Physiological and Biochemical Adaptation of Common Garden Plants to Inorganic Nitrogen-Laden Fine Particulate Matter Stress
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. Experimental Design
2.3. Test Equipment
2.4. Sample Collection and Sample Analysis
2.5. Statistical Analyses
3. Results
3.1. Effects of Inorganic Nitrogen-Laden Fine PM on Antioxidant Systems in Iris germanica L. and Portulaca grandiflora Hook.
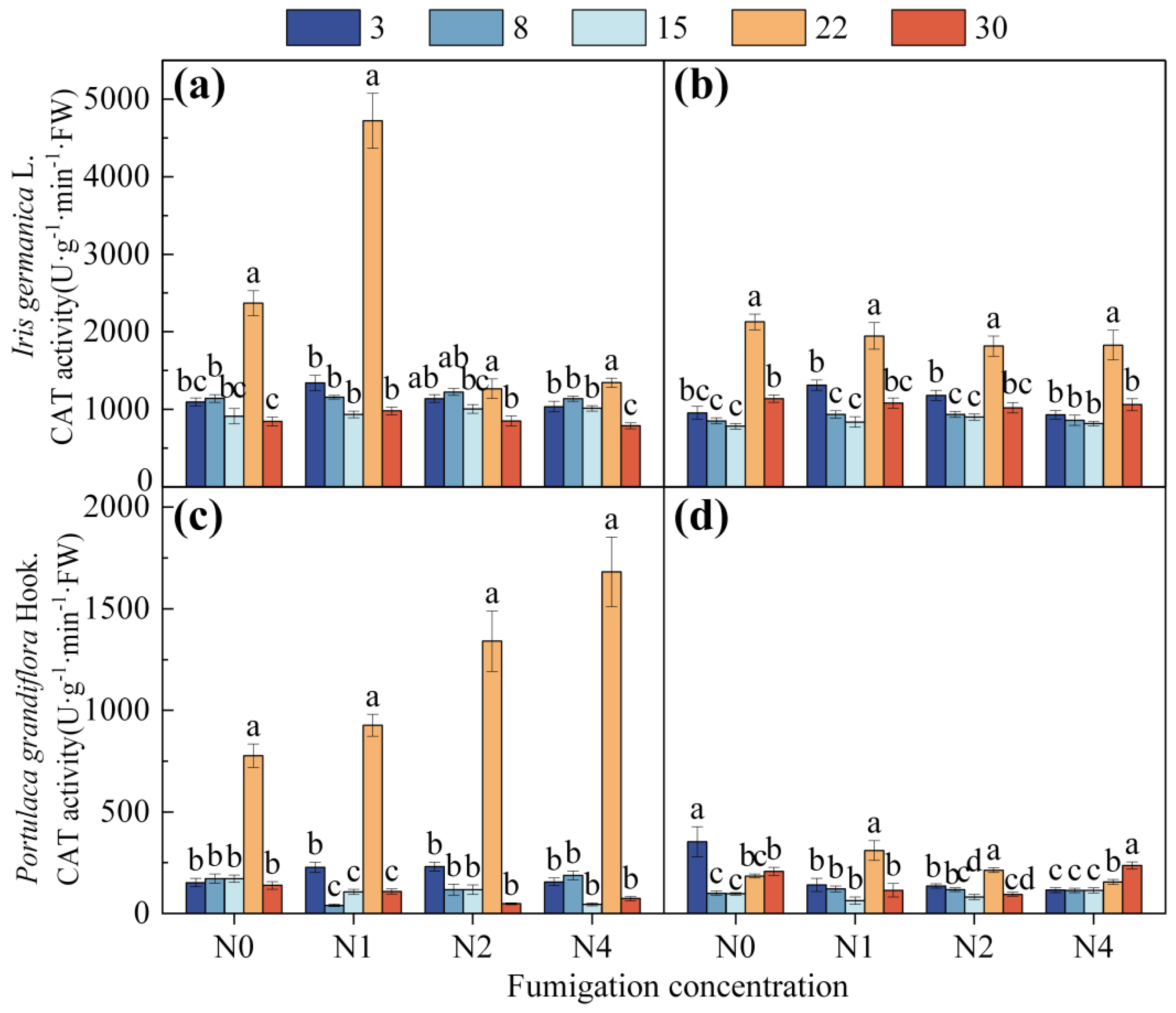
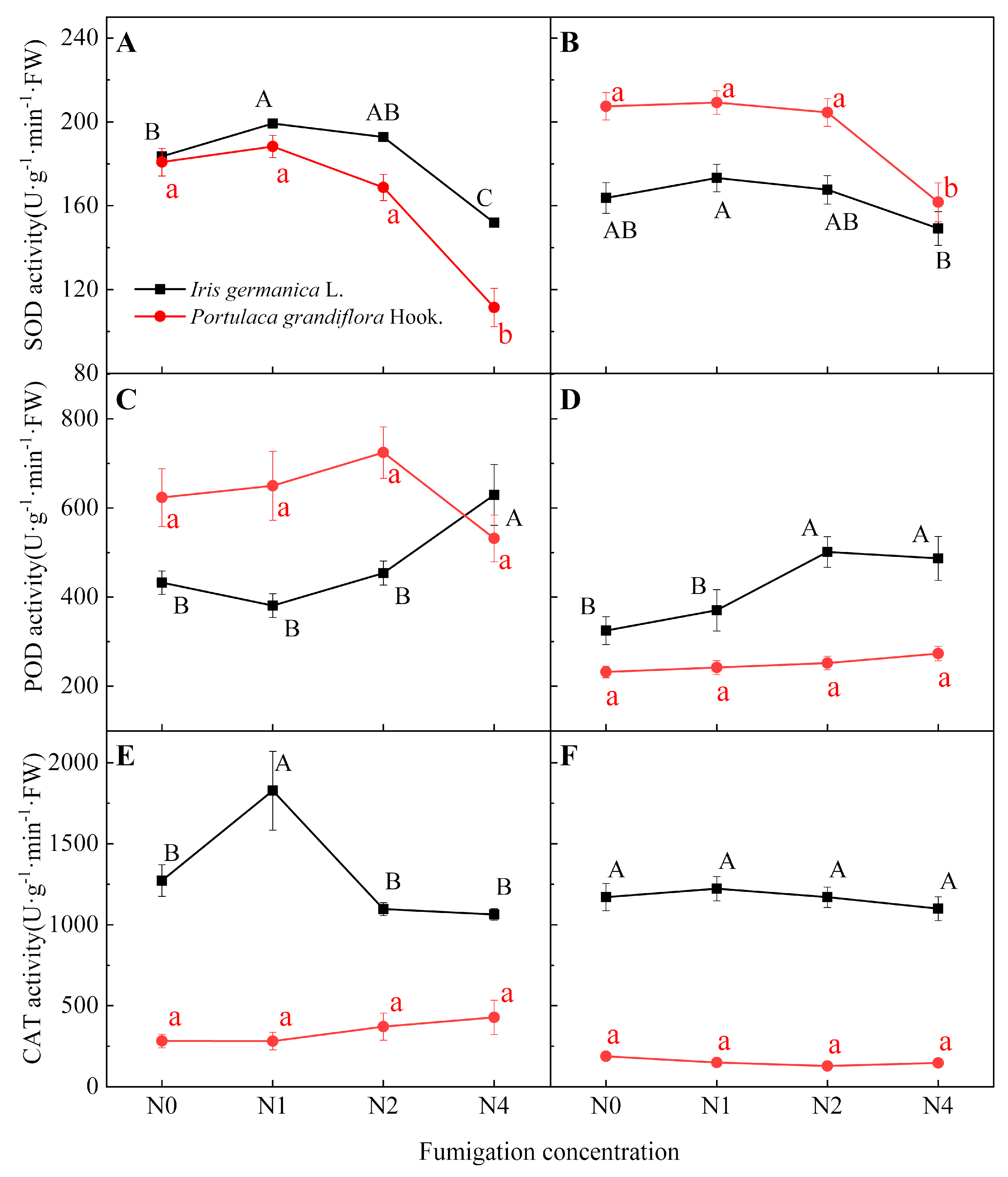
3.1.1. Effect of Fumigation Time on Antioxidant Enzyme Activity
3.1.2. Effect of Fumigation Concentration on Antioxidant Enzyme Activity
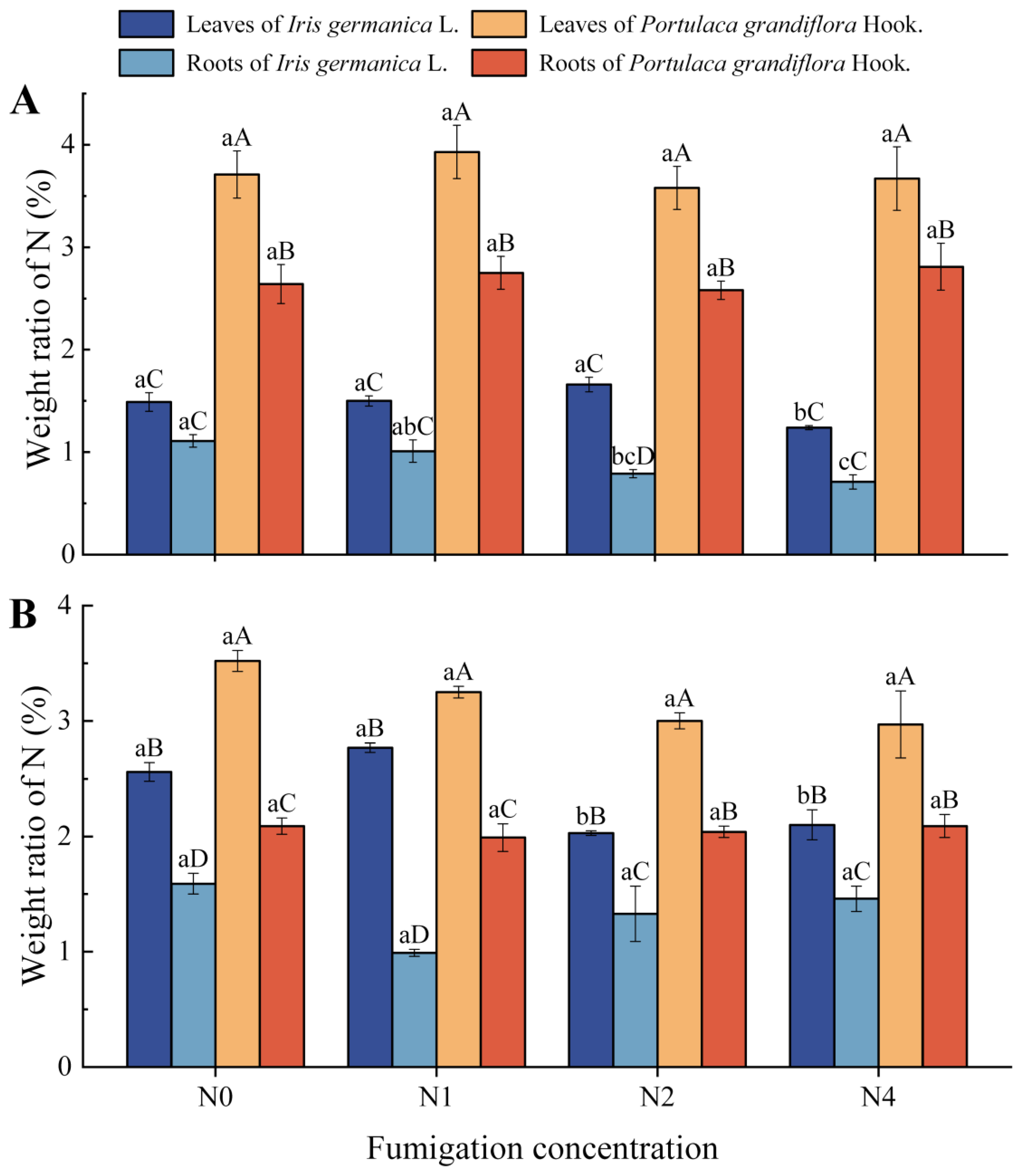
3.2. Effects of Inorganic Nitrogen-Laden PM on Nitrogen Storage Patterns in Iris germanica L. and Portulaca grandiflora Hook.
4. Discussion
4.1. Antioxidant Response to Inorganic Nitrogen PM2.5 in Iris germanica L. and Portulaca grandiflora Hook.
4.2. Nitrogen Storage in Iris germanica L. and Portulaca grandiflora Hook. Under Nitrogen-Laden PM2.5 Stress
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Ackerman, D.; Millet, D.B.; Chen, X. Global Estimates of Inorganic Nitrogen Deposition Across Four Decades. Glob. Biogeochem. Cycles 2019, 33, 100–107. [Google Scholar]
- Song, C.; Wang, K.; Song, Y.; Zhang, N. Effects of nitrogen deposition on soil respiration in regional forests of the central Yunnan Plateau. J. Nanjing For. Univ. (Nat. Sci. Ed.) 2024. [Google Scholar]
- Borer, E.T.; Stevens, C.J. Nitrogen deposition and climate: An integrated synthesis. Trends Ecol. Evol. 2022, 37, 541–552. [Google Scholar]
- Hong, W.Y. Meteorological variability and predictive forecasting of atmospheric particulate pollution. Sci. Rep. 2024, 14, 14. [Google Scholar]
- Zhao, C.; Peng, S.; Ruan, H.; Zhang, Y. Effects of nitrogen deposition on soil microbes. J. Nanjing For. Univ. (Nat. Sci. Ed.) 2015, 39, 149–155. [Google Scholar]
- Stevens, C.J. Nitrogen in the environment. Science 2019, 363, 578–580. [Google Scholar] [PubMed]
- Wu, L.; Yue, S.; Shi, Z.; Hu, W.; Chen, J.; Ren, H.; Deng, J.; Ren, L.; Fang, Y.; Yan, H.; et al. Source forensics of inorganic and organic nitrogen using δ15N for tropospheric aerosols over Mt. Tai. npj Clim. Atmos. Sci. 2021, 4, 8. [Google Scholar]
- Anas, M.; Liao, F.; Verma, K.K.; Sarwar, M.A.; Mahmood, A.; Chen, Z.; Li, Q.; Zeng, X.; Liu, Y.; Li, Y. Fate of nitrogen in agriculture and environment: Agronomic, eco-physiological and molecular approaches to improve nitrogen use efficiency. Biol. Res. 2020, 53, 47. [Google Scholar]
- Noor, H.; Yan, Z.; Sun, P.; Zhang, L.; Ding, P.; Li, L.; Ren, A.; Sun, M.; Gao, Z. Effects of Nitrogen on Photosynthetic Productivity and Yield Quality of Wheat (Triticum aestivum L.). Agronomy 2023, 13, 1448. [Google Scholar] [CrossRef]
- Cui, X.; Yue, P.; Wu, W.; Gong, Y.; Li, K.; Misselbrook, T.; Goulding, K.; Liu, X. The Growth and N Retention of Two Annual Desert Plants Varied Under Different Nitrogen Deposition Rates. Front. Plant Sci. 2019, 10, 356. [Google Scholar]
- Cun, Z.; Zhang, J.-Y.; Wu, H.-M.; Zhang, L.; Chen, J.-W. High nitrogen inhibits photosynthetic performance in a shade-tolerant and N-sensitive species Panax notoginseng. Photosynth. Res. 2021, 147, 283–300. [Google Scholar] [CrossRef]
- Yuan, J.; Peng, M.; Tang, G.; Wang, Y. Fine root production, mortality, and turnover in response to simulated nitrogen deposition in the subtropical Abies georgei (Orr) forest. Sci. Total Environ. 2024, 923, 171404. [Google Scholar] [CrossRef] [PubMed]
- Khan, A.; Sun, J.; Zarif, N.; Khan, K.; Jamil, M.A.; Yang, L.; Clothier, B.; Rewald, B. Effects of Increased N Deposition on Leaf Functional Traits of Four Contrasting Tree Species in Northeast China. Plants 2020, 9, 1231. [Google Scholar] [CrossRef]
- Zhang, L.; He, X. Nitrogen Utilization Mechanism in C3 and C4 Plants. Chin. Bull. Bot. 2020, 55, 228–239. [Google Scholar]
- Niu, S.; Liu, W.; Wan, S. Different growth responses of C3 and C4 grasses to seasonal water and nitrogen regimes and competition in a pot experiment. J. Exp. Bot. 2008, 59, 1431–1439. [Google Scholar] [CrossRef]
- Lin, G.; Chen, H.; Chen, B.; Yang, Y. Characterization of temporal PM2.5, nitrate, and sulfate using deep learning techniques. Atmos. Pollut. Res. 2022, 13, 101260. [Google Scholar] [CrossRef]
- Xie, M.; Feng, W.; He, S.; Wang, Q.G. Seasonal variations, temperature dependence, and sources of size-resolved PM components in Nanjing, east China. J. Environ. Sci. 2022, 121, 175–186. [Google Scholar] [CrossRef]
- Luo, W.; Wang, X.; Sardans, J.; Wang, Z.; Dijkstra, F.A.; Lü, X.-T.; Peñuelas, J.; Han, X. Higher capability of C3 than C4 plants to use nitrogen inferred from nitrogen stable isotopes along an aridity gradient. Plant Soil 2018, 428, 93–103. [Google Scholar] [CrossRef]
- Xu, X.; Xia, J.; Gao, Y.; Zheng, W. Additional focus on particulate matter wash-off events from leaves is required: A review of studies of urban plants used to reduce airborne particulate matter pollution. Urban For. Urban Green. 2020, 48, 126559. [Google Scholar] [CrossRef]
- Lu, T.; Lin, X.; Chen, J.; Huang, D.; Li, M. Atmospheric particle retention capacity and photosynthetic responses of three common greening plant species under different pollution levels in Hangzhou. Glob. Ecol. Conserv. 2019, 20, e00783. [Google Scholar] [CrossRef]
- Nawaz, M.F.; Rashid, M.H.U.; Saeed-Ur-Rehman, M.; Gul, S.; Farooq, T.H.; Sabir, M.A.; Iftikhar, J.; Abdelsalam, N.R.; Dessoky, E.S.; Alotaibi, S.S. Effect of Dust Types on the Eco-Physiological Response of Three Tree Species Seedlings: Eucalyptus camaldulensis, Conocarpus erectus and Bombax ceiba. Atmosphere 2022, 13, 1010. [Google Scholar] [CrossRef]
- Singh, S.; Pandey, B.; Roy, L.B.; Shekhar, S.; Singh, R.K. Tree responses to foliar dust deposition and gradient of air pollution around opencast coal mines of Jharia coalfield, India: Gas exchange, antioxidative potential and tolerance level. Environ. Sci. Pollut. Res. 2020, 28, 8637–8651. [Google Scholar] [CrossRef] [PubMed]
- Peng, L.; Yang, Z.; Liu, M.; Huang, M.; Zhang, C.; Yongli, D. Relationship between Net Photosynthetic Rate of Green Plants and Air Pollutants. Ecol. Environ. Sci. 2015, 24, 1166–1170. [Google Scholar] [CrossRef]
- He, C.; Zhang, Z.F.; Wang, Q.; Zhang, Y.P.; Wei, C.H.; Zhang, L.; Li, Z.; Yu, H.Q.; Chang, C.Y.; Zhang, Y.L. Evaluation of air pollution tolerance index of urban roadside young leaf and the correlation with its capturing capacity for water-insoluble fine particulate matters. Air Qual. Atmos. Health 2024, 17, 2675–2691. [Google Scholar] [CrossRef]
- Mo, L.; Ma, Z.Y.; Xu, Y.S.; Sun, F.B.; Lun, X.X.; Liu, X.H.; Chen, J.G.; Yu, X.X. Assessing the Capacity of Plant Species to Accumulate Particulate Matter in Beijing, China. PLoS ONE 2015, 10, e0140664. [Google Scholar] [CrossRef]
- Singh, S.K.; Singh, R.K.; Singh, R.S.; Pal, D.; Singh, K.K.; Singh, P.K. Screening potential plant species for arresting particulates in Jharia coalfield, India. Sustain. Environ. Res. 2019, 29, 37. [Google Scholar] [CrossRef]
- Yuan, Y.; Sun, Y.; Zhao, Y.; Liu, C.; Chen, X.; Li, F.; Bao, J. Identification of Floral Scent Profiles in Bearded Irises. Molecules 2019, 24, 1773. [Google Scholar] [CrossRef]
- Budiawan, A.; Purwanto, A.; Puradewa, L.; Cahyani, E.D.; Purwaningsih, C.E. Wound healing activity and flavonoid contents of purslane (Portulaca grandiflora) of various varieties. RSC Adv. 2023, 13, 9871–9877. [Google Scholar] [CrossRef]
- Yousefsani, B.S.; Boozari, M.; Shirani, K.; Jamshidi, A.; Dadmehr, M. A review on phytochemical and therapeutic potential of Iris germanica. J. Pharm. Pharmacol. 2021, 73, 611–625. [Google Scholar] [CrossRef]
- Zhang, J.W.; Huang, D.Z.; Zhao, X.J.; Zhang, M. Evaluation of drought resistance and transcriptome analysis for the identification of drought-responsive genes in. Sci. Rep. 2021, 11, 16308. [Google Scholar] [CrossRef]
- Borsai, O.; Hassan, M.A.; Negrușier, C.; Raigón, M.D.; Boscaiu, M.; Sestraș, R.E.; Vicente, O. Responses to Salt Stress in Portulaca: Insight into Its Tolerance Mechanisms. Plants 2020, 9, 1660. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.P.; Li, T.Y.; Cheng, Y.F.; Wang, F.J.; Zhao, X.J. Morphological and metabolic responses of four cultivars under salinity stress. Sci. Hortic. 2021, 281, 109960. [Google Scholar]
- Sun, L.Y.; Li, B.; Ma, Y.C.; Wang, J.Y.; Xiong, Z.Q. Year-Round Atmospheric Wet and Dry Deposition of Nitrogen and Phosphorus on Water and Land Surfaces in Nanjing, China. Water Environ. Res. 2013, 85, 514–521. [Google Scholar]
- JI, Y.; Zhang, J.; Song, J. Analysis of PM2.5 Pollution in Jiangsu Province and Determination of Building Outdoor PM2.5 Design Concentration. J. Change Univ. (Nat. Sci. Ed.) 2018, 30, 53–58. [Google Scholar]
- Ge, Z.; Ma, Y.; Xing, W.; Wu, Y.; Peng, S.; Mao, L.; Miao, Z. Inorganic Nitrogen-Containing Aerosol Deposition Caused “Excessive Photosynthesis” of Herbs, Resulting in Increased Nitrogen Demand. Plants 2022, 11, 2225. [Google Scholar] [CrossRef]
- Giannopolitis, C.N.; Ries, S.K. Superoxide dismutases: I. Occurrence in higher plants. Plant Physiol. 1977, 59, 309–314. [Google Scholar] [PubMed]
- Zheng, X.; Van Huystee, R. Peroxidase-regulated elongation of segments from peanut hypocotyls. Plant Sci. 1992, 81, 47–56. [Google Scholar]
- Change, B.; Maehly, A. Assay of catalases and peroxidase. Methods Enzym. 1955, 2, 764–775. [Google Scholar]
- Mauchly, J.W. Significance Test for Sphericity of a Normal n-Variate Distribution. Ann. Math. Stat. 1940, 11, 204–209. [Google Scholar] [CrossRef]
- Nadarajah, K.K. ROS Homeostasis in Abiotic Stress Tolerance in Plants. Int. J. Mol. Sci. 2020, 21, 5208. [Google Scholar] [CrossRef]
- Lal, M.K.; Tiwari, R.K.; Altaf, M.A.; Kumar, A.; Kumar, R. Abiotic and biotic stress in horticultural crops: Insight into recent advances in the underlying tolerance mechanism. Front. Plant Sci. 2023, 14, 1212982. [Google Scholar]
- He, A.; Dean, J.M.; Lodhi, I.J. Peroxisomes as cellular adaptors to metabolic and environmental stress. Trends Cell Biol. 2021, 31, 656–670. [Google Scholar] [PubMed]
- García, G.; Clemente-Moreno, M.J.; Díaz-Vivancos, P.; García, M.; Hernández, J.A. The Apoplastic and Symplastic Antioxidant System in Onion: Response to Long-Term Salt Stress. Antioxidants 2020, 9, 67. [Google Scholar] [CrossRef]
- Ramzan, M.; Gillani, M.; Shah, A.A.; Shah, A.N.; Kauser, N.; Jamil, M.; Ahmad, R.T.; Ullah, S. Triticum aestivum: Antioxidant gene profiling and morpho-physiological studies under salt stress. Mol. Biol. Rep. 2023, 50, 2569–2580. [Google Scholar]
- Verma, D.; Kaushal, N.; Balhara, R.; Singh, K. Genome-wide analysis of Catalase gene family reveal insights into abiotic stress response mechanism in Brassica juncea and B. rapa. Plant Sci. 2023, 330, 111620. [Google Scholar] [CrossRef]
- Trivedi, V.L.; Nautiyal, M.C.; Sati, J.; Attri, D.C. Antioxidant enzyme activities in male and female plants of Hippophae salicifolia D. Don in different pheno-phases. Acta Physiol. Plant. 2020, 42, 64. [Google Scholar]
- Yu, L.; Song, M.; Xia, Z.; Korpelainen, H.; Niinemets, Ü.; Li, C. Elevated temperature differently affects growth, photosynthetic capacity, nutrient absorption and leaf ultrastructure of Abies faxoniana and Picea purpurea under intra- and interspecific competition. Tree Physiol. 2019, 39, 1342–1357. [Google Scholar]
- Ye, W.; Xiong, D.; Yang, Z.; Zhang, Q.; Liu, X.; Gao, Y.; Xu, C.; Yang, Y. Effects of atmospheric warming on physiological characteristics of leaves and fine roots of Cunninghamia lanceolata saplings. Acta Ecol. Sin. 2020, 40, 7681–7689. [Google Scholar]
- Sonmez, M.C.; Ozgur, R.; Uzilday, B.; Turkan, I.; Ganie, S.A. Redox regulation in C3 and C4 plants during climate change and its implications on food security. Food Energy Secur. 2023, 12, e387. [Google Scholar]
- Burlacot, A.; Peltier, G. Energy crosstalk between photosynthesis and the algal CO2-concentrating mechanisms. Trends Plant Sci. 2023, 28, 795–807. [Google Scholar]
- Epstein, H.E.; Gill, R.A.; Paruelo, J.M.; Lauenroth, W.K.; Jia, G.J.; Burke, I.C. The relative abundance of three plant functional types in temperate grasslands and shrublands of North and South America: Effects of projected climate change. J. Biogeogr. 2002, 29, 875–888. [Google Scholar] [CrossRef]
- Nayyar, H.; Gupta, D. Differential sensitivity of C3 and C4 plants to water deficit stress: Association with oxidative stress and antioxidants. Environ. Exp. Bot. 2006, 58, 106–113. [Google Scholar] [CrossRef]
- Uzilday, B.; Turkan, I.; Sekmen, A.H.; Ozgur, R.; Karakaya, H.C. Comparison of ROS formation and antioxidant enzymes in (C3) and (C4) under drought stress. Plant Sci. 2012, 182, 59–70. [Google Scholar] [CrossRef]
- Gao, P.; Xue, P.; Dong, J.; Zhang, X.; Sun, H.; Geng, L.; Luo, S.; Zhao, J.; Liu, W. Contribution of PM2.5-Pb in atmospheric fallout to Pb accumulation in Chinese cabbage leaves via stomata. J. Hazard. Mater. 2021, 407, 124356. [Google Scholar] [CrossRef]
- Zhai, S.; Jacob, D.J.; Wang, X.; Liu, Z.; Wen, T.; Shah, V.; Li, K.; Moch, J.M.; Bates, K.H.; Song, S.; et al. Control of particulate nitrate air pollution in China. Nat. Geosci. 2021, 14, 389–395. [Google Scholar] [CrossRef]
- Hu, Y.; Peuke, A.D.; Zhao, X.; Yan, J.; Li, C. Effects of simulated atmospheric nitrogen deposition on foliar chemistry and physiology of hybrid poplar seedlings. Plant Physiol. Biochem. 2019, 143, 94–108. [Google Scholar] [CrossRef] [PubMed]
- He, X.; Chi, Q.; Cai, Z.; Cheng, Y.; Zhang, J.; Müller, C. 15N tracing studies including plant N uptake processes provide new insights on gross N transformations in soil-plant systems. Soil Biol. Biochem. 2020, 141, 107666. [Google Scholar] [CrossRef]
- Yuan, Z.Y.; Chen, H.Y.H. Negative effects of fertilization on plant nutrient resorption. Ecology 2015, 96, 373–380. [Google Scholar] [CrossRef]
- Abid, M.; Tian, Z.; Ata-Ul-Karim, S.T.; Cui, Y.; Liu, Y.; Zahoor, R.; Jiang, D.; Dai, T. Nitrogen Nutrition Improves the Potential of Wheat (Triticum aestivum L.) to Alleviate the Effects of Drought Stress during Vegetative Growth Periods. Front. Plant Sci. 2016, 7, 981. [Google Scholar] [CrossRef]
- Wei, S.; Wang, X.; Shi, D.; Li, Y.; Zhang, J.; Liu, P.; Zhao, B.; Dong, S. The mechanisms of low nitrogen induced weakened photosynthesis in summer maize (Zea mays L.) under field conditions. Plant Physiol. Biochem. 2016, 105, 118–128. [Google Scholar] [CrossRef]
- Liu, N.; Feng, Y.; Wei, L.; Liu, F. Responses of plant carbon and nitrogen assimilations to nitrogen addition in a subtropical forest: Canopy addition vs. understory addition. Ecotoxicol. Environ. Saf. 2023, 266, 115545. [Google Scholar] [CrossRef]
- Li, Y.; Liu, H.; Huang, G. The Effect of Nitrogen Rates on Yields and Nitrogen Use Efficiencies during Four Years of Wheat–Maize Rotation Cropping Seasons. Agron. J. 2016, 108, 2076–2088. [Google Scholar]
- Li, Z.; Wen, X.; Hu, C.; Li, X.; Li, S.; Zhang, X.; Hu, B. Regional simulation of nitrate leaching potential from winter wheat-summer maize rotation croplands on the North China Plain using the NLEAP-GIS model. Agric. Ecosyst. Environ. 2020, 294, 106861. [Google Scholar]
- Mancuso, G.; Bencresciuto, G.F.; Lavrnić, S.; Toscano, A. Diffuse Water Pollution from Agriculture: A Review of Nature-Based Solutions for Nitrogen Removal and Recovery. Water 2021, 13, 1893. [Google Scholar] [CrossRef]
- Su, W.; Kamran, M.; Xie, J.; Meng, X.; Han, Q.; Liu, T.; Han, J. Shoot and root traits of summer maize hybrid varieties with higher grain yields and higher nitrogen use efficiency at low nitrogen application rates. PeerJ 2019, 7, e7294. [Google Scholar] [PubMed]
- Green, T.H.; Mitchell, R.J. Effects of nitrogen on the response of loblolly pine to water stress I. Photosynthesis and stomatal conductance. New Phytol. 1992, 122, 627–633. [Google Scholar]
- Shen, H.; Dong, S.K.; DiTommaso, A.; Li, S.; Xiao, J.N.; Yang, M.Y.; Zhang, J.; Gao, X.X.; Xu, Y.D.; Zhi, Y.L.; et al. Eco-physiological processes are more sensitive to simulated N deposition in leguminous forbs than non-leguminous forbs in an alpine meadow of the Qinghai-Tibetan Plateau. Sci. Total Environ. 2020, 744, 140612. [Google Scholar] [PubMed]
- Ion, A.C.; Vermeylen, R.; Kourtchev, I.; Cafmeyer, J.; Chi, X.; Gelencsér, A.; Maenhaut, W.; Claeys, M. Polar organic compounds in rural PM2.5 aerosols from K-puszta, Hungary, during a 2003 summer field campaign: Sources and diel variations. Atmos. Chem. Phys. 2005, 5, 1805–1814. [Google Scholar]
- Lu, J.; Zhong, Y. Effects of Exogenous GA3 and SA on Salt Resistance of Iris germanica. Acta Agric. Jiangxi 2011, 23, 49–56. [Google Scholar]
- Reeves, G.; Grangé-Guermente, M.J.; Hibberd, J.M. Regulatory gateways for cell-specific gene expression in C4 leaves with Kranz anatomy. J. Exp. Bot. 2016, 68, 107–116. [Google Scholar]
- Majeran, W.; Cai, Y.; Sun, Q.; van Wijk, K.J. Functional Differentiation of Bundle Sheath and Mesophyll Maize Chloroplasts Determined by Comparative Proteomics. Plant Cell 2005, 17, 3111–3140. [Google Scholar] [CrossRef] [PubMed]
- Sage, R.F.; Monson, R.K.; Ehleringer, J.R.; Adachi, S.; Pearcy, R.W. Some like it hot: The physiological ecology of C4 plant evolution. Oecologia 2018, 187, 941–966. [Google Scholar] [CrossRef] [PubMed]
- Togawa-Urakoshi, Y.; Ueno, O. Photosynthetic nitrogen- and water-use efficiencies in C3 and C4 subtype grasses grown under two nitrogen supply levels. Plant Prod. Sci. 2021, 25, 183–194. [Google Scholar] [CrossRef]



| Indexes | SOD | POD | CAT | |||
|---|---|---|---|---|---|---|
| F | P | F | P | F | P | |
| Stage (S) | 13.496 | *** | 389.110 | *** | 101.999 | *** |
| Plant (P) | 9.675 | ** | 0.343 | NS | 3545.908 | *** |
| Concentration (C) | 115.293 | *** | 15.283 | *** | 26.914 | *** |
| Time (T) | 104.232 | *** | 185.639 | *** | 442.188 | *** |
| S × P | 161.042 | *** | 221.821 | *** | 1.303 | NS |
| S × C | 7.706 | *** | 0.961 | NS | 16.166 | *** |
| S × T | 41.691 | *** | 151.212 | *** | 78.761 | *** |
| P × C | 12.998 | *** | 21.516 | *** | 45.363 | *** |
| P × T | 42.304 | *** | 59.682 | *** | 54.925 | *** |
| C × T | 5.098 | *** | 5.749 | *** | 19.204 | *** |
| S × P × C | 0.695 | NS | 15.087 | *** | 31.297 | *** |
| S × P × T | 23.368 | *** | 23.783 | *** | 14.019 | *** |
| S × C × T | 4.969 | *** | 5.499 | *** | 15.425 | *** |
| P × C × T | 4.846 | *** | 10.407 | *** | 32.018 | *** |
| S × P × C × T | 2.351 | ** | 6.046 | *** | 33.128 | *** |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xiao, K.; Wang, Y.; Wang, R.; Hu, Z.; Peng, S.; Miao, Z.; Ge, Z. Physiological and Biochemical Adaptation of Common Garden Plants to Inorganic Nitrogen-Laden Fine Particulate Matter Stress. Horticulturae 2025, 11, 337. https://doi.org/10.3390/horticulturae11030337
Xiao K, Wang Y, Wang R, Hu Z, Peng S, Miao Z, Ge Z. Physiological and Biochemical Adaptation of Common Garden Plants to Inorganic Nitrogen-Laden Fine Particulate Matter Stress. Horticulturae. 2025; 11(3):337. https://doi.org/10.3390/horticulturae11030337
Chicago/Turabian StyleXiao, Keqin, Yiying Wang, Rongkang Wang, Zhanpeng Hu, Sili Peng, Zimei Miao, and Zhiwei Ge. 2025. "Physiological and Biochemical Adaptation of Common Garden Plants to Inorganic Nitrogen-Laden Fine Particulate Matter Stress" Horticulturae 11, no. 3: 337. https://doi.org/10.3390/horticulturae11030337
APA StyleXiao, K., Wang, Y., Wang, R., Hu, Z., Peng, S., Miao, Z., & Ge, Z. (2025). Physiological and Biochemical Adaptation of Common Garden Plants to Inorganic Nitrogen-Laden Fine Particulate Matter Stress. Horticulturae, 11(3), 337. https://doi.org/10.3390/horticulturae11030337






