Impact of Salinity on Sugar Composition and Partitioning in Relation to Flower Fertility in Solanum lycopersicum and Solanum chilense
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material and Growth Conditions
2.2. Morphological Measurements and Reproductive Development Assessment
2.3. Photosynthesis Parameters
2.4. Carbohydrates Analysis
2.4.1. Carbohydrates Extraction from Leaves and Flowers
2.4.2. Apical and Foliar Phloem Sap Collection
2.4.3. Carbohydrate Analysis
2.5. Gene Expression by qRT-PCR
2.6. Statistical Treatments
3. Results
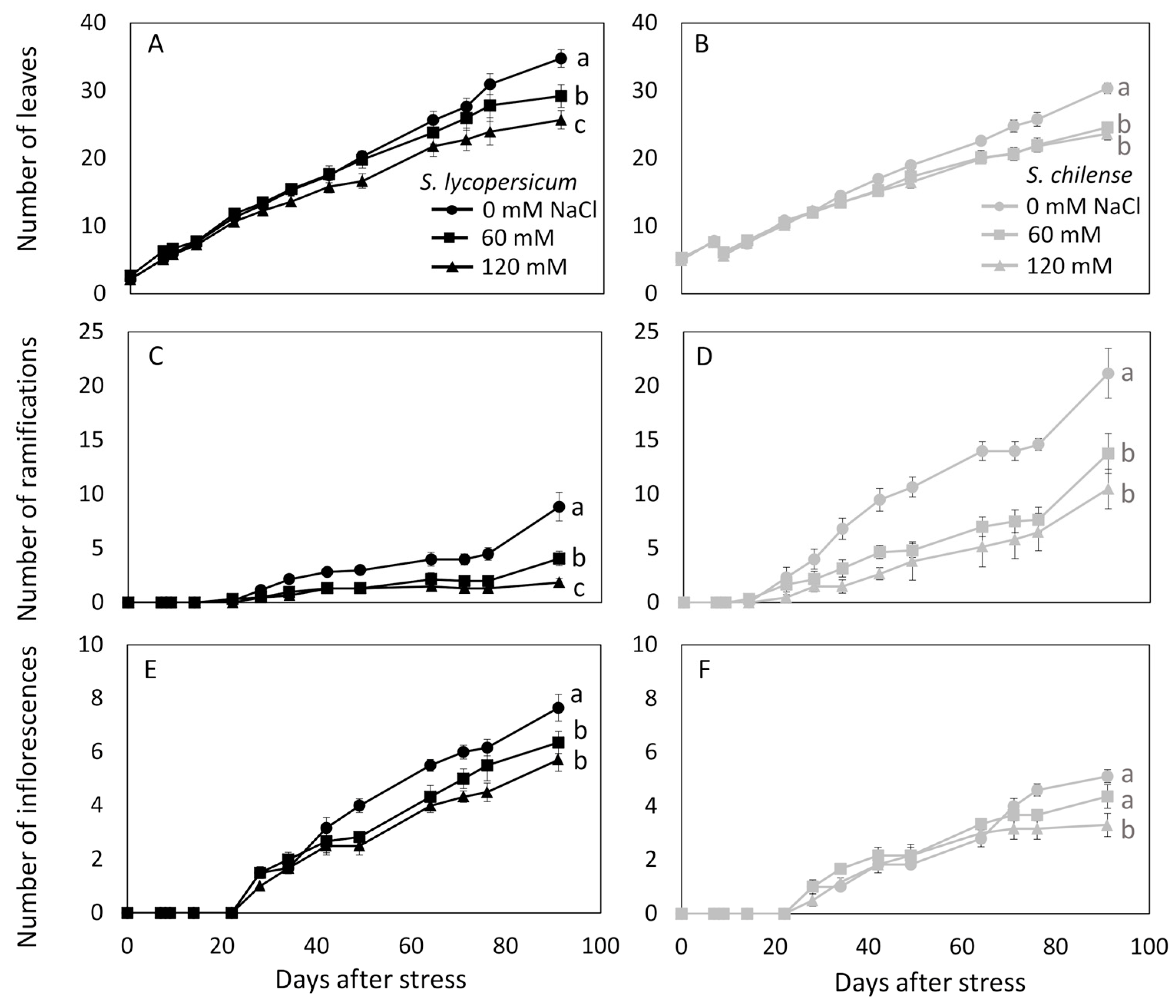
3.1. Vegetative and Reproductive Developments Under Control Conditions
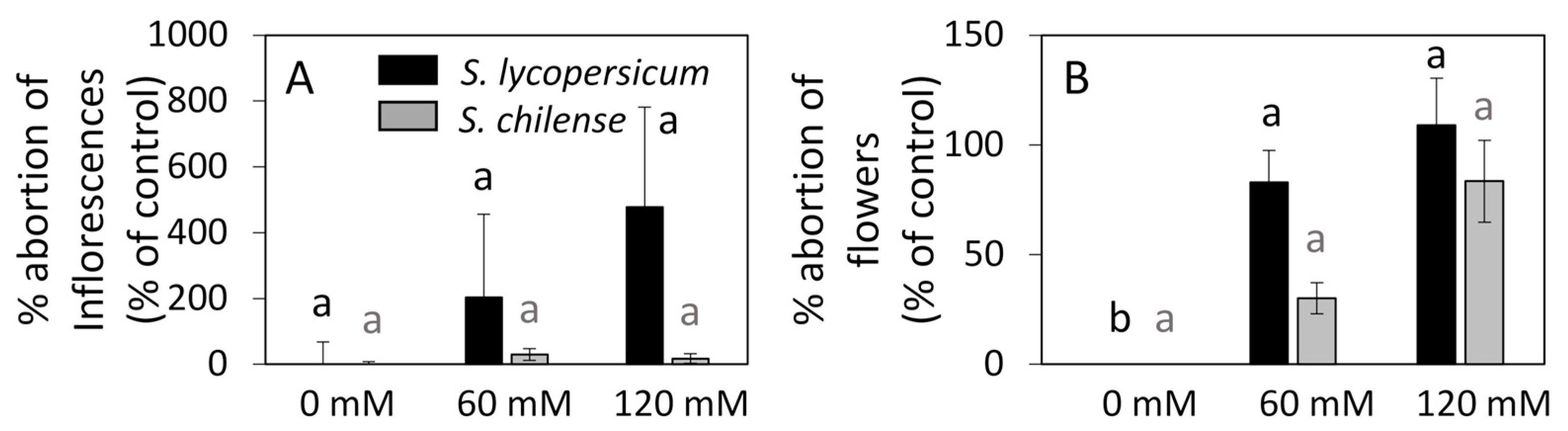
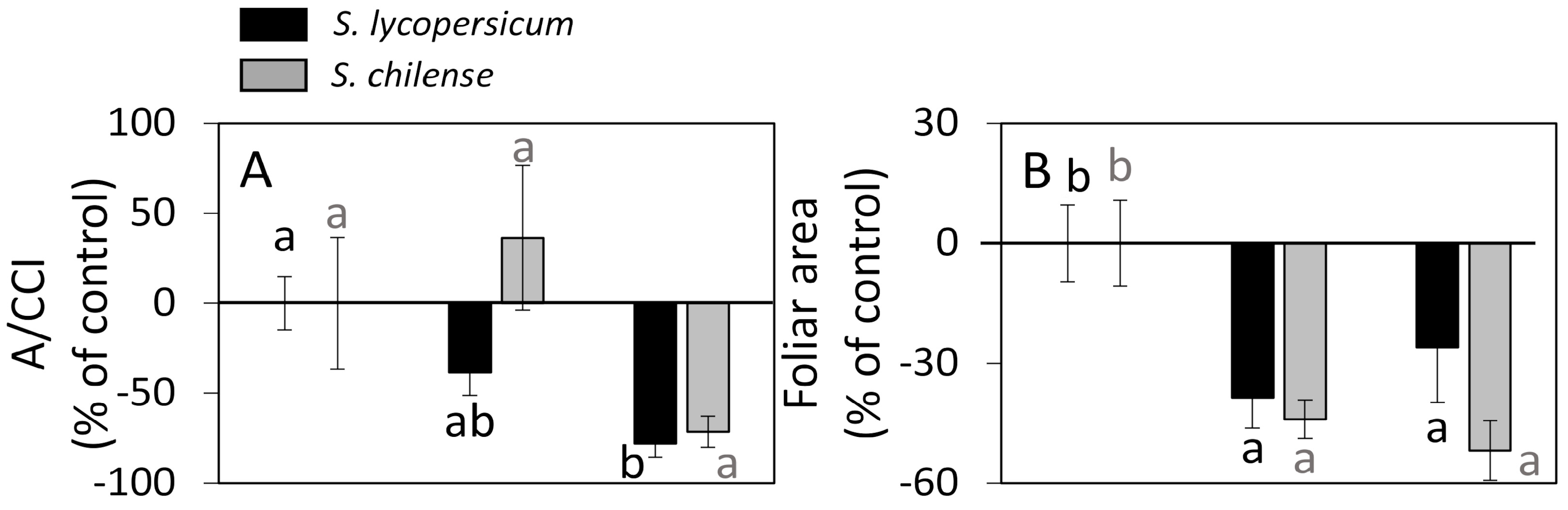
3.2. Vegetative and Reproductive Developments Under Salt Conditions
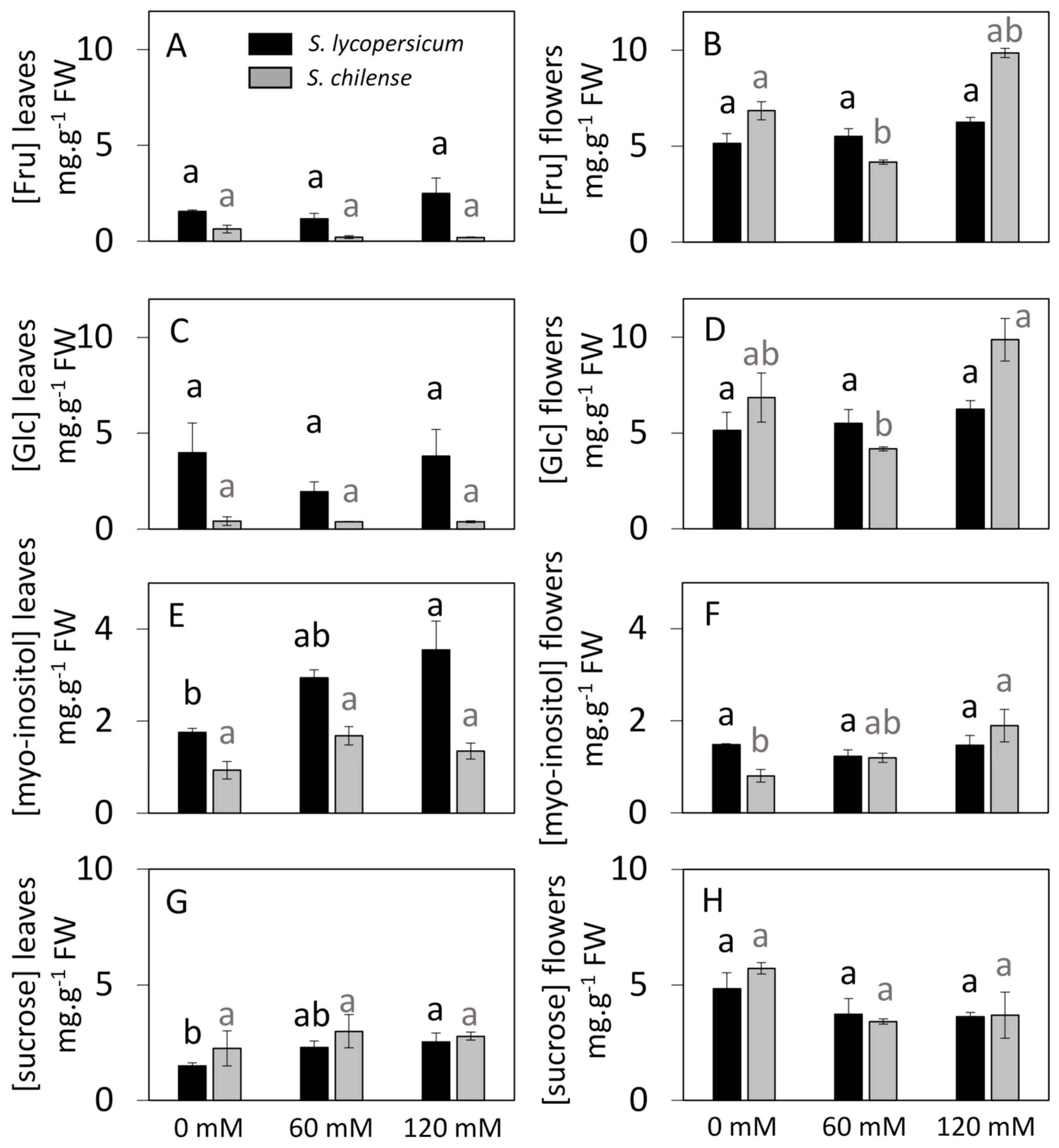
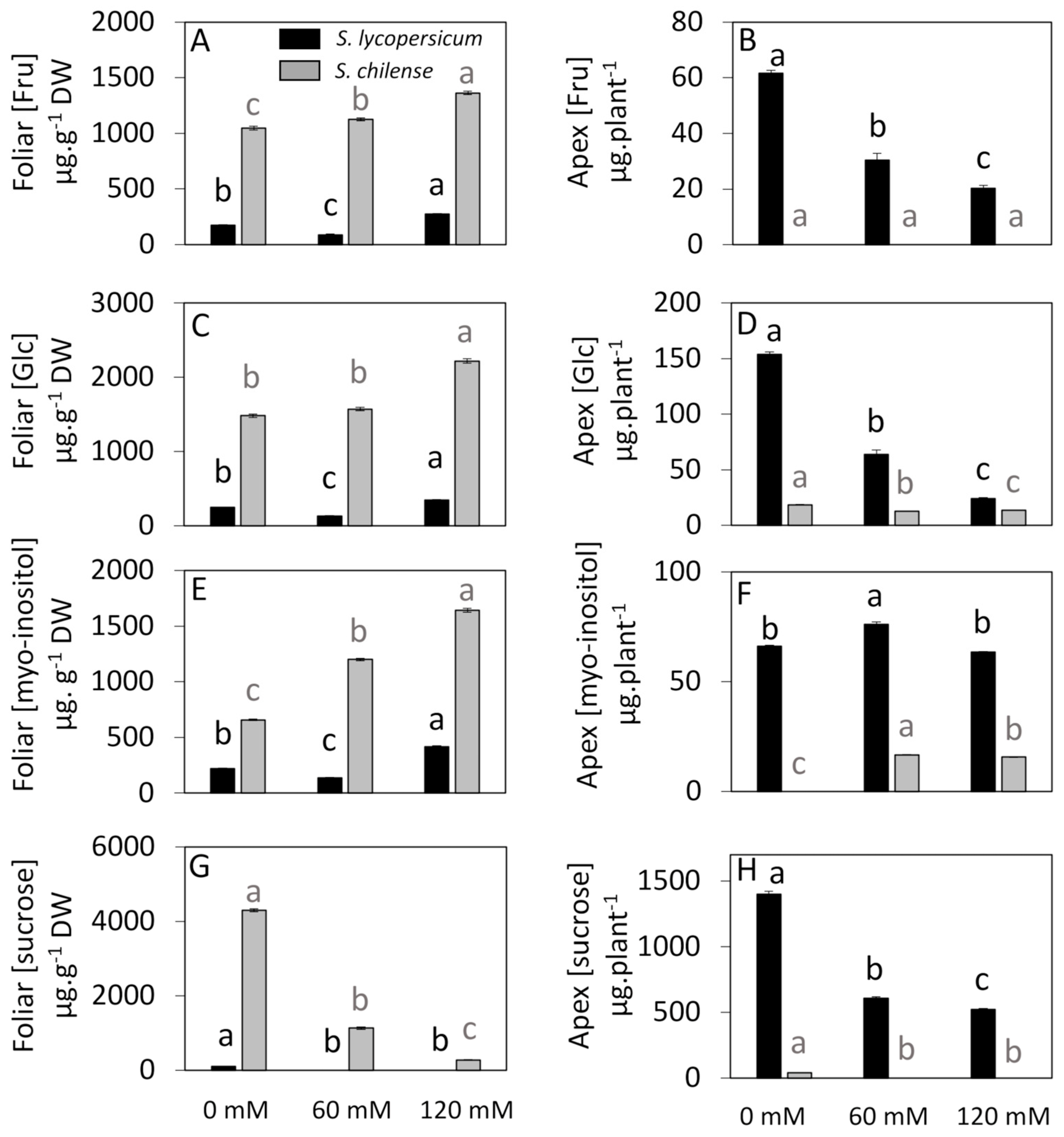
3.3. Sugar Concentrations and Distribution Under Control and Salt Conditions
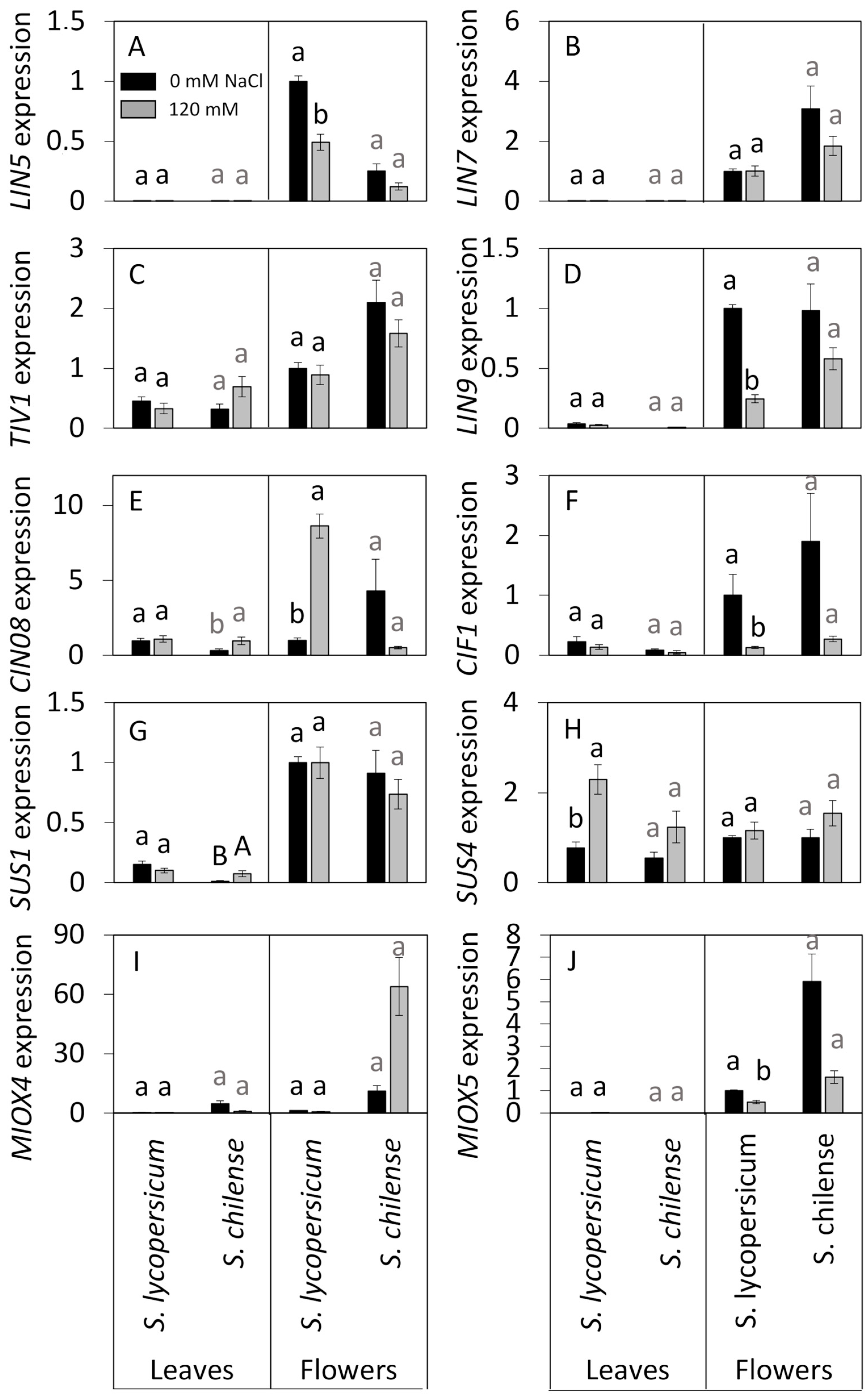
3.4. Expression Levels of Genes Involved in Sugar Metabolism Under Control and Salt Conditions
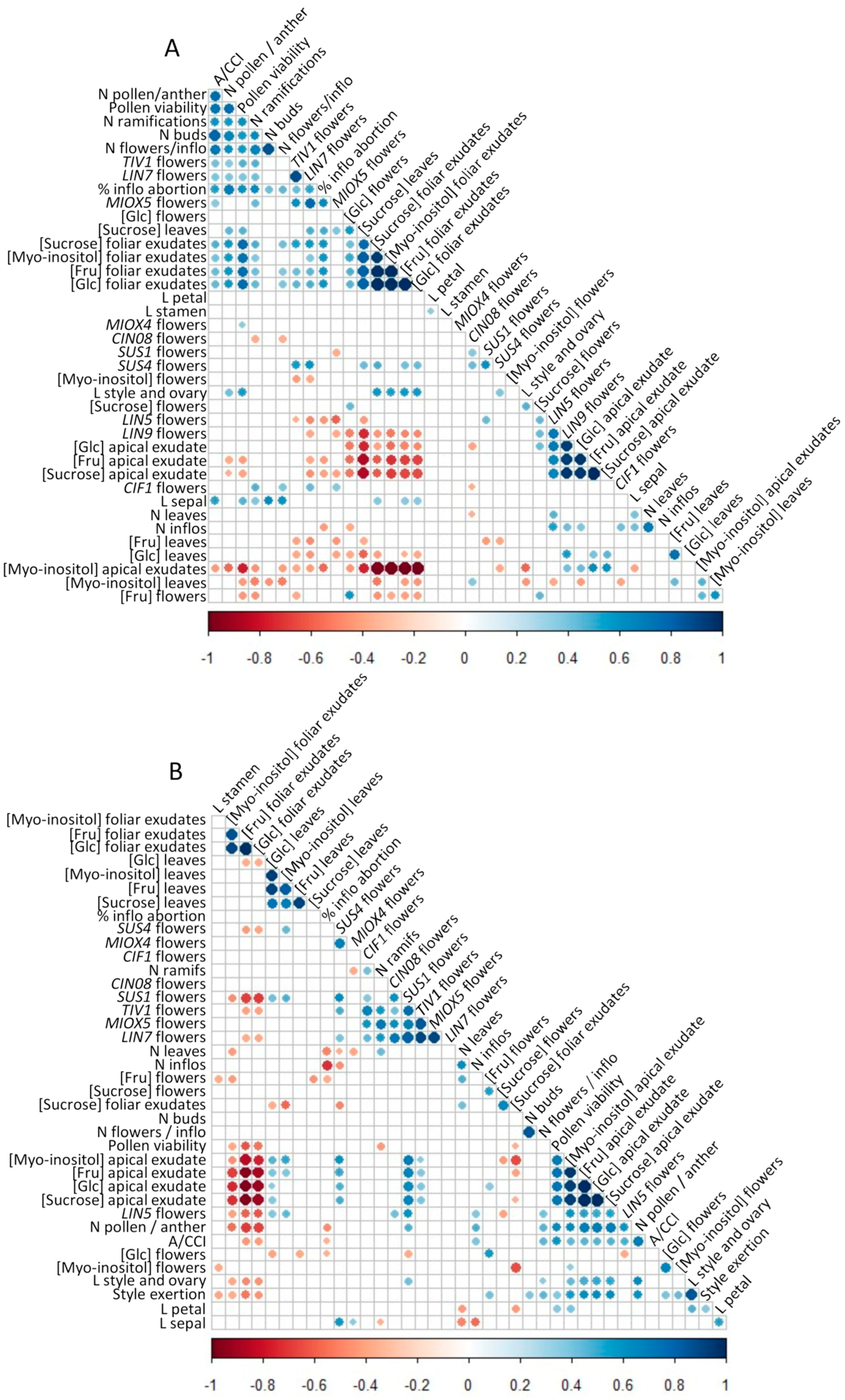
3.5. Correlations About Sugar Concentrations, Gene Expressions and Flower Fertility Parameters
4. Discussion
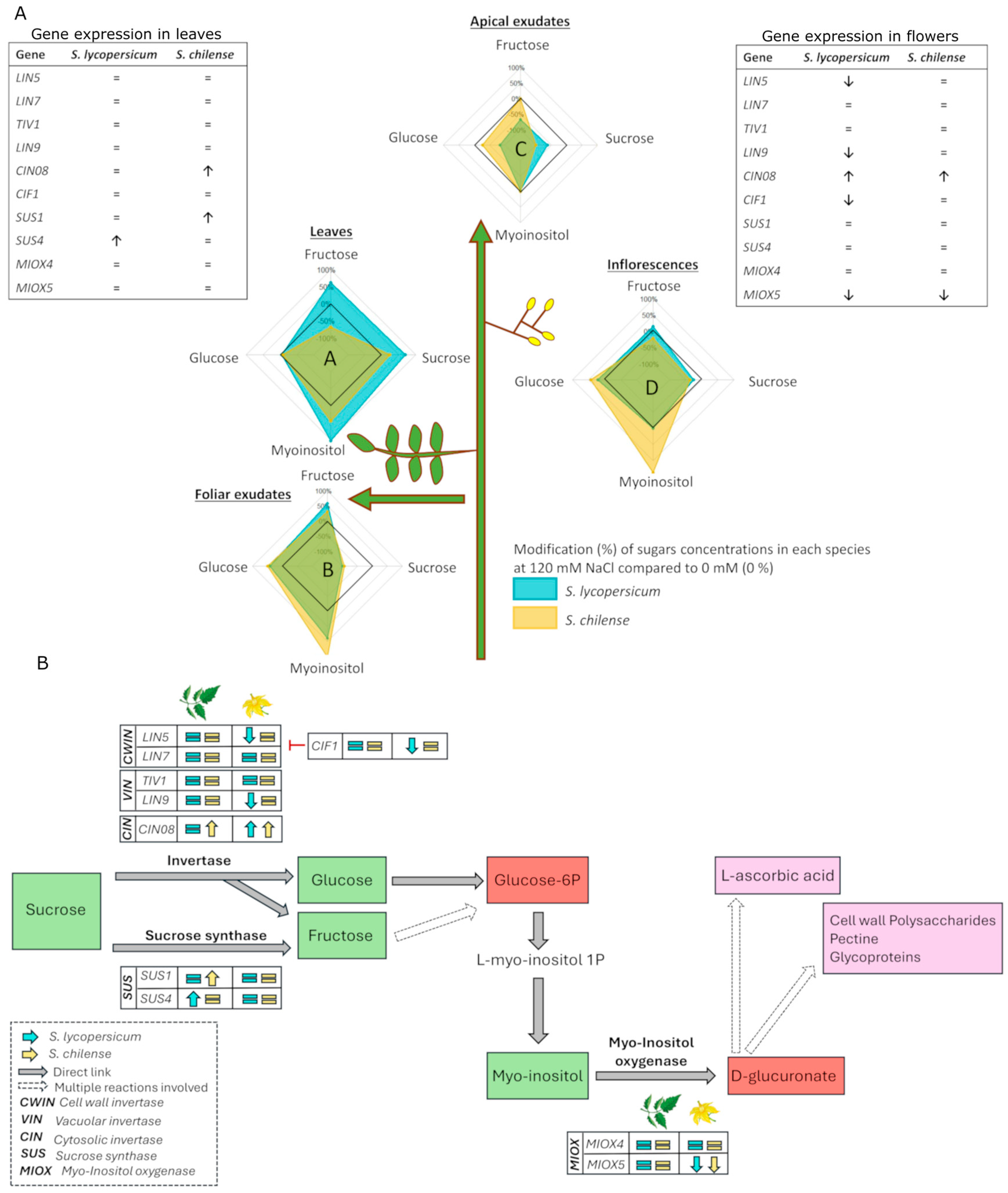
4.1. The Synthesis of Sugars Is Perturbed by Salt Stress and This Leads to a Modified Export of Sucrose from the Leaves
4.2. Salt Stress Induces a Slightly Modified Composition of Sugars in Flowers and Perturbs Flower Fertility
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Earley, E.J.; Ingland, B.; Winkler, J.; Tonsor, S.J. Inflorescences Contribute More than Rosettes to Lifetime Carbon Gain in Arabidopsis thaliana (Brassicaceae). Am. J. Bot. 2009, 96, 786–792. [Google Scholar] [CrossRef]
- Brazel, A.J.; Ó’Maoiléidigh, D.S. Photosynthetic Activity of Reproductive Organs. J. Exp. Bot. 2019, 70, 1737–1754. [Google Scholar] [CrossRef] [PubMed]
- Borghi, M.; Fernie, A.R. Outstanding Questions in Flower Metabolism. Plant J. 2020, 103, 1275–1288. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Palmer, W.M.; Martin, A.P. High Invertase Activity in Tomato Reproductive Organs Correlates with Enhanced Sucrose Import into, and Heat Tolerance of, Young Fruit. J. Exp. Bot. 2012, 63, 1155–1166. [Google Scholar] [CrossRef]
- Beckles, D.M.; Hong, N.; Stamova, L.; Luengwilai, K. Biochemical Factors Contributing to Tomato Fruit Sugar Content: A Review. Fruits 2012, 67, 49–64. [Google Scholar] [CrossRef]
- Osorio, S.; Ruan, Y.-L.; Fernie, A.R. An Update on Source-to-Sink Carbon Partitioning in Tomato. Front. Plant Sci. 2014, 5, 0516. [Google Scholar] [CrossRef]
- Ruan, Y.-L.; Jin, Y.; Yang, Y.-J. Sugar Input, Metabolism, and Signaling Mediated by Invertase: Roles in Development, Yield Potential, and Response to Drought and Heat. Mol. Plant 2010, 3, 942–955. [Google Scholar] [CrossRef]
- Smith, M.R.; Rao, I.M.; Merchant, A. Source-Sink Relationships in Crop Plants and Their Influence on Yield Development and Nutritional Quality. Front. Plant Sci. 2018, 9, 1889. [Google Scholar] [CrossRef]
- Munns, R.; Tester, M. Mechanisms of salinity tolerance. Annu. Rev. Plant Biol. 2008, 59, 651–681. [Google Scholar] [CrossRef]
- Munns, R.; Day, D.A.; Fricke, W. Energy costs of salt tolerance in crop plants. New Phytol. 2020, 225, 1072–1090. [Google Scholar] [CrossRef]
- Khan, H.A.; Siddique, K.H.M.; Colmer, T.D. Vegetative and Reproductive Growth of Salt-Stressed Chickpea Are Carbon-Limited: Sucrose Infusion at the Reproductive Stage Improves Salt Tolerance. J. Exp. Bot. 2017, 68, 2001–2011. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Su, Z.; Ma, H. Molecular Genetic Analyses of Abiotic Stress Responses during Plant Reproductive Development. J. Exp. Bot. 2020, 71, 2870–2885. [Google Scholar] [CrossRef] [PubMed]
- Sato, S.; Kamiyama, M.; Iwata, T. Moderate Increase of Mean Daily Temperature Adversely Affects Fruit Set of Lycopersicon Esculentum by Disrupting Specific Physiological Processes in Male Reproductive Development. Ann. Bot. 2006, 97, 731–738. [Google Scholar] [CrossRef]
- Martínez, J.-P.; Antúnez, A.; Pertuzé, R.; Acosta, M.D.P.; Palma, X.; Fuentes, L.; Ayala, A.; Araya, H.; Lutts, S. Effects of Saline Water on Water Status, Yield and Fruit Quality of Wild (Solanum chilense) and Domesticated (Solanum lycopersicum Var. cerasiforme) Tomatoes. Exp. Agric. 2012, 48, 573–586. [Google Scholar] [CrossRef]
- Ghanem, M.E.; Elteren, J.; Albacete, A. Impact of Salinity on Early Reproductive Physiology of Tomato (Solanum lycopersicum) in Relation to a Heterogeneous Distribution of Toxic Ions in Flower Organs. Funct. Plant Biol. 2009, 36, 125–136. [Google Scholar] [CrossRef] [PubMed]
- Romero-Aranda, M.R.; González-Fernández, P.; Pérez-Tienda, J. Na+ Transporter HKT1;2 Reduces Flower Na+ Content and Considerably Mitigates the Decline in Tomato Fruit Yields under Saline Conditions. Plant Physiol. Biochem. 2020, 154, 341–352. [Google Scholar] [CrossRef]
- Bigot, S.; Pongrac, P.; Šala, M.; van Elteren, J.T.; Martínez, J.-P.; Lutts, S.; Quinet, M. The Halophyte Species Solanum chilense Dun. Maintains Its Reproduction despite Sodium Accumulation in Its Floral Organs. Plants 2022, 11, 672. [Google Scholar] [CrossRef]
- Martínez, J.P.; Fuentes, R.; Farías, K. Effects of salt stress on fruit antioxidant capacity of wild (Solanum chilense) and domesticated (Solanum lycopersicum var. cerasiforme) tomatoes. Agronomy 2020, 10, 1481. [Google Scholar] [CrossRef]
- Tassoni, A.; Franceschetti, M.; Bagni, N. Polyamines and Salt Stress Response and Tolerance in Arabidopsis thaliana Flowers. Plant Physiol. Biochem. 2008, 46, 607–613. [Google Scholar] [CrossRef]
- Ma, L.; Liu, X.; Lv, W.; Yang, Y. Molecular Mechanisms of Plant Responses to Salt Stress. Front. Plant Sci. 2022, 13, 934877. [Google Scholar] [CrossRef]
- Sulpice, R.; Tsukaya, H.; Nonaka, H.; Mustardy, L.; Chen, T.H.H.; Murata, N. Enhanced Formation of Flowers in Salt-Stressed Arabidopsis after Genetic Engineering of the Synthesis of Glycine Betaine. Plant J. 2003, 36, 165–176. [Google Scholar] [CrossRef] [PubMed]
- Khatun, S.; Flowers, T.J. Effects of Salinity on Seed Set in Rice. Plant Cell Environ. 1995, 18, 61–67. [Google Scholar] [CrossRef]
- Albacete, A.A.; Martínez-Andújar, C.; Pérez-Alfocea, F. Hormonal and Metabolic Regulation of Source–Sink Relations under Salinity and Drought: From Plant Survival to Crop Yield Stability. Biotechnol. Adv. 2014, 32, 12–30. [Google Scholar] [CrossRef]
- Li, Q.; Chai, L.; Tong, N. Potential Carbohydrate Regulation Mechanism Underlying Starvation-Induced Abscission of Tomato Flower. IJMS 2022, 23, 1952. [Google Scholar] [CrossRef] [PubMed]
- Ko, H.-Y.; Tseng, H.-W.; Ho, L.-H. Hexose Translocation Mediated by SlSWEET5b Is Required for Pollen Maturation in Solanum lycopersicum. Plant Physiol. 2022, 189, 344–359. [Google Scholar] [CrossRef]
- Balibrea, M.E.; Dell’Amico, J.; Bolarín, M.C.; Pérez-Alfocea, F. Carbon Partitioning and Sucrose Metabolism in Tomato Plants Growing under Salinity. Physiol. Plant 2000, 110, 503–511. [Google Scholar] [CrossRef]
- Gharbi, E.; Martínez, J.-P.; Benahmed, H.; Lepoint, G.; Vanpee, B.; Quinet, M.; Lutts, S. Inhibition of Ethylene Synthesis Reduces Salt-Tolerance in Tomato Wild Relative Species Solanum chilense. J. Plant Physiol. 2017, 210, 24–37. [Google Scholar] [CrossRef]
- Chetelat, R.T.; Pertuzé, R.A.; Faúndez, L.; Graham, E.B.; Jones, C.M. Distribution, Ecology and Reproductive Biology of Wild Tomatoes and Related Nightshades from the Atacama Desert Region of Northern Chile. Euphytica 2009, 167, 77–93. [Google Scholar] [CrossRef]
- Martínez, J.P.; Antúnez, A.; Araya, H. Salt Stress Differently Affects Growth, Water Status and Antioxidant Enzyme Activities in Solanum lycopersicum and Its Wild Relative Solanum chilense. Aust. J. Bot. 2014, 62, 359–368. [Google Scholar] [CrossRef]
- Gharbi, E.; Martínez, J.-P.; Benahmed, H. Salicylic Acid Differently Impacts Ethylene and Polyamine Synthesis in the Glycophyte Solanum lycopersicum and the Wild-Related Halophyte Solanum chilense Exposed to Mild Salt Stress. Physiol. Plant 2016, 158, 152–167. [Google Scholar] [CrossRef]
- Gharbi, E.; Lutts, S.; Dailly, H.; Quinet, M. Comparison between the Impacts of Two Different Modes of Salicylic Acid Application on Tomato (Solanum lycopersicum) Responses to Salinity. Plant Signal Behav. 2018, 13, e1469361. [Google Scholar] [CrossRef]
- Brukhin, V.; Hernould, M.; Gonzalez, N.; Chevalier, C.; Mouras, A. Flower Development Schedule in Tomato Lycopersicon esculentum Cv. Sweet Cherry. Sex. Plant Reprod. 2003, 15, 311–320. [Google Scholar] [CrossRef]
- Ayenan, M.A.T.; Danquah, A.; Ampomah-Dwamena, C.; Hanson, P.; Asante, I.K.; Danquah, E.Y. Optimizing Pollencounter for High Throughput Phenotyping of Pollen Quality in Tomatoes. MethodsX 2020, 7, 100977. [Google Scholar] [CrossRef] [PubMed]
- Alexander, M.P. Differential Staining of Aborted and Nonaborted Pollen. Stain. Technol. 1969, 44, 117–122. [Google Scholar] [CrossRef]
- Dafni, A.; Maués, M.M. A Rapid and Simple Procedure to Determine Stigma Receptivity. Sex. Plant Reprod. 1998, 11, 177–180. [Google Scholar] [CrossRef]
- Paponov, I.A.; Engels, C. Effect of Nitrogen Supply on Leaf Traits Related to Photosynthesis during Grain Filling in Two Maize Genotypes with Different N Efficiency. Z. Pflanzenernähr Bodenk 2003, 166, 756–763. [Google Scholar] [CrossRef]
- Lejeune, P.; Bernier, G.; Requier, M.-C.; Kinet, J.-M. Sucrose Increase during Floral Induction in the Phloem Sap Collected at the Apical Part of the Shoot of the Long-Day Plant Sinapis alba L. Planta 1993, 190, 71–74. [Google Scholar] [CrossRef]
- Dielen, V.; Quinet, M.; Chao, J. UNIFLORA, a Pivotal Gene That Regulates Floral Transition and Meristem Identity in Tomato (Lycopersicon esculentum). New Phytol. 2004, 161, 393–400. [Google Scholar] [CrossRef]
- Descamps, C.; Quinet, M.; Jacquemart, A.-L. Climate Change–Induced Stress Reduce Quantity and Alter Composition of Nectar and Pollen From a Bee-Pollinated Species (Borago Officinalis, Boraginaceae). Front. Plant Sci. 2021, 12, 755843. [Google Scholar] [CrossRef]
- Jin, Y.; Ni, D.; Ruan, Y. Posttranslational Elevation of Cell Wall Invertase Activity by Silencing Its Inhibitor in Tomato Delays Leaf Senescence and Increases Seed Weight and Fruit Hexose Level. Plant Cell 2009, 21, 2072–2089. [Google Scholar] [CrossRef]
- Goren, S.; Huber, S.C.; Granot, D. Comparison of a Novel Tomato Sucrose Synthase, SlSUS4, with Previously Described SlSUS Isoforms Reveals Distinct Sequence Features and Differential Expression Patterns in Association with Stem Maturation. Planta 2011, 233, 1011–1023. [Google Scholar] [CrossRef]
- Stein, O.; Granot, D. An Overview of Sucrose Synthases in Plants. Front. Plant Sci. 2019, 10, 95. [Google Scholar] [CrossRef]
- Alok, A.; Singh, S.; Kumar, P.; Bhati, K.K. Potential of Engineering the Myo-Inositol Oxidation Pathway to Increase Stress Resilience in Plants. Mol. Biol. Rep. 2022, 49, 8025–8035. [Google Scholar] [CrossRef] [PubMed]
- Zouine, M.; Maza, E.; Djari, A. TomExpress, a Unified Tomato RNA-Seq Platform for Visualization of Expression Data, Clustering and Correlation Networks. Plant J. 2017, 92, 727–735. [Google Scholar] [CrossRef] [PubMed]
- Hall, T. BioEdit: A User-Friendly Biological Sequence Alignment Editor and Analysis Program for Windows 95/98/NT. Nucl. Acids 1999, 41, 95–98. [Google Scholar]
- Untergasser, A.; Nijveen, H.; Rao, X. Primer3Plus, an Enhanced Web Interface to Primer3. Nucleic Acids Res. 2007, 35, W71–W74. [Google Scholar] [CrossRef] [PubMed]
- Ye, J.; Coulouris, G.; Zaretskaya, I. Primer-BLAST: A Tool to Design Target-Specific Primers for Polymerase Chain Reaction. BMC Bioinform. 2012, 13, 134. [Google Scholar] [CrossRef]
- Leelatanawit, R.; Saetung, T.; Phuengwas, S. Selection of Reference Genes for Quantitative Real-Time PCR in Postharvest Tomatoes (Lycopersicon esculentum) Treated by Continuous Low-Voltage Direct Current Electricity to Increase Secondary Metabolites. Int. J. Food Sci. Technol. 2017, 52, 1942–1950. [Google Scholar] [CrossRef]
- Cho, Y.-G.; Kang, K.-K. Functional Analysis of Starch Metabolism in Plants. Plants 2020, 9, 1152. [Google Scholar] [CrossRef]
- Smith, A.M.; Stitt, M. Coordination of Carbon Supply and Plant Growth. Plant Cell Environ. 2007, 30, 1126–1149. [Google Scholar] [CrossRef]
- Jha, D.; Shirley, N.; Tester, M.; Roy, S.J. Variation in Salinity Tolerance and Shoot Sodium Accumulation in Arabidopsis Ecotypes Linked to Differences in the Natural Expression Levels of Transporters Involved in Sodium Transport. Plant Cell Environ. 2010, 33, 793–804. [Google Scholar] [CrossRef] [PubMed]
- Durand, M.; Mainson, D.; Porcheron, B.; Maurousset, L.; Lemoine, R.; Pourtau, N. Carbon Source–Sink Relationship in Arabidopsis Thaliana: The Role of Sucrose Transporters. Planta 2018, 247, 587–611. [Google Scholar] [CrossRef]
- Smillie, R.M.; Hetherington, S.E.; Davies, W.J. Photosynthetic Activity of the Calyx, Green Shoulder, Pericarp, and Locular Parenchyma of Tomato Fruit. J. Exp. Bot. 1999, 50, 707–718. [Google Scholar] [CrossRef]
- Lemoine, R.; La Camera, S.; Atanassova, R.; Dédaldéchamp, F.; Allario, T.; Pourtau, N.; Bonnemain, J.-L.; Laloi, M.; Coutos-Thévenot, P.; Maurousset, L.; et al. Source-to-Sink Transport of Sugar and Regulation by Environmental Factors. Front. Plant Sci. 2013, 4, 272. [Google Scholar] [CrossRef]
- Zhang, J.; Lyu, H.; Chen, J.; Cao, X.; Du, R.; Ma, L.; Wang, N.; Zhu, Z.; Rao, J.; Wang, J.; et al. Releasing a Sugar Brake Generates Sweeter Tomato without Yield Penalty. Nature 2024, 635, 647–656. [Google Scholar] [CrossRef]
- Sheoran, I.S.; Saini, H.S. Drought-Induced Male Sterility in Rice: Changes in Carbohydrate Levels and Enzyme Activities Associated with the Inhibition of Starch Accumulation in Pollen. Sex. Plant Reprod. 1996, 9, 161–169. [Google Scholar] [CrossRef]
- Fridman, E.; Zamir, D. Functional Divergence of a Synthenic Invertase Gene Family in Tomato, Potato, and Arabidopsis. Plant Physiol. 2003, 131, 603–609. [Google Scholar] [CrossRef] [PubMed]
- Wei, H.; Chai, S.; Ru, L.; Pan, L.; Cheng, Y.; Ruan, M.; Ye, Q.; Wang, R.; Yao, Z.; Zhou, G.; et al. New Insights into the Evolution and Expression Dynamics of Invertase Gene Family in Solanum lycopersicum. Plant Growth Regul. 2020, 92, 205–217. [Google Scholar] [CrossRef]
- Liu, S.; Li, Z.; Wu, S.; Wan, X. The Essential Roles of Sugar Metabolism for Pollen Development and Male Fertility in Plants. Crop J. 2021, 9, 1223–1236. [Google Scholar] [CrossRef]
- Zhang, Z.; Hu, M.; Xu, W.; Wang, Y.; Huang, K.; Zhang, C.; Wen, J. Understanding the Molecular Mechanism of Anther Development under Abiotic Stresses. Plant Mol. Biol. 2021, 105, 1–10. [Google Scholar] [CrossRef]
- Munir, S.; Mumtaz, M.; Ahiakpa, J.; Liu, G.; Chen, W.; Zhou, G.; Zheng, W.; Ye, Z.; Zhang, Y. Genome-Wide Analysis of Myo-Inositol Oxygenase Gene Family in Tomato Reveals Their Involvement in Ascorbic Acid Accumulation. BMC Genom. 2020, 21, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Smirnoff, N.; Conklin, P.L.; Loewus, F.A. Biosynthesis of Ascorbic Acid in Plants: A Renaissance. Annu. Rev. Plant. Physiol. Plant. Mol. Biol. 2001, 52, 437–467. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Tien, H.; Wen, M.; Yen, H.E. Myo-inositol Transport and Metabolism Participate in Salt Tolerance of Halophyte Ice Plant Seedlings. Physiol. Plant 2021, 172, 1619–1629. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Guo, Y. Elucidating the Molecular Mechanisms Mediating Plant Salt-Stress Responses. New Phytol. 2018, 217, 523–539. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bigot, S.; Martínez, J.P.; Lutts, S.; Quinet, M. Impact of Salinity on Sugar Composition and Partitioning in Relation to Flower Fertility in Solanum lycopersicum and Solanum chilense. Horticulturae 2025, 11, 285. https://doi.org/10.3390/horticulturae11030285
Bigot S, Martínez JP, Lutts S, Quinet M. Impact of Salinity on Sugar Composition and Partitioning in Relation to Flower Fertility in Solanum lycopersicum and Solanum chilense. Horticulturae. 2025; 11(3):285. https://doi.org/10.3390/horticulturae11030285
Chicago/Turabian StyleBigot, Servane, Juan Pablo Martínez, Stanley Lutts, and Muriel Quinet. 2025. "Impact of Salinity on Sugar Composition and Partitioning in Relation to Flower Fertility in Solanum lycopersicum and Solanum chilense" Horticulturae 11, no. 3: 285. https://doi.org/10.3390/horticulturae11030285
APA StyleBigot, S., Martínez, J. P., Lutts, S., & Quinet, M. (2025). Impact of Salinity on Sugar Composition and Partitioning in Relation to Flower Fertility in Solanum lycopersicum and Solanum chilense. Horticulturae, 11(3), 285. https://doi.org/10.3390/horticulturae11030285







