A Predictive Model of the Photosynthetic Rate of Chili Peppers Using Support Vector Regression and Environmental Multi-Factor Analysis
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Materials
2.2. Multienvironmental Factor Nested Experimental Design
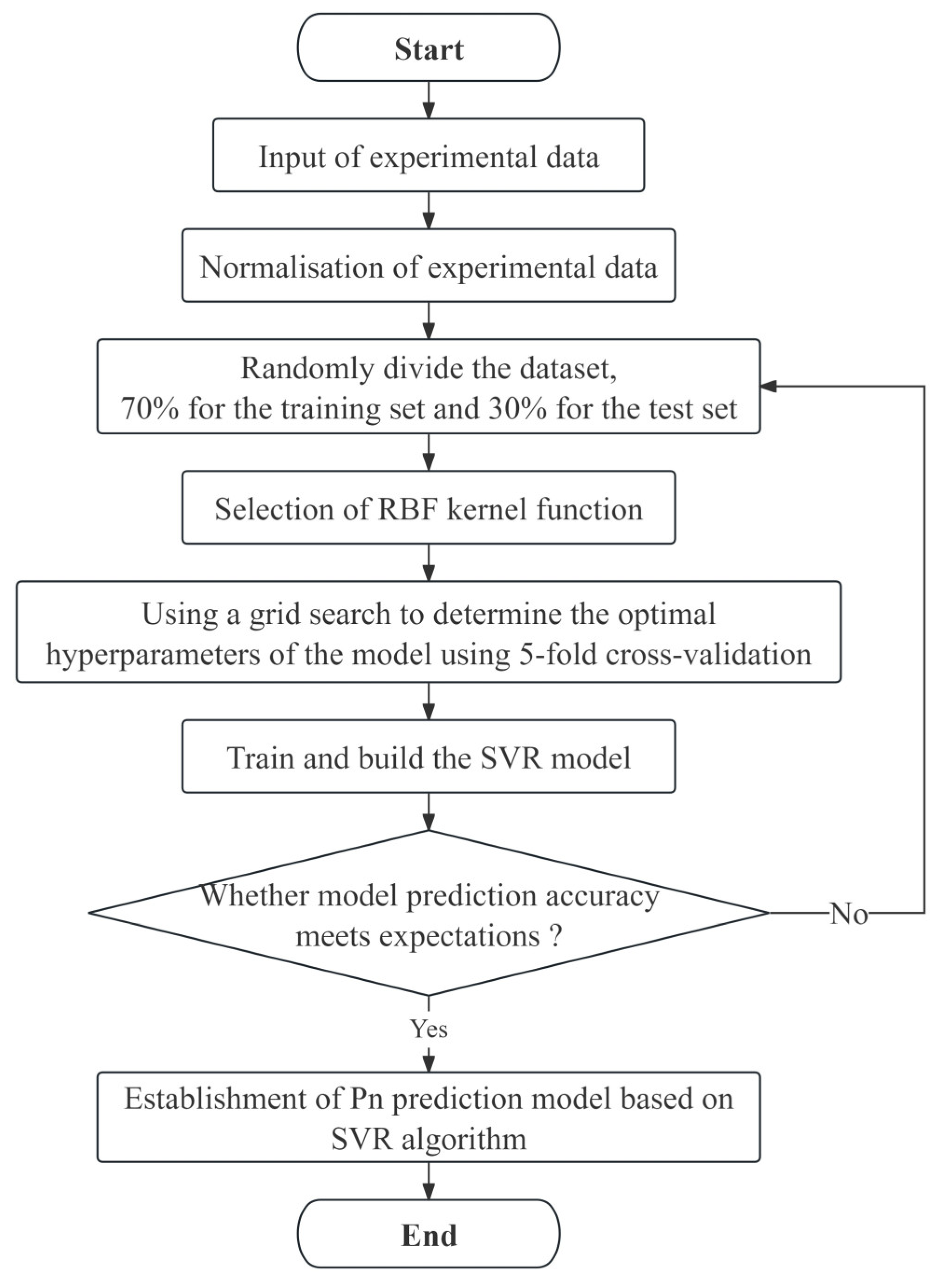
2.3. Construction of the Photosynthetic Rate Prediction Model
2.3.1. Data Preprocessing
2.3.2. Model Construction
2.3.3. Model Evaluation Metrics
2.4. Software Implementation and Data Visualization
3. Results
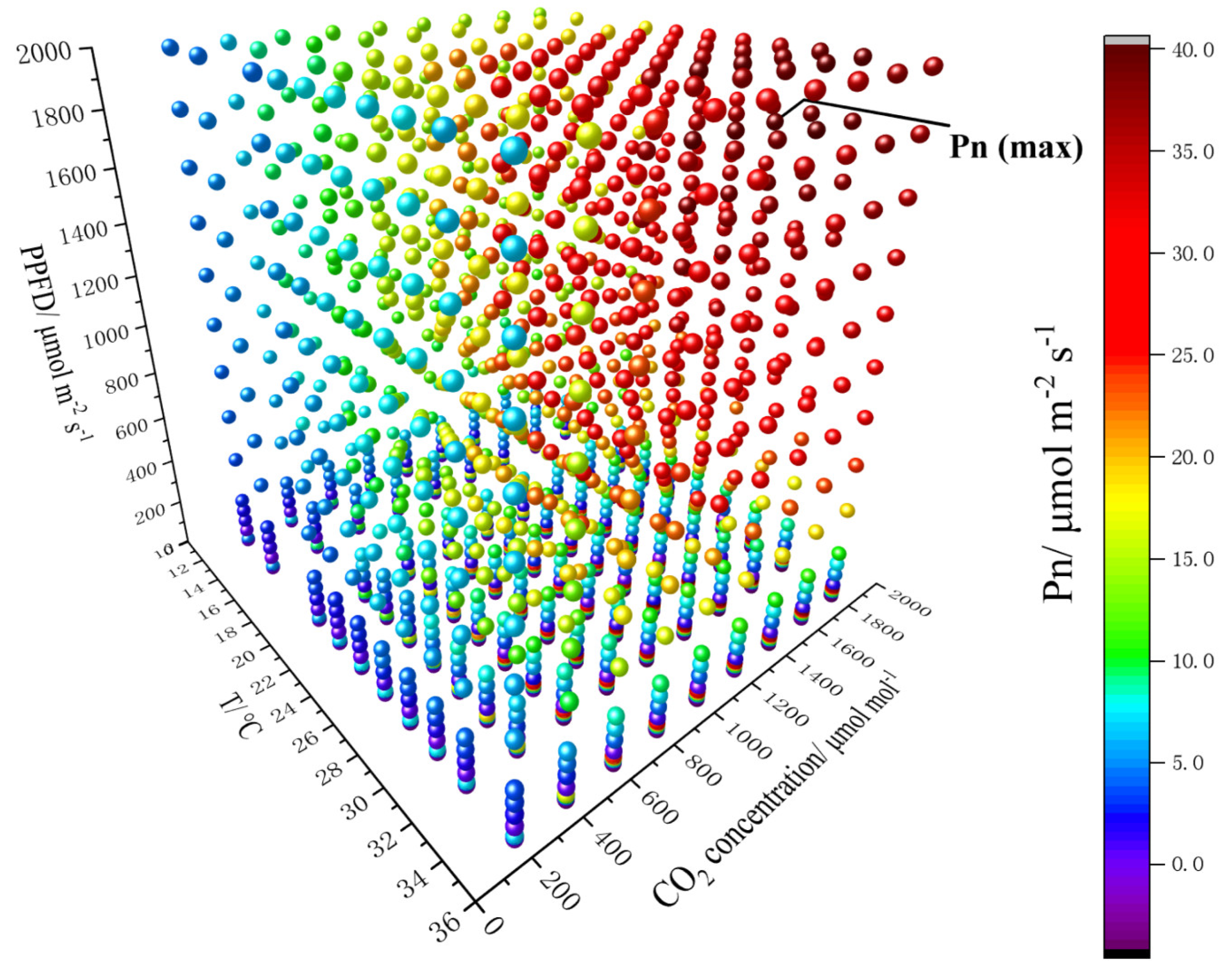
3.1. Impact of Environmental Factors on the Net Photosynthetic Rate
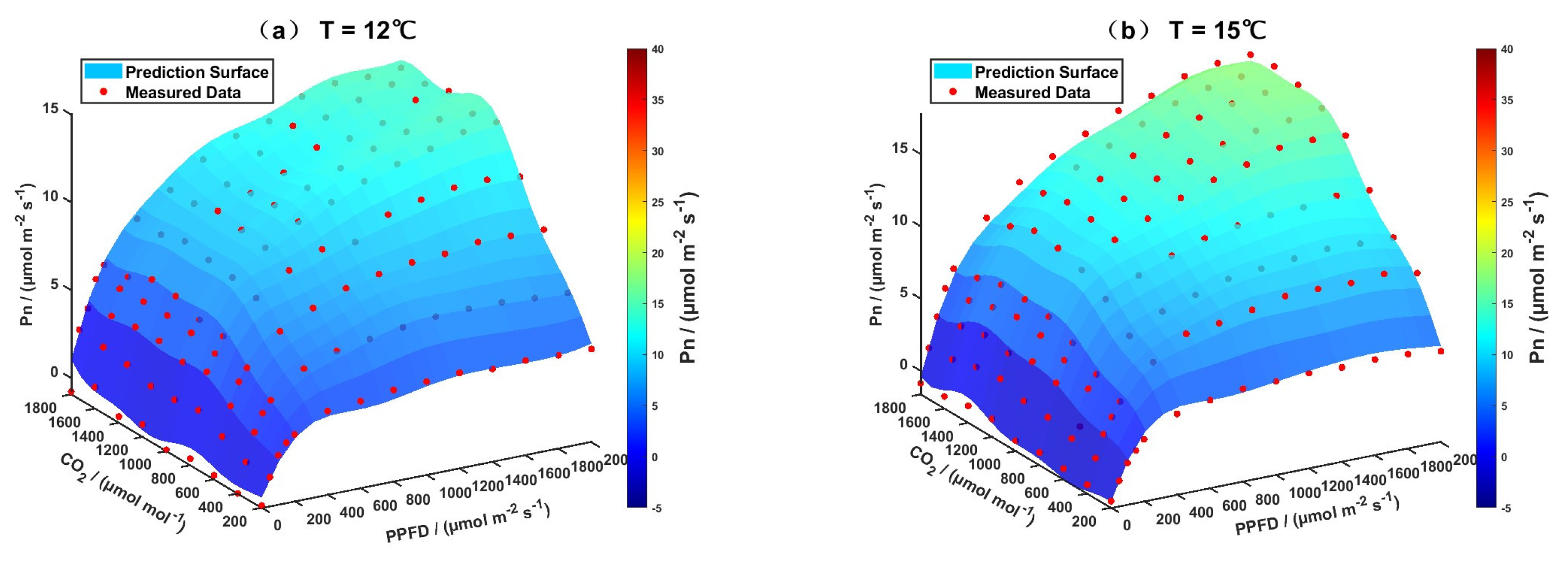
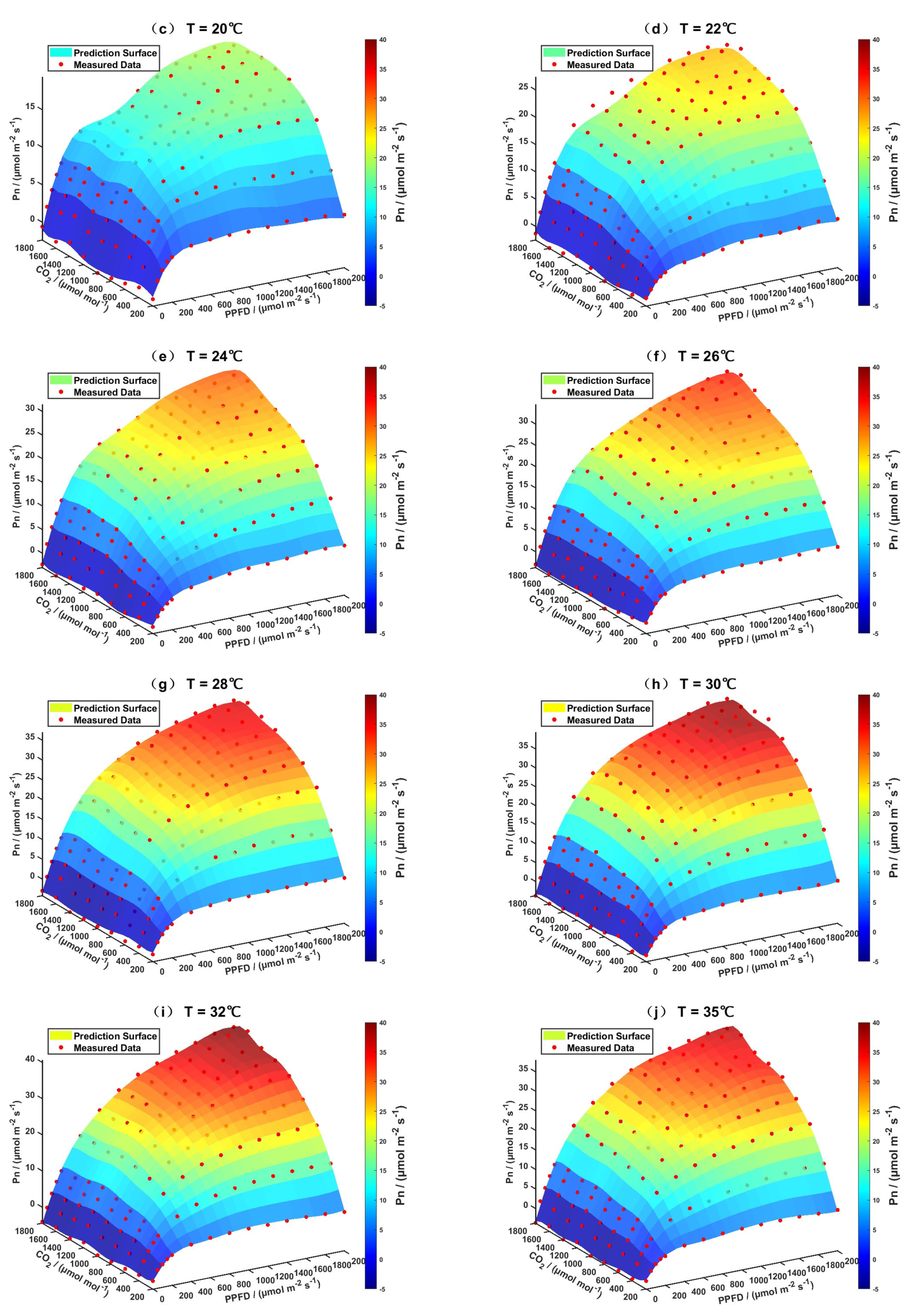
3.2. Model Prediction Results
3.2.1. Evaluation of the Support Vector Regression Model
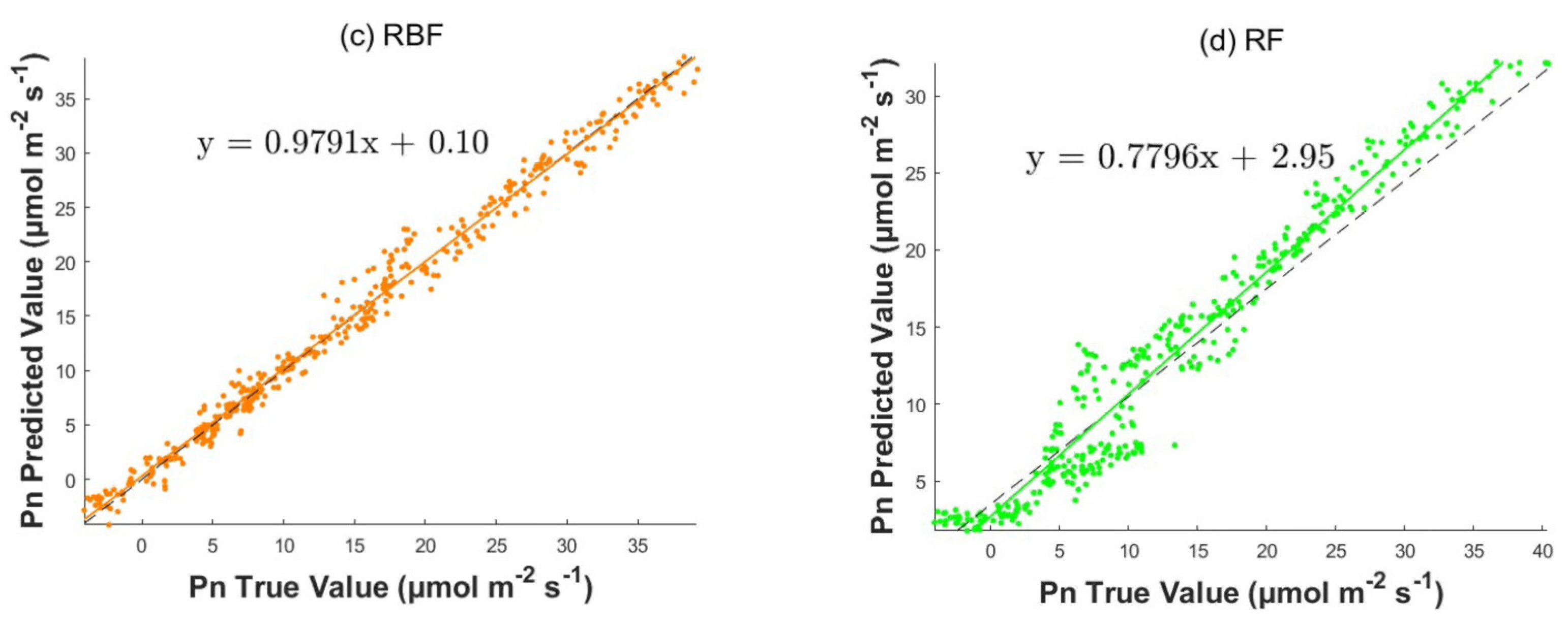
3.2.2. Comparative Analysis of Prediction Models
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Hernández-Morales, C.A.; Luna-Rivera, J.; Perez-Jimenez, R.J.C.C. Design and deployment of a practical IoT-based monitoring system for protected cultivations. Comput. Commun. 2022, 186, 51–64. [Google Scholar] [CrossRef]
- Xu, Y.; Li, J.; Wan, J.J.T.C.J. Agriculture and crop science in China: Innovation and sustainability. Crop J. 2017, 5, 95–99. [Google Scholar] [CrossRef]
- Wang, H.; Meng, X.Y.; Chen, Z.R.; Zhang, X.H.; Cheng, R.F.; Zhang, Y.; Li, W.; Song, W.X.; Zhang, Y. A feedback control method for plant factory environment based on photosynthetic rate prediction model. Comput. Electron. Agric. 2023, 211, 108007. [Google Scholar] [CrossRef]
- Jamil, F.; Ibrahim, M.; Ullah, I.; Kim, S.; Kahng, H.K.; Kim, D.-H.J.C.; Agriculture, E.i. Optimal smart contract for autonomous greenhouse environment based on IoT blockchain network in agriculture. Comput. Electron. Agric. 2022, 192, 106573. [Google Scholar] [CrossRef]
- Jo, W.J.; Shin, J.H. Development of a transpiration model for precise tomato (Solanum lycopersicum L.) irrigation control under various environmental conditions in greenhouse. Plant Physiol. Biochem. 2021, 162, 388–394. [Google Scholar] [CrossRef]
- Xin, P.P.; Zhang, H.H.; Hu, J.; Shao, Z.C.; Peters, R.T. CO2 control system design based on optimized regulation model. Appl. Eng. Agric. 2019, 35, 377–388. [Google Scholar] [CrossRef]
- Smith, E.N.; van Aalst, M.; Tosens, T.; Niinemets, Ü.; Stich, B.; Morosinotto, T.; Alboresi, A.; Erb, T.J.; Gómez-Coronado, P.A.; Tolleter, D.J.M. Improving photosynthetic efficiency toward food security: Strategies, advances, and perspectives. Mol. Plant 2023, 16, 1547–1563. [Google Scholar] [CrossRef]
- Gao, P.; Lu, M.; Yang, Y.; Li, H.; Tian, S.; Hu, J.J.C.; Agriculture, E.I. A predictive model of photosynthetic rates for eggplants: Integrating physiological and environmental parameters. Comput. Electron. Agric. 2025, 234, 110241. [Google Scholar] [CrossRef]
- Niu, Y.; Lyu, H.; Liu, X.; Zhang, M.; Li, H. Photosynthesis prediction and light spectra optimization of greenhouse tomato based on response of red–blue ratio. Sci. Hortic. 2023, 318, 112065. [Google Scholar] [CrossRef]
- Chen, D.Y.; Zhang, J.H.; Zhang, Z.X.; Lu, Y.Q.; Zhang, H.H.; Hu, J. A high efficiency CO2 concentration interval optimization method for lettuce growth. Sci. Total Environ. 2023, 879, 162731. [Google Scholar] [CrossRef]
- Pan, T.; Wang, Y.; Wang, L.; Ding, J.; Cao, Y.; Qin, G.; Yan, L.; Xi, L.; Zhang, J.; Zou, Z. Increased CO2 and light intensity regulate growth and leaf gas exchange in tomato. Physiol. Plant. 2020, 168, 694–708. [Google Scholar] [CrossRef]
- Orujova, T.Y.; Bayramov, S.M.; Gurbanova, U.A.; Babayev, H.G.; Aliyeva, M.N.; Guliyev, N.M.; Feyziyev, Y.M. Diurnal temperature-related variations in photosynthetic enzyme activities of two C4 species of Chenopodiaceae grown in natural environment. Photosynthetica 2018, 56, 1107–1112. [Google Scholar] [CrossRef]
- Dickinson, K.E.; Lalonde, C.G.; McGinn, P.J. Effects of spectral light quality and carbon dioxide on the physiology of Micractinium inermum: Growth, photosynthesis, and biochemical composition. J. Appl. Phycol. 2019, 31, 3385–3396. [Google Scholar] [CrossRef]
- Galmés, J.; Capó-Bauçà, S.; Niinemets, Ü.; Iñiguez, C. Potential improvement of photosynthetic CO2 assimilation in crops by exploiting the natural variation in the temperature response of Rubisco catalytic traits. Curr. Opin. Plant Biol. 2019, 49, 60–67. [Google Scholar] [CrossRef] [PubMed]
- Gao, P.; Lu, M.; Xu, J.H.; Zhang, H.M.; Li, Y.F.; Hu, J. IPECM Platform: An open-source software for greenhouse environment regulation using machine learning and optimization algorithm. Comput. Electron. Agric. 2024, 217, 108564. [Google Scholar] [CrossRef]
- Ye, Z.P.; Duan, S.H.; Chen, X.M.; Duan, H.L.; Gao, C.P.; Kang, H.J.; An, T.; Zhou, S.X. Quantifying light response of photosynthesis: Addressing the long-standing limitations of non-rectangular hyperbolic model. Photosynthetica 2021, 59, 185–191. [Google Scholar] [CrossRef]
- Yin, X.; Busch, F.A.; Struik, P.C.; Sharkey, T.D. Evolution of a biochemical model of steady-state photosynthesis. Plant Cell Environ. 2021, 44, 2811–2837. [Google Scholar] [CrossRef]
- Gao, P.; Tian, Z.W.; Lu, Y.Q.; Lu, M.; Zhang, H.H.; Wu, H.R.; Hu, J. A decision-making model for light environment control of tomato seedlings aiming at the knee point of light-response curves. Comput. Electron. Agric. 2022, 198, 107103. [Google Scholar] [CrossRef]
- Filippi, P.; Jones, E.J.; Wimalathunge, N.S.; Somarathna, P.D.S.N.; Pozza, L.E.; Ugbaje, S.U.; Jephcott, T.G.; Paterson, S.E.; Whelan, B.M.; Bishop, T.F.A. An approach to forecast grain crop yield using multi-layered, multi-farm data sets and machine learning. Precis. Agric. 2019, 20, 1015–1029. [Google Scholar] [CrossRef]
- Xin, P.P.; Zhang, H.H.; Hu, J.; Wang, Z.Y.; Zhang, Z.; Tao, Y.R. An Improved Photosynthesis Prediction Model Based on Artificial Neural Networks Intended for Cucumber Growth Control. Appl. Eng. Agric. 2018, 34, 769–788. [Google Scholar] [CrossRef]
- Hu, J.; Xin, P.P.; Zhang, S.W.; Zhang, H.H.; He, D.J. Model for tomato photosynthetic rate based on neural network with genetic algorithm. Int. J. Agric. Biol. Eng. 2019, 12, 179–185. [Google Scholar] [CrossRef]
- Kmet, T.; Kmetova, M.J.I.S. Adaptive critic design and Hopfield neural network based simulation of time delayed photosynthetic production and prey–predator model. Inf. Sci. 2015, 294, 586–599. [Google Scholar] [CrossRef]
- Santos, C.E.D.; Sampaio, R.C.; Coelho, L.D.; Bestard, G.A.; Llanos, C.H. Multi-objective adaptive differential evolution for SVM/SVR hyperparameters selection. Pattern Recognit. 2021, 110, 107649. [Google Scholar] [CrossRef]
- Sun, Y.T.; Ding, S.F.; Zhang, Z.C.; Jia, W.K. An improved grid search algorithm to optimize SVR for prediction. Soft Comput. 2021, 25, 5633–5644. [Google Scholar] [CrossRef]
- Abdollahpour, S.; Kosari-Moghaddam, A.; Bannayan, M.J.I.P.i.A. Prediction of wheat moisture content at harvest time through ANN and SVR modeling techniques. Inf. Process. Agric. 2020, 7, 500–510. [Google Scholar] [CrossRef]
- Xin, P.P.; Li, B.; Zhang, H.H.; Hui, J. Optimization and control of the light environment for greenhouse crop production. Sci. Rep. 2019, 9, 8650. [Google Scholar] [CrossRef]
- Lu, M.; Gao, P.; Li, H.M.; Sun, Z.T.; Yang, N.; Hu, J. An optimization approach for environmental control using quantum genetic algorithm and support vector regression. Comput. Electron. Agric. 2023, 215, 108432. [Google Scholar] [CrossRef]
- Mohammadi, B.; Mehdizadeh, S. Modeling daily reference evapotranspiration via a novel approach based on support vector regression coupled with whale optimization algorithm. Agric. Water Manag. 2020, 237, 106145. [Google Scholar] [CrossRef]
- Wei, Z.C.; Wan, X.B.; Lei, W.Y.; Yuan, K.K.; Lu, M.; Li, B.; Gao, P.; Wu, H.R.; Hu, J. A Cucumber Photosynthetic Rate Prediction Model in Whole Growth Period with Time Parameters. Agriculture 2023, 13, 204. [Google Scholar] [CrossRef]
- Hou, Z.; Niu, J.; Zhu, J.; Lu, L. Flow Prediction of a Measurement and Control Gate Based on an Optimized Back Propagation Neural Network. Appl. Sci. 2023, 13, 12313. [Google Scholar] [CrossRef]
- Pu, L.R.; Li, Y.F.; Gao, P.; Zhang, H.H.; Hu, J. A photosynthetic rate prediction model using improved RBF neural network. Sci. Rep. 2022, 12, 9563. [Google Scholar] [CrossRef] [PubMed]
- Li, J.H.; Zhu, D.S.; Li, C.X. Comparative analysis of BPNN, SVR, LSTM, Random Forest, and LSTM-SVR for conditional simulation of non-Gaussian measured fluctuating wind pressures. Mech. Syst. Signal Process. 2022, 178, 109285. [Google Scholar] [CrossRef]
- Lin, L.; Liu, X.J.C.; Agriculture, E.i. Mixture-based weight learning improves the random forest method for hyperspectral estimation of soil total nitrogen. Comput. Electron. Agric. 2022, 192, 106634. [Google Scholar] [CrossRef]
- Niu, Y.Y.; Lyu, H.H.; Liu, X.Y.; Zhang, M.; Li, H. Effects of supplemental lighting duration and matrix moisture on net photosynthetic rate of tomato plants under solar greenhouse in winter. Comput. Electron. Agric. 2022, 198, 107102. [Google Scholar] [CrossRef]
- Hou, J.; Li, Y.; Sun, Z.; Wang, H.; Lu, M.; Hu, J.; Wu, H. A cooperative regulation method for greenhouse soil moisture and light using Gaussian curvature and machine learning algorithms. Comput. Electron. Agric. 2023, 215, 108452. [Google Scholar] [CrossRef]
- Jiang, Q.; Zhu, L.; Shu, C.; Sekar, V. An efficient multilayer RBF neural network and its application to regression problems. Neural Comput. Appl. 2022, 34, 4133–4150. [Google Scholar] [CrossRef]
- Niu, G.; Masabni, J.J.H. Plant production in controlled environments. Horticulturae 2018, 4, 28. [Google Scholar] [CrossRef]
- Santosh, D.; Tiwari, K.; Singh, V.K.; Reddy, A.R.G.; Sciences, A. Micro climate control in greenhouse. Int. J. Curr. Microbiol. Appl. Sci. 2017, 6, 1730–1742. [Google Scholar] [CrossRef]
- Kang, J.; Chu, Y.; Ma, G.; Zhang, Y.; Zhang, X.; Wang, M.; Lu, H.; Wang, L.; Kang, G.; Ma, D.J.T.C.J. Physiological mechanisms underlying reduced photosynthesis in wheat leaves grown in the field under conditions of nitrogen and water deficiency. Crop J. 2023, 11, 638–650. [Google Scholar] [CrossRef]
- Shi, Y.; Ke, X.; Yang, X.; Liu, Y.; Hou, X. Plants response to light stress. J. Genet. Genom. 2022, 49, 735–747. [Google Scholar] [CrossRef]
- Fukayama, H.; Mizumoto, A.; Ueguchi, C.; Katsunuma, J.; Morita, R.; Sasayama, D.; Hatanaka, T.; Azuma, T. Expression level of Rubisco activase negatively correlates with Rubisco content in transgenic rice. Photosynth. Res. 2018, 137, 465–474. [Google Scholar] [CrossRef] [PubMed]
- Pang, Y.; Liao, Q.; Peng, H.; Qian, C.; Wang, F.J.P. CO2 mesophyll conductance regulated by light: A review. Planta 2023, 258, 11. [Google Scholar] [CrossRef] [PubMed]
- van de Ven, G.M.; Siegelmann, H.T.; Tolias, A.S. Brain-inspired replay for continual learning with artificial neural networks. Nat. Commun. 2020, 11, 4069. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.Y.; Jiang, Z.H.; Tai, Q.L.; Shen, C.S.; Rao, Y.; Zhang, W. Construction of a photosynthetic rate prediction model for greenhouse strawberries with distributed regulation of light environment. Math. Biosci. Eng. 2022, 19, 12774–12791. [Google Scholar] [CrossRef]
- Vincenzi, E.; Ji, Y.; Kerstens, T.; Lai, X.; Deelen, S.; de Beer, E.; Millenaar, F.; Marcelis, L.F.; Heuvelink, E.J.S.H. Duration, not timing during the photoperiod, of far-red application determines the yield increase in tomato. Sci. Hortic. 2024, 338, 113553. [Google Scholar] [CrossRef]

| Regression Algorithm | R2 | RMSE (μmol m−2 s−1) | MAE (μmol m−2 s−1) | MBE (μmol m−2 s−1) | MAPE |
|---|---|---|---|---|---|
| SVR | 0.9975 | 0.4897 | 0.3937 | −0.0202 | 0.0717 |
| BP | 0.9899 | 1.0681 | 0.8108 | 0.0711 | 0.1321 |
| RBF | 0.9879 | 1.1857 | 0.8894 | −0.0006 | 0.1376 |
| RF | 0.9428 | 2.6056 | 2.1055 | −0.0059 | 0.5393 |
| Regression Algorithm | R2 | RMSE (μmol m−2 s−1) | MAE (μmol m−2 s−1) | MBE (μmol m−2 s−1) | MAPE |
|---|---|---|---|---|---|
| SVR | 0.9941 | 0.6988 | 0.5166 | −0.0375 | 0.0907 |
| BP | 0.9887 | 1.1977 | 0.9062 | −0.0557 | 0.1309 |
| RBF | 0.9867 | 1.2739 | 0.9679 | 0.0098 | 0.1671 |
| RF | 0.9391 | 2.6522 | 2.1055 | −0.2399 | 0.4405 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, B.; Qiao, B.; Zhao, Q.; Yang, D.; Zhu, R.; Wang, Z.; Yang, Y. A Predictive Model of the Photosynthetic Rate of Chili Peppers Using Support Vector Regression and Environmental Multi-Factor Analysis. Horticulturae 2025, 11, 502. https://doi.org/10.3390/horticulturae11050502
Li B, Qiao B, Zhao Q, Yang D, Zhu R, Wang Z, Yang Y. A Predictive Model of the Photosynthetic Rate of Chili Peppers Using Support Vector Regression and Environmental Multi-Factor Analysis. Horticulturae. 2025; 11(5):502. https://doi.org/10.3390/horticulturae11050502
Chicago/Turabian StyleLi, Bin, Bo Qiao, Qianyu Zhao, Dan Yang, Rongcheng Zhu, Zhexuan Wang, and Yujie Yang. 2025. "A Predictive Model of the Photosynthetic Rate of Chili Peppers Using Support Vector Regression and Environmental Multi-Factor Analysis" Horticulturae 11, no. 5: 502. https://doi.org/10.3390/horticulturae11050502
APA StyleLi, B., Qiao, B., Zhao, Q., Yang, D., Zhu, R., Wang, Z., & Yang, Y. (2025). A Predictive Model of the Photosynthetic Rate of Chili Peppers Using Support Vector Regression and Environmental Multi-Factor Analysis. Horticulturae, 11(5), 502. https://doi.org/10.3390/horticulturae11050502





