Abstract
Cucumber cultivation suffers from severe yield and quality decline due to cold damage. Cucurbita ficifolia is used as a rootstock for grafting cucumber to improve resistance due to its excellent resistance. This study used Yunnan C. ficifolia as the rootstock and Jingyan Xiamei 2 cucumber as the scion, simulating common low-temperature stress of 15 °C, 10 °C, and 5 °C in an artificial climate chamber, to study the mechanism of cucumber-grafted seedlings responding to low temperatures in terms of growth and development, photosynthesis, osmotic regulation substances, antioxidant system, and gene expression. The results showed that the growth and development of grafted seedlings of C. ficifolia was less inhibited, the chlorophyll content was less reduced, and the content of osmoregulatory substances was significantly increased under low-temperature stress. The activity of antioxidant enzymes in grafted seedlings of C. ficifolia was significantly upregulated at low temperatures, and the expression of antioxidant enzyme genes was consistently higher in grafted seedlings of C. ficifolia. These results confirmed that C. ficifolia as a rootstock could improve the cold resistance of cucumber. Collectively, this study provides the basis for revealing the physiological and molecular mechanism of grafting seedlings to improve cucumber’s low temperature tolerance.
1. Introduction
Cucumber is one of the main vegetable crops in the world and an indispensable fresh fruit in the diet [1]. Cucumber is a typical cold-sensitive plant with a shallow root system, large leaves, and thermophilic and cold-intolerant characteristics. In China, cucumbers are mainly cultivated in plastic greenhouses (without heating equipment). Most of the northern regions of China belong to a temperate monsoon climate, with cold and dry winter, low temperatures, and insufficient light. Cucumber cultivation generally faces the problem of long-term partial critical low temperatures (<20 °C/8~12 °C, day/night) and short-term critical low temperatures (15 °C/4~8 °C, day/night) [2,3]. Low-temperature stress can cause tissue damage in cucumber seedlings during different developmental stages [4,5]. It also disrupts normal physiological and biochemical functions and metabolic processes within the plant, inhibiting plant growth (plant height, stem diameter, plant weight, etc.) [6]. Under low-temperature stress, activities linked to nutrient transport, energy conversion, protein synthesis, and signal transmission are altered due to changes in plant cell membrane permeability [7] and cell membrane structure [8]. In cucumber seedlings, low temperature and light decrease the contents of chlorophyll a and b and inhibit photosynthetic feedback [9], without affecting the structure and function of chloroplasts in leaves [10]. Low-temperature stress causes plants to produce many protective substances, such as soluble proteins, soluble sugars, and proline [11,12]. These substances protect cells by regulating osmotic pressure, stabilizing cell membranes and protoplasts [13], and lowering the freezing point of the cytoplasm. Low-temperature stress also increases electrolyte leakage and malondialdehyde (MDA) content in cucumber plants [14]. When plants are exposed to low-temperature stress, the activity of antioxidant enzymes such as superoxide dismutase (SOD), peroxidase (POD), catalase (CAT), and ascorbate peroxidase (APX) is enhanced. This provides an effective antioxidant protection mechanism that helps plants adapt to adverse environmental conditions [15]. Therefore, it is crucial to study how to improve the cold tolerance of cucumber in cucumber cultivation.
Grafting plays an important role in crop improvement and seedling propagation and is widely used for the propagation of many fruits and vegetables. Grafted plants have better yield, stress resistance, fruit flavor, and quality than plants grown by seeds [16]. Cultivating grafted cucumbers can effectively improve disease resistance and water absorption capacity, resulting in increased yields [17]. The rootstocks, such as pumpkin, have good cold tolerance; their use as rootstock of cucumber will significantly improve cold tolerance [18]. C. ficifolia is often used as a rootstock for grafting cucumber due to its superior traits such as cold and disease resistance. Cucumber grafted onto C. ficifolia could maintain higher soluble sugar and manganese (Mn) contents, higher SOD and POD activities, and could improve plant tolerance to major nutrient-induced salinity compared with self-grafted plants [19]. C. ficifolia, as a good rootstock, can significantly promote growth, fruiting, disease resistance, and nutrient absorption capacity [20]. However, there are almost no relevant reports on the effect of C. ficifolia grafting on the cold tolerance of cucumber seedlings. This study aims to reveal the physiological and molecular response mechanisms by which C. ficifolia rootstock improves the low-temperature tolerance of cucumber.
Here, we evaluated the response of cucumber seedlings grafted onto C. ficifolia rootstocks to low-temperature stress. We identified the important role of antioxidant enzyme activity and explored the molecular basis of the mechanism. This research provides a theoretical basis for cucumber physiological and molecular research under adverse conditions and offers a reference for selecting better rootstocks for cucumber grafting.
2. Materials and Methods
2.1. Plant Materials
Experiments were performed in a greenhouse at Yunnan Agricultural University (25°13′ N latitude, 102°74′ E longitude, 1890 m altitude). C. ficifolia seeds were obtained from Kunming, Yunnan Province (the College of Landscape and Horticulture, Yunnan Agricultural University provided materials). Jingyan Xiamei No. 2 cucumber seeds were obtained from Jingyan Yinong Beijing Seed Industry Technology Co., Ltd., Beijing, China. Jingyan Xiamei No. 2 is an early maturing cultivar suitable for open field cultivation (in spring, summer, and autumn) that has vigorous growth potential and is resistant to premature aging and heat.
2.2. Experimental Design
Three different types of grafted seedlings were chosen in this experiment, including cucumber self-rooted (ZG), cucumber self-grafted (HH), and C. ficifolia-grafted (NH), and we set ZG as the control group to compare the differences among NH, HH, and ZG. Seeds were soaked in warm water at 55 °C and disinfected by dipping in 0.1% potassium permanganate for 15 min. Then, seeds were spread flat in a culture dish lined with two layers of wet filter paper to perform light avoidance germination in the incubator at 28 °C. For grafting, scion seeds were sown two days earlier than rootstock seeds [21]. Once both the rootstock and scion had fully developed their first true leaves, we removed the growth points of the rootstock. Then, we made a 35° to 45° incision on approximately half of the rootstock’s hypocotyl using a blade. We made an opposing angle incision on the scion’s hypocotyl, aligned the two cut hypocotyls together, and used a clamp to assist with the healing process. The grafted seedlings were placed in an internal artificial climate chamber with a temperature of 28/18 °C (day/night), humidity of 90%, photon flux density (PFD) of 150 µmol·m−2·s−1, and photoperiod of 12/12 h. The seedlings were transferred to a climate chamber for chilling stress (Table 1) at the three-leaf stage. There were 3 replicates per treatment and 6 seedlings per replicate. Morphological indexes were measured 7 days later, and leaf samples were harvested to measure electrolyte leakage (EL), malondialdehyde (MDA), proline (Pro), and SOD. The collected samples were frozen in liquid nitrogen and stored at −80 °C. Three replicates were set for each physiological indicator measurement, with six seedlings in each replicate.

Table 1.
Treatment conditions at different temperatures.
2.3. Determination of Morphological Indicators
Seedlings were oven-dried at 105 °C for 0.5 h and then at 80 °C until a constant weight was achieved. Fresh and dry weights of seedlings were weighed using an electronic scale. Plant height and stem diameter were measured with straightedge and vernier caliper, respectively.
2.4. Chlorophyll Determination
First, 0.1 g fresh leaves was collected and immediately frozen in liquid nitrogen. The frozen leaves were ground with a mortar and transferred to a test tube. Then, 5 mL of 95% ethanol was added and the solution was extracted in the dark for 48 h. The mixture was then centrifuged at 12,000 rpm at 4 °C for 5 min. Absorbance at 649 nm and 665 nm was measured using a spectrophotometer.
The following formulae were used to calculate the chlorophyll contents:
Chla(mg/g FW) = (13.95 × OD665 − 6.88 × OD649) × 0.005/0.1
Chlb(mg/g FW) = (24.96 × OD649 − 7.32 × OD665) × 0.005/0.1
Chl(mg/g FW) = Chla + Chlb = (18.08 × OD649 − 6.63 × OD665) × 0.005/0.1
Chla, Chlb, and Chl represent the content of chlorophyll a, chlorophyll B, and total chlorophyll, respectively. FW represents the fresh weight of the plants. OD665 and OD649 represent the absorbance of photosynthetic pigment extract at wavelengths of 665 nm and 649 nm, respectively.
2.5. Determination of Electrolyte Leakage Rate and Malondialdehyde Content
Weigh 1.0 g of cucumber leaves and place them in a test tube. Add 20 mL of distilled water, then let the mixture sit at room temperature for 10 h. Using distilled water as a control to measure blank conductivity values. Seal the test tube and boil it in a water bath for 20 min, then remove it and let it cool to room temperature. Measure the final conductivity value three times and calculate the electrolyte permeability according to the formula electrolyte leakage rate (EL) = (initial conductance value − blank conductance value)/(final conductance value − blank conductance value) × 100%.
The content of malondialdehyde was measured by thiobarbituric acid (AKFA013C of Beijing Boxbio Science&Technology Co., Ltd., Beijing, China). Weigh 1.0 g of cucumber leaves, add 1 mL extraction solution, solution, and grind into a homogenate. Centrifuge the homogenate at 8000× g for 10 min at 4 °C. Extract the supernatant and mix 0.2 mL of it with 0.8 mL of reaction solution. Heat the mixture at 100 °C for 60 min, then cool to room temperature. Centrifuge again at 10,000× g for 10 min at room temperature. Take the supernatant and measure the absorbance values at 450 nm, 532 nm, and 600 nm, respectively. The following formula was used to calculate the results:
MDA content (mmol kg−1) = 5 × (6.45 × (OD532 − OD600) − 0.56 × OD450) ÷ W
OD450, OD532, and OD600 represent the absorbance of photosynthetic pigment extract at wavelengths of 450, 532, and 600 nm, respectively, while W is the weight of sample (g).
2.6. Osmotic Regulatory Substance Content Determination
The contents of free Pro, soluble protein (SP), and soluble sugar (SS) were detected using AKAM003C, AKPR015, and AKPL008C (Beijing Boxbio Science & Technology Co., Ltd., Beijing, China) kits, respectively, according to the manufacturer’s instructions. To analyze Pro, 1 g of cucumber leaf tissue was added to 10 mL of water, ground into homogenate, and extracted at 100 °C for 10 min. The mixture was cooled to room temperature, centrifuged at 8000× g for 10 min, and the supernatant was collected. Distilled water and L-Proline were used as reagent blank and standard, respectively. The concentration of Pro was determined using the ninhydrin method by measuring absorbance at 520 nm. SP content was estimated using the Bradford method with absorbance recorded at 595 nm. For SS determination, the anthrone method was used and the absorption value was measured at 620 nm [22,23].
2.7. Detection of Antioxidant Enzyme Activities
The activities of SOD, POD, CAT, and APX were measured by colorimetry using specific kits (AKAO001C-50S, AKAO005C, AKAO003-2C, and AKVI006U) from Beijing Boxbio Science & Technology Co., Ltd., Beijing, China, respectively, according to the manufacturer’s instructions.
2.8. RNA Extraction and qRT–PCR
Total RNA was extracted from seedling leaves using the Plant RNA Extraction Kit (TaKaRa, Beijing, China) according to the manufacturer’s protocol, and its quality was detected by 1% agarose gel electrophoresis. For RT-qPCR, cDNA was synthesized from total RNA using the Star Script III All-in-one RT Mix with gDNA Remover kit. RT-qPCR analysis was performed on a CFX96 Real-Time PCR Detection System using SYBR®Premix EX TaqTM II (TaKARa, China). PCR amplification was performed at 95 °C with a denaturation step for 30 s, followed by 40 cycles of 95 °C for 5 s and 60 °C for 34 s. For each sample, three independent biological replicates were used. Actin was used as the internal control. The comparative CT (2−ΔΔCT) method [24] was used to quantify the relative gene expression levels. Primers were designed using CDS sequences of genes and Primer 5 software, which were synthesized by Tsingke Biotechnology Co., Ltd. Beijing, China, as shown in Table 2.

Table 2.
List of primers for RT-qPCR.
2.9. Statistical Analysis
All the data were statistically analyzed using SPSS (29.0, IBM, Armonk, NY, USA), and the least significant difference (LSD) and Duncan’s method were used to test the significance of differences (p < 0.05). The data were expressed as the mean ± standard error of the mean (SEM), and graphs were drawn using GraphPad Prism (8.0, GraphPad Software, San Diego, CA, USA).
3. Results
3.1. Seedlings with C. ficifolia Rootstock Showed Enhanced Resistance Against Low-Temperature Stress
After being treated at 5 °C for 3 days, ZG and HH exhibited wilting leaves and plant lodging. In contrast, NH showed wilting leaves but remained upright, demonstrating significant differences from the ZG and HH (Figure 1).

Figure 1.
Different cucumber seedlings under low-temperature treatment. (a) ZG before treatment. (b) HH before treatment. (c) NH before treatment. (d) ZG treated with 5 °C for 3 days. (e) HH with 5 °C for 3 days. (f) NH with 5 °C for 3 days.
There were no significant differences in fresh weight, dry weight, plant height, or stem diameter between NH, HH, and ZG at room temperature. After low-temperature stress, the fresh weight, dry weight, plant height, and stem diameter of ZG, HH, and NH were all inhibited, but the overall effect of low-temperature inhibition on grafted seedlings was relatively small. Under low-temperature treatment at 15 °C, NH increased fresh weight, dry weight, and stem diameter by 11.8%, 16.5%, and 0.6%, respectively, compared with ZG. The differences between HH and ZG are relatively small. Under low-temperature treatment at 10 °C, there was no significant difference in fresh weight, dry weight, plant height, and stem diameter among ZG, HH, and NH. Under low-temperature treatment at 5 °C, the fresh weight, dry weight, plant height, and stem diameter of NH were 2.6%, 5.4%, −5.3%, and 2.1% higher than those of ZG, respectively. The fresh weight, dry weight, plant height, and stem diameter of HH were 3.2%, 10.1%, −3.2%, and 2.3% higher than those of ZG. These results suggest that grafting can mitigate the adverse effects of low temperatures on cucumber plant growth (Table 3).

Table 3.
Effect of low-temperature stress on the growth and development of different cucumber seedlings.
3.2. C. ficifolia, as Grafting Rootstock, Increased the Light and Pigment Content of Cucumber
The contents of chlorophyll a, chlorophyll b, and total chlorophyll a + b in NH increased by 11.9%, 20.6%, and 14.8%, respectively, while those in HH increased by 7.6%, 11.8%, and 9.0%, respectively, compared to ZG at room temperature. At 15 °C, the contents of chlorophyll a, chlorophyll b, and total chlorophyll a + b in NH increased by 23.9%, 29.1%, and 25.5%, respectively, while those in HH increased by 11.1%, 17.8%, and 13.3%, respectively, compared to ZG. At 10 °C, the contents of chlorophyll a, chlorophyll b, and total chlorophyll a + b in NH increased by 19.7%, 9.6%, and 16.5%, respectively, while those in HH increased by 13.6%, 9.8%, and 12.4%, respectively, compared to ZG. Additionally, at 5 °C, the contents of chlorophyll a, chlorophyll b, and total chlorophyll a + b in NH increased by 17.0%, 20.9%, and 18.4%, respectively, while those in HH increased by 11.1%, 13.6%, and 12.0%, respectively, compared to ZG. Overall, the use of C. ficifolia as a cucumber grafting rootstock enhanced the photosynthetic pigment content and photosynthetic performance of the plants to some extent at low temperatures (Figure 2).
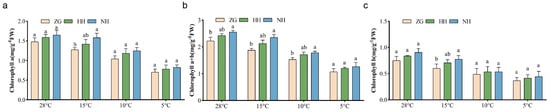
Figure 2.
Effect of low-temperature stress on chlorophyll content of different cucumber seedlings. (a) Chlorophyll a content. (b) Total chlorophyll content. (c) Chlorophyll b content. Data are expressed as mean ± SEM; n = 3. Different letters indicate significant differences in chlorophyll content among in different cucumber seedlings (p < 0.05).
3.3. The Content of Osmoregulatory Substances in NH Was Higher than That in ZG
Under the conditions of 15 °C, 10 °C, and 5 °C, the free Pro content of NH was significantly higher than that of ZG, by 31.1%, 18.3%, and 21.8%, respectively; the Pro content of HH was significantly higher than that of ZG, while there was no significant difference in Pro content between NH and HH. (Figure 3a). The soluble protein (SP) content of grafted seedlings was higher than that of ZG and increased with decreasing temperature (Figure 3b). At a low temperature of 15 °C, the SP content of grafted seedlings was higher than ZG, and the SP content of NH and HH was increased by 32.2% and 30.7%, respectively, compared with ZG. The NH had the highest SP content, while the HH had slightly higher SP content than ZG at 10 °C. At 5 °C, the SP contents of NH was significantly increased by 18.4% compared with that of ZG, and the samples of the HH group had intermediate values of SP compared to NH and ZG. As the temperature decreased, the SS content of ZG and grafted seedling leaves gradually increased, and the SS of grafted seedling leaves was always higher than that of ZG (Figure 3c). The SS content of NH leaves was 27.7% higher than that of ZG at 15 °C low temperature. The difference in leaf SS content between NH, HH, and ZG was not significant at 10 °C and 5 °C, but the grafted seedlings had higher leaf SS content than that of ZG.
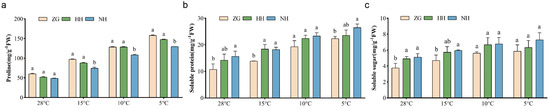
Figure 3.
Effect of low-temperature stress on the content of osmoregulatory substances in different cucumber seedlings. (a) The free Pro content. (b) The SP content. (c) The SS content. Data are expressed as mean ± SEM; n = 3. Different letters indicate significant differences in chlorophyll content among in different cucumber seedlings (p < 0.05).
3.4. The Electrical Conductivity Rate and Malondialdehyde Content of NH Were Always Lower than Those of ZG
The electrical conductivity (EL) value of the leaves of NH was significantly lower than that of ZG before low-temperature stress, and there was no significant difference between the two grafted seedlings. The EL value of leaves of NH and HH were 31.1% and 26.6% lower than that of ZG, respectively, at 15 °C. However, the differences in the leaf EL values of NH, HH, and ZG were not significant at 10 °C, and the EL value of leaves of NH at 5 °C was significantly lower than that of ZG, decreased by 25.1% (Figure 4a).
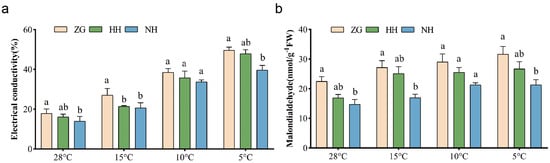
Figure 4.
Effect of low-temperature stress on electrolyte leakage rate and malondialdehyde content in different cucumber seedlings. (a) Electrical conductivity. (b) Malondialdehyde content. mean ± SEM; n = 3. Different letters indicate significant differences in chlorophyll content among different cucumber seedlings (p < 0.05).
Under low-temperature stress, the content of Malondialdehyde (MAD) in NH, HH, and ZG was increased. More particularly, the MDA content of NH was significantly lower than that of ZG, by 60.1% and 48.5%, respectively, at 15 °C and 5 °C. At 10 °C, the differences in MDA content among NH, HH, and ZG were not yet significant (Figure 4b).
3.5. The Activity of Antioxidant Enzymes Was Significantly Enhanced in NH
Under low-temperature adversity, the accumulation of reactive oxygen radicals occurred in the cells of plants, and SOD, as a key enzyme in the Calvin cycle, was able to scavenge reactive oxygen radicals effectively, which was an important factor in response to the ability of plants to resist low-temperature stress.
At room temperature, the SOD, POD, and CAT activities of grafted cucumber seedlings were significantly higher than those of ZG, while APX had no significant difference. The activity of these enzymes changed under low temperatures. At 15 °C, the SOD activity results of NH and HH were 30.4% and 24% higher than ZG, respectively. The CAT activity of NH was significantly higher than that of ZG, and the APX activity was 29.7% and 18.3% higher than HH and ZG. However, there was no significant difference in POD activity. At 10 °C, the POD and APX activity of NH were 26.5% and 35.2% higher than that of ZG, and the samples of the HH group had intermediate POD values compared to NH and ZG. The CAT activity of NH was significantly higher than HH and ZG by 9.5% and 15.3%, respectively. Moreover, the SOD activity of NH and HH was significantly higher than that of ZG by 40.7% and 26.9%, respectively. The APX activity of NH was significantly increased by 43.6% compared than ZG; however, there was no significant difference in POD and CAT activity compared with 15 °C and 10 °C. The results indicated that the NH showed better resistance to low-temperature stress and antioxidant stress compared with HH and ZG (Figure 5).
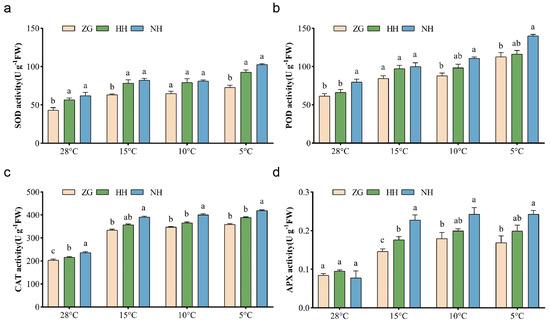
Figure 5.
Effect of low-temperature stress on antioxidant enzyme activity of different cucumber seedlings. (a) The SOD activity. (b) The POD activity (c) The CAT activity. (d) The APX activity. Data are expressed as mean ± SEM; n = 3. Different letters indicate significant differences in chlorophyll content among different cucumber seedlings (p < 0.05).
3.6. Grafting Resists Oxidative Damage Caused by Up-Regulating the Expression of Antioxidant System Genes
Then, we extracted RNA from the seedlings, and RT-qPCR was used to detect the expression of antioxidant enzyme genes, including SOD, POD, CAT, and APX in the leaves of seedlings. As shown in Figure 6a, under room temperature, SOD gene expression in leaves of NH and HH increased by 30.7% and 20.7% compared with the CK group. At 15 °C, 10 °C, and 5 °C, the SOD gene expression of ZG was lower than those of the two grafted seedlings. At low temperatures, POD gene expression in leaves of NH was significantly increased (Figure 6b) and 75.4% higher than that of the CK group at 15 °C, and it was significantly increased by 51.7% in ZG. The POD gene expression of HH peaked at 5 °C, which was significantly increased by 89.1%. After different low-temperature treatments, the CAT gene expression of NH was increased by 62.4%, 73.1%, and 77.3%, respectively, and the CAT gene expression of HH was increased by 46.1%, 46.3%, and 63.9%. The increase in CAT gene expression of ZG was lower than that of grafted seedings (Figure 6c). For the APX gene, after different low-temperature treatments, the gene expression of NH was increased by 28.3%, 42.1%, and 58.8%, respectively, which was higher than that of HH, and the increased amplitude of APX gene expression of ZG was the lowest (Figure 6d). These evidences showed that the expression of SOD, POD, CAT, and APX genes in grafted seedlings was higher than that in ZG and the control after different low-temperature treatments.
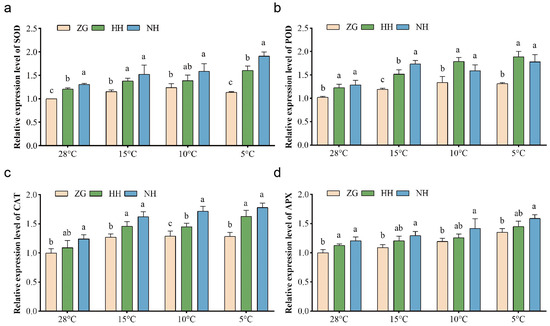
Figure 6.
Effects of low-temperature stress on gene expression of antioxidant system in different cucumber seedlings. (a) The SOD gene expression level. (b) The POD gene expression level. (c) The CAT gene expression level. (d) The APX gene expression level. Data are expressed as mean ± SEM; n = 3. Different letters indicate significant differences in chlorophyll content among different cucumber seedlings (p < 0.05).
4. Discussion
The inhibition of plant growth and development is the direct indicator of low-temperature stress. The influence of low-temperature stress can be estimated by measuring plant fresh weight, dry weight, plant height, and stem diameter. Further, chlorophyll plays the main role in photosynthesis and is the most important factor constituting plant productivity [25]. Under low-temperature stress, plant antioxidants are changed to cope with changing temperatures. Here, we measured various growth and development parameters of cucumber seedlings at the seedling stage for three different types: ZG, HH, and NH.
Cucumber seedlings growth was reduced at 5 °C, and further lower temperatures significantly influenced plant height, stem diameter, and root growth [26]. In another study, under mild low temperatures (21/15 °C, day/night), the plant height, leaf area, and leaf weight (dry and fresh) were significantly lower than those following moderate-temperature stress [27]. In this study, we found that there was no significant difference in plant height between grafted seedlings and self-rooted seedlings under different low-temperature stresses, which may be the spindling of self-rooted seedlings at low temperatures. However, the fresh weight, dry weight, and stem diameter of NH were better than those of ZG. Therefore, we surmise that resistance to low temperatures can be enhanced by grafting. NH showed higher chlorophyll contents than HH and ZH. The observed effects may be due to grafting, which increases the structure and stability of chloroplasts, reduces chlorophyll decomposition, and assists in maintaining higher photosynthetic activity. Grafting also decreases transpiration and water loss while increasing cucumber tolerance to low temperatures. However, the levels of chlorophyll a, chlorophyll b, and total chlorophyll a + b decreased as the temperature dropped. This reduction can be explained by stomatal restrictions and the decreased photosynthetic rate caused by low temperatures, which limit the transport of CO2 to the chloroplasts [28].
The structure of the cell membrane changes and permeability increases when plants are subjected to low-temperature stress [29]. Plants regulate cell osmotic balance through osmoregulatory substances to prevent excessive cell water loss and stabilize organelle structure [30], and high levels of osmoregulatory substances can improve plant cold resistance [31]. Our study found that the EL rate of both self-rooted and grafted seedlings gradually increased under low temperatures. Interestingly, the electrolyte leakage rate of NH seedings was lower than that of ZH, which is consistent with previous findings [32]. Metabolism and other important physiological activities of plant cells are a series of redox reactions, in which reactive oxygen species (ROS) are widely involved as the second messenger. Under low-temperature stress, the balance between production and removal of ROS in plants is damaged, and many ROS are generated, resulting in oxidative damage to the membrane system [33,34,35]. Grafting can reduce the accumulation of ROS caused by low temperatures in plants and reduce membrane damage [36]. The accumulation of MDA was used to assess the degree of membrane damage, and our results showed that the MDA content of grafted cucumber seedlings was lower than that of ZG, suggesting that grafting alleviated the oxidative damage caused by low temperatures to a certain extent.
There is a set of effective enzymatic active oxygen scavenging systems in plants, including SOD, CAT, POD, and APX. In the mechanism of ROS clearance, SOD is the first activator, which removes superoxide anions to produce H2O2 and O2, followed by CAT, APX, and POD enzymes to remove H2O2 [37,38]. Low-temperature stress tolerance is positively correlated with the activity of the above-mentioned antioxidant enzymes [39,40,41]. We examined the enzyme activities of SOD, POD, CAT, and APX in different cucumber seedlings at low temperatures. It is noteworthy that the enzyme activities of HH are higher than those of ZG, and the enzyme activities of NH are particularly prominent.
Subsequently, we observed the effect of low temperatures on the expression levels of genes linked with SOD, POD, CAT, and APX in cucumber seedlings. The literature reports that abiotic stress can trigger the overexpression of antioxidant enzyme genes in plants [42], while the overexpression of SOD, APX, GR, and other genes can enhance the cold resistance of plants [43]. Our study showed that the expression of SOD, POD, CAT, and APX genes in grafted seedlings increased with decreasing temperature, which was higher than that in ZG. This result suggested that the higher expression of antioxidant enzyme genes in grafted seedlings, especially in NH, was an important reason for enhancing the resistance of cucumber seedlings to oxidative damage.
In addition, grafting is a form of vegetative reproduction in plants, involving the attachment of a scion from one plant to the rootstock of another plant. The two parts are tightly joined to form a new, unified plant. During this process, the junction between the scion and rootstock is subjected to mechanical stress, which arises from the physical pressure and tension generated by the tissues of both parts as they integrate. Lichtenhaler [44] appointed that appropriate levels of stress can enhance plant physiological activities. Additionally, mechanical stress has been shown to impact the plant’s antioxidant system [45,46]. The antioxidant system comprises a group of enzymes and non-enzymatic substances that plants use to combat oxidative stress, playing a crucial role in maintaining the stability of plant cell structure and function. We observed differences in physiological indicators between HH and ZG. The SOD activity in HH was significantly higher than that in ZG. Similarly, the POD, CAT, and APX activities were also higher in HH than ZG. These differences may be related to the mechanical stress generated during grafting.
5. Conclusions
Use of C. ficifolia as a rootstock for cucumber reduced the effects of low temperatures on seedlings’ growth. Measurement of relative gene expression levels and enzyme activities in different cucumber seedlings under different temperatures suggested that C. ficifolia rootstock can improve the photosynthetic capacity of cucumber. Further, it can enhance its antioxidant capacity by promoting the expression of antioxidant enzyme genes, thus improving its cold resistance. Our study supports the development of Yunnan C. ficifolia as a stock for cold-tolerant cucumbers and provides a new reference for cucumber grafted stock.
Author Contributions
Conceptualization, H.X.; methodology, Z.Y.; software, H.X.; validation, W.D. and H.X.; formal analysis, W.D.; investigation, S.S.; resources, Z.Y.; data curation, W.D.; writing—original draft preparation, H.X. and C.G.; writing—review and editing, B.A.; writing—review and editing, H.X.; supervision, S.H.; project administration, Z.Y.; funding acquisition, Z.Y. and S.H. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by The Zhang Xiaolan Expert Workstation Project of Yunnan Province, China (Yunnan Provincial Science and Technology Department, China. 202205AF150021), the Major Science and Technology Projects of Yunnan Province, China (Yunnan Provincial Science and Technology Department, China. 202402AE090012), and the Key Laboratory of Vegetables of Yunnan Province Biology Construction Project, China (Yunnan Provincial Science and Technology Department, China. 202402AN360008).
Data Availability Statement
The original contributions presented in the study are included in the article, further inquiries can be directed to the corresponding author.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| EL | Electrolyte leakage |
| MDA | Malondialdehyde |
| Pro | Proline |
| SP | Soluble protein |
| SS | Soluble sugar |
| SOD | Superoxide dismutase |
| POD | Peroxidase |
| CAT | Catalase |
| APX | Ascorbate peroxidase |
References
- Zhang, J.; Feng, S.; Yuan, J.; Wang, C.; Lu, T.; Wang, H.; Yu, C. The formation of fruit quality in Cucumis sativus L. Front. Plant Sci. 2021, 12, 729448. [Google Scholar] [CrossRef] [PubMed]
- Li, H. Low temperature disorders and preventive measures in cucumber. Mod. Rural Technol. 2016, 4, 27. [Google Scholar]
- Wang, Y.; Zhang, F.; Xu, Y.; Chen, Q.; Zhang, H. The main progress in the research on the mechanism and application of cucumber’s low temperature and weak light tolerance. China Veg. 2005, 7–12. [Google Scholar] [CrossRef]
- Cabrera, R.M.; Saltveit, M.E.; Owens, K. Cucumber cultivars differ in their response to chilling temperatures. J. Am. Soc. Hortic. Sci. 1992, 117, 802–807. [Google Scholar] [CrossRef]
- Hao, J.; Li, Y.; Meng, S.; Zhao, B. Effects of night low temperature on sugar accumulation and sugar-metabolizing enzyme activities in melon fruit. Sci. Agric. Sin. 2009, 42, 3592–3599. [Google Scholar]
- Pei, Z.-Q.; Ma, C.; Dong, C.-Y.; Xu, T.-T.; Chai, C.-H.; Zhu, Q.; Wang, J.; Zheng, S.; Zhang, T.-G. Target of rapamycin coordinates auxin are involved in exogenous melatonin regulated low temperature tolerance in cucumber seedlings. Plant Physiol. Biochem. 2024, 215, 109055. [Google Scholar] [CrossRef]
- Jian, L. Advances of the studies on the mechanism of plant cold hardiness. Chin. Bull. Bot. 1992, 9, 17–22+16. [Google Scholar]
- Ando, Y.; Maeda, Y.; Mizutani, K.; Wakatsuki, N.; Hagiwara, S.; Nabetani, H. Impact of blanching and freeze-thaw pretreatment on drying rate of carrot roots in relation to changes in cell membrane function and cell wall structure. LWT-Food Sci. Technol. 2016, 71, 40–46. [Google Scholar] [CrossRef]
- Amin, B.; Atif, M.J.; Kandegama, W.; Nasar, J.; Alam, P.; Fang, Z.; Cheng, Z. Low temperature and high humidity affect dynamics of chlorophyll biosynthesis and secondary metabolites in Cucumber. BMC Plant Biol. 2024, 24, 903. [Google Scholar] [CrossRef] [PubMed]
- Blum, A. Plant Breeding for Stress Environments; CRC Press: Boca Raton, FL, USA, 1988. [Google Scholar]
- Kaplan, F.; Kopka, J.; Haskell, D.W.; Zhao, W.; Schiller, K.C.; Gatzke, N.; Sung, D.Y.; Guy, C.L. Exploring the Temperature-Stress Metabolome of Arabidopsis. Plant Physiol. 2004, 136, 4159–4168. [Google Scholar] [CrossRef] [PubMed]
- Kaplan, F.; Kopka, J.; Sung, D.Y.; Zhao, W.; Popp, M.; Porat, R.; Guy, C.L. Transcript and metabolite profiling during cold acclimation of Arabidopsis reveals an intricate relationship of cold-regulated gene expression with modifications in metabolite content. Plant J. 2007, 50, 967–981. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Dang, P.; Liu, L.; He, C. Cold acclimation by the CBF–COR pathway in a changing climate: Lessons from Arabidopsis thaliana. Plant Cell Rep. 2019, 38, 511–519. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Feng, Y.; Jing, T.; Liu, X.; Ai, X.; Bi, H. Melatonin Promotes the Chilling Tolerance of Cucumber Seedlings by Regulating Antioxidant System and Relieving Photoinhibition. Front. Plant Sci. 2021, 12, 789617. [Google Scholar] [CrossRef]
- Pan, D.-Y.; Fu, X.; Zhang, X.-W.; Liu, F.-J.; Bi, H.-G.; Ai, X.-Z. Hydrogen sulfide is required for salicylic acid–induced chilling tolerance of cucumber seedlings. Protoplasma 2020, 257, 1543–1557. [Google Scholar] [CrossRef]
- Nie, W.; Wen, D. Study on the applications and regulatory mechanisms of grafting on vegetables. Plants 2023, 12, 2822. [Google Scholar] [CrossRef] [PubMed]
- Robledo-Torres, V.; González-Cortés, A.; Luna-García, L.R.; Mendoza-Villarreal, R.; Pérez-Rodríguez, M.Á.; Camposeco-Montejo, N. Histological Variations in Cucumber Grafted Plants and Their Effect on Yield. Agronomy 2024, 14, 1377. [Google Scholar] [CrossRef]
- Fu, X.; Lv, C.-Y.; Zhang, Y.-Y.; Ai, X.-Z.; Bi, H.-G. Comparative transcriptome analysis of grafting to improve chilling tolerance of cucumber. Protoplasma 2023, 260, 1349–1364. [Google Scholar] [CrossRef]
- Huang, Y.; Bie, Z.; He, S.; Hua, B.; Zhen, A.; Liu, Z. Improving cucumber tolerance to major nutrients induced salinity by grafting onto Cucurbita ficifolia. Environ. Exp. Bot. 2010, 69, 32–38. [Google Scholar] [CrossRef]
- Zeng, Y.; Zhu, Y.; Wang, B.; Yang, L. Effects of Cucurbita ficifolia as rootstock on growth, fruit setting, disease resistance and leaf nutrient element contents in Cucumis sativus. J. Plant Resour. Environ. 2004, 13, 15–19. [Google Scholar]
- Miao, L.; Li, S.; Bai, L.; Anwar, A.; Li, Y.; He, C.; Yu, X. Effect of grafting methods on physiological change of graft union formation in cucumber grafted onto bottle gourd rootstock. Sci. Hortic. 2019, 244, 249–256. [Google Scholar] [CrossRef]
- Liu, Y.; Yan, Y.; Ma, L.; Cao, D.; Jin, X. Physiological and metabolomic analyses reveal the resistance response mechanism to tea aphid infestation in new shoots of tea plants (Camellia sinensis). Plant Stress 2024, 13, 100545. [Google Scholar] [CrossRef]
- Cheng, H.; Chang, S.; Shi, X.; Chen, Y.; Cong, X.; Cheng, S.; Li, L. Molecular mechanisms of the effects of sodium selenite on the growth, Nutritional quality, and species of organic selenium in Dandelions. Horticulturae 2024, 10, 209. [Google Scholar] [CrossRef]
- Schmittgen, T.D.; Livak, K.J. Analyzing real-time PCR data by the comparative CT method. Nat. Protoc. 2008, 3, 1101–1108. [Google Scholar] [CrossRef] [PubMed]
- Yuan, L.; Gu, W.; Sun, J.; Wang, Y. Regulation of DCPTA treatment on chlorophyll content and fluorescence parameters of maize seedlings leaves under low-temperature stress. Crops 2012, 5, 63–67. [Google Scholar] [CrossRef]
- Zhou, Q.; Wang, J.; Chen, X. Effect of durative low temprature on morphological and physiological characteristics of cucumber seedling. North. Hortic. 2010, 16, 1–3. [Google Scholar]
- Xiong, Z.; Yuan, W.; Chen, X.; Xu, Q.; Chen, C. Influence of sub-low temperature on the growth of cucumber (Cucumis sativus L.) grown in greenhouse. China Veg. 2007, 19–21. [Google Scholar] [CrossRef]
- Jurczyk, B.; Grzesiak, M.; Pociecha, E.; Wlazło, M.; Rapacz, M. Diverse stomatal behaviors mediating photosynthetic acclimation to Low temperatures in hordeum vulgare. Front. Plant Sci. 2019, 9, 1963. [Google Scholar] [CrossRef] [PubMed]
- Lu, M.; Lou, Q.; Chen, J. A review on chilling injury and cold tolerance in Cucumis sativus L. Chin. Bull. Bot. 2004, 21, 578–586. [Google Scholar]
- Ren, J.; Huang, Z.; Zeng, L.; Shi, Z. Review of physiological reaction mechanism of plants exposed to low temperature stress. World For. Res. 2013, 26, 15–20. [Google Scholar] [CrossRef]
- Zhang, M.; Wang, X.; Zhang, Y.; Wei, M. Effects of chlorophyllin-iron on osmotic adjustment and activities of antioxidantive enzymes in cucumber seedlings under suboptimal temperature. Chin. J. Appl. Ecol. 2014, 25, 3527–3532. [Google Scholar] [CrossRef]
- Xu, X. Study on the Physiological Response and Molecular Mechanism of Grafted Cucumber Seedlings Under Low Temperature Stress. Ph.D. Thesis, Gansu Agricultural University, Lanzhou, China, 2016. [Google Scholar]
- Dat, J.; Vandenabeele, S.; Vranová, E.; Van Montagu, M.; Inzé, D.; Van Breusegem, F. Dual action of the active oxygen species during plant stress responses. Cell. Mol. Life Sci. 2000, 57, 779–795. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Li, T.; Gao, X. Active oxygen metabolism and ascorbate-glutathione cycle of tomato leaves under low nocturnal temperature. Acta Bot. Boreali-Occident. Sin. 2011, 31, 707–714. [Google Scholar]
- Yu, J.; Zhang, G.; Feng, Z.; Li, X. Effects of low temperature and weak light on Anti-oxidative enzyme activities and Plasm- membrane permeability of pepper seedlings. Acta Bot. Boreali-Occident. Sin. 2005, 25, 2478–2483. [Google Scholar]
- Wang, H.; Ai, X.; Zheng, N.; Jiang, F. Effects of graft on lipid peroxidation and antioxidative enzyme activities of Capsicum annum seedlings under low temperature and weak light intensity. Chin. J. Appl. Ecol. 2010, 21, 1289–1294. [Google Scholar]
- Erich, F. Oxygen activation and oxygen toxicity. Annu. Rev. Plant Physiol. 1982, 33, 73–96. [Google Scholar] [CrossRef]
- Bowler, C.; Vanmontagu, M.; Inze, D. Superoxide dismutase and stress tolerance. Annu. Rev. Plant Physiol. Plant Mol. Biol. 1992, 43, 83–116. [Google Scholar] [CrossRef]
- Wang, R.; Shi, L.; Tang, G.; Liang, Y. Effect of osmotic stress on activities of protective enzymes system in Agropyron mongolicum seedling. Chin. Bull. Bot. 2003, 20, 330–335. [Google Scholar]
- Yang, S.; Chen, X.; Hui, W.; Ren, Y. Progress in responses of antioxidant enzyme systems in plant to environmental stresses. J. Fujian Agric. For. Univ. 2016, 45, 481–489. [Google Scholar]
- Zhang, Z.; Liu, S.; Wang, Z.; Zhang, Q.; Meng, F.; Yu, X. Effect of low temperature stress on physiological and biochemical indices of cucumber seedlings grafted on different rootstocks. Shandong Agric. Sci. 2009, 36–40. [Google Scholar] [CrossRef]
- Wang, Y.; Gao, P.; Huang, M.; Chen, H. Effects of high temperature on the activity and expression of antioxidative enzymes in rice flag leaves during the flowering stage. Plant Sci. J. 2015, 33, 355–361. [Google Scholar]
- Ma, D.; Sun, Q. Effects of temperature stress on membrane protective system of cucumber seedlings. Acta Bot. Boreali-Occident. Sin. 2001, 656–661. [Google Scholar] [CrossRef]
- Lichtenthaler, H.K.; Wenzel, O.; Buschmann, C.; Gitelson, A. Plant Stress Detection by Reflectance and Fluorescence. Ann. N. Y. Acad. Sci. 1998, 851, 271–285. [Google Scholar] [CrossRef]
- Miller, A.R.; Kelley, T.J. Mechanical Stress Stimulates Peroxidase Activity in Cucumber Fruit. HortScience 1989, 24, 650–652. [Google Scholar] [CrossRef]
- AbdElgawad, H.; Zinta, G.; Hegab, M.M.; Pandey, R.; Asard, H.; Abuelsoud, W. High Salinity Induces Different Oxidative Stress and Antioxidant Responses in Maize Seedlings Organs. Front. Plant Sci. 2016, 7, 276. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).