Ball-Milling-Modified Biochar with Additives Enhances Soil Cd Passivation, Increases Plant Growth and Restrains Cd Uptake by Chinese Cabbage
Abstract
1. Introduction
2. Materials and Methods
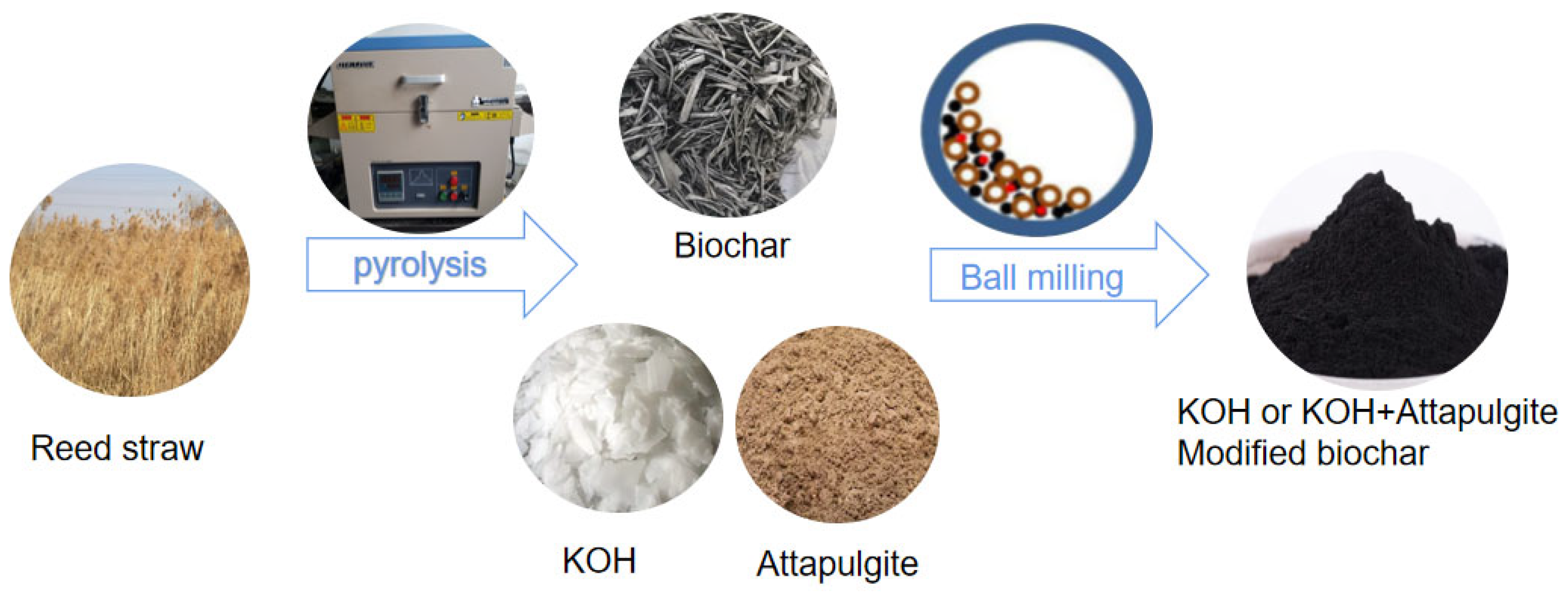
2.1. Modified Biochar Preparation
2.2. Characterization and Analysis of Modified Biochar
2.3. Batch Experiments
2.4. Pot Experiment
2.5. Soil Analysis
2.6. Data Statistical Analysis
3. Results and Discussions
3.1. Characteristics of Modified Biochar
3.1.1. Elemental Analysis and BET Nitrogen Adsorption
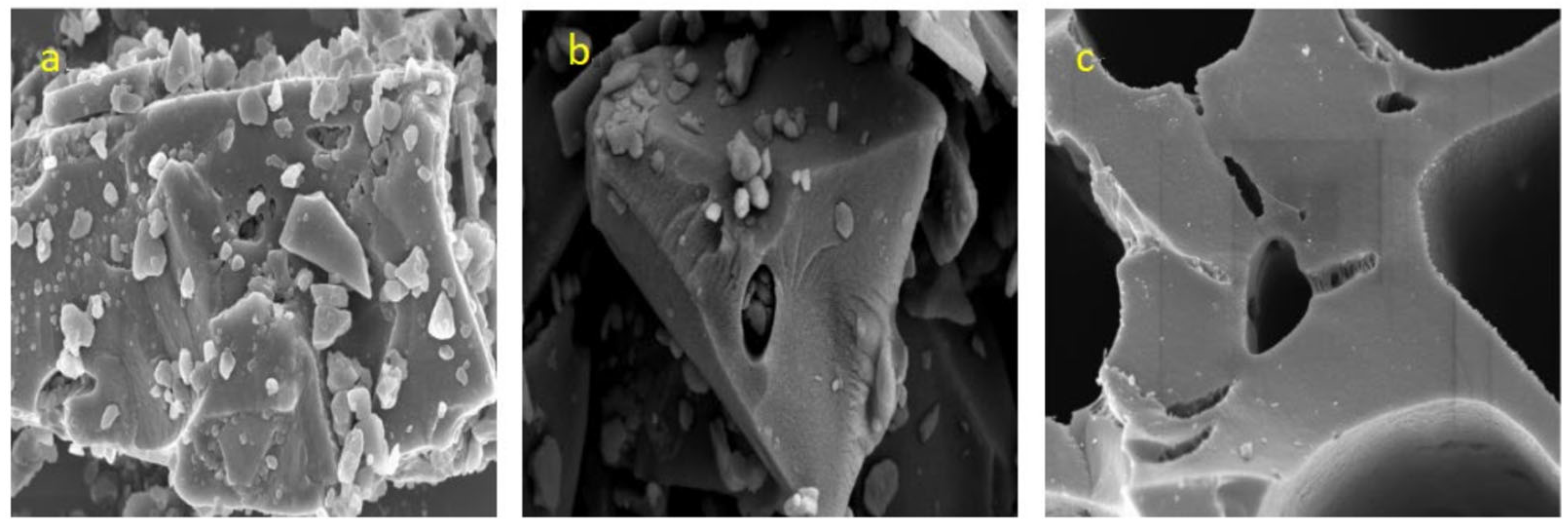
3.1.2. Analysis of Surface Morphology
3.1.3. Analysis of Infrared Spectrum
3.1.4. XRD Pattern Analysis
3.2. Kinetics and Isotherms of Cd
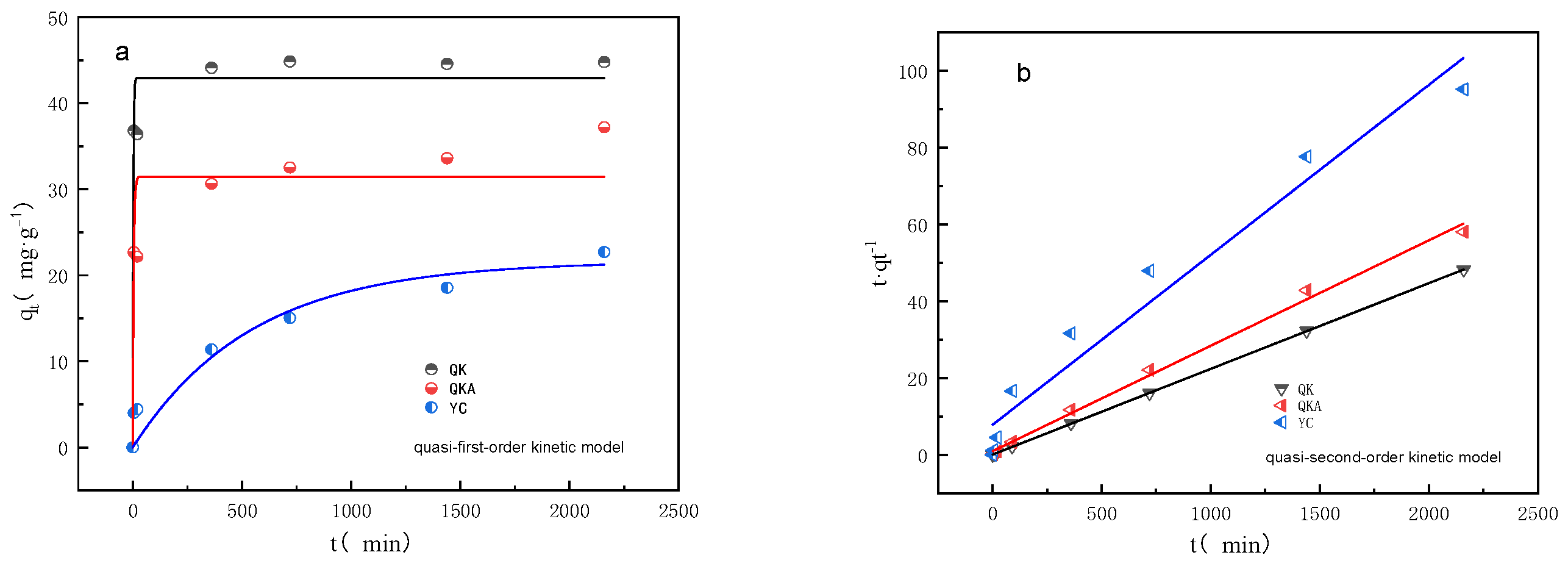
3.2.1. Adsorption Kinetics
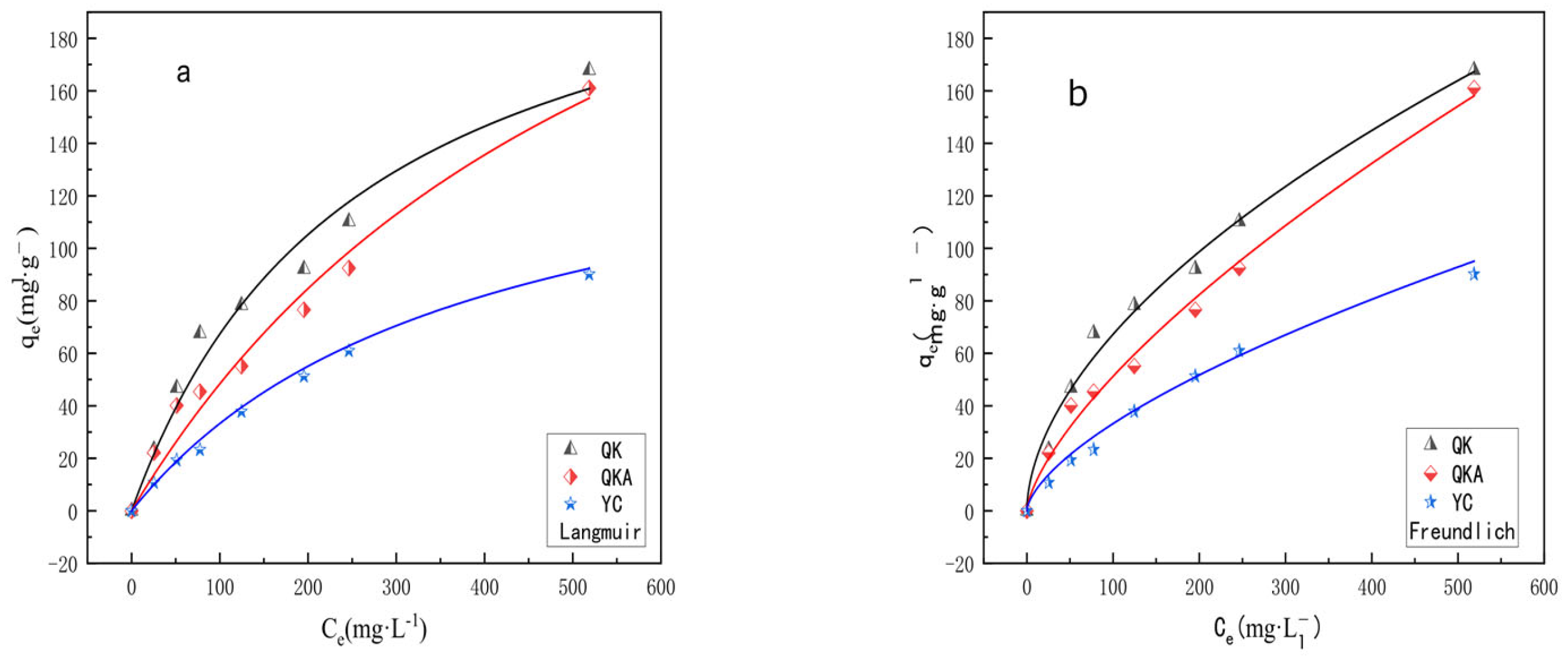
3.2.2. Adsorption Isotherm
3.3. Factors Influencing Cd Adsorption in the Solution
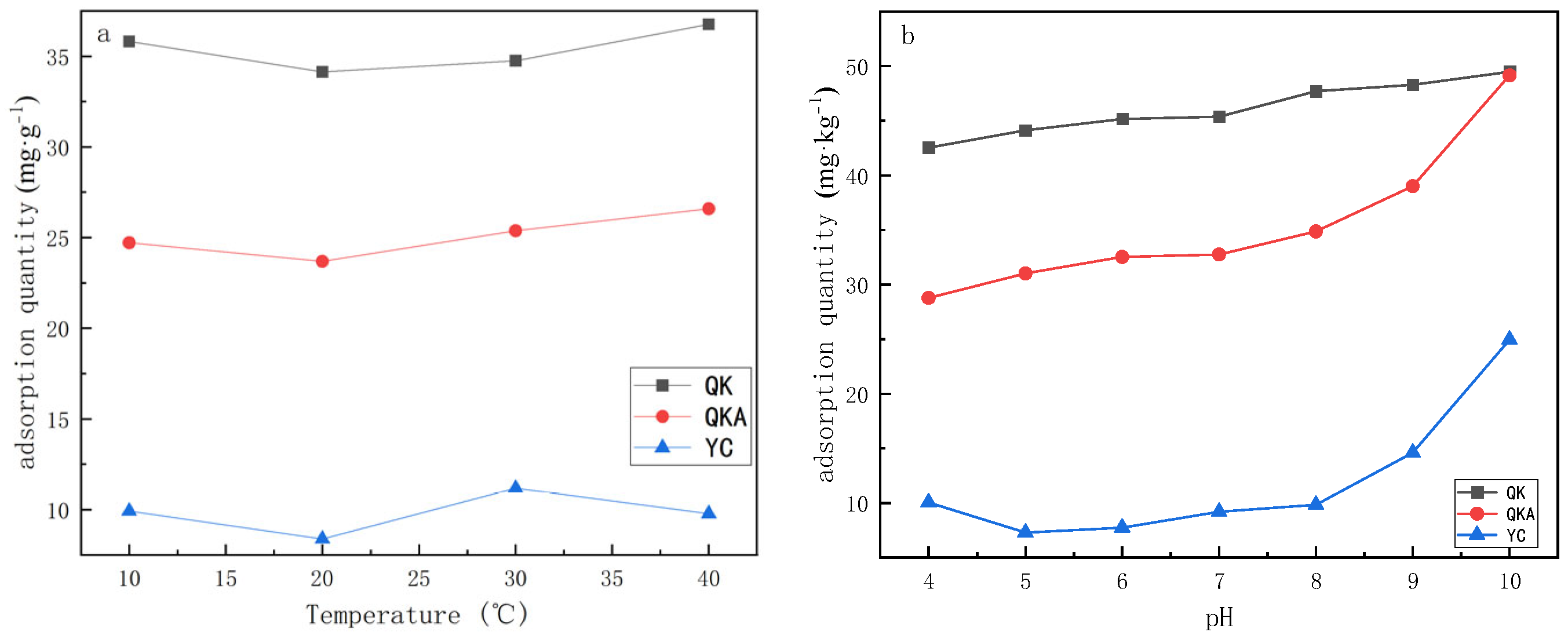
3.3.1. Influence of Temperature on the Adsorption Capacity of Modified Biochar
3.3.2. Effect of pH on Cd Adsorption Capacity by Modified Biochar
3.4. Effects of Modified Biochar on Soil Properties
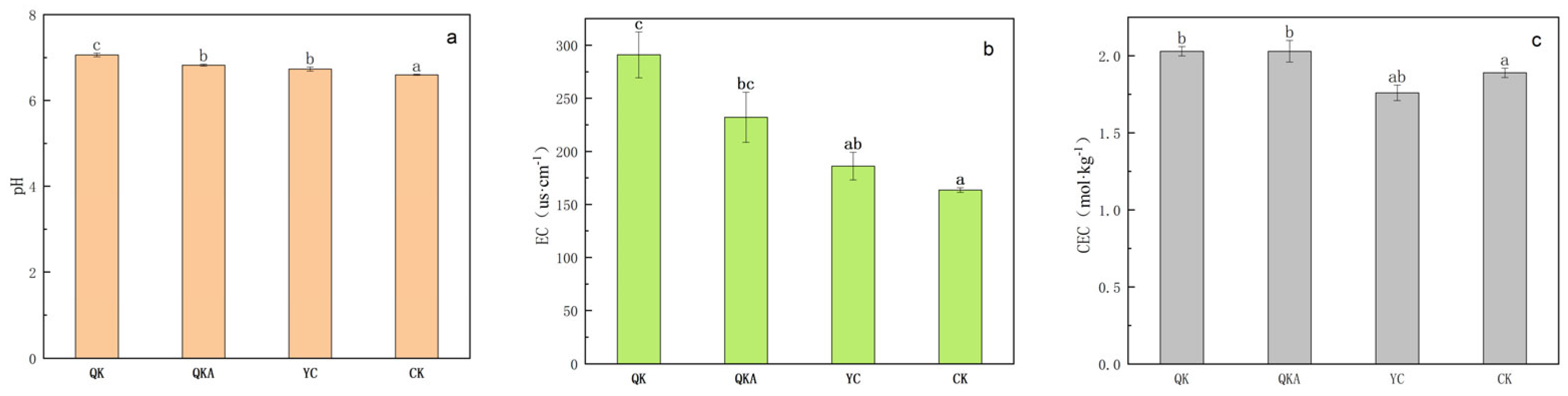
3.4.1. Changes in the Soil pH, EC and CEC
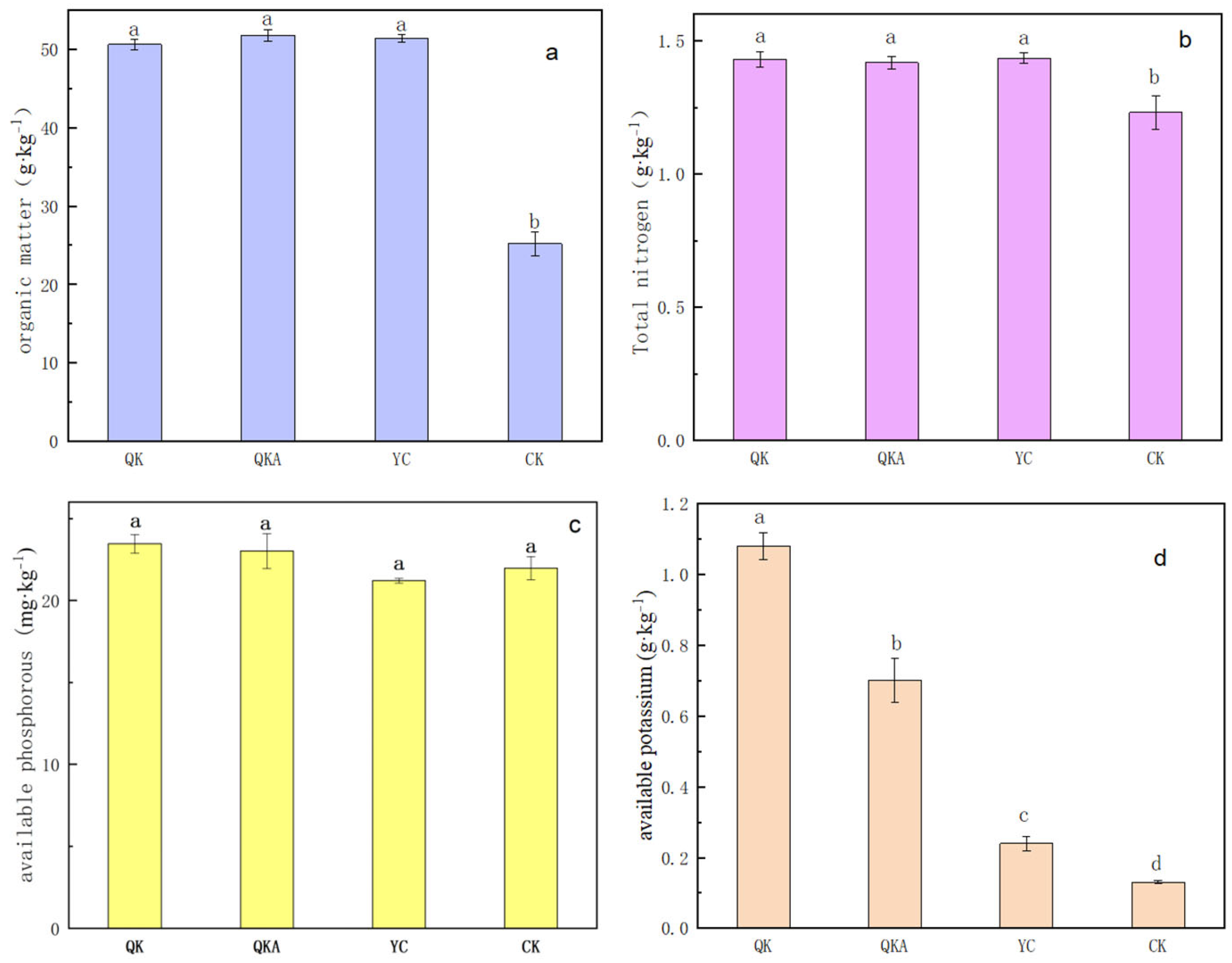
3.4.2. Change in Soil Fertility
3.5. Influence of Modified Biochar on Cd Bioavailability in Soils
3.6. Influence of Modified Biochar on Plant Growth and Cd Accumulation in Plants
3.6.1. Influence of Modified Biochar on Plant Height, Root Length and Biomass of Chinese Cabbage
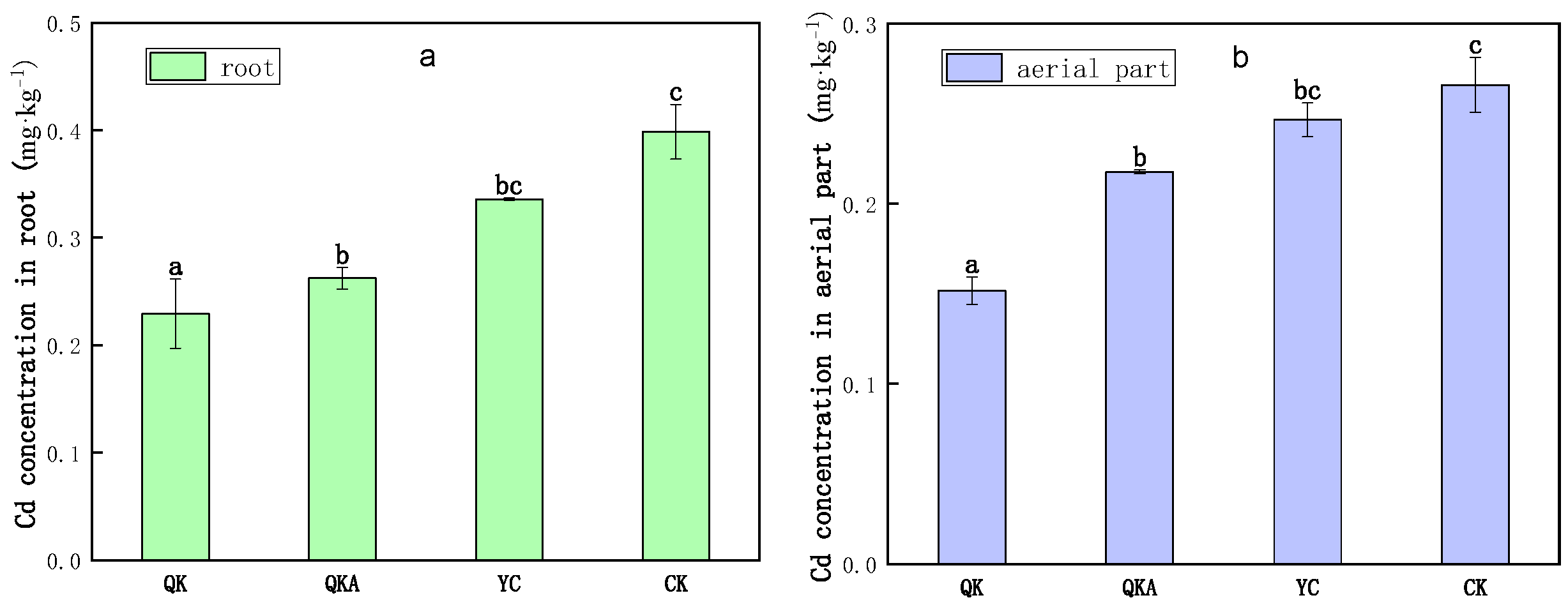
3.6.2. Influence of Modified Biochar on Cd Accumulation by Chinese Cabbage
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Kumar, A.; Subrahmanyam, G.; Mondal, R.; Cabral-Pinto, M.M.S.; Shabnam, A.A.; Jigyasu, D.K.; Malyan, S.K.; Kishor-Fagodiya, R.; Khan, S.A.; Kumar, A.; et al. Bio-remediation approaches for alleviation of cadmium contamination in natural resources. Chemosphere 2021, 268, 128855. [Google Scholar] [CrossRef]
- Zhang, H.J.; Zhang, X.Z.; Li, T.X.; Huang, F. Variation of cadmium uptake, translocation among rice lines and detecting for potential cadmium-safe cultivars. Environ. Earth Sci. 2014, 71, 277–286. [Google Scholar]
- He, Y.; Li, B.B.; Zhang, K.N.; Li, Z.; Chen, Y.G.; Ye, W.M. Experimental and numerical study on heavy metal contaminant migration and retention behavior of engineered barrier in tailings pond. Environ. Pollut. 2019, 252, 1010–1018. [Google Scholar] [CrossRef]
- Wu, H.W.; Liu, Q.Y.; Ma, J.; Liu, L.L.; Qu, Y.J.; Gong, Y.W.; Yang, S.H.; Luo, T. Heavy Metal(loids) in typical Chinese tobacco-growing soils: Concentrations, influence factors and potential health risks. Chemosphere 2020, 245, 125591. [Google Scholar] [CrossRef] [PubMed]
- Li, H.N.; Tian, Y.L.; Liu, W.; Long, Y.J.; Ye, J.; Li, B.X.; Li, N.; Yan, M.M.; Zhu, C.X. Impact of electrokinetic remediation of heavy metal contamination on antibiotic resistance in soil. Chem. Eng. J. 2020, 400, 125866. [Google Scholar] [CrossRef]
- Huang, S.H.; Rao, G.S.; Ashraf, U.; He, L.X.; Zhang, Z.Z.; Zhang, H.L.; Mo, Z.W.; Pan, S.G.; Tang, X.G. Application of inorganic passivators reduced Cd contents in brown rice in oilseed rape-rice rotation under Cd contaminated soil. Chemosphere 2020, 259, 127404. [Google Scholar] [CrossRef]
- Ma, H.; Wei, M.; Wang, Z.; Hou, S.; Li, X.; Xu, H. Bioremediation of cadmium polluted soil using a novel cadmium immobilizing plant growth promotion strain Bacillus sp. TZ5 loaded on biochar. J. Hazard. Mater. 2020, 388, 122065. [Google Scholar] [CrossRef]
- Haris, M.; Amjad, Z.; Usman, M.; Saleem, A.; Dyussenova, A.; Mahmood, Z.; Dina, K.; Guo, J.; Wang, W. A review of crop residue-based biochar as an efficient adsorbent to remove trace elements from aquatic systems. Biochar 2024, 6, 47. [Google Scholar] [CrossRef]
- Chen, H.M.; Du, X.F.; Lai, M.Q.; Nazhafati, M.; Li, C.; Qi, W.Q. Biochar Improves Sustainability of Green Roofs via Regulate of Soil Microbial Communities. Agriculture 2021, 11, 620. [Google Scholar] [CrossRef]
- Me’ndez, A.; Go’mez, A.; Paz-Ferreiro, J.; Gasco’, G. Effects of sewage sludge biochar on plant metal availability after application to a Mediterranean soil. Chemosphere 2012, 89, 1354–1359. [Google Scholar] [CrossRef]
- Pan, X.; Kuang, S.P.; Wang, X.; Ullah, H.; Rao, Z.P.; Ali, E.F.; Abbas, Q.; Lee, S.S.; Shaheen, S.M. Functionalization of sawdust biochar using Mg-Fe-LDH and sodium dodecyl sulfonate enhanced its stability and immobilization capacity for Cd and Pb in contaminated water and soil. Biochar 2025, 7, 16. [Google Scholar] [CrossRef]
- Wu, J.W.; Wang, T.; Wang, J.W.; Zhang, Y.S.; Pan, W. A novel modified method for the efficient removal of Pb and Cd from wastewater by biochar: Enhanced the ion exchange and precipitation capacity. Sci. Total Environ. 2021, 754, 142150. [Google Scholar] [CrossRef]
- Sun, T.; Gao, G.; Yang, W.H.; Sun, Y.B.; Huang, Q.Q.; Wang, L.; Liang, X.F. High-efficiency remediation of Hg and Cd co-contaminated paddy soils by Fe-Mn oxide modifed biochar and its microbial community responses. Biochar 2024, 6, 57. [Google Scholar] [CrossRef]
- Fakhar, A.; Snowie, J.C.G.; Ronley, C.C.; Mazhar, R.; Rubab, S.; Aitazaz, A.F.; Muhammad, I.K. Advancing modifed biochar for sustainable agriculture: A comprehensive review on characterization, analysis, and soil performance. Biochar 2025, 7, 8. [Google Scholar] [CrossRef] [PubMed]
- Kumar, M.; Xiong, X.; Wan, Z.; Sun, Y.; Tsang, D.C.W.; Gupta, J.; Gao, B.; Cao, X.; Tang, J.; Ok, Y.S. Ball milling as a mechanochemical technology for fabrication of novel biochar nanomaterials. Bioresour. Technol. 2020, 312, 123613. [Google Scholar] [CrossRef]
- Zhang, P.; Xue, B.; Jiao, L.; Meng, X.; Zhang, L.; Li, B.; Sun, H. Preparation of ball-milled phosphorus-loaded biochar and its highly efective remediation for Cd- and Pb-contaminated alkaline soil. Sci. Total Environ. 2022, 813, 152648. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhao, G.; Xuan, Y.; Gan, L.; Pan, M. Enhanced photocatalytic performance for phenol degradation using ZnO modified with nano-biochar derived from cellulose nanocrystals. Cellulose 2021, 28, 991–1009. [Google Scholar] [CrossRef]
- Sun, Y.; Lyu, H.; Cheng, Z.; Wang, Y.; Tang, J. Insight into the mechanisms of ball-milled biochar addition on soil tetracycline degradation enhancement: Physicochemical properties and microbial community structure. Chemosphere 2022, 291, 132691. [Google Scholar] [CrossRef]
- Montinaro, S.; Concas, A.; Pisu, M.; Cao, G. Immobilization of heavy metals in contaminated soils through ball milling with and without additives. Chem. Eng. J. 2008, 142, 271–284. [Google Scholar] [CrossRef]
- Zhan, G.Y.; Chen, Z.N.; Tong, F.; Shen, H.G.; Gao, Y.; Liu, L.Z.; Zhang, Z.H.; Lu, X. Properties of biochar and its effect on soil heavy metals under different straw materials and preparation techniques. J. Ecol. Rural Environ. 2021, 37, 86–95, (In Chinese with English Abstract). [Google Scholar]
- Xu, M.L.; Dai, W.J.; Zhao, Z.L.; Zheng, J.T.; Huang, F.; Mei, C.; Huang, S.T.; Liu, C.F.; Wang, P.; Xiao, R.B. Effect of rice straw biochar on three different levels of Cd-contaminated soils: Cd availability, soil properties, and microbial communities. Chemosphere 2022, 301, 134551. [Google Scholar] [CrossRef]
- Sun, L.J.; Gong, P.Y.; Sun, Y.F.; Qin, Q.; Song, K.; Ye, J.; Zhang, H.; Zhou, B.; Xue, Y. Modified chicken manure biochar enhanced the adsorption for Cd2+ in aqueous and immobilization of Cd in contaminated agricultural soil. Sci. Total Environ. 2022, 851, 158252. [Google Scholar] [CrossRef]
- GB/T 23739-2009; Soil Quality—Analysis of Available Lead and Cadmium Contents in Soils—Atomic Absorption Spectrometry. Standards Press of China: Beijing, China, 2009.
- Fathollahzadeh, H.; Kaczala, F.; Bhatnagar, A.; Hogland, W. Speciation of metals in contaminated sediments from oskarshamn harbor, oskarshamn, Sweden. Environ. Sci. Pollut. Res. 2014, 21, 2455–2464. [Google Scholar] [CrossRef]
- Lu, X.; Liu, L.Z.; Fan, R.Q.; Luo, J.; Yan, S.H.; Rengel, Z.; Zhang, Z. Dynamics of copper and tetracyclines during composting of water hyacinth biomass amended with peat or pig manure. Environ. Sci. Pollut. Res. 2017, 24, 23584–23597. [Google Scholar] [CrossRef]
- Sparks, D.L. Methods of Soil Analysis; Part 3; Soil Society of American: Madison, WI, USA, 1996. [Google Scholar]
- Liu, G.; Guo, W.; Pei, M.; Meng, F.; Wang, L. Synthesis of bentonite grafted by cationic polymer for the adsorption of Amido black 10B. Colloid Polym. Sci. 2016, 294, 2005–2012. [Google Scholar] [CrossRef]
- Han, L.; Li, J.; Zhang, T.; Qu, C.; Yu, T.; Yang, B.; Shao, Z. Removal of Cr(VI) by biochar derived via co-pyrolysis of oily sludge and corn stalks. Sci. Rep. 2022, 12, 9821. [Google Scholar] [CrossRef] [PubMed]
- Luo, J.W.; Li, X.; Ge, C.J.; Müller, K.; Yu, H.; Huang, P.; Li, J.T.; Tsang, D.C.W.; Bolan, N.; Rinklebe, J.; et al. Sorption of norfloxacin, sulfamerazine and oxytetracycline by KOH-modified biochar under single and ternary systems. Bioresour. Technol. 2018, 385, 385–392. [Google Scholar] [CrossRef]
- Kołodyńska, D.; Bąk, J.; Kozioł, M.; Pylypchuk, L.V. Investigations of Heavy Metal Ion Sorption Using Nanocomposites of Iron-Modified Biochar. Nanoscale Res. Lett. 2017, 12, 467. [Google Scholar] [CrossRef]
- Song, X.L.; Liu, H.Y.; Cheng, L.; Qu, Y.X. Surface modification of coconut-based activated carbon by liquid-phase oxidation and its effects on lead ion adsorption. Desalination 2010, 255, 78–83. [Google Scholar] [CrossRef]
- Muñoz-Batista, M.J.; Rodriguez-Padron, D.; Puente-Santiago, A.R.; Luque, R. Mechanochemistry: Toward sustainable design of advanced nanomaterials for electrochemical energy storage and catalytic applications. ACS Sustainable Chem. Eng. 2018, 6, 9530–9544. [Google Scholar] [CrossRef]
- Ehsan, M.; Barakat, M.A.; Husein, D.Z.; Ismail, S.M. Immobilization of Ni and Cd in Soil by Biochar Derived From Unfertilized Dates. Water Air Soil Pollut. 2014, 225, 2123. [Google Scholar] [CrossRef]
- Zhong, M.T.; Li, W.D.; Jiang, M.H.; Wang, J.G.; Shi, X.Y.; Song, J.H.; Zhang, W.X.; Wang, H.J.; Cui, J. Improving the ability of straw biochar to remediate Cd contaminated soil: KOH enhanced the modification of K3PO4 and urea on biochar. Ecotoxicol. Environ. Saf. 2023, 262, 115317. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharyya, K.G.; Gupta, S.S. Removal of Cu(II) by natural and acid-activated clays: An insight of adsorption isotherm, kinetic and thermodynamics. Desalination 2011, 272, 66–75. [Google Scholar] [CrossRef]
- Shilani, B.; Mehdipour, R.; Mousazadeh, B.; Noruzi, Y.; Hosseini, S.; Al-Saedi, H.N.; Mohealdeen, S.M. Utilizing triethylenetetramine-functionalized MIP-206 for highly efficient removal of Pb(II) from wastewater. Sci. Rep. 2024, 14, 15586. [Google Scholar] [CrossRef]
- Jiang, M.Y.; He, L.Z.; Niazi, N.K.; Wang, H.L.; Gustave, W.; Vithanage, M.; Geng, K.; Shang, H.; Zhang, X.L.; Wang, Z.Y. Nanobiochar for the remediation of contaminated soil and water: Challenges and opportunitie. Biochar 2023, 5, 2. [Google Scholar] [CrossRef]
- Edebali, S.; Pehlivan, E. Evaluation of chelate and cation exchange resins to remove copper ions. Powder Technol. 2016, 301, 520–525. [Google Scholar] [CrossRef]
- Van, Z.; Wieten, L.; Kimber, S.; Morris, S.; Chan, K.Y.; Downie, A.; Rust, J.; Joseph, S.; Cowie, A. Effects of biochar from slow pyrolysis of papermill waste on agronomic performance and soil fertility. Plant Soil 2010, 327, 235–246. [Google Scholar]
- Gao, R.L.; Tang, M.; Fu, Q.L.; Guo, G.G.; Xiao, L.; Hu, H.Q. Fractions transformation of heavy metals in compound contaminated soil treated with biochar, montmorillonite and mixed addition. Environ. Sci. 2017, 38, 361–367. [Google Scholar]
- Jia, Y.H.; Li, J.; Zeng, X.B.; Zhang, N.; Wen, J.; Liu, J.; Jiku, M.A.S.; Wu, C.X.; Su, S.M. The performance and mechanism of cadmium availability mitigation by biochars differ among soils with different pH: Hints for the reasonable choice of passivators. J. Environ. Manag. 2022, 312, 114903. [Google Scholar] [CrossRef]
- Qiao, J.T.; Liu, T.X.; Wang, X.Q.; Li, F.B.; Lv, Y.H.; Cui, J.H.; Zeng, X.D.; Yuan, Y.Z.; Liu, C.P. Simultaneous alleviation of cadmium and arsenic accumulation in rice by applying zero-valent iron and biochar to contaminated paddy soils. Chemosphere 2018, 195, 260–271. [Google Scholar] [CrossRef]
- Lyu, H.H.; Zhao, H.; Tang, J.C.; Gong, Y.Y.; Huang, Y.; Wu, Q.H.; Gao, B. Immobilization of hexavalent chromium in contaminated soils using biochar supported nanoscale iron sulfide composite. Chemosphere 2017, 194, 360–369. [Google Scholar] [CrossRef]
- Noronha, F.R.; Manikandan, S.K.; Nair, V. Role of coconut shell biochar and earthworm (Eudrilus euginea) in bioremediation and palak spinach (Spinacia oleracea L.) growth in cadmium-contaminated soil. J. Environ. Manag. 2022, 302, 114057. [Google Scholar] [CrossRef]
- Xie, S.; Wang, L.; Xu, Y.; Lin, D.; Zheng, S. Performance and mechanisms of immobilization remediation for Cd contaminated water and soil by hydroxy ferric combined acid-base modified sepiolite (hyfe/absep). Sci. Total Environ. 2020, 740, 140009. [Google Scholar] [CrossRef]
- Huang, D.; Deng, R.; Wan, J.; Zeng, G.; Xue, W.; Wen, X.; Zhou, C.; Hu, L.; Liu, X.; Xu, P. Remediation of lead-contaminated sediment by biochar-supported nano-chlorapatite: Accompanied with the change of available phosphorus and organic matters. J. Hazard. Mater. 2018, 348, 109–116. [Google Scholar] [CrossRef] [PubMed]
- Lai, C.; Muon, R.; Touch, V.; Hin, S.; Podwojewski, P.; Ket, P.; Jouquet, P.; Degré, A.; Ann, V. Impact of Biochar from Rice Husk on Nutrient Distribution and Rice Growth and Yield: A Soil Column Experiment. J. Soil Sci. Plant Nutr. 2024, 24, 159–171. [Google Scholar] [CrossRef]
- Jamieson, T.; Sager, E.; Gueguen, C. Characterization of biochar-derived dissolved organic matter using UV visible absorption and excitation-emission fluorescence spectroscopies. Chemosphere 2014, 103, 197–204. [Google Scholar] [CrossRef]
- Wan, H.; Hou, J.; Wei, Z.; Liu, F. Contrasting maize responses to soil phosphorus and potassium availability driven by biochar under reduced irrigation. Plant Soil 2024, 1–24. [Google Scholar] [CrossRef]
- Vuong, X.; Vu, L.; Duong, A.; Duong, H.; Hoang, T.; Luu, M.; Nguyen, T.; Nguyen, V.; Nguyen, T.; Van, T. Speciation and environmental risk assessment of heavy metals in soil from a lead/zinc mining site in Vietnam. Int. J. Environ. Sci. Technol. 2023, 20, 5295–5310. [Google Scholar] [CrossRef]
- Gujre, N.; Rangan, L.; Mitra, S. Occurrence, geochemical fraction, ecological and health risk assessment of cadmium, copper and nickel in soils contaminated with municipal solid wastes. Chemosphere 2021, 271, 129573. [Google Scholar] [CrossRef]
- Dong, M.Y.; Jiang, M.Y.; He, L.Z.; Zhang, Z.R.; Gustave, W.; Vithanage, M.; Niazi, N.K.; Chen, B.; Zhang, X.K.; Wang, H.L.; et al. Challenges in safe environmental applications of biochar: Identifying risks and unintended consequence. Biochar 2025, 7, 12. [Google Scholar] [CrossRef]
- Zhu, Y.G.; Chen, S.B.; Yang, J.C. Effects of soil amendments on lead uptake by two vegetable crops from a lead-contaminated soil from Anhui, China. Environ. Int. 2004, 30, 351–356. [Google Scholar] [CrossRef] [PubMed]
- Tang, B.; Xu, H.P.; Song, F.M.; Ge, H.G.; Chen, L.; Yue, S.Y.; Yang, W.S. Effect of biochar on immobilization remediation of Cd-contaminated soil and environmental quality. Environ. Res. 2022, 204, 111840. [Google Scholar] [CrossRef]
- Yuan, C.; Gao, B.; Peng, Y.; Gao, X.; Fan, B.; Chen, Q. A meta-analysis of heavy metal bioavailability response to biochar aging: Importance of soil and biochar properties. Sci. Total Environ. 2021, 756, 144058. [Google Scholar] [CrossRef] [PubMed]
- Gong, H.B.; Zhao, L.; Rui, X.; Hu, J.W.; Zhu, N. A review of pristine and modifed biochar immobilizing typical heavy metals in soil: Applications and challenges. J. Hazard. Mater. 2022, 432, 128668. [Google Scholar] [CrossRef]
- Liang, X.; Su, Y.; Wang, X.; Liang, C.; Tang, C.; Wei, J.; Liu, K.; Ma, J.; Yu, F.; Li, Y. Insights into the heavy metal adsorption and immobilization mechanisms of CaFelayered double hydroxide corn straw biochar: Synthesis and application in a combined heavy metal-contaminated environment. Chemosphere 2023, 313, 137467. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.F.; Dai, Z.M.; Tang, C.X.; Xu, J.M. The immobilization, plant uptake and translocation of cadmium in a soil-pakchoi (Brassica chinensis L.) system amended with various sugarcane bagasse-based materials. Environ. Pollut. 2022, 311, 119946. [Google Scholar] [CrossRef]
- Pathak, H.K.; Seth, C.S.; Chauhan, P.K.; Dubey, G.; Singh, G.; Jain, D.; Upadhyay, S.K.; Dwivedi, P.; Khoo, K.S. Recent advancement of nano-biochar for the remediation of heavy metals and emerging contaminants: Mechanism, adsorption kinetic model, plant growth and development. Environ. Res. 2024, 255, 119136. [Google Scholar] [CrossRef]
- Su, J.Z.; Zhang, M.Y.; Xu, W.H.; Xu, W.M.; Liu, C.; Rui, S.; Tuo, Y.F.; He, X.H.; Xiang, P. Preparation and applications of iron/biochar composites in remediation of heavy metal contaminated soils: Current status and further perspectives. Environ. Technol. Innov. 2024, 35, 10367. [Google Scholar] [CrossRef]
- Wang, L.; Wang, Y.J.; Ma, F.; Tankpa, V.; Bai, S.S.; Guo, X.M.; Wang, X. Mechanisms and reutilization of modified biochar used for removal of heavy metals from wastewater: A review. Sci. Total Environ. 2019, 668, 1298–1309. [Google Scholar] [CrossRef]
- Agbna, G.H.D.; She, D.L.; Liu, Z.P.; Elshaikh, N.A.; Shao, G.C.; Timm, L.C. Effects of deficit irrigation and biochar addition on the growth, yield, and quality of tomato. Sci. Hortic. 2017, 222, 90–101. [Google Scholar] [CrossRef]
- Wang, M.; Wu, J.; Zhou, T.; Liang, Y.; Zheng, L.X.; Sun, Y.X. Effects of copper and florfenicol on nirS- and nirK-type denitrifier communities and related antibiotic resistance in vegetable soils. Ecotoxicol. Environ. Saf. 2021, 213, 112001. [Google Scholar] [CrossRef]
- Khaliq, H.; Anwar, S.; Shafiq, F.; Ashraf, M.; Zhang, L.; Haider, I.; Khan, S. Interactive effects of soil and foliar-applied nanobiochar on growth, metabolites, and nutrient composition in Daucus carota. J. Plant Growth Regul. 2023, 42, 3715–3729. [Google Scholar] [CrossRef]
- Rasul, M.; Cho, J.; Shin, H.S.; Hur, J. Biochar-induced priming effects in soil via modifying the status of soil organic matter and microflora: A review. Sci. Total Environ. 2022, 805, 150304. [Google Scholar] [CrossRef] [PubMed]
- Mariyam, S.; Upadhyay, S.K.; Chakraborty, K.; Verma, K.K.; Duhan, J.S.; Muneer, S.; Meena, M.; Sharma, R.K.; Ghodake, G.; Seth, S.C. Nanotechnology, a frontier in agricultural science, a novel approach in abiotic stress management and convergence with new age medicine-A review. Sci. Total Environ. 2024, 912, 169097. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Kang, Z.; Yang, Y.; Li, Y.; Qiu, R.; Qin, J.; Li, H. Interplanting of rice cultivars with high and low Cd accumulation can achieve the goal of “repairing while producing” in Cd-contaminated soil. Sci. Total Environ. 2022, 851, 158229. [Google Scholar] [CrossRef]
- GB2762-2022; National Food Safety Standard—Limits of Contaminants in Food. Standards Press of China: Beijing, China, 2022.
- Tan, W.T.; Zhou, H.; Tang, S.F.; Chen, Q.; Zhou, X.; Liu, X.H.; Zeng, P.; Gu, J.F.; Liao, B.H. Simultaneous alleviation of Cd availability in contaminated soil and accumulation in rice (Oryza sativa L.) by Fe-Mn oxide-modified biochar. Sci. Total Environ. 2023, 858, 159730. [Google Scholar] [CrossRef]
- Yan, M.F.; Li, H.J.; Si, B.C.; Xu, C.Y.; Li, M. Parameters Optimization and Physicochemical Properties Characterization of Nanobiochar Prepared from Apple Branches by Ball Milling. Nano 2024, 19, 2450031. [Google Scholar] [CrossRef]

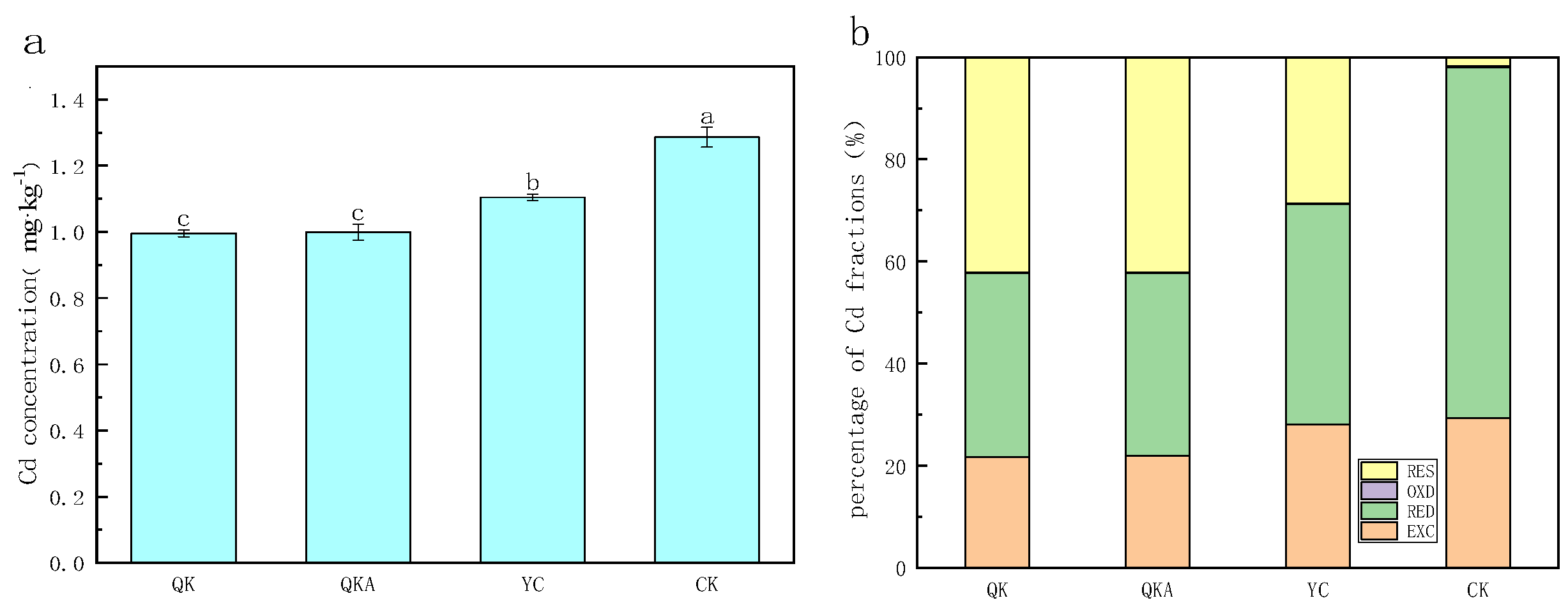
| Sample | pH | EC (µS cm−1) | Organic Matter (g kg−1) | Nitrogen (N) (g kg−1) | Available Potassium (g kg−1) | Available Phosphorus (mg·kg−1) | Cadmium Content (mg·kg−1) |
|---|---|---|---|---|---|---|---|
| Soil | 6.63 ± 0.03 d | 163.56 ± 2.28 d | 23.95 ± 1.5 c | 1.18 ± 0.09 c | 0.24 ± 0.06 d | 21.65 ± 1.41 b | 2.16 ± 0.09 a |
| QK | 10.54 ± 0.05 b | 6120.4 ± 18.5 a | 1054.05 ± 40.7 b | 5.14 ± 0.27 b | 43.07 ± 1.96 a | 29.87 ± 2.75 a | 0.042 ± 0.00 b |
| QKA | 11.21 ± 0.08 a | 2689.7 ± 17.9 b | 1067.67 ± 54.2 b | 5.22 ± 0.19 ab | 21.89 ± 1.18 b | 30.16 ± 2.59 a | 0.071 ± 0.01 b |
| YC | 9.46 ± 0.03 c | 1196.5 ± 13.4 c | 1219.21 ± 58.6 a | 5.94 ± 0.32 a | 10.44 ± 0.67 c | 28.14 ± 2.04 ab | 0.046 ± 0.01 b |
| Treatment | pH | O (%) | N (%) | C (%) | H (%) | S (%) | Specific Surface Area (m2·g−1) | Roe Volume (cc·g−1) | Pore Diameter (nm) |
|---|---|---|---|---|---|---|---|---|---|
| QK | 10.54 ± 0.05 b | 19.27 ± 0.12 a | 0.51 ± 0.03 b | 61.14 ± 1.02 b | 2.04 ± 0.14 a | 0.43 ± 0.02 a | 21.454 ± 0.97 b | 0.014 ± 0.002 b | 1.351 ± 0.095 a |
| QKA | 11.21 ± 0.08 a | 18.89 ± 0.09 b | 0.52 ± 0.03 b | 61.93 ± 1.36 b | 1.94 ± 0.09 a | 0.25 ± 0.03 b | 30.802 ± 1.01 a | 0.018 ± 0.001 a | 1.351 ± 0.078 a |
| YC | 9.46 ± 0.03 c | 16.32 ± 0.10 c | 0.59 ± 0.02 a | 70.72 ± 1.11 a | 1.99 ± 0.09 a | 0.25 ± 0.01 b | 31.120 ± 1.04 a | 0.017 ± 0.001 a | 1.348 ± 0.052 a |
| Quasi-First-Order Dynamics Equation | Quasi-Second-Order Dynamics Equation | ||||||
|---|---|---|---|---|---|---|---|
| Treatment | qe exp | qe | Kl | R2 | qe | K2 | R2 |
| QK | 44.10 | 42.78 | 0.392 | 0.978 | 44.80 | 1.38 × 103 | 0.995 |
| QKA | 30.64 | 30.63 | 0.251 | 0.976 | 36.43 | 2.75 × 104 | 0.996 |
| YC | 22.70 | 21.15 | 0.002 | 0.986 | 22.59 | 6.5 × 10 | 0.961 |
| Treatment | Langmuir | Freundlich | ||||
|---|---|---|---|---|---|---|
| Qm | KI | R2 | Kf | 1/n | R2 | |
| QK | 240.67 | 3.9 × 10−3 | 0.978 | 3.88 | 0.612 | 0.983 |
| QKA | 339.97 | 1.7 × 10−3 | 0.976 | 3.03 | 0.624 | 0.984 |
| YC | 160.41 | 2.6 × 10−3 | 0.989 | 1.09 | 0.084 | 0.981 |
| Treatments | Plant Height (cm) | Root Length (cm) | Arial Part Fresh Weight (g pot−1) | Root Fresh Weight (g pot−1) |
|---|---|---|---|---|
| QKA | 20.89 ± 0.18 ab | 9.75 ± 0.73 ab | 107.13 ± 3.14 a | 12.81 ± 0.33 ab |
| QK | 21.20 ± 0.43 a | 10.16 ± 1.01 a | 108.26 ± 5.54 a | 13.23 ± 0.29 a |
| YC | 21.28 ± 1.50 a | 8.48 ± 1.00 ab | 106.53 ± 4.17 ab | 12.73 ± 0.29 ab |
| CK | 19.70 ± 0.35 b | 7.96 ± 0.49 b | 96.70 ± 3.79 b | 11.76 ± 0.33 b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lu, X.; Sun, J.; Pan, G.; Qi, W.; Zhang, Z.; Xing, J.; Gao, Y. Ball-Milling-Modified Biochar with Additives Enhances Soil Cd Passivation, Increases Plant Growth and Restrains Cd Uptake by Chinese Cabbage. Horticulturae 2025, 11, 168. https://doi.org/10.3390/horticulturae11020168
Lu X, Sun J, Pan G, Qi W, Zhang Z, Xing J, Gao Y. Ball-Milling-Modified Biochar with Additives Enhances Soil Cd Passivation, Increases Plant Growth and Restrains Cd Uptake by Chinese Cabbage. Horticulturae. 2025; 11(2):168. https://doi.org/10.3390/horticulturae11020168
Chicago/Turabian StyleLu, Xin, Jiawan Sun, Guojun Pan, Weicong Qi, Zhenhua Zhang, Jincheng Xing, and Yan Gao. 2025. "Ball-Milling-Modified Biochar with Additives Enhances Soil Cd Passivation, Increases Plant Growth and Restrains Cd Uptake by Chinese Cabbage" Horticulturae 11, no. 2: 168. https://doi.org/10.3390/horticulturae11020168
APA StyleLu, X., Sun, J., Pan, G., Qi, W., Zhang, Z., Xing, J., & Gao, Y. (2025). Ball-Milling-Modified Biochar with Additives Enhances Soil Cd Passivation, Increases Plant Growth and Restrains Cd Uptake by Chinese Cabbage. Horticulturae, 11(2), 168. https://doi.org/10.3390/horticulturae11020168







