Spatial Distribution Characteristics and Relationships of Salt-Based Ions and Nutrients in Old Protected Vegetable Fields
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. Design of the Experiment
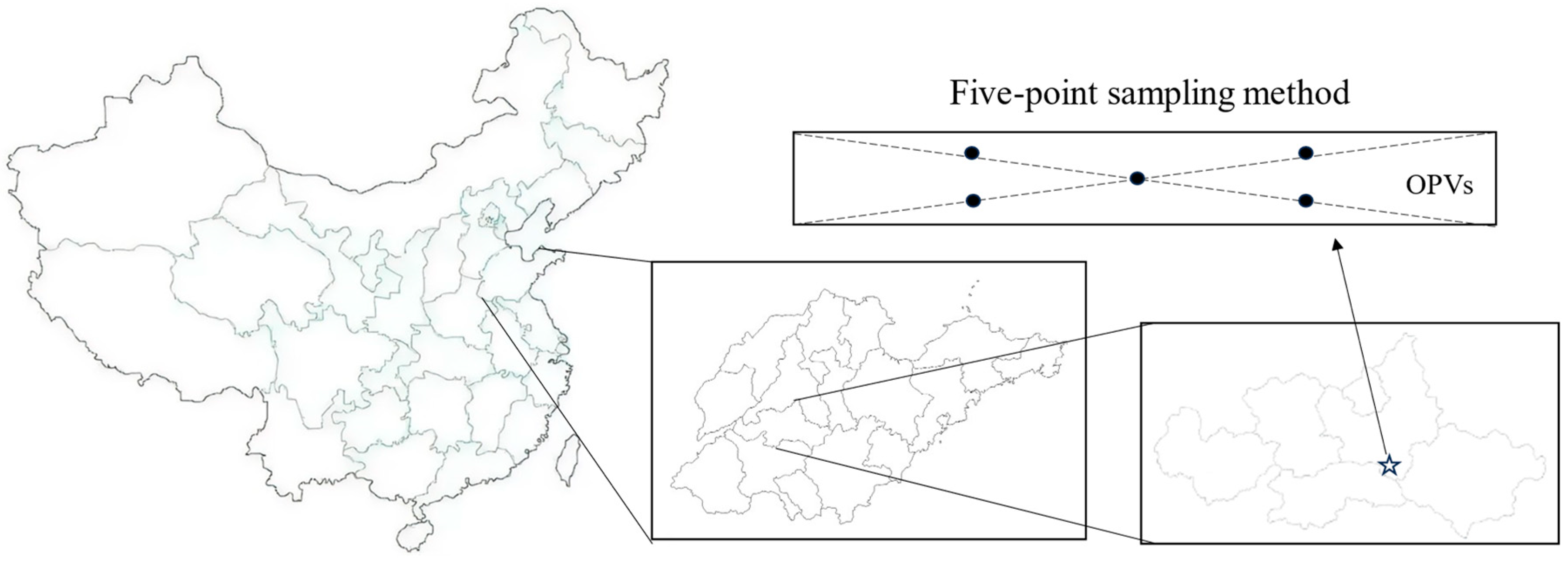
2.3. Measurement Indicators
2.4. Studies Analysis
3. Results
3.1. Soil pH at Different Depths in OPVs
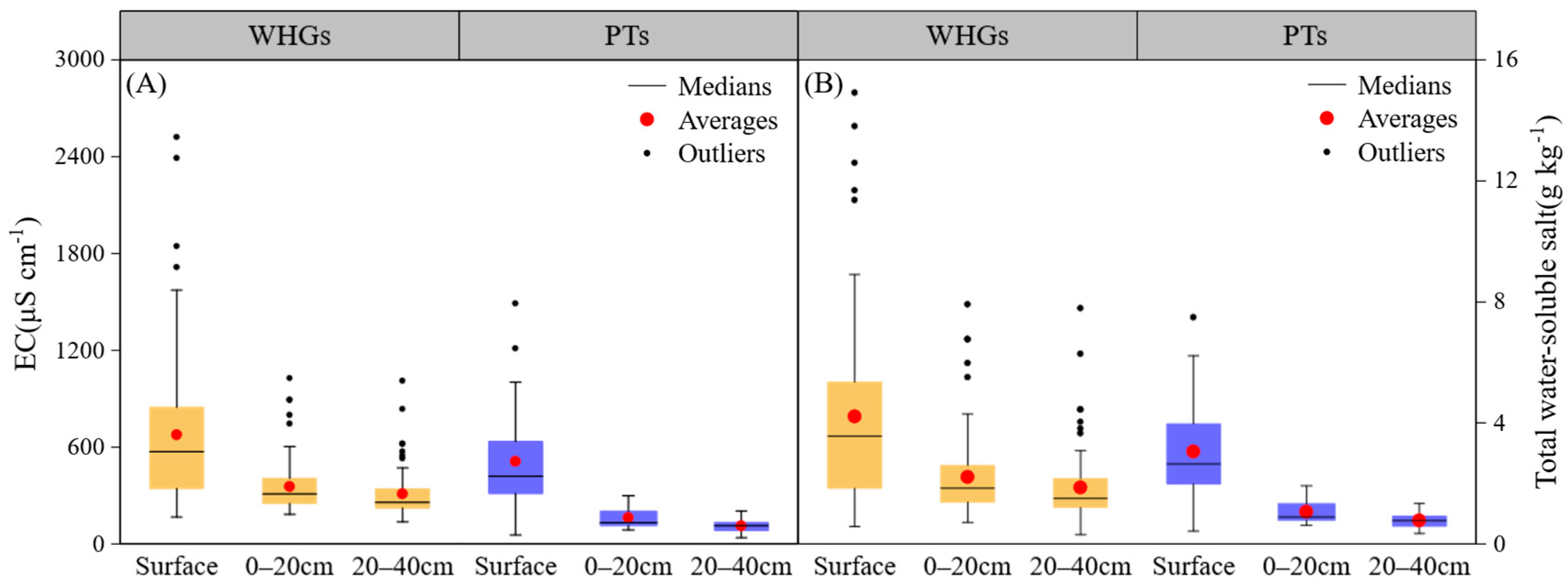
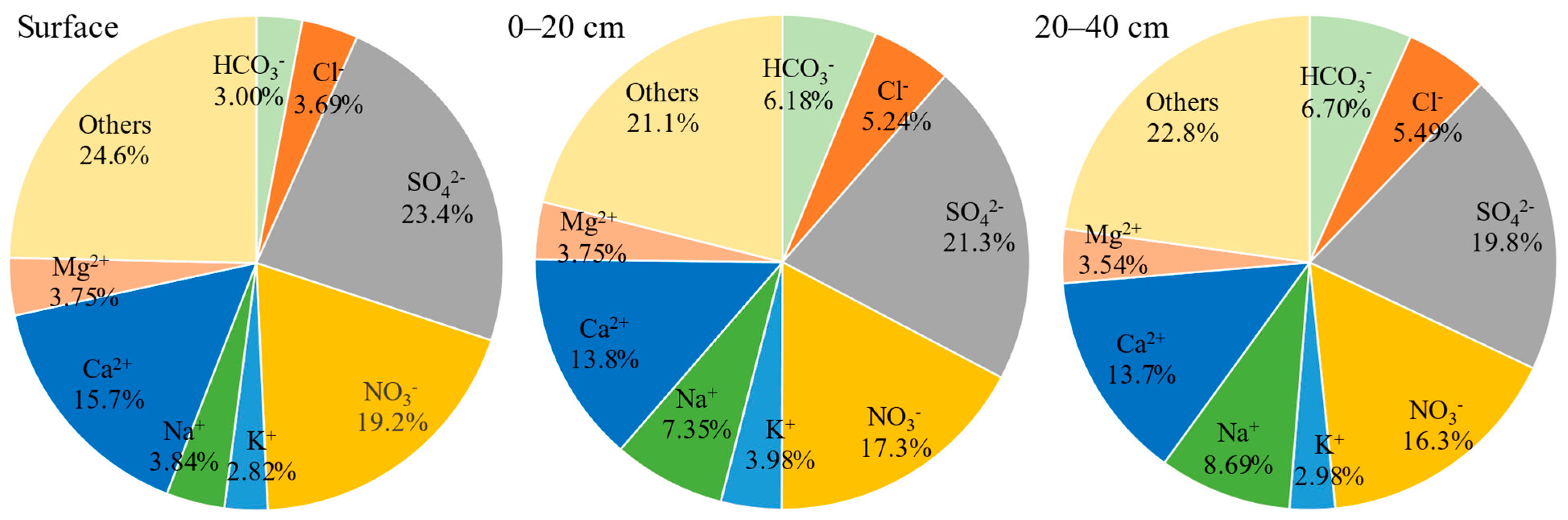
3.2. Soil Salinity Conditions at Different Depths in OPVs
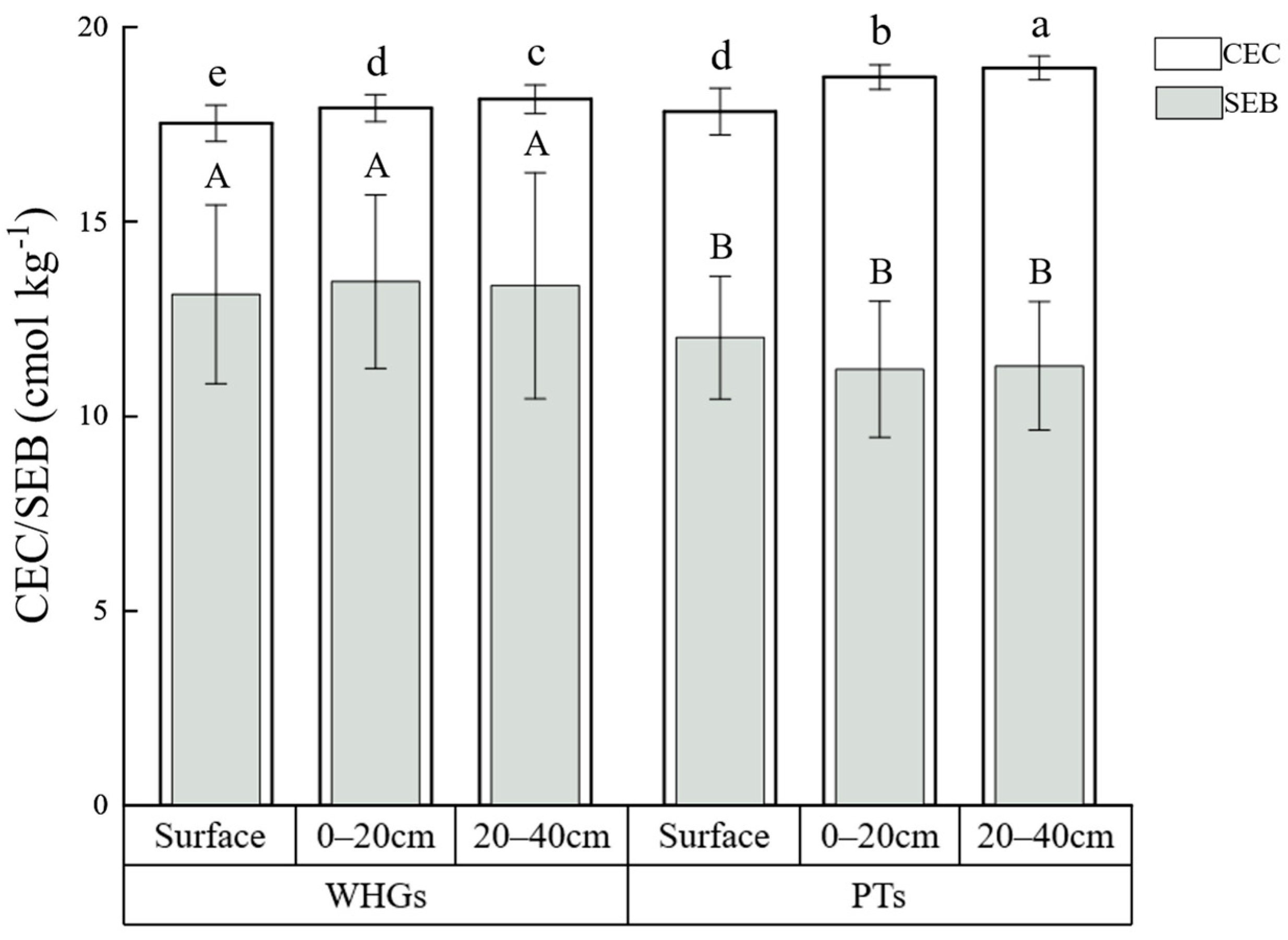
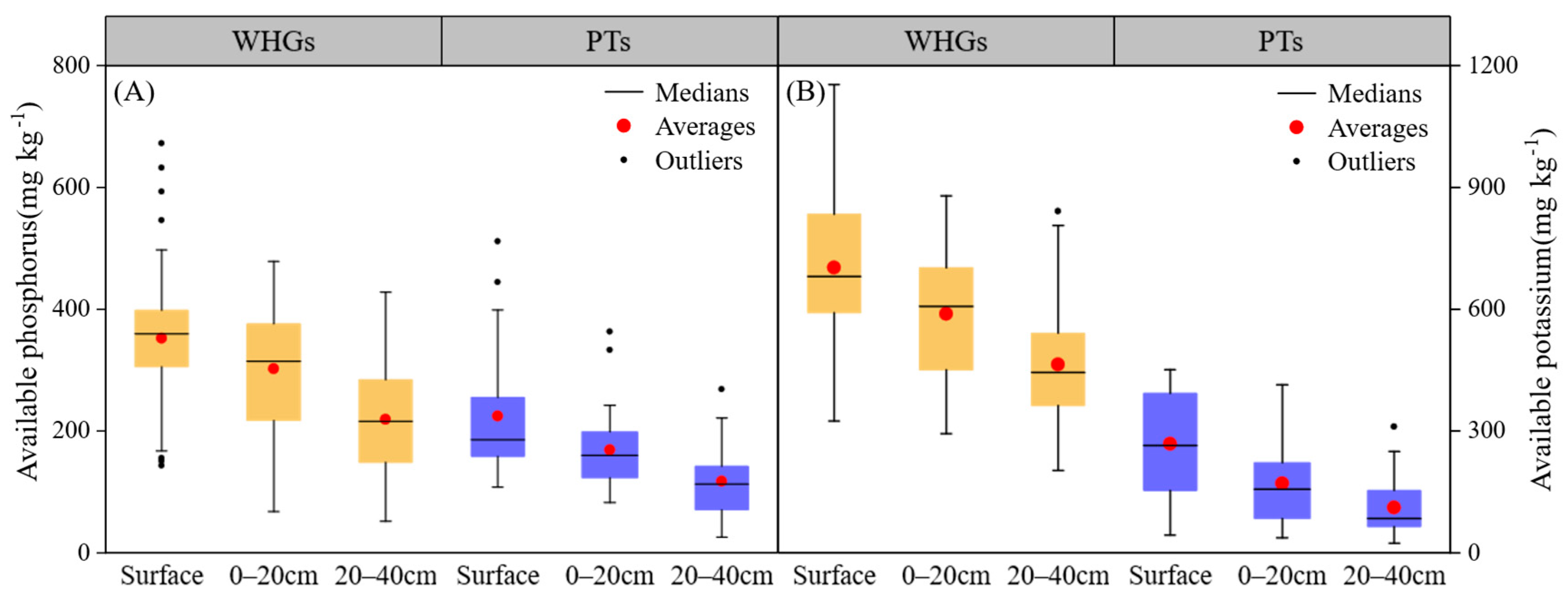
3.3. Soils Base Nutrient Conditions at Different Depths in OPVs
3.4. Correlation Analysis of Soil pH, Nutrients, and Salinity in OPVs
3.5. Principal Component Analysis of Soil Nutrients in OPVs
4. Discussion
4.1. Soil Acidification in OPVs
4.2. Secondary Soil Salinization in OPVs
4.3. Nutrient Imbalance in OPVs
4.4. Soil Multi-Factor Barrier and Outlook for OPVs
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Wang, L.; Yang, J. Introduction to Facility Agriculture; Chemical Industry Press: Beijing, China, 2017. [Google Scholar] [CrossRef]
- Li, T.L. Development status of China’s facility vegetable industry and outlook. China Veg. 2023, 1, 1–6. [Google Scholar]
- Zhang, Z.; Sun, D.; Tang, Y.; Zhu, R.; Li, X.; Gruda, N.; Dong, J.; Duan, Z. Plastic shed soil salinity in China: Current status and next steps. J. Clean. Prod. 2021, 296, 126453. [Google Scholar] [CrossRef]
- Li, J.; Wan, X.; Liu, X.; Chen, Y.; Slaughter, L.C.; Weindorf, D.C.; Dong, Y. Changes in soil physical and chemical characteristics in intensively cultivated greenhouse vegetable fields in North China. Soil Tillage Res. 2019, 195, 104366. [Google Scholar] [CrossRef]
- Wu, R.; Sun, H.; Xue, J.; Yan, D.; Liu, Y.; Gui, D.; Wang, X.; Yang, J. Acceleration of soil salinity accumulation and soil degradation due to greenhouse cultivation: A survey of farmers’ practices in China. Environ. Monit. Assess. 2020, 192, 399. [Google Scholar] [CrossRef]
- Han, J.; Luo, Y.; Yang, L.; Liu, X.; Wu, L.; Xu, J. Acidification and salinization of soils with different initial pH under green-house vegetable cultivation. J. Soils Sediments. 2014, 14, 1683–1692. [Google Scholar] [CrossRef]
- Pavlů, L.; Borůvka, L.; Drábek, O.; Nikodem, A. Effect of natural and anthropogenic acidification on aluminium distribution in forest soils of two regions in the Czech Republic. J. For. Res. 2021, 32, 363–370. [Google Scholar] [CrossRef]
- Lv, H.; Zhao, Y.; Wang, Y.; Wan, L.; Wang, J.; Butterbach-Bahl, K.; Lin, S. Conventional flooding irrigation and over fertilization drives soil pH decrease not only in the top- but also in subsoil layers in solar greenhouse vegetable production systems. Geoderma 2020, 363, 114156. [Google Scholar] [CrossRef]
- Kianpoor Kalkhajeh, Y.; Huang, B.; Hu, W.; Ma, C.; Gao, H.; Thompson, M.L.; Bruun Hansen, H.C. Environmental soil quality and vegetable safety under current greenhouse vegetable production management in China. Agric. Ecosyst. Environ. 2021, 307, 107230. [Google Scholar] [CrossRef]
- Li, T.; Yu, L.; Wu, Y.; Wan, G.; Li, J. Secondary Salinization of Greenhouse Vegetable Soils and Its Affecting Factors in Shandong Province of China. Acta Pedol. Sin. 2018, 55, 100–110. [Google Scholar]
- Cai, Z. Scientific and Technological Issues of Nutrient Management under Greenhouse Cultivation in China. Acta Pedol. Sin. 2019, 56, 36–43. [Google Scholar]
- Li, D.L.; Ren, Z.J.; Yan, L.M.; Bian, X.Y.; Zhao, Z.T. Pottiaceae in Shandong Province, China. J. Shandong Univ. (Sci. Ed.) 2016, 51, 11–15. [Google Scholar] [CrossRef]
- Xiong, Y.; Li, Q. Soils in China, 2nd ed.; Science Press: Beijing, China, 1987. [Google Scholar]
- Bao, S. Soil Agrochemical Analysis, 3rd ed.; China Agriculture Press: Beijing, China, 2000. [Google Scholar]
- Lever, J.; Krzywinski, M.; Altman, N. Principal component analysis. Nat. Methods 2017, 14, 641–642. [Google Scholar] [CrossRef]
- Guo, Y.; Geng, J.; Cheng, S.; Fang, H.; Li, X.; Yang, Y.; Li, Y.; Zhou, Y. Soil acidification and ammonia-oxidizing archaeal abundance dominate the contrasting responses of soil N2O emissions to NH4+ and NO3− enrichment in a subtropical plantation. Eur. J. Soil Biol. 2023, 116, 103491. [Google Scholar] [CrossRef]
- Ren, M.; Li, C.; Gao, X.; Niu, H.; Cai, Y.; Wen, H.; Yang, M.; Siddique, K.H.M.; Zhao, X. High nutrients surplus led to deep soil nitrate accumulation and acidification after cropland conversion to apple orchards on the Loess Plateau, China. Agric. Ecosyst. Environ. 2023, 351, 108482. [Google Scholar] [CrossRef]
- Huang, C.; Xu, J. Soil Science, 3rd ed.; China Agriculture Press: Beijing, China, 2010. [Google Scholar]
- Hong, J. Soil Pollution and Prevention, 4th ed.; China Agriculture Press: Beijing, China, 2019. [Google Scholar]
- Chen, L.; Hao, J.M.; Chen, A.Q.; Li, M.; Gao, Y.; Guan, Q.C.; Wang, H.L. Study on the water conservation function of cultivated land based on dualistic water cycle in Huang-Huai-Hai Plain. Acta Ecol. Sin. 2017, 37, 5871–5881. [Google Scholar] [CrossRef][Green Version]
- Zhang, G.; Yang, Z.; Li, G.; Zheng, X.; Mao, C. Hydrogeochemical characterization of Tai’an Pumped Storage Power Station reservoir and the implications for Reservoir Water Leakage. IOP Conf. Ser. Earth Environ. Sci. 2021, 826, 012018. [Google Scholar] [CrossRef]
- Litalien, A.; Zeeb, B. Curing the earth: A review of anthropogenic soil salinization and plant-based strategies for sustainable mitigation. Sci. Total Environ. 2020, 698, 134235. [Google Scholar] [CrossRef] [PubMed]
- Audette, Y.; Smith, D.S.; Parsons, C.T.; Chen, W.; Rezanezhad, F.; Van Cappellen, P. Phosphorus binding to soil organic matter via ternary complexes with calcium. Chemosphere 2020, 260, 127624. [Google Scholar] [CrossRef]
- Wan, L.; Lv, H.; Qasim, W.; Xia, L.; Yao, Z.; Hu, J.; Zhao, Y.; Ding, X.; Zheng, X.; Li, G.; et al. Heavy metal and nutrient concentrations in top- and sub-soils of greenhouses and arable fields in East China—Effects of cultivation years, management, and shelter. Environ. Pollut. 2022, 307, 119494. [Google Scholar] [CrossRef]
- Ma, Q.; Qian, Y.; Yu, Q.; Cao, Y.; Tao, R.; Zhu, M.; Ding, J.; Li, C.; Guo, W.; Zhu, X. Controlled-release nitrogen fertilizer application mitigated N losses and modified microbial community while improving wheat yield and N use efficiency. Agric. Ecosyst. Environ. 2023, 349, 108445. [Google Scholar] [CrossRef]
- Yuan, M.; Gu, Z.; Xia, S.; Zhao, J.; Wang, X. In-situ remediation of zinc contaminated soil using phosphorus recovery product: Hydroxyapatite/calcium silicate hydrate (HAP/C–S–H). Chemosphere 2022, 286, 131664. [Google Scholar] [CrossRef]
- Wang, R.; Zhong, Y.; Li, H.; Shi, W.; Li, Y. Research Progresses on Environmental and Agriculture Thresholds of Soil Phosphorus in High-input Vegetable Fields. Soils 2022, 54, 1–8. [Google Scholar] [CrossRef]
- Ma, J.; Ma, Y.; Wei, R.; Chen, Y.; Weng, L.; Ouyang, X.; Li, Y. Phosphorus transport in different soil types and the contribution of control factors to phosphorus retardation. Chemosphere 2021, 276, 130012. [Google Scholar] [CrossRef] [PubMed]
- Wakeel, A.; Ishfaq, M. Potash Use and Dynamics in Agriculture; Springer: Singapore, 2022. [Google Scholar] [CrossRef]
- Neina, D. The Role of Soil pH in Plant Nutrition and Soil Remediation. Appl. Environ. Soil Sci. 2019, 5794869. [Google Scholar] [CrossRef]
- Fan, Y.N.; Zhang, Y.; Chen, Z.; Wang, X.; Huang, B. Comprehensive assessments of soil fertility and environmental quality in plastic greenhouse production systems. Geoderma 2021, 385, 114899. [Google Scholar] [CrossRef]
- Chen, Y.; Huang, L.; Zhang, R.; Ma, J.; Guo, Z.; Zhao, J.; Weng, L.; Li, Y. Retardation factors in controlling the transport of inorganic, organic, and particulate phosphorus in fluvo-aquic soil. Ecotoxicol. Environ. Saf. 2023, 249, 114402. [Google Scholar] [CrossRef]
- Sun, X.; Li, Q.; Kong, X.; Cai, W.; Zhang, B.; Lei, M. Spatial Characteristics and Obstacle Factors of Cultivated Land Quality in an Intensive Agricultural Region of the North China Plain. Land 2023, 12, 1552. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, X.; Wang, J.; Shi, H.; Chen, N.; Hu, Q. Improved yield-salinity relationship considering salt and root distribution dynamics. Eur. J. Agron. 2023, 151, 127003. [Google Scholar] [CrossRef]
- Fan, Y.N.; Zhang, Y.; Hess, F.; Huang, B.; Chen, Z. Nutrient balance and soil changes in plastic greenhouse vegetable production. Nutr. Cycl. Agroecosyst. 2020, 117, 77–92. [Google Scholar] [CrossRef]



| Indicators | Content |
|---|---|
| pH Organic matter | 6.4 |
| 0.75% | |
| Alkaline hydrolyzable nitrogen | 45.3 mg kg−1 |
| Total nitrogen | 0.50 g kg−1 |
| Available phosphorus | 4.50 mg kg−1 |
| Total phosphorus | 0.80 g kg−1 |
| Available potassium | 72.0 mg kg−1 |
| Cation exchange capacity (CEC) | 16.9 cmol kg−1 |
| Soil salt base saturation (BS) | 98.4% |
| Measuring Items | Measuring Methods |
|---|---|
| Alkaline hydrolyzable nitrogen | NaOH hydrolysis—boric acid absorption—acid titration |
| NO3−-N, NH4+-N | KCl extraction–spectrophotometric method |
| Available phosphorus | NaHCO3 extraction, Mo-Sb Anti-spectrophotometric |
| Available potassium | NH4Ac extraction–flame photometry |
| Total nitrogen | H2SO4 digestion–Kjeldahl method |
| Total phosphorus | H2SO4-HClO4 digestion, Mo-Sb Anti-spectrophotometric |
| Items | Winter-Warm Heliogreenhouses | Plastic Tunnels | ||||
|---|---|---|---|---|---|---|
| Surface | 0–20 cm | 20–40 cm | Surface | 0–20 cm | 20–40 cm | |
| Mean (SD) | 6.13 ± 0.56 | 6.24 ± 0.53 | 6.29 ± 0.58 | 6.35 ± 0.44 | 6.08 ± 0.61 | 5.78 ± 0.70 |
| pH < 5.5 | 16.0% | 7.41% | 9.88% | 7.69% | 19.2% | 50.0% |
| 5.5 ≤ pH < 7.0 | 77.8% | 84.0% | 80.2% | 88.5% | 77.0% | 42.3% |
| pH ≥ 7.0 | 6.17% | 8.64% | 9.88% | 3.85% | 3.85% | 7.69% |
| Saturated Leachate EC25 (dS m−1) | Salinity (g kg−1) | Degree of Salinity | Plant Response |
|---|---|---|---|
| 0–2 | <1.0 | Non-Saline Soils | No salt damage to crops |
| 2–4 | 1–3 | Saline Soils | Crop yields for species highly susceptible to salinity stress are likely to be negatively impacted |
| 4–8 | 3–5 | Moderately Saline Soils | Yields of salt-sensitive crops are affected, but salt-tolerant crops (e.g., alfalfa, cotton, sugar beets, sorghum, cereals) are not significantly affected |
| 8–16 | 5–10 | Heavy Saline Soils | While only salt-tolerant crops were successfully harvested, seed germination was negatively impacted, resulting in seedling deficits and substantial yield reductions |
| >16 | >10 | Extremely Saline Soils | Plant growth is restricted to highly salt-tolerant species, such as salt-tolerant pasture grasses, shrubs, and trees |
| Components | Initial Eigenvalues | Extract Sums of Squared Loadings | ||||
|---|---|---|---|---|---|---|
| Total | Percentage of Variance (%) | Cumulative (%) | Total | Percentage of Variance (%) | Cumulative (%) | |
| 1 | 3.849 | 54.99 | 54.99 | 3.849 | 54.99 | 54.99 |
| 2 | 1.129 | 16.13 | 71.13 | 1.129 | 16.13 | 71.13 |
| 3 | 0.916 | 13.09 | 84.21 | 0.916 | 13.09 | 84.21 |
| 4 | 0.600 | 8.568 | 92.78 | |||
| 5 | 0.346 | 4.947 | 97.73 | |||
| 6 | 0.152 | 2.170 | 99.90 | |||
| 7 | 0.007 | 0.101 | 100.0 | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhan, N.; Yang, H.; Li, J.; Shi, X.; Yang, B.; Sun, Y.; Guo, G.; Cui, X. Spatial Distribution Characteristics and Relationships of Salt-Based Ions and Nutrients in Old Protected Vegetable Fields. Horticulturae 2025, 11, 126. https://doi.org/10.3390/horticulturae11020126
Zhan N, Yang H, Li J, Shi X, Yang B, Sun Y, Guo G, Cui X. Spatial Distribution Characteristics and Relationships of Salt-Based Ions and Nutrients in Old Protected Vegetable Fields. Horticulturae. 2025; 11(2):126. https://doi.org/10.3390/horticulturae11020126
Chicago/Turabian StyleZhan, Nanbiao, Haotian Yang, Jiayang Li, Xiaodi Shi, Binhao Yang, Yuhang Sun, Gengzi Guo, and Xiumin Cui. 2025. "Spatial Distribution Characteristics and Relationships of Salt-Based Ions and Nutrients in Old Protected Vegetable Fields" Horticulturae 11, no. 2: 126. https://doi.org/10.3390/horticulturae11020126
APA StyleZhan, N., Yang, H., Li, J., Shi, X., Yang, B., Sun, Y., Guo, G., & Cui, X. (2025). Spatial Distribution Characteristics and Relationships of Salt-Based Ions and Nutrients in Old Protected Vegetable Fields. Horticulturae, 11(2), 126. https://doi.org/10.3390/horticulturae11020126





