Abstract
Static magnetic field (SMF) treatment is a new type of physical preservation method. In this study, SMF treatment was applied to fresh-cut lotus root to investigate its effects and possible mechanisms in terms of preserving color and maintaining freshness, with the goal of developing a preservation method for fresh-cut lotus root. Fresh-cut lotus root was treated with a magnetic field strength of 3 mt and stored for 14 days under cold conditions (temperature 4 °C, humidity 70%, wind speed 0.1–0.3 m/s, and no light). The control group received no SMF treatments. The effects of the SMF on the color, hardness, browning, weight loss, soluble solids content, vitamin C (Vit. C) content, and polyphenol content, as well as the activities of MDA, POD, PPO, and PAL and the contents of flavor substances of the fresh-cut lotus root were monitored every 2 days throughout the storage period. The results showed that the SMF treatment significantly slowed the decline in the sensory quality of fresh-cut lotus root (p < 0.05). After 6 days of storage, the degree of browning in the control group was 1.96 times that in the SMF group. The SMF treatment also significantly delayed reductions in the Vit. C and polyphenol contents in fresh-cut lotus root (p < 0.05). After 8 days of storage, the polyphenol content in the SMF group was 1.54 times that in the control group. After 12 days of storage, the Vit. C content of the SMF group was 1.45 times that of the control group. When the storage time reached 12 days, the L* and ΔE values of the control group were 1.89 times and 1.44 times those of the SMF group, respectively. The SMF treatment significantly reduced the activities of PPO and POD oxidases, as well as the MDA content (p < 0.05). After 12 days of storage, the activities of PPO and POD and the MDA content in the control group were 2.04 times, 1.42 times, and 1.71 times higher than those in the SMF group, respectively. After 14 days of storage, the weight loss rate in the control group was 1.65 times that in the SMF group, while the hardness of the SMF group was 1.23 times that of the control group. The SMF treatment increased the contents of esters, aldehydes, and ketones in fresh-cut lotus root compared with the control group. The contents of esters, aldehydes, and ketones in the SMF group were 1.04 times, 1.41 times, and 1.49 times higher than those in the control group, respectively. Moreover, using SMF treatment as a new preservation method for fresh-cut lotus root provides a promising strategy for preserving other fresh-cut fruits and vegetables.
1. Introduction
Lotus root (Nelumbo nucifera Gaertn.) is a perennial aquatic herbaceous plant in the genus Nelmbo of the Nymphaeaceae family that is widely cultivated as a vegetable in China, Japan, India, and other Asian countries [1,2]. Lotus root is rich in nutrients, including starches, fats, proteins, flavonoids, polyphenols, polysaccharides, vitamins, minerals, and other bioactive components [3,4,5], and it exhibits antioxidant, free-radical-scavenging, immune-regulating, hypoglycemic, and weight loss effects [6,7,8]. At present, the processing of lotus root products is limited, and only a few deep-processed products are made from lotus root [9]. Lotus root is commonly processed into fresh-cut slices, which are either eaten raw or used in cooking [10]. However, during fresh-cutting and storage, lotus root undergoes water loss, nutrient depletion, browning, and microbial growth, leading to sensory degradation, reduced nutrient value, and microbial spoilage, ultimately causing deterioration [10,11]. Preservation methods such as [12,13] air-conditioning storage [14], heat treatment, film treatment, ultrasonic treatment [3], supercritical carbon dioxide technology, cold plasma treatment [15], and pulsed electric field treatment [16], among other advanced technologies, have been explored by researchers to extend the shelf life of fresh-cut lotus root, achieving improved preservation results. However, these technologies still face challenges. For instance, the energy consumption of air-conditioning storage and heat treatment is too large, and the preservation time of film treatment is limited. The processing space required for ultrasonic treatment, supercritical carbon dioxide technology, cold plasma treatment, and pulsed electric field treatment limits their suitability for continuous production, and they are difficult to integrate with low-temperature storage [3,15,16]. SMF treatment is a novel physical preservation method characterized by deep penetration and no reagent residue [17,18]. SMF treatment can eliminate pests and pathogenic bacteria from the surface of fruits and vegetables, thereby reducing their harmful effects [19]. After harvesting, fresh fruits and vegetables undergo rapid water loss and tissue softening, reducing their quality, especially under unsuitable storage conditions. SMF treatment enhances the hydrogen bonding of water molecules in fruits and vegetables, improving water activity and slowing down tissue softening [20]. Enzymatic browning is an important factor affecting fresh-cut fruits and vegetables during storage [11], which SMF treatment can inhibit by reducing enzyme activities [21]. In addition, an SMF can influence bacterial growth and gene expression, thereby inhibiting the growth of bacteria, including E. coli [22,23]. The research and application of SMF technology for storing freshly picked fruits and vegetables is well developed and effective, but its application for preserving fresh-cut lotus root has rarely been reported.
To study the effects of SMF treatment on the preservation of freshness in fresh-cut lotus root, we examined the color, hardness, browning, weight loss, soluble solids content, Vit. C content, polyphenol content, MDA activity, POD activity, PPO activity, and PAL activity of SMF-treated fresh-cut lotus root during storage. We also determined the contents of flavor substances in the fresh-cut lotus root after 14 days of storage. Furthermore, the effects of the SMF treatment on the quality of the fresh-cut lotus root during storage were evaluated by means of comparison with the quality indicators of refrigerated fresh-cut lotus root.
2. Materials and Methods
2.1. Plant Materials and Chemical Reagents
Mature lotus roots (Elian 5) were picked in late July, for a total of 5–6 main roots, about 110 cm long, weighing 3–5 kg each. These were obtained from local (Bozhou, China) farmers’ markets. The lotus roots were selected to be harvested within 24 h and were fresh and free from mechanical damage. The selected lotus root nodes were of similar size and maturity, measuring 15–20 cm in length and 5–8 cm in diameter. The lotus root nodes were washed with deionized water to remove surface dirt and stains and were then drained. A thin layer of skin was removed from the lotus roots using a paring knife, and the lotus roots were then sliced into 3 mm pieces. The fresh-cut lotus root was stored in deionized water for 5 min at 25 °C. Then, the fresh-cut lotus root was removed and drained at 4 °C until no surface moisture remained. All chemicals used in this study, including oxalic acid, 2,6-dichloroindophenol, sodium carbonate, gallic acid, foraminol, and sodium sulfate, were analytically pure and purchased from Tianjin Yongda Chemical Reagent Co., Ltd. (Tianjin, China). MDA, POD, PPO, and PAL activity assay kits were purchased from Nanjing jiancheng bioengineering institute (Nanjing, China).
2.2. Fresh-Cut Lotus Root Pretreatment
The fresh-cut lotus root was randomly divided into 2 equal portions (200 lotus root slices per portion). One sample was packed in a sealed storage container and placed in a hermetically sealed magnetic field chamber (MFHL10, Induce+ (Wuxi) Induction Technology Co., Ltd., Wuxi, China) for preservation under the following conditions: magnetic field strength, 3 mt [24]; temperature, 4 °C; humidity, 70%; wind speed, 0.1–0.3 m/s; and protection from light. The other sample served as the control group and was stored in a plastic box at 4 °C under light-protected conditions. Samples were randomly collected on days 0, 2, 4, 6, 8, 10, 12, and 14 to measure their quality parameters.
2.3. Surface Color Test
The surface color of the fresh-cut lotus root was measured using a benchtop spectroscopic grating colorimeter (YS6060, Shenzhen threenh technology Co., Ltd., Shenzhen, China) according to the method described by Shahab et al. [25]. The CIELAB color values (also referred to as L*, a*, and b*) of the fresh-cut lotus root were obtained on 5 different individual fresh-cut lotus root slices, and the average values were used. A standard plate (L = 94.59, a = −0.88, and b = 0.56) was used as the background. ΔL, Δa, and Δb denote the differences between the values for the fresh-cut lotus root slice and the standard. L and ΔE represent the chromaticity difference of the samples [26], and ΔE was calculated as shown in Equation (1):
where ΔL = L* − L, Δa = a* − a, and Δb = b* − b.
2.4. Browning Degree Test
A 10.0 g sample of fresh-cut lotus root was weighed, added to 0 °C distilled water at 1:10 (w/w), and homogenized using a tissue masher (WB550, Wiggens Technology (Beijing) Co., Ltd., Beijing, China). The supernatant was centrifuged at 4000 rpm for 10 min. Its absorbance was measured spectrophotometrically (UV1902, Shanghai lengguang technology Co., Ltd., Shanghai, China) at a wavelength of 410 nm, and deionized water was used as a blank. The result was recorded as A [27]. The browning degree B was calculated as shown in Equation (2).
2.5. Hardness Test
Hardness tests on the fresh-cut lotus root were performed with a physical property tester (Universal TA, Shanghai tengba instrument technology Co., Ltd., Shanghai, China) at room temperature (25 °C). The measurement conditions were set as follows: a compression ratio of 30%; an interval of 2 × 2 s; and a trigger force of 5 g. The pre-test, test, and post-test speeds were all set to 2 mm/s, with a displacement of 10 mm [28]. The results were averaged from 5 replicates for each sample and are expressed in N.
2.6. Weight Loss Test
Weight loss was determined using the gravimetric method [3]. Ten lotus root slices were used per sample. The initial mass was measured and recorded as m0. The sample was then reweighed on days 2, 4, 6, 8, 10, 12, and 14, with the recorded mass denoted by m1. The weight loss rate W was calculated using Equation (3).
2.7. Total Soluble Solid (TSS) Test
A 10.0 g sample of fresh-cut lotus root was weighed, ground under ice-cold conditions, homogenized using a tissue masher, and then subjected to diafiltration. The total soluble solid (TSS) content (°Brix) of the lotus root was determined using a refractometer (MyBrix, Mettler Toledo Technology (China) Co., Ltd., Shanghai, China).
2.8. Vit. C Content Test
A 20.0 g sample of fresh-cut lotus root was weighed and placed in a tissue homogenizer, then homogenized in 20 mL of 2% oxalic acid solution. The homogenate was then filtered. The filtrate was poured into a 100 mL volumetric flask and diluted to volume with 2% oxalic acid solution. Then, 2 mL of the filtrate was mixed with 9 mL of 2, 6-dichloroindophenol (0.15 mg mL−1), and its absorbance was measured at 515 nm. The Vit. C content of the sample was calculated on the basis of the calibration curve and is expressed in mg 100 g−1 [29].
2.9. Polyphenol Content Test
A 10.0 g sample of fresh-cut lotus root was weighed, ground under ice-cold conditions, homogenized using a tissue homogenizer, and then extracted with 100 mL of 60% ethanol. Polyphenols were extracted using an ultrasonic cleaner (SCIENTZ-250C, Wiggens Technology (Beijing) Co., Ltd., Beijing, China). The extraction conditions were set at a temperature of 40 °C, an ultrasonic power of 300 W, and an extraction duration of 30 min. The extract was centrifuged at 4000 rpm for 10 min using a refrigerated centrifuge (GTR318C, Hunan Kecheng Instrument and Equipment Co., Ltd., Changsha, China) at 4 °C. The Folin–Ciocalteu method was used to quantify the polyphenol content, which is expressed in mg g−1 [30]. A mixture of 1 mL of extract, 5 mL of distilled water, 3 mL of 7.5% sodium carbonate solution, and 1 mL of Folin–Ciocalteu reagent was added to a 10 mL centrifuge tube and incubated in the dark at room temperature (25 °C) for 2 h. The absorbance was measured at 765 nm, with gallic acid as the standard reference.
2.10. Polyphenol Oxidase (PPO), Peroxidase (POD), L-Phenylalanin Ammonia Lyase (PAL), and Malondialdehyde (MDA) Activity Tests
A 10.0 g sample of fresh-cut lotus root was weighed, ground under ice-cold conditions, and homogenized using a tissue masher. A 0.1 g sample of lotus root homogenate was taken, and PPO was extracted using a PPO assay kit (Nanjing jiancheng bioengineering institute, Nanjing, China). The PPO activity was measured spectrophotometrically at 420 nm using a PPO assay kit and is expressed in U/g. A 0.1 g sample of lotus root homogenate was mixed with 0.9 mL of 0.9% NaCl solution, extracted for 3 min, and then centrifuged at 3500 rpm for 10 min in a refrigerated centrifuge at 4 °C. A 0.1 mL sample of the supernatant was taken, and the POD activity, expressed in U/g, was measured spectrophotometrically at 420 nm using a POD assay kit (Nanjing jiancheng bioengineering institute, Nanjing, China). A 0.1 g sample of lotus root homogenate was taken, and PAL was extracted using a PAL assay kit (Nanjing jiancheng bioengineering institute, Nanjing, China). The PAL activity was measured spectrophotometrically at 290 nm using a PAL assay kit and is expressed in U/g. A 0.1 g sample of lotus root homogenate was mixed with 0.9 mL of anhydrous ethanol, extracted for 3 min, and then centrifuged at 4000 rpm for 10 min in a refrigerated centrifuge at 4 °C. A 0.2 mL sample of supernatant was taken, and the MDA activity, expressed as a percentage, was measured spectrophotometrically at 532 nm using an MDA kit (Nanjing jiancheng bioengineering institute, Nanjing, China).
2.11. Gas Chromatography–Mass Spectrometry (GC-MS) Analysis
Sample preparation: A 50.0 g sample of fresh-cut lotus root was weighed, ground under ice-cold conditions, mixed with 15 g of sodium chloride, and homogenized using a tissue masher. A 30 g sample of the homogenate was transferred to a screw-necked bottle. An extract was obtained by adding n-pentane, followed by shaking and filtering, and, subsequently, freezing at −80 °C in an ultra-low-temperature freezer (Haier, Qingdao, China). The appropriate amount of anhydrous sodium sulfate was added to the frozen filtrate, which was then shaken and filtered using static filtration. The extract was concentrated to 0.5 mL using a nitrogen blowing apparatus [28]. The GC-MS analysis of the flavor extract was performed using a gas chromatography–triple quadrupole mass spectrometer (8890+7000D, Agilent Technology Co., Ltd., New York, NY, USA).
The GC conditions were set as follows: A chromatographic column (DB-5, 30 m × 0.5 mm × 0.5 mm) was used. The initial temperature was set to 40 °C and maintained for 5 min, then warmed up to 70 °C at a rate of 5 °C/min and maintained for another 5 min. The temperature was further increased to 180 °C at 3 °C/min and maintained for 3 min, followed by a final ramp up to 240 °C at 5 °C/min, where it was maintained for 10 min. The injector temperature was set to 250 °C. Additionally, helium (He) was used as the carrier gas at a flow rate of 1 mL/min, with a split ratio of 10:1 [31].
The MS conditions were set as follows: electron ionization (EI) was used with an electron energy of 70 eV; the ion source temperature was set to 200 °C; the emission current was 200 μA; the interface temperature was 250 °C; and the detection voltage was 350 V [31].
2.12. Data Analysis
All data are expressed as the mean ± standard error (SE). The statistical analysis was performed using SAS software ver.8.1. Mean comparisons were conducted using Duncan’s multiple range test. Statistical significance was set at p < 0.05.
3. Results and Discussion
3.1. Effect of SMF on Surface Color, Browning Degree, Hardness, and Weight Loss of Fresh-Cut Lotus Root
Fresh-cut lotus root undergoes oxidation when exposed to air, leading to browning and quality deterioration [32]. As shown in Figure 1a, the samples in the control and SMF groups exhibited increased browning, which intensified over time, with the SMF group maintaining a better appearance compared to the control group. As shown in Figure 1b, the degree of browning in the SMF group was significantly lower than that in the control group (p < 0.05). The above results are consistent with the trend observed in the surface color changes of the fresh-cut lotus root, shown in Table 1. The L* value is a key indicator of browning, with lower values corresponding to increased browning [28]. As shown in Table 1, the L* values of the control and SMF groups decreased over the storage period. After 12 days of storage, the L* value of the control group was significantly lower than that of the SMF group, being 1.89 times lower (p < 0.05). The ΔE values of both the control and SMF groups increased over the storage period, with the ΔE value of the SMF group increasing at a slower rate than that of the control group. After 12 days of storage, the ΔE value reached 38.79 in the control group, whereas it was 27.03 in the SMF group, indicating significantly lower browning in the SMF group. The degree of browning in both the control and SMF groups increased over the storage period, with significantly higher values observed in the control group than in the SMF group. On day 6 of storage, the degree of browning in the control group was 1.96 times higher than that in the SMF group. Therefore, SMF treatment effectively controls browning and enhances color retention.
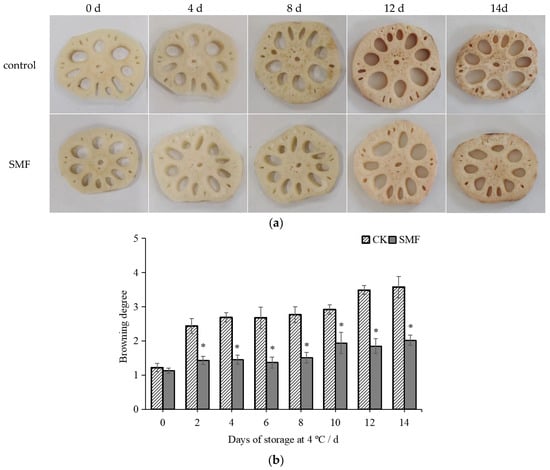
Figure 1.
Effects of SMF on sensory quality of fresh-cut lotus root. Note: (a) appearance changes of fresh-cut lotus root; (b) browning degree. * indicates a statistically significant difference (p < 0.05).

Table 1.
The surface color of fresh-cut lotus root.
During cutting, lotus root undergoes mechanical damage, leading to accelerated respiration, cell structure destruction, juice leakage, and water evaporation, ultimately increasing the weight loss rate [33]. As shown in Figure 2a, the weight loss rate was higher in the control group than in the SMF group. After 8 days of storage, the control group exhibited a significantly higher weight loss rate than the SMF group (p < 0.05). On day 14 of storage, the weight loss rate in the control group was 1.65 times higher than that in the SMF group. SMF treatment can influence enzymatic activity in stored plant tissues, leading to inhibited respiration. SMF treatment can also alter water properties and reduce water loss through evaporation [34,35]. Therefore, SMF treatment can effectively reduce water loss in fresh-cut lotus root. Figure 2b shows that the hardness of the SMF group was significantly higher than that of the control group (p < 0.05). On day 14 of storage, the hardness of the SMF group was 1.23 times higher than that of the control group. Fruits and vegetables are highly susceptible to heat from respiratory metabolism and storage conditions [28]. The respiration of fresh-cut lotus root causes water loss and enhances hydrolysis, leading to cell wall degradation, structural changes, and reduced hardness. Therefore, while the hardness of the fresh-cut lotus root gradually decreased during storage, the SMF treatment mitigated this effect.
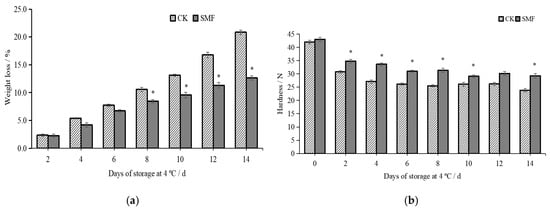
Figure 2.
Effects of SMF on weight loss and hardness of fresh-cut lotus root. Note: (a) weight loss; (b) hardness. * indicates a statistically significant difference between different groups (in the same column) (p < 0.05).
3.2. Effect of SMF on TSS Content, Vit. C Content, and Polyphenol Content of Fresh-Cut Lotus Root
The TSS content is one of the main indicators for assessing the quality of fruits and vegetables [36]. As shown in Figure 3a, the SMF group exhibited higher TSS content than the control group during the first 2 days of storage. After 4 days of storage, the TSS content in the SMF group was lower than that in the control group. Fresh-cut lotus cells were mechanically damaged, and enzymatic activity led to an increase in the TSS content during the first 10 days of storage. Sugars are a major component of the soluble solids present in lotus root. During the storage of fresh-cut lotus root, cellular respiration consumes sugars and organic acids, leading to a decrease in TSS after 10 days of storage [37].
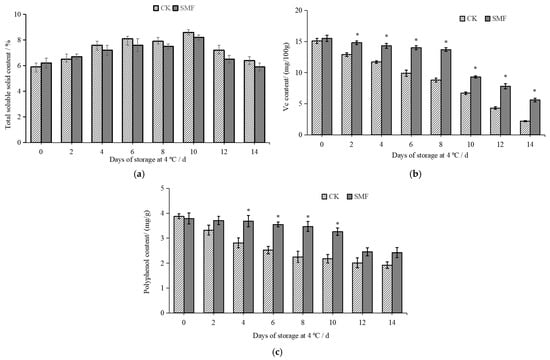
Figure 3.
Effect of SMF on TSS content, Vit. C content, and polyphenol content of fresh-cut lotus root. Note: (a) TSS content; (b) Vit. C content; (c) polyphenol content. * indicates a statistically significant difference between different groups (in the same column) (p < 0.05).
Vit. C is a well-known antioxidant, and its content serves as an important indicator for assessing the nutritional value of vegetables [38]. As shown in Figure 3b, as the storage time increased, the Vit. C content in both the control and SMF groups gradually decreased. However, the SMF group maintained a higher Vit. C content than the control group. After 2 days of storage, the Vit. C content in the SMF group was significantly higher than that in the control group (p < 0.05); this may be because SMF treatment can inhibit the respiration of fresh-cut lotus root [28]. In addition to preserving the Vit. C content, the SMF treatment influenced the texture of the fresh-cut lotus root. On day 12 of storage, the hardness of the SMF group was 1.45 times higher than that of the control group.
The polyphenol content in the fresh-cut lotus root gradually declined during storage, as illustrated in Figure 3c. As the storage time increased, the polyphenol content in both the control and SMF groups gradually decreased. On days 4, 6, 8, and 10 of storage, the polyphenol content in the SMF group was significantly higher than that in the control group (p < 0.05). On day 8 of storage, the polyphenol content in the SMF group was 1.54 times higher than that in the control group. This is because Vit. C is a strong reducing agent that inhibits the activity of polyphenol oxidase, and our results show that the SMF prevented Vit. C decline. Polyphenols exhibit antioxidant properties, including free radical scavenging. A high polyphenol content can extend the shelf life of fresh-cut lotus root, whereas polyphenol loss accelerates its deterioration [39].
3.3. Effect of SMF on Oxidase Activity (PPO, POD, PAL, and MDA Activity) of Fresh-Cut Lotus Root
The activities of PPO, POD, and PAL are related to the browning of fresh-cut lotus root [40]. As shown in Figure 4a, the PPO activity in the fresh-cut lotus root increased as the storage time increased but was reduced by the SMF treatment. On day 6 of fresh-cut lotus root storage, the PPO activity in the SMF treatment group was 17.38 U/g, which was 104% lower than that in the control group (p < 0.05). Furthermore, the SMF treatment effectively inhibited PPO accumulation in the fresh-cut lotus root. As shown in Figure 4b, as the storage time increased, the POD activity in both the SMF and control groups initially increased and then decreased. After slicing, the cell structure of the lotus root was disrupted, causing polyphenols to react with oxidative enzymes, leading to an increase in POD activity and accelerating the browning process [41]. Subsequently, the PPO activity in the fresh-cut lotus root gradually decreased, likely due to the inhibitory effects of low temperature and the SMF on enzyme activity. On days 4 and 6 of storage, the POD activity in the control group was 1.63 and 1.42 times higher than that in the SMF group. As shown in Figure 4c, the PAL activity in fresh-cut lotus root in both the SMF and control groups initially increased and then decreased as the storage time increased. The PAL activity in the fresh-cut lotus root in the SMF group was higher than that in the control group, reaching 1.21 times higher at 6 days of storage. The MDA content is an index used to evaluate the degree of oxidation. In general, a higher MDA content indicates greater cell membrane damage. Reducing MDA accumulation can improve the cold resistance and storage stability of fresh-cut lotus root [42]. As shown in Figure 4d, the MDA activity in the fresh-cut lotus root increased as the storage time increased but was reduced by the SMF treatment. On day 6 of storage, the MDA activity in the SMF treatment group was 0.90 U/g, while that in the control group was 1.71 times higher (p < 0.05).
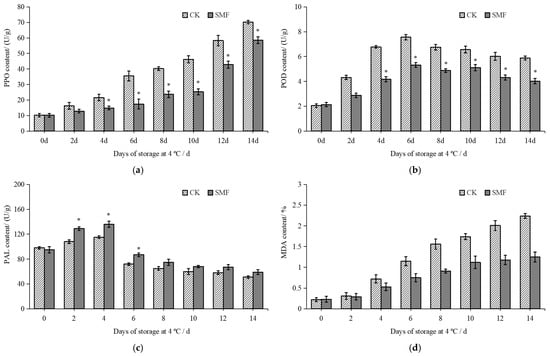
Figure 4.
Effects of SMF on PPO, POD, PAL, and MDA activity of fresh-cut lotus root. Note: (a) PPO activity; (b) POD activity; (c) PAL activity; (d) MDA activity. * indicates a statistically significant difference between different groups (in the same column) (p < 0.05).
3.4. Effect of SMF on Flavor Compounds of Fresh-Cut Lotus Root
The flavor compounds in fresh-cut lotus root are among the main factors that determine its quality. After the SMF treatment, the aroma components of the fresh-cut lotus root were obtained using GC-MS, as presented in Table 2. The flavor components of the lotus root were highly complex, and the types and contents of flavor compounds in the SMF and control groups differed significantly. A total of 55 flavor components were identified (Table 2)—16 esters, 13 alkanes, 7 aldehydes, 13 aromatic hydrocarbons, 3 ketones, and 3 ethers—along with other minor compounds. As shown in Table 2, the alkanes had the largest variety and the highest relative content among the flavor substances in fresh-cut lotus root in the SMF and control groups, but they contributed the least to the overall flavor profile. Aldehydes, esters, ketones, and other components were present in lower concentrations and fewer varieties but exerted a significant influence on the flavor of the lotus root and played an important role in its aroma [28]. On day 14 of storage, the alkane content in the SMF group was lower than that in the control group by 1.24 times. However, the contents of esters, aldehydes, and ketones in the SMF group were higher than those in the control group by 1.04 times, 1.41 times, and 1.49 times, respectively. Changes in the flavor components in fresh-cut lotus root influence the levels of aromatic compounds, thereby affecting its aroma. This effect is attributed to the hydrogen bond network of water molecules in SMF-treated fresh-cut lotus root, which increases free radical production, thereby accelerating aldol condensation and other chemical reactions. Simultaneously, the SMF treatment changed the cell membrane permeability and enzyme activity in the fresh-cut lotus root, as well as the dissolution and decomposition rates of its aroma components.

Table 2.
The flavor compounds of fresh-cut lotus root.
4. Conclusions
This study demonstrated that SMF treatment effectively preserves fresh-cut lotus root, thereby extending its shelf life. It is an efficient and eco-friendly preservation method for fresh-cut fruits and vegetables. Compared with the control group, the SMF treatment slowed the decline in sensory quality, delayed reductions in the contents of Vit. C and polyphenols, and suppressed the activity of PPO, POD, MDA, and PAL oxidases in fresh-cut lotus root. The SMF treatment also increased the levels of esters, aldehydes, and ketones in fresh-cut lotus root compared to the control group, and altered the trend of changes in the flavor composition and overall quality of fresh-cut lotus root during storage. Although SMF treatment is a widely studied technique, its industrial application is constrained by the lack of large-scale low-temperature SMF processing equipment for industrial applications. Therefore, the research and development of large-scale low-temperature SMF processing equipment for industrial use is a key focus for advancing SMF-based fruit and vegetable preservation.
Author Contributions
X.X.: conceptualization, methodology, validation, writing—original draft, and data curation; D.Z.: software, formal analysis, and methodology; X.X. and J.W.: writing—review and editing, supervision, funding acquisition, project administration; Z.M.: writing—review and editing and supervision; R.Z. and X.L.: investigation, resources, and conceptualization. All authors contributed to the conceptualization of this review, the study selection, and the quality assessment process, and they all also read and approved the final publication. All authors have read and agreed to the published version of the manuscript.
Funding
The authors are grateful for the support of the Excellent Top Talents Cultivation Funding Project of Anhui Universities (grant number gxyqZD2022086), the Provincial Quality Engineering Program for Higher Education Institutions in Anhui Province (grant number 2023zygzts103), the Natural Science Research Project of Anhui Universities (grant number 2022AH052410), and the Provincial Quality Engineering Program for Higher Education Institutions in Anhui Province (2023xjzlts084).
Data Availability Statement
No new data were created for the production of this manuscript. All of the data here discussed and presented are available in the relative references here cited and listed.
Acknowledgments
The authors are grateful for the support of the Anhui provincial department of education.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Qian, C.L.; Jiang, Y.Y.; Sun, Y.; Yin, X.D.; Zhang, M.; Kan, J.; Liu, J.; Xiao, L.X.; Jin, C.H.; Qi, X.H.; et al. Changes in the Texture and Flavor of Lotus Root after Different Cooking Methods. Foods 2023, 10, 2012. [Google Scholar] [CrossRef]
- Yan, Y.Y.; Wang, Q.; Sun, L.H.; Zhang, X.F. Extraction, preparation, and carboxymethyl of polysaccharide from Lotus root. Food Sci. Technol. 2022, 42, 17822. [Google Scholar] [CrossRef]
- Li, S.Y.; He, X.; Li, J.S.; Sun, Y.W.; Yi, Y.; Lamikanra, O. Effect of ultraviolet treatment on shelf life of fresh lotus root. J. Food Biochem. 2020, 44, 13223. [Google Scholar] [CrossRef]
- Peng, K.D.; Li, Y.; Sun, Y.; Xu, W.; Wang, H.X.; Zhang, R.; Yi, Y. Lotus Root Polysaccharide-Phenol Complexes: Interaction, Structure, Antioxidant, and Anti-Inflammatory Activities. Foods 2023, 12, 577. [Google Scholar] [CrossRef] [PubMed]
- Ji, L.; Gao, W.; Wei, J.; Pu, L.; Yang, J.; Guo, C. In vivo antioxidant properties of lotus root and cucumber: A pilot comparative study in aged subjects. J. Nutr. Health Aging 2015, 19, 765–770. [Google Scholar] [CrossRef] [PubMed]
- Hu, W.C.; Jiang, Y.Y.; Xue, Q.; Sun, F.T.; Zhang, J.M.; Zhou, J.; Niu, Z.Q.; Li, Q.T.; Li, F.; Shen, T. Structural characterisation and immunomodulatory activity of a polysaccharide isolated from lotus (Nelumbo nucifera Gaertn.) root residues. J. Funct. Foods. 2019, 60, 103457. [Google Scholar] [CrossRef]
- Yi, Y.; Huang, X.Y.; Zhong, Z.T.; Huang, F.; Li, S.Y.; Wang, L.M.; Min, T.; Wang, H.X. Structural and biological properties of polysaccharides from lotus root. Int. J. Biol. Macromol. 2019, 130, 454–461. [Google Scholar] [CrossRef]
- Yi, Y.; Lamikanra, O.; Sun, J.; Wang, L.M.; Min, T.; Wang, H.X. Activity diversity structure-activity relationship of polysaccharides from lotus root varieties. Carbohydr. Polym. 2018, 190, 67–76. [Google Scholar] [CrossRef]
- Yi, Y.; Tang, H.S.; Sun, Y.; Xu, W.; Min, T.; Wang, H.X. Comprehensive characterization of lotus root polysaccharide-phenol complexes. Food Chem. 2022, 366, 130693. [Google Scholar] [CrossRef]
- Xu, Y.H.; Bao, Y.Q.; Chen, J.H.; Yi, Y.; Ai, Y.W.; Hou, W.F.; Wang, L.M.; Wang, H.X.; Min, T. Mechanisms of ethanol treatment on controlling browning in fresh-cut lotus roots. Sci. Hortic. 2023, 310, 111708. [Google Scholar] [CrossRef]
- Chen, J.H.; Xu, Y.H.; Yi, Y.; Hou, W.F.; Wang, L.M.; Ai, Y.W.; Wang, H.X.; Min, T. Regulations and mechanisms of 1-methylcyclopropene treatment on browning and quality of fresh-cut lotus (Nelumbo nucifera Gaertn.) root slices. Postharvest Biol. Technol. 2022, 185, 111782. [Google Scholar] [CrossRef]
- Liu, X.S.; Lai, H.; Yan, Z.L.; Zhang, Y.; Feng, Y.L.; Yu, X. Effect of chitosan-based compound natural preservative on preserving quality of fresh-cut lotus root slices during refrigeration. J. Hubei Norm. Univ. Nat. Sci. 2022, 10, 27–34. [Google Scholar]
- Li, J.S.; Bai, J.; Li, S.Y.; Zhu, Z.Z.; Yi, Y.; Wang, H.X.; Lamikanra, O. Effect of lactic acid bacteria on the postharvest properties of fresh lotus root. Postharvest Biol. Technol. 2020, 160, 110983. [Google Scholar] [CrossRef]
- Gao, H.; Sun, J.L.; Gao, Y.J.; Nan, H.J.; Liu, Q. Effect of modified atmosphere packaging on preservation of fresh-cut lotus roots. Food Sci. 2008, 10, 612–614. [Google Scholar]
- Zheng, H.; Miao, T.; Shi, J.; Tian, M.; Wang, L.; Geng, X.; Zhang, Q. Effect of Cold Plasma Treatment on the Quality of Fresh-Cut Hami Melons during Chilling Storage. Horticulturae 2024, 10, 735. [Google Scholar] [CrossRef]
- Li, J.H.; Shi, J.Y.; Huang, X.W.; Wang, T.T.; Zou, X.B.; Li, Z.H.; Zhang, D.; Zhang, W.; Xu, Y.W. Effects of pulsed electric field pretreatment on frying quality of fresh-cut lotus root slices. LWT 2020, 132, 109873. [Google Scholar] [CrossRef]
- Miñano, H.L.A.; Silva, A.C.D.S.; Souto, S.; Costa, E.J.X. Magnetic fields in food processing perspectives, applications and action models. Processes 2020, 8, 814. [Google Scholar] [CrossRef]
- Guo, L.N.; Azam, S.M.R.; Guo, Y.T.; Liu, D.D.; Ma, H.L. Germicidal efficacy of the pulsed magnetic field against pathogens and spoilage microorganisms in food processing: An overview. Food Control. 2022, 136, 108496. [Google Scholar] [CrossRef]
- Navone, L.; Moffitt, K.; Hansen, K.; Blinco, J.; Payne, A.; Speight, R. Closing the textile loop: Enzymatic fibre separation and recycling of wool/polyester fabric blends. Waste Manag. 2020, 102, 149–160. [Google Scholar] [CrossRef] [PubMed]
- Jha, P.K.; Xanthakis, E.; Jury, V.; Le-Bail, A. An overview on magnetic field and electric field interactions with ice crystallisation; application in the case of frozen food. Crystals 2017, 7, 229. [Google Scholar] [CrossRef]
- Jin, J.T.; Zheng, B.S.; Xiao, K.J. Effect of high-intensity pulsed magnetic field on in activation of srawberry peroxydase and sterilization of strawberry juice. Sci. Technol. Food Ind. 2010, 31, 99–101. [Google Scholar] [CrossRef]
- Filipic, J.; Kraigher, B.; Tepus, B.; Kokol, V.; Mandic-Mulec, I. Effects of low-density static magnetic fields on the growth and activities of wastewater bacteria Escherichia coli and Pseudomonas putida. Bioresour. Technol. 2012, 120, 225–232. [Google Scholar] [CrossRef] [PubMed]
- EI May, A.; Snoussi, S.; Ben Miloud, N.; Maatouk, I.; Abdelmelek, H.; Ben Aïssa, R.; Landoulsi, A. Effects of static magnetic field on cell growth, viability, and differential gene expression in Salmonella. Foodborne Pathog. Dis. 2009, 6, 547–552. [Google Scholar] [CrossRef]
- Du, M.T.; Hu, B.; Chen, B.; Li, J.G.; Li, K.; Bai, Y.H. Effect of Static Magnetic Field Assisted Superchilling on Quality of Pork. Mod. Food Sci. Technol. 2025, 2, 1–11. [Google Scholar] [CrossRef]
- Shahab, S.; Jun, T.K.; Eun, H.L.; Anil, K.; Gye, H.S. Fully transparent and flexible antibacterial packaging films based on regenerated cellulose extracted from ginger pulp. Ind. Crops Prod. 2023, 197, 116554. [Google Scholar] [CrossRef]
- Liu, L.; Sun, W.D.; Huang, L.H.; Zhang, Y.S.; Chen, S.; Zhang, Y.H. Physicochemical Properties and Structural Characteristics of a Chitosan/Pullulan/Polyvinyl Alcohol/Collagen Food Film. Mod. Food Sci. Technol. 2023, 39, 81–88. [Google Scholar] [CrossRef]
- Wu, P.; Yang, L.Q.; Sun, Y.; Wang, W.; Zhong, J.; Zhu, L.Q. Study on Compound Regent on Browning of Fresh-cut Lotus Roots. Food Res. Dev. 2015, 12, 114–116. [Google Scholar] [CrossRef]
- Luo, L.; Wang, S.M.; Xu, W.W.; Pan, W.J. Study on the quality change of fresh-cut lotus root during storage with ultrasound-heat treatment. Food Ferment. Ind. 2022, 48, 203–210. [Google Scholar] [CrossRef]
- Wang, X.H.; Qiao, Y.; Tie, J.Z.; Zhang, W.B.; Wei, B.H.; Liu, Z.C.; Yu, J.H.; Hu, L.L. Sheep Manure-Tail Vegetable-Corn Straw Co-Composting Improved the Yield and Quality of Mini Chinese Cabbage. Foods 2025, 14, 163. [Google Scholar] [CrossRef]
- Liu, Q.; Li, Z.; Ou, J.Q.; Huang, W.; Wang, Y.; Liu, Y. Optimization of Liquid Fermentation Medium of Morchella and Studies on Antioxidant Activity of Fermentation Products. J. Chin. Inst. Food Sci. Technol. 2023, 23, 41–50. [Google Scholar] [CrossRef]
- Ibrahim, H.; Uttu, A.J.; Sallau, M.S.; Iyun, O.R. Gas chromatography-mass spectrometry (GC-MS) analysis of ethyl acetate root bark extract of Strychnos innocua (Delile). Beni-Suef Univ. J. Basic Appl. Sci. 2021, 10, 65. [Google Scholar] [CrossRef]
- Jian, Y.B.; Xiao, X.X.; Qian, X.F.; Liang, M.; Chen, H.; Song, W. Preservative effects and mechanism of condensed tannins from Cercis chinensis Bunge leaves on fresh-cut lotus root. J. Food Sci. 2024, 90, 17638. [Google Scholar] [CrossRef]
- Li, C.A.; Li, Y.Q.; Shi, J.Z.; Yang, D.; Zhang, Y.B.; Fu, Y.B. Effect of Plasma Activated Water on Fresh-cut Lotus Root Freshness Preservation during Cold Storage. Packag. Eng. 2024, 45, 42–49. [Google Scholar] [CrossRef]
- You, K.Y.; Zhang, X.Y.; Wang, Y.; Nian, R.; Xiong, X.F.; Zhu, D.S.; Cao, X.H. Effect of ultrasound immersion pretreatment combined magnetic field assisted freezing on the quality of frozen blueberries. LWT 2024, 211, 116921. [Google Scholar] [CrossRef]
- Abie, S.M.; Münch, D.; Egelandsdal, B.; Bjerke, F.; Wergeland, I.; Martinsen, Ø.G. Combined 0.2 T static magnetic field and 20 kHz, 2 V/cm square wave electric field do not affect supercooling and freezing time of saline solution and meat samples. J. Food Eng. 2021, 311, 110710. [Google Scholar] [CrossRef]
- Li, W.Q.; Wang, Y.F.; Yu, Y.; Liu, J. Establishment and Optimization of the Hyperspectral Detection Model for Soluble Solids Content in Fortunella Margarita. Spectrosc. Spectr. Anal. 2025, 2, 492–500. [Google Scholar]
- Lei, Y.; Liu, Y.Z.; Zeng, W.F.; Deng, X.X. Physicochemical and molecular analysis of cell wall metabolism between two navel oranges (Citrus sinensis) with different mastication traits. J. Sci. Food Agric. 2010, 90, 1479–1484. [Google Scholar] [CrossRef]
- Sarmento, J.D.; de Morais, P.L.; Melo, N.J.D.A.; Pontes, F.M.; da Silva, A.R.; Amâncio, A.V.; de Brito, F.A.; da Costa Lucas, R.; Dias, N.d.S.; Brito, F.A.L.d. Postharvest behavior and bioactive compounds of red pitaya (Hylocereus polyrhizus) under room storage. Braz. J. Agric. Environ. Eng. 2025, 7, 289648. [Google Scholar]
- Zhang, X.Y.; Wang, J.H.; Sun, W.W.; Zhang, X.H.; Li, X.A.; Li, F.J. Optimization of Ultrasonic-Microwave Synergistic Extraction of Polyphenols from Apple Residue and Its Property Evaluation. Food Sci. Technol. 2022, 47, 185–191. [Google Scholar] [CrossRef]
- Rong, H.Z.; Tu, C.; Xie, D.Q.; Xu, Y.J.; Yu, Y.S.; Xiao, G.S.; Wen, J.; Zou, B.; Cheng, L.N. Effect of electrostatic field synergetic modified atmosphere packaging treatment on the quality of two varieties of litchi during cold storage. Sci. Technol. Food Ind. 2025, 2, 1–15. [Google Scholar] [CrossRef]
- Sulaiman, A.; Soo, M.J.; Farid, M.; Silva, F.V.M. Thermosonication for polyphenoloxidase inactivation in fruits: Modeling the ultrasound and thermal kinetics in pear, apple and strawberry purees at different temperatures. J. Food Eng. 2015, 165, 133–140. [Google Scholar] [CrossRef]
- Li, Y.L.; Cui, K.B.; Shi, L.; Zhu, Z.S.; Li, L.; Liu, Y.; Zhu, X. Effect of near freezing temperature storage on chilling injury and active oxygen metabolism of apricot fruit. Food Sci. 2020, 41, 177–183. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).