Ultrasound in Plant Life and Its Application Perspectives in Horticulture and Agriculture
Abstract
1. Introduction
2. Sound Perception in Plants
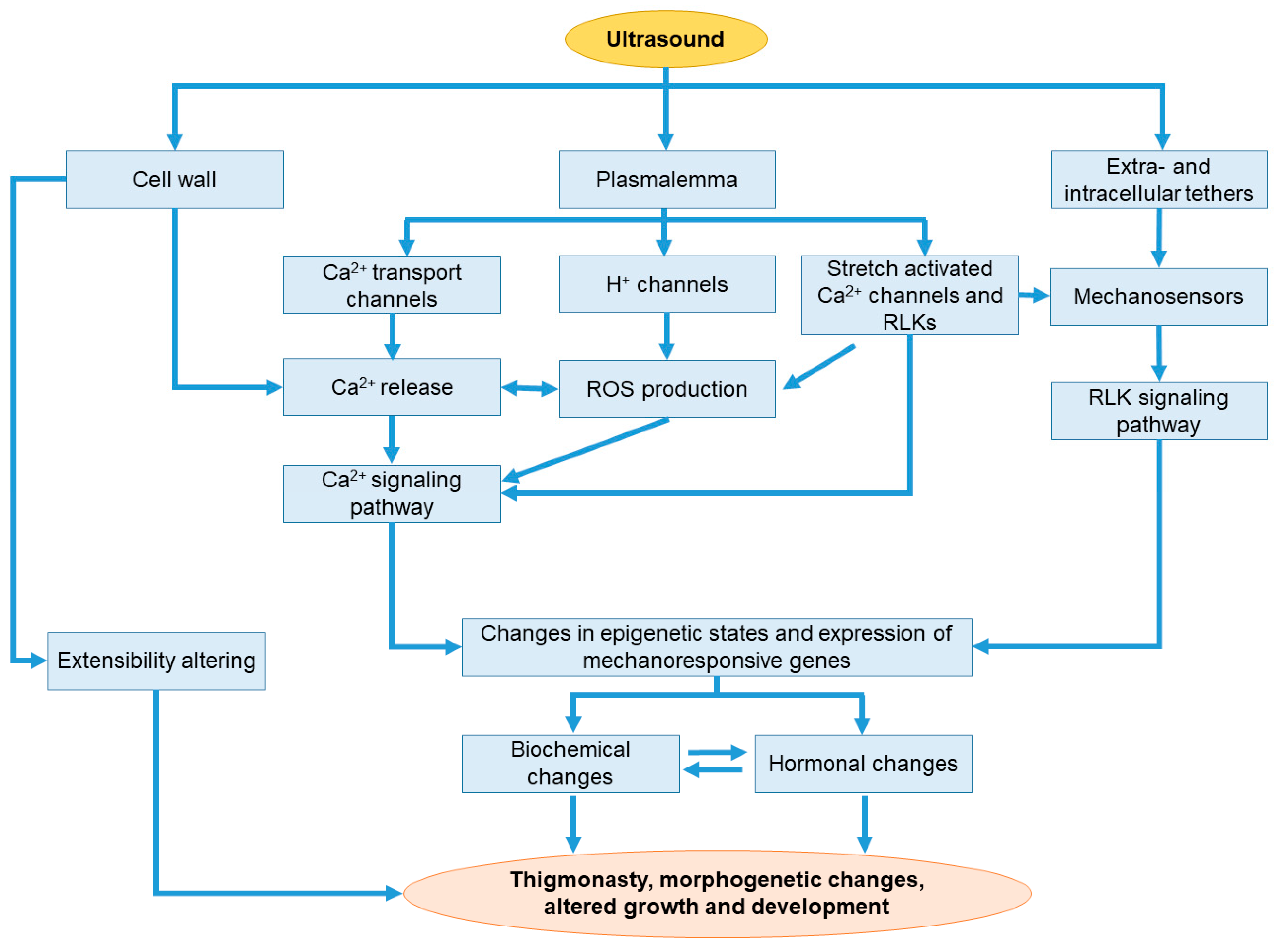
3. The Effect of Ultrasound on the Molecular and Physiological Functioning, Growth, and Development of the Plant
4. Ultrasound Emission by Plants
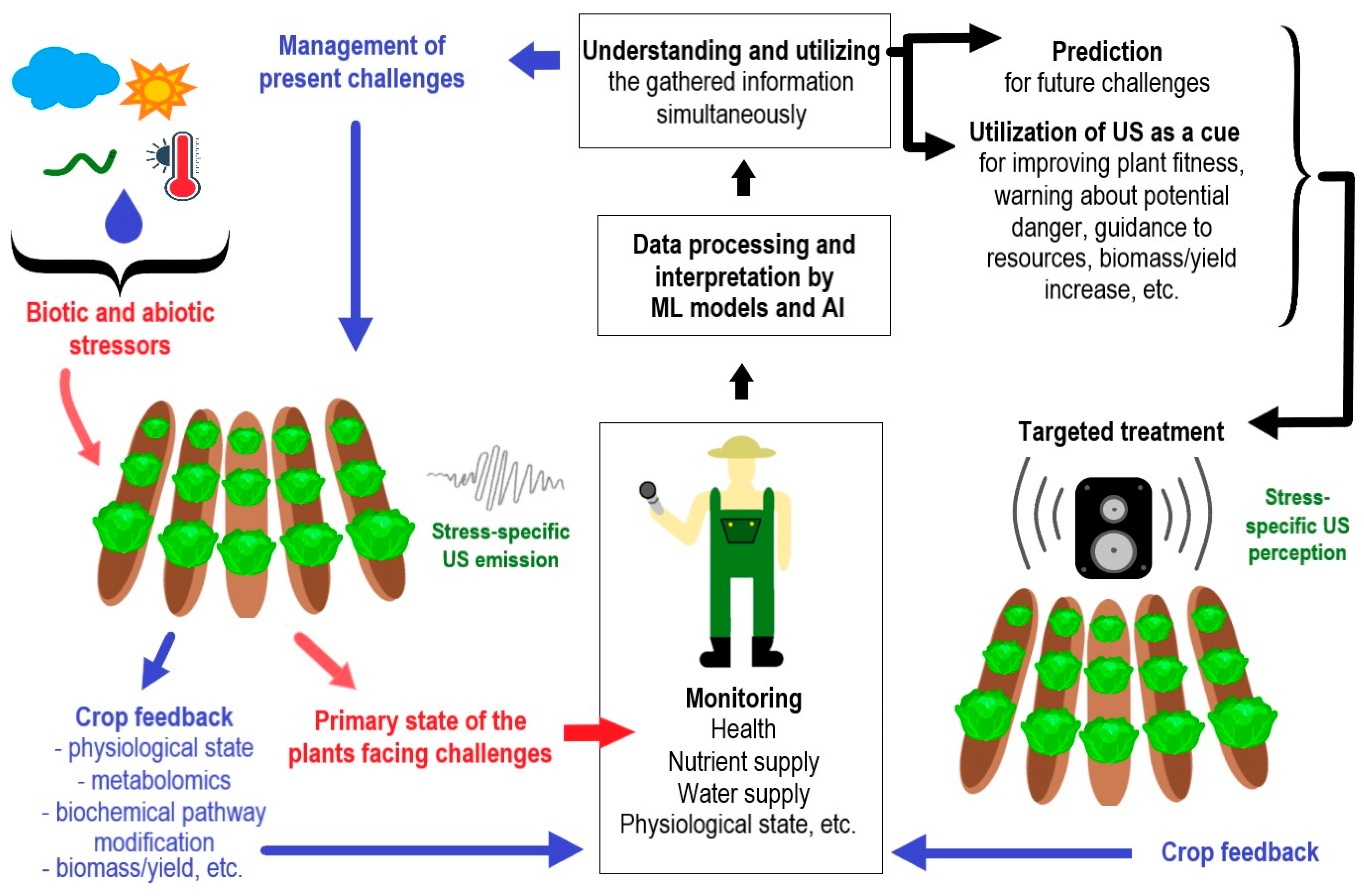
5. The Plant’s Benefit from Reflecting, Sensing, and Emitting Ultrasound
6. Perspectives on Ultrasonication and Ultrasound Detection in Horticulture and Crop Production
6.1. Use of Ultrasonication in Plant Tissue Culture for Enhancing In Vitro Growth and Development
6.2. Sonication-Assisted Agrobacterium-Mediated Transformation (SAAT)—A Methodological Application of Plant Ultrasonication in Gene Transformation
6.3. Advances and Perspectives on Ultrasound Applications in Agricultural and Horticultural Technologies
7. Concluding Remarks
Author Contributions
Funding
Conflicts of Interest
References
- Schneider, H.M. Characterization, costs, cues and future perspectives of phenotypic plasticity. Ann. Bot. 2022, 130, 131–148. [Google Scholar] [CrossRef] [PubMed]
- Demey, M.L.; Mishra, R.C.; Van Der Straeten, D. Sound perception in plants: From ecological significance to molecular understanding. Trends Plant Sci. 2023, 28, 825–840. [Google Scholar] [CrossRef] [PubMed]
- Khait, I.; Obolski, U.; Yovel, Y.; Hadany, L. Sound perception in plants. Semin. Cell Dev. Biol. 2019, 92, 134–138. [Google Scholar] [CrossRef] [PubMed]
- Jung, J.; Kim, S.K.; Kim, J.Y.; Jeong, M.J.; Ryu, C.M. Beyond chemical triggers: Evidence for sound-evoked physiological reactions in plants. Front. Plant Sci. 2018, 9, 25. [Google Scholar] [CrossRef]
- Gorai, S.; Hazra, S. Plant acoustic frequency technology: Sound waves in crop improvement. Agric. Food E-Newsl. 2020, 2, 37–40. [Google Scholar]
- Khait, I.; Lewin-Epstein, O.; Sharon, R.; Saban, K.; Goldstein, R.; Anikster, Y.; Zeron, Y.; Agassy, C.; Nizan, S.; Gayl, S.; et al. Sounds emitted by plants under stress are airborne and informative. Cell 2023, 186, 1328–1336. [Google Scholar] [CrossRef]
- Hussain, M.; u Rahman, M.K.; Mishra, R.C.; Van Der Straeten, D. Plants can talk: A new era in plant acoustics. Trends Plant Sci. 2023, 28, 987–990. [Google Scholar] [CrossRef]
- Allievi, S.; Arru, L.; Forti, L. A tuning point in plant acoustics investigation. Plant Sign. Behav. 2021, 16, 1919836. [Google Scholar] [CrossRef]
- Bhandawat, A.; Jayaswall, K. Biological relevance of sound in plants. Environ. Exp. Bot. 2022, 200, 104919. [Google Scholar] [CrossRef]
- Del Stabile, F.; Marsili, V.; Forti, L.; Arru, L. Is There a Role for Sound in Plants? Plants 2022, 11, 2391. [Google Scholar] [CrossRef]
- Nardini, A.; Cochard, H.; Mayr, S. Talk is cheap: Rediscovering sounds made by plants. Trends Plant Sci. 2024, 29, 662–667. [Google Scholar] [CrossRef]
- Son, J.S.; Jang, S.; Mathevon, N.; Ryu, C.M. Is plant acoustic communication fact or fiction? New Phytol. 2024, 242, 1876–1880. [Google Scholar] [CrossRef]
- Dobránszki, J.; Agius, D.R.; Berger, M.M.J.; Moschou, P.N.; Gallusci, P.; Martinelli, F. Plant memory and communication of encounters. Trends Plant Sci. 2025, 30, 199–212. [Google Scholar] [CrossRef]
- Yoneda, A.; Ohtani, M.; Katagiri, D.; Hosokawa, Y.; Demura, T. Hechtian strands transmit cell wall integrity signals in plant cells. Plants 2020, 9, 604. [Google Scholar] [CrossRef]
- Jia, H.; Chen, S.; Wang, X.; Shi, C.; Liu, K.; Zhang, S.; Li, J. Copper oxide nanoparticles alter cellular morphology via disturbing the actin cytoskeleton dynamics in Arabidopsis roots. Nanotoxicology 2020, 14, 127–144. [Google Scholar] [CrossRef]
- Simon, C.; Caorsi, V.; Campillo, C.; Sykes, C. Interplay between membrane tension and the actin cytoskeleton determines shape changes. Phys. Biol. 2018, 15, 065004. [Google Scholar] [CrossRef]
- Matsumura, M.; Nomoto, M.; Itaya, T.; Aratani, Y.; Iwamoto, M.; Matsuura, T.; Hayashi, Y.; Kinoshita, T.; Mori, I.C.; Suzuki, T.; et al. Mechanosensory trichome cells evoke a mechanical stimuli–induced immune response in plants. Nat. Commun. 2022, 13, 1216. [Google Scholar] [CrossRef]
- Liu, S.; Jiao, J.; Lu, T.J.; Xu, F.; Pickard, B.G.; Genin, G.M. Arabidopsis leaf trichomes as acoustic antennae. Biophys. J. 2017, 113, 2068–2076. [Google Scholar] [CrossRef]
- Kim, J.Y.; Lee, H.J.; Kim, J.A.; Jeong, M.J. Sound waves promote Arabidopsis thaliana root growth by regulating root phytohormone content. Int. J. Mol. Sci. 2020, 22, 5739. [Google Scholar] [CrossRef]
- Rodrigo-Moreno, A.; Bazihizina, N.; Azzarello, E.; Masi, E.; Tran, D.; Bouteau, F.; Baluska, F.; Mancuso, S. Root phonotropism: Early signalling events following sound perception in Arabidopsis roots. Plant Sci. 2017, 264, 9–15. [Google Scholar] [CrossRef] [PubMed]
- Hamant, O.; Haswell, E.S. Life behind the wall: Sensing mechanical cues in plants. BMC Biol. 2017, 15, 59. [Google Scholar] [CrossRef]
- Vogler, H.; Santos-Fernandez, G.; Mecchia, M.A.; Grossniklaus, U. To preserve or to destroy, that is the question: The role of the cell wall integrity pathway in pollen tube growth. Curr. Opin. Plant Biol. 2019, 52, 131–139. [Google Scholar] [CrossRef]
- Fruleux, A.; Verger, S.; Boudaoud, A. Feeling stressed or strained? A biophysical model for cell wall mechanosensing in plants. Front. Plant Sci. 2019, 10, 757. [Google Scholar] [CrossRef]
- Voxeur, A.; Höfte, H. Pectin-derived immune elicitors in response to lignin modification in plants. Proc. Natl. Acad. Sci. USA 2020, 117, 4442–4444. [Google Scholar] [CrossRef]
- Kaur, A.; Taneja, M.; Tyagi, S.; Sharma, A.; Singh, K.; Upadhyay, S.K. Genome-wide characterization and expression analysis suggested diverse functions of the mechanosensitive channel of small conductance-like (MSL) genes in cereal crops. Sci. Rep. 2020, 10, 16583. [Google Scholar] [CrossRef]
- Basu, D.; Haswell, E.S. Plant mechanosensitive ion channels: An ocean of possibilities. Curr. Opin. Plant Biol. 2017, 40, 43–48. [Google Scholar] [CrossRef]
- Nishii, K.; Möller, M.; Iida, H. Mix and match: Patchwork domain evolution of the land plant-specific Ca2+-permeable mechanosensitive channel MCA. PLoS ONE 2021, 16, e0249735. [Google Scholar] [CrossRef]
- Suzuki, Y.; Yamamura, H.; Imaizumi, Y.; Clark, R.B.; Giles, W.R. K+ and Ca2+ channels regulate Ca2+ signaling in chondrocytes: An illustrated review. Cells 2020, 9, 1577. [Google Scholar] [CrossRef]
- Kefauver, J.M.; Ward, A.B.; Patapoutian, A. Discoveries in structure and physiology of mechanically activated ion channels. Nature 2020, 587, 567–576. [Google Scholar] [CrossRef]
- Du, F.; Jiao, Y. Mechanical control of plant morphogenesis: Concepts and progress. Curr. Opin. Plant Biol. 2020, 57, 16–23. [Google Scholar] [CrossRef]
- Meena, M.K.; Prajapati, R.; Krishna, D.; Divakaran, K.; Pandey, Y.; Reichelt, M.; Mathew, M.K.; Boland, W.; Mithöfer, A.; Vadassery, J. The Ca2+ channel CNGC19 regulates Arabidopsis defense against Spodoptera herbivory. Plant Cell 2019, 31, 1539–1562. [Google Scholar] [CrossRef]
- Gangwar, S.P.; Green, M.N.; Michard, E.; Simon, A.A.; Feijó, J.A.; Sobolevsky, A.I. Structure of the Arabidopsis glutamate receptor-like channel GLR3. 2 ligand-binding domain. Structure 2021, 29, 161–169. [Google Scholar] [CrossRef]
- Fang, X.; Liu, B.; Shao, Q.; Huang, X.; Li, J.; Luan, S.; He, K. AtPiezo plays an important role in root cap mechanotransduction. Int. J. Mol. Sci. 2021, 22, 467. [Google Scholar] [CrossRef]
- Zhang, Z.; Tateda, C.; Jiang, S.C.; Shrestha, J.; Jelenska, J.; Speed, D.J.; Greenberg, J.T. A suite of receptor-like kinases and a putative mechano-sensitive channel are involved in autoimmunity and plasma membrane–based defenses in Arabidopsis. Mol. Plant-Microb. Interact. 2017, 30, 150–160. [Google Scholar] [CrossRef]
- Ghosh, R.; Mishra, R.C.; Choi, B.; Kwon, Y.S.; Bae, D.W.; Park, S.C.; Jeong, M.-J.; Bae, H. Exposure to sound vibrations lead to transcriptomic, proteomic and hormonal changes in Arabidopsis. Sci. Rep. 2016, 6, 33370. [Google Scholar] [CrossRef]
- Peiter, E.; Maathuis, F.J.; Mills, L.N.; Knight, H.; Pelloux, J.; Hetherington, A.M.; Sanders, D. The vacuolar Ca2+-activated channel TPC1 regulates germination and stomatal movement. Nature 2005, 434, 404–408. [Google Scholar] [CrossRef]
- Pérez-Sancho, J.; Vanneste, S.; Lee, E.; McFarlane, H.E.; Esteban del Valle, A.; Valpuesta, V.; Friml, J.; Botella, M.A.; Rosado, A. The Arabidopsis synaptotagmin1 is enriched in endoplasmic reticulum-plasma membrane contact sites and confers cellular resistance to mechanical stresses. Plant Physiol. 2015, 168, 132–143. [Google Scholar] [CrossRef]
- Lee, C.P.; Maksaev, G.; Jensen, G.S.; Murcha, M.W.; Wilson, M.E.; Fricker, M.; Hell, R.; Haswell, E.S.; Harvey Millar, A.; Sweetlove, L.J. MSL 1 is a mechanosensitive ion channel that dissipates mitochondrial membrane potential and maintains redox homeostasis in mitochondria during abiotic stress. Plant J. 2016, 88, 809–825. [Google Scholar] [CrossRef]
- Ali, S.; Tyagi, A.; Park, S.; Bae, H. Understanding the Mechanobiology of Phytoacoustics Through Molecular Lens: Mechanisms and Future Perspectives. J. Adv. Res. 2024, 65, 47–72. [Google Scholar] [CrossRef]
- Wang, K.; Yang, Z.; Qing, D.; Ren, F.; Liu, S.; Zheng, Q.; Liu, J.; Zhang, W.; Dai, C.; Wu, M.; et al. Quantitative and functional posttranslational modification proteomics reveals that TREPH1 plays a role in plant touch-delayed bolting. Proc. Natl. Acad. Sci. USA 2018, 115, E10265–E10274. [Google Scholar] [CrossRef]
- Pesti-Asbóth, G.; Molnár-Bíróné, P.; Forgács, I.; Remenyik, J.; Dobránszki, J. Ultrasonication affects the melatonin and auxin levels and the antioxidant system in potato in vitro. Front. Plant Sci. 2022, 13, 979141. [Google Scholar] [CrossRef]
- Dobránszki, J.; Asbóth, G.; Homoki, D.; Bíró-Molnár, P.; Teixeira da Silva, J.A.; Remenyik, J. Ultrasonication of in vitro potato single node explants: Activation and recovery of antioxidant defence system and growth responses. Plant Phys. Biochem. 2017, 121, 153–160. [Google Scholar] [CrossRef]
- Kadota, Y.; Shirasu, K.; Zipfel, C. Regulation of the NADPH oxidase RBOHD during plant immunity. Plant Cell Physiol. 2015, 56, 1472–1480. [Google Scholar] [CrossRef]
- Ghosh, R.; Gururani, M.A.; Ponpandian, L.N.; Mishra, R.C.; Park, S.C.; Jeong, M.J.; Bae, H. Expression analysis of sound vibration-regulated genes by touch treatment in Arabidopsis. Front. Plant Sci. 2017, 8, 100. [Google Scholar] [CrossRef]
- Mishra, R.C.; Ghosh, R.; Bae, H. Plant acoustics: In the search of a sound mechanism for sound signaling in plants. J. Exp. Bot. 2016, 67, 4483–4494. [Google Scholar] [CrossRef]
- Besson-Bard, A.; Pugin, A.; Wendehenne, D. New insights into nitric oxide signaling in plants. Annu. Rev. Plant Biol. 2008, 59, 21–39. [Google Scholar] [CrossRef]
- Mishra, V.; Singh, V.P. Implication of nitric oxide and hydrogen sulfide signalling in alleviating arsenate stress in rice seedlings. Environ. Pollut. 2021, 291, 117958. [Google Scholar] [CrossRef]
- Ali, S.; Tyagi, A.; Bae, H. ROS interplay between plant growth and stress biology: Challenges and future perspectives. Plant Physiol. Biochem. 2023, 203, 108032. [Google Scholar] [CrossRef]
- Sparke, M.A.; Wünsche, J.N. Mechanosensing of plants. Hortic. Rev. 2020, 47, 43–83. [Google Scholar]
- Feng, W.; Kita, D.; Peaucelle, A.; Cartwright, H.N.; Doan, V.; Duan, Q.; Liu, M.-C.; Maman, J.; Steinhorst, L.; Schmitz-Thom, I.; et al. The FERONIA receptor kinase maintains cell-wall integrity during salt stress through Ca2+ signaling. Curr. Biol. 2018, 28, 666–675. [Google Scholar] [CrossRef]
- Behnami, S.; Bonetta, D. With an ear up against the wall: An update on Mechanoperception in Arabidopsis. Plants 2021, 10, 1587. [Google Scholar] [CrossRef]
- Wolf, S. Plant cell wall signalling and receptor-like kinases. Biochem. J. 2017, 474, 471–492. [Google Scholar] [CrossRef]
- Teixeira da Silva, J.; Dobránszki, J. Sonication (ultrasound) affects in vitro growth of hybrid Cymbidium. Bot. Lith. 2015, 20, 121–130. [Google Scholar] [CrossRef][Green Version]
- Farrokhzad, Y.; Rezaei, A. Effects of ultrasound, tryptophan and proline on embryogenesis and regeneration of grape (Vitis vinifera L.). Azarian J. Agric. 2016, 3, 112–118. [Google Scholar]
- Koochani, M.; Majd, A.; Arbabian, S.; Ghanati, F.; Marandi, S.J. A comparative study on the effects of ultrasound and some growth factors on somatic embryogenesis and artificial seed production in cucumber (Cucumis sativus L.). Not. Bot. Horti Agrobot. 2020, 48, 1915–1928. [Google Scholar] [CrossRef]
- Muratova, S.A.; Papikhin, R. The effect of ultrasound irradiation on induction of callus formation and morphogenesis from the leaf discs of apple clonal rootstocks. J. Pharm. Sci. Res. 2018, 10, 2592–2596. [Google Scholar]
- Firoozi, B.; Zare, N.; Sofalian, O.; Sheikhzade-Mosadegh, P. In vitro indirect somatic embryogenesis and secondary metabolites production in the saffron: Emphasis on ultrasound and plant growth regulators. J. Agric. Sci. 2019, 25, 1–10. [Google Scholar] [CrossRef]
- Dobránszki, J.; Hidvégi, N.; Gulyás, A.; Teixeira da Silva, J.A. mRNA transcription profile of potato (Solanum tuberosum L.) exposed to ultrasound during different stages of in vitro plantlet development. Plant Mol. Biol. 2019, 100, 511–525. [Google Scholar] [CrossRef]
- Dobránszki, J.; Hidvégi, N.; Gulyás, A.; Tóth, B.; Teixeira da Silva, J.A. Abiotic stress elements in in vitro potato (Solanum tuberosum L.) exposed to air-based and liquid-based ultrasound: A comparative transcriptomic assessment. Prog. Biophys. Mol. Biol. 2020, 158, 47–56. [Google Scholar] [CrossRef] [PubMed]
- Teixeira da Silva, J.; Norbert, H.; Gulyás, A.; Bianka, T.; Judit, D. Transcriptomic response of in vitro potato (Solanum tuberosum L.) to piezoelectric ultrasound. Plant Mol. Biol. Rep. 2020, 38, 404–418. [Google Scholar] [CrossRef]
- Naqvi, B.; Mehboob, S.; Makhani, K.; Yousuf, K. Effect of physical, chemical and physiochemical treatments of surface sterilisation on medicinal plants Salvadora persica and Solanum surattense for in vitro propagation. Pak. J. Sci. Ind. Res. B Biol. Sci. 2017, 60, 141–144. [Google Scholar] [CrossRef]
- Sharififar, A.; Nazari, M.; Asghari, H.R. Effect of ultrasonic waves on seed germination of Atriplex lentiformis, Cuminum cyminum, and Zygophyllum eurypterum. J. Appl. Res. Med. Aromat. Plants 2015, 2, 102–104. [Google Scholar] [CrossRef]
- López-Ribera, I.; Vicient, C.M. Use of ultrasonication to increase germination rates of Arabidopsis seeds. Plant Methods 2017, 13, 31. [Google Scholar] [CrossRef] [PubMed]
- Babaei-Ghaghelestany, A.; Alebrahim, M.T.; MacGregor, D.R.; Khatami, S.A.; Farzaneh, R.H.N. Evaluation of ultrasound technology to break seed dormancy of common lambsquarters (Chenopodium album). Food Sci. Nutr. 2020, 8, 2662–2669. [Google Scholar] [CrossRef] [PubMed]
- Mihaylova, E.; Marcheva, M.; Peruhov, N. Ultrasound seed treatment for organic farming. Bulg. J. Agric. Sci. 2021, 27, 78–84. [Google Scholar]
- Najafi, R.; Rezaei, A.; Talei, D. Potential of ultrasound and nicotinic acid to improve physiological responses and trigonelline biosynthesis in fenugreek (Trigonella foenum-graecum L.). Ind. Crops Prod. 2022, 182, 114815. [Google Scholar] [CrossRef]
- Babaei, M.; Pirdashti, H.; Bakhshandeh, E. Ultrasonic waves improve aged seed germination of castor bean (Ricinus communis L.) under drought and salt stresses. Acta Physiol. Plant 2023, 45, 90. [Google Scholar] [CrossRef]
- El-Sattar, A.M.A.; Tawfik, E. Effects of ultrasonic waves on seedling growth, biochemical constituents, genetic stability of fenugreek (Trigonella foenum-graecum) under salinity stress. Vegetos 2023, 36, 1427–1436. [Google Scholar] [CrossRef]
- Gong, M.; Kong, M.; Huo, Q.; He, J.; He, J.; Yan, Z.; Lu, C.; Jiang, Y.; Song, J.; Han, W.; et al. Ultrasonic treatment can improve maize seed germination and abiotic stress resistance. BMC Plant Biol. 2024, 24, 758. [Google Scholar] [CrossRef]
- Xia, Q.; Tao, H.; Li, Y.; Pan, D.; Cao, J.; Liu, L.; Zhou, X.; Barba, F.J. Characterizing physicochemical, nutritional and quality attributes of wholegrain Oryza sativa L. subjected to high intensity ultrasound-stimulated pre-germination. Food Control 2020, 108, 106827. [Google Scholar] [CrossRef]
- Zeng, Z.; Liu, X.; Deng, Q.; Ashraf, U.; Chen, J.; Shen, W. Transcriptome analysis revealed mechanisms involved in improved germination and growth of sugarcane by ultrasonic treatment. Ind. Crops Prod. 2023, 192, 116104. [Google Scholar] [CrossRef]
- Yu, Z.; Gao, Y.; Duan, H.; Zheng, D.; Shang, Z.; Zhang, L.; Chen, Y. Ultrasound-assisted germination of red kidney beans: Enhancements in physicochemical and nutritional profiles. Food Chem. 2024, 454, 139829. [Google Scholar] [CrossRef]
- Younesian, A.; Gholipoor, M.; Ajam Norouzi, H. Alleviation of drought stress effects on red bean by ultrasonication and foliar application of 24-epi-brassinolid. Int. J. Plant Prod. 2017, 11, 505–514. [Google Scholar]
- Zeng, Z.; Cai, H.; Chen, J.; Liu, X.; Li, Y.; Zhang, Y.; Chen, J.; Rao, D.; Shen, W. Improving sugarcane agronomy: Field evidence for ultrasonic treatment enhancing yield, growth, and physiological and biochemical characteristics. Ind. Crops Prod. 2024, 211, 118276. [Google Scholar] [CrossRef]
- Lo Porto, C.; Ziuzina, D.; Los, A.; Boehm, D.; Palumbo, F.; Favia, P.; Tiwari, B.; Bourke, P.; Cullen, P.J. Plasma activated water and airborne ultrasound treatments for enhanced germination and growth of soybean. Innov. Food Sci. Emerg. Technol. 2018, 49, 13–19. [Google Scholar] [CrossRef]
- Wu, Y.; He, S.; Pan, T.; Miao, X.; Xiang, J.; Ye, Y.; Cao, X.; Sun, H. Enhancement of γ-aminobutyric acid and relevant metabolites in brown glutinous rice (Oryza sativa L.) through salt stress and low-frequency ultrasound treatments at pre-germination stage. Food Chem. 2023, 410, 135362. [Google Scholar] [CrossRef]
- Jamshidi, A.R.; Ghazanfari Moghaddam, A.; Ommani, A.R. Effect of ultrasonic atomizer on the yield and yield components of tomato grown in a vertical aeroponic planting system. Int. J. Hortic. Sci. 2019, 6, 237–246. [Google Scholar]
- Kawakami, D.; Yoshida, T.; Kanemaru, Y.; Zaquinaula, M.H.H.; Mizukami, T.; Arimoto, M.; Shibata, T.; Goto, A.; Enami, Y.; Amano, H.; et al. Induction of resistance to diseases in plant by aerial ultrasound irradiation. J. Pestic. Sci. 2019, 44, 41. [Google Scholar] [CrossRef]
- Yakupoğlu, G. Effects of magnetic field and ultrasound applications on endogenous melatonin content and drought stress tolerance of pepper seedlings. Horticulturae 2023, 9, 704. [Google Scholar] [CrossRef]
- Rizwan, H.M.; He, J.; Nawaz, M.; Cheng, K.-W.; Wang, M. Comprehensive in silico characterization of soybean (Glycine max L.) isoflavone reductase genes and their expressions in response to spermidine and ultrasonication. Plant Stress 2024, 11, 100392. [Google Scholar] [CrossRef]
- Yu, S.; Zhang, S.; Lu, L.; Liu, L.; Liang, J.; Lang, S.; Wang, C.; Wang, L.; Li, Z. Effects of combined ultrasound and calcium ion pretreatments on polyphenols during mung bean germination: Exploring underlying mechanisms. Food Res. Int. 2024, 195, 114947. [Google Scholar] [CrossRef] [PubMed]
- Hidvégi, N.; Gulyás, A.; Dobránszki, J. Ultrasound, as a hypomethylating agent, remodels DNA methylation and alters mRNA transcription in winter wheat (Triticum aestivum L.) seedlings. Physiol. Plant. 2022, 174, e13777. [Google Scholar] [CrossRef]
- Pszczółkowski, P.; Sawicka, B. Ultrasound application in potato cultivation: Potential for enhanced yield and sustainable agriculture. Sustainability 2024, 16, 108. [Google Scholar] [CrossRef]
- Milburn, J.A.; Johnson, R.P.C. The conduction of sap. II. Detection of vibrations produced by sap cavitation in Ricinus xylem. Planta 1966, 69, 43–52. [Google Scholar] [CrossRef]
- Ritman, K.T.; Milburn, J.A. Acoustic emissions from plants: Ultrasonic and audible compared. J. Exp. Bot. 1988, 39, 1237–1248. [Google Scholar] [CrossRef]
- Perks, M.P.; Irvine, J.; Grace, J. Xylem acoustic signals from mature Pinus sylvestris during an extended drought. Ann. For. Sci. 2004, 61, 1–8. [Google Scholar] [CrossRef]
- Ponomarenko, A.; Vincent, O.; Pietriga, A.; Cochard, H.; Badel, E.; Marmottant, P. Ultrasonic emissions reveal individual cavitation bubbles in water-stressed wood. J. R. Soc. Interface 2014, 11, 20140480. [Google Scholar] [CrossRef]
- Kikuta, S.B.; Lo Gullo, M.A.; Nardini, A.; Richter, H.; Salleo, S. Ultrasound acoustic emissions from dehydrating leaves of deciduous and evergreen trees. Plant Cell Environ. 1997, 20, 1381–1390. [Google Scholar] [CrossRef]
- Laschimke, R.; Burger, M.; Vallen, H. Acoustic emission analysis and experiments with physical model systems reveal a peculiar nature of the xylem tension. J. Plant Physiol. 2006, 163, 996–1007. [Google Scholar] [CrossRef]
- Zweifel, R.; Zeugin, F. Ultrasonic acoustic emissions in drought-stressed trees–more than signals from cavitation? New Phytol. 2008, 179, 1070–1079. [Google Scholar] [CrossRef]
- Vergeynst, L.L.; Dierick, M.; Bogaerts, J.A.; Cnudde, V.; Steppe, K. Cavitation: A blessing in disguise? New method to establish vulnerability curves and assess hydraulic capacitance of woody tissues. Tree Physiol. 2015, 35, 400–409. [Google Scholar] [CrossRef] [PubMed]
- Gagliano, M.; Mancuso, S.; Robert, D. Towards understanding plant bioacoustics. Trends Plant Sci. 2012, 17, 323–325. [Google Scholar] [CrossRef] [PubMed]
- Vergeynst, L.L.; Sause, M.G.; Hamstad, M.A.; Steppe, K. Deciphering acoustic emission signals in drought stressed branches: The missing link between source and sensor. Front. Plant Sci. 2015, 6, 494. [Google Scholar] [CrossRef] [PubMed]
- Simon, R.; Holderied, M.W.; Koch, C.U.; Helversen, O. Floral acoustics: Conspicuous echoes of a dish-shaped leaf attract bat pollinators. Science 2011, 333, 631–633. [Google Scholar] [CrossRef]
- Schöner, M.G.; Simon, R.; Schöner, C.R. Acoustic communication in plant–animal interactions. Curr. Opin. Plant Biol. 2016, 32, 88–95. [Google Scholar] [CrossRef]
- Grafe, T.U.; Schöner, C.R.; Kerth, G.; Junaidi, A.; Schöner, M.G. A novel resource–service mutualism between bats and pitcher plants. Biol. Lett. 2011, 7, 436–439. [Google Scholar] [CrossRef]
- Robert, D. Plant bioacoustics: The sound expression of stress. Cell 2023, 186, 1307–1308. [Google Scholar] [CrossRef]
- Dalal, V.K. Understanding Acoustic Communication in Plants. J. Biomed. Res. 2021, 2, 815–820. [Google Scholar] [CrossRef]
- Jeong, M.J.; Cho, J.I.; Park, S.H.; Kim, K.H.; Lee, S.K.; Kwon, T.R.; Park, S.-C.; Siddiqui, Z.S. Sound frequencies induce drought tolerance in rice plant. Pak. J. Bot. 2020, 46, 2015–2020. [Google Scholar]
- Veits, M.; Khait, I.; Obolski, U.; Zinger, E.; Boonman, A.; Goldshtein, A.; Saban, K.; Seltzer, R.; Ben-Dor, U.; Estlein, P. Flowers respond to pollinator sound within minutes by increasing nectar sugar concentration. Ecol. Lett. 2019, 22, 1483–1492. [Google Scholar] [CrossRef]
- Trick, H.N.; Finer, J.J. SAAT: Sonication-assisted Agrobacterium-mediated transformation. Transgenic Res. 1997, 6, 329–336. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, Z.; Yang, R.; Li, G.; Guo, X.; Zhang, H.; Zhang, H.; Di, R.; Zhao, Q.; Zhang, M. Improvement of soybean transformation via Agrobacterium tumefaciens methods involving α-aminooxyacetic acid and sonication treatments enlightened by gene expression profile analysis. Plant Cell Rep. 2016, 35, 1259–1271. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.-M.; Zhang, H.-M.; Liu, Z.-H.; Guo, X.-L.; Li, H.-C.; Li, G.-L.; Jiang, C.-Z.; Zhang, M.-C. Inhibition of isoflavone biosynthesis enhanced T-DNA delivery in soybean by improving plant–Agrobacterium tumefaciens interaction. Plant Cell Tiss. Org. Cult. 2015, 121, 183–193. [Google Scholar] [CrossRef]
- Tang, W.; Xiao, B.; Fei, Y. Slash pine genetic transformation through embryo cocultivation with A. tumefaciens and transgenic plant regeneration. In Vitr. Cell Dev. Biol. Plant 2014, 50, 199–209. [Google Scholar] [CrossRef]
- Koetle, M.J.; Baskaran, P.; Finnie, J.F.; Soos, V.; Balázs, E.; Van Staden, J. Optimization of transient GUS expression of Agrobacterium-mediated transformation in Dierama erectum Hilliard using sonication and Agrobacterium. S. Afr. J. Bot. 2017, 111, 307–312. [Google Scholar] [CrossRef]
- Mohanan, S.; Satyanarayana, K.V.; Sridevi, V.; Gowda, K.; Giridhar, P.; Chandrashekar, A.; Ravishankar, G.A. Evaluating the effect and effectiveness of different constructs with a conserved sequence for silencing of Coffea canephora N-methyltransferases. J. Plant Biochem. Biotechnol. 2014, 23, 399–409. [Google Scholar] [CrossRef]
- Klimek-Chodacka, M.; Baranski, R. A protocol for sonication-assisted Agrobacterium rhizogenes-mediated transformation of haploid and diploid sugar beet (Beta vulgaris L.) explants. Acta Biochim. Pol. 2014, 61, 13–17. [Google Scholar] [CrossRef]
- Alam, P.; Khan, Z.A.; Abdin, M.Z.; Khan, J.A.; Ahmad, P.; Elkholy, S.F.; Sharaf-Eldin, M.A. Efficient regeneration and improved sonication-assisted Agrobacterium transformation (SAAT) method for Catharanthus roseus. 3 Biotech 2017, 7, 26. [Google Scholar] [CrossRef]
- Wu, X.; Shi, H.; Chen, X.; Liu, Y.; Guo, Z. Establishment of Agrobacterium-mediated transformation of seashore paspalum (Paspalum vaginatum O. Swartz). In Vitr. Cell Dev. Biol. Plant 2018, 54, 545–552. [Google Scholar] [CrossRef]
- da Silva, M.L.; Pinto, D.L.P.; Passos, A.B.; Marcelino-Guimaraes, F.C.; Rossi, A.A.B.; Krause, W.; de Carvalho, I.F.; Batista, D.S.; Rocha, D.I.; Otoni, W.C. Novel and efficient transformation of wild passion fruit (Passiflora cincinnata Mast.) using sonication-assisted Agrobacterium-mediated transformation. In Vitr. Cell Dev. Biol. Plant 2021, 57, 380–386. [Google Scholar] [CrossRef]
- Udayabhanu, J.; Huang, T.; Xin, S.; Cheng, J.; Hua, Y.; Huang, H. Optimization of the transformation protocol for increased efficiency of genetic transformation in Hevea brasiliensis. Plants 2022, 11, 1067. [Google Scholar] [CrossRef] [PubMed]
- Rüter, P.; Eeckhaut, T.; Dhooghe, E.; De Keyser, E.; Doan, M.H.; Bartels, J.; Winkelmann, T. Optimization of Rhizobium rhizogenes-mediated transformation, regeneration and characterization of Malus domestica Borkh. Ri lines. Plant Cell Tiss. Organ Cult. 2024, 157, 32. [Google Scholar] [CrossRef]
- Chen, J.; Wang, L.; Chen, J.; Huang, J.; Liu, F.; Guo, R.; Yang, L.; Grabon, A.; Zhao, K.; Kong, F.; et al. Agrobacterium tumefaciens-mediated transformation system for the important medicinal plant Dendrobium catenatum Lindl. In Vitro Cell Dev. Biol. Plant 2018, 54, 228–239. [Google Scholar] [CrossRef]
- Faraz, R.; Gokhale, M.; Gothalwal, R. Enhancement in production of baicalein through transformation in Oroxylum indicum (L.) Vent by Rhizobium rhizogenes. Vegetos 2024, 37, 305–320. [Google Scholar]
- Georgiev, M.I.; Radziszewska, A.; Neumann, M.; Marchev, A.; Alipieva, K.; Ludwig-Müller, J. Metabolic alterations of Verbascum nigrum L. plants and SAArT transformed roots as revealed by NMR-based metabolomics. Plant Cell Tiss. Org. 2015, 123, 349–356. [Google Scholar] [CrossRef]
- Stevens, M.E.; Pijut, P.M. Agrobacterium-mediated genetic transformation and plant regeneration of the hardwood tree species Fraxinus profunda. Plant Cell Rep. 2014, 33, 861–870. [Google Scholar] [CrossRef]
- Palla, K.J.; Pijut, P.M. Agrobacterium-mediated genetic transformation of Fraxinus americana hypocotyls. Plant Cell Tiss. Org. Cult. 2015, 120, 631–641. [Google Scholar] [CrossRef]
- Wang, Y.; Pijut, P. Improvement of Agrobacterium-mediated transformation and rooting of black cherry. In Vitr. Cell Dev. Biol. Plant 2014, 50, 307–316. [Google Scholar] [CrossRef]
- Mayavan, S.; Subramanyam, K.; Jaganath, B.; Sathish, D.; Manickavasagam, M.; Ganapathi, A. Agrobacterium-mediated in planta genetic transformation of sugarcane setts. Plant Cell Rep. 2015, 34, 1835–1848. [Google Scholar] [CrossRef]
- Kapildev, G.; Chinnathambi, A.; Sivanandhan, G.; Rajesh, M.; Vasudevan, V.; Mayavan, S.; Arun, M.; Jeyaraj, M.; Alharbi, S.A.; Selvaraj, N.; et al. High-efficient Agrobacterium-mediated in planta transformation in black gram (Vigna mungo (L.) Hepper). Acta Physiol. Plant. 2016, 38, 205. [Google Scholar] [CrossRef]
- Rani, A.; Panwar, A.; Sathe, M.; Kush, A. A modified in planta method of Agrobacterium-mediated transformation enhances the transformation efficiency in safflower (Carthamus tinctorius L.). J. Plant Biochem. Biotechnol. 2018, 27, 272–283. [Google Scholar] [CrossRef]
- Karthik, S.; Pavan, G.; Sathish, S.; Siva, R.; Periyasamy, S.K.; Manickavasagam, M. Genotype-independent and enhanced in planta Agrobacterium tumefaciens-mediated genetic transformation of peanut [Arachis hypogaea (L.)]. 3 Biotech 2018, 8, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Vasudevan, V.; Siva, R.; Krishnan, V.; Manickavasagam, M. Polyamines, sonication and vacuum infiltration enhances the Agrobacterium-mediated transformation in watermelon (Citrullus lanatus Thunb.). S. Afr. J. Bot. 2020, 128, 333–338. [Google Scholar] [CrossRef]
- Karthik, S.; Pavan, G.; Prasanth, A.; Sathish, S.; Appunu, C.; Manickavasagam, M. Improved in planta genetic transformation efficiency in bitter gourd (Momordica charantia L.). In Vitr. Cell Dev. Biol. 2021, 57, 190–201. [Google Scholar] [CrossRef]
- Vasudevan, V.; Sathish, D.; Ajithan, C.; Sathish, S.; Manickavasagam, M. Efficient Agrobacterium-mediated in planta genetic transformation of watermelon [Citrullus lanatus Thunb.]. Plant Biotechnol. Rep. 2021, 15, 447–457. [Google Scholar] [CrossRef]
- Zhang, Y.; Chunxiao, Q.; Shijia, L.; Xu, Y.; Li, Y.; Yongxue, Z.; Song, Y.; Meihong, S.; Chunxiang, F.; Zhi, Q.; et al. Establishment of an efficient Agrobacterium-mediated genetic transformation system in halophyte Puccinellia tenuiflora. Mol. Breed. 2021, 41, 55. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Luo, W.; Wu, Y.; Li, X.; Albert, N.W.; Zhang, Y.; Chen, X.; Lin-Wang, K.; Deng, C.H.; Hu, Z.; et al. A callus-derived regeneration and Agrobacterium-mediated gene transformation developed for bilberry, Vaccinium myrtillus. Plant Cell Tiss. Org. Cult. 2023, 154, 177–187. [Google Scholar] [CrossRef]
- Saravanan, K.; Vidya, N.; Appunu, C.; Gurusaravanan, P.; Arun, M. A simple and efficient genetic transformation system for soybean (Glycine max (L.) Merrill) targeting apical meristem of modified half-seed explant. 3 Biotech 2023, 13, 293. [Google Scholar] [CrossRef]
- Chandrasekaran, A.; Pilavadi, T.; Venkatachalam, V.; Umapathy, D.; Arockiam, A.J.V.; Singarayar, M.S.; Lee, G.-J.; Markandan, M. Bio-engineered As-Ag-TiO2 nanoparticles enhance the genetic transformation of Pisum sativum L. via proton-coupled electron transfer-dependent alternative protonation. Ind. Crops Prod. 2024, 215, 118604. [Google Scholar] [CrossRef]
- Prem Kumar, G.; Sivakumar, S.; Siva, G.; Vigneswaran, M.; Senthil Kumar, T.; Jayabalan, N. Optimization and establishment of genotype-independent seed-based in planta transformation system in cotton (Gossypium hirsutum L. cv. SVPR-2). Plant Gene 2021, 27, 100296. [Google Scholar] [CrossRef]
- Qi, F.; Tang, M.; Wang, W.; Liu, L.; Cao, Y.; Jing, T.; Zhan, Y. In vitro adventitious shoot regeneration system for Agrobacterium-mediated genetic transformation of Fraxinus mandshurica Rupr. Trees 2022, 36, 1387–1399. [Google Scholar] [CrossRef]
- King, J.L.; Finer, J.J.; McHale, L.K. Development and optimization of agroinfiltration for soybean. Plant Cell Rep. 2015, 34, 133–140. [Google Scholar] [CrossRef] [PubMed]
- Sivanandhan, G.; Kapil Dev, G.; Theboral, J.; Selvaraj, N.; Ganapathi, A.; Manickavasagam, M. Sonication, vacuum infiltration and thiol compounds enhance the Agrobacterium-mediated transformation frequency of Withania somnifera (L.) Dunal. PLoS ONE 2015, 10, e0124693. [Google Scholar] [CrossRef] [PubMed]
- Subramanyam, K.; Arunachalam, C.; Rasu, M.T.; Sulaiman, A.A.; Manickavasagam, M.; Ganapathi, A. Highly efficient Agrobacterium-mediated in planta genetic transformation of snake gourd (Tricosanthes cucumerina L.). Plant Cell Tiss. Org. Cult. 2015, 123, 133–142. [Google Scholar] [CrossRef]
- Manickavasagam, M.; Subramanyam, K.; Ishwarya, R.; Elayaraja, D.; Ganapathi, A. Assessment of factors influencing the tissue culture-independent Agrobacterium-mediated in planta genetic transformation of okra [Abelmoschus esculentus (L.) Moench]. Plant Cell Tiss. Org. 2015, 123, 309–320. [Google Scholar] [CrossRef]
| Plant (Species, Cultivar) | Treated Explant | Method of Ultrasonic Treatment | Main Impacts of Ultrasonication | Ref. |
|---|---|---|---|---|
| Hybrid Cymbidium Twilight Moon ‘Day Light’ | Half-PLBs | Bath sonicator (Iuchi®, Tokyo, Japan) for 0, 1, 5, 10, 20, and 45 min, at 60 Hz and at 25 °C | Total of 5 and 10 min of sonication increased neo-PLB formation after 60 days of culture, whereas 1, 20, or 45 min reduced neo-PLB formation. | [53] |
| Grape (Vitis vinifera L.) cv. ‘Kodori’ | Stem internodes | For 0, 60, 120, and 240 s at 20 kHz and 2 W | Ultrasonication for 120 s in combination with 100 µM Trp or Pro gave the best result, including callusing frequency (3.3 and 4.7), length (14.7 and 12.7 mm), width (9 mm each), fresh (6.9 and 6.0 g) and dry (0.28 and 0.23 g) weight of the callus, embryo number/callus (5.5 and 4.0), and the percentage of embryos (81 and 82%), respectively. | [54] |
| Toothbrush tree (Salvadora persica L.) Yellow-fruit nightshade (Solanum surattense L.) | Shoot tip and nodal explants | (1) Treatment with 10 and 15% sodium hypochlorite (chemical treatment); (2) sonication for 20 min (physical treatment) (frequency and other details are not specified); and (3) treatment with 10 and 15% sodium hypochlorite under sonication for 20 min (physiochemical treatment) | In S. surattense, the highest number of plants survived when treating explants with 15% sodium hypochlorite under 20 min sonication (mean explant number was 2.4 vs. 1.0 without sonication). In S. persica, it was 2.8 with 15% sodium hypochlorite, both with and without sonication. This was found as the most proper method for controlling contamination in both rough (S. surattense) and smooth (S. persica) texture plants. | [61] |
| Potato (Solanum tuberosum L.) cv. ‘Desirée’ | Nodal explants from 4-week-old in vitro plantlets | Ultrasonication for 20 min at 35 kHz and 70 W (ultrasound is transmitted by air, in homemade ultrasonicator) | After ultrasonication (at 0 and 24 h) the activity of SOD, APS, and GR, as well as the concentration of α-tocopherol, increased. AA concentration significantly decreased. Length and fresh weight of shoots increased by 20 and 24% compared to untreated plants, respectively. | [42] |
| Clonal apple rootstock B9 and clonal rootstock of Malus siboldy 14-1 | Isosceles triangle-shaped cuts of the lamina with the leafstalk and middle parts of lamina | UZDN-2T ultrasound installation for 60 s, at 22 kHz, power density: 1–1.2; 2–2.6; 3–3.6; 4–6.0; 5–10.0; 6–12.6; and 7–14.9 W/cm2 | Ultrasonication for 60 s activated the callus formation on the leaf disks and increased the frequency of regeneration of adventive shoots in apple clonal rootstocks by 2.5–3.5 times. | [56] |
| Saffron (Crocus sativus L.) | Corms of 1–2 cm in diameter | Bath sonicator (Bandelin electronic®, Berlin, Germany) for 5 min, at 35 kHz and 25 °C, repeated every two weeks for 1 min. Explants were treated with different combinations of PGRs | Ultrasonication with PGRs resulted in significantly increased in vitro callus induction (the highest percentage of callus induction was 100% and a growth of 4.3 g per explant of callus yield). | [57] |
| Potato (Solanum tuberosum L.) cv. ‘Desirée’ | Single-node segments of 4-week-old in vitro plantlets | Ultrasonication for 20 min, at 35 kHz and at 70 W (ultrasound is transmitted by air, in homemade ultrasonicator) | After ultrasonication (0 h), 29 DEGs were upregulated and 34 DEGs downregulated, after 24 h, 31 and 11, after 48 h, 15 and 64, after 1 week, 16 and 70, and after 4 weeks, 68 and 69 DEGs were up-, and downregulated, respectively. Most of the DEGs play a role in biosynthesis, carbohydrate metabolism, catabolism, cellular protein modification, and response to stress. | [58] |
| Potato (Solanum tuberosum L.) cv. ‘Desirée’ | Single-node stem segments | Ultrasonicator (Elmasonic X-tra 30 H; Elma Schmidbauer GmbH, Singen, Germany) (ultrasound is transmitted by liquid) for 20 min at 35 kHz, 70 W, and 25 °C | After ultrasonication, 138 (0 h), 72 (24 h), 18 (48 h), 5 (1 week), and 59 (4 weeks) DEGs were upregulated and 6 (0 h), 82 (24 h), 96 (48 h), 172 (1 week), and 107 (4 weeks) DEGs were downregulated. Most of the DEGs coding for universal stress protein, chitinase, catalase, zinc finger proteins, 21 transcription factors, glutathione S-transferase, and 17 heat shock proteins. | [60] |
| Cucumber (Cucumis sativus L.) cv. ‘Dastgerd Esfahan’ | Hypocotyl pieces form 2-week-old seedlings | Ultrasonic bath (Falc Instruments, Treviglio, Italy) for 0, 5, 10, and 15 min at 40 and 34/722 kHz, 320 Hz | Optimal ultrasonication parameters were 40 kHz and 10 min. The ultrasound treatment accelerated seed germination (in the case of the production of synthetic seeds derived from somatic embryos, it was 76.39 ± 8.17 compared to the control; 55.55 ± 13.37; in the case of the production of synthetic seeds from apical buds it was 86.67 ± 8.43 in comparison with the control: 83.33 ± 8.03). Ultrasonicated explants started the flowering stage faster than, and within two weeks of, the controls. | [55] |
| Potato (Solanum tuberosum L.) cv. ‘Desirée’ | One-node stem segments | (1) Homemade ultrasonicator, ultrasound is transmitted by air; and (2) ultrasound is transmitted by liquid (Elmasonic X-tra 30 H); for 20 min at 35 kHz, 70 W, and at 25 °C | DEGs coding for chitinase, peroxidase, glutathione-S-transferase, transcription factors of ethylene responsive factor, dehydration-responsive element-binding, WRKY, and MYB were significantly expressed in liquid-based ultrasonication, contrary to air-based. Liquid-based US is a stronger abiotic stressor than air-based US. | [59] |
| Potato (Solanum tuberosum L.) cv. ‘Desirée’ | In vitro single-node segments of stems excised from 4-week-old plantlets | (Elmasonic X-tra 30 H ultrasonicator) for 0, 20, and 30 min, at 35 kHz, 70 W, and at 20 °C | Due to the ultrasound treatment, the three-level antioxidant defense system activated, and the endogenous melatonin level decreased. Melatonin had an important role in the growth and development of plants, and in addition it acted as an antioxidant. | [41] |
| Plant (Species, Cultivar) | Treated Explant | Method of Ultrasonic Treatment | Main Impacts of Ultrasonication | Ref. |
|---|---|---|---|---|
| Big saltbrush (Atriplex lentiformis S. Wats), Cumin (Cuminum cyminum L.), Zygophyllum eurypterum L. Boiss. and Buhse | Seeds | Ultrasonication (Elma, e 30 h; Elmasonic, Singen, Germany) for 0, 1, 3, 5, 7, and 9 min at 42 kHz | Ultrasonication for 5 min had the highest effect on the germination percentage in A. lentiformis (68.00%) and Z. eurypterum (73%) seeds; furthermore, 7 min of ultrasonication was the most effective for gemination of C. cyminum (80%) seeds, in comparison with the controls (40%, 37.5%, and 44%, respectively). | [62] |
| Arabidopsis thaliana L. (Col-0 ecotype) | Seeds (fresh, artificially deteriorated, naturally aged) | Ultrasonic bath (USC-1400, Unique, São Paulo, Brazil) for 30 s to 64 min at 45 kHz and 0.028 W m−3 volumetric power, at 24 °C | Ultrasonication for 30 s significantly increased germination in artificially deteriorated and naturally aged seeds. More pores are present on the coat of treated seeds (vs. control), which can be the reason for the increased germination rate. | [63] |
| Red bean (Phaseolus vulgaris L.) | Seeds | Ultrasonic bath (digital ultrasonic, Model 4820-CD(Song Young International. Co., Taiwan.) for 3 min at 24 kHz, at 32 °C | 24-epi-brassinolid foliar application combined with ultrasonication increased grain yield by about 20%. | [73] |
| Soybean (Glycine max L.) Merr | Seeds | Airborne acoustic ultrasound generator for 30 min at 25 kHz combined with using plasma activated water (PAW) | Airborne ultrasonication can increase the hydration of plants and the ability of seeds to absorb water. When using plasma treated water, faster growth and taller plants were detected. | [75] |
| Tomato (Solanum lycopersicum L. cvs. ‘Momotaro’, ‘Moneymaker’ Cabbage (Brassica oleracea L. var. capitata cv. ‘Shikidori’ Rice (Oryza sativa L. cvs. ‘Aichi-asahi’, ‘Kinuhikari’ | 1- or 2-week-old plants | Aerial ultrasound oscillator (was developed) (1) Ultrasound at frequency of 40.5 kHz, ca. 100 dB for 2 weeks (24 h per day). The oscillator was set over the plants at a distance of 70 cm; (2) ultrasound at frequencies of 19.8 and 28.9 kHz | Ultrasound of 40.5 kHz increased disease resistance in tomatoes and rice and reduced the incidence of Fusarium wilt and blast diseases, respectively. | [78] |
| Tomato (Solanum lycopersicum L.) Lycopersicon esculentum (Mill.) | Whole plants | Fogging period for 10, 15, and 20 min at the ultrasonic frequency of 50 and 107 kHz, and 2.1 MHz, using an aeroponic system | Frequency of 50 kHz and a 15 min fogging period of the nutrient solution were the most advantageous parameters, which had significant effect on the plant height, root weight and length, fruit weight, fruit length, and plant yield. | [77] |
| Wholegrain brown rice (Oryza sativa L.) subsp. Japonica | Grains | Ultrasonic irradiator (Tianhua Co., Jining, China) for 5, 10, 15, and 30 min at 400 W (28 kHz, 17.83 W cm−2) | Ultrasonication for 5 min caused the highest germination rate. The starch content in pretreated grains decreased, while the reducing sugar content increased. Ultrasonication increased the accumulation of antioxidants, GABA (with 48.9% and 56.9%, in naturally germinated grains and HIU-stimulated grains, respectively), and Pro (17.6 mg/100 g in naturally germinated and 19.5 mg/100 g in HIU-treated samples). Nutritional index of free amino acids together with the in vitro bioaccessibility of Ca and Fe (from 202.1 mg/kg to 259.6 mg/kg) were also raised significantly. | [70] |
| Common lambsquarters (Chenopodium album L.) | Seeds | Ultrasound bath (Bandelin DT 255 H model with internal dimensions of 325 mm × 175 mm × 305 mm and volume of 5.5 L) for 0, 5, 10, 15, and 30 min at 35 kHz and 230 W | Ultrasound treatment improved the germination. The highest percentage of germination was 83.3% after 15 min ultrasonication, compared to the control (29.8%). | [64] |
| Winter wheat (Triticum aestivum L.) variety Zebrets | Seeds | Ultrasonic bath (CARRERA—SINUS, Model: 2501) (Supply voltage: 230 V; 50 Hz; 50 W) for 3, 9, and 21 min at 42 kHz | Ultrasonication of contaminated seeds for 3 min showed a significant rise in the germination (by 35%) and the germinating energy of seeds (by 46%). At a frequency of 42 kHz with a duration of more than 3 min ultrasonication can destroy pathogenic fungi on seeds. | [65] |
| Fenugreek (Trigonella foenum-graecum L.) | Seeds | Ultrasonic bath (FALC Instruments, Treviglio, Italy) for 0.5 and 10 min at 40 and 59 kHz and 300 W, at 25 ± 0.5 °C | Ultrasonication for 5 min improved germination-related properties. It enhanced the effect of nicotinic acid promoting defense responses and the production of secondary metabolites, including trigonelline biosynthesis. | [66] |
| Winter wheat (Triticum aestivum L.) cv. ‘SE15’ | Seeds | Ultrasonication for 5 min, at 30 kHz and 70 W | Ultrasonication of seeds increased the length and weight of roots (by 23–68%) and shoots (16–28%) of 7-day-old seedlings significantly. DEGs play a role in starch biosynthesis, indole-3-acetic acid biosynthesis, photosynthesis, and TCA cycle pathways (these were affected by changes in DNA-methylation, as well). It resulted in DNA hypomethylation, which altered the accessibility of some genes for transcription. | [82] |
| Sugarcane (Saccharum officinarum L.) varieties ROC22, LC05–136, YT93–159 | Buds | Plant seed production increase processor (JD-1 L, Guangzhou Golden Rice Agricultural Science & Technology Co., Ltd., Guangzhou, China). Ultrasonication for 1, 2, and 5 min at 20–40 kHz | Ultrasonic treatment for 2–5 min increased the length, diameter, and germination rate of buds and enhanced the antioxidant enzyme activities. It reduced the contents of auxin, abscisic acid, and jasmonic acid, and increased the ratio of auxin/abscisic acid. In ROC22 and YT93–159, 2 and 5 min of ultrasonication, respectively, increased the bud length by about 90%. In YT93–159 and LC05–136, 5 min of ultrasonication, enhanced the bud diameter and germination rate by about 30%. The gene expression of gibberellin synthesis was upregulated, while that of abscisic acid synthesis was downregulated. | [71] |
| Pepper (Capsicum annuum L.) cv. ‘Çetinel’ | Seeds | Ultrasonic bath (Elma-Elmasonic S, Singen, Germany) for 0, 15, and 30 min at 40 Hz with magnetic field treatment (0, 0.3, 0.9, and 1.1 Tesla) for 5 min | Magnetic field treatment with ultrasonication decreased the malondialdehyde (19% and 35%, respectively) and hydrogen peroxide (52% and 58%, respectively) content, and increased the catalase enzyme activity. The endogenous melatonin content increased, too, giving tolerance against drought stress. | [79] |
| Glutinous rice (Oryza sativa L.) | Germinated brown glutinous rice | Ultrasound device for 5 min pulse on and 25 min pulse off, with an ultrasonic generator (28 kHz), a power switch (30, 40, 50 W), temperature switch, and an automatic timing unit (330 mm × 150 mm × 330 mm (L × H × W)) Ultrasonication combined with 2% CaCl2 stress. | Higher contents of gamma-aminobutyric acid (3.29-fold), pyruvic acid (7.63-fold), glycerol (4.88-fold), glutamate (2.02-fold), and glucose (1.32-fold) were accessed due to the 30 W ultrasound treatment and 2.0% CaCl2 stress at 9 h pre-germination. | [76] |
| Castor bean (Ricinus communis L.) | Seeds | Ultrasonic bath (KS-150EI, Ningbo Haishu Kesheng Ultrasonic Equipment Co., Ltd., Ningbo, China) bath capacity of 4 L (300 × 150 × 100 mm) for 0, 3, 6, 9, 12, 15, 20, 25, 30, 35, 40, 50, and 60 min at 40 kHz and 150 W and at 15, 20, 25, 30, and 35, 40 °C | Ultrasound treatment for 12 min at 30 °C increased germination parameters in both aged and non-aged seeds especially under drought and salt stresses. Under normal conditions, GPmax was between 80 and 100% for, at most, 6 days of accelerated aging without, and, at most, 10 days with ultrasonication. When decreasing water potential by 1.2 MPa (using NaCl), GPmax remained above 40% for, at most, 2 days of accelerated aging without, and, at most, 6 days with ultrasonication. | [67] |
| Fenugreek (Trigonella foenum-graecum L.) | Germinated seeds | Bath ultrasonication (Digital ultrasonic cleaner, MFUC-80A, Biobase Meihua Trading Co., Ltd., Shangdong, China) for 10 and 20 min at 40 kHz and 25 °C | Ultrasonication for 10 and 20 min had a significant effect on seed germination, early seedling development, and biochemical components under normal and salinity stress, also increasing the porosity of seeds, enhancing their ability to absorb water and oxygen. | [68] |
| Soybean (Glycine max L.) | Seeds | (1) Ultrasonication (Ultrasonic Homogenizer, JY92-IIN, Shanghai Drawell Scientific Instrument Co., Ltd., Shanghai, China) for 30 min at 400 W and 20–25 kHz at 25 °C; (2) 250 μM spermidine treatment | qRT-PCR analysis demonstrated that most isoflavone reductase genes, (primarily GmIFR9/17 and GmIFR36) were remarkably upregulated in soybean cotyledon, followed by hypocotyl and root tissues under spermidine and ultrasonication treatments. | [80] |
| Red kidney bean (Phaseolus vulgaris L.) | Beans | Water bath high-intensity ultrasonication for 10 min at 20 kHz and 250, 350, and 450 W, combined with H2O2 treatment, at 25 °C | Ultrasonication for 10 min at 350 W resulted in the highest germination rate (77.1%), increased total and soluble protein and ash content, and while reducing the fat, starch, and soluble sugar content, increased the accumulation of phenolic and flavonoid compounds, AA, and GABA, antioxidative capacity, improved amino acid composition, and protein digestibility. | [72] |
| Mung bean (Vigna radiata L.) variety Ming Mung Bean | Seeds | Ultrasonic generator (KQ-250E, Kunshan Ultrasonic Instrument Co., Kunshan, China) for 3–15 min at 40 kHz and 240–360 W (1) Traditional germination (2) Ultrasonic pretreatment and germination (3) Ultrasonic-Ca2+ pretreatment and germination | Ultrasound and ultrasound- Ca2+ pretreatments significantly increased the polyphenol content and enhanced the antioxidant capacity during germination. Ultrasound pretreatment stimulated flavonoid biosynthesis, while ultrasound-Ca2+ pretreatment promoted the tyrosine synthesis pathway. | [81] |
| Maize (Zea mays L.) ZD958 | Seeds | Ultrasonic processor (5ZCG-T6; Guangzhou Jindao Agricultural Technology Co., Ltd., Guangzhou, China) for 10–60 s at 20–40 kHz and at 20–25 °C | Ultrasonication for 40 s increased the acid protease (by 96.4%), α-amylase (73.8%), and β-amylase (49.1%) content. Most of the DEGs play a role in ribosome, proteasome, and pyruvate metabolism, sesquiterpenoid, triterpenoid, and phenylpropanoid biosynthesis, and oxidative phosphorylation. The amount of auxin (by 5.5%), gibberellin (37.3%), and SA (28.9%), and different TFs, were enhanced. Seed germination and growth under salt, drought, and waterlogging stresses were improved. | [69] |
| Potato (Solanum tuberosum L.) cvs. Denar, Lord, Owajca, Vineta, Satina, Tajfun, Syrena, Zagloba | Tubers | Ultrasonication in aquatic environment in an ultrasonic bathtube devicet for 10 min (at 18 °C); 200 W; and 50 Hz | Both total and marketable tuber yields of ultrasonically pre-treated plants were increased (5.5%) but a cultivar-dependent way. | [83] |
| Sugarcane (Saccharum officinarum L.) varieties ROC22, LC05–136, YT93–159 | Buds | Ultrasonic treatment machine (developed by Guangzhou Jindao Agricultural Technology Co., Ltd.). Ultrasonication for 1, 2, and 5 min at 20–40 kHz, dry treatment | Optimal ultrasonication time was 2–5 min. Cane yield increased by 2.2–22.1% and sugar yield by 0.3–21.2% in 2021, while in 2022, by 1.2–12.6% and 0.38–8.4%, respectively. It was beneficial to photosynthesis, root systems, and caused a more rapid growth. | [74] |
| Plant (Species, Cultivar) | Treated Explants | Method of Ultrasonic Treatment | Main Impacts of Ultrasonication | Ref. |
|---|---|---|---|---|
| Slash pine (Pinus elliottii Engelm.) | Mature zygotic embryos | Bath sonicator (Model PC5, L&R Manufacturing Co, Keamy, NJ, USA) for 15, 30, 45, 60, 75, or 90 s at 55 kHz, using Agrobacterium at 0.3, 0.6, 0.9, 1.2, 1.5, or 1.8 OD600 nm | The highest frequency of embryos with GUS transient expression was obtained after ultrasonication for 50 s and infection with A. tumefaciens OD600 = 0.9. | [104] |
| Coffea canephora L. | Somatic embryos | Ultrasonication for 30 s at 20 kHz and 80% amplitude (Bandelin Sonoplus Ultrasonicator, Bandelin electronic GmbH & Co. KG, Berlin, Germany) | The transformation efficiency (0.5–4%) was recorded by observing the number of putatively transformed hygromycin-resistant somatic embryos. | [106] |
| Sugar beet (Beta vulgaris L.) | Petiole and midrib explants from two-week-old shoots | Ultrasonication for 15–240 s using Agrobacterium rhizogenes at 0.05 and 0.5 OD600 (frequency and other sound-related details not specified) | Ultrasonication for 15 s with Agrobacterium OD600 = 0.5 were the most effective conditions for transformation. The highest transformation efficiency was 54%. | [107] |
| Soybean (Glycine max L.) Merrill | Cotyledonary node | Bath sonicator (KH2200B, Kunshan Hechuang, Kunshan Ultrasonic Instruments Co., Ltd., Kunshan, China) for 0 and 3 min at 40 kHz, using Agrobacterium at 0.8 OD600 | Transient GUS expression was significantly higher (41.4%) than in not ultrasonicated (9.0%) samples. Ultrasonication created micro-wounds for Agrobacterium infection and increased transformation efficiency by disrupting the synthesis of isoflavones. | [103] |
| Dark mullein (Verbascum nigrum L.) | Hairy root cultures | Sonication for 45 s at 35 kHz (UCI-50Raypa® R. Espinar S.L., Barcelona, Spain), using Agrobacterium rhizogenes at 0.8 OD600 | Two weeks after the transformation, first neoplastic roots were detected at the wound sites of V. nigrum leaves and the infection frequency was 83%. | [115] |
| Soybean (Glycine max L.) Merrill | Cotyledonary-node explants | Bath sonicator (KH2200B, Kunshan Hechuang, Kunshan Ultrasonic Instruments Co., Ltd., Kunshan, China) for 0 and 15 s at 40 kHz | As a result of ultrasonication, the transient GUS expression was (54.2%), significantly higher than that of not the sonicated (11%). | [102] |
| Catharanthus roseus L. | Hypocotyls | Bath sonicator (Imeco Ultrasonics, IMECO Ultrasonic Cleaning Mashine Manufacturer, Maharashtra, India) for 10 min, using Agrobacterium at 0.8 OD600 (frequency and other sound-related details not specified) | The transformation efficiency was 6.0%, compared to 3.5% with the conventional method. | [108] |
| Dierama erectum Hilliard | Embryonic shoot apical meristems, hypocotyls, and organogenic callus | Ultrasonication (Julabo Labotechnik GmbH, Seelbach, West Germany) for 0, 10, 20, 30, 40, 50, and 60 s at 35 kHz, using Agrobacterium at 0, 0.2 0.4, 0.8, 1.6, and 2 OD600 | The optimal duration of ultrasonication was 30 s, with Agrobacterium at 1.6 OD600. The embryonic shoot apical meristems proved to be the best target tissue for transformation, yielding a GUS gene expression efficiency of about 67%, compared to about 40% yielded by calluses. | [105] |
| Dendrobium catenatum L. | Primary protocorms generated from seeds | Sonication (Scientz, Ningbo, China) for 1, 2, 3, 4, and 5 min at 300 W and 40 kHz, using Agrobacterium at 0.4, 0.6, and 0.8 OD600 Three surfactants (Tween® 20, Triton™ X-100, and Silwet®-77) (Solarbio) at either 0.001% (v/v) or 0.01% (v/v) concentration were added to the A. tumefaciens suspension | The most advantageous condition for ultrasonication was 300 W at 40 kHz for 3 min, and the optimal OD600 was 0.6. The combination of the ultrasound treatment for 1 min and 0.001% (v/v) Triton™ X 100 was the optimal pretreatment to reach the highest transformation frequency (35%, compared to 17%). | [113] |
| Seashore paspalum (Paspalum vaginatum O. Swart) cv. ‘Sea Spray’ | Embryogenic callus from multiple explants | Ultrasonication for 5 and 10 min at 40 kHz, using Agrobacterium at 0.1, 0.6, and 1.2 OD600 | A high transient transformation efficiency (about 95%) was observed when using Agrobacterium concentration of OD600 = 0.6 with 5 min of ultrasonication (compared 75% without ultrasonication). | [109] |
| Passion fruit (Passiflora cincinnata Mast.) | Somatic embryos at the cotyledonary stage | Bransonic Ultrasonic Cleaner (B1210E-Mt, Branson Ultrasonics Corp., Brookfield, WI, USA) for 15 and 30 s at 47 kHz and 80 W, using Agrobacterium at 0.5 OD600 | The highest transformation efficiency (21.4%) was observed by ultrasonicating SEs for 30 s, in contrast to 13.6% in the control. | [110] |
| Hevea rubber tree (Hevea brasiliensis Müll.Arg.) | Mature cotyledonary somatic embryos | Ultrasonication for 10, 30, 50, and 70 s at 40 kHz, using Agrobacterium at 0.45, 0.6, and 0.75 OD600 | The best transformation efficiency was observed by using Agrobacterium at a concentration of OD600 = 0.45, and 50 s of ultrasonication. | [111] |
| Oroxylum indicum L. | Embryonic axis, cotyledon, leaf, and callus | Ultrasonication (Citizen, Mumbai, India) for 10–100 s at 50 MHz and 80% amplitude, using Rhizobium rhizogenes | Ultrasonication increased transformation frequency by 2.2-fold (60%) compared to manual wounding. Ultrasonication with 15 mM CaCl2 caused the best transformation efficiency (84%). | [114] |
| Apple (Malus domestica Borkh.) genotype ‘M26’ | Whole leaves | Bath sonication (Bandelin Sonorex Super 10 P, type DK 102 P, Bandelin electronic GmbH & Co. KG, Berlin, Germany) for 5, 10 min, at 35 kHz and 60 W L−1, using Rhizobium rhizogenes at 0.5 OD600 | Wounding by 10 min of ultrasonication improved transformation efficiency. The 4.4 roots/explant measured with no wounding was increased to 9.3 with ultrasonication. | [112] |
| Plant (Species, Cultivar) | Treated Explants | Method of Ultrasonic Treatment | Main Impacts of Ultrasonication | Ref. |
|---|---|---|---|---|
| Black cherry (Prunus serotina Ehrh.) genotype BC3 | Leaves | Ultrasonication (Branson Ultrasonics, Brookfield, USA) for 0, 30, 60, and 90 s at 40 kHz, and VI for 0, 5, 10, and 15 min at 63.5 cm Hg, using Agrobacterium at 0.5, 1.0, 1.5, and 1.8 OD600 | Optimal parameters of treatment were 15 min of VI without ultrasonication with a transformation efficiency of 21.7%. In contrast, 60 and 90 s of ultrasonication yielded 6.7%. | [118] |
| Pumpkin ash (Fraxinus profunda (Bush) Bush | Mature hypocotyls from 3- to-7-day-old embryos | Sonication for 90 s and VI for 10 min at 62.5 cm Hg with Agrobacterium at 0.6–1 OD600 (frequency and other sound-related details not specified) | Pumpkin ash hypocotyls were successfully transformed with Agrobacterium by sonication combined with VI. | [116] |
| Soybean (Glycine max L.) cv. ‘Williams 82’ | Entire seedling | Ultrasonication (Sharpertek Ultrasonic cleaner, Model# SH180-6L; Sharpertek USA, Auburn Hills, MI, USA) for 0, 20, 30, and 40 s at 40 kHz with VI for 0, 5, and 15 min and 3 × 5 min at 483 mm of Hg, using Agrobacterium at 0.6, 0.8 OD600 | Ultrasonication for 30 s, combined with three 5-min VI, led to the highest total expression of GUS. | [132] |
| White ash (Fraxinus americana L.) | Hypocotyls from mature embryos | Sonication for 90 s, with VI at 62.5 cm of Hg for 10 min with Agrobacterium at 0.4–0.6 OD600 (frequency and other sound-related details not specified) | Sonication for 90 s with 10 min of VI treatment was first reported to successfully regenerate transgenic plants. | [117] |
| Ashwagandha (Withania somnifera L.) cv. ‘Dunal’ | Six-day-old nodal explants | Ultrasonication for 5, 10, 15, 20, and 25 s at 30 kHz and VI for 5, 10, 15, and 20 min, using Agrobacterium at 0.1, 0.2, 0.5, and 1.0 OD600 | Ultrasonication for 10 s, combined with 10 min VIand Agrobacterium at 0.2 OD600, enhanced transformation efficiency. GUS foci frequency was 84%. | [133] |
| Snake gourd var. AGMMB2 (Tricosanthes cucumerina L.) | Seeds (decoated, 12 h old) | Bath sonicator (model 1510 Branson, Branson Ultrasonics, Kanagawa, Japan) for 0, 10, 20, 30, 40, 50, and 60 min at 40 kHz with VI for 0, 1, 2, 3, 4, and 5 min at 750 mm of Hg, using Agrobacterium at 1.0 OD600 | Sonication for 30 min, combined with 3 min of VI, proved to be the most advantageous method; 39.3% of infected seeds were germinated. | [134] |
| Sugarcane (Saccharum officinarum L.) hybrid varieties Co 62175, Co 6304, Co 8021, Co 86032, Co 6907 | Nodal cuttings from 6-month-old plants | Bath sonicator (model 2510 Branson, Branson Ultrasonics, Kanagawa, Japan). Sonication for 0, 2, 4, 6, 8, and 10 min at 40 kHz, in combination with VI for 0, 1, 2, 3, and 4 min at 0, 50, 100, 250, 500, and 750 mm of Hg | Sonication for 6 min, followed by 2-min VI at 500, were found to be the optimum to achieve the maximum transformation efficiency of 29.6% (with var. Co 62175). | [119] |
| Okra (Abelmoschus esculentus L.) Moench, var. Arka Anamika | Seeds | Bath sonicator (model 1510 Branson, Branson Ultrasonics, Kanagawa, Japan) for 0, 10, 20, 30, 40, and 50 min at 40 kHz, in combination with VI for 0, 1, 2, 3, 4, and 5 min at 750 mm of Hg with Agrobacterium at 0.6 OD600 | The transformation efficiency of okra was significantly improved by 30 min of sonication and 3 min of VI; 54% of infected seeds were germinated. | [135] |
| Black gram (Vigna mungo L.) cv. ‘T9’ | Cotyledon with embryo axis | Sonication (Branson Ultrasonics, Brookfield, WI, USA) for 0, 1, 2, 3, 4, 5, and 6 min and VI for 0, 1, 2, 3, 4, 5, and 6 min at 100 mm of Hg, using Agrobacterium at 0.8 OD600 (frequency and other sound-related details not specified) | Sonication for 3 min and 2-min of VI enhanced transformation efficiency of (46.2%), when compared to the control (25.6%). | [120] |
| Safflower (Carthamus tinctorius L.) | Seeds (decoated, 3 days old) | Bath sonicator for 10, 20, 30, 40, 50, and 60 s and VI for 5, 10, and 15 min at 250, 500, 750, and 1000 mm of Hg, using Agrobacterium at 0.5 OD600 | A combination of sonication (30 s) and VI (750 mm of Hg for 10 min) increased the transformation efficiency to 10.8% from 6.8%. | [121] |
| Peanut (Arachis hypogaea L.) cv. ‘CO7’ | One-half of the seeds (cotyledon with full embryo) | Bath sonicator (1510 Branson, Branson Ultrasonics, Kanagawa, Japan) for 0, 2, 4, 6, 8, and 10 min at 40 kHz, combined with VI for 0, 1, 2, 3, 4, and 5 min at 750 mm of Hg, using Agrobacterium at 0.8 OD600 | Sonication for 6 min, with 3 min VI of seed explants, resulted in the highest transformation efficiency of 33.6%. (without sonication 19.6%) | [122] |
| Watermelon (Citrullus lanatus Thunb.) cv. ‘Arka manik’ | Cotyledonary node explants from 7-day-old in vitro grown plants | Bath sonicator (Model 2510 Branson, Branson Ultrasonics, Kanagawa, Japan) for 10–50 s at 40 kHz with VI for 1–5 min at 100 mmHg | Optimal duration of ultrasonication (30 s) and VI (2 min) increased transformation efficiency to 17.3% from 7.6% and 1.3%, with and without ultrasonication. | [123] |
| Bitter gourd (Momordica charantia L.) | Seeds | Bath sonication (1510 Branson model, Branson Ultrasonics, Kanagawa, Japan) for 0, 5, 10, 15, 20, and 25 min at 40 kHz, combined with VI for 0, 2, 4, 6, 8, and 10 min at 750 mm of Hg, using Agrobacterium at 0.8 OD600 | The highest transformation efficiency (37%) was recognized by using ultrasonication for 15 min with VI for 6 min, compared to 18.6% in case of the control. | [124] |
| Watermelon (Citrullus lanatus Thunb.) cv. ‘Arka manik’, ‘Sugar baby’, ‘Arka muthu’, ‘IIHR-14’ | Embryonic axis attached with single cotyledon | Bath sonication for 1–5 min with VI for 0–5 min at 100 mm of Hg (frequency and other sound-related details not specified) | Optimal durations were 3 min for sonication and 2 min for VI, which enhanced the transformation efficiency to 17.33%, compared to 8.00% in the untreated case. | [125] |
| Alkaligrass (Puccinellia tenuiflora Griesb.) Scribn. And Merr. | 8-week-old embryogenic calluses | Explants were incubated with Agrobacterium suspension (0.3–0.4 OD600) (1) for 30 min; (2) for 10 min, then under ultrasonication for 10 min, and after that, another incubation for 10 min; (3) under vacuum for 10 min, and next, under ultrasonication for 10 min, following vacuum incubation (frequency and other sound-related details not specified) | The highest transformation efficiency (18.6%) resulted from vacuum for 10 min, ultrasonication for 10 min, and then vacuum for 10 min, compared to about 6.7% for simple 30 min of soaking. | [126] |
| Cotton (Gossypium hirsutum L.) cv. ‘SVPR-2’ | Seeds | Ultrasonication (Model 2510 Branson, Branson Ultrasonics, Kanagawa, Japan) for 20, 40, 60, 80, and 100 s at 60 kHz, combined with VI for 30, 60, 90, 120, and 150 s at 250, 500, and 750 mmHg, using Agrobacterium at 0.4, 0.6, 0.8, 1.0, and 1.2 OD600 | Optimal parameters of transformation were 60 s of ultrasonication, combined with 90 s of VI at 500 mmHg, using Agrobacterium at 0.1–1.0 OD600, which resulted in 28.66% maximum transformation frequency. | [130] |
| Manchurian ash (Fraxinus mandshurica Rupr.) | Hypocotyls | Explants were (1) immersed in Agrobacterium suspension for 15 min; and (2) sonicated for 90 s and then vacuum-infiltrated at 0.8 MPa for 10 min, using Agrobacterium at 0.6 OD600 (frequency and other sound-related details not specified) | Sonication for 90 s and 10 min vacuum treatment resulted in the maximum number of regenerated shoots. The number of adventitious buds per hypocotyl was doubled from 33.2 to 77.7. | [131] |
| Bilberry (Vaccinium myrtillus L.) | Calluses | Sonication (Qsonica Part No. Q700, Shanghai, China) for 2 s at 20 kHz, combined with VI for 10 min | Sonication with VI significantly increased the transformation efficiency (up to 4.45% from 0.8%). | [127] |
| Soybean (Glycine max L.) Merrill cv. ‘JS335’ | The apical meristem of modified half-seed | Sonication for 0, 1, 10, 20, and 30 min with VI for 0, 1, 10, 20, and 30 min, using Agrobacterium at 1.0 OD600 (frequency and other sound-related details not specified) | Sonication for 10 min, combined with 10 min VI, resulted in the highest transformation efficiency of 38.0%, compared to the control of 6.6%. | [128] |
| Pea (Pisum sativum L.) cv. ‘Ageta 6’ | Cotyledonary nodes | Sonication (Branson, MO, USA) for 10–50 s at 40 kHz with VI for 1–6 min at 750 mm Hg, using Agrobacterium | The highest transformation efficiency was 12.3%, resulting from 30 s sonication combined with 3 min VI, compared to 6.3% yielded by the control. | [129] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Király, A.; Farkas, D.; Dobránszki, J. Ultrasound in Plant Life and Its Application Perspectives in Horticulture and Agriculture. Horticulturae 2025, 11, 318. https://doi.org/10.3390/horticulturae11030318
Király A, Farkas D, Dobránszki J. Ultrasound in Plant Life and Its Application Perspectives in Horticulture and Agriculture. Horticulturae. 2025; 11(3):318. https://doi.org/10.3390/horticulturae11030318
Chicago/Turabian StyleKirály, Anita, Dóra Farkas, and Judit Dobránszki. 2025. "Ultrasound in Plant Life and Its Application Perspectives in Horticulture and Agriculture" Horticulturae 11, no. 3: 318. https://doi.org/10.3390/horticulturae11030318
APA StyleKirály, A., Farkas, D., & Dobránszki, J. (2025). Ultrasound in Plant Life and Its Application Perspectives in Horticulture and Agriculture. Horticulturae, 11(3), 318. https://doi.org/10.3390/horticulturae11030318








