Abstract
Coconut (Cocos nucifera L.) is an important tropical palm globally, but due to declining production, it is failing to meet the rapidly increasing market demand. In vitro technologies, including embryo culture, cryopreservation, and clonal propagation, are being explored to increase productivity and to conserve unique varieties. Despite the development of these technologies, there is still a need to improve the acclimatization process used to produce the large numbers of high-quality coconut seedlings needed for planting. Enhancing seedling growth and development during acclimatization, using an elevated concentration of atmospheric carbon dioxide (CO2), and applying appropriate modifications to the light environment were the objectives of this study. A series of four semi-automated acclimatization chambers, which could control atmospheric CO2 concentration, relative humidity and temperature, and light intensity, quality, and photoperiod, were designed to undertake this study. An initial experiment demonstrated that a concentration of 1600 μmol mol−1 CO2 in combination with an elevated light intensity of 600 μmol m−2 s−1 could significantly enhance the growth and development of seedlings as compared to ambient CO2 concentrations and standard illumination conditions. Further experimentation demonstrated that continuous illumination with a combination of red and blue light (7:3) gave superior growth and development. This study found that the 6-month-old age is the ideal age for accelerating the acclimatization process using CO2. Overall, the maximum effectiveness of the photomixotrophic growth system was seen when CO2 (1600 μmol mol−1) was applied to 6- to 7-month-old seedlings under a constant (24/0 h photoperiod) but elevated light intensity (600 μmol m−2 s−1) of red and blue light in the ratio of 7:3.
1. Introduction
Coconut (Cocos nucifera L.) is one of the most important crops in the tropical world, providing essential resources such as various food and drink products, building materials, and other necessary items for people living in both tropical and subtropical regions [1]. In vitro biotechnologies (embryo culture, somatic embryogenesis, and cryopreservation) are being developed to confront various production problems (e.g., pathogens and pest attacks, environmental stresses, genetic erosion, etc.) found within the coconut industry [2]. The effectiveness of these in vitro culture biotechnologies is gauged not by the number of regenerated seedlings coming from embryo culture or plantlets coming from somatic embryogenesis but by the quantity and quality of plants that become successfully established in the field [3]. Seedlings produced through in vitro culture are highly vulnerable to the environmental changes experienced upon deflasking [4]. Furthermore, coconut seedlings produced through in vitro methods exhibit lower photosynthetic efficiency as compared to seedlings grown naturally from fruit [5]. Thus, there is a critical phase that determines the survival of in vitro-derived coconut seedlings during the transition from heterotrophic to autotrophic growth conditions [6].
The net photosynthetic rate of in vitro-growing seedlings is low due to the relatively low atmospheric CO2 concentration that is present in the partially air-tight culture vessel [7] and the low light levels used for culture. To counteract this, forced ventilation of the culture vessel with CO2 has been used to promote seedling photosynthesis [8]. An earlier study by Samosir and Adkins (2014) showed that an elevated CO2 concentration (1600 μmol mol−1), when applied during the illumination phase of culture, can significantly improve seedling growth, but other concentrations of CO2 were not studied in their work.
Light is another critical driver of photosynthesis and growth during the transfer of seedlings from in vitro to ex vitro conditions, but for palm species, this is poorly studied. The key aspects of the light environment that need to be considered are the intensity of the light applied, the quality of the light, and the exposure duration (photoperiod) [9]. When below the light saturation point, increasing the light intensity is known to enhance the photosynthesis and growth of in vitro seedlings [10]. Broad-spectrum fluorescent lamps, which emit light in the 380 to 750 nm wavelength band, provided at low light intensities (<100 μmol m−2 s−1) have been commonly used to support in vitro seedling growth of several plant species [11], but this may not be optimal for coconut seedlings. For date palm (Phoenix dactylifera L.), in vitro-cultured seedlings grew well under both broad-spectrum fluorescent lamps and under enriched red-to-blue (9:1) LED (light-emitting diode) lighting [12]. A second date palm study also demonstrated that a red/blue LED treatment could also boost in vitro seedling production [13].
The photoperiod can also affect the growth of in vitro seedlings. For date palm and peach palm (Bactris gasipaes Kunth.) seedlings, a gradual transition from a 12/12 h (day/night) to a 16/8 h (day/night) photoperiod was found to increase seedling growth [14,15,16]. In another study, a 12/12 h photoperiod yielded the best results for in vitro-cultured oil palm (Elaeis guineensis L.) seedlings [17].
The present study aims to enhance the quantity and quality of coconut embryo-cultured seedlings coming through acclimatization by making several environmental modifications to the in vitro-culture system. Specifically, this study evaluates the use of elevated atmospheric CO2 treatments, under two different light intensities, and four different light qualities, under two photoperiods, applied to seedlings of different ages.
2. Materials and Methods
2.1. Plant Material Preparation
Approximately 500 mature Malayan Yellow Dwarf (MYD) fruit were obtained from the National Tropical Palm Germplasm Nursery in Wenchang City, Hainan, China. The fruit were split, and the endosperm plugs containing the embryos were extracted in the field using a sterile cork. The endosperm plugs were washed with mild detergent, rinsed with tap water for 5 min, and surface-sterilized with 75% (v/v) ethanol for 1 min, followed by a 20 min agitation in a 2.1% (v/v) sodium hypochlorite (NaOCl) solution, then rinsed three times with sterile water. Batches of 20 endosperm plugs were dried on filter paper, placed in clean glass containers, and transported to the laboratory of the Chinese Academy of Tropical Agricultural Science (CATAS) in Wenchang City. In the laboratory, the embryos were transferred to a laminar airflow (LAF) hood, where they were carefully isolated from the endosperm plugs using clean forceps and a scalpel blade. The embryos were then surface-sterilized by shaking in a 0.5% NaOCl solution (v/v) for 10 min, washed three times with sterile water, and then dried on a clean paper towel inside the LAF hood. Once dry, the embryos were placed individually (haustorium down) into a solidified medium (3 g L−1 Gelzan) containing Y3 basal medium (Eeuwens, 1976), 1-naphthaleneacetic acid (NAA; 2.68 μM), sucrose (60 g L −1), and Murashige and Skoog vitamins and activated charcoal (2 g L−1) within transparent culture vessels (Techno-Plas polypropylene; 50 mL). After 1 month of incubation in darkness at a constant 27 ± 2 °C, the culture tubes with germinated embryos were placed under a 14/10 h (day/night) photoperiod with a daytime white light intensity of ca. 25 μmol m−2 s−1 and a constant temperature of 27 ± 2 °C. Five months later, the cultured seedlings (seedlings of other ages were also used in some experiments) measuring ca. 10 to 12 cm in height, with one to two leaves and with a small primary root, were ready for experimentation.
2.2. Design of the Experimental Apparatus
To investigate several potential enhancements to the in vitro acclimatization process, four versatile, small-scale, portable acclimatization chambers (Figure 1) were developed. These chambers were preliminarily tested using several batches of 6-month-old MYD coconut seedlings to ensure their operational effectiveness. The critical features tested included their ability to maintain specific atmospheric CO2 concentrations (Figure 1C,F), being able to apply a range of light intensities, light qualities (Figure 1B,D), and photoperiods, and their ability to control relative humidity (RH) and temperature conditions, all while keeping the cultured seedlings sterile. The acclimatization chamber was designed in Australia (UQ), and the units were manufactured by Chengdu Feiyan Ltd. (Chengdu City, Sichuan Province, China). The main body of each chamber was made from a transparent plastic acrylic sheet (5 mm thick). Each chamber consisted of two components (Figure 1G): the outer acrylic casing and the inner growth maintenance system (Figure 1E,H,J). The inner growth maintenance system consisted of three components: the lighting component, the CO2 enrichment component, and the environmental control component (Figure 1I). One entire chamber, with all components in place but without any medium or plant materials, weighed 18 kg.

Figure 1.
The components of the experimental apparatus used for the acclimatization of coconut seedlings. (A) One of the four acclimatization chambers used to study the growth of embryo-cultured coconut seedlings. (B) An aerial view of how the chamber was subdivided into two sub-chambers, each with three nutrient channels, and the positioning of the LED light strips. (C) The CO2 supply system, with a valve controlling gas flow. (D) The customized lighting system in operation. (E) The planform of the main body where seedlings from embryo culture could be placed. (F) The transmitters for CO2 and air humidity monitoring purposes. (G) The liquid medium supply and plant supporting system. (H) The customized plastic basket for seedling support. (I) The environmental control computer for data monitoring and recording purposes. (J) The customized sponge cylinder (to support the seedlings in the baskets).
The inner growth system of each chamber had two sides, each with three water-tight channels capable of holding six seedlings and three different types of liquid medium. At the bottom of each channel, an air bubble bar (Belos 10 cm; Belos Aquarium Ltd., Wenchang City, Hainan, China) was placed, connected to an external air pump through a sealed tube. The lighting system was mounted on the underside of the removable acrylic plastic lids of each chamber, allowing the transmission of culture room light if necessary. This lighting system included six 18 W, 60 cm long strips, each with 96 LED units (bright LEDs, type 2835; KingWua LED, Dongguan, Guangdong Province, China). The LED strips could be configured to provide specific light quality combinations suitable for the experimentation. This study utilized four different LED light combinations: red (700 nm) 30% + blue (450 nm) 70%, red 50% + blue 50%, red 70% + blue 30%, and 100% white (400 to 750 nm). The environmental control system comprised two main components: the monitoring system and the CO2 supply system. For atmospheric monitoring, two detectors were installed in each half of the acclimatization chamber (RS-CO2 485, Shandong Renke Control Technology Co., Ltd., Jinan, Shandong, China), one on each side of the dividing wall. These detectors were capable of measuring CO2 levels from 0 to 5000 μmol mol−1, a culture temperature from 0 to +40 °C, and an RH from 0 to 95%. Positioned on the back wall of each chamber, the detectors transmitted data to the computerized environment control system via a Bluetooth connection to a computer, providing real-time environmental factor information from both sides of the chamber. To ensure proper air circulation, two 8 cm diameter fans (Taida AFB0824SH, 24 V, 0.33 A) were installed on the back wall of each chamber. The CO2 supply system delivered CO2 generated through a chemical reaction (using an aquarium CO2 generator; Crazy Stone 1808311904, Wenchang City, Hainan, China), with the generated CO2 having a purity range of 97 to 99%, as specified in the generator’s manual. The overall environmental control system utilized a Jianda Renke RS-XZJ-100-Y environmental control computer to monitor and regulate chamber RH, lighting, CO2 concentration, and temperature. The data were also transmitted to a mobile phone for 24/7 monitoring.
2.3. The Liquid Culture Medium
When the chamber was used with a liquid culture medium, the medium could be administered or withdrawn individually from each of the six channels located at the base of each chamber via valves mounted on its front wall. In one operational mode, and to ensure proper root aeration of the test seedlings, the seedlings were placed into one of 36 small baskets (70 mm in diameter and height; Lvyibang PE Product, Baoding, Hebei Province, China) that dipped into the liquid medium and were clipped to the side of the channel, allowing the bottom of the baskets to become submerged into the liquid medium. Each basket contained a custom-made foam cylinder (autoclavable; 49 mm in diameter; Lvyibang PE Product, Hebei Province, China) with a slot cut to hold a single seedling, enabling its roots to extend into the liquid medium. Both foam cylinders and baskets were sterilized and reused in subsequent experiments after autoclaving.
2.4. Seedling Preparation
Unless otherwise stated, 5-month-old seedlings were removed from their embryo culture vessels, and in a LAF hood, their roots were washed with sterile water to eliminate any adhering medium components. Seedlings of uniform size and good health were individually placed into foam cylinders moistened with a liquid medium and then into the baskets. The baskets were then transferred to the previously surface-sterilized acclimatization chamber. The inner surfaces of the acclimatization chambers were cleaned by first washing with tap water, followed by a strong detergent, and then sterile water. Once dry, the surfaces of the chamber were wiped twice with adhesive-bonded fabric wipes containing 70% (v/v) alcohol. All other components were washed with sodium hypochlorite 2.1% (v/v) for 7 min, followed by a single rinse with sterile water. After cleaning, the chambers and components were moved from the LAF hood to a culture room, recleaned with 70% ethanol, and exposed to UV light overnight to complete the sterilization process.
2.5. Experiment 1: Influence of Atmospheric CO2 Concentration and Light Intensity
The four acclimatization chambers (Figure 1) were placed onto two shelves in a tissue culture room, where the electronic components of the chambers had been previously installed. A total of 144 in vitro-grown, 5-month-old MYD coconut seedlings were randomly allocated to the four chambers (36 seedlings per chamber). For this experiment, each chamber was provided with a liquid medium consisting of Y3 basal medium containing Murashige and Skoog vitamins supplemented with 100 μM NAA and 15 g L−1 sucrose. Two LED white light intensity treatments (300 and 600 μmol m2 s−1) were applied to one-half of each chamber using a 14/10 h (day/night) photoperiod. These treatments were maintained for 1 month, after which the seedlings were moved to the culture room and exposed to either a 300 or a 600 μmol m2 s−1 light treatment at a temperature of 27 ± 2 °C for an additional month before assessment of their growth and quality. The chambers were provided with a constant supply of CO2 at one of four concentrations (400, 800, 1600, or 3000 μmol mol−1), with the 400 μmol mol−1 treatment considered to be the control (Table 1). In this experiment, the measurements made included the percentage of seedlings surviving the treatments, their fresh weight, height, leaf number, and chlorophyll content, and the width and thickness of their first fully expanded leaf. The data were determined from three replications of six seedlings per treatment.

Table 1.
The treatments applied to seedlings in CO2 and light intensities.
2.6. Experiment 2: Influence of Light Quality and Photoperiod
Four acclimatization chambers (Figure 1) were used for this experiment, involving 144 in vitro-grown, 5-month-old MYD coconut seedlings, with 36 seedlings randomly allocated to each chamber. In this experiment, the seedlings were placed individually into glass tubes (2.5 × 25.0 cm; diameter/height) and placed within the channels at the base of each chamber. Each tube contained 25 mL of a solidified culture medium (3 g L−1 Gelzan) containing Y3 basal medium, Murashige and Skooge vitamins 100 μM NAA, and 15 g L−1 sucrose. Each tube was closed with a 22 μm pore clear plastic membrane (BKMAM, Changde City, Hunan Province, China) that allowed CO2 to permeate into the vessels while preventing microorganism contamination. The experiment tested seedling growth under two photoperiod conditions [either a 14/10 h (day/night) photoperiod or a 24/0 h continuous light treatment] applied at four different light quality treatments (pure white LED light, or red and blue LED light applied at three different ratios of 3:7, 1:1, and 7:3) and the predetermined best light intensity (600 μmol m−2 s−1; Experiment 1). These treatments were maintained for 1 month, after which all seedlings, still in their culture tubes, were moved to the culture room and exposed to a 14/10 h (day/night) photoperiod at 27 ± 2 °C for an additional month before being assessed (Table 2). The measurements included the number of seedlings surviving the treatments, their fresh weight, height, and leaf number, and the width and thickness of the first fully expanded leaf. To assess health, the chlorophyll content was estimated in the first fully expanded leaf. The data were determined from three replications of six seedlings per treatment.

Table 2.
The two different photoperiods and four light qualities that were applied to seedlings.
2.7. Experiment 3: Influence of CO2 Applied at Different Stages of Seedling Development
Three acclimatization chambers (Figure 2) were utilized for this experiment, which used 108 in vitro-grown MYD coconut seedlings (of various ages), with 36 seedlings randomly allocated to each chamber. The seedlings were placed individually into glass vessels (2.5 × 25.0 cm; diameter/height) and placed within the channels at the base of each chamber. Each tube contained 25 mL of solidified culture medium (3 g L−1 Gelzan) containing Y3 basal medium, Murashige and Skoog vitamins, NAA (100 μM), and sucrose (15 g L−1). Each tube was closed with a 22 μm pore size clear plastic membrane (BKMAM, Changde City, Hunan Province, China) that allowed CO2 to permeate while preventing microorganism contamination. The experiment tested seedling performance under two different levels of atmospheric CO2 (400 and 1600 μmol mol−1) applied at three different stages of seedling development (4 months, 6 months, and 7 to 9 months of age), the best light intensity (600 μmol m−2 s−1) (Table 3), as determined in Experiment 1, and the light quality (red/blue, 7:3) and photoperiod (24/0 h, continuous light), as determined from Experiment 2. These treatments were maintained for 1 month, after which the seedlings were moved to the culture room and exposed to continuous white light (100 μmol m−2 s−1 at a temperature of 27 ± 2 °C) for an additional month before assessment. The measurements taken included the number of seedlings surviving the treatment, their fresh weight, height, and leaf number, and the width and thickness of the first fully expanded leaf. To assess health, the chlorophyll content was estimated in the first fully expanded leaf. The data were determined from three replications of six seedlings per treatment.
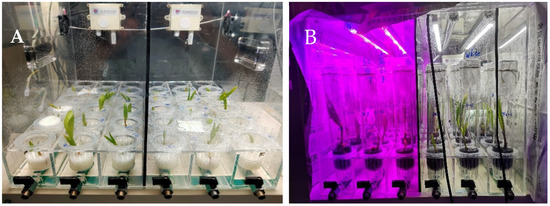
Figure 2.
The specific usage of the experimental equipment in the acclimatization of coconut seedlings. (A) For Experiment 1, 5-month-old embryo-cultured coconut seedlings were placed into an acclimatization chamber. (B) For Experiment 2, the chamber was fitted with a carbon dioxide transmitter monitor and an environmental control unit. The seedlings were grown in tall culture tubes covered with a gas-permeable membrane and illuminated with white light or a combination of red (70%) and blue (30%) light. There was a light-proof barrier between the two halves of the chamber.

Table 3.
Two CO2 concentrations that were applied at various seedling growth stages.
2.8. Measurements
The following seedling traits were measured after 2 months of culture: seedling height, as determined from the root/shoot junction for the seedling to the tip of the tallest leaf; the number of fully formed leaves; the width, thickness, and an estimate of chlorophyll content of the first fully formed leaf; the number of primary roots formed; the fresh weight gain; and the percentage of seedlings surviving the treatment. Leaf width was measured using a steel ruler, and thickness was measured using a SanLiang precision digital caliper (Model 19100822). The chlorophyll content was determined by an SPAD meter (SPAD-502 Plus, Konica, Minolta, Tokyo, Japan).
2.9. Statistical Analysis
All datasets were determined from three replications of six seedlings and underwent statistical analysis using an Analysis of Variance (ANOVA) test. The mean values were then compared using Fisher’s Least Significant Difference (LSD) test, utilizing the Statistical Analysis System (SAS; Version 9.4). Since the germination data did not conform to the assumptions of normality and equal variance, a square root transformation was applied prior to performing the ANOVA.
3. Results
3.1. Atmospheric CO2 Concentration and Light Intensity on Acclimatization
To determine the influence of atmospheric CO2 concentration and light intensity on the growth and development of the in vitro-derived coconut seedlings during acclimatization, different treatments were used, as previously described (Section 2.7). Of all the treatments tried in this experiment, Treatment 6 (1600 μmol mol−1 CO2 and 600 μmol m−2 s−1 light intensity) gave significant improvement in most of seedling growth parameters, such as survival rate, seedling height, chlorophyll content, leaf thickness, root length and fresh weight (Figure 3), when compared to the control (Treatment 2: 400 μmol mol−1 CO2) at 600 μmol m−2 s−1 light intensity. Although other treatments gave significant improvements in some growth attributes, in all cases, they significantly reduced the seedling survival rate and fresh weight when compared to the control. Furthermore, increasing the CO2 concentration to 3000 μmol mol−1 did not offer any additional benefits to that observed for the 1600 μmol mol−1 concentration (Figure 3). Overall, these findings indicate that an elevated CO2 concentration (1600 μmol mol−1) combined with an enhanced light intensity (600 μmol m−2 s−1) can significantly enhance the growth and development of embryo-cultured seedlings during acclimatization.
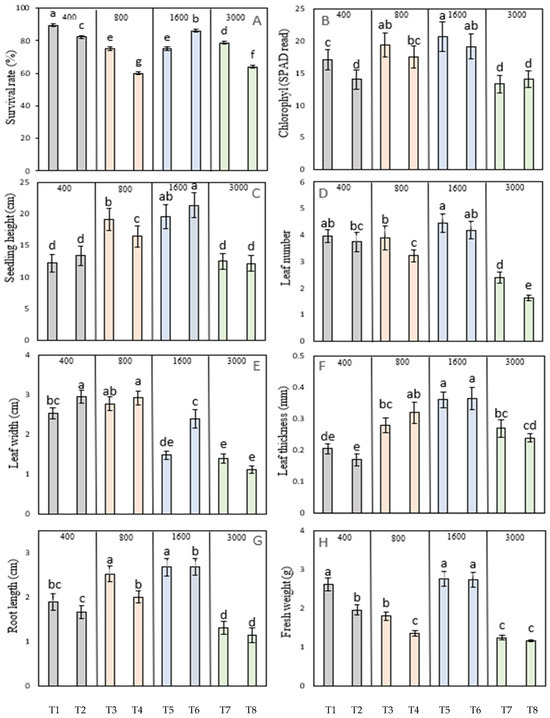
Figure 3.
The influence of atmospheric carbon dioxide concentration (400, 800, 1600, or 3000 μmol mol−1) and light intensity at 300 (odd treatment numbers) and 600 μmol m2 s−1 (even treatment numbers) on the growth and development of 5-month-old MYD in vitro-grown seedlings. (A) Percentage seedling survival rate. (B) Leaf chlorophyl content as assessed by SPAD. (C) Seedling height. (D) Leaf number. (E) Leaf width. (F) Leaf thickness. (G) Root length. (H) Seedling fresh weight. Each dataset includes three replications of six seedlings in each treatment, and bars represent the mean with two standard errors. The letter above the bars indicates a significant difference within that dataset. Treatments marked with the same letter are not significantly different from each other, while treatments marked with different letters are significantly different (p < 0.05). T1 to T8 represented Treatment 1 to Treatment 8, as mentioned in Table 1.
3.2. Influence of Light Quality and Photoperiod on Acclimatization
To determine the influence of light quality and photoperiod on the growth and development of the in vitro-derived coconut seedlings during acclimatization, different treatments were used, as mentioned in Section 2.8. Of all the treatments used, Treatment 4 (14/0 h photoperiod + red/blue at a ratio of 7:3) and Treatment 8 (24/10 h photoperiod + red/blue at a ratio of 7:3) gave the best improvements in chlorophyll content, leaf number, leaf width, root length, and seedling height when compared to the control (Treatment 1: 14/10 h photoperiod and white light; Figure 4). Specifically, the findings suggest that a light regime comprising red/blue (7:3) LED light, combined with either a 14/10 h or continuous photoperiod, can enhance the growth characteristics of embryo-cultured seedlings. However, a continuous photoperiod (Treatment 8) significantly improved the seedling survival rate when compared to the control conditions, unlike Treatment 4, which exhibited a lower survival rate as compared to the control. Also, Treatment 8 exhibited a significantly better survival rate, leaf width, and seedling height as compared to Treatment 4. Overall, these findings indicate that an extended photoperiod (24/0 h) combined with an enriched red/blue LED light ratio (7:3) support superior seedling growth and development.
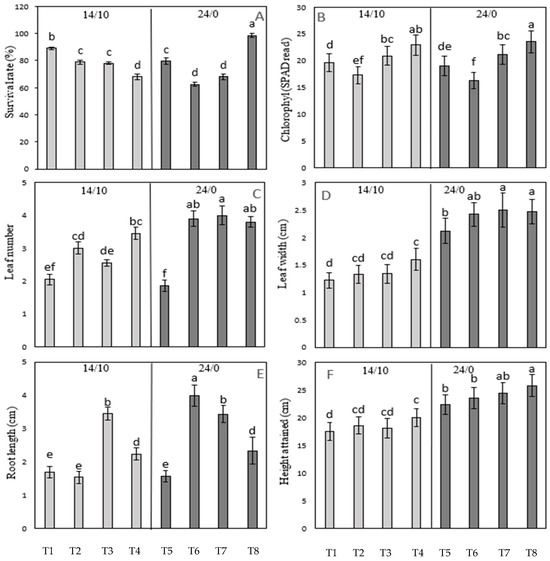
Figure 4.
The influence of photoperiod (either 14/10 h day/night or 24/0 constant light) and light quality [either pure white light (1 and 5) or red and blue light applied at three different ratios of 3:7 (2 and 6), 1:1 (3 and 7), and 7:3 (4 and 8)] on the growth and development of 5-month-old MYD in vitro-grown seedlings. (A) Percentage seedling survival rate. (B) Leaf chlorophyl content as assessed by SPAD. (C) Leaf number. (D) Leaf width. (E) Root length. (F) Seedling height. Each dataset includes three replications of six seedlings, and the bars represent the mean with two standard errors. The letter above the bars indicates a significant difference within that dataset. Treatments marked with the same letter are not significantly different from each other, while treatments marked with different letters are significantly different (p < 0.05). T1 to T8 represented Treatment 1 to Treatment 8, as mentioned in Table 2.
3.3. Influence of CO2 Applied at Different Stages of Seedling Development to Accelerate the Acclimatization Process
To accelerate the acclimatization process, the influence of CO2 on the growth and development of in vitro-derived coconut seedlings, when applied at different developmental stages, was tested, as mentioned in Section 2.9. The survival rate and growth traits of the 7- to 9-month-old seedlings were found to be unaffected by the CO2 at both concentrations 400 and 1600 μmol mol−1 CO2 applied in Treatments 3 and 6, respectively (Figure 5). Treatments 3 (400 μmol mol−1 CO2 applied at 7–9 months of development) and 6 (1600 μmol mol−1 CO2 applied at the same developmental stage) significantly enhanced seedling growth as compared to their respective controls. Similarly, Treatment 5 (1600 μmol mol−1 CO2 applied to 6-month-old seedlings) gave the best improvement in all aspects of seedling growth measured (seedling height, leaf number, leaf width, and root length; Figure 5) when compared to Treatment 2 (400 μmol mol−1 CO2 and 6 months of age; Figure 5). These results suggest that CO2 plays a minor role in the acclimatization process when the seedlings are older (7 to 9 months of age) and had minor improvement at 4 months of age (Figure 5).
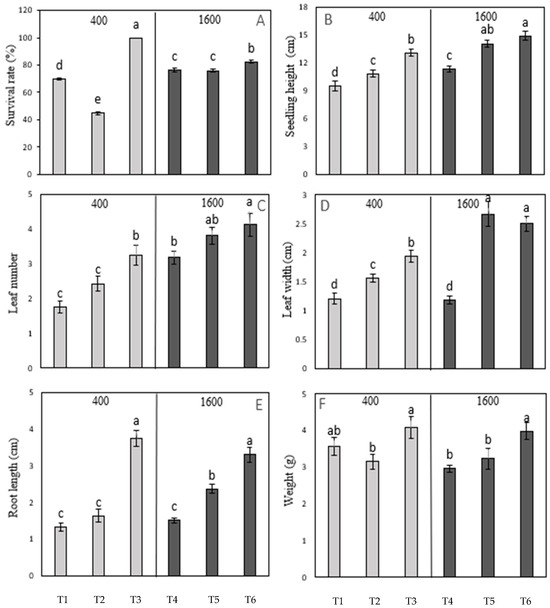
Figure 5.
The influence of atmospheric CO2 concentration (at 400 and 1600 μmol mol−1) when applied to seedlings of different ages [either 4 months old (1 and 4), 6 months old (2 and 5), or 7 to 9 months of age (3 and 6), all applied at the previously determined best light intensity (600 μmol m−2 s−1) and light quality (red/blue ratio 7:3) on the growth and development of MYD in vitro-grown seedlings. (A) Percentage seedling survival rate. (B) Seedling height attainment. (C) Leaf number. (D) Leaf width. (E) Root length. (F) Fresh weight. Each dataset includes three replications of six seedlings, and the bars represent the mean with two standard errors. The letter above the bars indicates a significant difference within that dataset. Treatments marked with the same letter are not significantly different from each other, while treatments marked with different letters are significantly different (p < 0.05). T1 to T6 represented Treatment 1 to Treatment 6, as mentioned in Table 3.
The application of CO2 led to notable improvements in seedling height attainment, leaf number and width, root length, and overall survival. Additionally, Treatment 6 further increased the seedlings’ fresh weight. In contrast, no significant improvements in the survival or growth parameters were observed when seedlings at younger stages of development were treated. These findings suggest that transferring seedlings at a more advanced stage of development (7–9 months) is more effective than transferring seedlings at younger stages. The letters above the bars in Figure 3, Figure 4 and Figure 5 indicate significant differences between treatments within each dataset. Treatments marked with the same letter are not significantly different from each other (i.e., they belong to the same homogeneous group), while treatments marked with different letters are significantly different (p < 0.05). This statistical analysis helped to identify which treatments had a significant impact on the measured growth parameters.
4. Discussion
The effectiveness of any in vitro culture system is determined not only by the number of regenerated seedlings coming from the cultured materials but also by the quantity and quality of the plants that become successfully established from those cultures in soil [3]. Coconut seedlings produced in vitro from either embryo culture or somatic embryogenesis are highly vulnerable to the abrupt environmental changes that occur during the acclimatization step. Thus, acclimatization remains a critical step in the transition of seedlings from heterotrophic to autotrophic growth conditions [9]. The present study aimed to enhance the quantity and quality of coconut embryo-cultured seedlings coming through the acclimatization process by manipulating the culture vessel CO2 concentration and the illumination system, viz., the intensity, quality, and photoperiod, when applied to seedlings of different ages.
The present study showed that the maximum effectiveness of the photomixotrophic growth system was when a CO2 concentration of 1600 μmol mol−1 (Figure 3), a constant 24 h photoperiod (Figure 4), an elevated light intensity (600 μmol m−2 s−1; Figure 3), and a quality of light consisting of a combination of red and blue light in the ratio of 7:3 (Figure 4) were applied to 6-month-old seedlings (Figure 5).
In previous studies, the influence of headspace CO2 concentration has been shown to stimulate coconut seedling growth. Samosir and Adkins (2014) [18] demonstrated that a headspace CO2 concentration of 1600 μmol mol−1 could promote the growth and development of seedlings, reducing the time necessary for them to remain in vitro. Similar effects of elevated CO2 application have been observed in other plant species with improved growth outcomes, viz., for banana (Musa acuminata L.), coffee (Coffea arabica L.) [19], and eucalyptus (Eucalyptus tereticornis Sm) [20] seedlings, when under in vitro culture conditions. Carbon dioxide enrichment was thought to be beneficial to seedling growth as it stimulated various morphological and physiological trait changes, including leaf chlorophyll content [21,22], stomatal density [23], seedling fresh and dry weight, leaf area, shoot length [24,25], and root production [26].
The light intensity used during the in vitro acclimatization process of many species is another important factor that has been shown to affect the rate of growth and development of plants when placed ex vitro. Several species have been shown to prefer higher light intensities during acclimatization, including micro-propagated grape (Vitis vinifera L.) [27], banana (Musa spp.) [28], tobacco (Nicotiana tabacum L.) [29], and chili ancho pepper (Capsicum annuum L.) [30]. Light-emitting diode lighting systems can deliver a much wider range of light intensities as well as a variety of different light qualities; however, LED lighting systems have not been commonly used for in vitro coconut acclimatization. Moderate LED light intensities, coupled with an elevated headspace CO2 concentration, have led to increased growth in several other species [31].
Light quality is known to affect the growth and development of many plant tissues through the phytochrome system [32]. It can be inferred from the present results that a combination of red and blue light (especially in the ratio red/blue ratio 7:3) can promote growth when compared to pure white LED light. Similar results have been demonstrated for potato (Solanum tuberosum L.) in vitro seedlings, where a red/blue treatment ratio of 45:55 improved growth [10]. A combination of a red/blue ratio of 9:1 LED lighting on date palm seedlings in vitro resulted in a significant increase in the number of shoots formed [12].
The photoperiod is another factor that can significantly affect the growth and development of seedlings coming through in vitro culture. For date palm and peach palm seedlings, a transition from a short-day photoperiod (12/12 h; day/night) to a long-day photoperiod (16/8 h day/night) was found to increase seedling growth [33,34]. In the present study, the use of a 24/0 h (day/night) photoperiod showed greater improvements in all growth traits than a 16/8 h photoperiod [35].
5. Conclusions
This study provides valuable insights into the ways to improve the success rates of ex vitro coconut plant production and streamline the acclimatization process through targeted in vitro treatments. The findings demonstrate that enriching the culture vessel headspace CO2 concentration to 1600 μmol mol−1, combined with a moderate light intensity of 600 μmol m−2 s−1, significantly improved the survival and growth traits of embryo-cultured seedlings. Moreover, a red/blue light ratio of 7:3, paired with a continuous 24 h photoperiod, was identified as the most effective combination for increasing seedling survival rates, while both 14/10 and 24/0 light/dark cycles enhanced growth traits. Notably, the application of these treatments is most effective when seedlings have reached at least 6 months of age. These findings contribute to the field by offering practical guidelines for optimizing coconut seedling acclimatization and advancing tissue culture techniques, thereby addressing critical challenges in coconut propagation and production.
Author Contributions
Conceptualization, S.W.A. and Z.M.; methodology, Z.M. and S.W.A.; data collection, Z.L. and Z.M.; formal analysis, Z.M.; writing—original draft preparation, Z.M.; writing—review and editing, Z.M., N.P.C.N., S.K., E.K., J.M.B., S.W.A., A.B. and Z.L.; research supervision, S.K., S.W.A. and J.M.B.; project administration, S.K. and S.W.A. All authors have read and agreed to the published version of the manuscript.
Funding
This research was sponsored by the Australian Center for International Agricultural Research (ACIAR), grant number HORT/2017/025, the Coconut Research Institute of Chinese Academy of Tropical Agricultural Sciences (CATAS), and a Hainan International Science and Technology Cooperation R&D Project (GHYF2023005).
Data Availability Statement
Data are contained within the article.
Acknowledgments
We acknowledge ACIAR, CATAS, and CSC-UQ scholarship for their support of this research.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Mu, Z.; Tran, B.-M.; Xu, H.; Yang, Z.; Qamar, U.Z.; Wang, X.; Xiao, Y.; Luo, J. Exploring the potential application of coconut water in healthcare and biotechnology: A review. Beverage Plant Res. 2024, 4, e018. [Google Scholar] [CrossRef]
- Mu, Z.; Yang, S.; Xu, H.; Yang, Z.; Haque, M.M.; Tran, B.-M.; Chen, J.; Wang, X.; Peng, H.; Luo, J. The Influence of Maturity, Storage, and Embryo Size on Coconut Callus Induction Success. Forests 2024, 15, 764. [Google Scholar] [CrossRef]
- Cueto, C.; Rivera, R.; Kim, H.; Kong, H.; Baek, H.; Sebastian, L.; Park, H. Development of cryopreservation protocols for cryobanks of coconut zygotic embryos. Acta Hortic. 2014, 1039, 297–302. [Google Scholar] [CrossRef]
- Pospíšilová, J.; Synková, H.; Haisel, D.; Semorádová, S. Acclimation of Plantlets to Ex Vitro Conditions: Effects of Air Humidity, Irradiance, CO2 Concentration and Abscisic Acid (a Review). Acta Hortic. 2007, 748, 29–38. [Google Scholar] [CrossRef]
- Mu, Z.; Guo, X.; Biddle, J.; Foale, M.; Li, Z.; Adkins, S. A Newly Designed Chamber for the Acclimatization of Coconut Plantlets Coming from In Vitro. In Vitro Cell. Dev. Biol.-Anim. 2020, 56, 44. [Google Scholar]
- Triques, K.; Rival, A.; Beulé, T.; Dussert, S.; Hocher, V.; Verdeil, J.-L.; Hamon, S. Developmental changes in carboxylase activities in in vitro cultured coconut zygotic embryos: Comparison with corresponding activities in seedlings. Plant Cell Tissue Organ Cult. 1997, 49, 227–231. [Google Scholar] [CrossRef]
- Kozai, T.; Kubota, C. Developing a photoautotrophic micropropagation system for woody plants. J. Plant Res. 2001, 114, 525–537. [Google Scholar] [CrossRef]
- Nguyen, Q.T.; Kozai, T.; Van Nguyen, U. Effects of sucrose concentration, supporting material and number of air exchanges of the vessel on the growth of in vitro coffee plantlets. Plant Cell Tissue Organ Cult. 1999, 58, 51–57. [Google Scholar] [CrossRef]
- Mu, Z. Overcoming Bottlenecks in the Pathway of Clonal Propagation of Coconut (Cocos nucifera L.). Ph.D. Thesis, The University of Queensland, St Lucia, QLD, Australia, 2022. [Google Scholar]
- Jao, R.-C.; Fang, W. Growth of potato plantlets in vitro is different when provided concurrent versus alternating blue and red light photoperiods. HortScience 2004, 39, 380–382. [Google Scholar] [CrossRef]
- Seelye, J.F.; Mullan, A.C. Light-Emitting Diode Lights: The Future of Plant Lighting©. In Combined Proceedings of the International Plant Propagators’ Society; International Plant Propagator’s Society: Monroe, LA, USA, 2010; Volume 60, p. 172. [Google Scholar]
- Al-Mayahi, A.M.W. Effect of red and blue light emitting diodes “CRB-LED” on in vitro organogenesis of date palm (Phoenix dactylifera L.) cv. Alshakr. World J. Microbiol. Biotechnol. 2016, 32, 160. [Google Scholar] [CrossRef] [PubMed]
- Al-Khayri, J.M.; Naik, P.M. Date palm micropropagation: Advances and applications. Cienc. E Agrotecnologia 2017, 41, 347–358. [Google Scholar] [CrossRef]
- Heringer, A.S.; Steinmacher, D.A.; Fraga, H.P.; Vieira, L.N.; Montagna, T.; Quinga, L.A.; Quoirin, M.G.; Jiménez, V.M.; Guerra, M.P. Improved high-efficiency protocol for somatic embryogenesis in peach palm (Bactris gasipaes Kunth) using RITA® temporary immersion system. Sci. Hortic. 2014, 179, 284–292. [Google Scholar] [CrossRef]
- Jain, S.M.; Al-Khayri, J.M.; Johnson, D.V. Date Palm Biotechnology; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2011. [Google Scholar]
- Zayed, Z.E. Enhanced indirect somatic embryogenesis from shoot-tip explants of date palm by gradual reductions of 2, 4-D concentration. In Date Palm Biotechnology Protocols Volume I; Springer: Berlin/Heidelberg, Germany, 2017; pp. 77–88. [Google Scholar]
- Gomes, H.T.; Bartos, P.M.C.; Balzon, T.A.; Scherwinski-Pereira, J.E. Regeneration of somatic embryos of oil palm (Elaeis guineensis) using temporary immersion bioreactors. Ind. Crops Prod. 2016, 89, 244–249. [Google Scholar] [CrossRef]
- Samosir, Y.M.S.; Adkins, S. Improving acclimatization through the photoautotrophic culture of coconut (Cocos nucifera) seedlings: An in vitro system for the efficient exchange of germplasm. Vitr. Cell. Dev. Biol.-Plant 2014, 50, 493–501. [Google Scholar] [CrossRef]
- Nguyen, Q.T.; Kozai, T.; Niu, G.; Van Nguyen, U. Photosynthetic characteristics of coffee (Coffea arabusta) plantlets in vitro in response to different CO2 concentrations and light intensities. Plant Cell Tissue Organ Cult. 1998, 55, 133–139. [Google Scholar] [CrossRef]
- Khan, P.S.V.; Kozai, T.; Nguyen, Q.; Kubota, C.; Dhawan, V. Growth and net photosynthetic rates of Eucalyptus tereticornis Smith under photomixotrophic and various photoautotrophic micropropagation conditions. Plant Cell Tissue Organ Cult. 2002, 71, 141–146. [Google Scholar] [CrossRef]
- Afreen, F.; Zobayed, S.M.A.; Kozai, T. Photoautotrophic culture of Coffea arabusta somatic embryos: Development of a bioreactor for large-scale plantlet conversion from cotyledonary embryos. Ann. Bot. 2002, 90, 21–29. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, A.; Tanaka, R. Chlorophyll metabolism. Curr. Opin. Plant Biol. 2006, 9, 248–255. [Google Scholar] [CrossRef] [PubMed]
- Apel, P. Influence of CO2 on stomatal numbers. Biol. Plant. 1989, 31, 72–74. [Google Scholar] [CrossRef]
- Zobayed, S.M.A.; Kubota, C.; Kozai, T. Mass propagation of Eucalyptus camaldulensis in a scaled-up vessel under in vitro photoautotrophic condition. Ann. Bot. 2000, 85, 587–592. [Google Scholar] [CrossRef]
- Nguyen, Q.T.; Kozai, T. Growth of In vitro banana (Musa spp.) shoots under photomixotrophic and photoautotrophic conditions. Vitr. Cell. Dev. Biol.-Plant 2001, 37, 824–829. [Google Scholar] [CrossRef]
- Kirdmanee, C.; Kitaya, Y.; Kozai, T. Effects of CO2 enrichment and supporting materialin vitro on photoautotrophic growth ofEucalyptus plantlets in vitro andex vitro. In Vitro Cell. Dev. Biol.-Plant 1995, 31, 144–149. [Google Scholar] [CrossRef]
- Krishna, H.; Singh, S.; Sharma, R.; Khawale; Grover, M.; Patel, V. Biochemical changes in micropropagated grape (Vitis vinifera L.) plantlets due to arbuscular-mycorrhizal fungi (AMF) inoculation during ex vitro acclimatization. Sci. Hortic. 2005, 106, 554–567. [Google Scholar] [CrossRef]
- Aragón, C.E.; Escalona, M.; Capote, I.; Pina, D.; Cejas, I.; Rodriguez, R.; Cañal, M.J.; Sandoval, J.; Roels, S.; Debergh, P.; et al. Photosynthesis and carbon metabolism in plantain (Musa AAB) plantlets growing in temporary immersion bioreactors and during ex vitro acclimatization. In Vitro Cell. Dev. Biol.-Plant 2005, 41, 550–554. [Google Scholar] [CrossRef]
- Pospíšilová, J.; Wilhelmová, N.A.; Synkova, H.; Čatský, J.; Krebs, D.; Ticha, I.; Hanáčková, B.; Snopek, J. Acclimation of tobacco plantlets to ex vitro conditions as affected by application of abscisic acid. J. Exp. Bot. 1998, 49, 863–869. [Google Scholar] [CrossRef]
- Estrada-Luna, A.A.; Davies, F.T., Jr. Arbuscular mycorrhizal fungi influence water relations, gas exchange, abscisic acid and growth of micropropagated chile ancho pepper (Capsicum annuum) plantlets during acclimatization and post-acclimatization. J. Plant Physiol. 2003, 160, 1073–1083. [Google Scholar] [CrossRef] [PubMed]
- Zuo, W.-Y.; He, J.-S.; Han, M.; Ji, C.-J.; Flynn, D.F.; Fang, J.-Y. Responses of plant stomata to elevated CO2 and temperature: Observations from 10 plant species grown in temperature and CO2 gradients. Acta Ecol. Sin. 2005, 25, 565–574. [Google Scholar]
- Olle, M.; Viršile, A. The effects of light-emitting diode lighting on greenhouse plant growth and quality. Agric. Food Sci. 2013, 22, 223–234. [Google Scholar] [CrossRef]
- Assy Bah, B.; Durand-Gasselin, T.; Pannetier, C. Use of zygotic embryo culture to collect germplasm of coconut (Cocos nucifera L.) 1987. Available online: https://agritrop.cirad.fr/438165/ (accessed on 4 November 2024).
- Sáenz, L.; Chan, J.L.; Narvaez, M.; Oropeza, C. Protocol for the Micropropagation of Coconut from Plumule Explants. In Plant Cell Culture Protocols; Springer: Berlin/Heidelberg, Germany, 2018; pp. 161–170. [Google Scholar]
- Khierallah, H.S.; Bader, S.M. Micropropagation of date palm (Phoenix dactylifera L.) var. Maktoom through direct organogenesis. II Int. Date Palm Conf. 2006, 736, 213–224. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).