Biogenic Zinc Oxide Nanoparticles Protect Tomato Plants Against Pseudomonas syringae pv. tomato
Abstract
1. Introduction
2. Materials and Methods
2.1. Duckweed Growth Condition and Biogenic Zinc Oxide Nanoparticle (ZnO-NP) Synthesis
2.2. Bacterial Growth and Inoculum Preparation
2.3. Seed Germination and Seedling Growth
2.4. ZnO-NPs’ Activity In Vitro Antimicrobial Assay
2.5. Effect of ZnO-NPs in Protecting Tomato Plants Against Pst
2.6. ZnO-NP Impact on Bacterial Growth in Planta
2.7. Plant Defense Gene Expression in ZnO-NP-Treated Plants
2.8. H2O2, MDA and Antioxidant Determination
2.9. Statistical Analysis
3. Results and Discussion
3.1. ZnO-NPs’ Activity in Vitro Antimicrobial Assay
3.2. Effect of ZnO-NPs in Protecting Tomato Plants Against Pst
3.3. ZnO-NPs’ Impact on Bacterial Growth in Planta
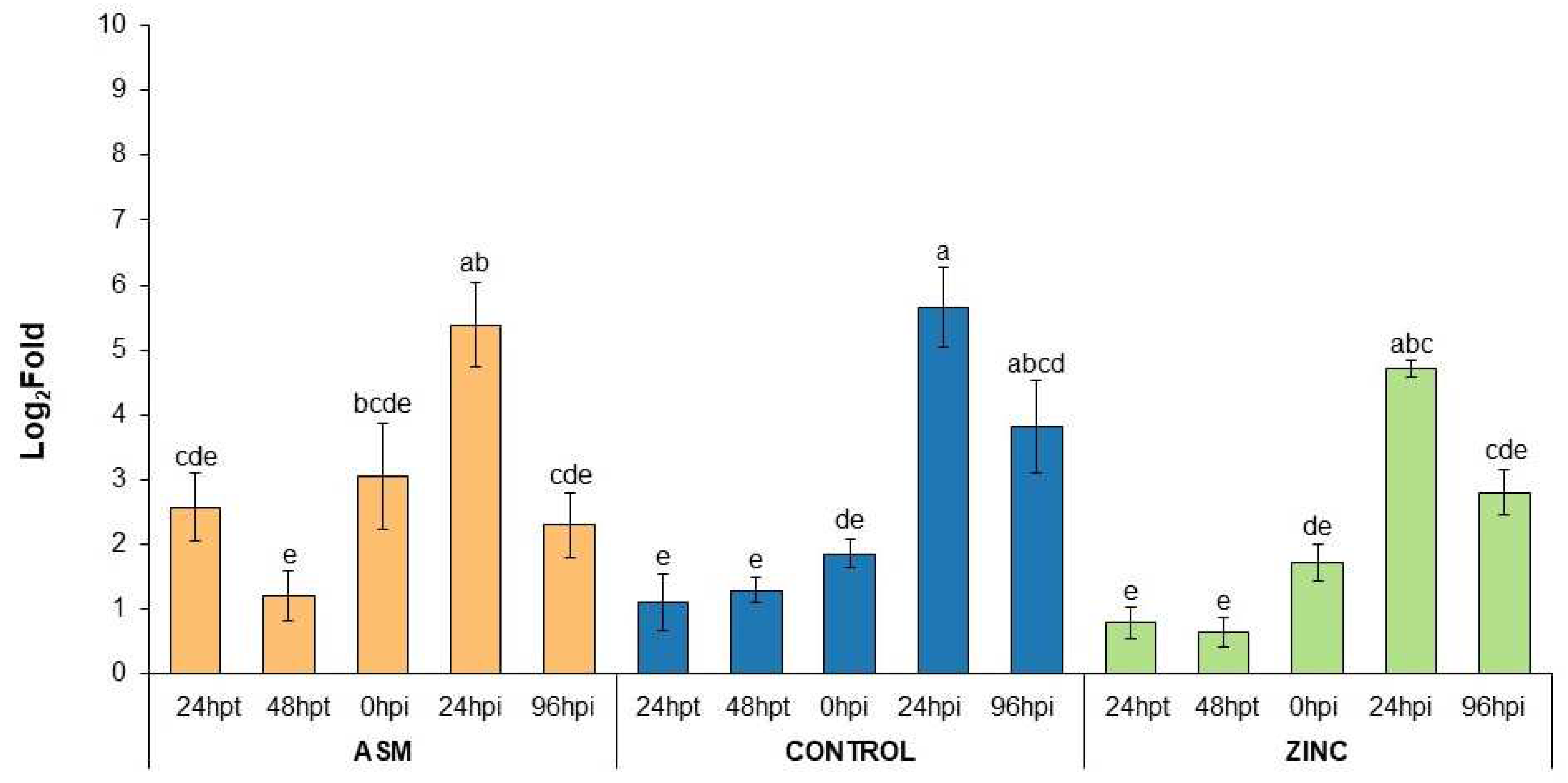
3.4. Plant Defense Gene Expression in ZnO-NP-Treated Plants
3.5. Oxidative Stress and Antioxidants
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- FAO FAOSTAT: Statistical Database. Available online: https://www.fao.org/faostat/en/ (accessed on 2 July 2024).
- Bhowmik, D.; Kumar, K.P.S.; Paswan, S.; Srivastava, S. Tomato-a natural medicine and its health benefits. J. Pharmacogn. Phytochem. 2012, 1, 33–43. [Google Scholar]
- Cheng, H.M.; Koutsidis, G.; Lodge, J.K.; Ashor, A.W.; Siervo, M.; Lara, J. Lycopene and tomato and risk of cardiovascular diseases: A systematic review and meta-analysis of epidemiological evidence. Crit. Rev. Food Sci. Nutr. 2019, 59, 141–158. [Google Scholar] [CrossRef]
- Panno, S.; Davino, S.; Caruso, A.G.; Bertacca, S.; Crnogorac, A.; Mandić, A.; Noris, E.; Matić, S. A review of the most common and economically important diseases that undermine the cultivation of tomato crop in the Mediterranean basin. Agronomy 2021, 11, 2188. [Google Scholar] [CrossRef]
- Schneider, R.W.; Grogan, R.G. Bacterial speck of tomato: Sources of inoculum and establishment of a resident population. Phytopathology 1977, 67, 388–394. [Google Scholar] [CrossRef]
- Yunis, H.; Bashan, Y.; Okon, Y.; Henis, Y. Weather dependence, yield losses, and control of bacterial speck of tomato caused by Pseudomonas tomato. Plant Dis. 1980, 64, 937–939. [Google Scholar] [CrossRef]
- Preston, G.M. Pseudomonas syringae pv. tomato: The right pathogen, of the right plant, at the right time. Mol. Plant Pathol. 2000, 1, 263–275. [Google Scholar] [CrossRef]
- Sundin, G.; Castiblanco, L.; Yuan, X.; Zeng, Q.; Yang, C.-H. Bacterial disease management: Challenges, experience, innovation, and future prospects. Mol. Plant Pathol. 2016, 17, 1506–1518. [Google Scholar] [CrossRef]
- Griffin, K.; Gambley, C.; Brown, P.; Li, Y. Copper-tolerance in Pseudomonas syringae pv. tomato and Xanthomonas spp. and the control of diseases associated with these pathogens in tomato and pepper. A systematic literature review. Crop Prot. 2017, 96, 144–150. [Google Scholar] [CrossRef]
- Abrahamian, P.; Jones, J.B.; Vallad, G.E. Efficacy of copper and copper alternatives for management of bacterial spot on tomato under transplant and field production. Crop Prot. 2019, 126, 104919. [Google Scholar] [CrossRef]
- Worrall, E.A.; Hamid, A.; Mody, K.T.; Mitter, N.; Pappu, H.R. Nanotechnology for plant disease management. Agronomy 2018, 8, 285. [Google Scholar] [CrossRef]
- Elmer, W.; White, J.C. The future of nanotechnology in plant pathology. Annu. Rev. Phytopathol. 2018, 56, 111–133. [Google Scholar] [CrossRef] [PubMed]
- Fu, L.; Wang, Z.; Dhankher, O.P.; Xing, B. Nanotechnology as a new sustainable approach for controlling crop diseases and increasing agricultural production. J. Exp. Bot. 2020, 71, 507–519. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.R.; Siddiqui, Z.A.; Fang, X. Potential of metal and metal oxide nanoparticles in plant disease diagnostics and management: Recent advances and challenges. Chemosphere 2022, 297, 134114. [Google Scholar] [CrossRef]
- Kumar, A.; Choudhary, A.; Kaur, H.; Guha, S.; Mehta, S.; Husen, A. Potential applications of engineered nanoparticles in plant disease management: A critical update. Chemosphere 2022, 295, 133798. [Google Scholar] [CrossRef]
- Hyder, S.; Ul-Nisa, M.; Shahzadi; Shahid, H.; Gohar, F.; Gondal, A.S.; Riaz, N.; Younas, A.; de los Santos-Villalobos, S.; Montoya-Martínez, A.C.; et al. Recent trends and perspectives in the application of metal and metal oxide nanomaterials for sustainable agriculture. Plant Physiol. Biochem. 2023, 202, 107960. [Google Scholar] [CrossRef] [PubMed]
- Vallee, B.L.; Auld, D.S. Zinc coordination, function, and structure of zinc enzymes and other proteins. Biochemistry 1990, 29, 5647–5659. [Google Scholar] [CrossRef]
- Vallee, B.L.; Auld, D.S. Zinc: Biological functions and coordination motifs. Acc. Chem. Res. 1993, 26, 543–551. [Google Scholar] [CrossRef]
- Frassinetti, S.; Bronzetti, G.L.; Caltavuturo, L.; Cini, M.; Croce, C.D. The role of zinc in life: A review. J. Environ. Pathol. Toxicol. Oncol. 2006, 25, 597–610. [Google Scholar] [CrossRef]
- Küpper, H.; Andresen, E. Mechanisms of metal toxicity in plants. Metallomics 2016, 8, 269–285. [Google Scholar] [CrossRef]
- Kaur, H.; Garg, N. Zinc toxicity in plants: A review. Planta 2021, 253, 129. [Google Scholar] [CrossRef]
- Khan, S.T.; Malik, A.; Alwarthan, A.; Shaik, M.R. The enormity of the zinc deficiency problem and available solutions; an overview. Arab. J. Chem. 2022, 15, 103668. [Google Scholar] [CrossRef]
- Whittaker, P. Iron and zinc interactions in humans. Am. J. Clin. Nutr. 1998, 68, 442S–446S. [Google Scholar] [CrossRef] [PubMed]
- Kalia, A.; Abd-Elsalam, K.; Kuca, K. Zinc-based nanomaterials for diagnosis and management of plant diseases: Ecological safety and future prospects. J. Fungi 2020, 6, 222. [Google Scholar] [CrossRef]
- Rehman, F.U.; Paker, N.P.; Rehman, S.U.; Javed, M.T.; Farooq Hussain Munis, M.; Chaudhary, H.J. Zinc oxide nanoparticles: Biogenesis and applications against phytopathogens. J. Plant Pathol. 2024, 106, 45–65. [Google Scholar] [CrossRef]
- Siddiqi, K.S.; Ur Rahman, A.; Tajuddin; Husen, A. Properties of zinc oxide nanoparticles and their activity against microbes. Nanoscale Res. Lett. 2018, 13, 141. [Google Scholar] [CrossRef] [PubMed]
- Cai, L.; Liu, C.; Fan, G.; Liu, C.; Sun, X. Preventing viral disease by ZnONPs through directly deactivating TMV and activating plant immunity in: Nicotiana benthamiana. Environ. Sci. Nano 2019, 6, 3653–3669. [Google Scholar] [CrossRef]
- Sofy, A.R.; Sofy, M.R.; Hmed, A.A.; Dawoud, R.A.; Alnaggar, A.E.-A.M.; Soliman, A.M.; El-Dougdoug, N.K. Ameliorating the adverse effects of tomato mosaic tobamovirus infecting tomato plants in Egypt by boosting immunity in tomato plants using zinc oxide nanoparticles. Molecules 2021, 26, 1337. [Google Scholar] [CrossRef]
- Ahmad, H.; Venugopal, K.; Rajagopal, K.; De Britto, S.; Nandini, B.; Pushpalatha, H.G.; Konappa, N.; Udayashankar, A.C.; Geetha, N.; Jogaiah, S. Green synthesis and characterization of zinc oxide nanoparticles using Eucalyptus globules and their fungicidal ability against pathogenic fungi of apple orchards. Biomolecules 2020, 10, 425. [Google Scholar] [CrossRef]
- Siddiqui, Z.; Hashmi, A.; Khan, M.; Parveen, A. Management of bacteria Pectobacterium carotovorum, Xanthomonas campestris pv. carotae, and fungi Rhizoctonia solani, Fusarium solani and Alternaria dauci with silicon dioxide nanoparticles on carrot. Int. J. Veg. Sci. 2019, 26, 1–11. [Google Scholar] [CrossRef]
- Sardar, M.; Ahmed, W.; Al Ayoubi, S.; Nisa, S.; Bibi, Y.; Sabir, M.; Khan, M.M.; Ahmed, W.; Qayyum, A. Fungicidal synergistic effect of biogenically synthesized zinc oxide and copper oxide nanoparticles against Alternaria citri causing citrus black rot disease. Saudi J. Biol. Sci. 2022, 29, 88–95. [Google Scholar] [CrossRef]
- Malandrakis, A.A.; Kavroulakis, N.; Chrysikopoulos, C.V. Zinc nanoparticles: Mode of action and efficacy against boscalid-resistant Alternaria alternata isolates. Sci. Total Environ. 2022, 829, 154638. [Google Scholar] [CrossRef]
- Abdelaziz, A.M.; Salem, S.S.; Khalil, A.M.A.; El-Wakil, D.A.; Fouda, H.M.; Hashem, A.H. Potential of biosynthesized zinc oxide nanoparticles to control fusarium wilt disease in eggplant (Solanum melongena) and promote plant growth. Biometals 2022, 35, 601–616. [Google Scholar] [CrossRef] [PubMed]
- Mosquera-Sánchez, L.P.; Arciniegas-Grijalba, P.A.; Patiño-Portela, M.C.; Guerra–Sierra, B.E.; Muñoz-Florez, J.E.; Rodríguez-Páez, J.E. Antifungal effect of zinc oxide nanoparticles (ZnO-NPs) on Colletotrichum sp., causal agent of anthracnose in coffee crops. Biocatal. Agric. Biotechnol. 2020, 25, 101579. [Google Scholar] [CrossRef]
- Abdallah, Y.; Liu, M.; Ogunyemi, S.O.; Ahmed, T.; Fouad, H.; Abdelazez, A.; Yan, C.; Yang, Y.; Chen, J.; Li, B. Bioinspired green synthesis of chitosan and zinc oxide nanoparticles with strong antibacterial activity against rice pathogen Xanthomonas oryzae pv. oryzae. Molecules 2020, 25, 4795. [Google Scholar] [CrossRef] [PubMed]
- Ogunyemi, S.O.; Zhang, M.; Abdallah, Y.; Ahmed, T.; Qiu, W.; Ali, M.A.; Yan, C.; Yang, Y.; Chen, J.; Li, B. The Bio-Synthesis of three metal oxide nanoparticles (ZnO, MnO2, and MgO) and their antibacterial activity against the bacterial leaf blight pathogen. Front. Microbiol. 2020, 11, 588326. [Google Scholar] [CrossRef]
- Khan, M.; Siddiqui, Z. Role of zinc oxide nanoparticles in the nanagement of disease complex of beetroot (Beta vulgaris L.) caused by Pectobacterium betavasculorum, Meloidogyne incognita and Rhizoctonia solani. Hortic. Environ. Biotechnol. 2020, 62, 225–241. [Google Scholar] [CrossRef]
- Şahin, B.; Soylu, S.; Kara, M.; Türkmen, M.; Aydin, R.; Çetin, H. Superior antibacterial activity against seed-borne plant bacterial disease agents and enhanced physical properties of novel green synthesized nanostructured ZnO using Thymbra spicata plant extract. Ceram. Int. 2021, 47, 341–350. [Google Scholar] [CrossRef]
- Jiang, H.; Lv, L.; Ahmed, T.; Jin, S.; Shahid, M.; Noman, M.; Osman, H.-E.H.; Wang, Y.; Sun, G.; Li, X.; et al. Effect of the nanoparticle exposures on the tomato bacterial wilt disease control by modulating the rhizosphere bacterial community. Int. J. Mol. Sci. 2022, 23, 414. [Google Scholar] [CrossRef] [PubMed]
- Parveen, A.; Siddiqui, Z.A. Zinc oxide nanoparticles affect growth, photosynthetic pigments, proline content and bacterial and fungal diseases of tomato. Arch. Phytopathol. Plant Prot. 2021, 54, 1519–1538. [Google Scholar] [CrossRef]
- Elsharkawy, M.; Derbalah, A.; Hamza, A.; El-Shaer, A. zinc oxide nanostructures as a control strategy of bacterial speck of tomato caused by Pseudomonas syringae in Egypt. Environ. Sci. Pollut. Res. Int. 2020, 27, 19049–19057. [Google Scholar] [CrossRef]
- Khan, R.A.A.; Tang, Y.; Naz, I.; Alam, S.S.; Wang, W.; Ahmad, M.; Najeeb, S.; Rao, C.; Li, Y.; Xie, B.; et al. Management of Ralstonia solanacearum in tomato using ZnO nanoparticles synthesized through Matricaria chamomilla. Plant Dis. 2021, 105, 3224–3230. [Google Scholar] [CrossRef] [PubMed]
- Fraga, F.S.; Silva, A.C.A.; Dantas, N.O.; Tebaldi, N.D.; Luz, J.M.Q. Doped zinc-oxide nanocrystals for the control of tomato bacterial spot and Xanthomonas gardneri in seeds. Trop. Plant Pathol. 2021, 46, 406–413. [Google Scholar] [CrossRef]
- Tolisano, C.; Del Buono, D. Biobased: Biostimulants and biogenic nanoparticles enter the scene. Sci. Total Environ. 2023, 885, 163912. [Google Scholar] [CrossRef]
- Wang, Q.; Xu, S.; Zhong, L.; Zhao, X.; Wang, L. Effects of zinc oxide nanoparticles on growth, development, and flavonoid synthesis in Ginkgo biloba. Int. J. Mol. Sci. 2023, 24, 15775. [Google Scholar] [CrossRef]
- Naranjo, E.; Merfa, M.V.; Santra, S.; Ozcan, A.; Johnson, E.; Cobine, P.A.; De La Fuente, L. Zinkicide is a ZnO-based nanoformulation with bactericidal activity against Liberibacter crescens in batch cultures and in microfluidic chambers simulating plant vascular systems. Appl. Environ. Microbiol. 2020, 86, 1–18. [Google Scholar] [CrossRef]
- Shantharaj, D.; Naranjo, E.; Merfa, M.V.; Cobine, P.A.; Santra, S.; De La Fuente, L. Zinc oxide-based nanoformulation zinkicide mitigates the xylem-limited pathogen Xylella fastidiosa in tobacco and southern highbush blueberry. Plant Dis. 2023, 107, 1096–1106. [Google Scholar] [CrossRef]
- Abdullaeva, Z. Synthesis of Nanoparticles and Nanomaterials; Springer International Publishing: Cham, Switzerland, 2017; ISBN 978-3-319-54074-0. [Google Scholar]
- Singh, T.; Singh, A.; Wang, W.; Yadav, D.; Kumar, A.; Singh, P.K. Biosynthesized nanoparticles and its implications in agriculture. In Biological Synthesis of Nanoparticles and Their Applications; CRC Press: Boca Raton, FL, USA, 2020; ISBN 978-0-429-26523-5. [Google Scholar]
- Omran, B.A.; Baek, K.-H. Valorization of agro-industrial biowaste to green nanomaterials for wastewater treatment: Approaching green chemistry and circular economy principles. J. Environ. Manag. 2022, 311, 114806. [Google Scholar] [CrossRef] [PubMed]
- Anadebe, V.C.; Chukwuike, V.I.; Ebenso, E.E.; Barik, R.C. Chapter 14—Trends and perspectives in waste-derived nanoparticles and circular economy. In Waste-Derived Nanoparticles; Aslam, J., Aslam, R., Hussain, C.M., Eds.; Elsevier: Amsterdam, The Netherlands, 2024; pp. 367–379. ISBN 978-0-443-22337-2. [Google Scholar]
- Del Buono, D.; Di Michele, A.; Costantino, F.; Trevisan, M.; Lucini, L. Biogenic ZnO nanoparticles synthesized using a novel plant extract: Application to enhance physiological and biochemical traits in maize. Nanomaterials 2021, 11, 1270. [Google Scholar] [CrossRef]
- Buonaurio, R.; Stravato, V.M.; Cappelli, C. Occurrence of Pseudomonas syringae pv. tomato Race 1 in Italy on Pto gene-bearing tomato plants. J. Phytopathol. 1996, 144, 437–440. [Google Scholar] [CrossRef]
- Orfei, B.; Pothier, J.F.; Fenske, L.; Blom, J.; Moretti, C.; Buonaurio, R.; Smits, T.H.M. Race-specific genotypes of Pseudomonas syringae pv. tomato are defined by the presence of mobile DNA elements within the genome. Front. Plant Sci. 2023, 14, 1197706. [Google Scholar] [CrossRef]
- King, E.O.; Ward, M.K.; Raney, D.E. Two simple media for the demonstration of pyocyanin and fluorescin. J. Lab. Clin. Med. 1954, 44, 301–307. [Google Scholar] [PubMed]
- Horsfall, J.G.; Barratt, R.W. An improved grading system for measuring plant diseases. Phytopathology 1945, 35, 655. [Google Scholar]
- Molinari, S. Systemic acquired resistance activation in solanaceous crops as a management strategy against root-knot nematodes. Pest Manag. Sci. 2014, 72, 888–896. [Google Scholar] [CrossRef] [PubMed]
- Scarponi, L.; Buonaurio, R.; Martinetti, L. Persistence and translocation of a benzothiadiazole derivative in tomato plants in relation to systemic acquired resistance against Pseudomonas syringae pv. tomato. Pest Manag. Sci. 2001, 57, 262–268. [Google Scholar] [CrossRef]
- Soylu, S.; Baysal, O.; Soylu, E.M. Induction of disease resistance by the plant activator, acibenzolar-S-methyl (ASM), against bacterial canker (Clavibacter michiganensis subsp. michiganensis) in tomato seedlings. Plant Sci. 2003, 165, 1069–1075. [Google Scholar] [CrossRef]
- Pfaffl, M.W. A new mathematical model for relative quantification in Real-Time RT-PCR. Nucleic Acids Res. 2001, 29, e45. [Google Scholar] [CrossRef]
- Vandesompele, J.; De Preter, K.; Pattyn, F.; Poppe, B.; Van Roy, N.; De Paepe, A.; Speleman, F. Accurate normalization of Real-Time Quantitative RT-PCR data by geometric averaging of multiple internal control genes. Genome Biol. 2002, 3, research0034.1. [Google Scholar] [CrossRef]
- Velikova, V.; Yordanov, I.; Edreva, A. Oxidative stress and some antioxidant systems in acid rain-treated bean plants: Protective role of exogenous polyamines. Plant Sci. 2000, 151, 59–66. [Google Scholar] [CrossRef]
- Lichtenthaler, H.K.; Buschmann, C. Chlorophylls and carotenoids: Measurement and characterization by UV-VIS spectroscopy. Curr. Protoc. Food Anal. Chem. 2001, 1, F4.3.1–F4.3.8. [Google Scholar] [CrossRef]
- Parađiković, N.; Vinković, T.; Vinković Vrček, I.; Žuntar, I.; Bojić, M.; Medić-Šarić, M. Effect of natural biostimulants on yield and nutritional quality: An example of sweet yellow pepper (Capsicum annuum L.) plants. J. Sci. Food Agric. 2011, 91, 2146–2152. [Google Scholar] [CrossRef]
- Streibig, J.C.; Rudemo, M.; Jensen, J.E. Dose-response curves and statistical models. In Herbicide Bioassays; Streibig, J.C., Kudsk, P., Eds.; CRC Press Inc.: Boca Raton, FL, USA, 1993; pp. 29–55. [Google Scholar]
- Onofri, A. BIOASSAY97: A new Excel VBA macro to perform statistical analyses on herbicide dose-response data. Ital. J. Agrometeorol. 2001, 3, 40–45. [Google Scholar]
- Orfei, B.; Moretti, C.; Loreti, S.; Tatulli, G.; Onofri, A.; Scotti, L.; Aceto, A.; Buonaurio, R. Silver nanoclusters with Ag2+/3+ oxidative states are a new highly effective tool against phytopathogenic bacteria. Appl. Microbiol. Biotechnol. 2023, 107, 4519–4531. [Google Scholar] [CrossRef]
- Onofri, A.; Pannacci, E. Spreadsheet tools for biometry classes in crop science programmes. Commun. Biometry Crop Sci. 2014, 9, 3–13. [Google Scholar]
- R Core Team. R: A Language and Environment for Statistical Computing. 2024. Available online: https://www.R-project.org/ (accessed on 2 July 2024).
- Al-Momani, H.; Al Balawi, D.; Hamed, S.; Albiss, B.A.; Almasri, M.; AlGhawrie, H.; Ibrahim, L.; Al Balawi, H.; Al Haj Mahmoud, S.; Pearson, J.; et al. The impact of biosynthesized ZnO nanoparticles from Olea Europaea (common olive) on Pseudomonas aeruginosa growth and biofilm formation. Sci. Rep. 2023, 13, 5096. [Google Scholar] [CrossRef] [PubMed]
- Fadwa, A.O.; Alkoblan, D.K.; Mateen, A.; Albarag, A.M. Synergistic Effects of zinc oxide nanoparticles and various antibiotics combination against Pseudomonas aeruginosa clinically isolated bacterial strains. Saudi J. Biol. Sci. 2021, 28, 928–935. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.-H.; Kim, Y.-G.; Cho, M.H.; Lee, J. ZnO nanoparticles inhibit Pseudomonas aeruginosa biofilm formation and virulence factor production. Microbiol. Res. 2014, 169, 888–896. [Google Scholar] [CrossRef]
- Soni, D.; Gandhi, D.; Tarale, P.; Bafana, A.; Pandey, R.A.; Sivanesan, S. Oxidative stress and genotoxicity of zinc oxide nanoparticles to Pseudomonas species, human promyelocytic leukemic (HL-60), and blood cells. Biol. Trace Elem. Res. 2017, 178, 218–227. [Google Scholar] [CrossRef]
- Huang, C.-H.; Vallad, G.E.; Zhang, S.; Wen, A.; Balogh, B.; Figueiredo, J.F.L.; Behlau, F.; Jones, J.B.; Momol, M.T.; Olson, S.M. Effect of application frequency and reduced rates of Acibenzolar-S-Methyl on the field efficacy of induced resistance against bacterial spot on tomato. Plant Dis. 2012, 96, 221–227. [Google Scholar] [CrossRef]
- Abdelkhalek, A.; Al-Askar, A.A. Green synthesized ZnO nanoparticles mediated by Mentha spicata extract induce plant systemic resistance against tobacco mosaic virus. Appl. Sci. 2020, 10, 5054. [Google Scholar] [CrossRef]
- Abou-Zeid, H.M.; Ismail, G.S.M.; Abdel-Latif, S.A. Influence of seed priming with ZnO nanoparticles on the salt-induced damages in wheat (Triticum aestivum L.) plants. J. Plant Nutr. 2021, 44, 629–643. [Google Scholar] [CrossRef]
- Farhana; Munis, M.F.H.; Alamer, K.H.; Althobaiti, A.T.; Kamal, A.; Liaquat, F.; Haroon, U.; Ahmed, J.; Chaudhary, H.J.; Attia, H. ZnO nanoparticle-mediated seed priming induces biochemical and antioxidant changes in chickpea to alleviate Fusarium wilt. J. Fungi 2022, 8, 753. [Google Scholar] [CrossRef] [PubMed]
- Mazhar, M.W.; Ishtiaq, M.; Hussain, I.; Parveen, A.; Bhatti, K.H.; Azeem, M.; Thind, S.; Ajaib, M.; Maqbool, M.; Sardar, T.; et al. Seed nano-priming with zinc oxide nanoparticles in rice mitigates drought and enhances agronomic profile. PLoS ONE 2022, 17, e0264967. [Google Scholar]
- Rexlin, J.; Vijayakumar, S.; Nilavukkarasi, M.; Vidhya, E.; Alharthi, N.S.; Sajjad, M.; Punitha, V.N.; Praseetha, P.K. Bioengineered ZnO nanoparticles as a nano priming agent in Cyamopsis tetragonoloba (L.).Taub. to improve yield and disease resistance. Appl. Nanosci. 2023, 13, 5993–6001. [Google Scholar] [CrossRef]
- Tyagi, S.; Shah, A.; Karthik, K.; Rathinam, M.; Rai, V.; Chaudhary, N.; Sreevathsa, R. Reactive oxygen species in plants: An invincible fulcrum for biotic stress mitigation. Appl. Microbiol. Biotechnol. 2022, 106, 5945–5955. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Xiao, Z.; Chen, F.; Yue, L.; Zou, H.; Lyu, J.; Wang, Z. Metallic oxide nanomaterials act as antioxidant nanozymes in higher plants: Trends, meta-analysis, and prospect. Sci. Total Environ. 2021, 780, 146578. [Google Scholar] [CrossRef]
- Del Buono, D.; Luzi, F.; Tolisano, C.; Puglia, D.; Di Michele, A. Synthesis of a lignin/zinc oxide hybrid nanoparticles system and its application by nano-priming in maize. Nanomaterials 2022, 12, 568. [Google Scholar] [CrossRef]
- Shen, Y.; Li, J.; Gu, R.; Yue, L.; Wang, H.; Zhan, X.; Xing, B. Carotenoid and superoxide dismutase are the most effective antioxidants participating in ROS scavenging in phenanthrene accumulated wheat leaf. Chemosphere 2018, 197, 513–525. [Google Scholar] [CrossRef]
- Siddiqui, Z.A.; Parveen, A.; Ahmad, L.; Hashem, A. Effects of graphene oxide and zinc oxide nanoparticles on growth, chlorophyll, carotenoids, proline contents and diseases of carrot. Sci. Hortic. 2019, 249, 374–382. [Google Scholar] [CrossRef]
- Daayf, F.; El Hadrami, A.; El-Bebany, A.F.; Henriquez, M.A.; Yao, Z.; Derksen, H.; El-Hadrami, I.; Adam, L.R. Phenolic compounds in plant defense and pathogen counter-defense mechanisms. In Recent Advances in Polyphenol Research; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2012; pp. 191–208. ISBN 978-1-118-29975-3. [Google Scholar]
- Agati, G.; Azzarello, E.; Pollastri, S.; Tattini, M. Flavonoids as antioxidants in plants: Location and functional significance. Plant Sci. 2012, 196, 67–76. [Google Scholar] [CrossRef]
- Duffy, G.M.; Pillai, S.C.; McCormack, D.E. The Effect of the rate of precursor production on the purity and aggregation morphology of nanoparticulate zinc oxide. J. Mater. Chem. 2006, 17, 181–184. [Google Scholar] [CrossRef]
- Cabot, C.; Martos, S.; Llugany, M.; Gallego, B.; Tolrà, R.; Poschenrieder, C. A role for zinc in plant defense against pathogens and herbivores. Front. Plant Sci. 2019, 10, 1171. [Google Scholar] [CrossRef] [PubMed]
- Lei, X.J.; Liu, Z.Z.; Park, J.H.; Kim, I.H. Novel zinc sources as antimicrobial growth promoters for monogastric animals: A Review. J. Anim. Sci. Technol. 2022, 64, 187–196. [Google Scholar] [CrossRef] [PubMed]
- Faizan, M.; Hayat, S.; Pichtel, J. Effects of zinc oxide nanoparticles on crop plants: A perspective analysis. In Sustainable Agriculture Reviews 41: Nanotechnology for Plant Growth and Development; Hayat, S., Pichtel, J., Faizan, M., Fariduddin, Q., Eds.; Springer International Publishing: Cham, Switzerland, 2020; pp. 83–99. ISBN 978-3-030-33996-8. [Google Scholar]
- Castagna, A.; Ederli, L.; Pasqualini, S.; Mensuali-Sodi, A.; Baldan, B.; Donnini, S.; Ranieri, A. The tomato ethylene receptor LE-ETR3 (NR) is not involved in mediating ozone sensitivity: Causal relationships among ethylene emission, oxidative burst and tissue damage. New Phytol. 2007, 174, 342–356. [Google Scholar] [CrossRef] [PubMed]
- Goyal, R.K.; Fatima, T.; Topuz, M.; Bernadec, A.; Sicher, R.; Handa, A.K.; Mattoo, A.K. Pathogenesis-related protein 1b1 (PR1b1) is a major tomato fruit protein responsive to chilling temperature and upregulated in high polyamine transgenic genotypes. Front. Plant Sci. 2016, 7, 901. [Google Scholar] [CrossRef]
- Fowler, J.H.; Narváez-Vásquez, J.; Aromdee, D.N.; Pautot, V.; Holzer, F.M.; Walling, L.L. Leucine aminopeptidase regulates defense and wound signalling in tomato downstream of jasmonic acid. Plant Cell 2009, 21, 1239–1251. [Google Scholar] [CrossRef]
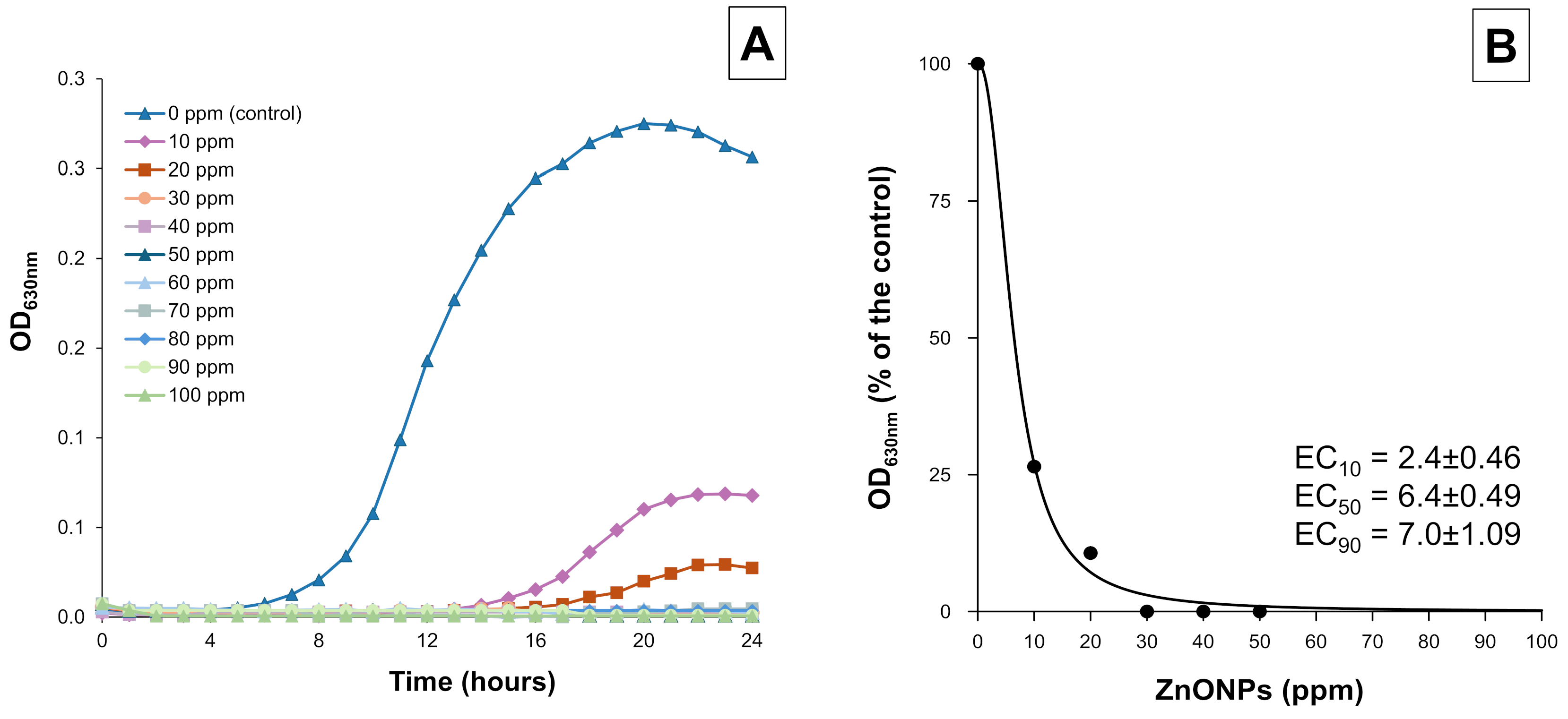
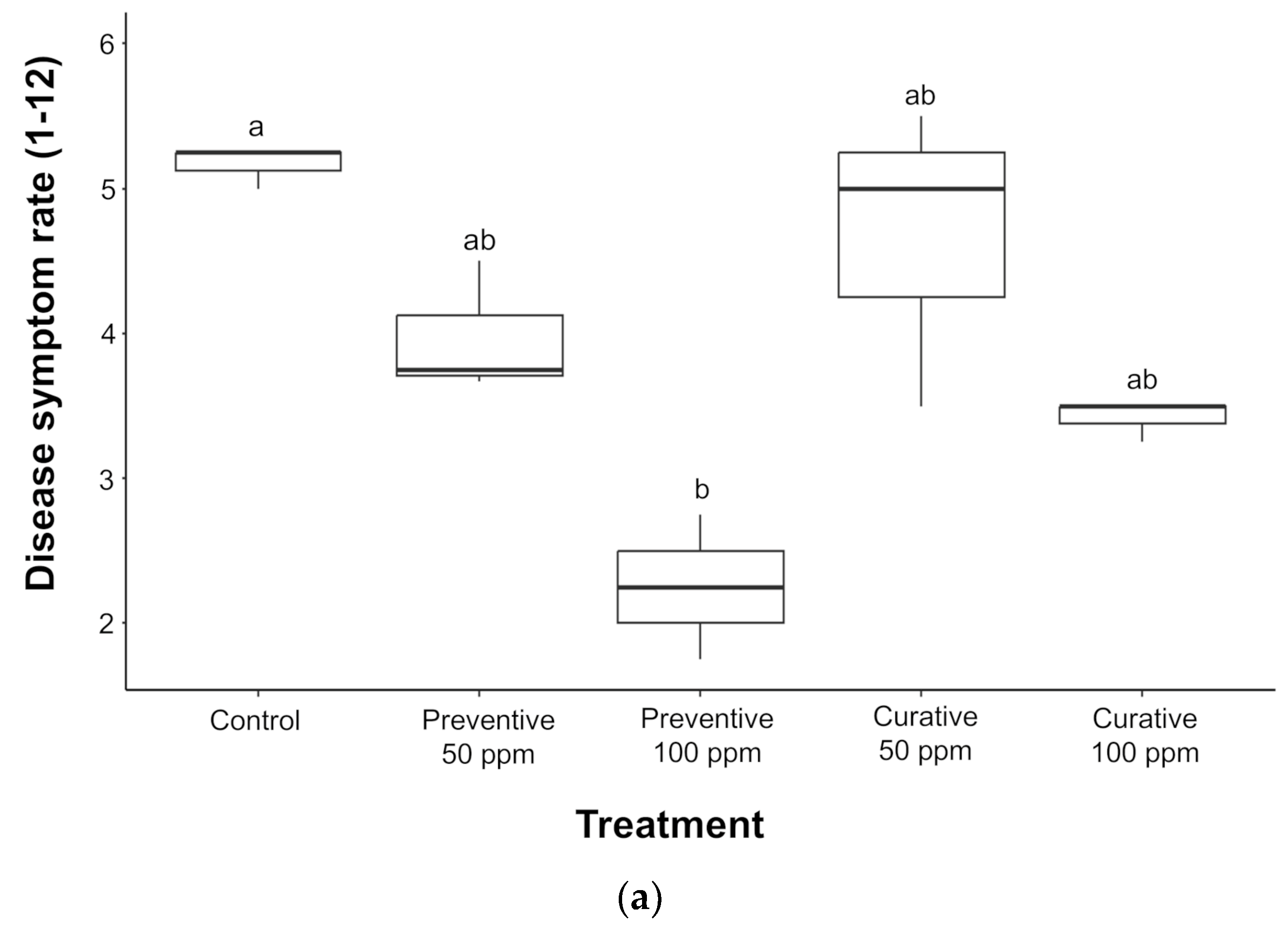
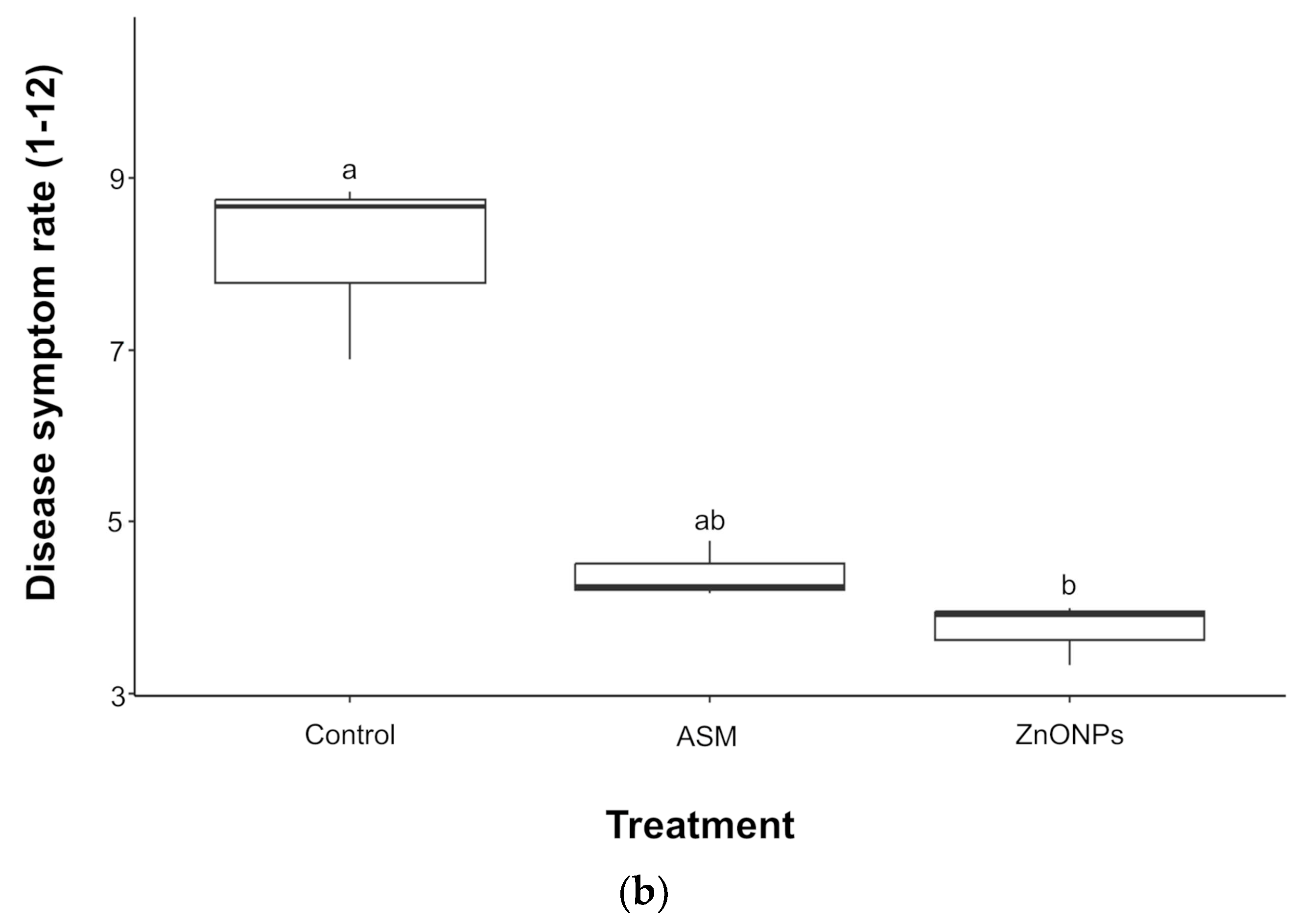

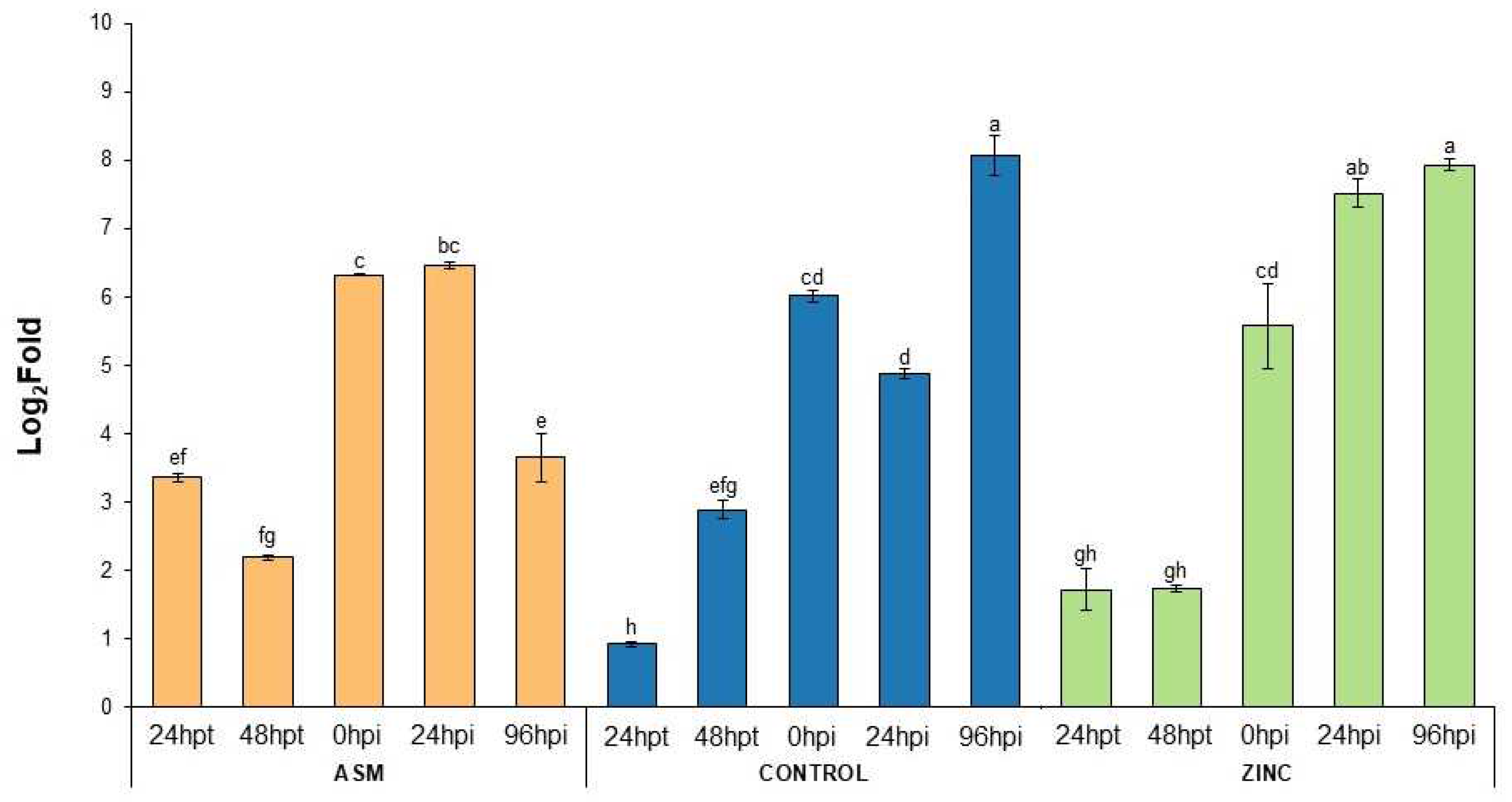
| Time | Treatment | H2O2 (nmol g−1 FW) | MDA (nmol−1 FW) |
|---|---|---|---|
| 72 h | Control | 32.5 ± 7.5 a | 8.11 ± 1.11 a |
| ZnO-NPs | 29.6 ± 4.3 a | 7.32 ± 1.38 a | |
| ZnO-NPs+Pst | 32.0 ± 1.9 a | 7.40 ± 0.36 a | |
| Pst | 37.4 ± 3.0 a | 8.70 ± 0.40 a | |
| 96 h | Control | 32.3 ± 4.0 b | 7.35 ± 0.16 b |
| ZnO-NPs | 28.9 ± 3.4 b | 6.81 ± 0.27 b | |
| ZnO-NPs+Pst | 32.9 ± 4.5 b | 7.30 ± 0.98 b | |
| Pst | 41.6 ± 3.5 a | 9.02 ± 0.75 a |
| Time | Treatment | Carotenoids (mg g−1 FW) | Anthocyanin (µg g−1 FW) | TPC (mg GAE g−1 FW) | TFC (mg CE g−1 FW) |
|---|---|---|---|---|---|
| 72 h | Control | 0.030 ± 0.005 b | 0.93 ± 0.10 a | 0.24 ± 0.02 bc | 1.44 ± 0.02 a |
| ZnO-NPs | 0.040 ± 0.002 a | 0.71 ± 0.07 a | 0.28 ± 0.01 a | 1.23 ± 0.20 ab | |
| ZnO-NPs+Pst | 0.044 ± 0.002 a | 0.90 ± 0.08 a | 0.26 ± 0.01 ab | 1.04 ± 0.15 b | |
| Only Pst | 0.030 ± 0.001 b | 0.81 ± 0.18 a | 0.22 ± 0.03 c | 1.03 ± 0.20 b | |
| 96 h | Control | 0.030 ± 0.001 b | 0.84 ± 0.20 a | 0.25 ± 0.03 b | 1.09 ± 0.04 ab |
| ZnO-NPs | 0.032 ± 0.003 b | 0.83 ± 0.02 b | 0.30 ± 0.01 a | 1.18 ± 0.08 a | |
| ZnO-NPs+Pst | 0.059 ± 0.005 a | 1.01 ± 0.25 a | 0.25 ± 0.01 b | 1.14 ± 0.11 ab | |
| Only Pst | 0.040 ± 0.013 a | 0.89 ± 0.13 ab | 0.19 ± 0.01 c | 1.00 ± 0.04 b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Orfei, B.; Scian, A.; Del Buono, D.; Paglialunga, M.; Tolisano, C.; Priolo, D.; Moretti, C.; Buonaurio, R. Biogenic Zinc Oxide Nanoparticles Protect Tomato Plants Against Pseudomonas syringae pv. tomato. Horticulturae 2025, 11, 431. https://doi.org/10.3390/horticulturae11040431
Orfei B, Scian A, Del Buono D, Paglialunga M, Tolisano C, Priolo D, Moretti C, Buonaurio R. Biogenic Zinc Oxide Nanoparticles Protect Tomato Plants Against Pseudomonas syringae pv. tomato. Horticulturae. 2025; 11(4):431. https://doi.org/10.3390/horticulturae11040431
Chicago/Turabian StyleOrfei, Benedetta, Anna Scian, Daniele Del Buono, Michela Paglialunga, Ciro Tolisano, Dario Priolo, Chiaraluce Moretti, and Roberto Buonaurio. 2025. "Biogenic Zinc Oxide Nanoparticles Protect Tomato Plants Against Pseudomonas syringae pv. tomato" Horticulturae 11, no. 4: 431. https://doi.org/10.3390/horticulturae11040431
APA StyleOrfei, B., Scian, A., Del Buono, D., Paglialunga, M., Tolisano, C., Priolo, D., Moretti, C., & Buonaurio, R. (2025). Biogenic Zinc Oxide Nanoparticles Protect Tomato Plants Against Pseudomonas syringae pv. tomato. Horticulturae, 11(4), 431. https://doi.org/10.3390/horticulturae11040431






