Tomato Production with Organic Fertilizer from Soluble Bonito Fish Waste in Hydroponic Cultivation Systems
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Materials and Seedling Preparation
2.2. Growth Conditions in the Greenhouse
2.3. Treatments and Measurements
2.4. Fruit Quality Analysis
2.5. Statistical Analysis
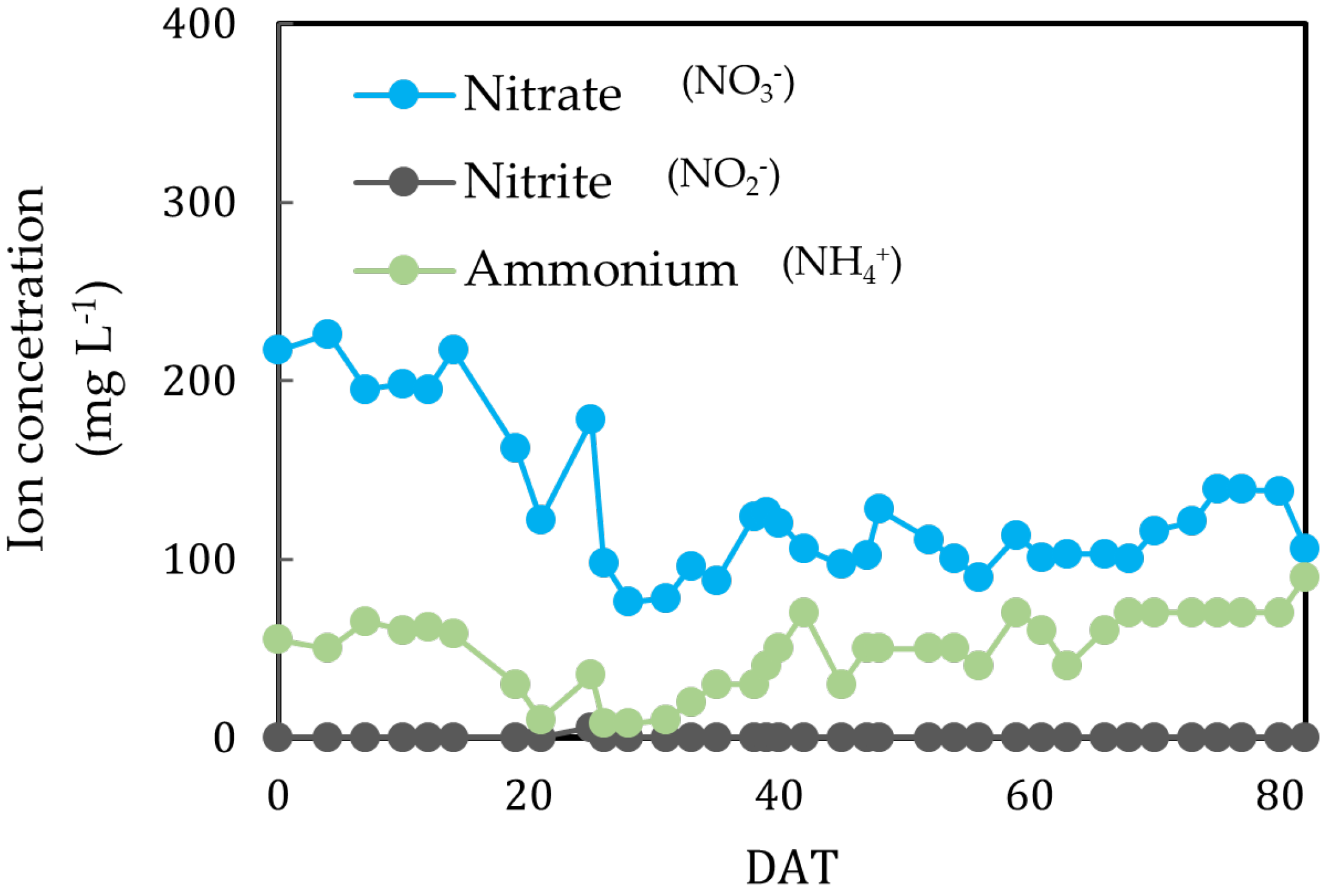
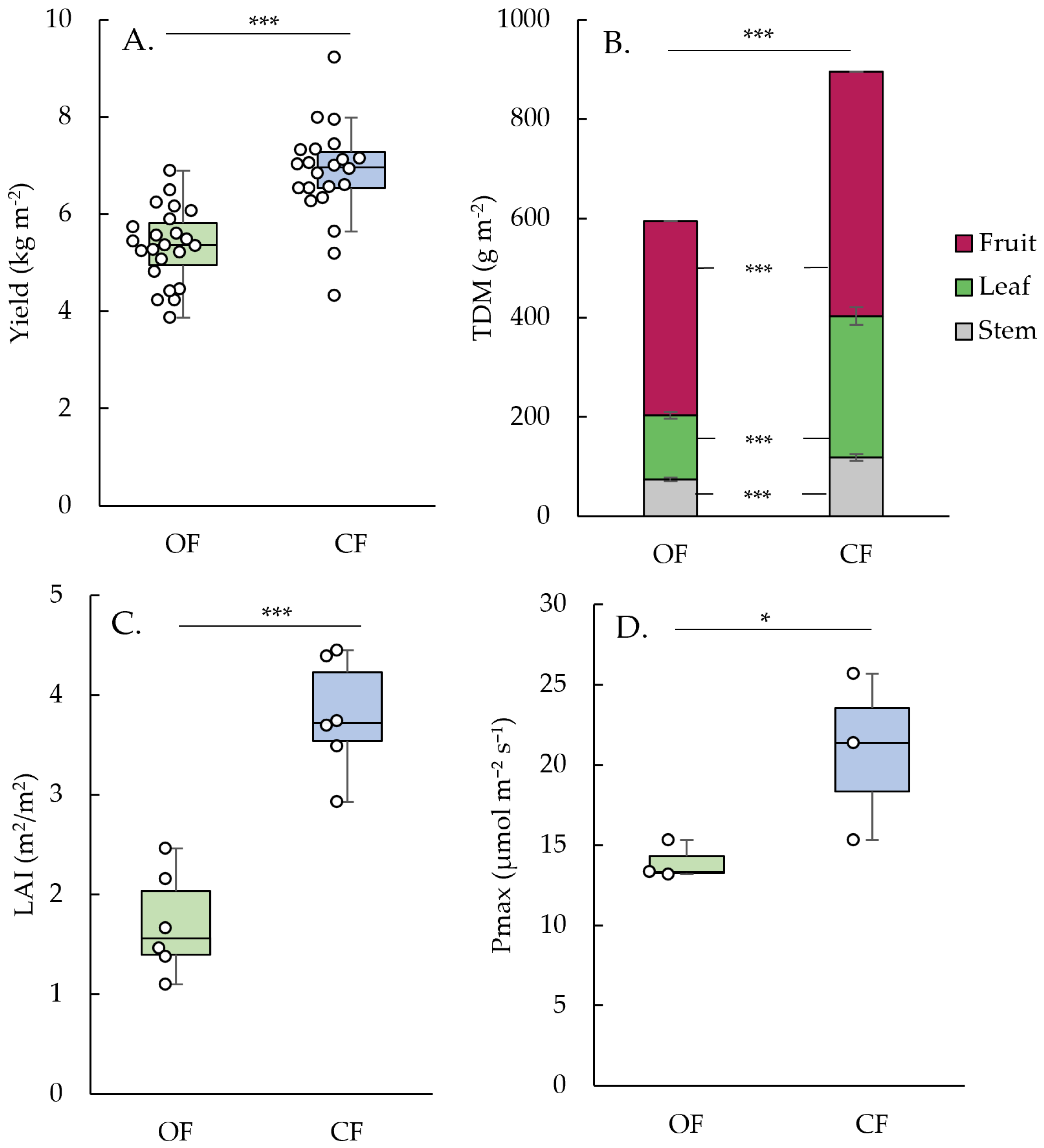
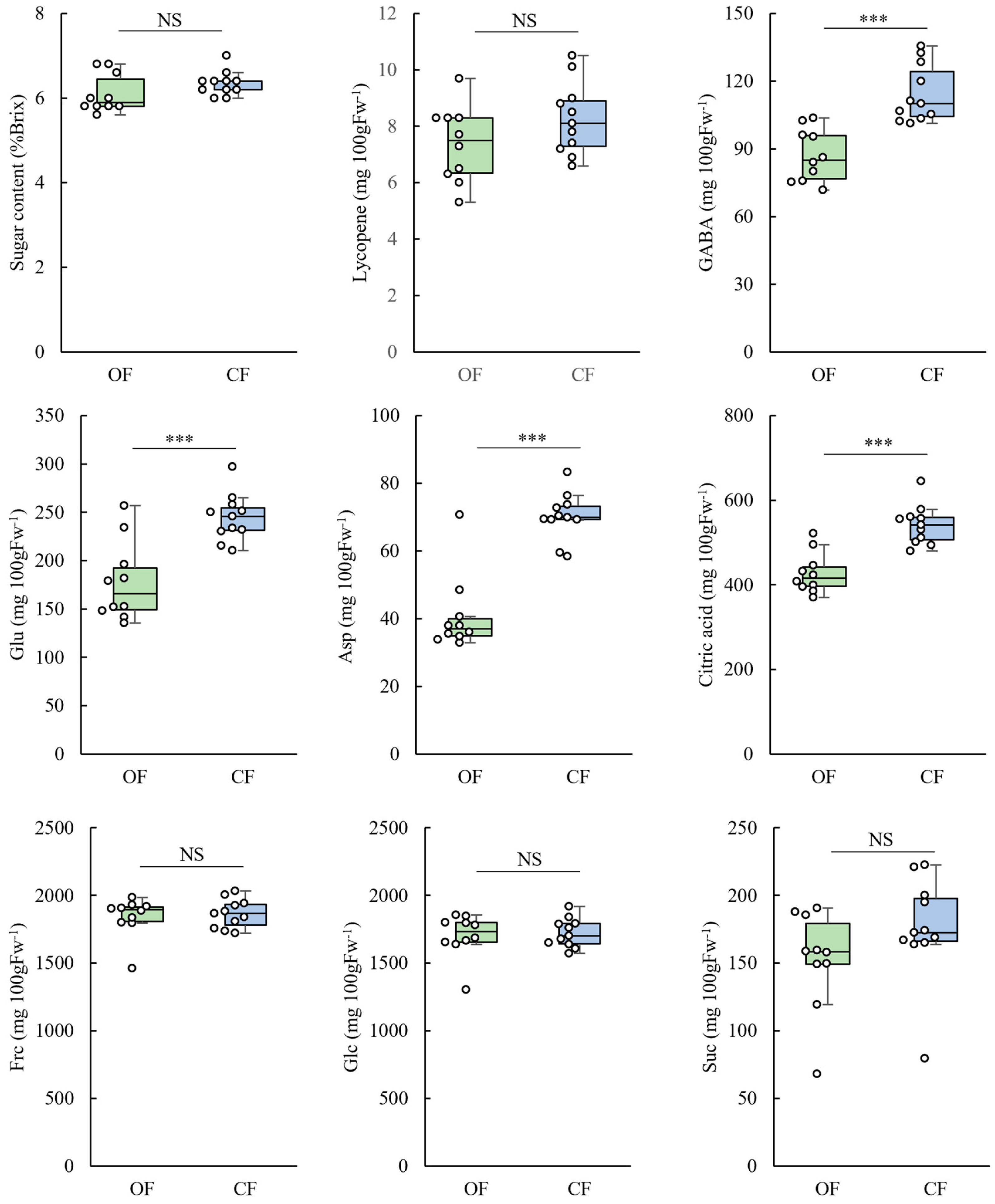
3. Results
3.1. Microbial Preprocessing of Organic Materials
3.2. Aboveground Plant Biomass
3.3. Fruit Quality
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Food and Agricultural Organization of the United Nations (FAO). World Production of Tomato in 2023. Available online: https://www.fao.org/faostat/en/#data/QCL (accessed on 12 March 2025).
- Alhammad, Z.; Awaideh, M. Exploring the role of tomato value chains in enhancing food security in Jordan. Am.-Eurasian J. Sustain. Agric. 2023, 17, 1–7. [Google Scholar] [CrossRef]
- Wu, X.; Yu, L.; Pehrsson, P.R. Are processed tomato products as nutritious as fresh tomatoes? Scoping review on the effects of industrial processing on nutrients and bioactive compounds in tomatoes. Adv. Nutr. 2022, 13, 138–151. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.; Kuthiala, T.; Thakur, K.; Thatai, K.S.; Singh, G.; Kumar, P.; Arya, S.K. Kitchen waste: Sustainable bioconversion to value-added product and economic challenges. Biomass Convers. Biorefinery 2025, 15, 1749–1770. [Google Scholar] [CrossRef]
- Chavan, S.; Yadav, B.; Atmakuri, A.; Tyagi, R.D.; Wong, J.W.C.; Drogui, P. Bioconversion of organic wastes into value-added products: A review. Bioresour. Technol. 2022, 344, 126398. [Google Scholar] [CrossRef]
- Awasthi, M.K.; Sarsaiya, S.; Patel, A.; Juneja, A.; Singh, R.P.; Yan, B.; Awasthi, S.K.; Jain, A.; Liu, T.; Duan, Y. Refining biomass residues for sustainable energy and bio-products: An assessment of technology, its importance, and strategic applications in circular bio-economy. Renew. Sustain. Energy Rev. 2020, 127, 109876. [Google Scholar] [CrossRef]
- Bouhia, Y.; Hafidi, M.; Ouhdouch, Y.; Boukhari, M.E.M.E.; Mphatso, C.; Zeroual, Y.; Lyamlouli, K. Conversion of waste into organo-mineral fertilizers: Current technological trends and prospects. Rev. Environ. Sci. Biotechnol. 2022, 21, 425–446. [Google Scholar] [CrossRef]
- Bhatia, T.; Sindhu, S.S. Sustainable management of organic agricultural wastes: Contributions in nutrients availability, pollution mitigation and crop production: A review. Discov. Agric. 2024, 2, 130. [Google Scholar] [CrossRef]
- Shi, T.; Scott, L.C.; Kailiang, Y.; Josep, P.; Jordi, S.; Hailing, L.; Jian-Sheng, Y. A global meta-analysis on the effects of organic and inorganic fertilization on grasslands and croplands. Nat. Commun. 2024, 15, 3411. [Google Scholar] [CrossRef]
- García Castellanos, B.; García García, B.; García García, J. Economic and Environmental Effects of Replacing Inorganic Fertilizers with Organic Fertilizers in Three Rainfed Crops in a Semi-Arid Area. Sustainability 2023, 15, 16897. [Google Scholar] [CrossRef]
- Shinohara, M.; Aoyama, C.; Fujiwara, K.; Watanabe, A.; Ohmori, H.; Uehara, Y.; Takano, M. Microbial mineralization of organic nitrogen into nitrate to allow the use of organic fertilizer in hydroponics. Soil Sci. Plant Nutr. 2011, 57, 190–203. [Google Scholar] [CrossRef]
- Ehret, D.L.; Menzies, J.G.; Helmer, T. Production and quality of greenhouse roses in recirculating nutrient systems. Sci. Hortic. 2005, 106, 103–113. [Google Scholar] [CrossRef]
- Shinohara, M. Hydroponics with organic fertilizers—A method for building an ecological system of microorganisms in culture liquid by a parallel mineralization method. Agric. Hortic. 2006, 81, 753–764. (In Japanese) [Google Scholar]
- Kano, K.; Kitazawa, H.; Suzuki, K.; Widiastuti, A.; Odani, H.; Zhou, S.; Chinta, Y.D.; Eguchi, Y.; Shinohara, M.; Sato, T. Effects of Organic Fertilizer on Bok Choy Growth and Quality in Hydroponic Cultures. Agronomy 2021, 11, 491. [Google Scholar] [CrossRef]
- Kechasov, D.; Verheul, M.J.; Paponov, M.; Panosyan, A.; Paponov, I.A. Organic Waste-Based Fertilizer in Hydroponics Increases Tomato Fruit Size but Reduces Fruit Quality. Front. Plant Sci. 2021, 12, 680030. [Google Scholar] [CrossRef]
- Ezziddine, M.; Liltved, H.; Seljåsen, R. Hydroponic Lettuce Cultivation Using Organic Nutrient Solution from Aerobic Digested Aquacultural Sludge. Agronomy 2021, 11, 1484. [Google Scholar] [CrossRef]
- Phibunwatthanawong, T.; Riddech, N. Liquid organic fertilizer production for growing vegetables under hydroponic condition. Int. J. Recycl. Org. Waste Agric. 2019, 8, 369–380. [Google Scholar] [CrossRef]
- Rachma, D.F.; Maeda, K.; Yamanouchi, Y.; Ando, A.; Ueda, H.; Shinohara, M.; Ahn, D.H. Hydroponic tomato cultivation using organic fertilizer from bonito fish-based soluble. Acta Hortic. 2025, 1416, 137–140. [Google Scholar] [CrossRef]
- Kawamura-Aoyama, C.; Fujiwara, K.; Shinohara, M.; Takano, M. Study on the hydroponic culture of lettuce with microbially degraded solid food waste as a nitrate source. Jpn. Agric. Res. Q. 2014, 48, 71–76. [Google Scholar]
- Ahmed, Z.F.R.; Alnuaimi, A.K.H.; Askri, A.; Tzortzakis, N. Evaluation of Lettuce (Lactuca sativa L.) Production Under Hydroponic System: Nutrient Solution Derived from Fish Waste vs. Inorganic Nutrient Solution. Horticulturae 2021, 7, 292. [Google Scholar] [CrossRef]
- World Bank Group. What a Waste Global Database (Country Level Dataset). 2024. Available online: https://datacatalog.worldbank.org/search/dataset/0039597 (accessed on 12 March 2025).
- Illera-Vives, M.; Labandeira, S.S.; Brito, L.M.; Lopez-Fabal, A.; Lopez-Mosquera, M.E. Evaluation of compost from seaweed and fish waste as a fertilizer for horticultural use. Sci. Hortic. 2015, 186, 101–107. [Google Scholar] [CrossRef]
- Hosseinzadeh, S.; Verheust, Y.; Bonarrigo, G.; Van Hulle, S. Closed hydroponic systems: Operational parameters, root exudates occurrence and related water treatment. Rev. Environ. Sci. Bio-Technol. 2017, 16, 59–79. [Google Scholar] [CrossRef]
- Djangsou, H.; Francia, E.; Ronga, D.; Buti, M. Blossom end-rot in tomato (Solanum lycopersicum L.): A multi-disciplinary overview of inducing factors and control strategies. Sci. Hortic. 2019, 249, 49–58. [Google Scholar] [CrossRef]
- Hidekazu, I. New Ways to Evaluate the Quality of Vegetables Using Instruments (A Review). Jpn. Agric. Res. Q. 2014, 48, 111–120. Available online: http://www.jircas.affrc.go.jp (accessed on 23 December 2024).
- Horie, H. Analysis for the taste compounds in various vegetables by capillary electrophoresis. Bunseki Kagaku 2009, 58, 1063–1066. (In Japanese) [Google Scholar] [CrossRef][Green Version]
- Wang, Z.; Akira, A.; Atsuko, T.; Hiroshi, U. Effects of Cooking Conditions on the Relationships Among Oxalate, Nitrate, and Lutein in Spinach. Food Sci. Technol. Res. 2018, 24, 421–425. [Google Scholar] [CrossRef]
- Hideki, H. Production of Guanylic Acid by Heating Vegetables. J. Jpn. Culin. Sci. Soc. 2012, 45, 346–351. (In Japanese) [Google Scholar]
- Ikeda, H.; Osawa, T. Nitrate- and ammonium-N absorption by vegetables from nutrient solution containing ammonium nitrate and the resultant change of solution pH. J. Jpn. Soc. Hort. Sci. 1981, 50, 225–230. [Google Scholar] [CrossRef]
- Strayer, R.F.; Finger, B.W.; Alazraki, M.P. Evaluation of an anaerobic digestion system for processing CELSS crop residues for resource recovery. Adv. Space Res. 1997, 20, 2009–2015. [Google Scholar] [CrossRef]
- Britto, D.T.; Kronzucker, H.J. NH4+ toxicity in higher plants: A critical review. J. Plant Physiol. 2002, 159, 567–584. [Google Scholar] [CrossRef]
- Liu, G.; Du, Q.; Li, J. Interactive effects on nitrate-ammonium ratios and temperatures on growth, photosynthesis, and nitrogen metabolism of tomato seedlings. Sci. Hortic. 2016, 214, 41–50. [Google Scholar] [CrossRef]
- Knowles, T. Denitrification. Microbial. Rev. 1982, 46, 43–70. [Google Scholar]
- Mulder, A.; van de Graaf, A.A.; Robertson, L.A.; Kuenen, J.G. Anaerobic ammonium oxidation discovered in a denitrifying fluidized bed reactor. FEMS Microbiol. Ecol. 1995, 16, 177–183. [Google Scholar] [CrossRef]
- Higashide, T.; Heuvelink, E. Physiological and Morphological Changes over the Past 50 Years in Yield Components in Tomato. J. Amer. Soc. Hort. Sci. 2009, 134, 460–465. [Google Scholar]
- Jian, S.; Liao, Q.; Song, H.; Liu, Q.; Lepo, J.E.; Guan, C.; Zhang, J.; Ismail, A.M.; Zhang, Z. NRT1.1-Related NH4+ toxicity is associated with disturbed balance between NH4+ uptake and assimilation. Plant Physiol. 2018, 178, 1473–1488. [Google Scholar] [CrossRef]
- Huang, W.T.; Xie, Y.Z.; Chen, X.F.; Zhang, J.; Chen, H.H.; Ye, X.; Guo, J.; Yang, L.T.; Chen, L.S. Growth, mineral nutrients, photosynthesis and related physiological parameters of Citrus in response to nitrogen deficiency. Agronomy 2021, 11, 1859. [Google Scholar] [CrossRef]
- Jia, Y.; Xu, H.; Wang, Y.; Ye, X.; Lai, N.; Huang, Z.; Yang, L.; Li, Y.; Chen, L.S.; Guo, J. Differences in morphological and physiological features of citrus seedlings are related to Mg transport from the parent to branch organs. BMC Plant Biol. 2021, 21, 239. [Google Scholar]
- Chen, H.; Hu, W.; Wang, Y.; Zhang, P.; Zhou, Y.; Yang, L.; Li, Y.; Chen, L.; Guo, J. Declined photosynthetic nitrogen use efficiency under ammonium nutrition is related to photosynthetic electron transport chain disruption in citrus plants. Sci. Hortic. 2022, 308, 111594. [Google Scholar] [CrossRef]
- Barreto, R.F.; Prado, R.M.; Leal, A.J.F.; Troleis, M.J.B.; Silva Junior, G.B.; Monteiro, C.C.; Santos, L.C.N.; Carvalho, R.F. Mitigation of ammonium toxicity by silicon in tomato depends on the ammonium concentration. Acta Agric. Scand. Sect. B-Soil Plant Sci. 2016, 66, 483–488. [Google Scholar] [CrossRef][Green Version]
- Shiyab, S.M.; Shatnawi, M.A.; Shibli, R.A.; Al Smeirat, N.G.; Ayad, J.; Akas, M.W. Growth, nutrient acquisition, and physiological responses of hydroponic grown tomato to sodium chloride salt induced stress. J. Plant Nutr. 2013, 36, 665–676. [Google Scholar] [CrossRef]
- Sato, S.; Yanagisawa, S. Characterization of metabolic states of Arabidopsis thaliana under diverse carbon and nitrogen nutrient conditions via targeted metabolomic analysis. Plant Cell Physiol. 2014, 55, 306–319. [Google Scholar] [CrossRef]
- Setien, I.; Fuertes-Mendizabal, T.; Gonzalez, A.; Aparicio-Tejo, P.M.; Gonzalez-Murua, C.; Gonzalez-Moro, M.B.; Estavillo, J.M. High irradiance improves ammonium tolerance in wheat plants by increasing N assimilation. J. Plant Phisiol. 2013, 170, 758–771. [Google Scholar] [CrossRef] [PubMed]
- De la Pena, M.; Gonzalez-Moro, M.B.; Marino, D. Providing carbon skeletons to sustain amide synthesis in roots underlines the suitability of Brachypodium distachyon fo the study of ammonium stress in cereals. AoB Plants 2019, 11, plz029. [Google Scholar] [CrossRef] [PubMed]
- Vega-Mas, I.; Cukier, C.; Coleto, I.; Gonzalez-Murua, C.; Limami, A.M.A.M.; Gonzalez-Moro, M.B.B.; Marino, D. Isotopic labelling reveals the efficient adaptation of wheat root TCA cycle flux modes to match carbon demand under ammonium nutrition. Sci. Rep. 2019, 9, 8925. [Google Scholar] [CrossRef]
- Ghiasi, S.; Lehmann, M.M.; Badeck, F.W.; Ghashghaie, J.; Hansch, R.; Meinen, R.; Streb, S.; Hudig, M.; Ruckle, M.E.; Carrera, D.; et al. Nitrate and ammonium differ in their impact on δ13C of plant metabolites and respired CO2 from tobacco leaves. Isot. Environ. Health Stud. 2020, 57, 11–34. [Google Scholar] [CrossRef]
- Li, S.; Yan, L.; Riaz, M.; White, P.J.; Yi, C.; Wang, S.; Shi, L.; Xu, F.; Wang, C.; Cai, H.; et al. Integrated transcriptome and metabolome analysis reveals the physiological and molecular responses of allotetraploid rapeseed to ammonium toxicity. Environ. Exp. Bot. 2021, 189, 104550. [Google Scholar] [CrossRef]
- Vega-Mas, I.; Marino, D.; de la Pena, M.; Fuertez-Mendizabal, T.; Gonzalez-Murua, C.; Estavillo, J.M.; Gonzalez-Moro, M.B. Enhanced photorespiratory and TCA pathways by elevated CO2 to manage ammonium nutrition in tomato leaves. Plant Physiol. Biochem. 2024, 217, 109216. [Google Scholar] [CrossRef]
- Magalhaes, J.R.; Wilcox, G.E. Growth, free amino acids and mineral composition of tomato plants in relation to nitrogen form and growing media. J. Amer. Soc. Hort. Sci. 1984, 109, 406–411. [Google Scholar] [CrossRef]
- Gama, P.B.; Inanaga, S.; Tanaka, K.; Nakazawa, R. Physiological response of common bean (Phaseolus vulgaris) seedlings to salinity stress. Afr. J. Biotechnol. 2007, 6, 79–88. [Google Scholar]
- Sato, S.; Sakaguchi, S.; Furukawa, H.; Ikeda, H. Effects of NaCl application to hydroponic nutrient solution on fruit characteristics of tomato (Lycopersicon esculentum Mill.). Sci. Horticul. 2006, 109, 248–253. [Google Scholar]
- Wu, H.; Xu, K.; He, X.; Wang, X. Removal of Nitrogen by Three Plant Species in Hydroponic Culture: Plant Uptake and Microbial Degradation. Water Air Soil Pollut. 2016, 227, 324. [Google Scholar] [CrossRef]
- Zhang, P.; Senge, M.; Dai, Y. Effect of salinity stress on growth, yield, fruit quality and water use efficiency of tomato under hydroponics system. Rev. Agric. Sci. 2016, 4, 46–55. [Google Scholar] [CrossRef]
- Shinohara, M. Manufacturing Method of Inorganic Fertilizer. JP Patent No. 6156821B2, 5 July 2017. Available online: https://patents.google.com/patent/JP6156821B2/ja (accessed on 17 March 2025).
- Vega-Mas, I.; Marino, D.; Sanchez-Zabala, J.; Gonzalez-Murua, C.; Estavillo, J.M.; Gonzalez-Moro, M.B. CO2 Enrichment modulates ammonium nutrition in tomato adjusting carbon and nitrogen metabolism to stomatal conductance. Plant Sci. 2015, 241, 32–44. [Google Scholar] [CrossRef]
| Composition | Unit | OF Raw | OF (MPM) | CF |
|---|---|---|---|---|
| EC | mS cm−1 | 15.4 | 0.66 | 2.4 |
| Cl− | mg L−1 | 3600 | 79 | - |
| NO3−-N | mg L−1 | <0.22 | 20.71 | 239 |
| SO42−-S | mg L−1 | 130 | 5.47 | - |
| PO43−-P | mg L−1 | 3000 | 48.86 | 70.25 |
| NH4+-N | mg L−1 | 120 | 20.43 | 6 |
| K+ | mg L−1 | 1800 | 43.43 | 398.45 |
| Na+ | mg L−1 | 1100 | 37.71 | - |
| Ca2+ | mg L−1 | 770 | 22.14 | 164.22 |
| Mg2+ | mg L−1 | 60 | 8.06 | 36.18 |
| Fe | mg L−1 | 40 | 0.28 | 2.3 |
| Mn | mg L−1 | 10 | 0.11 | 0.58 |
| Mo | mg L−1 | 0.11 | <0.05 | - |
| Zn | mg L−1 | 3.8 | 0.28 | 0.09 |
| Cu | mg L−1 | 0.94 | 0.10 | <0.05 |
| B | mg L−1 | 4.9 | 0.13 | 0.34 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rachma, D.F.; Maeda, K.; Yamanouchi, Y.; Ueda, H.; Shinohara, M.; Ahn, D.-H. Tomato Production with Organic Fertilizer from Soluble Bonito Fish Waste in Hydroponic Cultivation Systems. Horticulturae 2025, 11, 381. https://doi.org/10.3390/horticulturae11040381
Rachma DF, Maeda K, Yamanouchi Y, Ueda H, Shinohara M, Ahn D-H. Tomato Production with Organic Fertilizer from Soluble Bonito Fish Waste in Hydroponic Cultivation Systems. Horticulturae. 2025; 11(4):381. https://doi.org/10.3390/horticulturae11040381
Chicago/Turabian StyleRachma, Dannisa Fathiya, Kazuya Maeda, Yuta Yamanouchi, Hiroshi Ueda, Makoto Shinohara, and Dong-Hyuk Ahn. 2025. "Tomato Production with Organic Fertilizer from Soluble Bonito Fish Waste in Hydroponic Cultivation Systems" Horticulturae 11, no. 4: 381. https://doi.org/10.3390/horticulturae11040381
APA StyleRachma, D. F., Maeda, K., Yamanouchi, Y., Ueda, H., Shinohara, M., & Ahn, D.-H. (2025). Tomato Production with Organic Fertilizer from Soluble Bonito Fish Waste in Hydroponic Cultivation Systems. Horticulturae, 11(4), 381. https://doi.org/10.3390/horticulturae11040381





