-Q.; Zhao, T.-M.; Hou, X.-L.; Xiong, A.-S.; You, X. Low Light Intensity Elongates Period and Defers Peak Time of Photosynthesis: A Computational Approach to Circadian-Clock-Controlled Photosynthesis in Tomato. Hortic. Res. 2023, 10, uhad077. [Google Scholar] [CrossRef] [PubMed]
- Pokhilko, A.; Mas, P.; Millar, A.J. Modelling the Widespread Effects of TOC1 Signalling on the Plant Circadian Clock and Its Outputs. BMC Syst. Biol. 2013, 7, 23. [Google Scholar] [CrossRef]
- Hsu, P.Y.; Harmer, S.L. Wheels within Wheels: The Plant Circadian System. Trends Plant Sci. 2014, 19, 240–249. [Google Scholar] [CrossRef]
- Creux, N.; Harmer, S. Circadian Rhythms in Plants. Cold Spring Harb. Perspect. Biol. 2019, 11, a034611. [Google Scholar] [CrossRef]
- Nakamichi, N.; Kiba, T.; Henriques, R.; Mizuno, T.; Chua, N.-H.; Sakakibara, H. PSEUDO-RESPONSE REGULATORS 9, 7, and 5 Are Transcriptional Repressors in the Arabidopsis Circadian Clock. Plant Cell 2010, 22, 594–605. [Google Scholar] [CrossRef] [PubMed]
- Ohara, T.; Fukuda, H.; Tokuda, I.T. An Extended Mathematical Model for Reproducing the Phase Response of Arabidopsis Thaliana under Various Light Conditions. J. Theor. Biol. 2015, 382, 337–344. [Google Scholar] [CrossRef] [PubMed]
- De Caluwé, J.; Xiao, Q.; Hermans, C.; Verbruggen, N.; Leloup, J.-C.; Gonze, D. A Compact Model for the Complex Plant Circadian Clock. Front. Plant Sci. 2016, 7, 74. [Google Scholar] [CrossRef]
- Pay, M.L.; Kim, D.W.; Somers, D.E.; Kim, J.K.; Foo, M. Modelling of Plant Circadian Clock for Characterizing Hypocotyl Growth under Different Light Quality Conditions. In Silico Plants 2022, 4, diac001. [Google Scholar] [CrossRef]
- Zhang, R.; Gonze, D.; Hou, X.; You, X.; Goldbeter, A. A Computational Model for the Cold Response Pathway in Plants. Front. Physiol. 2020, 11, 591073. [Google Scholar] [CrossRef]
- Akman, O.E.; Watterson, S.; Parton, A.; Binns, N.; Millar, A.J.; Ghazal, P. Digital clocks: Simple Boolean models can quantitatively describe circadian systems. J. R. Soc. Interface 2012, 9, 2365–2382. [Google Scholar] [CrossRef]
- Dixon, L.E.; Knox, K.; Kozma-Bognar, L.; Southern, M.M.; Pokhilko, A.; Millar, A.J. Temporal Repression of Core Circadian Genes Is Mediated through EARLY FLOWERING 3 in Arabidopsis. Curr. Biol. 2011, 21, 120–125. [Google Scholar] [CrossRef]
- Fogelmark, K.; Troein, C. Rethinking Transcriptional Activation in the Arabidopsis Circadian Clock. PLoS Comput. Biol. 2014, 10, e1003705. [Google Scholar] [CrossRef] [PubMed]
- Solanas, G.; Welz, P.-S. (Eds.) Circadian Regulation: Methods and Protocols; Methods in Molecular Biology; Humana Press: New York, NY, USA, 2022; ISBN 978-1-0716-2249-0. [Google Scholar]
- Schaffer, A.A. Improving the Accuracy of PSI-BLAST Protein Database Searches with Composition-Based Statistics and Other Refinements. Nucleic Acids Res. 2001, 29, 2994–3005. [Google Scholar] [CrossRef] [PubMed]
- Hu, Z.-H.; Zhang, N.; Qin, Z.-Y.; Li, J.-W.; Tao, J.-P.; Yang, N.; Chen, Y.; Kong, J.-Y.; Luo, W.; Chen, X.; et al. Circadian Rhythm Response and Its Effect on Photosynthetic Characteristics of the Lhcb Family Genes in Tea Plant. BMC Plant Biol. 2024, 24, 333. [Google Scholar] [CrossRef] [PubMed]
- Green, R.M.; Tingay, S.; Wang, Z.-Y.; Tobin, E.M. Circadian Rhythms Confer a Higher Level of Fitness to Arabidopsis Plants. Plant Physiol. 2002, 129, 576–584. [Google Scholar] [CrossRef]
- Dodd, A.N.; Salathia, N.; Hall, A.; Kévei, E.; Tóth, R.; Nagy, F.; Hibberd, J.M.; Millar, A.J.; Webb, A.A.R. Plant Circadian Clocks Increase Photosynthesis, Growth, Survival, and Competitive Advantage. Science 2005, 309, 630–633. [Google Scholar] [CrossRef]
- Mandal, R.; Dutta, G. From Photosynthesis to Biosensing: Chlorophyll Proves to Be a Versatile Molecule. Sens. Int. 2020, 1, 100058. [Google Scholar] [CrossRef]
- Gao, F.; Guo, J.; Shen, Y. Advances from Chlorophyll Biosynthesis to Photosynthetic Adaptation, Evolution and Signaling. Plant Stress 2024, 12, 100470. [Google Scholar] [CrossRef]
- Greenwood, M.; Tokuda, I.T.; Locke, J.C.W. A Spatial Model of the Plant Circadian Clock Reveals Design Principles for Coordinated Timing. Mol. Syst. Biol. 2022, 18, e10140. [Google Scholar] [CrossRef]
- Wang, Z.-Y.; Tobin, E.M. Constitutive Expression of the CIRCADIAN CLOCK ASSOCIATED 1 (CCA1) Gene Disrupts Circadian Rhythms and Suppresses Its Own Expression. Cell 1998, 93, 1207–1217. [Google Scholar] [CrossRef]
- Das, A.; Guyer, L.; Hörtensteiner, S. Chlorophyll and Chlorophyll Catabolite Analysis by HPLC. In Plant Senescence; Guo, Y., Ed.; Methods in Molecular Biology; Springer: New York, NY, USA, 2018; Volume 1744, pp. 223–235. ISBN 978-1-4939-7670-6. [Google Scholar]
- Steed, G.; Ramirez, D.C.; Hannah, M.A.; Webb, A.A.R. Chronoculture, Harnessing the Circadian Clock to Improve Crop Yield and Sustainability. Science 2021, 372, eabc9141. [Google Scholar] [CrossRef] [PubMed]
- Hwang, Y.; Han, S.; Yoo, C.Y. Anterograde signaling controls plastid transcription via sigma factors separately from nuclear photosynthesis genes. Nat. Commun. 2022, 13, 7440. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Yu, C.; Xiang, B.; Niu, J.; Zheng, Y. Processing tomato chlorophyll a/b-binding protein 1C interacts with CMV 2b protein. Physiol. Mol. Plant Pathol. 2022, 120, 101857. [Google Scholar] [CrossRef]
- Liu, T.; Duan, W.; Chen, Z.; Yuan, J.; Xiao, D.; Hou, X.; Li, Y. Enhanced photosynthetic activity in pak choi hybrids is associated with increased grana thylakoids in chloroplasts. Plant J. 2020, 103, 2211–2224. [Google Scholar] [CrossRef]
- Müller, L.M.; Von Korff, M.; Davis, S.J. Connections between Circadian Clocks and Carbon Metabolism Reveal Species-Specific Effects on Growth Control. J. Exp. Bot. 2014, 65, 2915–2923. [Google Scholar] [CrossRef]
- Pan, W.-J.; Wang, X.; Deng, Y.-R.; Li, J.-H.; Chen, W.; Chiang, J.Y.; Yang, J.-B.; Zheng, L. Nondestructive and Intuitive Determination of Circadian Chlorophyll Rhythms in Soybean Leaves Using Multispectral Imaging. Sci. Rep. 2015, 5, 11108. [Google Scholar] [CrossRef] [PubMed]
- Dodd, A.N.; Dalchau, N.; Gardner, M.J.; Baek, S.; Webb, A.A.R. The Circadian Clock Has Transient Plasticity of Period and Is Required for Timing of Nocturnal Processes in Arabidopsis. New Phytol. 2014, 201, 168–179. [Google Scholar] [CrossRef]
- Covington, M.F.; Maloof, J.N.; Straume, M.; Kay, S.A.; Harmer, S.L. Global Transcriptome Analysis Reveals Circadian Regulation of Key Pathways in Plant Growth and Development. Genome Biol. 2008, 9, R130. [Google Scholar] [CrossRef]
- Joo, Y.; Fragoso, V.; Yon, F.; Baldwin, I.T.; Kim, S.-G. Circadian Clock Component, LHY, Tells a Plant When to Respond Photosynthetically to Light in Nature. J. Integr. Plant Biol. 2017, 59, 572–587. [Google Scholar] [CrossRef]


| Photoperiod | 3L:3D | 6L:6D | 12L:12D | 24L:0D |
|---|---|---|---|---|
| Chlorophyll (SPAD) | 30.83 0.32 a | 24.00 0.44 b | 23.33 0.78 b | 30.40 0.46 a |
| Nitrogen (mg/g) | 9.80 0.10 a | 8.13 0.21 b | 8.03 0.15 a | 9.33 0.15 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lv, H.; Chen, C.; Luo, J.; Xiao, M.; Xiong, A.; Hou, X.; You, X. Skeleton Photoperiod Enhances Photosynthetic Yield in Celery via Circadian-Regulated Metabolic Coordination. Horticulturae 2025, 11, 520. https://doi.org/10.3390/horticulturae11050520
Lv H, Chen C, Luo J, Xiao M, Xiong A, Hou X, You X. Skeleton Photoperiod Enhances Photosynthetic Yield in Celery via Circadian-Regulated Metabolic Coordination. Horticulturae. 2025; 11(5):520. https://doi.org/10.3390/horticulturae11050520
Chicago/Turabian StyleLv, Hengmin, Chen Chen, Jian Luo, Mengting Xiao, Aisheng Xiong, Xilin Hou, and Xiong You. 2025. "Skeleton Photoperiod Enhances Photosynthetic Yield in Celery via Circadian-Regulated Metabolic Coordination" Horticulturae 11, no. 5: 520. https://doi.org/10.3390/horticulturae11050520
APA StyleLv, H., Chen, C., Luo, J., Xiao, M., Xiong, A., Hou, X., & You, X. (2025). Skeleton Photoperiod Enhances Photosynthetic Yield in Celery via Circadian-Regulated Metabolic Coordination. Horticulturae, 11(5), 520. https://doi.org/10.3390/horticulturae11050520







