Dynamic Traits of Intracellular Water and Salt Based on Electrophysiological Measurements During Adaptations of Three Mangrove Species Under Salinity Stresses
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Materials and Treatments
2.2. Plant Height Increment and Leaf Water Potential (ΨLw)
2.3. Electrophysiological Parameters Measurement and Dynamics Model Parameters Calculation
2.4. Photosynthetic Gas Exchange Parameters
2.5. Statistical Analysis
3. Results
3.1. Effects of Salinity on Growth Indices and Leaf Water Potential (ΨLw)
3.2. Effects of Salinity on Electrophysiological Parameters
3.3. Effects of Salinity on Photosynthetic Gas Exchange Parameters
3.4. Principal Component Analysis
4. Discussion
4.1. Responses of Rhizophora Stylosa to Salinity
4.2. Responses of Kandelia Candel to Salinity
4.3. Response of Aegiceras Corniculatum to Salinity
4.4. Comparison of the Salt Adaptation Mechanisms Among Three Mangrove Plants According to the Electrophysiological Information
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Kauffman, J.; Arifanti, V.; Basuki, I.; Kurnianto, S.; Novita, N.; Murdiyarso, D.; Donato, D.; Warren, M. Protocols for the measurement, monitoring, and reporting of structure, biomass, carbon stocks and greenhouse gas emissions in tropical peat swamp forests. Environ. Sci. 2016, 221, 006429. [Google Scholar] [CrossRef]
- Wang, W.; Wang, M. The Mangroves of China; Science Press: Beijing, China, 2007. [Google Scholar]
- Reef, R.; Lovelock, C.E. Regulation of Water Balance in Mangroves. Ann. Bot. 2015, 115, 385–395. [Google Scholar] [CrossRef] [PubMed]
- Fu, Z.W.; Feng, Y.R.; Gao, X.; Ding, F.; Li, J.H.; Yuan, T.T.; Lu, Y.T. Salt stress-induced chloroplastic hydrogen peroxide stimulates pdTPI sulfenylation and methylglyoxal accumulation. Plant Cell 2023, 35, 1593–1616. [Google Scholar] [CrossRef]
- Munns, R.; Tester, M. Mechanisms of Salinity Tolerance. Annu. Rev. Plant Biol. 2008, 59, 651–681. [Google Scholar] [CrossRef]
- Krauss, K.W.; Ball, M.C. On the Halophytic Nature of Mangroves. Trees 2013, 27, 7–11. [Google Scholar] [CrossRef]
- Nizam, A.; Meera, S.P.; Kumar, A. Genetic and Molecular Mechanisms Underlying Mangrove Adaptations to Intertidal Environments. iScience 2022, 25, 103547. [Google Scholar] [CrossRef] [PubMed]
- Tomlinson, P.B. The Botany of Mangroves; Cambridge University Press: New York, NY, USA, 1986. [Google Scholar]
- Sreeshan, A.; Meera, S.P.; Augustine, A. A Review on Transporters in Salt Tolerant Mangroves. Trees-Struct. Funct. 2014, 28, 957–960. [Google Scholar] [CrossRef]
- Lee, S.Y.; Primavera, J.H.; Dahdouh-Guebas, F.; McKee, K.; Bosire, J.O.; Cannicci, S.; Diele, K.; Fromard, F.; Koedam, N.; Marchand, C.; et al. Ecological Role and Services of Tropical Mangrove Ecosystems: A Reassessment. Glob. Ecol. Biogeogr. 2014, 23, 726–743. [Google Scholar] [CrossRef]
- Liang, M.; Hu, F.; Xie, D.; Chen, Z.; Zheng, Q.; Xie, Q.; Zheng, F.; Liu, D.; Jian, S.; Chen, H.; et al. Physiological Measurements and Transcriptome Survey Reveal How Semi-Mangrove Clerodendrum inerme Tolerates Saline Adversity. Front. Plant Sci. 2022, 13, 882884. [Google Scholar] [CrossRef]
- Wu, X.; Li, J.; Song, L.Y.; Zeng, L.L.; Guo, Z.J.; Ma, D.N.; Wei, M.Y.; Zhang, L.D.; Wang, X.X.; Zheng, H.L. NADPH oxidase-dependent H2O2 production mediates salicylic acid-induced salt tolerance in mangrove plant Kandelia obovata by regulating Na+/K+ and redox homeostasis. Plant J. 2024, 118, 1119–1135. [Google Scholar] [CrossRef]
- Zeng, L.L.; Song, L.Y.; Wu, X.; Ma, D.N.; Song, S.W.; Wang, X.X.; Zheng, H.L. Brassinosteroid enhances salt tolerance via S-nitrosoglutathione reductase and nitric oxide signaling pathway in mangrove Kandelia obovata. Plant Cell Environ. 2024, 47, 511–526. [Google Scholar] [CrossRef] [PubMed]
- Ball, M.C. Salinity Tolerance in the Mangroves Aegiceras corniculatum and Avicennia marina. I. Water Use in Relation to Growth, Carbon Partitioning, and Salt Balance. Funct. Plant Biol. 1988, 15, 447–464. [Google Scholar] [CrossRef]
- Feng, X.; Li, G.; Xu, S.; Wu, W.; Chen, Q.; Shao, S.; Liu, M.; Wang, N.; Zhong, C.; He, Z.; et al. Genomic Insights into Molecular Adaptation to Intertidal Environments in the Mangrove Aegiceras corniculatum. New Phytol. 2021, 231, 2346–2358. [Google Scholar] [CrossRef] [PubMed]
- Miryeganeh, M.; Saze, H. The First De Novo Transcriptome Assembly and Transcriptomic Dynamics of the Mangrove Tree Rhizophora stylosa Griff. (Rhizophoraceae). Int. J. Mol. Sci. 2021, 22, 11964. [Google Scholar] [CrossRef] [PubMed]
- Basyuni, M.; Baba, S.; Kinjo, Y.; Putri, L.A.P.; Hakim, L.; Oku, H. Salt-Dependent Increase in Triterpenoids Is Reversible upon Transfer to Fresh Water in Mangrove Plants Kandelia candel and Bruguiera gymnorrhiza. J. Plant Physiol. 2012, 169, 1903–1908. [Google Scholar] [CrossRef]
- Huang, H.; Zhuang, L.; Tang, H.; Guo, Z.; Li, Q.; Lin, Z.; Dai, M.; Wang, X.; Wang, Y.; Zheng, H.; et al. Biosynthesis-Based Spatial Metabolome of Condensed Tannin Reveals Its Role in Salt Tolerance of Non-Salt-Secretor Mangrove Kandelia obovata. Plant Cell Environ. 2024, 48, 39503313. [Google Scholar] [CrossRef]
- Ali Solangi, K.; Wu, Y.; Xing, D.; Ahmed Qureshi, W.; Hussain Tunio, M.; Ali Sheikh, S.; Shabbir, A. Can Electrophysiological Information Reflect the Response of Mangrove Species to Salt Stress? A Case Study of Rewatering and Sodium Nitroprusside Application. Plant Signal. Behav. 2022, 17, 2073420. [Google Scholar] [CrossRef]
- Mimura, T.; Kura-Hotta, M.; Tsujimura, T.; Ohnishi, M.; Miura, M.; Okazaki, Y.; Mimura, M.; Maeshima, M.; Washitani-Nemoto, S. Rapid Increase of Vacuolar Volume in Response to Salt Stress. Planta 2003, 216, 397–402. [Google Scholar] [CrossRef]
- Ali Solangi, K.; Wu, Y.; Chen, Q.; Ahmed Qureshi, W.; Xing, D.; Hussain Tunio, M.; Ali Shaikh, S. The Differential Responses of Aegiceras corniculatum and Kandelia candel under Salt Stress and Re-Watering Phase. A Study of Leaf Electrophysiological and Growth Parameters. J. Plant Interact. 2021, 16, 307–320. [Google Scholar] [CrossRef]
- Lou, C.H. Messenger transmission of electrochemical waves in higher plants. Acta Biophys. Sin. 1996, 4, 739–745. (In Chinese) [Google Scholar]
- Sinyukhin, A.M.; Britikov, E.A. Action Potentials in the Reproductive System of Plants. Nature 1967, 215, 1278–1280. [Google Scholar] [CrossRef]
- Zhang, C.; Wu, Y.; Su, Y.; Xing, D.; Dai, Y.; Wu, Y.; Fang, L. A Plant’s Electrical Parameters Indicate Its Physiological State: A Study of Intracellular Water Metabolism. Plants 2020, 9, 1256. [Google Scholar] [CrossRef] [PubMed]
- Gallé, A.; Lautner, S.; Flexas, J.; Fromm, J. Environmental Stimuli and Physiological Responses: The Current View on Electrical Signalling. Environ. Exp. Bot. 2015, 114, 15–21. [Google Scholar] [CrossRef]
- Blackman, C.; Brodribb, T. Two Measures of Leaf Capacitance: Insights into the Water Transport Pathway and Hydraulic Conductance in Leaves. Funct. Plant Biol. 2011, 38, 118–126. [Google Scholar] [CrossRef]
- Shi, L. Distribution, growth environment and environmental adaptability of mangroves in China. Mar. Inf. 2002, 4, 14–18. (In Chinese) [Google Scholar] [CrossRef]
- Zhang, M.; Wu, Y.; Xing, D.; Zhao, K.; Yu, R. Rapid Measurement of Drought Resistance in Plants Based on Electrophysiological Properties. Trans. ASABE (Am. Soc. Agric. Biol. Eng.) 2015, 58, 1441–1446. [Google Scholar] [CrossRef]
- Javed, Q.; Wu, Y.; Xing, D.; Azeem, A.; Ullah, I.; Zaman, M. Re-Watering: An Effective Measure to Recover Growth and Photosynthetic Characteristics in Salt-Stressed Brassica napus L. Chil. J. Agric. Res. 2017, 77, 78–86. [Google Scholar] [CrossRef]
- Wei, P.; Li, H.; Wu, Y.; Zhang, C. Association of the electrical parameters and photosynthetic characteristics of the tea tree manifests its response to simulated karst drought. Plant Signal. Behav. 2024, 19, 2359258. [Google Scholar] [CrossRef]
- Zhang, C.; Su, Y.; Wu, Y.; Li, H.; Zhou, Y.; Xing, D. Comparison on the Nutrient Plunder Capacity of Orychophragmus Violaceus and Brassica Napus L. Based on Electrophysiological Information. Horticulturae 2021, 7, 206. [Google Scholar] [CrossRef]
- Chen, T.; Wu, Y.; Xing, D.; Duan, R. Effects of NaHSO3 on Cellular Metabolic Energy, Photosynthesis and Growth of Iris pseudacorus L. Horticulturae 2022, 8, 185. [Google Scholar] [CrossRef]
- Duan, R.; Xing, D.; Chen, T.; Wu, Y. Effects of Different Inorganic Nitrogen Sources of Iris pseudacorus and Iris japonica on Energy Distribution, Nitrogen, and Phosphorus Removal. HortScience 2022, 57, 698–707. [Google Scholar] [CrossRef]
- Zhao, S.; Zhang, Q.; Liu, M.; Zhou, H.; Ma, C.; Wang, P. Regulation of Plant Responses to Salt Stress. Int. J. Mol. Sci. 2021, 22, 4609. [Google Scholar] [CrossRef] [PubMed]
- De Jong, S.M.; Addink, E.A.; Doelman, J.C. Detecting Leaf-Water Content in Mediterranean Trees Using High-Resolution Spectrometry. Int. J. Appl. Earth Obs. Geoinf. 2014, 27, 128–136. [Google Scholar] [CrossRef]
- Nguyen, C.T.; Kurenda, A.; Stolz, S.; Chételat, A.; Farmer, E.E. Identification of Cell Populations Necessary for Leaf-to-Leaf Electrical Signaling in a Wounded Plant. Proc. Natl. Acad. Sci. USA 2018, 115, 10178–10183. [Google Scholar] [CrossRef]
- Theerawitaya, C.; Samphumphaung, T.; Cha-um, S.; Yamada, N.; Takabe, T. Responses of Nipa Palm (Nypa fruticans) Seedlings, a Mangrove Species, to Salt Stress in Pot Culture. FLORA 2014, 209, 597–603. [Google Scholar] [CrossRef]
- Slama, I.; Abdelly, C.; Bouchereau, A.; Flowers, T.; Savouré, A. Diversity, Distribution and Roles of Osmoprotective Compounds Accumulated in Halophytes under Abiotic Stress. Ann. Bot. 2015, 115, 433–447. [Google Scholar] [CrossRef]
- Flowers, T.J.; Colmer, T.D. Salinity Tolerance in Halophytes. New Phytol. 2008, 179, 945–963. [Google Scholar] [CrossRef]
- Zhang, J.; Shi, H. Physiological and Molecular Mechanisms of Plant Salt Tolerance. Photosynth. Res. 2013, 115, 1–22. [Google Scholar] [CrossRef]
- Kosová, K.; Prášil, I.T.; Vítámvás, P. Protein Contribution to Plant Salinity Response and Tolerance Acquisition. Int. J. Mol. Sci. 2013, 14, 6757–6789. [Google Scholar] [CrossRef]
- Fang, F.; Gui, H.; Li, Z.; Zhang, X. Physiological Adaptation of Six Mangrove Seedlings to Different Salinity. Bull. Bot. Res. 2023, 43, 881–889. (In Chinese) [Google Scholar]
- Ankit, A.; Kamali, S.; Singh, A. Genomic & Structural Diversity and Functional Role of Potassium (K+) Transport Proteins in Plants. Int. J. Biol. Macromol. 2022, 208, 844–857. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Chen, S.; Zhou, X.; Li, C.; Shao, J.; Wang, R.; Fritz, E.; Hüttermann, A.; Polle, A. Effect of NaCl on Photosynthesis, Salt Accumulation and Ion Compartmentation in Two Mangrove Species, Kandelia candel and Bruguiera gymnorhiza. Aquat. Bot. 2008, 88, 303–310. [Google Scholar] [CrossRef]
- Yuan, F.; Xu, Y.; Leng, B.; Wang, B. Beneficial Effects of Salt on Halophyte Growth: Morphology, Cells, and Genes. Open Life Sci. 2019, 14, 191–200. [Google Scholar] [CrossRef]
- Alhaddad, F.A.; Abu-Dieyeh, M.H.; ElAzazi, E.-S.M.; Ahmed, T.A. Salt Tolerance of Selected Halophytes at the Two Initial Growth Stages for Future Management Options. Sci. Rep. 2021, 11, 10194. [Google Scholar] [CrossRef]
- Xu, H.M.; Tam, N.F.Y.; Zan, Q.J.; Bai, M.; Shin, P.K.S.; Vrijmoed, L.L.P.; Cheung, S.G.; Liao, W.B. Effects of Salinity on Anatomical Features and Physiology of a Semi-Mangrove Plant Myoporum bontioides. Mar. Pollut. Bull. 2014, 85, 738–746. [Google Scholar] [CrossRef]
- Parida, A.K.; Das, A.B.; Mittra, B. Effects of NaCl Stress on the Structure, Pigment Complex Composition, and Photosynthetic Activity of Mangrove Bruguiera parviflora Chloroplasts. Photosynthetica 2003, 41, 191–200. [Google Scholar] [CrossRef]
- Hao, G.; Jones, T.J.; Luton, C.; Zhang, Y.; Manzane, E.; Scholz, F.G.; Bucci, S.J.; Cao, K.; Goldstein, G. Hydraulic Redistribution in Dwarf Rhizophora mangle Trees Driven by Interstitial Soil Water Salinity Gradients: Impacts on Hydraulic Architecture and Gas Exchange. Tree Physiol. 2009, 29, 697–705. [Google Scholar] [CrossRef]
- Chen, X.; Wegner, L.H.; Gul, B.; Yu, M.; Shabala, S. Dealing with Extremes: Insights into Development and Operation of Salt Bladders and Glands. Crit. Rev. Plant Sci. 2023, 43, 158–170. [Google Scholar] [CrossRef]
- Sugihara, K.; Hanagata, N.; Dubinsky, Z.; Baba, S.; Karube, I. Molecular Characterization of cDNA Encoding Oxygen Evolving Enhancer Protein 1 Increased by Salt Treatment in the Mangrove Bruguiera gymnorrhiza. Plant Cell Physiol. 2000, 41, 1279–1285. [Google Scholar] [CrossRef]
- Chen, C.I.; Lin, K.H.; Huang, M.Y.; Wong, S.L.; Liao, T.S.; Chen, M.N.; Weng, J.H.; Hsueh, M.L.; Lai, Y.H.; Wang, C.W. Photosynthesis in Response to Different Salinities and Immersions of Two Native Rhizophoraceae Mangroves. Cells 2022, 11, 3054. [Google Scholar] [CrossRef]

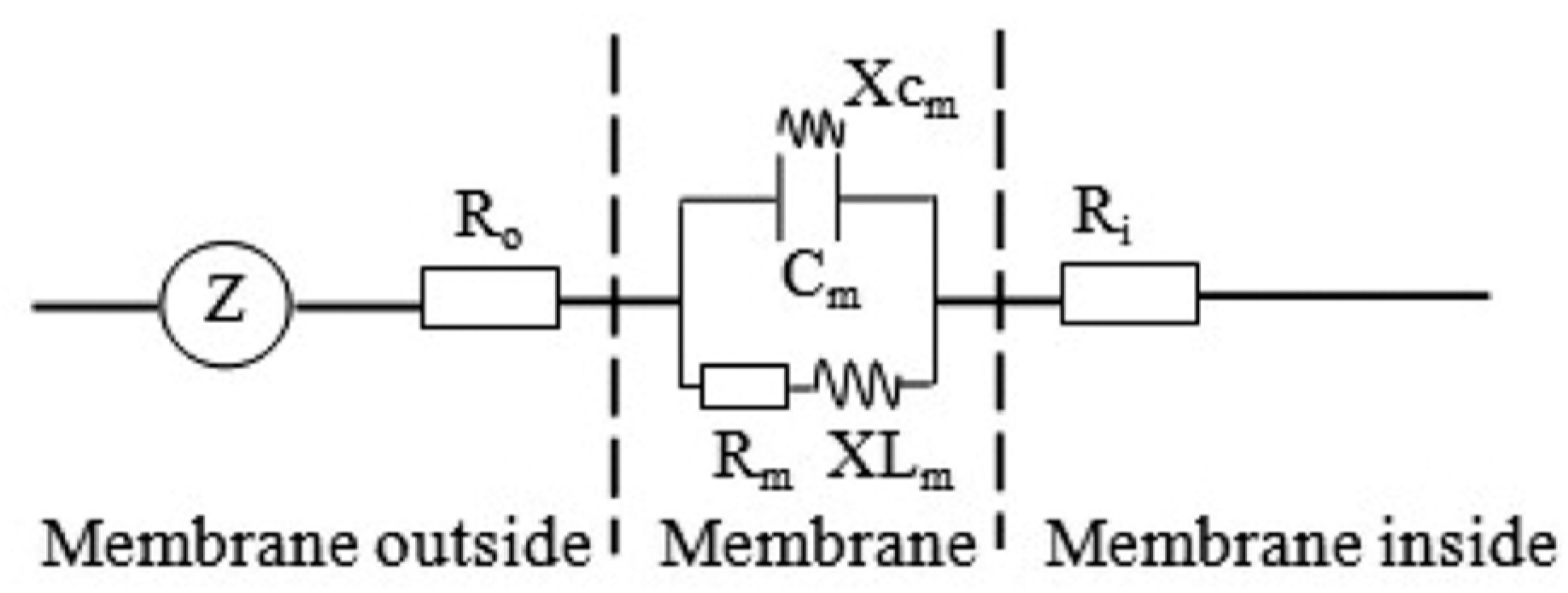
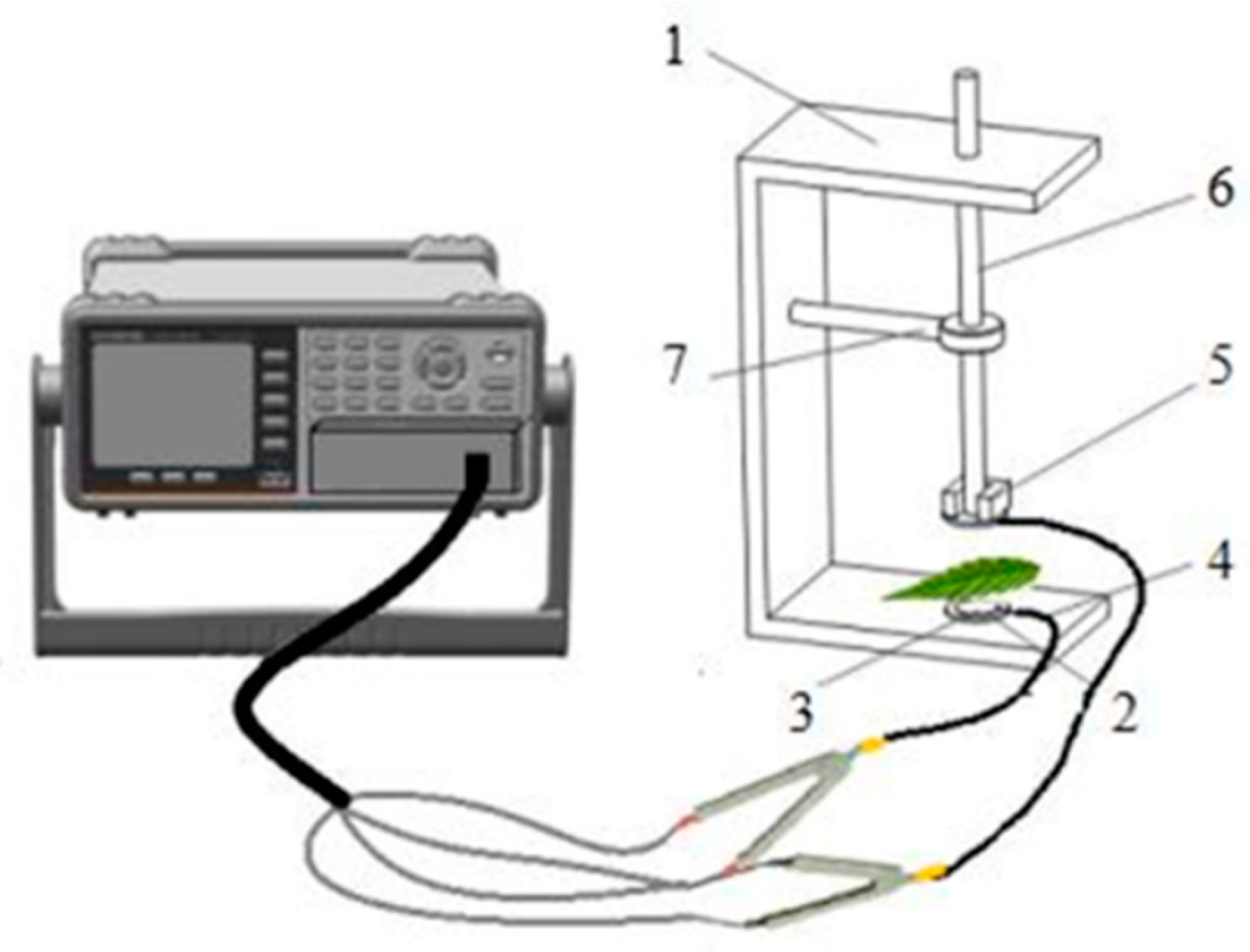
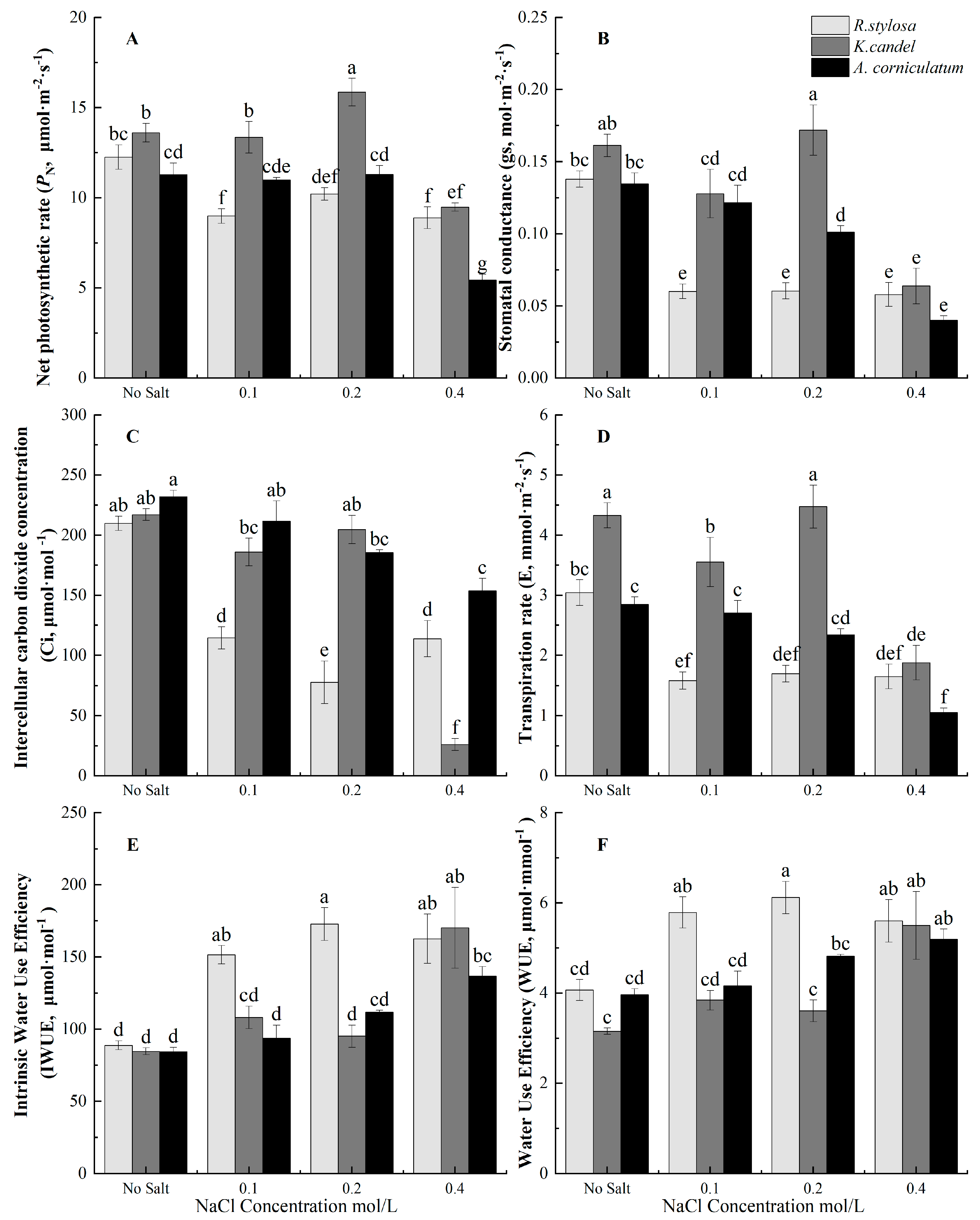
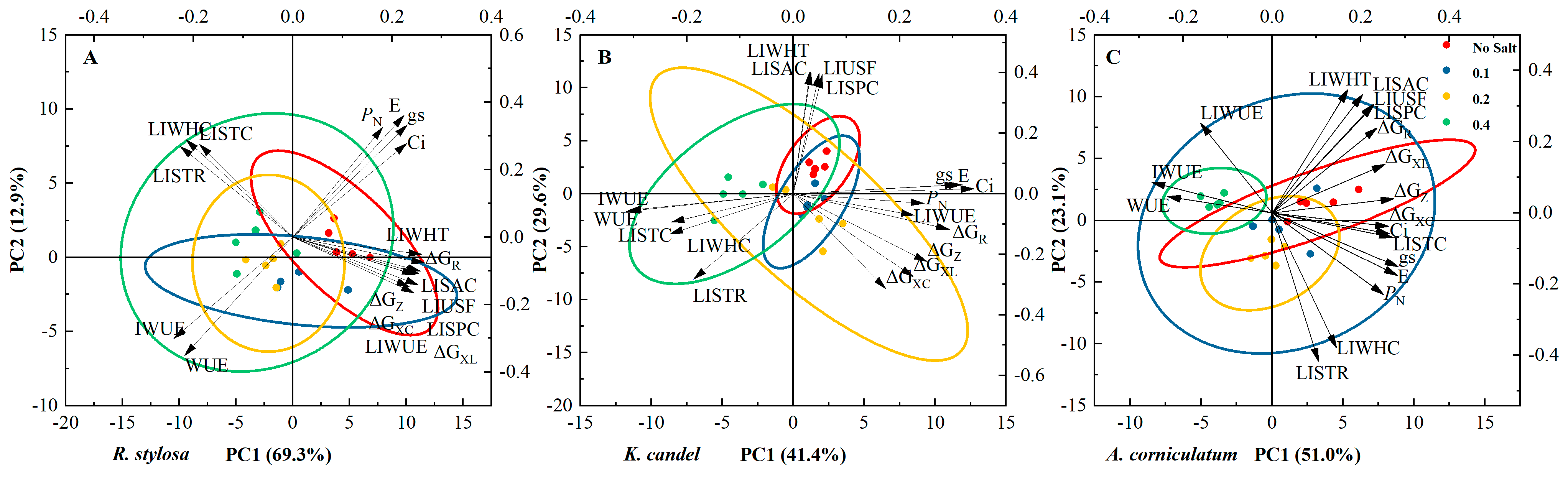
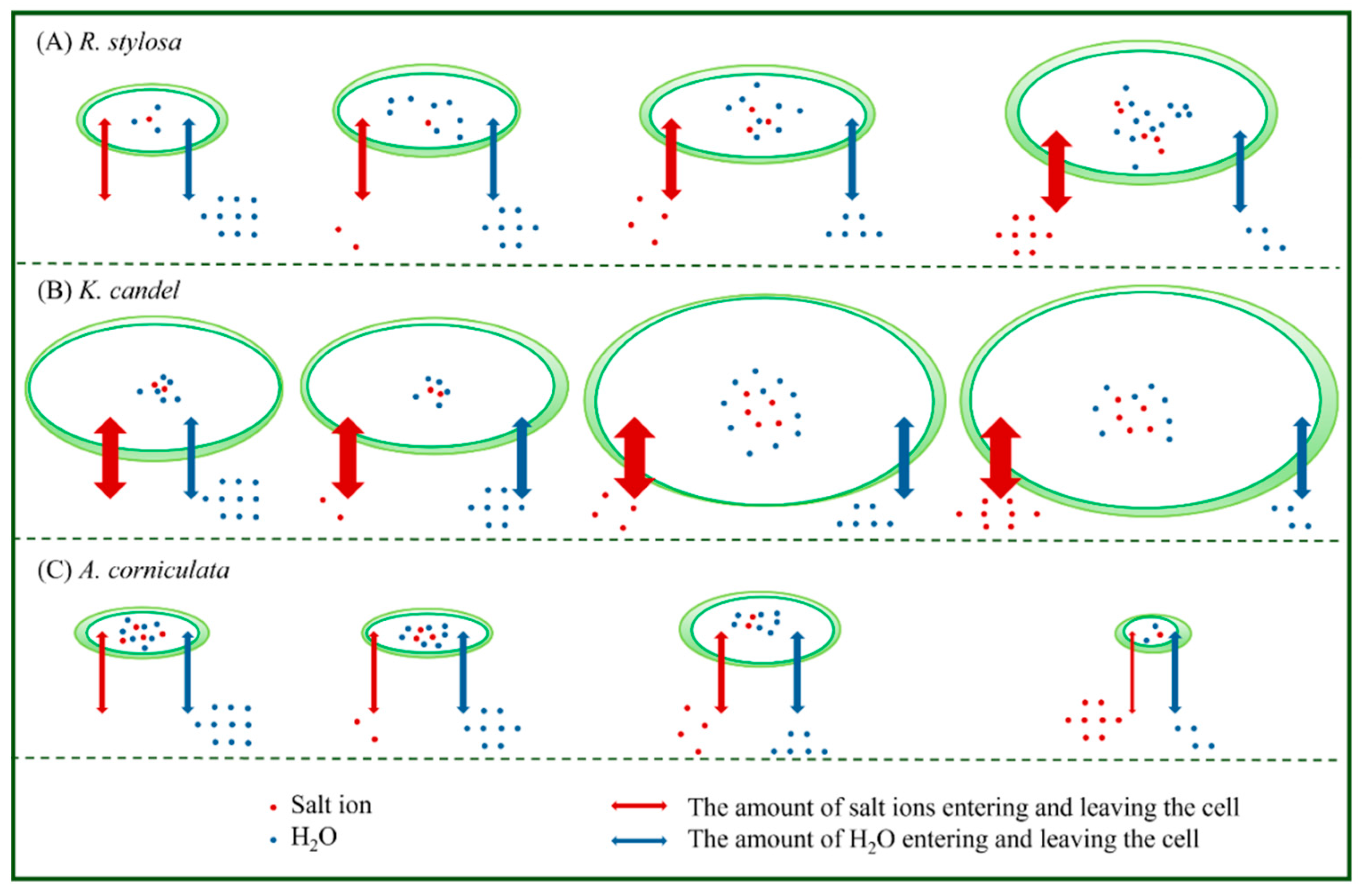
| Plant Species | Salt Balance Mechanisms | Habitats | Leaf Anatomy |
|---|---|---|---|
| R. stylosa | Salt exclusion | Mid-tidal Zone | Dorsiventral leaf with thick upper epidermal cuticle and high tannin content |
| K. candel | Salt exclusion | Low-mid tide Zone | Isobilateral leaf, thick upper and lower epidermal cuticle, white crystals in endodermis, fenestrated and spongy tissues, high tannin content |
| A. corniculatum | Salt exclusion Salt secretion | Low-mid tide Zone | Dorsiventral leaf, upper and lower epidermal cuticle thick and with salt glands, low tannin content |
| Species | NaCl Concentration/mol·L−1 | ∆H/mm | ΨLw/Mpa |
|---|---|---|---|
| R. stylosa | No salt | 35.00 ±2.89 cd | −1.55 ± 0.05 ab |
| 0.1 | 30.00 ±5.00 d | −1.59 ± 0.03 bc | |
| 0.2 | 46.67 ± 4.41 bc | −1.54 ± 0.04 ab | |
| 0.4 | 50.33 ± 3.18 b | −1.65 ± 0.04 bc | |
| K. candel | No salt | 46.67 ± 3.33 bc | −2.09 ± 0.14 d |
| 0.1 | 33.33 ± 1.67 cd | −1.70 ± 0.10 bc | |
| 0.2 | 76.67 ± 6.01 a | −1.63 ± 0. 06 bc | |
| 0.4 | 15.00 ± 7.64 e | −1.94 ± 0.31 cd | |
| A. corniculatum | No salt | 13.33 ± 4.41 e | −1.35 ± 0.03 ab |
| 0.1 | 13.33 ± 7.26 e | −1.32 ± 0.02 ab | |
| 0.2 | 26.67 ± 1.67 de | −1.18 ± 0.03 a | |
| 0.4 | 21.67 ± 1.67 de | −1.56 ± 0.17 ab |
| Species | NaCl Concentration/mol L−1 | LIWHC | LIWUE (×10−1) | LIWHT |
|---|---|---|---|---|
| R. stylosa | No salt | 313.80 ± 49.14 de | 1.28 ± 0.29 bcd | 48.41 ± 0.59 ab |
| 0.1 | 496.97 ± 49.29 de | 0.86 ± 0.14 cde | 46.54 ± 1.72 ab | |
| 0.2 | 667.72 ± 93.62 cd | 0.55 ± 0.06 de | 39.45 ± 0.86 c | |
| 0.4 | 1164.21 ± 154.21 b | 0.36 ± 0.08 e | 38.59 ± 1.39 c | |
| K. candel | No salt | 1183.06 ± 72.84 b | 0.57 ± 0.04 de | 38.54 ± 1.35 c |
| 0.1 | 1128.80 ± 122.25 bc | 0.78 ± 0.15 cde | 26.27 ± 2.02 d | |
| 0.2 | 2618.53 ± 319.66 a | 0.46 ± 0.12 de | 24.98 ± 3.28 d | |
| 0.4 | 2832.50 ± 400.19 a | 0.25 ± 0.03 e | 28.70 ± 2.77 d | |
| A. corniculatum | No salt | 170.22 ± 18.89 de | 1.69 ± 0.37 b | 51.39 ± 0.70 a |
| 0.1 | 182.90 ± 28.21 de | 1.45 ± 0.16 bc | 46.49 ± 2.14 ab | |
| 0.2 | 353.10 ± 37.80 de | 0.87 ± 0.12 cde | 40.29 ± 1.67 c | |
| 0.4 | 53.05 ± 6.72 e | 3.16 ± 0.67 a | 44.30 ± 1.84 bc |
| Species | NaCl Concentration/mol L−1 | LISAC | LISPC | LIUSF | LISTR | LISTC | LISAC/LIUSF (%) | LISPC/LIUSF (%) |
|---|---|---|---|---|---|---|---|---|
| R. stylosa | No salt | 0.79 ± 0.01 ab | 2.29 ± 0.16 bc | 3.09 ± 0.17 b | 6.52 ± 1.06 d | 19.56 ± 2.58 de | 25.71% | 74.29% |
| 0.1 | 0.77 ± 0.05 b | 2.12 ± 0.4 bcd | 2.89 ± 0.45 bc | 10.78 ± 1.15 cd | 29.58 ± 3.01 cd | 26.64% | 73.36% | |
| 0.2 | 0.63 ± 0.02 c | 1.18 ± 0.07 de | 1.82 ± 0.09 cde | 17.04 ± 2.62 cd | 30.59 ± 4.08 cd | 34.95% | 65.05% | |
| 0.4 | 0.63 ± 0.02 c | 1.15 ± 0.07 de | 1.77 ± 0.08 cde | 30.24 ± 3.86 bc | 52.92 ± 6.18 b | 35.28% | 64.72% | |
| K. candel | No salt | 0.60 ± 0.02 c | 1.08 ± 0.08 de | 1.68 ± 0.10 de | 30.94 ± 2.39 bc | 51.44 ± 3.21 b | 35.90% | 64.10% |
| 0.1 | 0.42 ± 0.03 d | 0.58 ± 0.06 e | 1.01 ± 0.09 e | 43.69 ± 5.57 b | 43.09 ± 4.70 bc | 41.93% | 58.07% | |
| 0.2 | 0.40 ± 0.05 d | 0.56 ± 0.09 e | 0.96 ± 0.14 e | 107.48 ± 10.83 a | 100.42 ± 13.63 a | 41.97% | 58.03% | |
| 0.4 | 0.46 ± 0.04 d | 0.67 ± 0.09 e | 1.12 ± 0.13 de | 103.77 ± 21.03 a | 109.84 ± 15.42 a | 40.67% | 59.33% | |
| A. corniculatum | No salt | 0.88 ± 0.02 a | 4.26 ± 0.75 a | 5.14 ± 0.77 a | 3.30 ± 0.33 d | 17.73 ± 4.31 de | 17.11% | 82.89% |
| 0.1 | 0.77 ± 0.05 b | 2.57 ± 0.78 b | 3.34 ± 0.83 b | 4.02 ± 0.78 d | 12.57 ± 2.90 de | 23.05% | 76.94% | |
| 0.2 | 0.65 ± 0.03 c | 1.26 ± 0.10 cde | 1.91 ± 0.12 cde | 8.74 ± 0.79 cd | 16.71 ± 2.00 de | 34.05% | 65.95% | |
| 0.4 | 0.71 ± 0.03 bc | 1.61 ± 0.23 bcde | 2.31 ± 0.26 bcd | 1.18 ± 0.11 d | 2.82 ± 0.51 e | 30.55% | 69.45% |
| Species | NaCl Concentration/mol L−1 | ΔGR | ΔGZ | ΔGXC | ΔGXL |
|---|---|---|---|---|---|
| R. stylosa | No salt | 76.28 ± 7.38 bc | 91.42 ± 9.79 bc | 95.81 ± 10.7 d | 80.85 ± 8.01 d |
| 0.1 | 61.09 ± 6.90 bc | 77.61 ± 8.91 bc | 82.02 ± 9.96 d | 66.59 ± 6.99 d | |
| 0.2 | 37.39 ± 4.81 c | 55.23 ± 5.04 bc | 65.76 ± 4.87 d | 48.13 ± 4.54 d | |
| 0.4 | 34.68 ± 8.40 c | 52.56 ± 9.48 c | 63.06 ± 9.62 d | 45.31 ± 8.22 d | |
| K. candel | No salt | 187.71 ± 11.55 a | 178.24 ± 6.83 a | 171.33 ± 6.82 bc | 178.37 ± 7.03 bc |
| 0.1 | 219.26 ± 19.35 a | 218.95 ± 15.50 a | 229.39 ± 14.80 ab | 225.31 ± 12.33 ab | |
| 0.2 | 196.13 ± 38.72 a | 229.01 ± 52.32 a | 284.72 ± 62.32 a | 253.92 ± 55.59 a | |
| 0.4 | 96.19 ± 12.79 b | 112.95 ± 11.83 b | 156.63 ± 19.11 c | 133.17 ± 13.78 c | |
| A. corniculatum | No salt | 75.50 ± 14.48 bc | 83.83 ± 15.70 bc | 85.50 ± 16.16 d | 75.77 ± 14.37 d |
| 0.1 | 47.97 ± 11.47 c | 70.50 ± 11.16 bc | 75.56 ± 12.22 d | 55.04 ± 10.98 d | |
| 0.2 | 39.27 ± 6.65 c | 64.92 ± 6.09 bc | 77.21 ± 6.46 d | 52.38 ± 6.43 d | |
| 0.4 | 38.65 ± 3.19 c | 38.96 ± 2.41 c | 39.41 ± 2.51 d | 38.52 ± 2.46 d |
| Plant Species | Leaf Intracellular Water-Use Parameters | Salt Transfer Parameters | Cellular Endogenous Convertible Energy | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| LIWHC | LIWUE | LIWHT | LISAC | LISPC | LIUSF | LISTR | LISTC | LISAC/ LIUSF | LISPC/ LIUSF | ΔGR | ΔGZ | ΔGXC | ΔGXL | |
| R. stylosa | low | low | high | middle | middle | middle | middle | middle | low | high | low | low | low | low |
| K. candel | high | low | low | low | low | low | high | high | high | low | high | high | high | high |
| A. corniculatum | low | high | high | high | high | high | low | low | low | high | low | low | low | low |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, J.; Wu, Y.; Xing, D.; Zhang, Q.; Quispe Puma, M.R.; Chen, Q. Dynamic Traits of Intracellular Water and Salt Based on Electrophysiological Measurements During Adaptations of Three Mangrove Species Under Salinity Stresses. Horticulturae 2025, 11, 309. https://doi.org/10.3390/horticulturae11030309
Wang J, Wu Y, Xing D, Zhang Q, Quispe Puma MR, Chen Q. Dynamic Traits of Intracellular Water and Salt Based on Electrophysiological Measurements During Adaptations of Three Mangrove Species Under Salinity Stresses. Horticulturae. 2025; 11(3):309. https://doi.org/10.3390/horticulturae11030309
Chicago/Turabian StyleWang, Jing, Yanyou Wu, Deke Xing, Qian Zhang, Melisa Romina Quispe Puma, and Qian Chen. 2025. "Dynamic Traits of Intracellular Water and Salt Based on Electrophysiological Measurements During Adaptations of Three Mangrove Species Under Salinity Stresses" Horticulturae 11, no. 3: 309. https://doi.org/10.3390/horticulturae11030309
APA StyleWang, J., Wu, Y., Xing, D., Zhang, Q., Quispe Puma, M. R., & Chen, Q. (2025). Dynamic Traits of Intracellular Water and Salt Based on Electrophysiological Measurements During Adaptations of Three Mangrove Species Under Salinity Stresses. Horticulturae, 11(3), 309. https://doi.org/10.3390/horticulturae11030309








