Influence of Supplementary Blue and Far-Red Light on the Morphology and Texture of Ocimum basilicum L. Grown in Controlled Environments
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Cultivation
2.2. Light Treatments
2.3. Determination of Biometric Indicators
2.4. Chlorophyll Fluorescence Measurements
2.5. Leaf Texture and Surface Density Analysis
2.6. Experimental Design and Data Analysis
3. Results
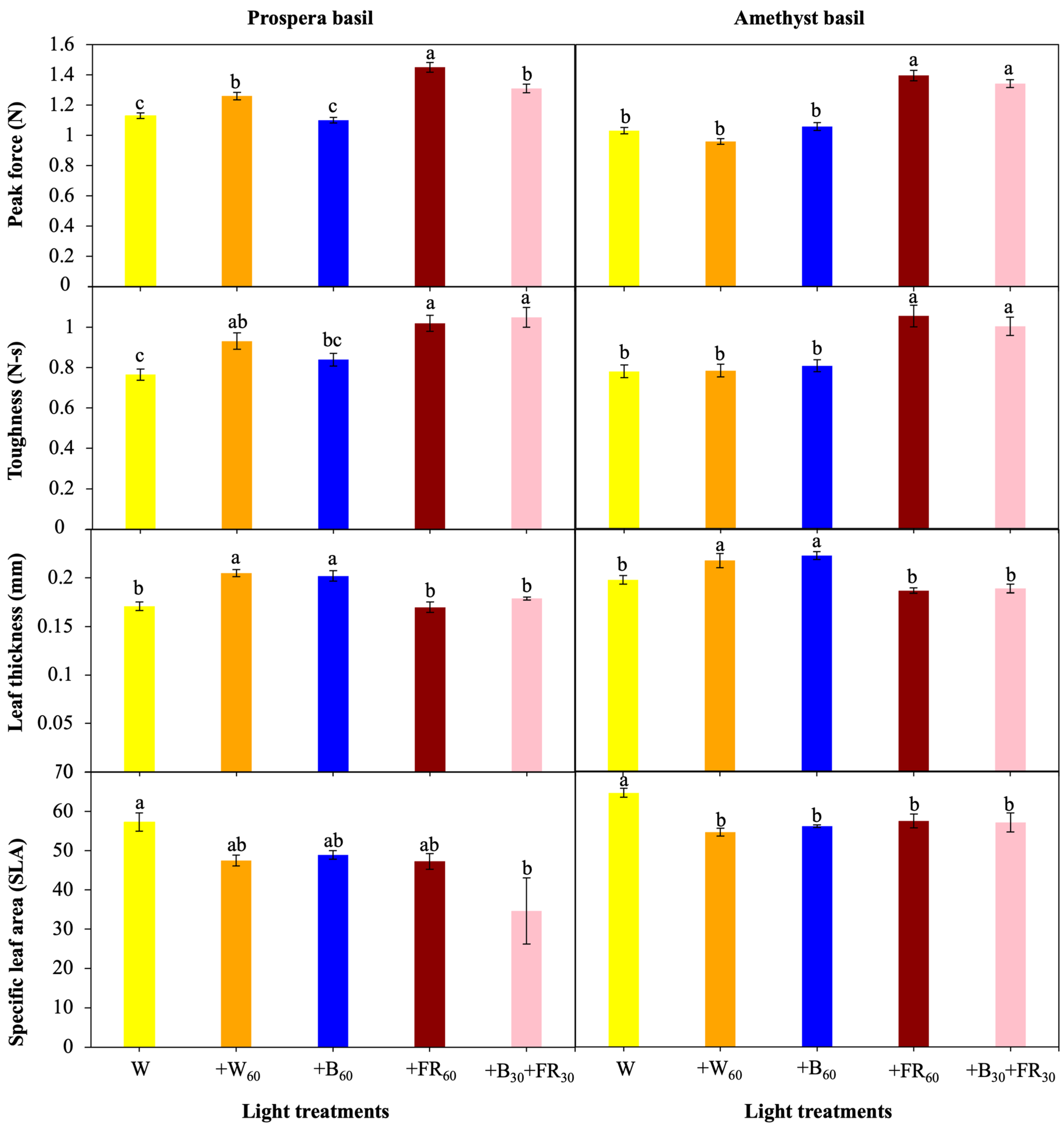
3.1. Biomass Accumulation and Plant Morphology
3.2. Leaf Texture and Thickness
3.3. Chlorophyll Fluorescence and Leaf Coloration
4. Discussion
4.1. Basil Growth and Morphology
4.2. Chlorophyll Fluorescence
4.3. Basil Leaf Coloration
4.4. Leaf Thickness and Texture
4.5. Future Studies
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Carvalho, S.D.; Schwieterman, M.L.; Abrahan, C.E.; Colquhoun, T.A.; Folta, K.M. Light quality dependent changes in morphology, antioxidant capacity, and volatile production in sweet basil (Ocimum basilicum). Front. Plant Sci. 2016, 7, 1328. [Google Scholar] [CrossRef] [PubMed]
- Filip, S. Basil (Ocimum basilicum L.) a source of valuable phytonutrients. Int. J. Clin. Nutr. Diet. 2017, 3, 118. [Google Scholar] [CrossRef] [PubMed]
- Singletary, K.W. Basil: A brief summary of potential health benefits. Nutr. Today 2018, 53, 92–97. [Google Scholar] [CrossRef]
- Rattanachaikunsopon, P.; Phumkhachorn, P. Antimicrobial activity of basil (Ocimum basilicum) oil against Salmonella Enteritidis in vitro and in food. Biosci. Biotechnol. Biochem. 2010, 74, 1200–1204. [Google Scholar] [CrossRef]
- Dou, H.; Niu, G.; Gu, M.; Masabni, J.G. Effects of light quality on growth and phytonutrient accumulation of herbs under controlled environments. Horticulturae 2017, 3, 36. [Google Scholar] [CrossRef]
- Sipos, L.; Balázs, L.; Székely, G.; Jung, A.; Sárosi, S.; Radácsi, P.; Csambalik, L. Optimization of basil (Ocimum basilicum L.) production in LED light environments—A review. Sci. Hortic. 2021, 289, 110486. [Google Scholar] [CrossRef]
- Dudai, N.; Nitzan, N.; Gonda, I. Ocimum basilicum L. (Basil). In Medicinal, Aromatic and Stimulant Plants; Novak, J., Blüthner, W.-D., Eds.; Springer International Publishing: Cham, Switzerland, 2020; pp. 377–405. [Google Scholar] [CrossRef]
- Baghalian, K.; Hajirezaei, M.-R.; Lawson, T. Editorial: Current and future perspectives for controlled environment agriculture (CEA) in the 21st century. Front. Plant Sci. 2023, 14, 1334641. [Google Scholar] [CrossRef]
- Walters, K.J.; Lopez, R.G. Hydroponic basil production: Temperature influences volatile organic compound profile, but not overall consumer preference. Horticulturae 2022, 8, 76. [Google Scholar] [CrossRef]
- Kelly, N.; Vaštakaitė-Kairienė, V.; Runkle, E.S. Chapter 18—Indoor lighting effects on plant nutritional compounds. In Plant Factory Basics, Applications and Advances; Kozai, T., Niu, G., Masabni, J., Eds.; Academic Press: Cambridge, MA, USA, 2022; pp. 329–349. [Google Scholar] [CrossRef]
- Kusuma, P.; Pattison, P.M.; Bugbee, B. Chapter 7—Photon efficacy in horticulture: Turning LED packages into LED luminaires. In Plant Factory Basics, Applications and Advances; Kozai, T., Niu, G., Masabni, J., Eds.; Academic Press: Cambridge, MA, USA, 2022; pp. 115–128. [Google Scholar] [CrossRef]
- Aldarkazali, M.; Rihan, H.Z.; Carne, D.; Fuller, M.P. The growth and development of sweet basil (Ocimum basilicum) and bush basil (Ocimum minimum) grown under three light regimes in a controlled environment. Agronomy 2019, 9, 743. [Google Scholar] [CrossRef]
- Yam, F.K.; Hassan, Z. Innovative advances in LED technology. Microelectron. J. 2005, 36, 129–137. [Google Scholar] [CrossRef]
- Zhang, X.; Bian, Z.; Yuan, X.; Chen, X.; Lu, C. A review on the effects of light-emitting diode (LED) light on the nutrients of sprouts and microgreens. Trends Food Sci. Technol. 2020, 99, 203–216. [Google Scholar] [CrossRef]
- Zheludev, N. The life and times of the LED—A 100-year history. Nat. Photonics 2007, 1, 189–192. [Google Scholar] [CrossRef]
- Zhen, S.; Kusuma, P.; Bugbee, B. Chapter 17—Toward an optimal spectrum for photosynthesis and plant morphology in LED-based crop cultivation. In Plant Factory Basics, Applications and Advances; Kozai, T., Niu, G., Masabni, J., Eds.; Academic Press: Cambridge, MA, USA, 2022; pp. 309–327. [Google Scholar] [CrossRef]
- Hendricks, S.B.; Borthwick, H.A. The function of phytochrome in regulation of plant growth. Proc. Natl. Acad. Sci. USA 1967, 58, 2125–2130. [Google Scholar] [CrossRef] [PubMed]
- Metallo, R.M.; Kopsell, D.A.; Sams, C.E.; Bumgarner, N.R. Influence of blue/red vs. white LED light treatments on biomass, shoot morphology, and quality parameters of hydroponically grown kale. Sci. Hortic. 2018, 235, 189–197. [Google Scholar] [CrossRef]
- Ahmad, M. Photocycle and signaling mechanisms of plant cryptochromes. Curr. Opin. Plant Biol. 2016, 33, 108–115. [Google Scholar] [CrossRef]
- Cashmore, A.R.; Jarillo, J.A.; Wu, Y.-J.; Liu, D. Cryptochromes: Blue Light Receptors for Plants and Animals. Science 1999, 284, 760–765. [Google Scholar] [CrossRef]
- Jin, W.; Urbina, J.L.; Heuvelink, E.; Marcelis, L.F.M. Adding far-red to red-blue light-emitting diode light promotes yield of lettuce at different planting densities. Front. Plant Sci. 2021, 11, 609977. [Google Scholar] [CrossRef]
- Park, C.H.; Park, Y.E.; Yeo, H.J.; Kim, J.K.; Park, S.U. Effects of light-emitting diodes on the accumulation of phenolic compounds and glucosinolates in Brassica juncea sprouts. Horticulturae 2020, 6, 77. [Google Scholar] [CrossRef]
- Yudina, L.; Sukhova, E.; Gromova, E.; Mudrilov, M.; Zolin, Y.; Popova, A.; Nerush, V.; Pecherina, A.; Grishin, A.A.; Dorokhov, A.A.; et al. Effect of duration of led lighting on growth, photosynthesis and respiration in lettuce. Plants 2023, 12, 442. [Google Scholar] [CrossRef]
- Hosseini, A.; Zare Mehrjerdi, M.; Aliniaeifard, S.; Seif, M. Photosynthetic and growth responses of green and purple basil plants under different spectral compositions. Physiol. Mol. Biol. Plants 2019, 25, 741–752. [Google Scholar] [CrossRef]
- Landi, M.; Zivcak, M.; Sytar, O.; Brestic, M.; Allakhverdiev, S.I. Plasticity of photosynthetic processes and the accumulation of secondary metabolites in plants in response to monochromatic light environments: A review. Biochim. Biophys. Acta BBA Bioenerg. 2020, 1861, 148131. [Google Scholar] [CrossRef] [PubMed]
- Zhen, S.; van Iersel, M.W. Far-red light is needed for efficient photochemistry and photosynthesis. J. Plant Physiol. 2017, 209, 115–122. [Google Scholar] [CrossRef]
- Hammock, H.A.; Sams, C.E. Variation in supplemental lighting quality influences key aroma volatiles in hydroponically grown ‘Italian Large Leaf’ basil. Front. Plant Sci. 2023, 14, 1184664. [Google Scholar] [CrossRef] [PubMed]
- Jeong, S.J.; Niu, G.; Zhen, S. Far-red light and temperature interactively regulate plant growth and morphology of lettuce and basil. Environ. Exp. Bot. 2024, 218, 105589. [Google Scholar] [CrossRef]
- Larsen, D.H.; Woltering, E.J.; Nicole, C.C.S.; Marcelis, L.F.M. Response of basil growth and morphology to light intensity and spectrum in a vertical farm. Front. Plant Sci. 2020, 11, 597906. [Google Scholar] [CrossRef]
- Litvin, A.G.; Currey, C.J.; Wilson, L.A. Effects of supplemental light source on basil, dill, and parsley growth, morphology, aroma, and flavor. J. Am. Soc. Hortic. Sci. J. Amer. Soc. Hort. Sci. 2020, 145, 18–29. [Google Scholar] [CrossRef]
- Lin, K.-H.; Huang, M.-Y.; Hsu, M.-H. Morphological and physiological response in green and purple basil plants (Ocimum basilicum) under different proportions of red, green, and blue LED lightings. Sci. Hortic. 2021, 275, 109677. [Google Scholar] [CrossRef]
- Rahman, M.M.; Vasiliev, M.; Alameh, K. LED Illumination spectrum manipulation for increasing the yield of sweet basil (Ocimum basilicum L.). Plants 2021, 10, 344. [Google Scholar] [CrossRef]
- Kannan, M.; Elavarasan, G.; Balamurugan, A.; Dhanusiya, B.; Freedon, D. Hydroponic farming—A state of art for the future agriculture. Mater. Today Proc. 2022, 68, 2163–2166. [Google Scholar] [CrossRef]
- Yue, C.; Vickers, Z.; Wang, J.; Anderson, N.O.; Wisdorf, L.; Brady, J.; Schermann, M.; Phelps, N.; Venturelli, P. Consumer acceptability of aquaponically grown basil. HortScience Horts 2020, 55, 841–850. [Google Scholar] [CrossRef]
- Kelly, N.; Choe, D.; Meng, Q.; Runkle, E.S. Promotion of lettuce growth under an increasing daily light integral depends on the combination of the photosynthetic photon flux density and photoperiod. Sci. Hortic. 2020, 272, 109565. [Google Scholar] [CrossRef]
- Bornhorst, E.R.; Luo, Y.; Park, E.; Zhou, B.; Turner, E.R.; Teng, Z.; Trouth, F.; Simko, I.; Fonseca, J.M. In search of optimum fresh-cut raw material: Using computer vision systems as a sensory screening tool for browning-resistant romaine lettuce accessions. Horticulturae 2024, 10, 731. [Google Scholar] [CrossRef]
- Ibraheem, N.; Hasan, M.; Khan, R.Z.; Mishra, P. Understanding color models: A review. ARPN J. Sci. Technol. 2012, 2, 265–275. [Google Scholar]
- Maxwell, K.; Johnson, G.N. Chlorophyll fluorescence—A practical guide. J. Exp. Bot. 2000, 51, 659–668. [Google Scholar] [CrossRef]
- Date, M.B.; Rivero, W.C.; Tan, J.; Specca, D.; Simon, J.E.; Salvi, D.A.; Karwe, M.V. Growth of hydroponic sweet basil (O. basilicum L.) using plasma-activated nutrient solution (PANS). Agriculture 2023, 13, 443. [Google Scholar] [CrossRef]
- Gutiérrez-Rodríguez, E.; Lieth, H.J.; Jernstedt, J.A.; Labavitch, J.M.; Suslow, T.V.; Cantwell, M.I. Texture, composition and anatomy of spinach leaves in relation to nitrogen fertilization. J. Sci. Food Agric. 2013, 93, 227–237. [Google Scholar] [CrossRef]
- Vile, D.; Garnier, É.; Shipley, B.; Laurent, G.; Navas, M.-L.; Roumet, C.; Lavorel, S.; Díaz, S.; Hodgson, J.G.; Lloret, F.; et al. Specific leaf area and dry matter content estimate thickness in laminar leaves. Ann. Bot. 2005, 96, 1129–1136. [Google Scholar] [CrossRef]
- Wickham, H.; François, R.; Henry, L.; Müller, K.; Vaughan, D. dplyr: A Grammar of Data Manipulation. Available online: https://cran.r-project.org/web/packages/dplyr/index.html (accessed on 1 February 2023).
- de Mendiburu, F. Agricolae: Statistical Procedures for Agricultural Research. Available online: https://cran.r-project.org/web/packages/agricolae/index.html (accessed on 1 February 2023).
- Franklin, K.A.; Quail, P.H. Phytochrome functions in Arabidopsis development. J. Exp. Bot. 2009, 61, 11–24. [Google Scholar] [CrossRef]
- Smith, H. Phytochromes and light signal perception by plants—An emerging synthesis. Nature 2000, 407, 585–591. [Google Scholar] [CrossRef]
- Ballaré, C.L.; Pierik, R. The shade-avoidance syndrome: Multiple signals and ecological consequences. Plant Cell Environ. 2017, 40, 2530–2543. [Google Scholar] [CrossRef]
- Casal, J.J. Photoreceptor signaling networks in plant responses to shade. Annu. Rev. Plant Biol. 2013, 64, 403–427. [Google Scholar] [CrossRef] [PubMed]
- Lorrain, S.; Allen, T.; Duek, P.D.; Whitelam, G.C.; Fankhauser, C. Phytochrome-mediated inhibition of shade avoidance involves degradation of growth-promoting bHLH transcription factors. Plant J. 2008, 53, 312–323. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; van Iersel, M.W. Photosynthetic physiology of blue, green, and red light: Light intensity effects and underlying mechanisms. Front. Plant Sci. 2021, 12, 619987. [Google Scholar] [CrossRef] [PubMed]
- Terashima, I.; Fujita, T.; Inoue, T.; Chow, W.S.; Oguchi, R. Green light drives leaf photosynthesis more efficiently than red light in strong white light: Revisiting the enigmatic question of why leaves are green. Plant Cell Physiol. 2009, 50, 684–697. [Google Scholar] [CrossRef]
- Ma, Y.; Ma, X.; Gao, X.; Wu, W.; Zhou, B. Light induced regulation pathway of anthocyanin biosynthesis in plants. Int. J. Mol. Sci. 2021, 22, 11116. [Google Scholar] [CrossRef]
- Shalitin, D.; Yu, X.; Maymon, M.; Mockler, T.; Lin, C. Blue light–dependent in vivo and in vitro phosphorylation of arabidopsis cryptochrome 1. Plant Cell 2003, 15, 2421–2429. [Google Scholar] [CrossRef]
- Christie, J.M.; Blackwood, L.; Petersen, J.; Sullivan, S. Plant flavoprotein photoreceptors. Plant Cell Physiol. 2014, 56, 401–413. [Google Scholar] [CrossRef]
- Gould, K.S. Nature’s swiss army knife: The diverse protective roles of anthocyanins in leaves. J. Biomed. Biotechnol. 2004, 2004, 314–320. [Google Scholar] [CrossRef]
- Hatier, J.-H.B.; Gould, K.S. Anthocyanin function in vegetative organs. In Anthocyanins: Biosynthesis, Functions, and Applications; Winefield, C., Davies, K., Gould, K., Eds.; Springer: New York, NY, USA, 2009; pp. 1–19. [Google Scholar] [CrossRef]
- Meng, Q.; Kelly, N.; Runkle, E.S. Substituting green or far-red radiation for blue radiation induces shade avoidance and promotes growth in lettuce and kale. Environ. Exp. Bot. 2019, 162, 383–391. [Google Scholar] [CrossRef]
- Liu, J.; van Iersel, M.W. Far-red light effects on lettuce growth and morphology in indoor production are cultivar specific. Plants 2022, 11, 2714. [Google Scholar] [CrossRef]
- Lee, M.; Xu, J.; Wang, W.; Rajashekar, C. The effect of supplemental blue, red, and far-red light on the growth and the nutritional quality of red and green leaf lettuce. Am. J. Plant Sci. 2019, 10, 2219–2235. [Google Scholar] [CrossRef]
- Zheng, L.; Van Labeke, M.-C. Long-term effects of red- and blue-light emitting diodes on leaf anatomy and photosynthetic efficiency of three ornamental pot plants. Front. Plant Sci. 2017, 8, 917. [Google Scholar] [CrossRef] [PubMed]
- Falcioni, R.; Moriwaki, T.; Perez-Llorca, M.; Munné-Bosch, S.; Gibin, M.S.; Sato, F.; Pelozo, A.; Pattaro, M.C.; Giacomelli, M.E.; Rüggeberg, M.; et al. Cell wall structure and composition is affected by light quality in tomato seedlings. J. Photochem. Photobiol. B Biol. 2020, 203, 111745. [Google Scholar] [CrossRef] [PubMed]
- Meng, Q.; Boldt, J.; Runkle, E.S. Blue Radiation Interacts with Green Radiation to Influence Growth and Predominantly Controls Quality Attributes of Lettuce. J. Am. Soc. Hortic. Sci. J. Amer. Soc. Hort. Sci. 2020, 145, 75–87. [Google Scholar] [CrossRef]
- Wadhera, D.; Capaldi-Phillips, E.D. A review of visual cues associated with food on food acceptance and consumption. Eat. Behav. 2014, 15, 132–143. [Google Scholar] [CrossRef]
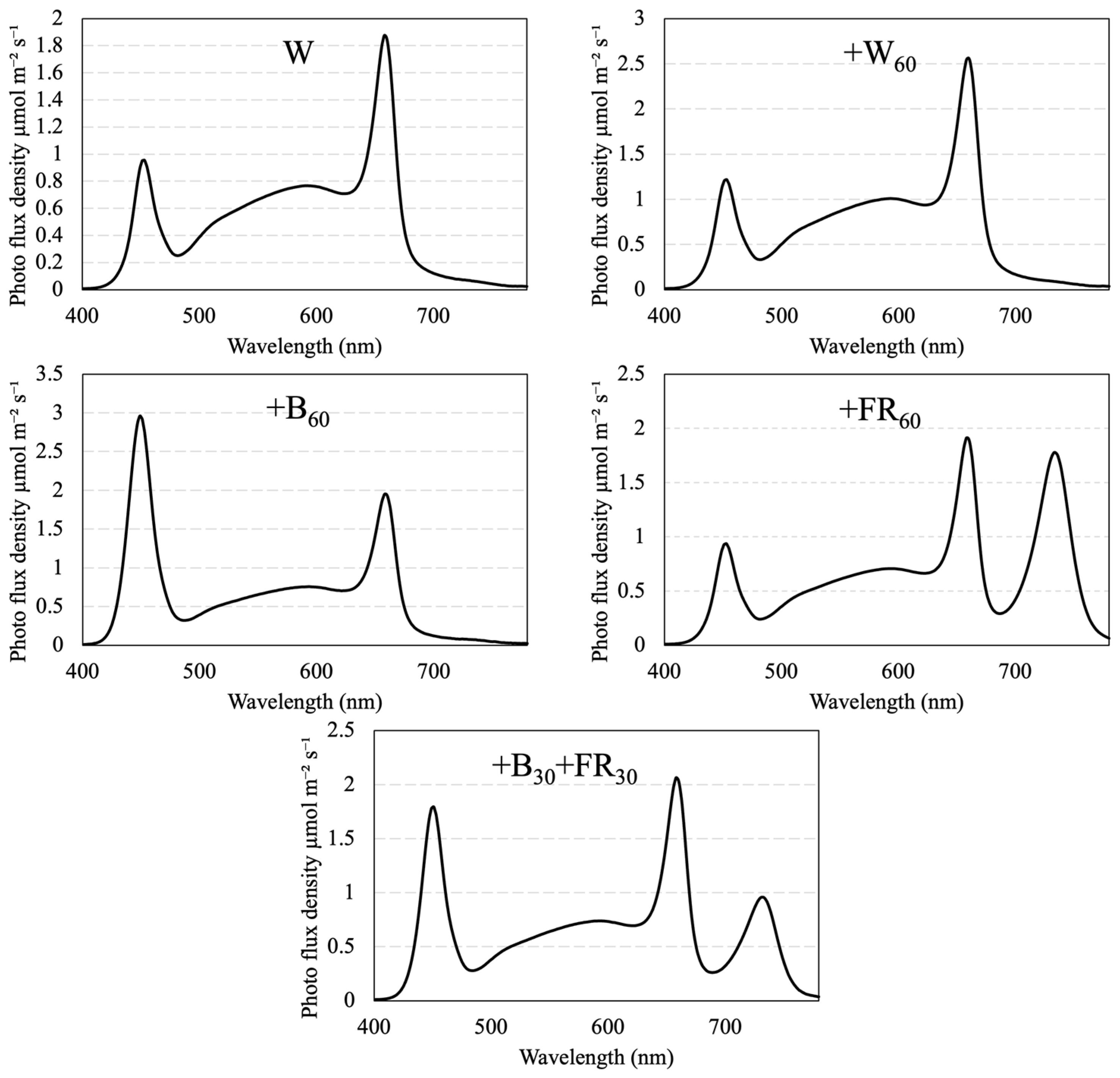
| Treatment | W | +W60 | +B60 | +FR60 | +B30+FR30 |
|---|---|---|---|---|---|
| TPFD | 181.55 | 242.57 | 239.68 | 240.51 | 239.79 |
| PPFD | 176.67 | 235.68 | 234.30 | 173.10 | 202.92 |
| B PFD | 32.65 | 42.63 | 88.83 | 32.02 | 55.14 |
| G PFD | 64.13 | 83.90 | 63.41 | 58.88 | 61.80 |
| R PFD | 79.90 | 109.14 | 82.06 | 82.20 | 85.99% |
| FR PFD | 4.70 | 6.64 | 5.14 | 67.19 | 36.65% |
| B% | 17.98% | 17.58% | 37.06% | 13.31% | 22.99% |
| G% | 35.32% | 34.59% | 26.46% | 24.48% | 25.77% |
| R% | 44.01% | 45.00% | 34.24% | 34.18% | 35.86% |
| FR% | 2.59% | 2.74% | 2.14% | 27.94% | 15.28% |
| Cultivar | Treatment | Wfresh (g) | Wdry (g) | Leaf Number | Stem Length (mm) | Stem Width (mm) |
|---|---|---|---|---|---|---|
| ‘Prospera’ | W | 8.43 c | 0.57 c | 15.7 b | 99.7 b | 4.32 c |
| +W60 | 12.5 a | 0.90 a | 21.8 a | 103.0 b | 5.31 ab | |
| +B60 | 10.4 b | 0.75 b | 18.6 b | 96.2 b | 4.92 b | |
| +FR60 | 13.1 a | 1.02 a | 23.1 a | 168.3 a | 5.16 ab | |
| +B30+FR30 | 13.2 a | 0.98 a | 22.0 a | 152.1 a | 5.35 a | |
| ‘Amethyst’ | W | 4.72 c | 0.31 c | 10.2 d | 73.1 c | 3.52 c |
| +W60 | 6.74 b | 0.44 ab | 15.6 b | 86.7 c | 4.05 b | |
| +B60 | 6.13 b | 0.38 bc | 12.7 c | 82.3 c | 3.93 bc | |
| +FR60 | 8.88 a | 0.50 a | 18.8 a | 158.7 a | 4.81 a | |
| +BFR60 | 8.47 a | 0.51 a | 16.6 ab | 131.0 b | 4.77 a |
| Cultivar | Treatment | Fs | Fm’ | ETR | phiPSII | Fo | Fm | Fv/Fm |
|---|---|---|---|---|---|---|---|---|
| ‘Prospera’ | W | 161.2 a | 353.4 b | 32.69 b | 0.534 c | 91.19 a | 464.6 a | 0.804 c |
| +W60 | 132.0 ab | 329.7 b | 61.82 a | 0.587 bc | 88.39 ab | 458.7 a | 0.807 c | |
| +B60 | 127.8 ab | 333.3 b | 59.80 a | 0.610 abc | 87.95 ab | 466.5 a | 0.811 bc | |
| +FR60 | 126.2 b | 403.2 a | 54.78 a | 0.688 a | 86.30 ab | 479.6 a | 0.820 a | |
| +B30+FR30 | 137.7 ab | 377.8 ab | 53.96 a | 0.635 ab | 83.32 b | 454.9 a | 0.817 ab | |
| ‘Amethyst’ | W | 99.0 a | 292.2 b | 42.58 b | 0.664 ab | 70.71 a | 362.0 b | 0.805 b |
| +W60 | 101.1 a | 252.9 c | 59.60 a | 0.605 b | 61.12 b | 310.8 d | 0.803 b | |
| +B60 | 86.07 a | 267.9 bc | 55.72 a | 0.675 ab | 65.35 b | 345.3 bc | 0.811 ab | |
| +FR60 | 107.7 a | 341.5 a | 50.82 ab | 0.685 a | 72.79 a | 391.6 a | 0.814 a | |
| +B30+FR30 | 86.6 a | 285.7 b | 55.23 a | 0.699 a | 64.86 b | 335.5 cd | 0.807 ab |
| Purple Section of Leaf | Green Section of Leaf | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Treatment | L* | a* | b* | Hue | Chroma | L* | a* | b* | Hue | Chroma |
| W | 26.1 bc | 2.86 c | 1.79 bc | 32.1 | 3.38 | 36.3 b | −0.8 a | 8.84 c | 95.2 | 8.75 |
| +W60 | 24.9 c | 3.11 c | 0.90 c | 16.3 | 3.24 | 36.0 b | −0.48 a | 10.4 c | 92.6 | 10.4 |
| +B60 | 25.4 c | 3.33 bc | 1.25 bc | 20.7 | 3.56 | 36.5 b | −1.08 a | 11.1 c | 95.6 | 11.0 |
| +FR60 | 28.1 a | 3.84 ab | 4.83 a | 51.5 | 6.17 | 38.1 a | −3.11 b | 16.4 a | 100.8 | 16.2 |
| +B30+FR30 | 26.9 ab | 4.42 a | 2.30 b | 27.6 | 4.99 | 37.3 a | −1.23 a | 13.7 b | 95.1 | 13.6 |
| Treatment | L* | a* | b* | Hue | Chroma |
|---|---|---|---|---|---|
| W | 40.9 b | −21.5 bc | 31.5 b | 124.2 | 38.2 |
| +W60 | 39.7 c | −20.7 a | 30.7 b | 124.0 | 37.1 |
| +B60 | 40.7 bc | −20.9 ab | 30.8 b | 124.1 | 37.3 |
| +FR60 | 44.0 a | −21.8 c | 35.5 a | 121.7 | 41.9 |
| +B30+FR30 | 44.3 a | −21.9 c | 35.6 a | 121.6 | 41.8 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Oehler, M.A.; Kelly, N.; Fonseca, J.M.; Evensen, E.; Park, E.; Gu, G.; Teng, Z.; Luo, Y. Influence of Supplementary Blue and Far-Red Light on the Morphology and Texture of Ocimum basilicum L. Grown in Controlled Environments. Horticulturae 2025, 11, 287. https://doi.org/10.3390/horticulturae11030287
Oehler MA, Kelly N, Fonseca JM, Evensen E, Park E, Gu G, Teng Z, Luo Y. Influence of Supplementary Blue and Far-Red Light on the Morphology and Texture of Ocimum basilicum L. Grown in Controlled Environments. Horticulturae. 2025; 11(3):287. https://doi.org/10.3390/horticulturae11030287
Chicago/Turabian StyleOehler, Madison A., Nathan Kelly, Jorge M. Fonseca, Ella Evensen, Eunhee Park, Ganyu Gu, Zi Teng, and Yaguang Luo. 2025. "Influence of Supplementary Blue and Far-Red Light on the Morphology and Texture of Ocimum basilicum L. Grown in Controlled Environments" Horticulturae 11, no. 3: 287. https://doi.org/10.3390/horticulturae11030287
APA StyleOehler, M. A., Kelly, N., Fonseca, J. M., Evensen, E., Park, E., Gu, G., Teng, Z., & Luo, Y. (2025). Influence of Supplementary Blue and Far-Red Light on the Morphology and Texture of Ocimum basilicum L. Grown in Controlled Environments. Horticulturae, 11(3), 287. https://doi.org/10.3390/horticulturae11030287







