Abstract
Cowpea is a nutritionally and economically valuable legume, known for its adaptability to adverse conditions. However, water stress negatively affects its development, requiring technologies to enhance resilience. This study aimed to induce tolerance to water deficit in cowpea through seed priming with polyethylene glycol 6000 (PEG 6000) and silicic acid. A completely randomized experiment was conducted in a phytotron chamber with two water regimes (W50 and W100) and six seed priming treatments, with four replications. Priming consisted of three water potentials induced by PEG 6000 (0 MPa, −0.4 MPa, and −0.8 MPa) and two silicon concentrations (0 and 200 mg L−1). Gas exchange parameters, including photosynthetic rate (A), transpiration rate (E), stomatal conductance (gs), intercellular CO2 concentration (Ci), instantaneous water use efficiency (WUEi), and instantaneous carboxylation efficiency (iCE), were evaluated. Seed priming with PEG 6000 and silicon improved A, WUEi, and iCE under water deficit. Treatments 2 (0 MPa + 200 mg L−1 Si), 3 (−0.4 MPa + 0 mg L−1 Si), and 4 (−0.4 MPa + 200 mg L−1 Si) enhanced gas exchange, suggesting an effective strategy for improving drought tolerance in cowpea and ensuring food security.
1. Introduction
Climate change across the world is causing damage due to reduced productivity in various crops, resulting in negative ecological, economic, and social impacts [1]. Water scarcity is one of the consequences of these changes, characterized as a primary abiotic stress and, therefore, one of the main challenges to be faced by society in the 21st century, as its negative impacts will be reflected in several human activities on the planet [2], with agriculture being the most affected, particularly compromising food and nutritional security [3]. In this regard, as an adaptation strategy to adverse conditions, a better understanding of plant plasticity will increase the possibility of selecting resilient plants to the expected climate fluctuations [4]. Thus, there is a need to cultivate species with a diversity of improved genotypes adapted to growing environments, such as cowpea [Vigna unguiculata (L.) Walp.], which possesses traits of interest to farmers [5].
Cowpea is a species characterized by its high yield potential and contains essential nutrients for health, such as proteins, amino acids, carbohydrates, minerals, fibers, and vitamins. It also contains bioactive compounds with anti-inflammatory, immunostimulant, neuroprotective, anticancer, antioxidant, and cardioprotective properties [6,7]. Moreover, its low cultivation cost and moderate drought tolerance make it a widely used crop by small-, medium-, and large-scale farmers [8]. However, despite showing moderate tolerance to several factors, drought remains one of the most limiting to its productivity [9,10]. Therefore, the implementation of technologies that enable cowpea production even under adverse conditions is necessary to ensure food security and a source of income for farmers.
Among the various available technologies, seed priming stands out for inducing tolerance to deleterious biotic and abiotic effects in plants through stress memory imposed during the seed conditioning process. This process results in physiological and metabolic improvements in the resulting plants, leading to individuals with greater vigor and resistance to stress [11,12]. Studies indicate that the seed priming technique has shown promising results in various crops such as maize [13], wheat [14], soybean [15], faba bean [16], and cowpea [17,18]. In general, the beneficial effects observed in these species are associated with increased regulation of primary and secondary metabolites, greater antioxidant capacity, and activation of osmoprotective and osmoregulatory mechanisms, providing plants with greater resistance to environmental stresses.
In addition to the aforementioned information, it is noteworthy that various inducing agents can be used in seed priming, such as silicon [19], spectral light radiation [20], and polyethylene glycol 6000 (PEG 6000) [21]. However, information on the combination of these factors in the osmotic conditioning of cowpea seeds is still scarce, and further studies are needed to elucidate the effectiveness of this type of treatment. In this context, the hypothesis is that physiological seed conditioning can mitigate the effects of drought in cowpea plants by promoting improvements in physiological responses. Therefore, the aim of this study was to induce drought tolerance in cowpea through physiological seed conditioning using polyethylene glycol 6000 and silicic acid.
2. Materials and Methods
2.1. Location and Conduct of the Experiment
The experiment was conducted in two stages, with Stage I (application of seed conditioning) being carried out at the Cultivated Plant Ecophysiology Laboratory—ECOLAB/UEPB, located in the state of Paraíba. Stage II of the experiment (plant development in a phytotron growth chamber) was carried out at the Experimental Station located at 07°12′42.99″ South latitude, 35°54′36.27″ West longitude, and an altitude of 521 m, belonging to the State University of Paraíba—UEPB.
2.2. Application of Seed Priming (Step I)
Cowpea seeds [Vigna unguiculata (L.) Walp.] of the cultivar BRS Pingo de Ouro were used, provided by the Laboratory of Ecophysiology of Cultivated Plants—ECOLAB/UEPB. Initially, the seeds were screened, and those showing physical or biological damage and/or malformation were discarded. Subsequently, the seeds were disinfected in a 1% sodium hypochlorite (NaClO) solution for 3 min, followed by rinsing with distilled water for 1 min. The seeds were then briefly dried at room temperature using sterilized paper [22].
Immediately after this process, 50 seeds with a moisture content of approximately 15% were placed in Gerbox®-type plastic boxes (11 × 11 × 3.5 cm in length, width, and height), arranged in an orderly manner. The substrate used inside the boxes consisted of two layers of Germitex® paper (non-toxic, neutral pH, grammage of 65 g m−2), previously autoclaved and moistened with the specific solutions for each priming treatment, in a volume equivalent to approximately 2.5 times the dry mass of the paper [20].
The boxes containing the seeds were placed in B.O.D.-type germination chambers (Marconi, Piracicaba, Brazil), adapted with LED strips to provide the required light conditions. The conditioning period lasted 5 h, which was sufficient for seed imbibition without radicle protrusion. Afterwards, the seeds were transferred to Gerbox® boxes containing two layers of dry germitest paper and subjected to the drying process under the same light and temperature conditions used during conditioning (Figure 1).
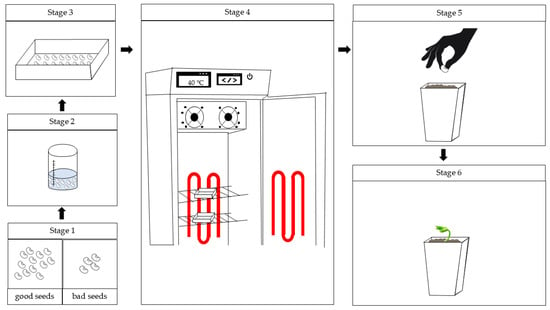
Figure 1.
Methodological flowchart for Step I. Stage 1: sorting the seeds; Stage 2: sterilization of the seeds in sodium hypochlorite solution; Stage 3: seeds placed in the substrate with the appropriate treatments; Stage 4: Gerboxes containing the seeds conditioned in a B.O.D. germination chamber adapted with LED strips; Stage 5: seeds obtained from conditioning sown in pots; Stage 6: conducting the experiment (Step II).
Seed conditioning (E) was carried out using combinations of three water potentials induced by polyethylene glycol 6000 (PEG 6000): no water deficit (Ψw = 0 MPa), moderate deficit (Ψw = −0.4 MPa), and severe deficit (Ψw = −0.8 MPa). These were used along with two concentrations of silicic acid (a commercial product containing 42% silicon): 0 and 200 mg L−1. The conditioning was conducted under high-light-intensity conditions, using red light with a wavelength between 600 and 680 nm (RL) and a constant temperature of 40 °C. All these conditions were employed to activate defense responses in the seeds, as described by Araújo et al. [23], Costa et al. [20], and Alencar et al. [22] (Table 1). After the treatment, the seeds were dried under the same light and temperature conditions until they returned to their original dry weight, with moisture content ranging from 12% to 15%.

Table 1.
Descriptive summary of the treatments used in the experiment.
2.3. Growing in a Fitotron-Type Growth Chamber (Step II)
The seeds obtained from the priming in Step I were sown in polyethylene pots with a capacity of 0.8 L, filled with 1 kg of substrate consisting of 75% homogenized soil and 25% cattle manure. After sowing, the pots were transferred to a Fitotron-type growth chamber (Weiss Technik, Technal, Reiskirchen-Lindenstruth, Germany), with the temperature set to 32 °C during the day and 28 °C at night, relative humidity maintained at 60%, and a 12 h photoperiod, aiming to simulate conditions similar to those found in the semi-arid region of Paraíba.
Water replacement levels were managed normally, using 100% of ETc (crop evapotranspiration), until 20 days after sowing. Water stress began at 21 DAS, when the irrigation rates were differentiated. Irrigation was daily, using the method of weighing the pots [22]. The water used was public-supply water from CAGEPA (Companhia de Água e Esgotos da Paraíba).
2.4. Variables Analyzed
The gas exchange assessments were conducted using an LI-6800 Portable Photosynthesis System (LI-COR Inc., Lincoln, USA). Throughout the evaluations, a constant photon flux density of 1200 μmol m−2 s−1 of actinic light was employed. The chamber’s relative humidity was maintained at 60%, and the CO2 concentration at 400 μmol mol−1. The air temperature within the chamber was monitored using a thermoelectric sensor located at its base, and it was set at 27 °C. Plants were evaluated for the following parameters: photosynthetic rate (A) (μmol CO2 m−2 s−1), transpiration rate (E) (mmol H2O m−2 s−1), stomatal conductance (gs) (mmol m−2 s−1), and intercellular CO2 concentration (Ci) (μmol CO2 m−2 s−1). These variables were measured utilizing the third fully expanded leaf, counted from the apex of the main branch, during the morning hours (between 7 and 11 a.m.).
With these data, the instantaneous efficiency of water use (WUEi) [(μmol CO2 m−2 s−1) (mmol H2O m−2 s−1)−1] was quantified through the ratio between photosynthetic rate (A) and transpiration rate (E) and instantaneous carboxylation efficiency (iCE) [(μmol CO2 m−2 s−1) (μmol CO2 m−2 s−1)−1], through the ratio between photosynthetic rate (A) and intercellular CO2 concentration (Ci).
2.5. Experimental Design and Statistical Analysis
The experiment was conducted in a completely randomized design (DIC), in a 6 × 2 factorial scheme. The factors consisted of six seed conditionings—control (40 °C + Ψw 0 MPa + 0 mg L−1 of Si + RL), seed priming 2 (40 °C + Ψw 0 MPa + 200 mg L−1 of Si + RL), seed priming 3 (40 °C + Ψw −0.4 MPa + 0 mg L−1 of Si + RL), seed priming 4 (40 °C + Ψw −0.4 MPa + 200 mg L−1 Si + RL), seed priming 5 (40 °C + Ψw −0.8 MPa + 0 mg L−1 Si + RL), and seed priming 6 (40 °C + Ψw −0.8 MPa + 200 mg L−1 Si + RL)—and two levels of crop evapotranspiration water replacement (100 and 50% of ETc), with four replications. The combination of these two factors resulted in 12 treatments, making up 48 experimental units.
The data obtained were subjected to the Shapiro–Wilk normality test [24], and when the assumptions of normality were met, analysis of variance was carried out (F test up to 5% probability). The t-test (LSD) was used to compare the means of reference evapotranspiration in order to assess the performance of the treatments when water restriction was imposed. And Tukey’s test was used to analyze the difference between the different seed priming combinations. The statistical software Sisvar 5.6 [25] was used to carry out the analyses.
3. Results
3.1. Photosynthetic Rate
Plants exposed to water restriction conditions (W50) showed reductions in their photosynthetic rate, regardless of treatment, when compared to the control under ideal irrigation conditions (W100). These reductions corresponded to 84, 39, 45, 50, 57, and 47%, respectively, for all treatments (Figure 2).
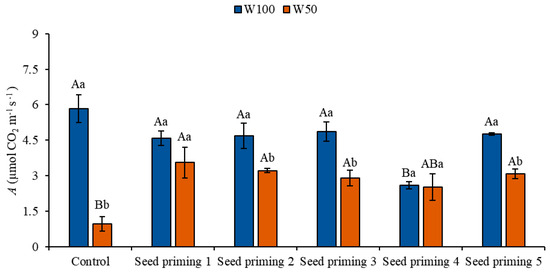
Figure 2.
Carbon assimilation (A) of the cowpea cultivar BRS Pingo de Ouro, conditioned to two irrigation rates (W100 and W50, corresponding to 100 and 50% of crop evapotranspiration water replacement) and seed priming combinations. Ψw: water potential in Mega Pascal (MPa); and Si: silicon in milligrams per liter (mg L−1). Capital letters differentiate between seed priming combinations (Tukey p ≤ 0.05). Lowercase letters differentiate between irrigation rates (t-Student p ≤ 0.05). Error bars represent the standard error (SE, n = 4).
However, among the plants under water restriction, an increase in carbon assimilation was observed in the priming treatments 2, 3, 4, and 6, with increments of 272, 236, 204, and 221%, respectively, compared to the stressed control. On the other hand, even under ideal irrigation conditions (W100), the plants subjected to priming showed a reduction in their carbon assimilation rate compared to the control (Figure 2).
3.2. Transpiration Rate
In the present study, when evaluating transpiration (E), it was observed that plants under water restriction conditions (W50) showed a reduction in transpiration rate, regardless of treatment, when compared to the control under ideal irrigation conditions (W100). The reductions observed were 88, 66, 55, 68, 75, and 72%, respectively, among the treatments (Figure 3).
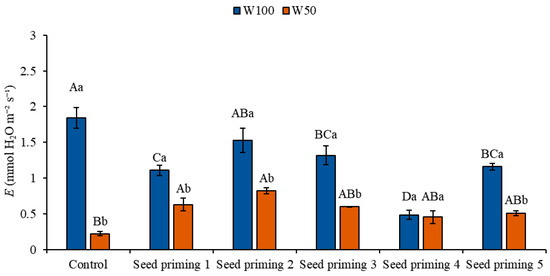
Figure 3.
Transpiration (E) of the cowpea cultivar BRS Pingo de Ouro, conditioned to two irrigation rates (W100 and W50, corresponding to 100 and 50% of crop evapotranspiration water replacement) and combinations of seed priming. Ψw: water potential in Mega Pascal (MPa); and Si: silicon in milligrams per liter (mg L−1). Capital letters differentiate between seed priming combinations (Tukey p ≤ 0.05). Lowercase letters differentiate between irrigation rates (t-Student p ≤ 0.05). Error bars represent the standard error (SE, n = 4).
However, notable increases of 187, 274, 173, 106, and 132% were observed in primings 2, 3, 4, 5, and 6, respectively, in relation to the control under W50 (Figure 3). Statistically significant differences were verified in primings 2 and 3 compared to the control under the same irrigation level. When analyzing the behavior of the treatments under ideal irrigation conditions (W100), a reduction in transpiration rate of 39, 16, 28, 73, and 37% was observed in primings 2, 3, 4, 5, and 6, respectively, compared to the control under W100 (Figure 3).
3.3. Stomatal Conductance
Water restriction also caused reductions in stomatal conductance (gs) when the stressed plants were compared to the control under optimal irrigation conditions (W100). These reductions corresponded to 89, 69, 66, 67, 77, and 74% in the different seed primings (Figure 4).
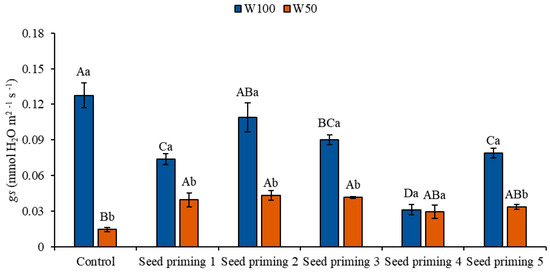
Figure 4.
Stomatal conductance (gs) of the cowpea cultivar BRS Pingo de Ouro, conditioned to two irrigation rates (W100 and W50, corresponding to 100 and 50% of crop evapotranspiration water replacement) and seed priming combinations. Ψw: water potential in Mega Pascal (MPa); and Si: silicon in milligrams per liter (mg L−1). Capital letters differentiate between seed priming combinations (Tukey p ≤ 0.05). Lowercase letters differentiate between irrigation rates (t-Student p ≤ 0.05). Error bars represent the standard error (SE, n = 4).
Under W100, a decrease in stomatal conductance was observed in all plants subjected to primings 2, 3, 4, 5, and 6, with reductions of 42, 15, 29, 76, and 38%, respectively, compared to the control under the same irrigation conditions (Figure 4). Under W50, when comparing the treatments to the control under the same water level, significant increases in stomatal conductance were observed in primings 2, 3, 4, 5, and 6, with increases of 172, 198, 186, 102, and 131%, respectively (Figure 4).
3.4. Intercellular Carbon Concentration
When evaluating internal carbon concentration (Ci), reductions were observed across all treatments subjected to water restriction (W50) compared to the irrigated control (W100), with particular emphasis on primings 2 and 6, which exhibited decreases of 21 and 22%, respectively. Under W50 conditions, the control plants showed the highest Ci values, whereas the remaining treatments presented reductions of 20, 13, 11, 18, and 20% for primings 2, 3, 4, 5, and 6, respectively, in comparison to the control under the same water replacement level. Under optimal irrigation conditions (W100), primings 2, 4, 5, and 6 did not result in increases in Ci, on the contrary, reductions of 7, 3, 21, and 6%, respectively, were recorded in comparison to the control plants under W100 (Figure 5).
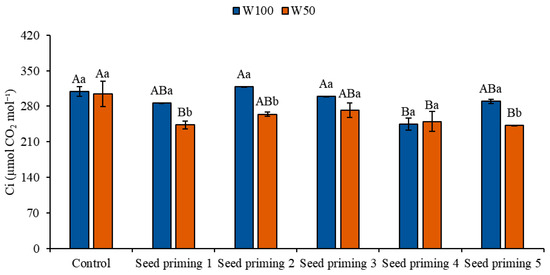
Figure 5.
Internal carbon (Ci) of the cowpea cultivar BRS Pingo de Ouro, conditioned to two irrigation rates (W100 and W50, corresponding to 100 and 50% of crop evapotranspiration water replacement) and seed priming combinations. Ψw: water potential in Mega Pascal (MPa); and Si: silicon in milligrams per liter (mg L−1). Capital letters differentiate between seed priming combinations (Tukey p ≤ 0.05). Lowercase letters differentiate between irrigation rates (t-Student p ≤ 0.05). Error bars represent the standard error (SE, n = 4).
3.5. Instantaneous Efficiency of Water Use
When evaluating instantaneous water use efficiency (WUEi), all priming treatments showed satisfactory results under water stress conditions (W50), with increases of 72, 22, 50, 73, and 86% in relation to the control under the same condition. Similarly, primings 2, 4, 5, and 6 also demonstrated increases in WUEi under optimal irrigation (W100), with improvements of 28, 20, 71, and 28%, respectively, when compared to the control under the same water replacement level (Figure 6).
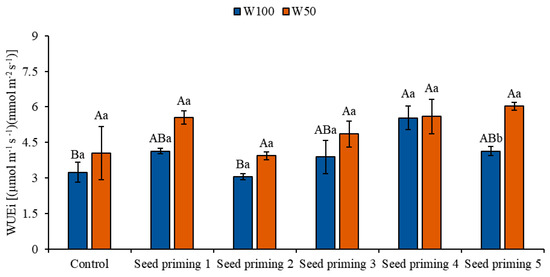
Figure 6.
Instantaneous water use efficiency (WUEi) of the cowpea cultivar BRS Pingo de Ouro, conditioned to two irrigation rates (W100 and W50, corresponding to 100 and 50% of crop evapotranspiration water replacement) and seed priming combinations. Ψw: water potential in Mega Pascal (MPa); and Si: silicon in milligrams per liter (mg L−1). Capital letters differentiate between seed priming combinations (Tukey p ≤ 0.05). Lowercase letters differentiate between irrigation rates (t-Student p ≤ 0.05). Error bars represent the standard error (SE, n = 4).
3.6. Instantaneous Carboxylation Efficiency
Water restriction also negatively affected the instantaneous carboxylation efficiency (iCE), with reductions observed in all priming treatments when compared to the control under full irrigation (W100). These reductions corresponded to 84, 21, 37, 42, 47, and 32% for primings 1, 2, 3, 4, 5, and 6, respectively. However, under stress conditions (W50), a marked increase in iCE values was observed for primings 2 (400%), 3 (300%), 4 (267%), 5 (233%), and 6 (333%) in relation to the control under the same irrigation level. Conversely, under W100 conditions, all primings presented lower iCE values when compared to the control within the same irrigation regime, with reductions of 16, 26, 16, 47, and 16% for primings 2, 3, 4, 5, and 6, respectively (Figure 7).
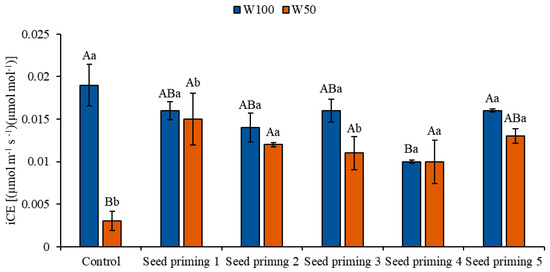
Figure 7.
Instantaneous carboxylation efficiency (iCE) of the cowpea cultivar BRS Pingo de Ouro, conditioned to two irrigation rates (W100 and W50, corresponding to 100 and 50% of crop evapotranspiration water replacement) and seed priming combinations. Ψw: water potential in Mega Pascal (MPa); and Si: silicon in milligrams per liter (mg L−1). Capital letters differentiate between seed priming combinations (Tukey p ≤ 0.05). Lowercase letters differentiate between irrigation rates (t-Student p ≤ 0.05). Error bars represent the standard error (SE, n = 4).
4. Discussion
Corroborating the results of the present study, negative changes in photosynthesis in cowpea were observed when water restriction was imposed [26,27]. These studies emphasize that, even though cowpea may show some tolerance to water restriction, this type of stress compromises the plant’s development.
The reductions examined in carbon assimilation are directly linked to stomatal closure. Through this mechanism, plant species are able to restrict water loss and overcome periods of drought, mainly to maintain cell turgor [26]. However, the plants that underwent priming showed significant increases in their photosynthetic rate, with increases of 272, 236, 204, and 221% in seed priming 2, 3, 4, and 6, respectively, when compared to the control under stress, reducing the effects of water restriction. On the other hand, this contributes to a decrease in CO2 assimilation and transpiration, as it is a common pathway for both. Furthermore, when this flow is restricted, less CO2 is available for fixation during photosynthesis, resulting in lower biomass production and limitations in plant growth and development [28,29].
Transpiration is a process closely related to photosynthesis, aiding in water absorption by the roots through a negative pressure gradient generated within the plant. This gradient maintains a continuous flow of water towards the atmosphere, against the force of gravity, through the conducting vessels, and can indirectly reflect the soil water status. The results obtained for the transpiration rate are related to reductions in stomatal conductance (gs), which also reflect the soil water status, indicating that the root system was not able to adequately supply the water demand. As a defense mechanism, the plant promotes partial stomatal closure, increasing stomatal resistance [29].
Furthermore, another explanation for the reduction in the photosynthetic process lies in the decrease in chlorophyll content caused by water stress, which results in significant reductions in gas exchange [30]. In this way, the results observed for this variable can be justified by the stomatal adjustment promoted by the osmotic conditioning of seeds, which aims to improve water use efficiency in plants (Figure 3).
The positive results obtained for stomatal conductance (gs) in the seed priming treatments were crucial, reflecting improvements in other variables assessed in this study, particularly transpiration (E) and net carbon assimilation (A). This is due to the fact that stomatal conductance is directly related to the functioning of stomata in gas exchange processes, such as the uptake of carbon dioxide (CO2) and the release of water vapor (H2O) between the plant and the environment.
Plants subjected to priming showed increases in A, E, and gs. This outcome may be associated with the action of PEG 6000, which, during the seed priming process, promotes water withdrawal from the cell wall of the seed. This osmotic stress induces solute accumulation, resulting in a greater cellular turgor potential at the moment of seed rehydration, thereby accelerating the emergence of the primary root. Such a mechanism may induce stress memory, placing the seedling in a state of alert for future adverse conditions. Additionally, it may stimulate the production of proline, a compound that assists in maintaining the plant’s water status, contributing to the preservation of photosynthetic activity [31].
Silicon also plays an important role in this context. Its use in seed treatment has expanded over the years, with proven effectiveness in various crops, particularly in mitigating the deleterious effects caused by water stress [32]. Specifically, silicon may act as a physical barrier by accumulating in the leaf apoplasts, contributing to the enhancement of photosynthesis [33]. Furthermore, this element actively participates in plant defense mechanisms, promoting increased activity of antioxidant enzymes such as superoxide dismutase and peroxidase, which constitute an effective line of defense against reactive oxygen species, helping to mitigate the adverse effects of drought [34].
The results obtained for internal carbon (Ci) suggest that the reduction in CO2 assimilation compromised this variable, which favored carbon accumulation and negatively influenced photosynthetic rates, as well as contributing to a decrease in instantaneous carboxylation efficiency. The increase in Ci can cause stomatal limitations, such as changes in their behavior and density [35]. Therefore, the internal concentration of CO2 in the leaf mesophyll is reduced by stomatal closure, with a consequent decrease in the rate of carbon assimilation [27].
WUEi is a measure that relates the rate of carbon assimilation in a plant to the amount of water lost through transpiration. It represents the efficiency with which the plant utilizes the available water to produce biomass via photosynthesis. This physiological variable reflects the plant’s ability to maximize the use of available water for its growth and development [36].
These variables are of utmost importance, as they reveal physiological mechanisms that enable plants to thrive even under adverse conditions. In this study, WUEi was influenced by water restriction, showing its lowest values under full irrigation (W100). This response is associated with a simultaneous increase in both carbon assimilation and transpiration rates. For this reason, WUEi showed relevant outcomes in plants derived from seed priming, as these demonstrated greater efficiency in stomatal control, better regulating water loss in relation to carbon assimilation [37,38].
Similarly to WUEi, the instantaneous carboxylation efficiency (iCE) reflects the efficiency of carbon fixation metabolic reactions and is expressed as the ratio between net CO2 assimilation and internal carbon concentration (Ci) [37]. When evaluating this variable, it can be inferred that the reductions in iCE likely occurred due to a decrease in stomatal conductance, which compromises the physiological processes related to CO2 assimilation, directly affecting carboxylation efficiency [37].
Nevertheless, it is evident that plants derived from primed seeds acquire some form of physiological memory, standing out for their greater tolerance to abiotic stress. The increases observed in WUEi under water restriction may be associated with the greater accumulation of proline induced by PEG 6000 treatment, an effect that is enhanced by the action of silicon, given that osmoregulation represents a key mechanism for improving this variable [22]. On the other hand, the observed increases in instantaneous carboxylation efficiency are mainly attributed to the higher internal CO2 concentration and the gains in assimilation rates observed in this study [27].
Moreover, osmotic priming with PEG 6000 can trigger improvements in the plant antioxidant system, promoting enhanced activity against reactive oxygen species (ROS) [39]. This effect is intensified by the presence of silicon, which contributes to the mitigation of the negative effects of water stress by reducing lipid peroxidation and plasma membrane permeability [40]. Overall, seeds treated with PEG 6000 and silicon exhibited a superior performance, even under limiting water conditions (Figure 8).
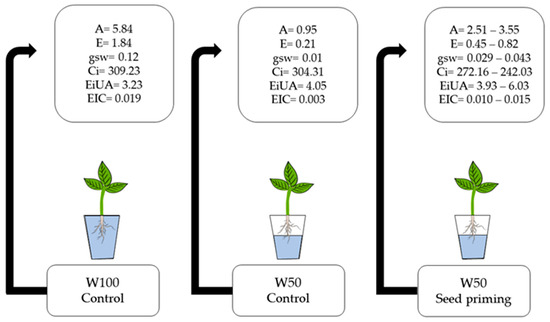
Figure 8.
W100 Control: represents the values obtained under normal irrigation conditions (100% field capacity); W50 Control: represents the values obtained under limited irrigation conditions (50% field capacity), without seed priming application; W50 Seed Priming: presents the minimum and maximum values of the variables photosynthetic rate (A), transpiration rate (E), stomatal conductance (gs), intercellular CO2 concentration (Ci), instantaneous water use efficiency (iWUE), and instantaneous carboxylation efficiency (iCE), regardless of the treatment, under water deficit conditions.
However, little is known about the combination of PEG 6000 and silicon in seed conditioning. Although the action of silicon in this type of treatment was observed by Özdemir [41] in purple maize, promoting improvements in gas exchange, especially in net carbon assimilation and transpiration rate, further studies are still needed. Bourioug et al. [42] also reported an increase in photosynthesis and transpiration in sunflower plants subjected to water stress after conditioning with PEG 6000.
These results reinforce the hypothesis that these agents may be used to induce drought stress tolerance. Furthermore, other factors may enhance the beneficial effects of osmotic seed conditioning, such as light conditions, as demonstrated in studies with Moringa oleifera Lam. [20]. However, studies addressing the combined effects of these factors in the seed treatment of cowpea are scarce.
Another relevant aspect is described by Saha et al. [43], in which the researchers discuss different seed priming techniques and their positive outcomes in inducing stress tolerance under field conditions, further supporting the potential of this technique in the present study. Moreover, seed priming is characterized by low application costs and high adaptive potential, being recommended not only for the physiological benefits it promotes in plants but also for its economic feasibility. Thus, the application of this technique to cowpea cultivation at the field level is plausible, both due to the promising results and the economic and logistical aspects involved.
5. Conclusions
Based on the above, it is concluded that seed priming with PEG 6000 and silicic acid in Vigna unguiculata (L.) Walp., cultivar BRS Pingo de Ouro, promoted increases in photosynthetic rates even under water restriction, primarily through the induction of greater instantaneous water use efficiency (WUEi) and carboxylation efficiency (iCE). Among the treatments, seed primings 2 (40 °C + Ψw 0 MPa + 200 mg L−1 Si + RL), 3 (40 °C + Ψw −0.4 MPa + 0 mg L−1 Si + RL), and 4 (40 °C + Ψw −0.4 MPa + 200 mg L−1 Si + RL) stood out for their greater effectiveness in improving gas exchange. However, seed priming 2 proved particularly promising, as it achieved remarkable results even in the absence of PEG 6000.
These findings suggest that the combination of thermal and light conditioning with silicon application represents an effective and viable strategy for the cultivation of cowpea in environments with limited water availability, contributing significantly to agricultural sustainability and food security in semi-arid regions.
Nonetheless, further research is needed to elucidate the biochemical and molecular mechanisms involved in drought stress tolerance induced by seed priming. Future studies should include biochemical analyses and gene expression profiling in order to deepen our understanding of the adaptive processes triggered by these treatments.
Author Contributions
Writing—review and editing, writing—original draft, methodology, formal analysis, data curation, conceptualization: G.F.D., R.S.d.A. and A.S.d.M.; resources, formal analysis: P.M.d.O.V., I.E.C., E.S.D.d.F., H.A.d.A., R.L.d.S.F., S.d.F.L. and S.I.B.; writing—review and editing: J.R.d.S.S. and C.F.d.L.; supervision, project administration, investigation, funding acquisition: A.S.d.M. All authors have read and agreed to the published version of the manuscript.
Funding
The authors acknowledge the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES) Brazil, finance code 001. Thanks are also due to the Conselho Nacional de Desenvolvimento Científico e Tecnológico—CNPq, for granting financial aid (Proc. 408952/2021-0 and 307559/2022-0) and the Fundação de Apoio à Pesquisa do Estado da Paraíba (Edital Fapesq-PB/CNPq no. 77/2022 and Edital no. 004/2018—SEIRHMACT/Fapesq-PB).
Data Availability Statement
Data will be made available on request.
Conflicts of Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Ferguson, J.N. Climate change and abiotic stress mechanisms in plants. Emerg. Top. Life Sci. 2019, 3, 165–181. [Google Scholar] [CrossRef]
- Sousa, L.I.S.; Brito, A.E.A.; Souza, L.C.; Teixeira, K.B.S.; Nascimento, V.R.; Albuquerque, G.D.P.; Oliveira Neto, C.F.; Okumura, R.S.; Nogueira, G.A.S.; Freitas, J.M.N.; et al. Does silicon attenuate PEG 6000-induced water deficit in germination and growth initial the seedlings corn. Braz. J. Biol. 2023, 83, e265991. [Google Scholar] [CrossRef] [PubMed]
- Mekonnen, T.W.; Gerrano, A.S.; Mbuma, N.W.; Labuschagne, M.T. Breeding of vegetable cowpea for nutrition and climate resilience in Sub-Saharan Africa: Progress, Opportunities, and Challenges. Plants 2022, 12, e1583. [Google Scholar] [CrossRef]
- Brooker, R.; Brown, L.K.; George, T.S.; Pakeman, R.J.; Palmer, S.; Ramsay, L.; Schöb, C.; Schurch, N.; Wilkinson, M.J. Active and adaptive plasticity in a changing climate. Trends Plant Sci. 2022, 27, 717–728. [Google Scholar] [CrossRef]
- Martey, E.; Etwire, P.M.; Adogoba, D.S.; Tengey, T.K. Farmers’ preferences for climate-smart cowpea varieties: Implications for crop breeding programmes. Clim. Dev. 2021, 14, 105–120. [Google Scholar] [CrossRef]
- Collado, E.; Klug, T.V.; Artés-Hernández, F.; Aguayo, E.; Artés, F.; Fernández, J.A.; Gómez, P.A. Quality changes in nutritional traits of fresh-cut and then microwaved cowpea seeds and pods. Food Bioprocess. Technol. 2019, 12, 338–346. [Google Scholar] [CrossRef]
- Fasuan, T.O.; Chukwu, C.T.; Uchegbu, N.N.; Olagunju, T.M.; Asadu, K.C.; Nwachukwu, M.C. Effects of pre-harvest synthetic chemicals on post-harvest bioactive profile and phytoconstituents of white cultivar of Vigna unguiculata grains. J. Food Process. Preserv. 2022, 46, e16187. [Google Scholar] [CrossRef]
- Guimarães, D.G.; Oliveira, L.M.; Guedes, M.O.; Ferreira, G.F.P.; Prado, T.R.; Amaral, C.L.F. Desempenho da cultivar de feijão-caupi BRS Novaera sob níveis de irrigação e adubação em ambiente protegido. Rev. Cult. Agron. 2020, 29, 61. [Google Scholar] [CrossRef][Green Version]
- Boukar, O.; Belko, N.; Charmarthi, S.; Togola, S.; Batieno, J.; Owusu, E.; Haruna, M.; Diallo, S.; Umar, M.L.; Olufajo, O.; et al. Cowpea (Vigna unguiculata): Genetics, genomics and breeding. Plant Breed. 2019, 138, 415–424. [Google Scholar] [CrossRef]
- Saka, J.O.; Agbeleye, O.A.; Ayoola, O.T.; Lawal, B.O.; Adetumbi, J.A.; Oloyede-Kamiyo, Q.O. Assessment of varietal diversity and production systems of cowpea [Vigna unguiculata (L.) Walp.] in Southwest Nigeria. J. Agric. Rural Dev. Trop. Subtrop. 2019, 119, 43–52. [Google Scholar] [CrossRef]
- Jatana, B.S.; Grover, S.; Ram, H.; Baath, G.S. Seed priming: Molecular and physiological mechanisms underlying biotic and abiotic stress tolerance. Agronomy 2024, 14, 2901. [Google Scholar] [CrossRef]
- Diya, A.; Beena, R.; Jayalekshmy, V.G. Physiological, Biochemical and Molecular Mechanisms of Seed Priming: A Review. Legume Res. 2024, 47, 159–166. [Google Scholar] [CrossRef]
- Kakar, H.A.; Ullah, S.; Shah, W.; Ali, B.; Satti, S.Z.; Ullah, R.; Muhammad, Z.; Eldin, S.M.; Ali, I.; Alwahibi, M.S.; et al. Seed priming modulates physiological and agronomic attributes of maize (Zea mays L.) under induced polyethylene glycol osmotic stress. ACS Omega 2023, 8, 22788–22808. [Google Scholar] [CrossRef]
- Alzoubi, O. Silicon Seed priming as a strategy for enhancing salt tolerance in wheat (Triticum aestivum L.): Insights Into Physiol. Biochem. adaptations. Egypt. J. Bot. 2025, 65, 151–166. [Google Scholar] [CrossRef]
- Vanitha, C.; Kathiravan, M.; Umarani, R.; Sathiya, K.; Menaka, C.; Yuvaraj, M.; Cyriac, J. Seed priming with nano silica alleviates drought stress through regulating antioxidant defense system and osmotic adjustment in soybean (Glycine max L.). Silicon 2024, 16, 2157–2170. [Google Scholar] [CrossRef]
- Aboellail, G.; Mahdy, A.; Badr Eldin, R.M. Use of silicon nanoparticles as a seed-priming solution for increasing the germination and growth parameters of faba bean (Vicia faba L.) seedling under salinity stress. Alex. J. Agric. Sci. 2023, 68, 273–287. [Google Scholar] [CrossRef]
- Boucelha, L.; Djebbar, R.; Abrous-Belbachir, O. Vigna unguiculata seed priming is related to redox status of plumule, radicle and cotyledons. Funct. Plant Biol. 2019, 46, 584–594. [Google Scholar] [CrossRef] [PubMed]
- Nabi, F.; Chaker-Haddadj, A.; Chebaani, M.; Ghalem, A.; Mebdoua, S.; Ounane, S.M. Influence of seed priming on early stages growth of cowpea [Vigna unguiculata (L.) Walp.] grown under salt stress conditions. Legume Res. Int. J. 2020, 43, 665–671. [Google Scholar] [CrossRef]
- Costa, A.A.; Paiva, E.P.; Torres, S.B.; Souza-Neta, M.L.; Pereira, K.T.O.; Leite, M.S.; Sá, F.V.S.; Benedito, C.P. Osmoprotection in Salvia hispanica L. seeds under water stress attenuators. Braz. J. Biol. 2022, 82, e233547. [Google Scholar] [CrossRef]
- Costa, P.S.; Ferraz, R.L.S.; Dantas-Neto, J.; Martins, V.D.; Viégas, P.R.A.; Meira, K.S.; Ndhlala, A.R.; Azevedo, C.A.V.; Melo, A.S. Seed priming with light quality and Cyperus rotundus L. extract modulate the germination and initial growth of Moringa oleifera Lam. seedlings. Braz. J. Biol. 2024, 84, e255836. [Google Scholar] [CrossRef]
- Vidak, M.; Lazarević, B.; Nekić, M.; Šatović, Z.; Carović-Stanko, K. effect of hormonal priming and osmopriming on germination of winter savory (Satureja montana L.) natural population under drought stress. Agronomy 2022, 12, 1288. [Google Scholar] [CrossRef]
- Alencar, R.S.; Dias, G.F.; Araujo, Y.M.L.; Oliveira-Viana, P.M.; Borborema, D.A.; Bonou, S.I.; Sales, J.R.S.; Cavalcante, I.E.; Barroso, V.S.F.; Schneider, R.; et al. Seed priming with residual silicon-glass microparticles mitigates water stress in cowpea. Sci. Hortic. 2024, 328, 112933. [Google Scholar] [CrossRef]
- Araújo, E.D.; Melo, A.S.; Rocha, M.D.S.; Carneiro, R.F.; Rocha, M.M. Germination and initial growth of cowpea cultivars under osmotic stress and salicylic acid. Rev. Caatinga 2018, 31, 80–89. [Google Scholar] [CrossRef]
- Shapiro, S.S.; Wilk, M.B. An analysis of variance test for normality (complete samples). Biometrika 1965, 52, 591–609. [Google Scholar] [CrossRef]
- Ferreira, D.F. SISVAR: A computer analysis system to fixed effects split plot type designs. Braz. J. Biometr. 2019, 37, 529–535. [Google Scholar] [CrossRef]
- Ferreira, D.P.; Sousa, D.P.; Nunes, H.G.G.C.; Pinto, J.V.N.; Farias, V.D.S.; Costa, D.L.P.; Moura, V.B.; Teixeira, E.; Sousa, A.M.L.; Pinheiro, H.A.; et al. Cowpea ecophysiological responses to accumulated water deficiency during the reproductive phase in northeastern Pará, Brazil. Horticulturae 2021, 7, 116. [Google Scholar] [CrossRef]
- Oliveira, A.P.S.; Melo, Y.L.; Alencar, R.S.; Viégas, P.R.A.; Dias, G.F.; Ferraz, R.L.S.; Sá, F.V.S.; Dantas Neto, J.; Magalhães, I.D.; Gheyi, H.R.; et al. Osmoregulatory and antioxidants modulation by salicylic acid and methionine in cowpea plants under the water restriction. Plants 2023, 12, 1341. [Google Scholar] [CrossRef]
- Costa, D.L.P.; Takaki, A.Y.; Silva Farias, V.D.; Oliveira Teixeira, E.; Nunes, H.G.G.C.; Souza, P.J.O.P. Stomatal conductance of cowpea submitted to different hydric regimes in Castanhal, Pará, Brazil. J. Agric. Stud. 2019, 8, 138–149. [Google Scholar] [CrossRef]
- Mndela, M.; Tjelele, J.T.; Madakadze, I.C.; Mangwane, M.; Samuels, I.M.; Muller, F.; Pule, H.T. A global meta-analysis of woody plant responses to elevated CO2: Implications on biomass, growth, leaf N content, photosynthesis and water relations. Ecol. Process. 2022, 11, 52. [Google Scholar] [CrossRef]
- Parveen, A.; Ashraf, M.A.; Hussain, I.; Parveen, S.; Rasheed, R.; Mahmood, Q. Promotion of growth and physiological characteristics in water-stressed Triticum aestivum in relation to foliar-application of salicylic acid. Water 2021, 13, 1316. [Google Scholar] [CrossRef]
- Costa, P.S.; Ferraz, R.L.S.; Dantas Neto, J.; Bonou, S.I.; Cavalcante, I.E.; Alencar, R.S.; Melo, Y.L.; Magalhães, I.D.; Ndhlala, A.R.; Schneider, R.; et al. Seed priming with glass waste microparticles and red light irradiation mitigates thermal and water stresses in seedlings of Moringa oleifera. Plants 2022, 11, 2510. [Google Scholar] [CrossRef]
- El Moukhtari, A.; Ksiaa, M.; Zorrig, W.; Cabassa, C.; Abdelly, C.; Farissi, M.; Savoure, A. How Silicon Alleviates the Effect of Abiotic Stresses During Seed Germination: A Review. J. Plant Growth Regul. 2023, 42, 3323–3341. [Google Scholar] [CrossRef]
- Merwad, A.R.M.A.; Desoky, E.S.; Rady, M.M. Response of water deficit-stressed Vigna unguiculata performances to silicon, proline or methionine foliar application. Sci. Hortic. 2018, 228, 132–144. [Google Scholar] [CrossRef]
- Raza, M.A.S.; Zulfiqar, B.; Iqbal, R.; Muzamil, M.N.; Aslam, M.U.; Muhammad, F.; Amin, J.; Aslam, H.M.U.; Ibrahim, M.A.; Uzair, M.; et al. Morpho-physiological and biochemical response of wheat to various treatments of silicon nano-particles under drought stress conditions. Sci. Rep. 2023, 13, 2700. [Google Scholar] [CrossRef]
- Lawson, T.; Milliken, A.L. Photosynthesis–beyond the leaf. New Phytol. 2023, 238, 55–61. [Google Scholar] [CrossRef] [PubMed]
- Freire, M.H.C.; Sousa, G.G.; Ceita, E.D.A.R.; Barbosa, A.S.; Goes, G.F.; Lacerda, C.F. Gas exchange of fava bean varieties under salinity conditions of irrigation water. Agrarian 2021, 14, 61–70. [Google Scholar] [CrossRef]
- Jacinto Júnior, S.G.; Moraes, J.G.L.; Silva, F.B.; Silva, B.N.; Sousa, G.G.; Oliveira, L.L.B.; Mesquita, R.O. Respostas fisiológicas de genótipos de fava (Phaseolus lunatus L.) submetidas ao estresse hídrico cultivadas no Estado do Ceará. Rev. Bras. Meteorol. 2019, 34, 413–422. [Google Scholar] [CrossRef]
- Jayawardhane, J.; Goyali, J.C.; Zafari, S.; Igamberdiev, A.U. The response of cowpea (Vigna unguiculata) plants to three abiotic stresses applied with increasing intensity: Hypoxia, salinity, and water deficit. Metabolites 2022, 12, 38. [Google Scholar] [CrossRef]
- Uddin, S.; Ullah, S.; Nafees, M. Effect of seed priming on growth and performance of Vigna radiata L. under induced drought stress. J. Agric. Food Res. 2021, 4, e100140. [Google Scholar] [CrossRef]
- Khan, A.; Khan, A.L.; Muneer, S.; Kim, Y.H.; Al-Rawahi, A.; Al-Harrasi, A. Silicon and salinity: Crosstalk in crop-mediated stress tolerance mechanisms. Front. Plant Sci. 2019, 10, 1429. [Google Scholar] [CrossRef]
- Özdemir, E. Silicon stimulated bioactive and physiological metabolisms of purple corn (Zea mays indentata L.) under deficit and well-watered conditions. 3 Biotech 2021, 11, 319. [Google Scholar] [CrossRef] [PubMed]
- Bourioug, M.; Ezzaza, K.; Bouabid, R.; Alaoui-Mhamdi, M.; Bungau, S.; Bougead, P.; Alaoui-Sossé, L.; Alaoui-Sossé, B.; Aleya, L. Influence of hydro-and osmo-priming on sunflower seeds to break dormancy and improve crop performance under water stress. Environ. Sci. Pollut. Res. 2020, 27, 13215–13226. [Google Scholar] [CrossRef] [PubMed]
- Saha, D.; Choyal, P.; Mishra, U.N.; Dey, P.; Bose, B.; Gupta, N.K.; Mehta, B.K.; Kumar, P.; Pandey, S.; Chauhan, J.; et al. Drought stress responses and inducing tolerance by seed priming approach in plants. Plant Stress 2022, 4, 100066. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).