The Effect of Cropping System and Irrigation Regime on the Plant Growth and Biochemical Profile of Cichorium spinosum
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Conditions
2.2. Nutritional Characterization
2.3. Organic Acids
2.4. Tocopherols
2.5. Sugars Composition
2.6. Mineral Composition
2.7. Fatty-Acid Composition
2.8. Phenolic Compounds and Bioactive Properties
2.8.1. Phenolic Compounds Profile
2.8.2. Antioxidant Properties
2.8.3. Antibacterial and Antifungal Properties
2.8.4. NO-Production Inhibition, Antiproliferative and Hepatotoxic Properties
2.9. Statistical Analysis
3. Results and Discussion
3.1. Growth Parameters
3.2. Chemical Composition
3.2.1. Nutritional Characterization
3.2.2. Organic Acids
3.2.3. Tocopherols
3.2.4. Sugars Composition
3.2.5. Mineral Composition
3.2.6. Fatty-Acid Composition
3.3. Phenolic Compounds and Bioactive Properties
3.3.1. Phenolic Compounds
3.3.2. Bioactive Properties
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ceccanti, C.; Landi, M.; Benvenuti, S.; Pardossi, A.; Guidi, L. Mediterranean wild edible plants: Weeds or “new functional crops”? Molecules 2018, 23, 2299. [Google Scholar] [CrossRef] [PubMed]
- Corrêa, R.C.G.; Di Gioia, F.; Ferreira, I.C.F.R.; Petropoulos, S.A. Wild greens used in the Mediterranean diet. In The Mediterranean Diet: An Evidence-based Approach; Preedy, V., Watson, R., Eds.; Academic Press: London, UK, 2020; pp. 209–228. ISBN 9788578110796. [Google Scholar]
- Sánchez-Mata, M.d.C.; Tardío, J. Mediterranean Wild Edible Plants; Springer: New York, NY, USA, 2016; ISBN 978-1-4939-3327-3. [Google Scholar]
- Chatzopoulou, E.; Carocho, M.; Di Gioia, F.; Petropoulos, S.A. The beneficial health effects of vegetables and wild edible greens: The case of the mediterranean diet and its sustainability. Appl. Sci. 2020, 10, 9144. [Google Scholar] [CrossRef]
- Borelli, T.; Hunter, D.; Powell, B.; Ulian, T.; Mattana, E.; Termote, C.; Pawera, L.; Beltrame, D.; Penafiel, D.; Tan, A.; et al. Born to eat wild: An integrated conservation approach to secure wild food plants for food security and nutrition. Plants 2020, 9, 1299. [Google Scholar] [CrossRef] [PubMed]
- Psaroudaki, A.; Nikoloudakis, N.; Skaracis, G.; Katsiotis, A. Genetic structure and population diversity of eleven edible herbs of Eastern Crete. J. Biol. Res. 2015, 22, 7. [Google Scholar] [CrossRef]
- Klados, E.; Tzortzakis, N. Effects of substrate and salinity in hydroponically grown Cichorium spinosum. J. Soil. Sci. Plant Nutr. 2014, 14, 211–222. [Google Scholar] [CrossRef]
- Petropoulos, S.A.; Karkanis, A.; Martins, N.; Ferreira, I.C.F.R. Edible halophytes of the Mediterranean basin: Potential candidates for novel food products. Trends Food Sci. Technol. 2018, 74, 69–84. [Google Scholar] [CrossRef]
- Vardavas, C.I.; Majchrzak, D.; Wagner, K.H.; Elmadfa, I.; Kafatos, A. The antioxidant and phylloquinone content of wildly grown greens in Crete. Food Chem. 2006, 99, 813–821. [Google Scholar] [CrossRef]
- Zegichi, S.; Kalithraka, S.; Simopoulos, A. Nutritional composition of molokhia (Corchorus olitorius) and stamnagathi (Cichorium spinosum). World Rev. Nutr. Diet. 2003, 91, 1–21. [Google Scholar]
- Brieudes, V.; Angelis, A.; Vougogiannopoulos, K.; Pratsinis, H.; Kletsas, D.; Mitakou, S.; Halabalaki, M.; Skaltsounis, L.A. Phytochemical analysis and antioxidant potential of the phytonutrient-rich decoction of Cichorium spinosum and C. intybus. Planta Med. 2016, 82, 1070–1078. [Google Scholar] [CrossRef]
- Petropoulos, S.A.; Fernandes, Â.; Tzortzakis, N.; Sokovic, M.; Ciric, A.; Barros, L.; Ferreira, I.C.F.R. Bioactive compounds content and antimicrobial activities of wild edible Asteraceae species of the Mediterranean flora under commercial cultivation conditions. Food Res. Int. 2019, 119, 859–868. [Google Scholar] [CrossRef]
- Chatzigianni, M.; Alkhaled, B.; Livieratos, I.; Stamatakis, A.; Ntatsi, G.; Savvas, D. Impact of nitrogen source and supply level on growth, yield and nutritional value of two contrasting ecotypes of Cichorium spinosum L. grown hydroponically. J. Sci. Food Agric. 2018, 98, 1615–1624. [Google Scholar] [CrossRef] [PubMed]
- Mancosu, N.; Snyder, R.L.; Kyriakakis, G.; Spano, D. Water scarcity and future challenges for food production. Water 2015, 7, 975–992. [Google Scholar] [CrossRef]
- Lovelli, S.; Perniola, M.; Scalcione, E.; Troccoli, A.; Ziska, L.H. Future climate change in the Mediterranean area: Implications for water use and weed management. Ital. J. Agron. 2012, 7, 44–49. [Google Scholar] [CrossRef]
- Adu, M.O.; Yawson, D.O.; Armah, F.A.; Asare, P.A.; Frimpong, K.A. Meta-analysis of crop yields of full, deficit, and partial root-zone drying irrigation. Agric. Water Manag. 2018, 197, 79–90. [Google Scholar] [CrossRef]
- Claro, A.M.; Fonseca, A.; Fraga, H.; Santos, J.A. Future Agricultural Water Availability in Mediterranean Countries under Climate Change: A Systematic Review. Water 2024, 16, 2484. [Google Scholar] [CrossRef]
- Fischer, G.; Tubiello, F.N.; van Velthuizen, H.; Wiberg, D.A. Climate change impacts on irrigation water requirements: Effects of mitigation, 1990–2080. Technol. Forecast. Soc. Change 2007, 74, 1083–1107. [Google Scholar] [CrossRef]
- Nikolaou, G.; Neocleous, D.; Christou, A.; Kitta, E.; Katsoulas, N. Implementing sustainable irrigation in water-scarce regions under the impact of climate change. Agronomy 2020, 10, 1120. [Google Scholar] [CrossRef]
- Neupane, J.; Guo, W. Agronomic basis and strategies for precision water management: A review. Agronomy 2019, 9, 87. [Google Scholar] [CrossRef]
- Savé, R.; de Herralde, F.; Aranda, X.; Pla, E.; Pascual, D.; Funes, I.; Biel, C. Potential changes in irrigation requirements and phenology of maize, apple trees and alfalfa under global change conditions in Fluvià watershed during XXIst century: Results from a modeling approximation to watershed-level water balance. Agric. Water Manag. 2012, 114, 78–87. [Google Scholar] [CrossRef]
- Galindo, A.; Collado-González, J.; Griñán, I.; Corell, M.; Centeno, A.; Martín-Palomo, M.J.; Girón, I.F.; Rodríguez, P.; Cruz, Z.N.; Memmi, H.; et al. Deficit irrigation and emerging fruit crops as a strategy to save water in Mediterranean semiarid agrosystems. Agric. Water Manag. 2018, 202, 311–324. [Google Scholar] [CrossRef]
- Ünlü, M.; Kanber, R.; Şenyigit, U.; Onaran, H.; Diker, K. Trickle and sprinkler irrigation of potato (Solanum tuberosum L.) in the Middle Anatolian Region in Turkey. Agric. Water Manag. 2006, 79, 43–71. [Google Scholar] [CrossRef]
- De Boni, A.; D’Amico, A.; Acciani, C.; Roma, R. Crop Diversification and Resilience of Drought-Resistant Species in Semi-Arid Areas: An Economic and Environmental Analysis. Sustainability 2022, 14, 9552. [Google Scholar] [CrossRef]
- Wang, X.; Li, X.; Zhao, W.; Hou, X.; Dong, S. Current views of drought research: Experimental methods, adaptation mechanisms and regulatory strategies. Front. Plant Sci. 2024, 15, 1371895. [Google Scholar] [CrossRef] [PubMed]
- Fita, A.; Rodríguez-Burruezo, A.; Boscaiu, M.; Prohens, J.; Vicente, O. Breeding and domesticating crops adapted to drought and salinity: A new paradigm for increasing food production. Front. Plant Sci. 2015, 6, 978. [Google Scholar] [CrossRef] [PubMed]
- Jia, P.Y.; Zhang, L.X.; Huang, Z.; Tian, F.P.; Hu, Y.; Wu, G.L. Physiological characteristics of three wild Sonchus species to prolonged drought tolerance in arid regions. Pakistan J. Bot. 2018, 50, 9–17. [Google Scholar]
- Guarise, M.; Borgonovo, G.; Bassoli, A.; Ferrante, A. The Effect of Drought on Sisymbrium officinale (L.) Wild Species for Potential Cultivation as a Leafy Vegetable. Horticulturae 2023, 9, 111. [Google Scholar] [CrossRef]
- Christoforidi, I.; Kollaros, D.; Manios, T.; Daliakopoulos, I.N. Drought- and Salt-Tolerant Plants of the Mediterranean and Their Diverse Applications: The Case of Crete. Land 2022, 11, 2038. [Google Scholar] [CrossRef]
- Litskas, V.D.; Chrysargyris, A.; Tzortzakis, N.; Stavrinides, M.C.; Petropoulos, S.A. Can the commercial cultivation of wild edible species contribute to sustainable food production? A case study of golden thistle (Scolymus hispanicus L.). Int. J. Life Cycle Assess. 2025, 30, 446–461. [Google Scholar] [CrossRef]
- Borelli, T.; Hunter, D.; Padulosi, S.; Amaya, N.; Meldrum, G.; de Oliveira Beltrame, D.M.; Samarasinghe, G.; Wasike, V.W.; Güner, B.; Tan, A.; et al. Local solutions for sustainable food systems: The contribution of orphan crops and wild edible species. Agronomy 2020, 10, 231. [Google Scholar] [CrossRef]
- Ray, A.; Ray, R.; Sreevidya, E.A. How Many Wild Edible Plants Do We Eat—Their Diversity, Use, and Implications for Sustainable Food System: An Exploratory Analysis in India. Front. Sustain. Food Syst. 2020, 4, 56. [Google Scholar] [CrossRef]
- Polyzos, N.; Paschoalinotto, B.H.; Pires, T.C.S.P.; Añibarro-Ortega, M.; Calhelha, R.; Ferreira, I.C.F.R.; Dias, M.I.; Barros, L.; Petropoulos, S.A. The Impact of Deficit Irrigation on the Agronomic Performance and Chemical Composition of Scolymus hispanicus L. Horticulturae 2024, 10, 479. [Google Scholar] [CrossRef]
- Calone, R.; Mircea, D.M.; González-Orenga, S.; Boscaiu, M.; Lambertini, C.; Barbanti, L.; Vicente, O. Recovery from Salinity and Drought Stress in the Perennial Sarcocornia fruticosa vs. the Annual Salicornia europaea and S. veneta. Plants 2022, 11, 1058. [Google Scholar] [CrossRef]
- Bowles, T.M.; Mooshammer, M.; Socolar, Y.; Calderón, F.; Cavigelli, M.A.; Culman, S.W.; Deen, W.; Drury, C.F.; Garcia y Garcia, A.; Gaudin, A.C.M.; et al. Long-Term Evidence Shows that Crop-Rotation Diversification Increases Agricultural Resilience to Adverse Growing Conditions in North America. One Earth 2020, 2, 284–293. [Google Scholar] [CrossRef]
- Li, J.; Huang, L.; Zhang, J.; Coulter, J.A.; Li, L.; Gan, Y. Diversifying crop rotation improves system robustness. Agron. Sustain. Dev. 2019, 39, 38. [Google Scholar] [CrossRef]
- Yu, T.; Mahe, L.; Li, Y.; Wei, X.; Deng, X.; Zhang, D. Benefits of Crop Rotation on Climate Resilience and Its Prospects in China. Agronomy 2022, 12, 436. [Google Scholar] [CrossRef]
- Preissel, S.; Reckling, M.; Schläfke, N.; Zander, P. Magnitude and farm-economic value of grain legume pre-crop benefits in Europe: A review. F. Crop. Res. 2015, 175, 64–79. [Google Scholar] [CrossRef]
- Lötjönen, S.; Ollikainen, M. Does crop rotation with legumes provide an efficient means to reduce nutrient loads and GHG emissions? Rev. Agric. Food Environ. Stud. 2017, 98, 283–312. [Google Scholar] [CrossRef]
- Reckling, M.; Hecker, J.M.; Bergkvist, G.; Watson, C.A.; Zander, P.; Schläfke, N.; Stoddard, F.L.; Eory, V.; Topp, C.F.E.; Maire, J.; et al. A cropping system assessment framework—Evaluating effects of introducing legumes into crop rotations. Eur. J. Agron. 2016, 76, 186–197. [Google Scholar] [CrossRef]
- Polyzos, N.; Paschoalinotto, B.H.; Compocholi, M.; Pinela, J.; Heleno, S.A.; Calhelha, R.C.; Dias, M.I.; Barros, L.; Petropoulos, S.A. Fertilization of pot-grown Cichorium spinosum L.: How it can affect plant growth, chemical profile, and bioactivities of edible parts? Horticulturae 2022, 8, 890. [Google Scholar] [CrossRef]
- AOAC. Official Methods of Analysis of Association of Official Analytical Chemists; Horwitz, W., Latimer, G., Eds.; AOAC Inter.: Gaithersburg, MD, USA, 2019; ISBN 0935584773. [Google Scholar]
- Mohamed, M.H.M.; Ali, M.M.E.; Zewail, R.M.Y.; Liava, V.; Petropoulos, S.A. The Mitigating Effects of Biostimulant Amendments on the Response of Purslane Plants Grown under Drought Stress Conditions. Horticulturae 2024, 10, 858. [Google Scholar] [CrossRef]
- Saheri, F.; Barzin, G.; Pishkar, L.; Boojar, M.M.A.; Babaeekhou, L. Correction to: Foliar spray of salicylic acid induces physiological and biochemical changes in purslane (Portulaca oleracea L.) under drought stress. Biologia 2020, 75, 2189–2200. [Google Scholar] [CrossRef]
- Delfine, S.; Fratianni, A.; D’Agostino, A.; Panfili, G. Influence of Drought Stress on Physiological Responses and Bioactive Compounds in Chicory (Cichorium intybus L.): Opportunity for a Sustainable Agriculture. Foods 2022, 11, 3725. [Google Scholar] [CrossRef] [PubMed]
- Nemecek, T.; von Richthofen, J.S.; Dubois, G.; Casta, P.; Charles, R.; Pahl, H. Environmental impacts of introducing grain legumes into European crop rotations. Eur. J. Agron. 2008, 28, 380–393. [Google Scholar] [CrossRef]
- Venter, Z.S.; Jacobs, K.; Hawkins, H.J. The impact of crop rotation on soil microbial diversity: A meta-analysis. Pedobiologia 2016, 59, 215–223. [Google Scholar] [CrossRef]
- Zhao, J.; Chen, J.; Beillouin, D.; Lambers, H.; Yang, Y.; Smith, P.; Zeng, Z.; Olesen, J.E.; Zang, H. Global systematic review with meta-analysis reveals yield advantage of legume-based rotations and its drivers. Nat. Commun. 2022, 13, 4926. [Google Scholar] [CrossRef]
- Jensen, C.R.; Joernsgaard, B.; Andersen, M.N.; Christiansen, J.L.; Mogensen, V.O.; Friis, P.; Petersen, C.T. The effect of lupins as compared with peas and oats on the yield of the subsequent winter barley crop. Eur. J. Agron. 2004, 20, 405–418. [Google Scholar] [CrossRef]
- Sánchez-Navarro, V.; Zornoza, R.; Faz, Á.; Fernández, J.A. Does the use of cowpea in rotation with a vegetable crop improve soil quality and crop yield and quality? A field study in SE Spain. Eur. J. Agron. 2019, 107, 10–17. [Google Scholar] [CrossRef]
- Cui, Z.; Yan, B.; Gao, Y.; Wu, B.; Wang, Y.; Xie, Y.; Xu, P.; Wang, H.; Wen, M.; Wang, Y.; et al. Crop yield and water use efficiency in response to long-term diversified crop rotations. Front. Plant Sci. 2022, 13, 1024898. [Google Scholar] [CrossRef]
- Ghadirnezhad Shiade, S.R.; Fathi, A.; Taghavi Ghasemkheili, F.; Amiri, E.; Pessarakli, M. Plants’ responses under drought stress conditions: Effects of strategic management approaches—A review. J. Plant Nutr. 2023, 46, 2198–2230. [Google Scholar] [CrossRef]
- Yousefvand, P.; Sohrabi, Y.; Mastinu, A.; Heidari, G.; Weisany, W. Salicylic acid altered the fatty acids compositions and nutrient status of shallot (Allium hirtifolium) grown under drought stress. J. Agric. Food Res. 2024, 18, 101502. [Google Scholar] [CrossRef]
- Allamine, H.M.; Buyuktas, D.; Karaca, C.; Aydinsakir, K.; Erdurmus, C. Effect of regulated deficit irrigation on productivity, evapotranspiration and quality of grain sorghum. Irrig. Sci. 2023, 41, 277–293. [Google Scholar] [CrossRef]
- Abdou, N.M.; Roby, M.H.H.; AL-Huqail, A.A.; Elkelish, A.; Sayed, A.A.S.; Alharbi, B.M.; Mahdy, H.A.A.; Abou-Sreea, A.I.B. Compost Improving Morphophysiological and Biochemical Traits, Seed Yield, and Oil Quality of Nigella sativa under Drought Stress. Agronomy 2023, 13, 1147. [Google Scholar] [CrossRef]
- Petropoulos, S.A.; Levizou, E.; Ntatsi, G.; Fernandes, Â.; Petrotos, K.; Akoumianakis, K.; Barros, L.; Ferreira, I.C.F.R. Salinity effect on nutritional value, chemical composition and bioactive compounds content of Cichorium spinosum L. Food Chem. 2017, 214, 129–136. [Google Scholar] [CrossRef]
- Petropoulos, S.A.; Fernandes, Â.; Dias, M.I.; Pereira, C.; Calhelha, R.C.; Chrysargyris, A.; Tzortzakis, N.; Ivanov, M.; Sokovic, M.D.; Barros, L.; et al. Chemical composition and plant growth of Centaurea raphanina subsp. mixta plants cultivated under saline conditions. Molecules 2020, 25, 2204. [Google Scholar] [CrossRef]
- Petropoulos, S.; Fernandes, Â.; Karkanis, A.; Antoniadis, V.; Barros, L.; Ferreira, I.C.F.R. Nutrient solution composition and growing season affect yield and chemical composition of Cichorium spinosum plants. Sci. Hortic. 2018, 231, 97–107. [Google Scholar] [CrossRef]
- Liava, V.; Fernandes, Â.; Reis, F.; Finimundy, T.; Mandim, F.; Pinela, J.; Stojković, D.; Ferreira, I.C.F.R.; Barros, L.; Petropoulos, S.A. How Does Domestic Cooking Affect the Biochemical Properties of Wild Edible Greens of the Asteraceae Family? Foods 2024, 13, 2677. [Google Scholar] [CrossRef] [PubMed]
- Al Muhairi, M.A.; Cheruth, A.J.; Kurup, S.S.; Rabert, G.A.; Al-Yafei, M.S. Effect of abscisic acid on biochemical constituents, enzymatic and non enzymatic antioxidant status of lettuce (Lactuca sativa L.) under varied irrigation regimes. Cogent Food Agric. 2015, 1, 1080888. [Google Scholar] [CrossRef]
- Skrypnik, L.; Maslennikov, P.; Antipina, M.; Katserov, D.; Feduraev, P. Comparative Study on the Response of Hyssop (Hyssopus officinalis L.), Salvia (Salvia officinalis L.), and Oregano (Origanum vulgare L.) to Drought Stress Under Foliar Application of Selenium. Plants 2024, 13, 2986. [Google Scholar] [CrossRef] [PubMed]
- Zhao, W.; Wu, K.; Wu, Y.; Yu, H.; Cao, W.; Ma, H. Effects of biochar amendment on greenhouse tomato quality, nutrient uptake and use efficiency under various irrigation and fertilization regimes. Sci. Hortic. 2024, 337, 113441. [Google Scholar] [CrossRef]
- Petropoulos, S.A.; Fernandes, Â.; Calhelha, R.C.; Di Gioia, F.; Kolovou, P.; Barros, L.; Ferreira, I.C.F.R. Chemical composition and bioactive properties of Cichorium spinosum L. in relation to nitrate/ammonium nitrogen ratio. J. Sci. Food Agric. 2019, 99, 6741–6750. [Google Scholar] [CrossRef]
- Petropoulos, S.A.; Fernandes, Â.; Ntatsi, G.; Levizou, E.; Barros, L.; Ferreira, I.C.F.R. Nutritional profile and chemical composition of Cichorium spinosum ecotypes. LWT-Food Sci. Technol. 2016, 73, 95–101. [Google Scholar] [CrossRef]
- Petropoulos, S.; Fernandes, Â.; Karkanis, A.; Ntatsi, G.; Barros, L.; Ferreira, I. Successive harvesting affects yield, chemical composition and antioxidant activity of Cichorium spinosum L. Food Chem. 2017, 237, 83–90. [Google Scholar] [CrossRef]
- Faizan, M.; Alam, P.; Rajput, V.D.; Shareen; Kaur, K.; Faraz, A.; Minkina, T.; Maqbool Ahmed, S.; Rajpal, V.R.; Hayat, S. Potential role of tocopherol in protecting crop plants against abiotic stresses. Physiol. Mol. Biol. Plants 2023, 29, 1563–1575. [Google Scholar] [CrossRef] [PubMed]
- Ali, E.; Hussain, S.; Hussain, N.; Kakar, K.U.; Shah, J.M.; Zaidi, S.H.R.; Jan, M.; Zhang, K.; Khan, M.A.; Imtiaz, M. Tocopherol as plant protector: An overview of Tocopherol biosynthesis enzymes and their role as antioxidant and signaling molecules. Acta Physiol. Plant. 2022, 44, 20. [Google Scholar] [CrossRef]
- Munné-Bosch, S.; Falk, J. New insights into the function of tocopherols in plants. Planta 2004, 218, 323–326. [Google Scholar] [CrossRef] [PubMed]
- Sadiq, M.; Aisha, N.; Muhammad, A.; Al, F.; Parvaiz, Q. Alpha-Tocopherol-Induced Regulation of Growth and Metabolism in Plants Under Non-stress and Stress Conditions. J. Plant Growth Regul. 2019, 38, 1325–1340. [Google Scholar] [CrossRef]
- Kapur, B.; Karaca, C.; Sarıdaş, M.A.; Ağçam, E.; Çeliktopuz, E.; Kargı, S.P. Enhancing secondary compounds in strawberry fruit through optimized irrigation and seaweed application. Sci. Hortic. 2024, 324, 112609. [Google Scholar] [CrossRef]
- Ibrahim, E.A.; Ebrahim, N.E.S.; Mohamed, G.Z. Mitigation of water stress in broccoli by soil application of humic acid. Sci. Rep. 2024, 14, 2765. [Google Scholar] [CrossRef]
- Thomas, A.; Beena, R.; Laksmi, G.; Soni, K.B.; Swapna, A.; Viji, M.M. Changes in sucrose metabolic enzymes to water stress in contrasting rice genotypes. Plant Stress 2022, 5, 100088. [Google Scholar] [CrossRef]
- Xu, J.; Li, X.; Wan, W.; Zhu, X.; Li, C.; Zhao, X.; Zhao, Y.; Pang, S.; Diao, M. Impact of regulated deficit irrigation on the dynamics of quality changes in processing tomato fruits during ripening. Agric. Water Manag. 2024, 304, 109068. [Google Scholar] [CrossRef]
- Götze, P.; Rücknagel, J.; Wensch-Dorendorf, M.; Märländer, B.; Christen, O. Crop rotation effects on yield, technological quality and yield stability of sugar beet after 45 trial years. Eur. J. Agron. 2017, 82, 50–59. [Google Scholar] [CrossRef]
- Papafilippaki, A.; Nikolaidis, N.P. Comparative study of wild and cultivated populations of Cichorium spinosum: The influence of soil and organic matter addition. Sci. Hortic. 2020, 261, 108942. [Google Scholar] [CrossRef]
- Gómez-Bellot, M.J.; Guerrero, L.; Yuste, J.E.; Vallejo, F.; Sánchez-Blanco, M.J. Identifying Bioactive Compounds in Common Bean (Phaseolus vulgaris L.) Plants under Water Deficit Conditions. Horticulturae 2024, 10, 663. [Google Scholar] [CrossRef]
- Obadi, A.; Alharbi, A.; Alomran, A.; Alghamdi, A.G.; Louki, I.; Alkhasha, A.; Alqardaeai, T. Enhancement in Tomato Yield and Quality Using Biochar Amendments in Greenhouse under Salinity and Drought Stress. Plants 2024, 13, 1634. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Zhang, H.; Yang, C.; Li, H.; Wu, J. Effects of water stress on nutrients and enzyme activity in rhizosphere soils of greenhouse grape. Front. Microbiol. 2024, 15, 1376849. [Google Scholar] [CrossRef] [PubMed]
- Alomari-Mheidat, M.; Corell, M.; Martín-Palomo, M.J.; Castro-Valdecantos, P.; Medina-Zurita, N.; de Sosa, L.L.; Moriana, A. Moderate Water Stress Impact on Yield Components of Greenhouse Tomatoes in Relation to Plant Water Status. Plants 2024, 13, 128. [Google Scholar] [CrossRef]
- Thompson, R.B.; Gallardo, M.; Valdez, L.C.; Fernández, M.D. Using plant water status to define threshold values for irrigation management of vegetable crops using soil moisture sensors. Agric. Water Manag. 2007, 88, 147–158. [Google Scholar] [CrossRef]
- Yfantopoulos, D.; Ntatsi, G.; Karkanis, A.; Savvas, D. Evaluation of the Role of Legumes in Crop Rotation Schemes of Organic or Conventionally Cultivated Cabbage. Agronomy 2024, 14, 297. [Google Scholar] [CrossRef]
- Haruna, S.I.; Nkongolo, N.V. Influence of cover crop, tillage, and crop rotation management on soil nutrients. Agriculture 2020, 10, 225. [Google Scholar] [CrossRef]
- Adesanya, T.; Zvomuya, F.; Fernandez, M.R.; Luce, M. Crop rotation diversity and tillage effects on soil and wheat grain nutrient concentration in an organically-managed system. J. Agric. Food Res. 2024, 18, 101411. [Google Scholar] [CrossRef]
- Dwivedi, S.L.; Nigam, S.N.; Nageswara Rao, R.C.; Singh, U.; Rao, K.V.S. Effect of drought on oil, fatty acids and protein contents of groundnut (Arachis hypogaea L.) seeds. F. Crop. Res. 1996, 48, 125–133. [Google Scholar] [CrossRef]
- Ghaffari, M.; Gholizadeh, A.; Rauf, S.; Shariati, F. Drought-stress induced changes of fatty acid composition affecting sunflower grain yield and oil quality. Food Sci. Nutr. 2023, 11, 7718–7731. [Google Scholar] [CrossRef] [PubMed]
- Harisha, C.B.; Rane, J.; Halagunde Gowda, G.R.; Chavan, S.B.; Chaudhary, A.; Verma, A.K.; Ravi, Y.; Asangi, H.; Halli, H.M.; Boraiah, K.M.; et al. Effect of Deficit Irrigation and Intercrop Competition on Productivity, Water Use Efficiency and Oil Quality of Chia in Semi-Arid Regions. Horticulturae 2024, 10, 101. [Google Scholar] [CrossRef]
- Upchurch, R.G. Fatty acid unsaturation, mobilization, and regulation in the response of plants to stress. Biotechnol. Lett. 2008, 30, 967–977. [Google Scholar] [CrossRef]
- Xu, L.; Han, L.; Huang, B. Membrane fatty acid composition and saturation levels associated with leaf dehydration tolerance and post-drought rehydration in Kentucky bluegrass. Crop Sci. 2011, 51, 273–281. [Google Scholar] [CrossRef]
- Stepien, A.; Wojtkowiak, K.; Pietrzak-Fiecko, R. Nutrient content, fat yield and fatty acid profile of winter rapeseed (Brassica napus L.) grown under different agricultural production systems. Chil. J. Agric. Res. 2017, 77, 266–272. [Google Scholar] [CrossRef]
- Mohammadi, K.; Ghalavand, A.; Aghaalikhani, M.; Heidari, G.; Shahmoradi, B.; Sohrabi, Y. Effect of different methods of crop rotation and fertilization on canola traits and soil microbial activity. Aust. J. Crop Sci. 2011, 5, 1261–1268. [Google Scholar]
- Wacal, C.; Ogata, N.; Basalirwa, D.; Sasagawa, D.; Kato, M.; Handa, T.; Masunaga, T.; Yamamoto, S.; Nishihara, E. Fatty Acid Composition of Sesame (Sesamum indicum L.) Seeds in Relation to Yield and Soil Chemical Properties on Continuously Monocropped Upland Fields Converted from Paddy Fields. Agronomy 2019, 9, 801. [Google Scholar] [CrossRef]
- Gokkus, M.K. Effects of urea fertilizer and nettle extract on the biochemical and morphological characteristics of ornamental peppers (Capsicum frutescens L.) under deficit irrigation conditions. Irrig. Drain. 2024; Early View. [Google Scholar] [CrossRef]
- Gharibi, S.; Tabatabaei, B.E.S.; Saeidi, G.; Goli, S.A.H. Effect of Drought Stress on Total Phenolic, Lipid Peroxidation, and Antioxidant Activity of Achillea Species. Appl. Biochem. Biotechnol. 2016, 178, 796–809. [Google Scholar] [CrossRef]
- Mahmood, S.; Afzal, B.; Bashir, R.; Shakoor, M.B.; Nisa, Z.U.; Rizwan, M.; Awais, M.; Azeem, M.; Wahid, A.; Yong, J.W.H. Melatonin priming could modulate primary and secondary metabolism of sunflower with better nutraceutical value and tolerance against water deficit environment. Plant Stress 2024, 13, 100533. [Google Scholar] [CrossRef]
- Albergaria, E.T.; Oliveira, A.F.M.; Albuquerque, U.P. The effect of water deficit stress on the composition of phenolic compounds in medicinal plants. South African J. Bot. 2020, 131, 12–17. [Google Scholar] [CrossRef]
- Muszyńska, E.; Dziurka, K.; Labudda, M. What Makes the Life of Stressed Plants a Little Easier? Defense Mechanisms against Adverse Conditions. Plants 2023, 12, 1040. [Google Scholar] [CrossRef]
- Mitchell, A.E.; Hong, Y.J.; Koh, E.; Barrett, D.M.; Bryant, D.E.; Denison, R.F.; Kaffka, S. Ten-year comparison of the influence of organic and conventional crop management practices on the content of flavonoids in tomatoes. J. Agric. Food Chem. 2007, 55, 6154–6159. [Google Scholar] [CrossRef] [PubMed]
- Buczek, J.; Jańczak-Pieniążek, M.; Harasim, E.; Kwiatkowski, C.A.; Kapusta, I. Effect of Cropping Systems and Environment on Phenolic Acid Profiles and Yielding of Hybrid Winter Wheat Genotypes. Agriculture 2023, 13, 834. [Google Scholar] [CrossRef]
- Siwek, P.; Bucki, P.; Domagała-Świątkiewicz, I.; Lalewicz, P. Effect of cover crops integration in crop rotation on the yield and chemical composition of edible parts of vegetables grown in an organic system in high tunnel. Sci. Hortic. 2024, 332, 4–10. [Google Scholar] [CrossRef]
- Gonçalves, S.; Moreira, E.; Andrade, P.B.; Valentão, P.; Romano, A. Effect of in vitro gastrointestinal digestion on the total phenolic contents and antioxidant activity of wild Mediterranean edible plant extracts. Eur. Food Res. Technol. 2019, 245, 753–762. [Google Scholar] [CrossRef]
- Sarker, U.; Oba, S. Drought Stress Effects on Growth, ROS Markers, Compatible Solutes, Phenolics, Flavonoids, and Antioxidant Activity in Amaranthus tricolor. Appl. Biochem. Biotechnol. 2018, 186, 999–1016. [Google Scholar] [CrossRef]
- Birsa, M.L.; Sarbu, L.G. Health Benefits of Key Constituents in Cichorium intybus L. Nutrients 2023, 15, 1322. [Google Scholar] [CrossRef]
- Petropoulos, S.; Fernandes, A.; Barros, L.; Ferreira, I. A comparison of the phenolic profile and antioxidant activity of different Cichorium spinosum L. ecotypes. J. Sci. Food Agric. 2017, 98, 183–189. [Google Scholar] [CrossRef]
- Sishu, N.K.; Selvaraj, C.I. Phytochemistry, Pharmacological Applications, and Therapeutic Effects of Green Synthesized Nanomaterials Using Cichorium Species—A Comprehensive Review; Springer: Berlin/Heidelberg, Germany, 2024; Volume 397, ISBN 0123456789. [Google Scholar]
- Petropoulos, S.; Fernandes, Â.; Stojković, D.; Pereira, C.; Taofiq, O.; Di Gioia, F.; Tzortzakis, N.; Soković, M.; Barros, L.; Ferreira, I. Cotton and cardoon byproducts as potential growing media components for Cichorium spinosum L. commercial cultivation. J. Clean. Prod. 2019, 240, 118254. [Google Scholar] [CrossRef]
- Alhaithloul, H.A.; Soliman, M.H.; Ameta, K.L.; El-Esawi, M.A.; Elkelish, A. Changes in ecophysiology, osmolytes, and secondary metabolites of the medicinal plants of Mentha piperita and Catharanthus roseus subjected to drought and heat stress. Biomolecules 2020, 10, 43. [Google Scholar] [CrossRef] [PubMed]


| Month | Mean Temperature (°C) | Mean Highest Temperature (°C) | Mean Lowest Temperature (°C) | Relative Humidity (%) | Rainfall (mm) |
|---|---|---|---|---|---|
| March | 9.15 | 15.47 | 3.07 | 51.75 | 55.90 |
| April | 13.56 | 20.16 | 7.08 | 52.63 | 21.90 |
| May | 20.95 | 28.52 | 12.91 | 45.81 | 21.50 |
| Mean | 14.55 | 21.38 | 7.69 | 50.06 | 33.10 |
| Treatments | Total Fat | Crude Protein | Ash | Total Dietary Fiber | Carbohydrates | Energy (kcal/100 g dw) | |
|---|---|---|---|---|---|---|---|
| Irrigation (I) | Control (C) | 3.34 ± 0.16 a | 21.25 ± 2.45 a | 15.22 ± 0.69 b | 49.37 ± 1.22 a | 10.82 ± 0.78 b | 257.10 ± 4.16 a |
| Deficit Irrigation (DI) | 3.04 ± 0.24 b | 21.81 ± 0.15 a | 15.22 ± 0.13 b | 44.40 ± 5.51 b | 15.5 ± 5.2 a | 265.5 ± 11.7 a | |
| Full Irrigation (FI) | 2.75 ± 0.06 c | 20.37 ± 0.71 a | 19.1 ± 0.8 a | 48.5 ± 1.3 a b | 9.32 ± 0.37 b | 240.5 ± 1.2 b | |
| Crop Management (CM) | Crop Rotation (CR) | 3.17 ± 0.32 a | 20.23 ± 1.26 b | 16.49 ± 1.42 a | 46.47 ± 5.34 a | 13.6 ± 5.0 a | 256.93 ± 15.47 a |
| No Crop Rotation (NCR) | 2.91 ± 0.22 a | 22.06 ± 1.17 a | 16.5 ± 2.5 a | 48.37 ± 0.92 a | 10.15 ± 0.89 a | 251.81 ± 9.38 a | |
| I × CM | C × CR | 3.5 ± 0.1 a | 19.1 ± 0.9 d | 15.8 ± 0.2 c | 50.4 ± 1.1 a | 11.21 ± 0.03 b | 253.4 ± 0.8 c |
| C × NCR | 3.19 ± 0.03 b | 23.4 ± 1.1 a | 14.6 ± 0.5 e | 48.3 ± 0.2 c | 10.44 ± 1.03 b | 260.8 ± 1.3 b | |
| DI × CR | 3.2 ± 0.1 b | 21.7 ± 0.2 bc | 15.3 ± 0.1 d | 39.4 ± 0.1 e | 20.3 ± 0.1 a | 276.3 ± 0.5 a | |
| DI × NCR | 2.8 ± 0.1 c | 21.8 ± 0.2 b | 15.1 ± 0.1 d | 49.4 ± 0.1 b | 10.8 ± 0.2 b | 254.8 ± 0.3 c | |
| FI × CR | 2.8 ± 0.1 cd | 19.7 ± 0.2 d | 18.4 ± 0.2 b | 49.7 ± 0.4 b | 9.4 ± 0.5 c | 241.1 ± 1.5 d | |
| FI × NCR | 2.7 ± 0.1 d | 20.9 ± 0.6 c | 19.76 ± 0.01 a | 47.3 ± 0.3 d | 9.2 ± 0.1 c | 239.8 ± 0.2 d |
| Treatments | Oxalic Acid | Quinic Acid | Shikimic Acid | Ascorbic Acid | Succinic Acid | Total Organic Acids | |
|---|---|---|---|---|---|---|---|
| Irrigation (I) | Control (C) | 4.90 ± 0.23 a | 6.65 ± 0.19 a | tr | tr | 1.55 ± 0.04 a | 13.11 ± 0.27 a |
| Deficit Irrigation (DI) | 5.04 ± 0.08 a | 6.8 ± 0.21 a | tr | tr | 1.40 ± 0.19 a | 13.25 ± 0.24 a | |
| Full Irrigation (FI) | 4.2 ± 0.6 b | 4.32 ± 1.75 b | tr | tr | 1.20 ± 0.48 a | 9.71 ± 2.84 b | |
| Crop Management (CM) | Crop Rotation (CR) | 4.83 ± 0.14 a | 6.43 ± 0.43 a | tr | tr | 1.60 ± 0.05 a | 12.86 ± 0.49 a |
| No Crop Rotation (NCR) | 4.60 ± 0.72 a | 5.41 ± 2.03 a | tr | tr | 1.18 ± 0.34 b | 11.19 ± 3.05 a | |
| I × CM | C × CR | 4.8 ± 0.2 bc | 6.6 ± 0.2 a | tr | tr | 1.57 ± 0.01 b | 12.97 ± 0.42 b |
| C × NCR | 5.04 ± 0.3 a | 6.7 ± 0.3 a | tr | tr | 1.52 ± 0.05 b | 13.26 ± 0.03 a | |
| DI × CR | 4.97 ± 0.02 ab | 6.8 ± 0.3 a | tr | tr | 1.57 ± 0.04 b | 13.3 ± 0.3 a | |
| FI × NCR | 5.11 ± 0.04 a | 6.8 ± 0.4 a | tr | tr | 1.2 ± 0.1 c | 13.11 ± 0.3 ab | |
| DI × CR | 4.7 ± 0.1 c | 5.9 ± 0.3 b | tr | tr | 1.64 ± 0.05 a | 12.24 ± 0.3 c | |
| FI × NCR | 3.65 ± 0.04 d | 2.69 ± 0.09 c | tr | tr | 0.75 ± 0.01 d | 7.09 ± 0.07 d |
| Treatments | α-Tocopherol | β-Tocopherol | Total Tocopherols | |
|---|---|---|---|---|
| Irrigation (I) | Control (C) | 0.047 ± 0.016 a | 0.107 ± 0.003 b | 0.153 ± 0.013 b |
| Deficit Irrigation (DI) | 0.04 ± 0.01 a | 0.15 ± 0.01 a | 0.19 ± 0.02 a | |
| Full Irrigation (FI) | 0.031 ± 0.005 b | 0.109 ± 0.041 b | 0.140 ± 0.036 b | |
| Crop Management (CM) | Crop Rotation (CR) | 0.044 ± 0.013 a | 0.10 ± 0.03 b | 0.149 ± 0.032 a |
| No Crop Rotation (NCR) | 0.038 ± 0.13 a | 0.138 ± 0.022 a | 0.176 ± 0.031 a | |
| I × CM | C × CR | 0.0617 ± 0.0003 a | 0.104 ± 0.003 e | 0.165 ± 0.003 c |
| C × NCR | 0.0319 ± 0.0001 d | 0.11 ± 0.003 d | 0.142 ± 0.003 d | |
| DI × CR | 0.0354 ± 0.0004 c | 0.141 ± 0.005 c | 0.177 ± 0.005 b | |
| FI × NCR | 0.055 ± 0.001 b | 0.158 ± 0.007 a | 0.214 ± 0.008 a | |
| DI × CR | 0.035 ± 0.001 c | 0.072 ± 0.001 f | 0.107 ± 0.003 e | |
| FI × NCR | 0.027 ± 0.001 e | 0.147 ± 0.004 b | 0.173 ± 0.003 b |
| Treatments | Fructose | Glucose | Sucrose | Trehalose | Total Sugars | |
|---|---|---|---|---|---|---|
| Irrigation (I) | Control (C) | 0.73 ± 0.06 ab | 2.67 ± 0.08 a | 2.4 ± 0.2 ab | 0.38 ± 0.02 a | 6.15 ± 0.17 a |
| Deficit Irrigation (DI) | 0.69 ± 0.09 b | 2.68 ± 0.09 a | 2.75 ± 0.57 a | 0.33 ± 0.06 b | 6.45 ± 0.52 a | |
| Full Irrigation (FI) | 0.82 ± 0.12 a | 2.23 ± 0.24 b | 2.06 ± 0.24 b | 0.41 ± 0.01 a | 5.51 ± 0.13 b | |
| Crop Management (CM) | Crop Rotation (CR) | 0.83 ± 0.08 a | 2.4 ± 0.3 a | 2.70 ± 0.45 a | 0.35 ± 0.06 b | 6.29 ± 0.56 a |
| No Crop Rotation (NCR) | 0.67 ± 0.04 b | 2.64 ± 0.16 a | 2.09 ± 0.19 b | 0.39 ± 0.0 a | 5.8 ± 0.3 b | |
| I × CM | C × CR | 0.79 ± 0.02 b | 2.6 ± 0.1 b | 2.55 ± 0.07 b | 0.36 ± 0.01 c | 6.31 ± 0.01 b |
| C × NCR | 0.68 ± 0.02 d | 2.73 ± 0.04 a | 2.20 ± 0.02 c | 0.40 ± 0.02 ab | 6.0 ± 0.1 c | |
| DI × CR | 0.78 ± 0.04 b | 2.61 ± 0.01 b | 3.3 ± 0.1 a | 0.27 ± 0.01 d | 6.9 ± 0.1 a | |
| FI × NCR | 0.61 ± 0.01 e | 2.75 ± 0.11 a | 2.23 ± 0.09 c | 0.39 ± 0.01 b | 5.97 ± 0.01 c | |
| DI × CR | 0.94 ± 0.04 a | 2.01 ± 0.11 d | 2.28 ± 0.06 c | 0.41 ± 0.03 a | 5.63 ± 0.01 d | |
| FI × NCR | 0.71 ± 0.01 c | 2.44 ± 0.08 c | 1.84 ± 0.04 d | 0.40 ± 0.01 ab | 5.39 ± 0.04 e |
| Treatments | K (g/Kg) | Na (mg/Kg) | Ca (g/Kg) | Mg (g/Kg) | Fe (mg/Kg) | Mn (mg/Kg) | Cu (mg/Kg) | Zn (mg/Kg) | |
|---|---|---|---|---|---|---|---|---|---|
| Irrigation (I) | Control (C) | 36.6 ± 6.6 ab | 6004 ± 1052 a | 11.98 ± 0.49 a | 3.70 ± 0.07 a | 849 ± 47 b | 105.7 ± 18.6 c | 9.37 ± 0.54 b | 45.8 ± 1.3 b |
| Deficit Irrigation (DI) | 37.8 ± 6.6 a | 5259 ± 694 a | 11.1 ± 0.3 b | 3.5 ± 0.2 a | 537 ± 32 b | 135.7 ± 9.7 b | 12.9 ± 0.5 a | 58.4 ± 1.4 a | |
| Full Irrigation (FI) | 29.5 ± 3.4 b | 3481 ± 572 b | 9.5 ± 0.7 c | 3.1 ± 0.4 b | 2915 ± 645 a | 183.3 ± 18.4 a | 12.3 ± 0.4 a | 58.8 ± 9.9 a | |
| Crop Management (CM) | Crop Rotation (CR) | 39.6 ± 5.4 a | 4212 ± 959 b | 10.7 ± 1.5 a | 3.3 ± 0.4 b | 1260 ± 113 a | 133.3 ± 14.9 a | 12.3 ± 3.1 a | 51.8 ± 5.9 a |
| No Crop Rotation (NCR) | 29.6 ± 2.5 b | 5617 ± 1300 a | 11.1 ± 0.7 a | 3.6 ± 1.2 a | 1608 ± 142 a | 149.8 ± 17.6 a | 10.8 ± 1.5 a | 56.8 ± 9.8 a | |
| I × CM | C × CR | 43 ± 2 a | 5049 ± 170 c | 12.3 ± 0.4 a | 3.7 ± 0.1 a | 888 ± 44 c | 88.7 ± 0.4 f | 8.9 ± 0.4 e | 46 ± 1 d |
| C × NCR | 30.6 ± 0.4 c | 6960 ± 154 a | 11.7 ± 0.6 b | 3.6 ± 0.1 a | 809 ± 4 d | 122.7 ± 1.3 e | 9.8 ± 0.4 d | 45 ± 2 d | |
| DI × CR | 44 ± 3 a | 4627 ± 82 d | 10.78 ± 0.06 c | 3.35 ± 0.13 b | 565 ± 25 e | 144.5 ± 0.3 c | 16.1 ± 0.5 a | 59 ± 1 b | |
| FI × NCR | 31.9 ± 1.1 bc | 5891 ± 62 b | 11.4 ± 0.1 b | 3.7 ± 0.2 a | 510 ± 10 f | 126.9 ± 2 d | 9.8 ± 0.2 d | 57 ± 1 b | |
| DI × CR | 32.6 ± 0.5 b | 2962 ± 117 f | 8.9 ± 0.2 e | 2.7 ± 0.1 c | 2327 ± 74 b | 166.7 ± 5.7 b | 11.9 ± 0.3 c | 50 ± 1 c | |
| FI × NCR | 26.3 ± 0.4 d | 4000 ± 89 e | 10.2 ± 0.3 d | 3.45 ± 0.05 b | 3504 ± 20 a | 199.9 ± 2.3 a | 12.7 ± 0.03 b | 68 ± 1 a |
| Irrigation (I) | Crop Management (CM) | I × CM | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| C | DI | FI | CR | NCR | C × CR | C × NCR | DI × CR | DI × NCR | FI × CR | FI × NCR | |
| C6:0 | tr | tr | tr | tr | tr | tr | tr | tr | tr | tr | tr |
| C11:0 | 0.139 ± 0.016 a | 0.26 ± 0.17 a | 0.193 ± 0.063 a | 0.123 ± 0.012 ba | 0.276 ± 0.118 a | 0.125 ± 0.003 e | 0.154 ± 0.003 v | 0.109 ± 0.003 f | 0.423 ± 0.004 a | 0.136 ± 0.004 d | 0.251 ± 0.008 b |
| C12:0 | 0.11 ± 0.05 a | 0.175 ± 0.101 a | 0.122 ± 0.063 a | 0.071 ± 0.009 b | 0.201 ± 0.051 | 0.066 ± 0.002 e | 0.156 ± 0.001 c | 0.084 ± 0.001 d | 0.268 ± 0.007 a | 0.065 ± 0.002 e | 0.181 ± 0.005 b |
| C13:0 | 0.79 ± 0.24 b | 1.010 ± 0.015 a | 0.662 ± 0.054 b | 0.875 ± 0.197 a | 0.769 ± 0.196 a | 1.01 ± 0.02 a | 0.576 ± 0.001 d | 1.002 ± 0.011 a | 1.02 ± 0.03 a | 0.613 ± 0.01 c | 0.712 ± 0.003 b |
| C14:0 | 0.994 ± 0.073 a | 1.116 ± 0.328 a | 1.146 ± 0.087 a | 0.94 ± 0.11 b | 1.232 ± 0.156 a | 0.927 ± 0.004 d | 1.061 ± 0.001 c | 0.82 ± 0.03 e | 1.42 ± 0.03 a | 1.07 ± 0.03 c | 1.22 ± 0.06 b |
| C14:1 | 0.33 ± 0.12 b | 0.437 ± 0.039 a | 0.261 ± 0.015 b | 0.37 ± 0.07 a | 0.31 ± 0.12 a | 0.44 ± 0.01 b | 0.218 ± 0.005 f | 0.4 ± 0.2 c | 0.47 ± 0.02 a | 0.27 ± 0.01 d | 0.248 ± 0.007 e |
| C15:0 | 0.168 ± 0.038 a | 0.154 ± 0.074 a | 0.209 ± 0.027 a | 0.151 ± 0.065 b | 0.203 ± 0.016 a | 0.134 ± 0.001 e | 0.204 ± 0.005 c | 0.086 ± 0.001 f | 0.222 ± 0.005 b | 0.234 ± 0.006 a | 0.185 ± 0.006 d |
| C15:1 | 0.222 ± 0.066 a | 0.228 ± 0.020 a | 0.203 ± 0.027 a | 0.23 ± 0.05 a | 0.205 ± 0.035 a | 0.283 ± 0.004 a | 0.162 ± 0.003 d | 0.246 ± 0.006 b | 0.21 ± 0.003 c | 0.163 ± 0.007 d | 0.243 ± 0.006 b |
| C16:0 | 23.39 ± 2.49 a | 24.36 ± 3.81 a | 25.67 ± 1.98 a | 21.97 ± 1.46 b | 27.0 ± 1.1 a | 21.1 ± 0.2 d | 25.6 ± 0.9 b | 20.88 ± 0.03 d | 27.84 ± 0.07 a | 23.8 ± 0.4 c | 27.4 ± 0.8 a |
| C16:1 | 2.68 ± 0.16 a | 2.83 ± 0.35 a | 3.009 ± 0.471 a | 2.54 ± 0.04 b | 3.14 ± 0.27 | 2.54 ± 0.05 d | 2.82 ± 0.1 c | 2.51 ± 0.05 d | 3.15 ± 0.02 b | 2.58 ± 0.03 d | 3.4 ± 0.1 a |
| C17:0 | 0.97 ± 0.22 a | 0.85 ± 0.24 a | 1.003 ± 0.365 a | 0.69 ± 0.06 b | 1.19 ± 0.12 a | 0.77 ± 0.04 d | 1.17 ± 0.02 b | 0.64 ± 0.01 f | 1.07 ± 0.02 c | 0.67 ± 0.01 e | 1.34 ± 0.01 a |
| C18:0 | 2.93 ± 0.41 a | 3.12 ± 0.65 a | 3.24 ± 0.53 a | 2.61 ± 0.11 b | 3.58 ± 0.21 a | 2.55 ± 0.06 d | 3.3 ± 0.07 b | 2.525 ± 0.001 d | 3.71 ± 0.01 a | 2.755 ± 0.004 c | 3.73 ± 0.05 a |
| C18:1n9c | 1.65 ± 0.33 c | 1.865 ± 0.036 b | 1.99 ± 0.027 a | 1.85 ± 0.15 a | 1.82 ± 0.15 a | 1.68 ± 0.01 c | 1.62 ± 0.03 d | 1.86 ± 0.05 b | 1.87 ± 0.07 b | 2.01 ± 0.02 a | 1.97 ± 0.03 a |
| C18:2n6c | 18.56 ± 1.52 b | 18.05 ± 1.46 b | 20.28 ± 0.80 a | 20.11 ± 0.72 a | 17.81 ± 1.32 b | 19.95 ± 0.02 b | 17.2 ± 0.2 d | 19.4 ± 0.2 c | 16.7 ± 0.1 e | 21.1 ± 0.2 a | 19.6 ± 0.1 c |
| C18:3n6 | 0.350 ± 0.039 a | 0.336 ± 0.056 a | 0.38 ± 0.04 a | 0.338 ± 0.059 a | 0.371 ± 0.022 a | 0.32 ± 0.01 d | 0.386 ± 0.009 b | 0.29 ± 0.01 e | 0.387 ± 0.002 b | 0.41 ± 0.02 a | 0.342 ± 0.008 c |
| C18:3n3 | 43.90 ± 1.63 a | 41.5 ± 4.4 a b | 39.01 ± 2.13 b | 43.95 ± 2.27 a | 39.0 ± 2.6 b | 45.4 ± 0.1 a | 42.4 ± 0.7 b | 45.6 ± 0.2 a | 37.5 ± 0.2 d | 40.9 ± 0.1 c | 37.1 ± 0.9 d |
| C22:0 | 0.843 ± 0.087 a | 0.93 ± 0.06 a | 0.90 ± 0.18 a | 0.904 ± 0.133 a | 0.88 ± 0.12 a | 0.76 ± 0.01 e | 0.92 ± 0.02 c | 0.881 ± 0.005 d | 0.99 ± 0.01 b | 1.07 ± 0.01 a | 0.73 ± 0.02 f |
| C22:2 | tr | tr | tr | tr | tr | tr | tr | tr | tr | tr | tr |
| C23:0 | 0.631 ± 0.138 | 0.952 ± 0.023 a | 0.62 ± 0.04 b | 0.78 ± 0.12 a | 0.69 ± 0.21 a | 0.76 ± 0.01 c | 0.51 ± 0.01 f | 0.93 ± 0.02 b | 0.972 ± 0.003 a | 0.66 ± 0.01 d | 0.59 ± 0.02 e |
| C24:0 | 1.36 ± 0.15 b | 1.758 ± 0.052 a | 1.089 ± 0.041 b | 1.49 ± 0.25 a | 1.312 ± 0.456 a | 1.23 ± 0.03 d | 1.51 ± 0.02 c | 1.8 ± 0.1 a | 1.72 ± 0.01 b | 1.46 ± 0.06 c | 0.72 ± 0.03 e |
| SFA | 32.32 ± 3.15 a | 34.7 ± 5.4 a | 34.86 ± 2.48 a | 30.61 ± 1.52 b | 37.31 ± 1.99 a | 29.5 ± 0.2 e | 35.2 ± 0.8 c | 29.75 ± 0.01 e | 39.65 ± 0.04 a | 32.6 ± 0.3 d | 37.1 ± 0.8 b |
| MUFA | 4.878 ± 0.075 b | 5.36 ± 0.38 a | 5.47 ± 0.48 a | 4.994 ± 0.052 b | 5.5 ± 0.5 a | 4.93 ± 0.07 d | 4.82 ± 0.07 e | 5.018 ± 0.005 c | 5.71 ± 0.07 b | 5.03 ± 0.02 c | 5.9 ± 0.2 a |
| PUFA | 62.8 ± 3.1 a | 59.9 ± 5.8 a | 59.67 ± 2.96 a | 64.40 ± 1.55 a | 57.21 ± 2.37 b | 65.6 ± 0.1 a | 60.001 ± 0.892 c | 65.235 ± 0.004 a | 54.64 ± 0.03 e | 62.3 ± 0.3 b | 57.001 ± 0.969 d |
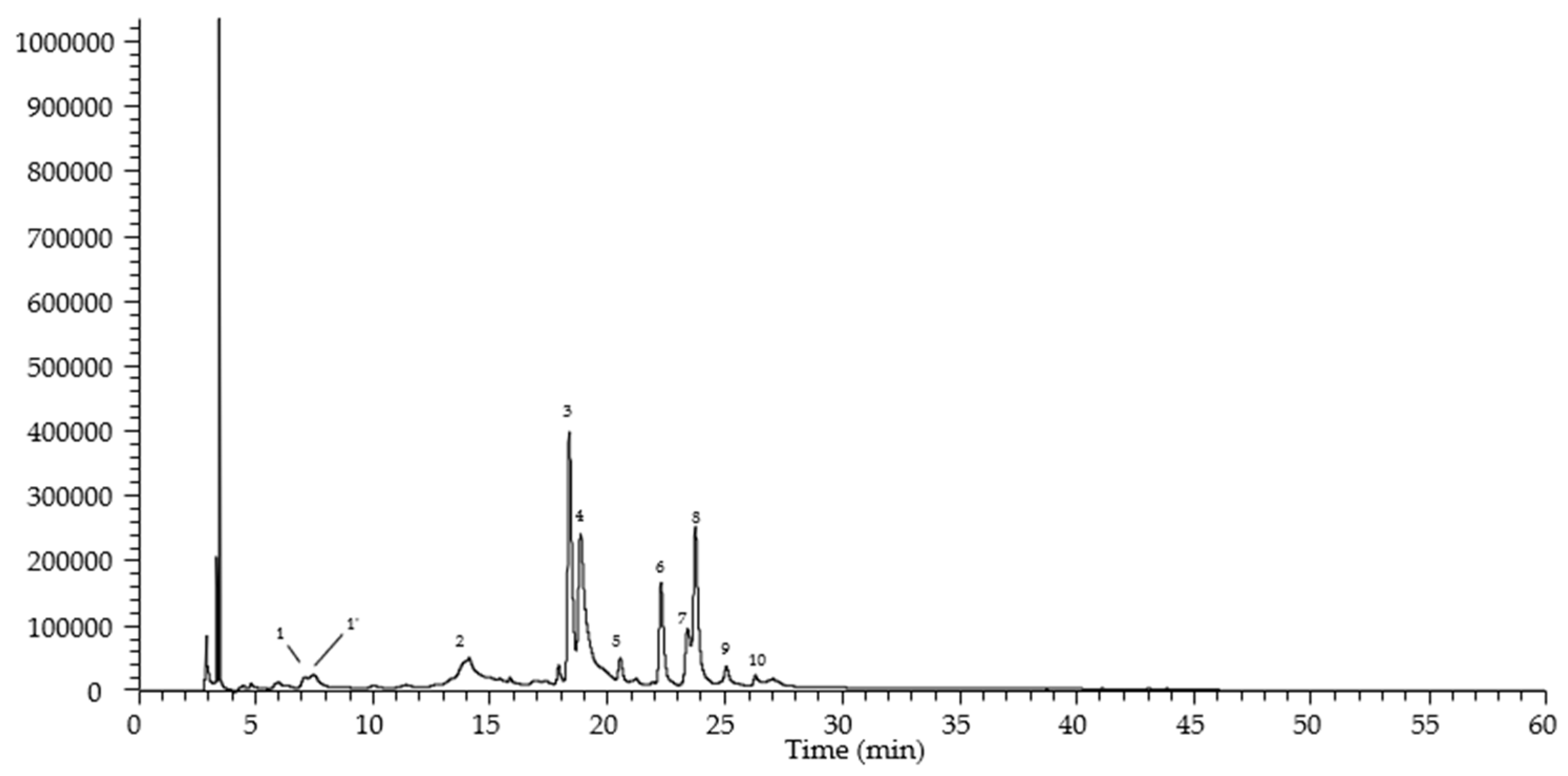
| Peak | Rt | λmax | [M-H]− | MS2 | Tentative Identification |
|---|---|---|---|---|---|
| 1 | 7.07 | 326 | 353 | 191 (100), 179 (23), 135(5) | cis 5-O-Caffeoylquinic acid |
| 1’ | 7.41 | 326 | 353 | 191 (100), 179 (12), 135 (5) | trans 5-O-Caffeoylquinic acid |
| 2 | 13.98 | 328 | 473 | 391 (39), 311 (100), 293 (28) | Chicoric acid |
| 3 | 18.22 | 353 | 477 | 301 (100) | Quercetin-O-hexurunoside |
| 4 | 18.73 | 343 | 461 | 285 (100) | Luteolin-O-hexurunoside |
| 5 | 20.4 | 335 | 505 | 463 (11), 301 (100) | Quercetin-O-acetylhexoside |
| 6 | 22.13 | 346 | 461 | 285 (100) | Kaempherol-O-hexurunoside |
| 7 | 23.32 | 333 | 445 | 269 (100) | Apigenin-O-hexurunoside |
| 8 | 23.59 | 345 | 491 | 315 (100) | Isorhamnetin-O-hexurunoside |
| 9 | 24.9 | 326 | 489 | 285 (100) | Kaempherol-O-acetylhexoside |
| 10 | 26.14 | 327 | 519 | 315 (100) | Isorhamnetin-O-acetylhexoside |
| Irrigation (I) | Crop Management (CM) | I × CM | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Tentative Identification | Control (C) | Deficit Irrigation (DI) | Full Irrigation (FI) | Crop Rotation (CR) | None Crop Rotation (NCR) | C × CR | C × NCR | DI × CR | DI × NCR | FI × CR | FI × NCR |
| cis 5-O-Caffeoylquinic acid | 0.54 ± 0.13 b | 0.91 ± 0.23 a | 0.413 ± 0.122 c | 0.613 ± 0.38 a | 0.63 ± 0.07 a | 0.41 ± 0.01 e | 0.66 ± 0.01 c | 1.13 ± 0.01 a | 0.69 ± 0.03 b | 0.29 ± 0.01 f | 0.53 ± 0.01 d |
| trans 5-O-Caffeoylquinic acid | 0.83 ± 0.25 b | 1.92 ± 0.39 a | 0.644 ± 0.067 c | 1.23 ± 0.78 a | 1.03 ± 0.37 a | 0.81 ± 0.03 d | 0.84 ± 0.02 c | 2.301 ± 0.002 a | 1.54 ± 0.05 b | 0.58 ± 0.03 f | 0.71 ± 0.03 e |
| Chicoric acid | 1.095 ± 0.361 b | 3.36 ± 0.57 a | 0.84 ± 0.18 c | 1.90 ± 1.46 a | 1.63 ± 0.85 a | 1.13 ± 0.01 c | 1.07 ± 0.04 d | 3.92 ± 0.01 a | 2.8 ± 0.05 b | 0.67 ± 0.01 f | 1.02 ± 0.02 e |
| Quercetin-O-hexurunoside | 1.310 ± 0.006 b | 3.38 ± 0.03 a | 1.51 ± 0.07 c | 2.05 ± 0.98 a | 2.08 ± 0.92 a | 1.31 ± 0.01 e | 1.31 ± 0.01 e | 3.41 ± 0.03 a | 3.354 ± 0.003 b | 1.45 ± 0.01 d | 1.575 ± 0.001 c |
| Luteolin-O-hexurunoside | 1.410 ± 0.098 c | 2.55 ± 0.02 a | 2.074 ± 0.318 b | 1.95 ± 0.46 a | 2.07 ± 0.55 a | 1.51 ± 0.02 e | 1.32 ± 0.03 f | 2.569 ± 0.004 a | 2.53 ± 0.03 b | 1.77 ± 0.04 d | 2.38 ± 0.02 c |
| Quercetin-O-acetylhexoside | 0.52 ± 0.07 b | 0.757 ± 0.027 a | 0.512 ± 0.005 b | 0.625 ± 0.118 a | 0.568 ± 0.122 a | 0.585 ± 0.002 c | 0.454 ± 0.003 f | 0.78 ± 0.02 a | 0.73 ± 0.01 b | 0.507 ± 0.003 e | 0.517 ± 0.004 d |
| Kaempherol-O-hexurunoside | 0.811 ± 0.062 c | 1.360 ± 0.006 a | 0.917 ± 0.122 b | 1.009 ± 0.252 a | 1.051 ± 0.255 a | 0.872 ± 0.004 d | 0.75 ± 0.01 f | 1.355 ± 0.004 b | 1.365 ± 0.006 a | 0.799 ± 0.009 e | 1.036 ± 0.008 c |
| Apigenin-O-hexurunoside | 1.066 ± 0.185 b | 1.46 ± 0.021 a | 1.57 ± 0.38 a | 1.369 ± 0.211 a | 1.36 ± 0.44 a | 1.25 ± 0.03 d | 0.89 ± 0.01 f | 1.66 ± 0.03 b | 1.26 ± 0.01 c | 1.2 ± 0.4 e | 1.939 ± 0.001 a |
| Isorhamnetin-O-hexurunoside | 1.03 ± 0.03 c | 1.47 ± 0.06 a | 1.164 ± 0.257 b | 1.113 ± 0.223 b | 1.336 ± 0.203 a | 1.01 ± 0.02 d | 1.06 ± 0.01 c | 1.42 ± 0.05 b | 1.53 ± 0.03 a | 0.91 ± 0.01 e | 1.41 ± 0.01 b |
| Kaempherol-O-acetylhexoside | 0.48 ± 0.04 b | 0.616 ± 0.014 a | 0.493 ± 0.033 b | 0.529 ± 0.059 a | 0.53 ± 0.08 a | 0.523 ± 0.002 d | 0.436 ± 0.003 f | 0.603 ± 0.001 b | 0.63 ± 0.004 a | 0.461 ± 0.001 e | 0.526 ± 0.001 c |
| Isorhamnetin-O-acetylhexoside | 0.425 ± 0.042 b | 0.491 ± 0.002 a | 0.421 ± 0.003 b | 0.459 ± 0.031 a | 0.432 ± 0.044 b | 0.466 ± 0.001 c | 0.383 ± 0.003 f | 0.4927 ± 0.0001 a | 0.4887 ± 0.0002 b | 0.4179 ± 0.0005 e | 0.4234 ± 0.0009 d |
| Total Phenolic Acids | 2.46 ± 0.12 b | 6.19 ± 1.19 a | 1.90 ± 0.37 c | 3.75 ± 0.62 a | 3.27 ± 0.26 a | 2.34 ± 0.05 d | 2.57 ± 0.01 c | 7.35 ± 0.01 a | 5.03 ± 0.03 b | 1.54 ± 0.02 f | 2.26 ± 0.02 e |
| Total Flavonoids | 7.06 ± 0.47 c | 12.09 ± 0.21 a | 8.66 ± 1.18 b | 9.1 ± 1.3 a | 9.43 ± 1.22 a | 7.51 ± 0.03 d | 6.602 ± 0.001 e | 12.29 ± 0.07 a | 11.89 ± 0.05 b | 7.51 ± 0.03 d | 9.81 ± 0.03 c |
| Total Phenolic compounds | 9.516 ± 0.353 c | 18.3 ± 1.4 a | 10.56 ± 1.55 b | 12.8 ± 1.9 a | 12.72 ± 3.26 a | 9.9 ± 0.1 d | 9.174 ± 0.009 e | 19.6 ± 0.1 a | 16.92 ± 0.02 b | 9.06 ± 0.05 f | 12.07 ± 0.01 c |
| Treatments | Irrigation (I) | Crop Management | I × CM | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Control (C) | Deficit Irrigation (DI) | Full Irrigation (FI) | Crop Rotation (CR) | None Crop Rotation (NCR) | C × CR | C × NCR | DI × CR | DI × NCR | FI × CR | FI × NCR | ||||||||||||
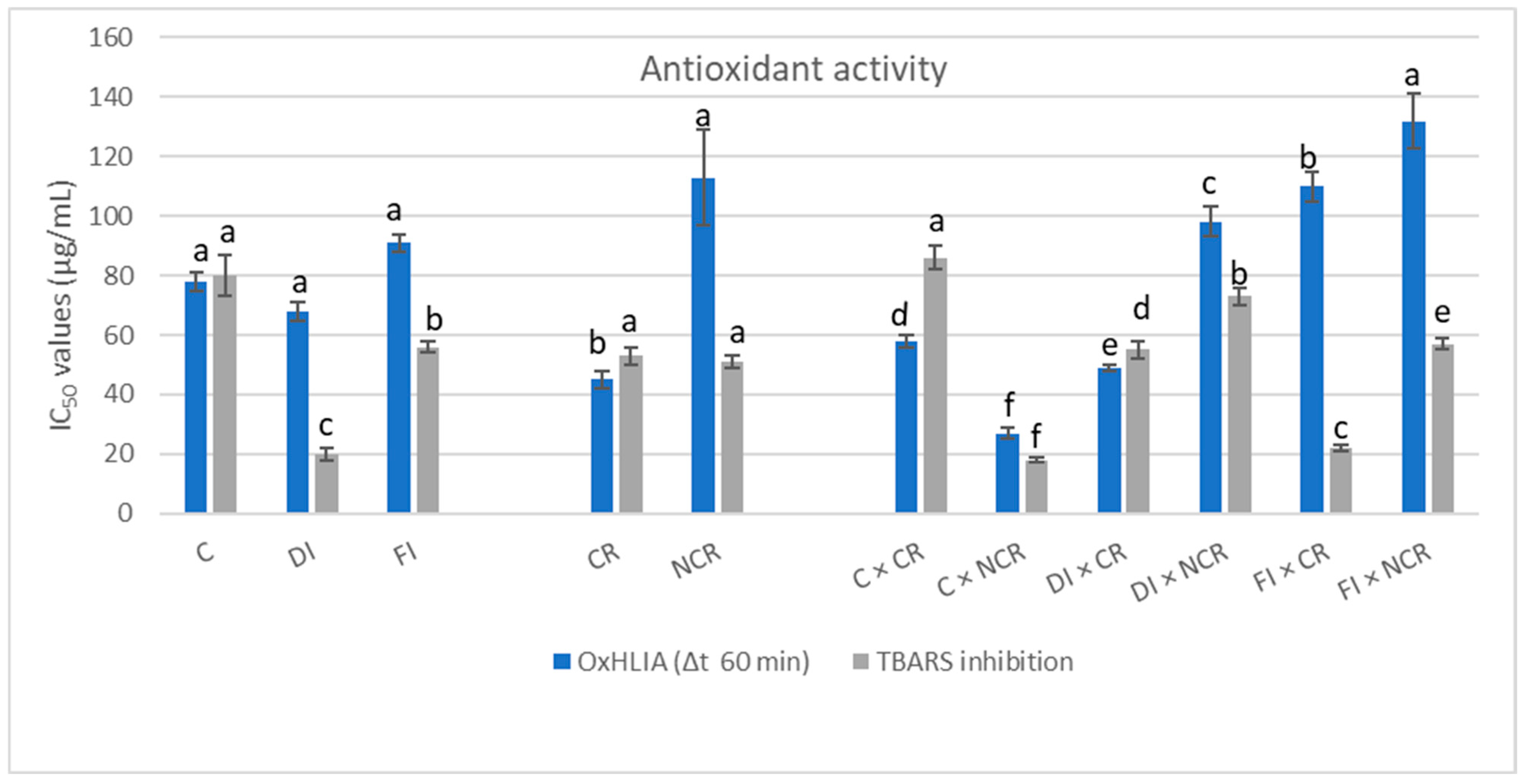
| Antioxidant activity IC50 values (µg/mL) A | ||||||||||||||||||||||
| OxHLIA (Δt 60 min) | 78 ± 3 a | 68 ± 3 a | 91 ± 3 a | 45 ± 3 b | 113 ± 16 a | 58 ± 2 d | 27 ± 2 f | 49 ± 1 e | 98 ± 5 c | 110 ± 5 b | 132 ± 9 a | |||||||||||
| TBARS inhibition | 80 ± 7 a | 20 ± 2 c | 56 ± 2 b | 53 ± 3 a | 51 ± 2 a | 86 ± 4 a | 18 ± 1 f | 55 ± 3 d | 73 ± 3 b | 22 ± 1 e | 57 ± 2 c | |||||||||||
| Antibacterial activity (mg/mL) B | ||||||||||||||||||||||
| MIC | MBC | MIC | MBC | MIC | MBC | MIC | MBC | MIC | MBC | MIC | MBC | MIC | MBC | MIC | MBC | MIC | MBC | MIC | MBC | MIC | MBC | |
| Food bacteria | ||||||||||||||||||||||
| Gram-negative bacteria | ||||||||||||||||||||||
| Enterobacter cloacae | 10 | >10 | 7.5 | >10 | >10 | >10 | >10 | >10 | >10 | >10 | 10 | >10 | 10 | >10 | 5 | >10 | 10 | >10 | >10 | >10 | 10 | >10 |
| Escherichia coli | 5 | >10 | 3.75 | >10 | 10 | >10 | 6.7 | >10 | 5.8 | >10 | 5 | >10 | 5 | >10 | 5 | >10 | 2.5 | >10 | 10 | >10 | 10 | >10 |
| Pseudomonas aeruginosa | 10 | >10 | 10 | >10 | >10 | >10 | >10 | >10 | 10 | >10 | 10 | >10 | 10 | >10 | 10 | >10 | 10 | >10 | >10 | >10 | 10 | >10 |
| Salmonella enterica | >10 | >10 | >10 | >10 | 10 | >10 | >10 | >10 | 5.58 | >10 | >10 | >10 | 5 | >10 | >10 | >10 | 2.5 | >10 | 10 | >10 | 10 | >10 |
| Yersinia enterocolitica | 10 | >10 | 7.5 | >10 | 10 | >10 | 10 | >10 | 8.3 | >10 | 10 | >10 | 10 | >10 | 10 | >10 | 5 | >10 | 10 | >10 | 10 | >10 |
| Gram-positive bacteria | ||||||||||||||||||||||
| Bacillus cereus | 10 | >10 | 7.5 | >10 | >10 | >10 | >10 | >10 | 10 | >10 | >10 | >10 | 10 | >10 | >10 | >10 | 10 | >10 | 10 | >10 | >10 | >10 |
| Listeria monocytogenes | 5 | >10 | 3.75 | >10 | 10 | >10 | 6.7 | >10 | 5.8 | >10 | >10 | >10 | 2.5 | >10 | 10 | >10 | 5 | >10 | 10 | >10 | 10 | >10 |
| Staphylococcus aureus | 10 | >10 | 10 | >10 | >10 | >10 | >10 | >10 | 10 | >10 | 10 | >10 | 2.5 | >10 | 10 | >10 | 5 | >10 | 10 | >10 | 10 | >10 |
| Clinical bacteria | ||||||||||||||||||||||
| Gram-negative bacteria | ||||||||||||||||||||||
| Escherichia coli | 5 | >10 | 3.75 | >10 | 10 | >10 | 6.7 | >10 | 7.5 | >10 | 5 | >10 | 5 | >10 | 5 | >10 | 2.5 | >10 | 10 | >10 | 10 | >10 |
| Klebsiella pneumoniae | >10 | >10 | 10 | >10 | >10 | >10 | >10 | >10 | >10 | >10 | >10 | >10 | >10 | >10 | 10 | >10 | 10 | >10 | >10 | >10 | >10 | >10 |
| Morganella morganii | >10 | >10 | >10 | >10 | >10 | >10 | >10 | >10 | >10 | >10 | >10 | >10 | >10 | >10 | >10 | >10 | >10 | >10 | >10 | >10 | >10 | >10 |
| Proteus mirabilis | >10 | >10 | >10 | >10 | >10 | >10 | >10 | >10 | >10 | >10 | >10 | >10 | >10 | >10 | >10 | >10 | >10 | >10 | >10 | >10 | >10 | >10 |
| Pseudomonas aeruginosa | 10 | >10 | >10 | >10 | >10 | >10 | >10 | >10 | >10 | >10 | 10 | >10 | 10 | >10 | >10 | >10 | 10 | >10 | >10 | >10 | 10 | >10 |
| Gram-positive bacteria | ||||||||||||||||||||||
| Enterococcus faecalis | >10 | >10 | >10 | >10 | >10 | >10 | >10 | >10 | >10 | >10 | >10 | >10 | 10 | >10 | >10 | >10 | 10 | >10 | 10 | >10 | >10 | >10 |
| Listeria monocytogenes | >10 | >10 | >10 | >10 | >10 | >10 | >10 | >10 | >10 | >10 | >10 | >10 | 2.5 | >10 | 10 | >10 | 5 | >10 | 10 | >10 | 10 | >10 |
| MRSA | 6.25 | >10 | 6.25 | >10 | 10 | >10 | 10 | >10 | 5 | >10 | 10 | >10 | 2.5 | >10 | 10 | >10 | 2.5 | >10 | 10 | >10 | 10 | >10 |
| Antifungal activity (mg/mL) B | ||||||||||||||||||||||
| MIC | MBC | MIC | MBC | MIC | MBC | MIC | MBC | MIC | MBC | MIC | MBC | MIC | MBC | MIC | MBC | MIC | MBC | MIC | MBC | MIC | MBC | |
| Aspergillus brasiliensis | >10 | >10 | >10 | >10 | >10 | >10 | >10 | >10 | >10 | >10 | >10 | >10 | >10 | >10 | >10 | >10 | >10 | >10 | >10 | >10 | >10 | >10 |
| Aspergillus fumigatus | 10 | >10 | 10 | >10 | 10 | >10 | 10 | >10 | 10 | >10 | 10 | >10 | 10 | >10 | 10 | >10 | 10 | >10 | 10 | >10 | 10 | >10 |
| NO-production inhibition (IC50 values μg/mL) C | ||||||||||||||||||||||
| RAW 264,7 | >400 | >400 | >400 | >400 | >400 | >400 | >400 | >400 | >400 | >400 | >400 | |||||||||||
| Antiproliferative Activity (GI50 values μg/mL) D | ||||||||||||||||||||||
| AGS | 291 ± 16.04 a | 221 ± 21 c | 240 ± 46 b | 264 ± 32 a | 237 ± 42 b | 289 ± 28 ab | 225 ± 23 c | 279 ± 26 b | 293 ± 22 a | 218 ± 14 c | 202 ± 13 d | |||||||||||
| Caco-2 | >400 | >400 | >400 | >400 | >400 | >400 | >400 | >400 | >400 | >400 | >400 | |||||||||||
| MCF7 | >400 | >400 | >400 | >400 | >400 | >400 | >400 | >400 | >400 | >400 | >400 | |||||||||||
| NCI-H460 | >400 | >400 | >400 | >400 | >400 | >400 | >400 | >400 | >400 | >400 | >400 | |||||||||||
| Hepatotoxicity (GI50 values μg/mL) D | ||||||||||||||||||||||
| PLP2 | >400 | >400 | >400 | >400 | >400 | >400 | >400 | >400 | >400 | >400 | >400 | |||||||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Paschoalinotto, B.H.; Polyzos, N.; Liava, V.; Mandim, F.; Pires, T.C.S.P.; Añibarro-Ortega, M.; Ferreira, I.C.F.R.; Dias, M.I.; Barros, L.; Petropoulos, S.A. The Effect of Cropping System and Irrigation Regime on the Plant Growth and Biochemical Profile of Cichorium spinosum. Horticulturae 2025, 11, 306. https://doi.org/10.3390/horticulturae11030306
Paschoalinotto BH, Polyzos N, Liava V, Mandim F, Pires TCSP, Añibarro-Ortega M, Ferreira ICFR, Dias MI, Barros L, Petropoulos SA. The Effect of Cropping System and Irrigation Regime on the Plant Growth and Biochemical Profile of Cichorium spinosum. Horticulturae. 2025; 11(3):306. https://doi.org/10.3390/horticulturae11030306
Chicago/Turabian StylePaschoalinotto, Beatriz H., Nikolaos Polyzos, Vasiliki Liava, Filipa Mandim, Tânia C. S. P. Pires, Mikel Añibarro-Ortega, Isabel C. F. R. Ferreira, Maria Inês Dias, Lillian Barros, and Spyridon A. Petropoulos. 2025. "The Effect of Cropping System and Irrigation Regime on the Plant Growth and Biochemical Profile of Cichorium spinosum" Horticulturae 11, no. 3: 306. https://doi.org/10.3390/horticulturae11030306
APA StylePaschoalinotto, B. H., Polyzos, N., Liava, V., Mandim, F., Pires, T. C. S. P., Añibarro-Ortega, M., Ferreira, I. C. F. R., Dias, M. I., Barros, L., & Petropoulos, S. A. (2025). The Effect of Cropping System and Irrigation Regime on the Plant Growth and Biochemical Profile of Cichorium spinosum. Horticulturae, 11(3), 306. https://doi.org/10.3390/horticulturae11030306













