Silicon Application Methods Differentially Modulate Nutrient Uptake and Morphophysiology in Passiflora edulis Seedlings Under Salt Stress
Abstract
1. Introduction
2. Materials and Methods
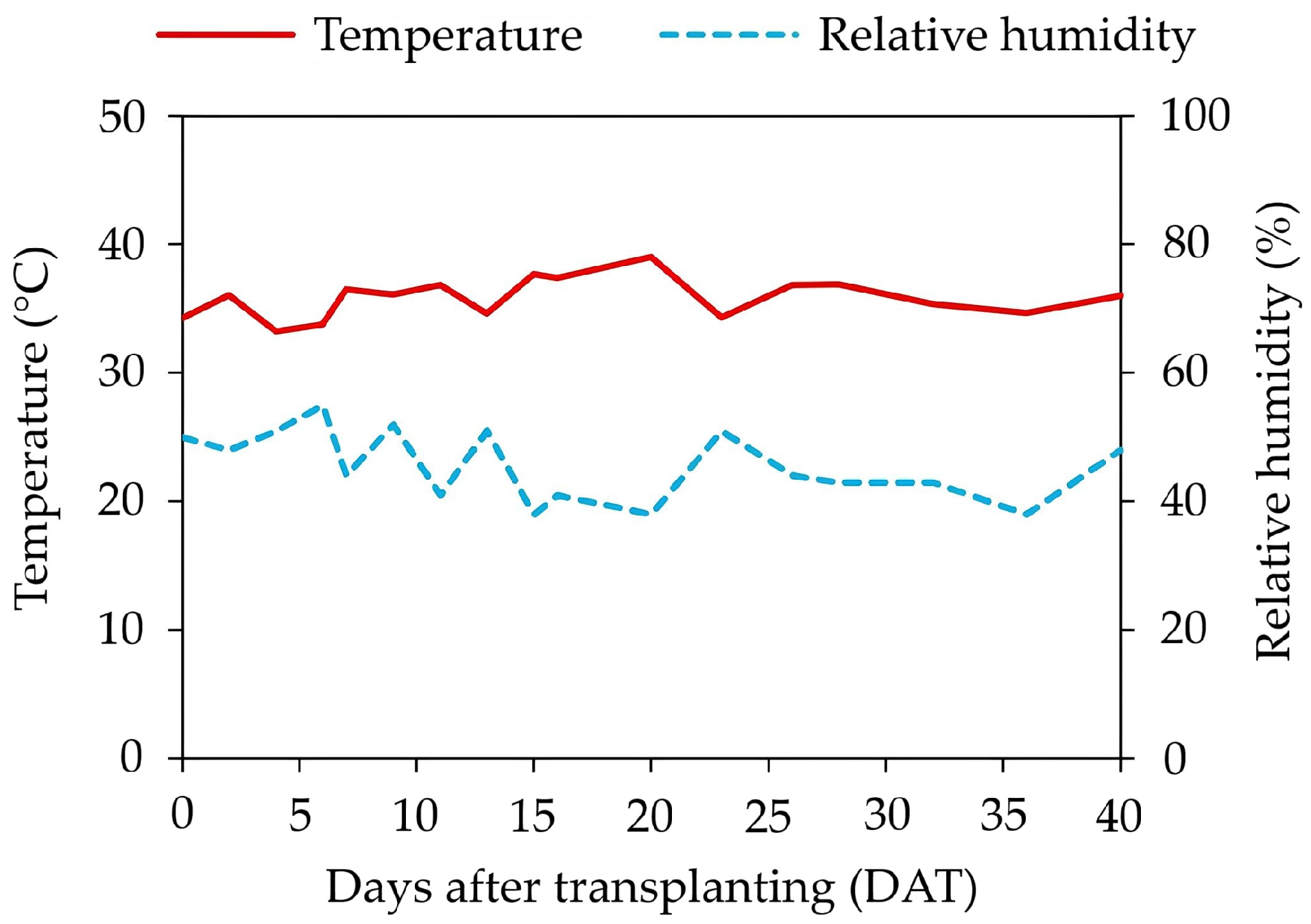
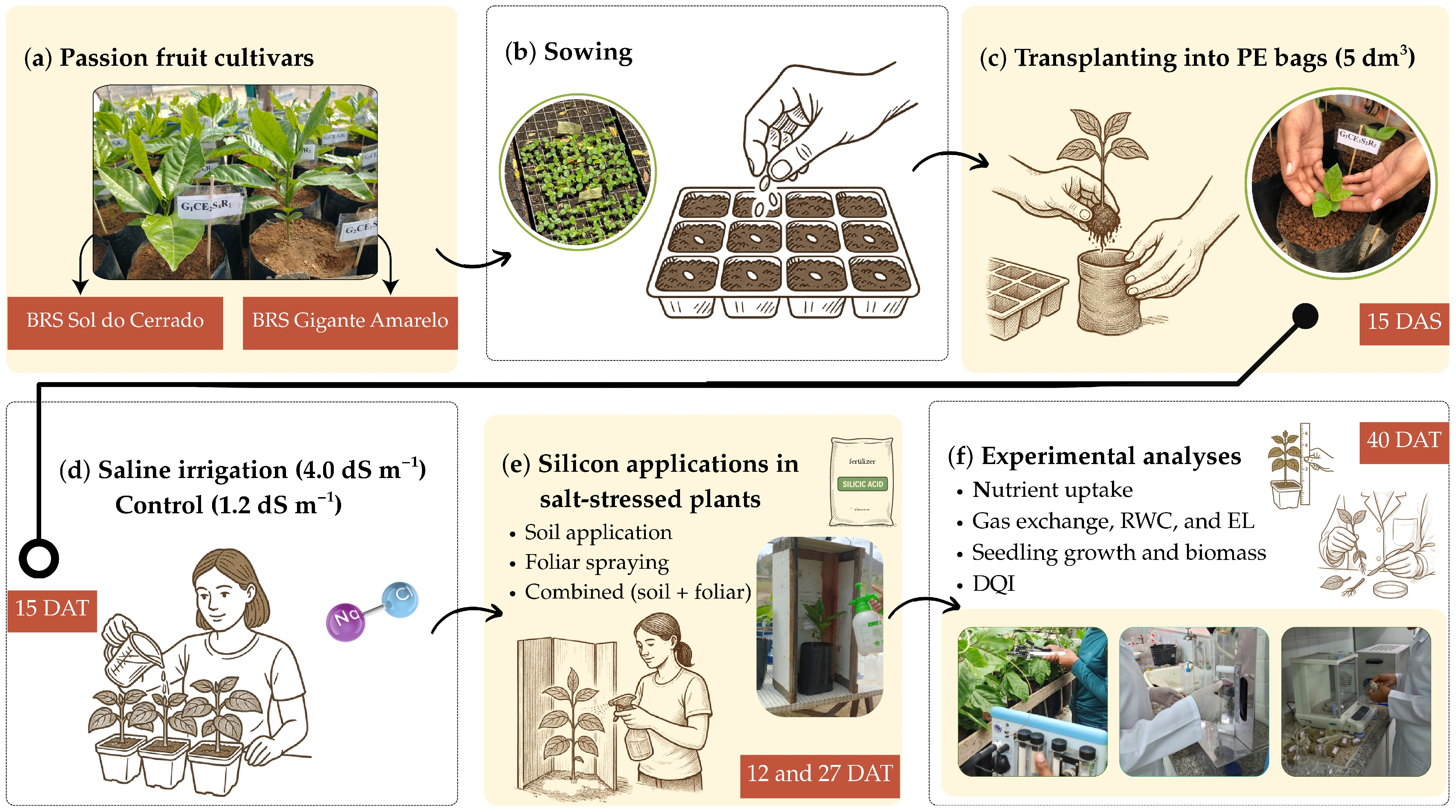
2.1. Experimental Conditions
2.2. Treatments and Experimental Design
2.3. Experimental Analyses
2.3.1. Foliar Nutrient Content Analysis
2.3.2. Physiological Traits Analysis
2.3.3. Seedling Growth and Quality Analysis
2.4. Statistical Analysis
3. Results
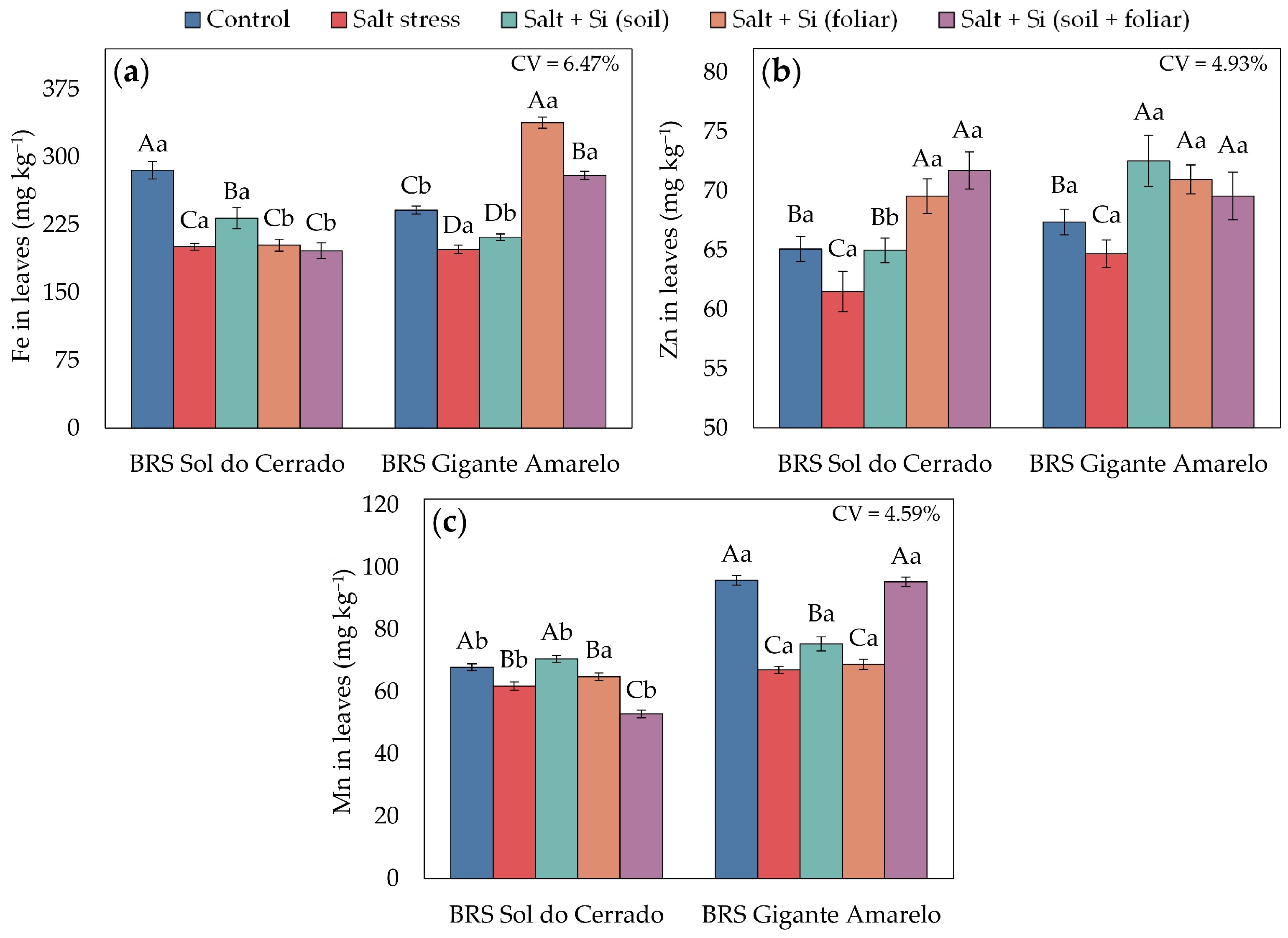
3.1. Foliar Mineral Element Content
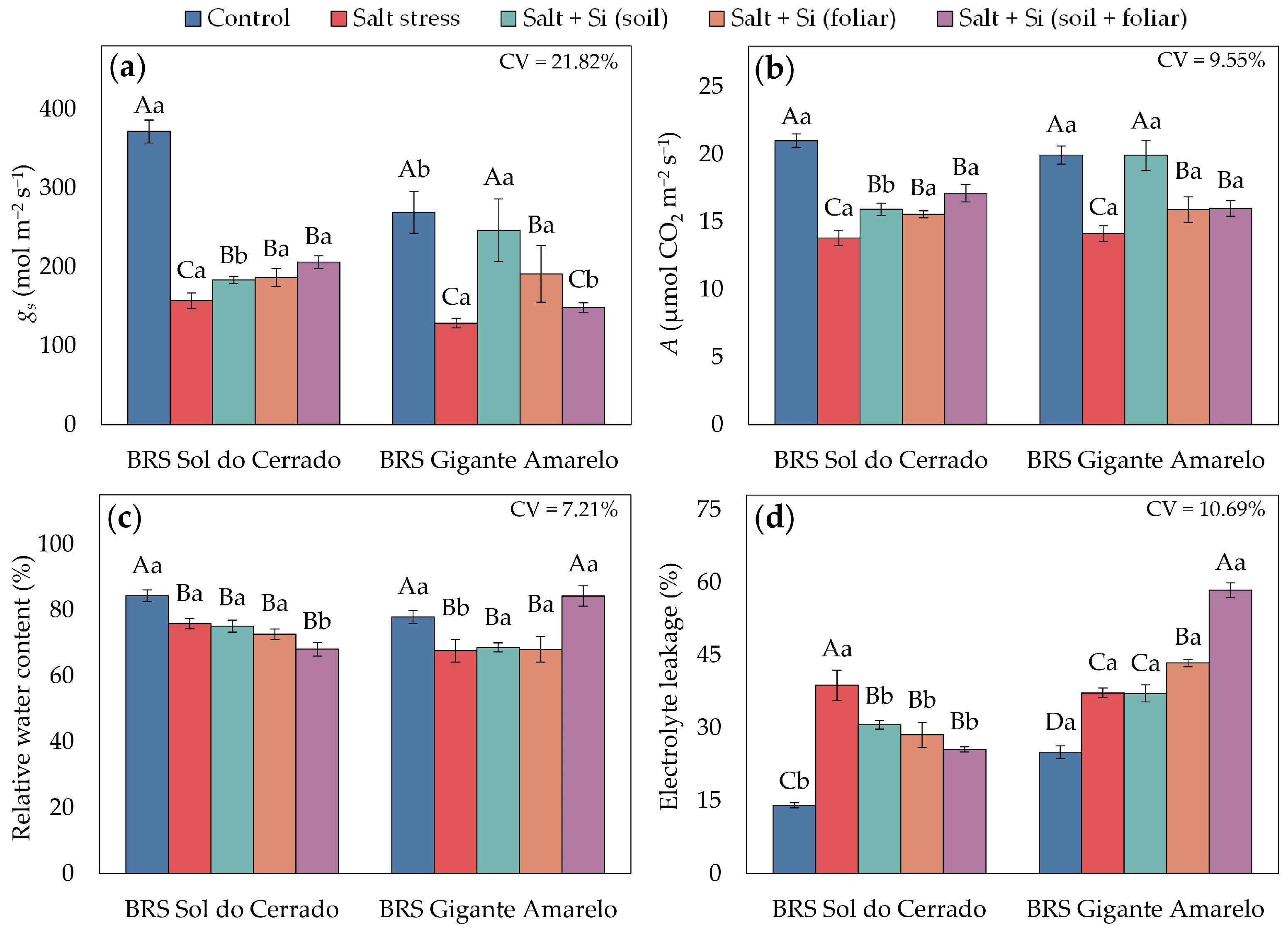
3.2. Physiological Traits
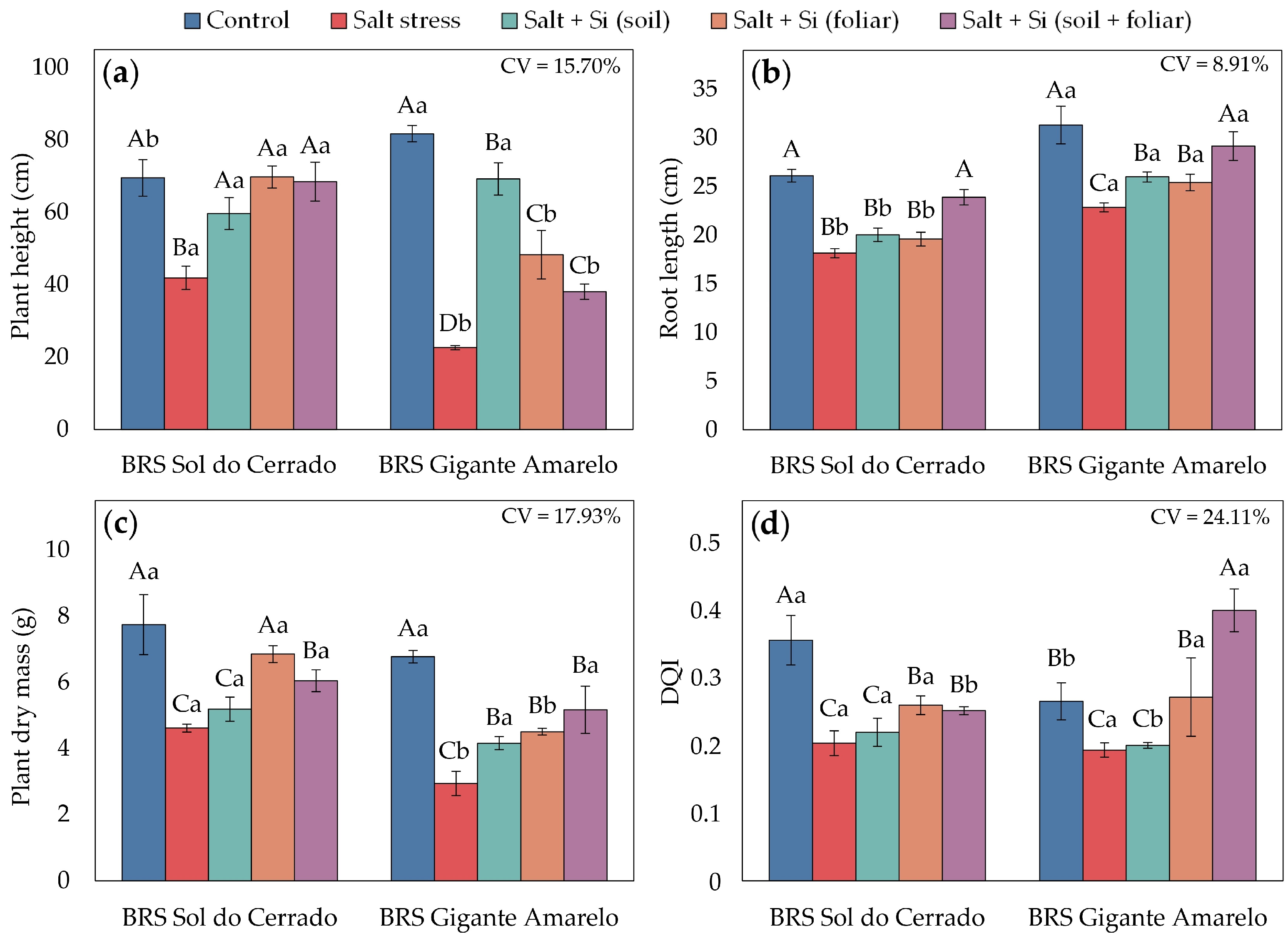
3.3. Seedling Growth and Quality
3.4. PCA
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| DQI | Dickson Quality Index |
| DAT | Days after transplanting |
| DAS | Days after sowing |
| PE | Polyethylene |
| RWC | Relative water content |
| EL | Electrolyte leakage |
| EC | Electrical conductivity |
| gs | Stomatal conductance |
| A | CO2 assimilation rate |
| IRGA | Infrared gas analyzer |
| FM | Fresh mass |
| TM | Turgid mass |
| DM | Dry mass |
| PH | Plant height |
| RL | Root length |
| PDM | Plant dry mass |
| SD | Stem diameter |
| SDM | Shoot dry mass |
| RDM | Root dry mass |
| ANOVA | Analysis of variance |
| PCA | Principal component analysis |
| CV | Coefficient of variation |
References
- IBGE—Brazilian Institute of Geography and Statistics. Passion Fruit Production; IBGE: Rio de Janeiro, Brazil, 2025. Available online: https://www.ibge.gov.br/explica/producao-agropecuaria/maracuja/br (accessed on 18 October 2025). (In Portuguese)
- Pereira, Z.C.; Cruz, J.M.A.; Corrêa, R.F.; Sanches, E.A.; Campelo, P.H.; Bezerra, J.A. Passion Fruit (Passiflora spp.) Pulp: A Review on Bioactive Properties, Health Benefits and Technological Potential. Food Res. Int. 2023, 166, 112626. [Google Scholar] [CrossRef] [PubMed]
- Ayers, R.S.; Westcot, D.W. The Quality of Water in Agriculture, 2nd ed.; UFPB: Campina Grande, Brazil, 1999; 153p. (In Portuguese) [Google Scholar]
- Almeida, C.J.S.; Dantas, J.S.; Mesquita, E.F.; Sousa, C.S.; Soares, V.C.S.; Diniz, J.P.C.; Pereira, R.F.; Lins, L.K.S.; Nogueira, V.F.B.; Silva Filho, I.P. Silicon as a Salt Stress Mitigator in Yellow Passion Fruit Seedlings. Agric. Res. Trop. 2024, 54, e80305. [Google Scholar] [CrossRef]
- Santos, I.S.; Jesus, O.N.; Sampaio, S.R.; Gonçalves, Z.S.; Soares, T.L.; Ferreira, J.R.; Lima, L.K.S. Salt Tolerance Strategy in Passion Fruit Genotypes During Germination and Seedling Growth and Spectrophotometric Quantification of Hydrogen Peroxide (H2O2). Sci. Hortic. 2024, 338, 113818. [Google Scholar] [CrossRef]
- Silva, A.C.Z.; Pereira, R.F.; Ferreira, R.S.; Alves, S.B.; Sousa, F.S.; Rodrigues, S.S.; Brito Neto, J.F.; Melo, A.S.; Silva, R.M.; Mesquita, E.F. Silicon and Potassium Synergistically Alleviate Salt Stress and Enhance Soil Fertility, Nutrition, and Physiology of Passion Fruit Seedlings. Front. Plant Sci. 2025, 16, 1685221. [Google Scholar] [CrossRef]
- Alkharabsheh, H.M.; Seleiman, M.F.; Hewedy, O.A.; Battaglia, M.L.; Jalal, R.S.; Alhammad, B.A.; Schillaci, C.; Ali, N.; Al-Doss, A. Field Crop Responses and Management Strategies to Mitigate Soil Salinity in Modern Agriculture: A Review. Agronomy 2021, 11, 2299. [Google Scholar] [CrossRef]
- Fu, H.; Yang, Y. How Plants Tolerate Salt Stress. Curr. Issues Mol. Biol. 2023, 45, 5914–5934. [Google Scholar] [CrossRef]
- Shabbir, S.; Nazir, Q.; Saleem, I.; Naz, R.; Azhar, S.; Rafay, M.; Usman, M. Soil Salinity Hinders Plant Growth and Development and Its Remediation: A Review. J. Agric. Res. 2023, 61, 189–200. [Google Scholar] [CrossRef]
- Xiao, F.; Zhou, H. Plant Salt Response: Perception, Signaling, and Tolerance. Front. Plant Sci. 2023, 13, 1053699. [Google Scholar] [CrossRef]
- Diniz, G.L.; Nobre, R.G.; Lima, G.S.; Soares, L.A.A.; Gheyi, H.R. Irrigation with Saline Water and Silicate Fertilization in the Cultivation of ‘Gigante Amarelo’ Passion Fruit. Rev. Caatinga 2021, 34, 199–207. [Google Scholar] [CrossRef]
- Sá, J.R.; Toledo, F.H.S.F.; Mariño, Y.A.; Soares, C.R.F.S.; Ferreira, E.V.O. Growth and Nutrition of Passiflora edulis Submitted to Saline Stress After Silicon Application. Rev. Bras. Frutic. 2021, 43, e-057. [Google Scholar] [CrossRef]
- Queiroz, L.L.G.; Mesquita, E.F.; Sousa, C.S.; Pereira, R.F.; Diniz, J.P.C.; Melo, A.S.; Alencar, R.S.; Dias, G.F.; Soares, V.C.S.; Mesquita, F.O.; et al. Foliar Silicon Alleviates Water Deficit in Cowpea by Enhancing Nutrient Uptake, Proline Accumulation, and Antioxidant Activity. Plants 2025, 14, 1241. [Google Scholar] [CrossRef]
- Khan, A.; Khan, A.L.; Muneer, S.; Kim, Y.H.; Al-Rawahi, A.; Al-Harrasi, A. Silicon and Salinity: Crosstalk in Crop-Mediated Stress Tolerance Mechanisms. Front. Plant Sci. 2019, 10, 1429. [Google Scholar] [CrossRef] [PubMed]
- Farouk, S.; Elhindi, K.M.; Alotaibi, M.A. Silicon Supplementation Mitigates Salinity Stress on Ocimum basilicum L. Via Improving Water Balance, Ion Homeostasis, and Antioxidant Defense System. Ecotoxicol. Environ. Saf. 2020, 206, 111396. [Google Scholar] [CrossRef] [PubMed]
- Rahmani, V.; Dehnavi, M.M.; Balouchi, H.; Yadavi, A.; Hamidian, M. Silicon Can Improve Nutrient Uptake and Performance of Black Cumin Under Drought and Salinity Stresses. Commun. Soil Sci. Plant Anal. 2022, 54, 297–310. [Google Scholar] [CrossRef]
- Singh, P.; Kumar, V.; Sharma, A. Interaction of Silicon with Cell Wall Components in Plants: A Review. J. Appl. Nat. Sci. 2023, 15, 480–497. [Google Scholar] [CrossRef]
- Gharbi, P.; Amiri, J.; Mahna, N.; Naseri, L.; Sadaghiani, M.R. Silicon-Induced Mitigation of Salt Stress in GF677 and GN15 RootStocks: Insights into Physiological, Biochemical, and Molecular Mechanisms. BMC Plant Biol. 2025, 25, 719. [Google Scholar] [CrossRef]
- Greger, M.; Landberg, T.; Vaculík, M. Silicon Influences Soil Availability and Accumulation of Mineral Nutrients in Various Plant Species. Plants 2018, 7, 41. [Google Scholar] [CrossRef]
- Pavlovic, J.; Kostic, L.; Bosnic, P.; Kirkby, E.A.; Nikolic, M. Interactions of Silicon with Essential and Beneficial Elements in Plants. Front. Plant Sci. 2021, 23, 697592. [Google Scholar] [CrossRef]
- Tripathi, P.; Subedi, S.; Khan, A.L.; Chung, Y.S.; Kim, Y. Silicon Effects on The Root System of Diverse Crop Species Using Root Phenotyping Technology. Plants 2021, 10, 885. [Google Scholar] [CrossRef]
- Ma, J.F.; Yamaji, N. A Cooperative System of Silicon Transport in Plants. Trends Plant Sci. 2015, 20, 435–442. [Google Scholar] [CrossRef]
- Mitani-Ueno, N.; Yamaji, N.; Huang, S.; Yoshioka, Y.; Miyaji, T.; Ma, J.F. A Silicon Transporter Gene Required for Healthy Growth of Rice on Land. Nat. Commun. 2023, 14, 6522. [Google Scholar] [CrossRef]
- Verma, K.K.; Song, X.-P.; Li, D.-M.; Singh, M.; Wu, J.-M.; Singh, R.K.; Sharma, A.; Zhang, B.-Q.; Li, Y.-R. Silicon and Soil Microorganisms Improve Rhizospheric Soil Health with Bacterial Community, Plant growth, Performance and Yield. Plant Signal. Behav. 2022, 17, 2104004. [Google Scholar] [CrossRef] [PubMed]
- Dutra, A.F.; Leite, M.R.L.; Melo, C.C.F.; Amaral, D.S.; Silva, J.L.F.; Prado, R.M.; Piccolo, M.S.; Miranda, R.S.; Silva Júnior, G.B.; Sousa, T.K.S.A.; et al. Soil and Foliar Si Fertilization Alters Elemental Stoichiometry and Increases Yield of Sugarcane Cultivars. Sci. Rep. 2023, 13, 16040. [Google Scholar] [CrossRef] [PubMed]
- Babu, P.M.; Thakuria, D.; Majumdar, S.; Kalita, H.C. Sources and Application Methods of Silicon for Rice in Acid Soil. Silicon 2025, 17, 499–515. [Google Scholar] [CrossRef]
- Baioui, R.; Hidri, R.; Zouari, S.; Hajji, M.; Falouti, M.; Bounaouara, F.; Borni, M.; Hamzaoui, A.H.; Abdelly, C.; Zorrig, W.; et al. Foliar Application of Silicon: An Innovative and Effective Strategy for Enhancing Tomato Yield in Hydroponic Systems. Agronomy 2025, 15, 1553. [Google Scholar] [CrossRef]
- Tedesco, M.J.; Gianello, C.; Bissani, C.A.; Bohnen, H.; Volkweiss, S.J. Soil, Plant, and Other Material Analysis, 2nd ed.; UFRS: Porto Alegre, Brazil, 1995; 174p. (In Portuguese) [Google Scholar]
- Meneghetti, A.M. Manual of Procedures for Sampling and Chemical Analysis of Plants, Soil, and Fertilizers; EDUTFPR: Curitiba, Brazil, 2018; 252p. (In Portuguese) [Google Scholar]
- Dickson, A.; Leaf, A.L.; Hosner, J.F. Quality Appraisal of White Spruce and White Pine Seedling Stock in Nurseries. For. Chron. 1960, 36, 10–13. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2025; Available online: https://www.r-project.org (accessed on 13 October 2025).
- Liu, C.; Jiang, X.; Yuan, Z. Plant Responses and Adaptations to Salt Stress: A Review. Horticulturae 2024, 10, 1221. [Google Scholar] [CrossRef]
- Dabravolski, S.A.; Isayenkov, S.V. The Physiological and Molecular Mechanisms of Silicon Action in Salt Stress Amelioration. Plants 2024, 13, 525. [Google Scholar] [CrossRef]
- Wadas, W.; Kondraciuk, T. The Role of Foliar-Applied Silicon in Improving the Growth and Productivity of Early Potatoes. Agriculture 2025, 15, 556. [Google Scholar] [CrossRef]
- Attia, E.A.; Elhawat, N. Combined Foliar and Soil Application of Silica Nanoparticles Enhances the Growth, Flowering Period and Flower Characteristics of Marigold (Tagetes erecta L.). Sci. Hortic. 2021, 282, 110015. [Google Scholar] [CrossRef]
- Souri, Z.; Khanna, K.; Karimi, N.; Ahmad, P. Silicon and Plants: Current Knowledge and Future Prospects. J. Plant Growth Regul. 2021, 40, 906–925. [Google Scholar] [CrossRef]
- Sheng, H.; Chen, S. Plant Silicon-Cell Wall Complexes: Identification, Model of Covalent Bond Formation and Biofunction. Plant Physiol. Biochem. 2020, 155, 13–19. [Google Scholar] [CrossRef]
- Yan, G.; Fan, X.; Zheng, W.; Gao, Z.; Yin, C.; Li, T.; Liang, Y. Silicon Alleviates Salt Stress-Induced Potassium Deficiency by Promoting Potassium Uptake and Translocation in Rice (Oryza sativa L.). J. Plant Physiol. 2021, 258, 153379. [Google Scholar] [CrossRef]
- Mahmood, M.Z.; Odeibat, H.A.; Ahmad, R.; Gatasheh, M.K.; Shahzad, M.; Abbasi, A.M. Low Apoplastic Na+ and Intracellular Ionic Homeostasis Confer Salinity Tolerance Upon Ca2SiO4 Chemigation in Zea mays L. Under Salt Stress. Front. Plant Sci. 2024, 14, 1268750. [Google Scholar] [CrossRef]
- Abbasi, H.; Jamil, M.; Haq, A.; Ali, S.; Ahmad, R.; Malik, Z.; Parveen, Z. Salt Stress Manifestation on Plants, Mechanism of Salt Tolerance and Potassium Role in Alleviating It: A Review. Zemdirb. Agric. 2016, 103, 229–238. [Google Scholar] [CrossRef]
- Li, H.; Zhu, Y.; Hu, Y.; Han, W.; Gong, H. Beneficial Effects of Silicon in Alleviating Salinity Stress of Tomato Seedlings Grown Under Sand Culture. Acta Physiol. Plant. 2015, 37, 71. [Google Scholar] [CrossRef]
- Shao, Y.; Li, S.; Gao, L.; Sun, C.; Hu, J.; Ullah, A.; Gao, J.; Li, X.; Jiang, D.; Cao, W.; et al. Magnesium Application Promotes Rubisco Activation and Contributes to High-Temperature Stress Alleviation in Wheat During the Grain Filling. Front. Plant Sci. 2021, 12, 675582. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, N.; Zhang, B.; Bozdar, B.; Chachar, S.; Rai, M.; Li, J.; Li, Y.; Hayat, F.; Chachar, Z.; Tu, P. The Power of Magnesium: Unlocking the Potential for Increased Yield, Quality, and Stress Tolerance of Horticultural Crops. Front. Plant Sci. 2023, 14, 1285512. [Google Scholar] [CrossRef] [PubMed]
- Souza, T.M.A.; Mendonça, V.; Sá, F.V.S.; Silva, M.J.; Dourado, C.S.T. Calcium Silicate as Salt Stress Attenuator in Seedlings of Yellow Passion Fruit cv. BRS GA1. Rev. Caatinga 2020, 33, 509–517. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ferreira, R.d.S.; Pereira, R.F.; Silva, A.C.Z.d.; Brito Neto, J.F.d.; Lins, L.K.S.; Sousa, C.d.S.; Diniz, J.P.C.; Fernandes, F.S.; Maia, O.S.; Gomes, E.A.; et al. Silicon Application Methods Differentially Modulate Nutrient Uptake and Morphophysiology in Passiflora edulis Seedlings Under Salt Stress. Horticulturae 2025, 11, 1396. https://doi.org/10.3390/horticulturae11111396
Ferreira RdS, Pereira RF, Silva ACZd, Brito Neto JFd, Lins LKS, Sousa CdS, Diniz JPC, Fernandes FS, Maia OS, Gomes EA, et al. Silicon Application Methods Differentially Modulate Nutrient Uptake and Morphophysiology in Passiflora edulis Seedlings Under Salt Stress. Horticulturae. 2025; 11(11):1396. https://doi.org/10.3390/horticulturae11111396
Chicago/Turabian StyleFerreira, Raquel da Silva, Rennan Fernandes Pereira, Alicia Camila Zeferino da Silva, José Félix de Brito Neto, Lays Klécia Silva Lins, Caio da Silva Sousa, José Paulo Costa Diniz, Fernanda Suassuna Fernandes, Orquídea Suassuna Maia, Elisângela Alencar Gomes, and et al. 2025. "Silicon Application Methods Differentially Modulate Nutrient Uptake and Morphophysiology in Passiflora edulis Seedlings Under Salt Stress" Horticulturae 11, no. 11: 1396. https://doi.org/10.3390/horticulturae11111396
APA StyleFerreira, R. d. S., Pereira, R. F., Silva, A. C. Z. d., Brito Neto, J. F. d., Lins, L. K. S., Sousa, C. d. S., Diniz, J. P. C., Fernandes, F. S., Maia, O. S., Gomes, E. A., Alves, R. A. S., Melo, A. S. d., & Mesquita, E. F. d. (2025). Silicon Application Methods Differentially Modulate Nutrient Uptake and Morphophysiology in Passiflora edulis Seedlings Under Salt Stress. Horticulturae, 11(11), 1396. https://doi.org/10.3390/horticulturae11111396











