Grafting Boosts Physiological Performance and Nutrient Acquisition of Cantaloupe Under Salt and Bicarbonate Stress in Soilless Culture
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Design and Location
2.2. Experimental Setup
2.3. Light-Absorbing Compounds in Photosynthetic Apparatus
2.4. Relative Chlorophyll Content (SPAD)
2.5. Plant Water Relations
2.6. Proline Concentration
2.7. Soluble Sugar Content
2.8. Protein Quantification
2.9. Vegetative Parameters
2.10. Nutrient Element Analysis
2.11. Statistical Analysis
3. Results
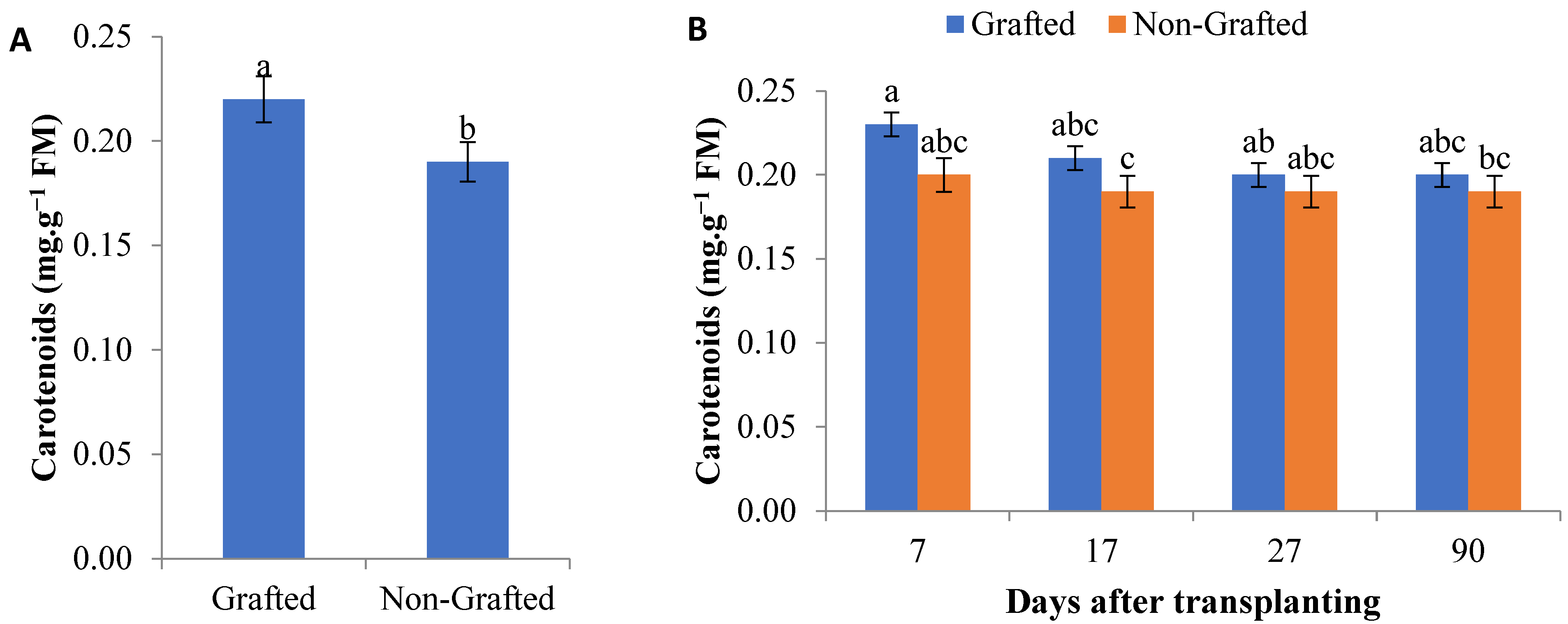
3.1. Photosynthetic Pigments
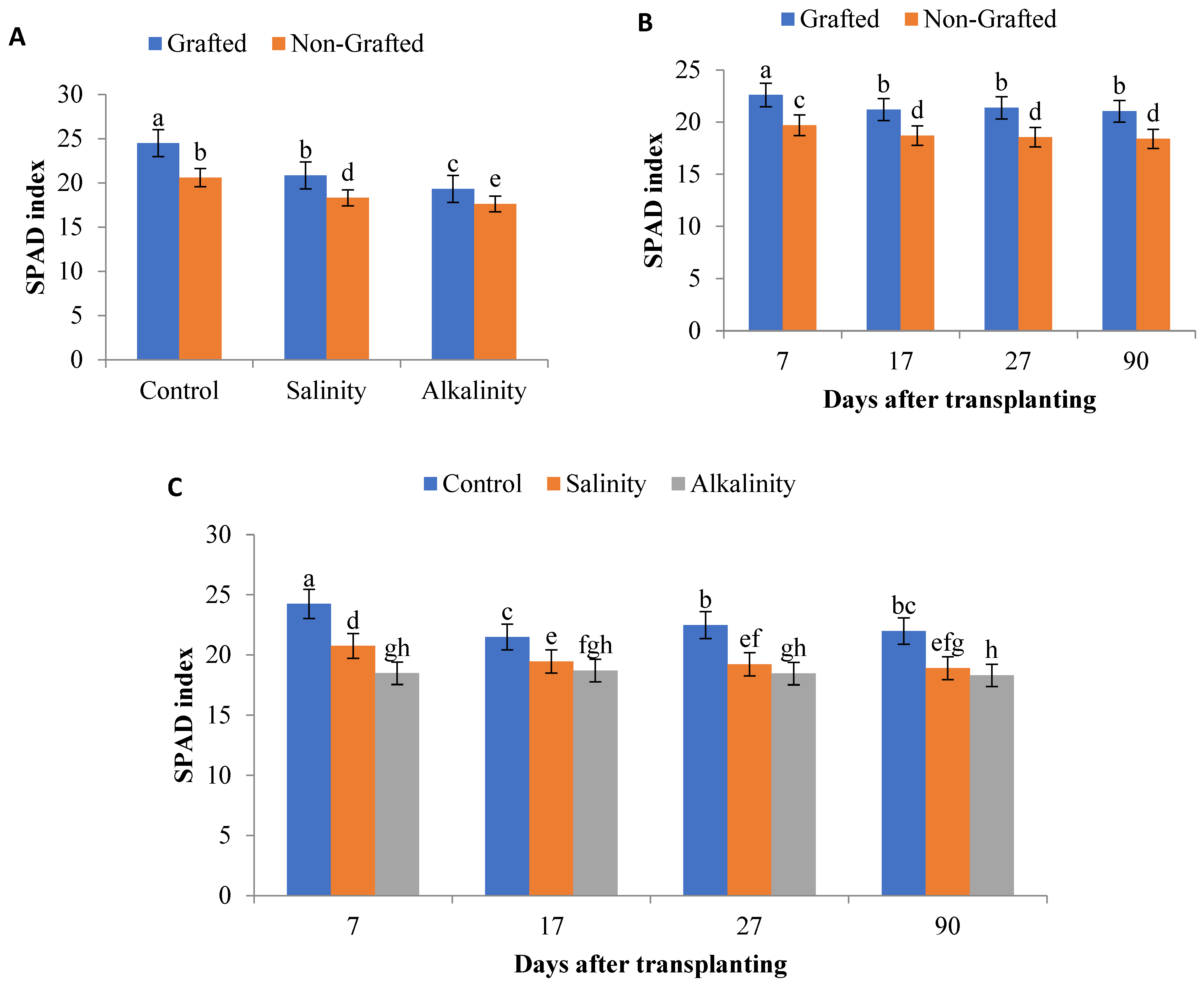
3.2. SPAD Index
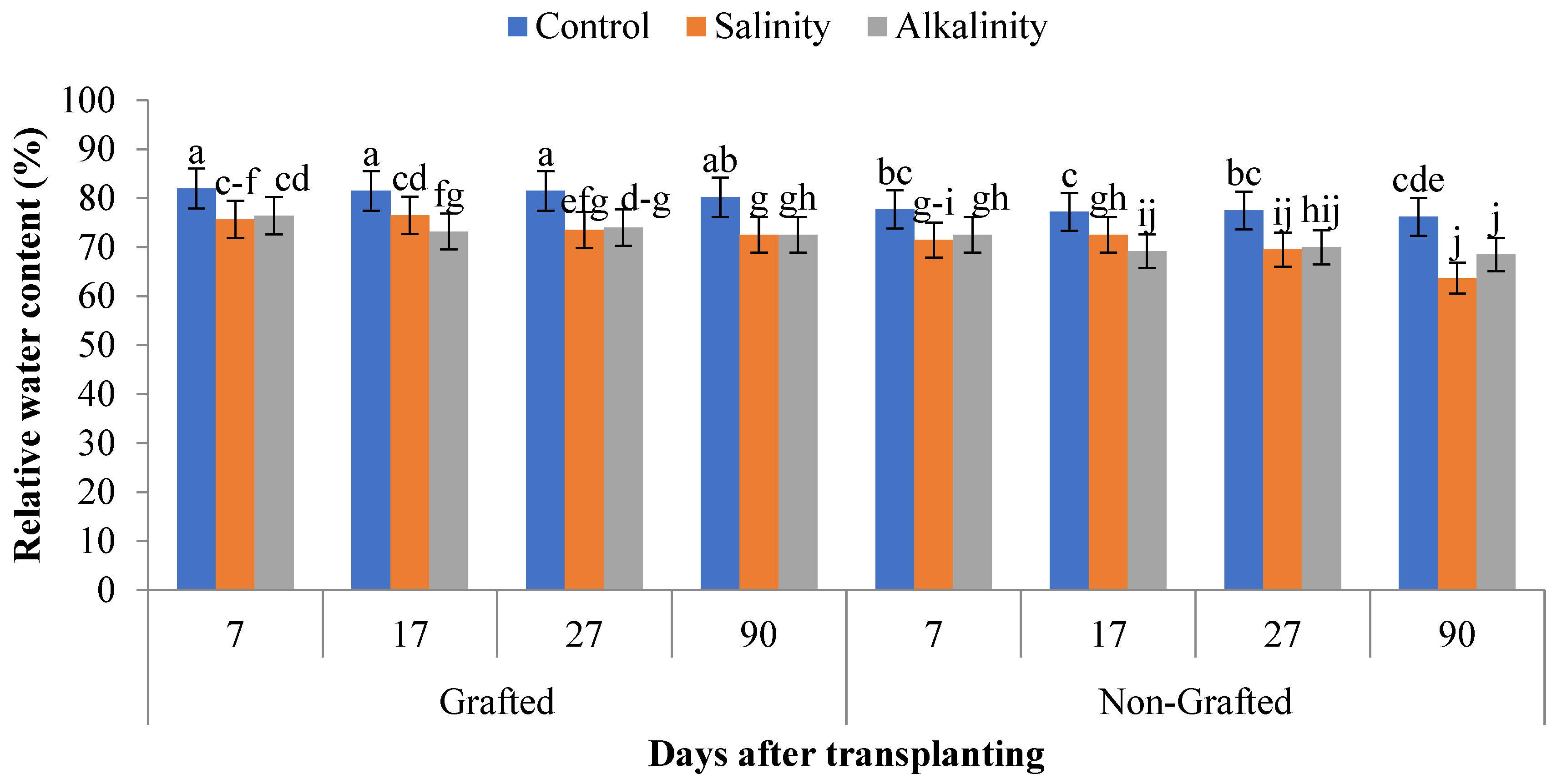
3.3. Relative Water Content
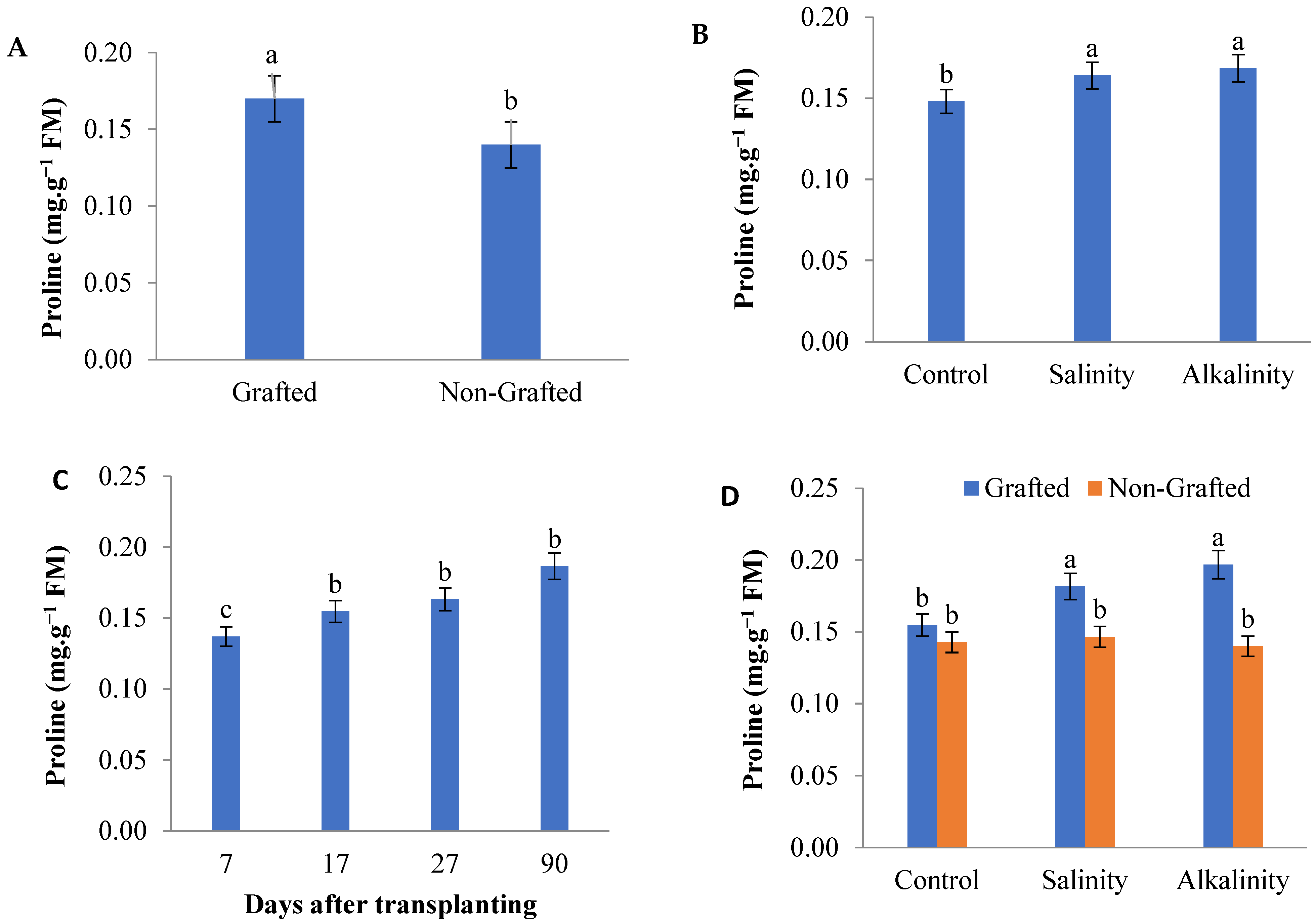
3.4. Proline Content
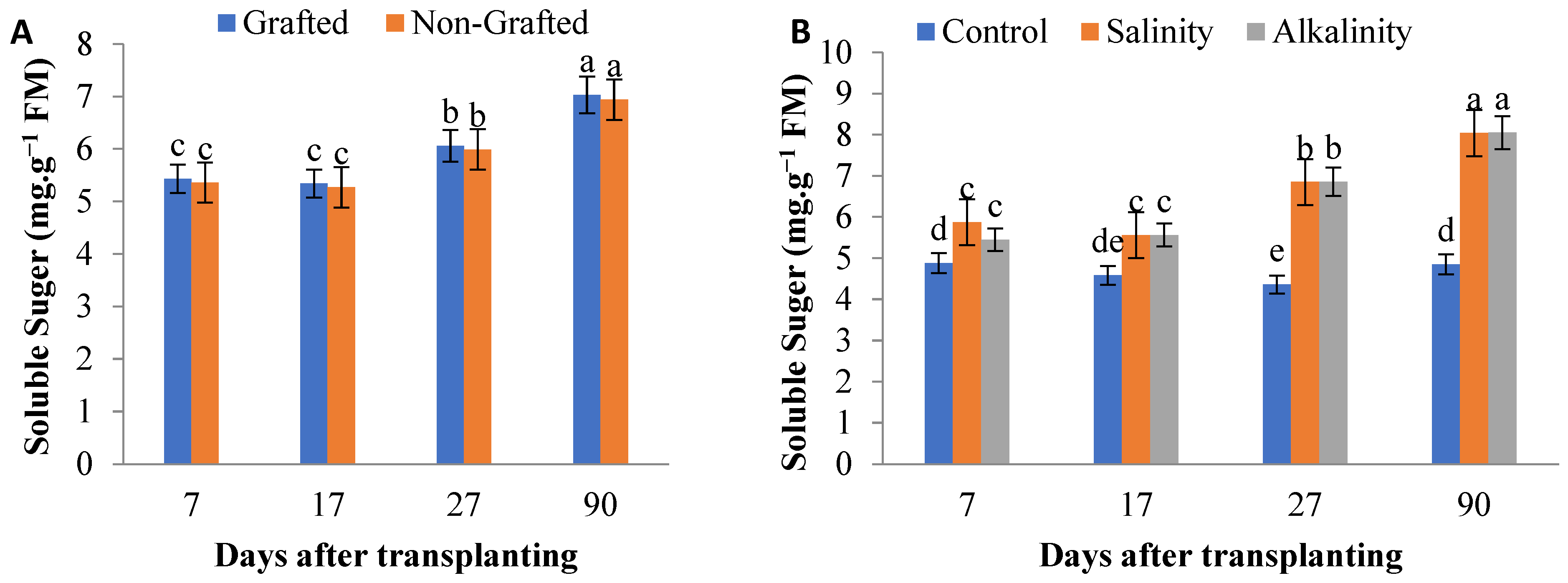
3.5. Soluble Sugars
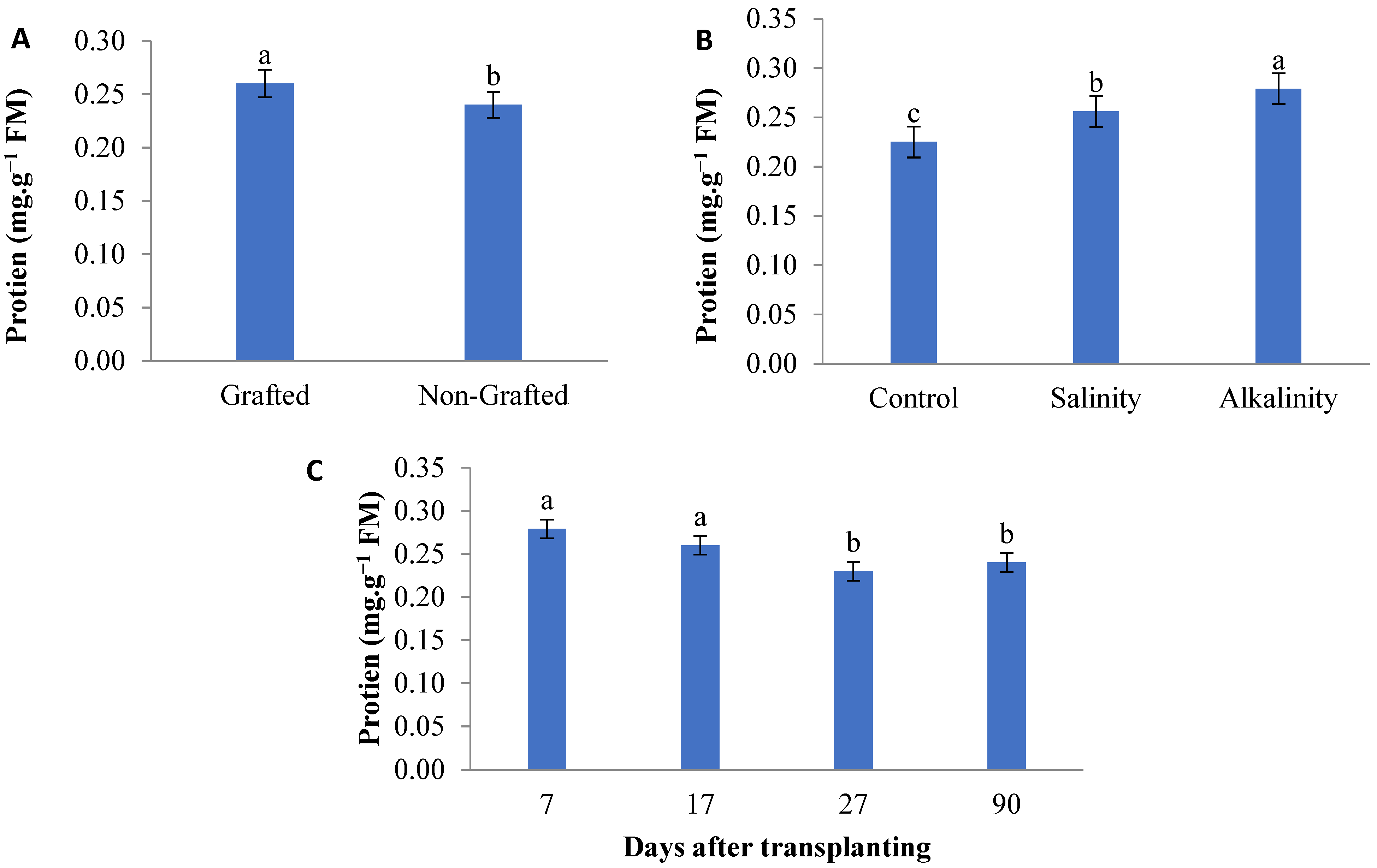
3.6. Protein Content
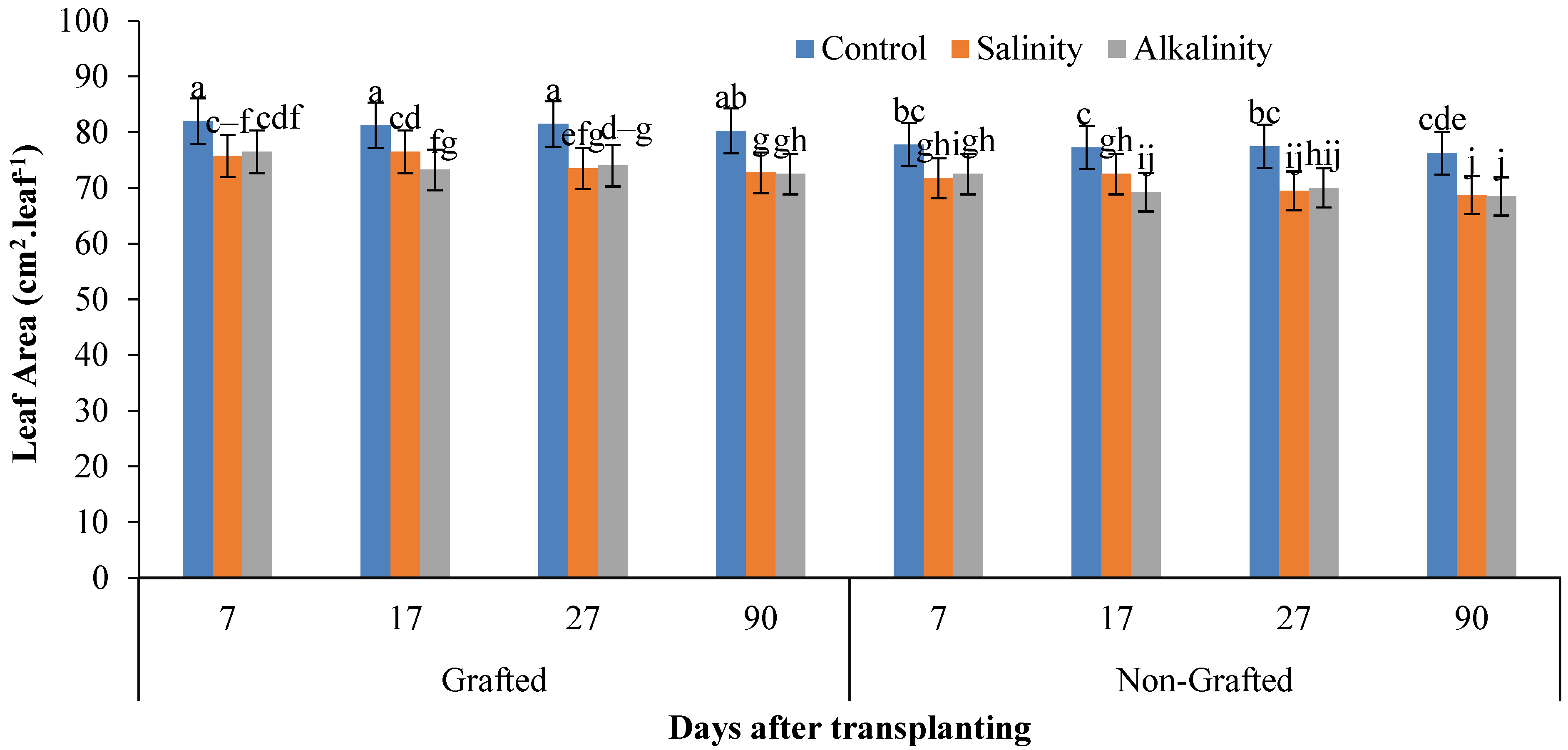
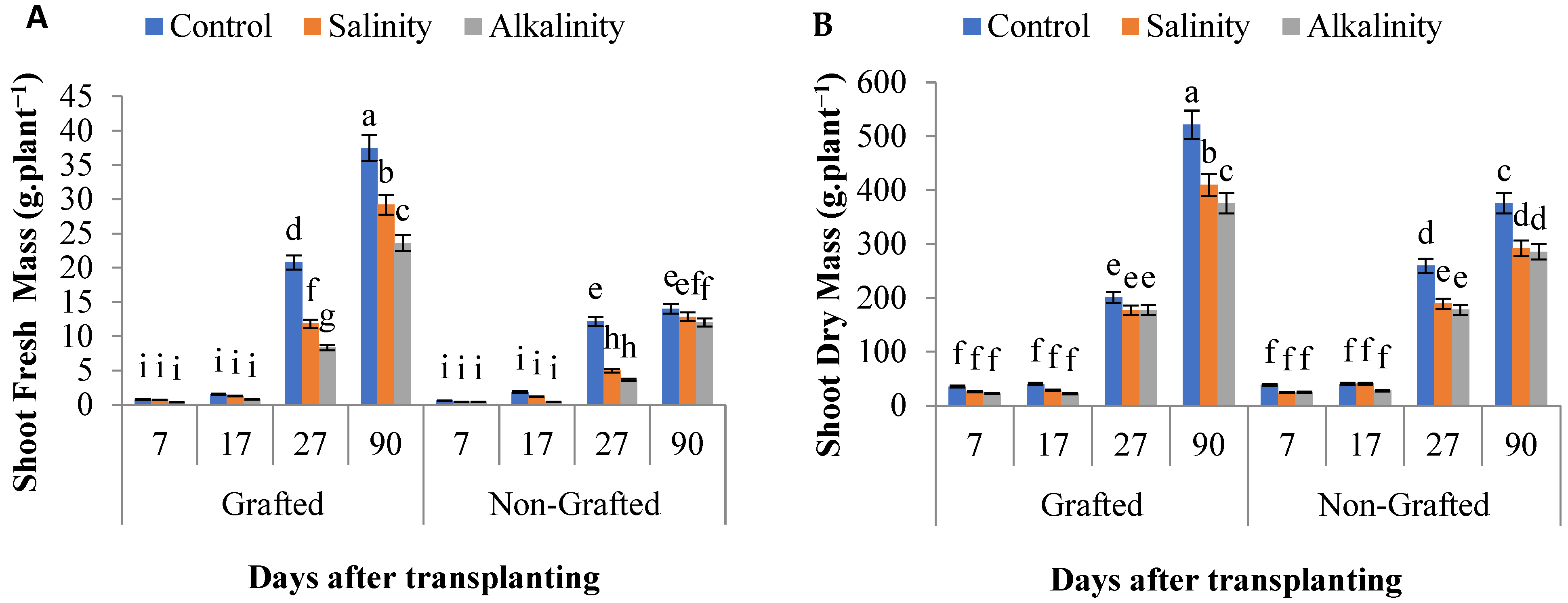
3.7. Plant Growth
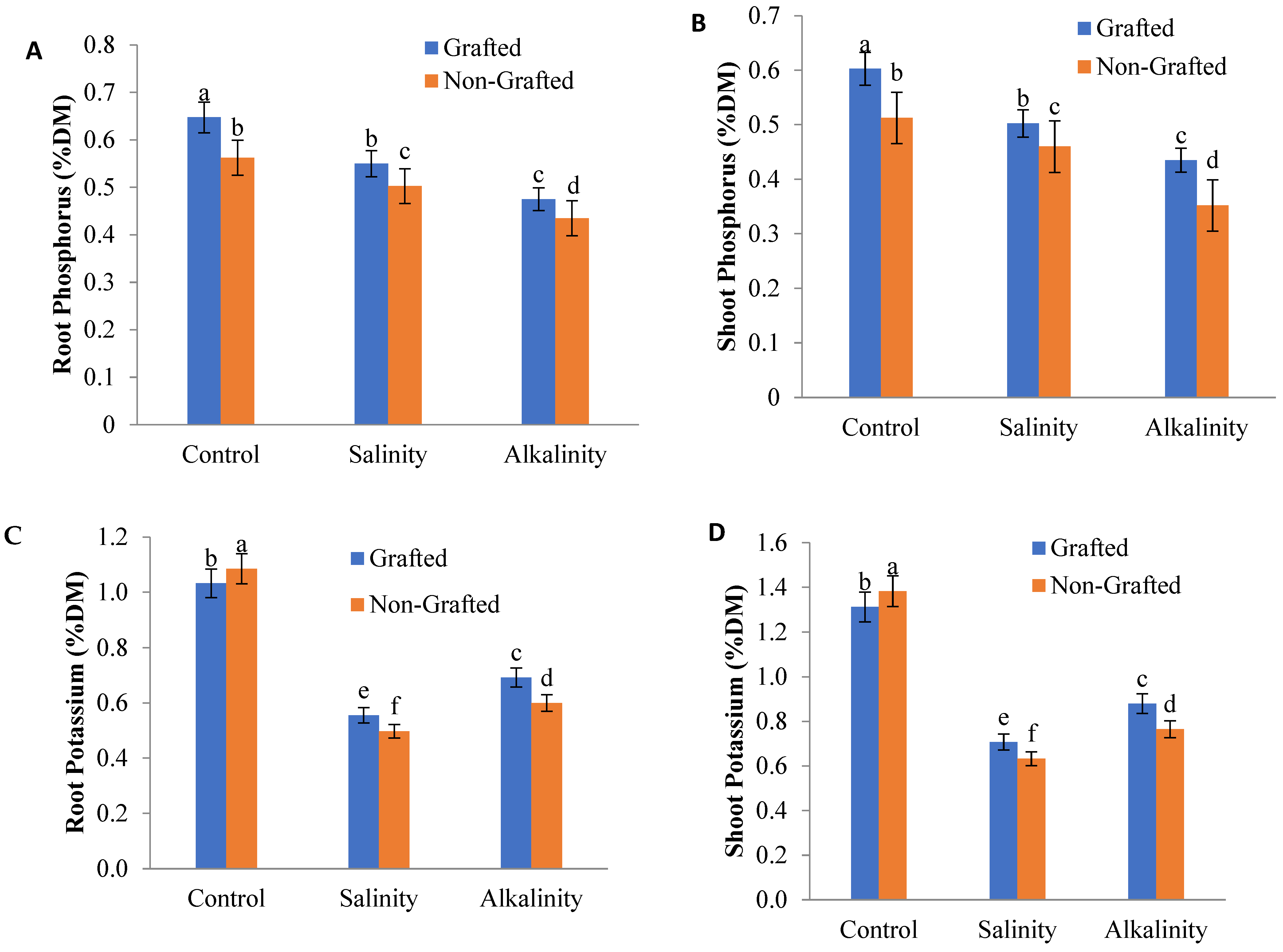
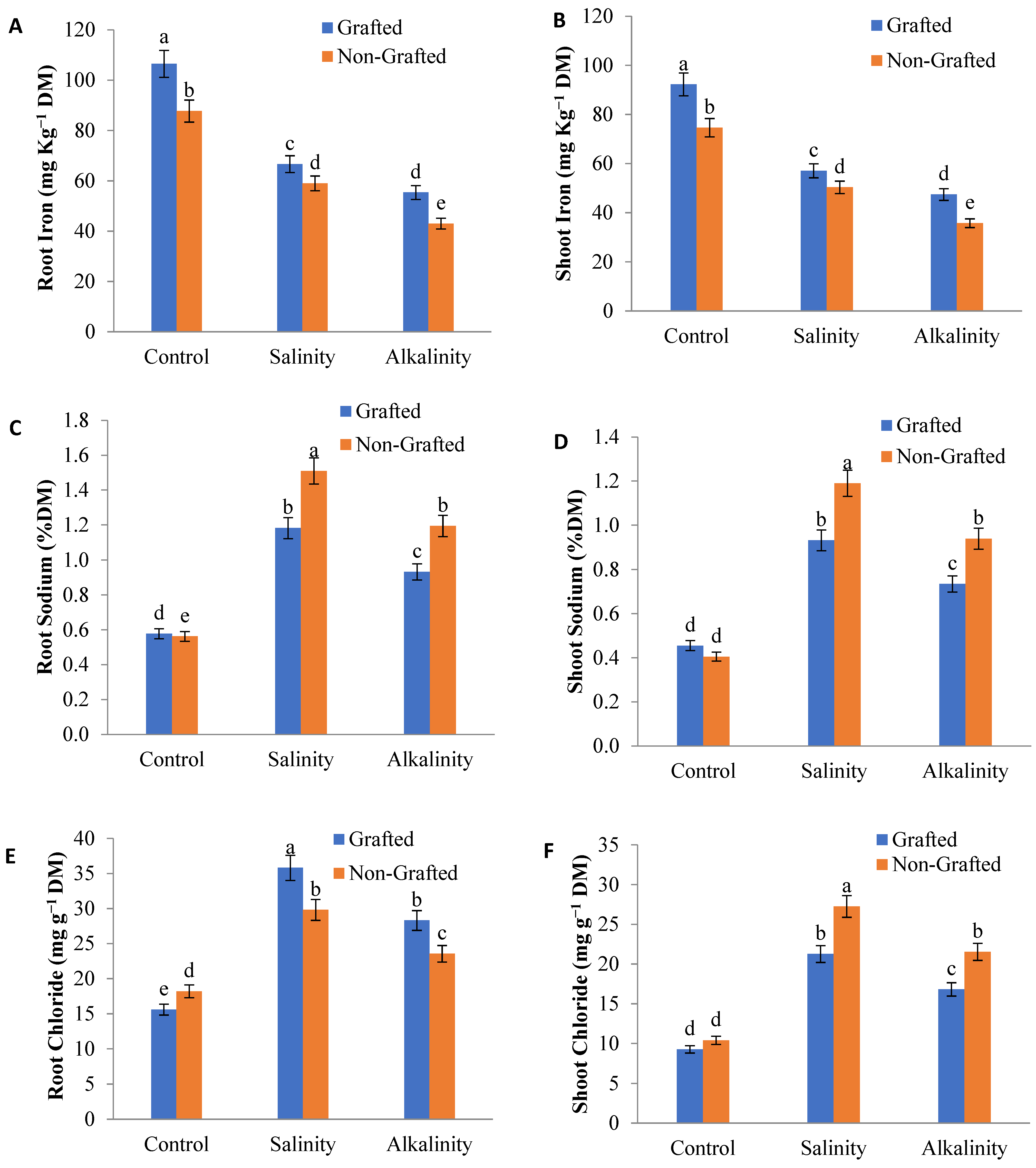
3.8. Nutrient Elements
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Dhillon, N.P.; Laenoi, S.; Srimat, S.; Pruangwitayakun, S.; Mallappa, A.; Kapur, A.; Yadav, K.K.; Hegde, G.; Schafleitner, R.; Schreinemachers, P. Sustainable cucurbit breeding and production in Asia using public–private partnerships by the world vegetable center. Agronomy 2020, 10, 1171. [Google Scholar] [CrossRef]
- Xu, L.; He, Y.; Tang, L.; Xu, Y.; Zhao, G. Genetics, genomics, and breeding in melon. Agronomy 2022, 12, 2891. [Google Scholar] [CrossRef]
- Canton, H. Food and agriculture organization of the United Nations—FAO. In The Europa Directory of International Organizations 2021; Routledge: London, UK, 2021; pp. 297–305. [Google Scholar]
- Raghami, M.; López-Sesé, A.I.; Hasandokht, M.R.; Zamani, Z.; Moghadam, M.R.F.; Kashi, A. Genetic diversity among melon accessions from Iran and their relationships with melon germplasm of diverse origins using microsatellite markers. Plant Syst. Evol. 2014, 300, 139–151. [Google Scholar] [CrossRef]
- Lija, M.; Beevy, S.S. A Review on the diversity of Melon. Plant Sci. Today 2021, 8, 995–1003. [Google Scholar] [CrossRef]
- Chikh-Rouhou, H.; Abdedayem, W.; Solmaz, I.; Sari, N.; Garcés-Claver, A. Melon (Cucumis melo L.): Genomics and breeding. In Smart Plant Breeding for Vegetable Crops in Post-Genomics Era; Springer: Berlin/Heidelberg, Germany, 2023; pp. 25–52. [Google Scholar]
- Yusuf, A.F.; Wibowo, W.A.; Subiastuti, A.S.; Daryono, B.S. Morphological studies of stability and identity of melon (Cucumis melo L.) ‘Hikapel’ and comparative cultivars. In Proceedings of the AIP Conference Proceedings, Yogyakarta, Indonesia, 24–25 June 2020. [Google Scholar]
- Maas, E.V.; Hoffman, G.J. Crop salt tolerance—Current assessment. J. Irrig. Drain. Div. 1977, 103, 115–134. [Google Scholar] [CrossRef]
- Roosta, H.R.; Bikdeloo, M. Nutritional responses of grafted cucumber on two types of Iranian local squash to alkalinity and salinity stresses. J. Plant Nutr. 2022, 45, 1275–1282. [Google Scholar] [CrossRef]
- Araújo, B.D.A.; Celin, E.F.; da Costa, R.S.; Calvet, A.S.; de Carvalho, H.H.; Bezerra, M.A. Development and quality of melon fruits grown under salt stress. Rev. Bras. Eng. Agric. E Ambient. Agriambi 2024, 28, e277374. [Google Scholar] [CrossRef]
- Gruda, N.S.; Dong, J.; Li, X. From salinity to nutrient-rich vegetables: Strategies for quality enhancement in protected cultivation. Crit. Rev. Plant Sci. 2024, 43, 327–347. [Google Scholar] [CrossRef]
- Akrami, M.; Arzani, A. Inheritance of fruit yield and quality in melon (Cucumis melo L.) grown under field salinity stress. Sci. Rep. 2019, 9, 7249. [Google Scholar] [CrossRef]
- Akrami, M.; Arzani, A. Physiological alterations due to field salinity stress in melon (Cucumis melo L.). Acta Physiol. Plant. 2018, 40, 91. [Google Scholar] [CrossRef]
- Pereira, F.A.d.L.; Medeiros, J.F.d.; Gheyi, H.R.; Dias, N.d.S.; Preston, W.; Vasconcelos, C.B. Tolerância de cultivares de melão à salinidade da água de irrigação. Rev. Bras. Eng. Agrícola Ambient. 2017, 21, 846–851. [Google Scholar] [CrossRef]
- Soufi, H.R.; Roosta, H.R.; Hamidpour, M. The plant growth, water and electricity consumption, and nutrients uptake are influenced by different light spectra and nutrition of lettuce. Sci. Rep. 2023, 13, 20766. [Google Scholar] [CrossRef]
- Yang, W.; Ling, Y.; Li, M.; Zhang, X.; Liu, B. Screening and identification of saline-tolerant germplasm in melon. Agriculture 2023, 13, 2051. [Google Scholar] [CrossRef]
- Soufi, H.R.; Roosta, H.R.; Fatehi, F.; Ghorbanpour, M. Spectral composition of LED light differentially affects biomass, photosynthesis, nutrient profile, and foliar nitrate accumulation of lettuce grown under various replacement methods of nutrient solution. Food Sci. Nutr. 2023, 11, 8143–8162. [Google Scholar] [CrossRef]
- Alizadeh, Z.; Ghasemnezhad, M.; Sangani, M.F.; Atak, A. The effects of high concentration of bicarbonate applications on kiwifruit genotypes with different ploidy levels on some growth parameters of leaves. Turk. J. Agric. For. 2023, 47, 436–447. [Google Scholar] [CrossRef]
- Naseer, M.N.; Rahman, F.U.; Hussain, Z.; Khan, I.A.; Aslam, M.M.; Aslam, A.; Waheed, H.; Khan, A.U.; Iqbal, S. Effect of salinity stress on germination, seedling growth, mineral uptake and chlorophyll contents of three Cucurbitaceae species. Braz. Arch. Biol. Technol. 2022, 65, e22210213. [Google Scholar] [CrossRef]
- Chen, C.; Yu, W.; Xu, X.; Wang, Y.; Wang, B.; Xu, S.; Lan, Q.; Wang, Y. Research advancements in salt tolerance of cucurbitaceae: From salt response to molecular mechanisms. Int. J. Mol. Sci. 2024, 25, 9051. [Google Scholar] [CrossRef]
- Alamri, S.; Hu, Y.; Mukherjee, S.; Aftab, T.; Fahad, S.; Raza, A.; Ahmad, M.; Siddiqui, M.H. Silicon-induced postponement of leaf senescence is accompanied by modulation of antioxidative defense and ion homeostasis in mustard (Brassica juncea) seedlings exposed to salinity and drought stress. Plant Physiol. Biochem. 2020, 157, 47–59. [Google Scholar] [CrossRef]
- Soufi, H.; Kalaji, H.M.; Hamidpour, M.; Malekzadeh, K. The roles of light in a plant factory: Photosynthesis efficiency and gas exchange parameters of lettuce as a function of light spectra. Greenh. Plant Prod. J. 2024, 1, 1–26. [Google Scholar] [CrossRef]
- Malekzadeh, M.R.; Roosta, H.R.; Esmaeilizadeh, M.; Dabrowski, P.; Kalaji, H.M. Improving strawberry plant resilience to salinity and alkalinity through the use of diverse spectra of supplemental lighting. BMC Plant Biol. 2024, 24, 252. [Google Scholar] [CrossRef]
- Colla, G.; Rouphael, Y.; Cardarelli, M.; Salerno, A.; Rea, E. The effectiveness of grafting to improve alkalinity tolerance in watermelon. Environ. Exp. Bot. 2010, 68, 283–291. [Google Scholar] [CrossRef]
- Colla, G.; Rouphael, Y.; Cardarelli, M.; Massa, D.; Salerno, A.; Rea, E. Yield, fruit quality and mineral composition of grafted melon plants grown under saline conditions. J. Hortic. Sci. Biotechnol. 2006, 81, 146–152. [Google Scholar] [CrossRef]
- Ulas, F.; Aydın, A.; Ulas, A.; Yetisir, H. The efficacy of grafting on alkali stressed watermelon cultivars under hydroponic conditions. Gesunde Pflanz. 2021, 73, 345–357. [Google Scholar] [CrossRef]
- Mousavi, S.A.; Roosta, H.R.; Esmaeilizadeh, M.; Eshghi, S. Alleviating the adverse effects of salinity and alkalinity stresses on some physiological traits by selenium and silicon foliar applications on cucumber (Cucumis sativus L.) plants. J. Plant Nutr. 2022, 46, 556–573. [Google Scholar] [CrossRef]
- Zribi, K.; Gharsalli, M. Effect of bicarbonate on growth and iron nutrition of pea. J. Plant Nutr. 2002, 25, 2143–2149. [Google Scholar] [CrossRef]
- Malekzadeh, M.R.; Roosta, H.R.; Kalaji, H.M. Enhancing strawberry resilience to saline, alkaline, and combined stresses with light spectra: Impacts on growth, enzymatic activity, nutrient uptake, and osmotic regulation. BMC Plant Biol. 2024, 24, 1038. [Google Scholar] [CrossRef]
- Qi, J.; Marshall, J.D.; Mattson, K.G. High soil carbon dioxide concentrations inhibit root respiration of Douglas fir. New Phytol. 1994, 128, 435–442. [Google Scholar] [CrossRef]
- Wang, Y.; Lu, Q.; Zhang, F.; Wang, W.; Wu, C. Effects of Biochar on the Yield of Melon and the Diversity of Rhizosphere Soil Microbial Communities Under Saline–Alkali Stress. Plants 2025, 14, 1423. [Google Scholar] [CrossRef]
- Ulas, A.; Aydin, A.; Ulas, F.; Yetisir, H.; Miano, T.F. Cucurbita rootstocks improve salt tolerance of melon scions by inducing physiological, biochemical and nutritional responses. Horticulturae 2020, 6, 66. [Google Scholar] [CrossRef]
- Colla, G.; Cardarelli, M.; Fiorillo, A.; Rouphael, Y.; Salerno, A.; Rea, E. Can grafting in watermelon plants enhance tolerance to bicarbonate in nutrient solution? In Proceedings of the XXVIII International Horticultural Congress on Science and Horticulture for People (IHC2010): International Symposium on 927, Lisbon, Portugal, 22–27 August 2010; pp. 323–329. [Google Scholar]
- Keshavarzi, M.; Raghami, M.; Roosta, H. Grafting effects on some morpho-physiological characteristics of a cantaloupe landrace (Shahpasand) under salinity and alkalinity stress in hydroponic system. J. Soil Plant Interact. Isfahan Univ. Technol. 2019, 9, 41–52. [Google Scholar] [CrossRef]
- Roosta, H.R.; Karimi, H.R. Effects of alkali-stress on ungrafted and grafted cucumber plants: Using two types of local squash as rootstock. J. Plant Nutr. 2012, 35, 1843–1852. [Google Scholar] [CrossRef]
- Bie, Z.; Azher Nawa, M.; Huang, Y.; Lee, J.M.; Colla, G. Introduction to vegetable grafting. In Vegetable Grafting: Principles and Practices; CABI: Wallingford, UK, 2017; pp. 1–21. [Google Scholar]
- Nie, W.; Wen, D. Study on the applications and regulatory mechanisms of grafting on vegetables. Plants 2023, 12, 2822. [Google Scholar] [CrossRef]
- Davis, A.R.; Perkins-Veazie, P.; Sakata, Y.; Lopez-Galarza, S.; Maroto, J.V.; Lee, S.-G.; Huh, Y.-C.; Sun, Z.; Miguel, A.; King, S.R. Cucurbit grafting. Crit. Rev. Plant Sci. 2008, 27, 50–74. [Google Scholar] [CrossRef]
- Abdelmageed, A.; Gruda, N. Influence of grafting on growth, development and some physiological parameters of tomatoes under controlled heat stress conditions. Eur. J. Hortic. Sci. 2009, 74, 16–20. [Google Scholar] [CrossRef]
- Kumar, P.; Rouphael, Y.; Cardarelli, M.; Colla, G. Vegetable grafting as a tool to improve drought resistance and water use efficiency. Front. Plant Sci. 2017, 8, 1130. [Google Scholar] [CrossRef]
- Soltani, S.; Arouiee, H.; Salehi, R.; Nemati, S.H.; Moosavi-Nezhad, M.; Gruda, N.S.; Aliniaeifard, S. Morphological, phytochemical, and photosynthetic performance of grafted tomato seedlings in response to different LED light qualities under protected cultivation. Horticulturae 2023, 9, 471. [Google Scholar] [CrossRef]
- Rasool, A.; Mansoor, S.; Bhat, K.; Hassan, G.; Baba, T.R.; Alyemeni, M.N.; Alsahli, A.A.; El-Serehy, H.A.; Paray, B.A.; Ahmad, P. Mechanisms underlying graft union formation and rootstock scion interaction in horticultural plants. Front. Plant Sci. 2020, 11, 590847. [Google Scholar] [CrossRef] [PubMed]
- Singh, H.; Kumar, P.; Kumar, A.; Kyriacou, M.C.; Colla, G.; Rouphael, Y. Grafting tomato as a tool to improve salt tolerance. Agronomy 2020, 10, 263. [Google Scholar] [CrossRef]
- Yavuz, N. Can grafting affect yield and water use efficiency of melon under different irrigation depths in a semi-arid zone? Arab. J. Geosci. 2021, 14, 1118. [Google Scholar] [CrossRef]
- Suansia, A.; Samal, K.C. Vegetable grafting: A sustainable and eco-friendly strategy for soil-borne pest and disease management. J. Pharmacogn. Phytochem 2021, 10, 1634–1642. [Google Scholar]
- Álvarez-Hernández, J.C. Grafting in Horticultural Crop Species: Effective Pest and Disease Management Technique with Potential in Michoacan, Mexico. In Horticultural Crops; IntechOpen: Rijeka, Croatia, 2019. [Google Scholar]
- Cohen, R.; Dombrovsky, A.; Louws, F.J. Grafting as agrotechnology for reducing disease damage. In Vegetable Grafting: Principles and Practices; CABI: Wallingford, UK, 2017; pp. 155–170. [Google Scholar]
- Xiong, M.; Liu, C.; Guo, L.; Wang, J.; Wu, X.; Li, L.; Bie, Z.; Huang, Y. Compatibility evaluation and anatomical observation of melon grafted onto eight Cucurbitaceae species. Front. Plant Sci. 2021, 12, 762889. [Google Scholar] [CrossRef]
- Ulas, F.; Aydin, A.; Ulas, A.; Yetisir, H. Rootstock effects on alkali stressed melon plants. Indian J. Hortic. 2019, 76, 112–117. [Google Scholar] [CrossRef]
- Bahadur, A.; Singh, P.M.; Rai, N.; Singh, A.K.; Singh, A.K.; Karkute, S.G.; Behera, T.K. Grafting in vegetables to improve abiotic stress tolerance, yield and quality. J. Hortic. Sci. Biotechnol. 2024, 99, 385–403. [Google Scholar] [CrossRef]
- Devi, P.; Perkins-Veazie, P.; Miles, C. Impact of grafting on watermelon fruit maturity and quality. Horticulturae 2020, 6, 97. [Google Scholar] [CrossRef]
- Muhamad Hassan, M.H.; Awang, Y.; Jaafar, J.N.; Sayuti, Z.; Othman Ghani, M.N.; Mohamad Sabdin, Z.H.; Nazli, M.H. Effects of salinity sources on growth, physiological process, yield, and fruit quality of grafted rock melon (Cucumis melo L.). Pertanika J. Trop. Agric. Sci. 2022, 45, 919–941. [Google Scholar] [CrossRef]
- Ulas, F.; Aydın, A.; Ulas, A.; Yetisir, H. Grafting for sustainable growth performance of melon (Cucumis melo) under salt stressed hydroponic condition. Eur. J. Sustain. Dev. 2019, 8, 201. [Google Scholar] [CrossRef]
- Kuşvuran, Ş.; Kaya, E.; Ellialtıoğlu, Ş.Ş. Role of Grafting in Tolerance to Salt Stress in Melon (Cucumis melo L.) Plants: Ion regulation and antioxidant defense systems. Biotech Stud. 2021, 30, 22–32. [Google Scholar] [CrossRef]
- Liu, Y.; Tan, Z.; Meng, L.; Li, Y.; Peng, Y. CmDUF239-1 improves the salt tolerance of grafted melon by enhancing antioxidant capacity and Na+/K+ homeostasis. Plants 2025, 14, 2670. [Google Scholar] [CrossRef]
- Rouphael, Y.; Cardarelli, M.; Rea, E.; Colla, G. Improving melon and cucumber photosynthetic activity, mineral composition, and growth performance under salinity stress by grafting onto Cucurbita hybrid rootstocks. Photosynthetica 2012, 50, 180–188. [Google Scholar] [CrossRef]
- Gallegos-Cedillo, V.M.; Nájera, C.; Signore, A.; Ochoa, J.; Gallegos, J.; Egea-Gilabert, C.; Gruda, N.S.; Fernández, J.A. Analysis of global research on vegetable seedlings and transplants and their impacts on product quality. J. Sci. Food Agric. 2024, 104, 4950–4965. [Google Scholar] [CrossRef] [PubMed]
- Tyson, R.; Simonne, E.; Treadwell, D.; Davis, M.; White, J. Effect of water pH on yield and nutritional status of greenhouse cucumber grown in recirculating hydroponics. J. Plant Nutr. 2008, 31, 2018–2030. [Google Scholar] [CrossRef]
- Arnon, D.I. Copper enzymes in isolated chloroplasts. Polyphenoloxidase in Beta vulgaris. Plant Physiol. 1949, 24, 1. [Google Scholar] [CrossRef]
- Paquin, R.; Lechasseur, P. Observations sur une méthode de dosage de la proline libre dans les extraits de plantes. Can. J. Bot. 1979, 57, 1851–1854. [Google Scholar] [CrossRef]
- Irigoyen, J.; Einerich, D.; Sánchez-Díaz, M. Water stress induced changes in concentrations of proline and total soluble sugars in nodulated alfalfa (Medicago sativa) plants. Physiol. Plant. 1992, 84, 55–60. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Olsen, S.R. Estimation of Available Phosphorus in Soils by Extraction with Sodium Bicarbonate; US Department of Agriculture: Washington, DC, USA, 1954. [Google Scholar]
- Pessarakli, M. Handbook of Photosynthesis; CRC Press: Boca Raton, FL, USA, 2024. [Google Scholar]
- Martins, T.; Barros, A.N.; Rosa, E.; Antunes, L. Enhancing health benefits through chlorophylls and chlorophyll-rich agro-food: A comprehensive review. Molecules 2023, 28, 5344. [Google Scholar] [CrossRef] [PubMed]
- Morelli, L.; Rodriguez-Concepcion, M. Open avenues for carotenoid biofortification of plant tissues. Plant Commun. 2023, 4, 100466. [Google Scholar] [CrossRef]
- Maoka, T. Carotenoids as natural functional pigments. J. Nat. Med. 2020, 74, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Tros, M.; Mascoli, V.; Shen, G.; Ho, M.-Y.; Bersanini, L.; Gisriel, C.J.; Bryant, D.A.; Croce, R. Breaking the red limit: Efficient trapping of long-wavelength excitations in chlorophyll-f-containing photosystem I. Chem 2021, 7, 155–173. [Google Scholar] [CrossRef]
- Li, X.; Zhang, W.; Niu, D.; Liu, X. Effects of abiotic stress on chlorophyll metabolism. Plant Sci. 2024, 342, 112030. [Google Scholar] [CrossRef]
- Wang, X.; Chen, Z.; Sui, N. Sensitivity and responses of chloroplasts to salt stress in plants. Front. Plant Sci. 2024, 15, 1374086. [Google Scholar] [CrossRef] [PubMed]
- Khan, I.; Rehman, A.; Akram, W.; Anjum, T.; Yasin, N.A.; Aftab, Z.-e.-H.; Munir, B.; Khan, W.U.; Li, G. Unlocking Salinity Stress Resilience in Turnip (Brassica rapa subsp. rapa) Plants Using Bacillus subtilis Z-12 and Bacillus aryabhattai Z-48. Microorganisms 2025, 13, 359. [Google Scholar] [CrossRef]
- Hundare, A.; Joshi, V.; Joshi, N. Salicylic acid attenuates salinity-induced growth inhibition in in vitro raised ginger (Zingiber officinale Roscoe) plantlets by regulating ionic balance and antioxidative system. Plant Stress 2022, 4, 100070. [Google Scholar] [CrossRef]
- Lu, C.; Li, L.; Liu, X.; Chen, M.; Wan, S.; Li, G. Salt stress inhibits photosynthesis and destroys chloroplast structure by downregulating chloroplast development–related genes in Robinia pseudoacacia seedlings. Plants 2023, 12, 1283. [Google Scholar] [CrossRef]
- Oraei, M.; Tabatabaie, S.J.; Fallahi, E. The effects of salinity stress and rootstock on the growth, photosynthetic rate, nutrient and sodium concentrations of almond (Prunus dulcis Mill.). J. Hortic. Sci. 2010, 23, 131–140. [Google Scholar]
- Mohsenian, Y.; Roosta, H.R. Effects of grafting on alkali stress in tomato plants: Datura rootstock improve alkalinity tolerance of tomato plants. J. Plant Nutr. 2015, 38, 51–72. [Google Scholar] [CrossRef]
- Ghodrati-Tazangi, M.J.; Samani, R.B.; Tavallali, V.; Alizadeh, A.; Honarvar, M. Responses of Hyssopus officinalis to bicarbonate stress and foliar application of green synthesized zinc nano-complex formed on Medicago sativa extract. Sci. Hortic. 2023, 319, 112197. [Google Scholar] [CrossRef]
- Shahsavandi, F.; Eshghi, S.; Gharaghani, A.; Ghasemi-Fasaei, R.; Jafarinia, M. Effects of bicarbonate induced iron chlorosis on photosynthesis apparatus in grapevine. Sci. Hortic. 2020, 270, 109427. [Google Scholar] [CrossRef]
- Massimi, M.; Radócz, L.; Kabashi, B. The response of chlorophyll content and ionic composition in tomato and pepper seedlings to foliar nutrition in growing chambers. Agronomy 2023, 13, 2234. [Google Scholar] [CrossRef]
- Tavakkoli, M.M.; Roosta, H.; Hamidpour, M. Effects of alkali stress and growing media on growth and physiological characteristics of gerbera plants. J. Agric. Sci. Technol. 2016, 18, 453–466. [Google Scholar]
- Yang, X.; Lu, M.; Wang, Y.; Wang, Y.; Liu, Z.; Chen, S. Response mechanism of plants to drought stress. Horticulturae 2021, 7, 50. [Google Scholar] [CrossRef]
- Deng, C.; Zhang, G.; Pan, X.; Zhao, K. Chlorophyll fluorescence and gas exchange responses of maize seedlings to saline-alkaline stress. Bulg. J. Agric. Sci. 2010, 16, 49–58. [Google Scholar]
- Kumar, K.; Jaiswal, A.; Koppolu, U.M.K.; Kumar, K.R.R. Alkaline stress disrupts growth, biochemistry, and ion homeostasis of chickpea (Cicer arietinum L.) roots. Front. Agron. 2024, 6, 1497054. [Google Scholar] [CrossRef]
- Yang, S.; Xu, Y.; Tang, Z.; Jin, S.; Yang, S. The Impact of Alkaline Stress on Plant Growth and Its Alkaline Resistance Mechanisms. Int. J. Mol. Sci. 2024, 25, 13719. [Google Scholar] [CrossRef]
- Saleem, A.; Zulfiqar, A.; Saleem, M.Z.; Ali, B.; Saleem, M.H.; Ali, S.; Tufekci, E.D.; Tufekci, A.R.; Rahimi, M.; Mostafa, R.M. Alkaline and acidic soil constraints on iron accumulation by Rice cultivars in relation to several physio-biochemical parameters. BMC Plant Biol. 2023, 23, 397. [Google Scholar] [CrossRef]
- Zhang, R.-R.; Wang, Y.-H.; Li, T.; Tan, G.-F.; Tao, J.-P.; Su, X.-J.; Xu, Z.-S.; Tian, Y.-S.; Xiong, A.-S. Effects of simulated drought stress on carotenoid contents and expression of related genes in carrot taproots. Protoplasma 2021, 258, 379–390. [Google Scholar] [CrossRef]
- Liu, D.; Ma, Y.; Rui, M.; Lv, X.; Chen, R.; Chen, X.; Wang, Y. Is high pH the key factor of alkali stress on plant growth and physiology? A case study with wheat (Triticum aestivum L.) seedlings. Agronomy 2022, 12, 1820. [Google Scholar] [CrossRef]
- Turan, S.; Tripathy, B.C. Salt-stress induced modulation of chlorophyll biosynthesis during de-etiolation of rice seedlings. Physiol. Plant. 2015, 153, 477–491. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Tang, W.; Hedtke, B.; Zhong, L.; Liu, L.; Peng, L.; Lu, C.; Grimm, B.; Lin, R. Tetrapyrrole biosynthetic enzyme protoporphyrinogen IX oxidase 1 is required for plastid RNA editing. Proc. Natl. Acad. Sci. USA 2014, 111, 2023–2028. [Google Scholar] [CrossRef] [PubMed]
- Poggi, G.M.; Corneti, S.; Aloisi, I.; Ventura, F. Environment-oriented selection criteria to overcome controversies in breeding for drought resistance in wheat. J. Plant Physiol. 2023, 280, 153895. [Google Scholar] [CrossRef]
- Quemada, C.; Pérez-Escudero, J.M.; Gonzalo, R.; Ederra, I.; Santesteban, L.G.; Torres, N.; Iriarte, J.C. Remote sensing for plant water content monitoring: A review. Remote Sens. 2021, 13, 2088. [Google Scholar] [CrossRef]
- Hatfield, J.L.; Dold, C. Water-use efficiency: Advances and challenges in a changing climate. Front. Plant Sci. 2019, 10, 103. [Google Scholar] [CrossRef] [PubMed]
- Sheng, M.; Tang, M.; Chen, H.; Yang, B.; Zhang, F.; Huang, Y. Influence of arbuscular mycorrhizae on photosynthesis and water status of maize plants under salt stress. Mycorrhiza 2008, 18, 287–296. [Google Scholar] [CrossRef] [PubMed]
- Ncisana, L.; Nyathi, M.K.; Mkhize, N.R.; Mabhaudhi, T.; Tjelele, T.J.; Mbambalala, L.; Modi, A.T. Water use efficiency (WUE) and nutrient concentration of selected fodder radish (Raphanus sativus L.) genotypes for sustainable diets. Sci. Rep. 2024, 14, 31315. [Google Scholar] [CrossRef] [PubMed]
- Kopittke, P.M.; Menzies, N.W. Effect of pH on Na induced Ca deficiency. Plant Soil 2005, 269, 119–129. [Google Scholar] [CrossRef]
- Poschenrieder, C.; Fernández, J.A.; Rubio, L.; Pérez, L.; Terés, J.; Barceló, J. Transport and use of bicarbonate in plants: Current knowledge and challenges ahead. Int. J. Mol. Sci. 2018, 19, 1352. [Google Scholar] [CrossRef]
- Wang, N.; Dong, X.; Chen, Y.; Ma, B.; Yao, C.; Ma, F.; Liu, Z. Direct and bicarbonate-induced iron deficiency differently affect iron translocation in Kiwifruit roots. Plants 2020, 9, 1578. [Google Scholar] [CrossRef]
- Yang, C.; Jianaer, A.; Li, C.; Shi, D.; Wang, D. Comparison of the effects of salt-stress and alkali-stress on photosynthesis and energy storage of an alkali-resistant halophyte Chloris virgata. Photosynthetica 2008, 46, 273–278. [Google Scholar] [CrossRef]
- Chen, D.; Mubeen, B.; Hasnain, A.; Rizwan, M.; Adrees, M.; Naqvi, S.A.H.; Iqbal, S.; Kamran, M.; El-Sabrout, A.M.; Elansary, H.O. Role of promising secondary metabolites to confer resistance against environmental stresses in crop plants: Current scenario and future perspectives. Front. Plant Sci. 2022, 13, 881032. [Google Scholar]
- Nawaz, M.; Sun, J.; Shabbir, S.; Khattak, W.A.; Ren, G.; Nie, X.; Bo, Y.; Javed, Q.; Du, D.; Sonne, C. A review of plants strategies to resist biotic and abiotic environmental stressors. Sci. Total Environ. 2023, 900, 165832. [Google Scholar] [CrossRef]
- Wennerström, H.; Oliveberg, M. On the osmotic pressure of cells. QRB Discov. 2022, 3, e12. [Google Scholar] [CrossRef]
- Khan, S.; Siraj, S.; Shahid, M.; Haque, M.M.; Islam, A. Osmolytes: Wonder molecules to combat protein misfolding against stress conditions. Int. J. Biol. Macromol. 2023, 234, 123662. [Google Scholar] [CrossRef]
- Tiwari, Y.K. Proline as a key player in heat stress tolerance: Insights from maize. Discov. Agric. 2024, 2, 121. [Google Scholar] [CrossRef]
- Pingle, S.N.; Suryawanshi, S.T.; Pawar, K.R.; Harke, S.N. The effect of salt stress on proline content in maize (Zea mays). Environ. Sci. Proc. 2022, 16, 64. [Google Scholar]
- Abdallah, M.M.-S.; El Sebai, T.N.; Ramadan, A.A.E.-M.; El-Bassiouny, H.M.S. Physiological and biochemical role of proline, trehalose, and compost on enhancing salinity tolerance of quinoa plant. Bull. Natl. Res. Cent. 2020, 44, 96. [Google Scholar] [CrossRef]
- Inayat, H.; Mehmood, H.; Danish, S.; Alharbi, S.A.; Ansari, M.J.; Datta, R. Impact of cobalt and proline foliar application for alleviation of salinity stress in radish. BMC Plant Biol. 2024, 24, 287. [Google Scholar] [CrossRef]
- Zhu, S.; Xu, R.; Engel, A.L.; Wang, Y.; McNeel, R.; Hurley, J.B.; Chao, J.R.; Du, J. Proline provides a nitrogen source in the retinal pigment epithelium to synthesize and export amino acids for the neural retina. J. Biol. Chem. 2023, 299, 105275. [Google Scholar] [CrossRef]
- Yin, H.; Yang, F.; He, X.; Du, X.; Mu, P.; Ma, W. Advances in the functional study of glutamine synthetase in plant abiotic stress tolerance response. Crop J. 2022, 10, 917–923. [Google Scholar] [CrossRef]
- Gao, Z.; Han, J.; Mu, C.; Lin, J.; Li, X.; Lin, L.; Sun, S. Effects of saline and alkaline stresses on growth and physiological changes in oat (Avena sativa L.) seedlings. Not. Bot. Horti Agrobot. 2014, 42, 357–362. [Google Scholar] [CrossRef]
- Shamsabad, M.R.M.; Esmaeilizadeh, M.; Roosta, H.R.; Dehghani, M.R.; Dąbrowski, P.; Kalaji, H.M. The effect of supplementary light on the photosynthetic apparatus of strawberry plants under salinity and alkalinity stress. Sci. Rep. 2022, 12, 13257. [Google Scholar] [CrossRef]
- Jeandet, P.; Formela-Luboińska, M.; Labudda, M.; Morkunas, I. The role of sugars in plant responses to stress and their regulatory function during development. Int. J. Mol. Sci. 2022, 23, 5161. [Google Scholar] [CrossRef] [PubMed]
- Atta, K.; Mondal, S.; Gorai, S.; Singh, A.P.; Kumari, A.; Ghosh, T.; Roy, A.; Hembram, S.; Gaikwad, D.J.; Mondal, S. Impacts of salinity stress on crop plants: Improving salt tolerance through genetic and molecular dissection. Front. Plant Sci. 2023, 14, 1241736. [Google Scholar] [CrossRef]
- Vergine, M.; Palm, E.R.; Salzano, A.M.; Negro, C.; Nissim, W.G.; Sabbatini, L.; Balestrini, R.; de Pinto, M.C.; Dipierro, N.; Gohari, G. Water and nutrient availability modulate the salinity stress response in Olea europaea cv. Arbequina. Plant Stress 2024, 14, 100648. [Google Scholar] [CrossRef]
- Balasubramaniam, T.; Shen, G.; Esmaeili, N.; Zhang, H. Plants’ response mechanisms to salinity stress. Plants 2023, 12, 2253. [Google Scholar] [CrossRef] [PubMed]
- Muhammad, M.; Waheed, A.; Wahab, A.; Majeed, M.; Nazim, M.; Liu, Y.-H.; Li, L.; Li, W.-J. Soil salinity and drought tolerance: An evaluation of plant growth, productivity, microbial diversity, and amelioration strategies. Plant Stress 2024, 11, 100319. [Google Scholar] [CrossRef]
- Chauhan, J.; Prathibha, M.; Singh, P.; Choyal, P.; Mishra, U.N.; Saha, D.; Kumar, R.; Anuragi, H.; Pandey, S.; Bose, B. Plant photosynthesis under abiotic stresses: Damages, adaptive, and signaling mechanisms. Plant Stress 2023, 10, 100296. [Google Scholar] [CrossRef]
- Ikram, M.; Khalid, B.; Batool, M.; Ullah, M.; Zitong, J.; Rauf, A.; Rao, M.J.; Rehman, H.U.; Kuai, J.; Xu, Z. Secondary metabolites as biostimulants in salt stressed plants: Mechanisms of oxidative defense and signal transduction. Plant Stress 2025, 16, 100891. [Google Scholar] [CrossRef]
- Alkharabsheh, H.M.; Seleiman, M.F.; Hewedy, O.A.; Battaglia, M.L.; Jalal, R.S.; Alhammad, B.A.; Schillaci, C.; Ali, N.; Al-Doss, A. Field crop responses and management strategies to mitigate soil salinity in modern agriculture: A review. Agronomy 2021, 11, 2299. [Google Scholar] [CrossRef]
- Hualpa-Ramirez, E.; Carrasco-Lozano, E.C.; Madrid-Espinoza, J.; Tejos, R.; Ruiz-Lara, S.; Stange, C.; Norambuena, L. Stress salinity in plants: New strategies to cope with in the foreseeable scenario. Plant Physiol. Biochem. 2024, 208, 108507. [Google Scholar] [CrossRef]
- Msimbira, L.A.; Smith, D.L. The roles of plant growth promoting microbes in enhancing plant tolerance to acidity and alkalinity stresses. Front. Sustain. Food Syst. 2020, 4, 106. [Google Scholar] [CrossRef]
- Navarro-León, E.; Grazioso, A.; Atero-Calvo, S.; Rios, J.J.; Esposito, S.; Blasco, B. Evaluation of the alkalinity stress tolerance of three Brassica rapa CAX1 TILLING mutants. Plant Physiol. Biochem. 2023, 198, 107712. [Google Scholar] [CrossRef]
- Li, H.; Lv, J.; Su, Y.; Wu, Y. Appropriate Sodium Bicarbonate Concentration Enhances the Intracellular Water Metabolism, Nutrient Transport and Photosynthesis Capacities of Coix lacryma-jobi L. Agronomy 2023, 13, 1790. [Google Scholar] [CrossRef]
- Aung, T.T.; Shi, F.; Zhai, Y.; Xue, J.; Wang, S.; Ren, X.; Zhang, X. Acidic and alkaline conditions affect the growth of tree peony plants via altering photosynthetic characteristics, limiting nutrient assimilation, and impairing ROS balance. Int. J. Mol. Sci. 2022, 23, 5094. [Google Scholar] [CrossRef]
- Zhang, H.; Li, X.; Che, Y.; Wang, Y.; Li, M.; Yi, R.; Xu, N.; Sun, G. A study on the effects of salinity and pH on PSII function in mulberry seedling leaves under saline–alkali mixed stress. Trees 2020, 34, 693–706. [Google Scholar]
- Jia, B.; Cui, H.; Zhang, D.; Hu, B.; Li, Y.; Shen, Y.; Cai, X.; Sun, X.; Sun, M. The conserved evolution of plant H+-ATPase family and the involvement of soybean H+-ATPases in sodium bicarbonate stress responses. Plant Physiol. Biochem. 2023, 204, 108133. [Google Scholar] [CrossRef]
- Roosta, H.R. Interaction between water alkalinity and nutrient solution pH on the vegetative growth, chlorophyll fluorescence and leaf magnesium, iron, manganese, and zinc concentrations in lettuce. J. Plant Nutr. 2011, 34, 717–731. [Google Scholar] [CrossRef]
- Savvas, D.; Ntatsi, G.; Barouchas, P. Impact of grafting and rootstock genotype on cation uptake by cucumber (Cucumis sativus L.) exposed to Cd or Ni stress. Sci. Hortic. 2013, 149, 86–96. [Google Scholar] [CrossRef]
- Yan, Y.; Wang, S.; Wei, M.; Gong, B.; Shi, Q. Effect of different rootstocks on the salt stress tolerance in watermelon seedlings. Hortic. Plant J. 2018, 4, 239–249. [Google Scholar] [CrossRef]
- Lu, J.; Cheng, F.; Huang, Y.; Bie, Z. Grafting watermelon onto pumpkin increases chilling tolerance by up regulating arginine decarboxylase to increase putrescine biosynthesis. Front. Plant Sci. 2022, 12, 812396. [Google Scholar] [CrossRef] [PubMed]
- Zuo, Y.; Ren, L.; Zhang, F.; Jiang, R.-F. Bicarbonate concentration as affected by soil water content controls iron nutrition of peanut plants in a calcareous soil. Plant Physiol. Biochem. 2007, 45, 357–364. [Google Scholar] [CrossRef]
- Edelstein, M.; Plaut, Z.; Ben-Hur, M. Sodium and chloride exclusion and retention by non-grafted and grafted melon and Cucurbita plants. J. Exp. Bot. 2011, 62, 177–184. [Google Scholar] [CrossRef]
- Huang, Y.; Bie, Z.; Liu, P.; Niu, M.; Zhen, A.; Liu, Z.; Lei, B.; Gu, D.; Lu, C.; Wang, B. Reciprocal grafting between cucumber and pumpkin demonstrates the roles of the rootstock in the determination of cucumber salt tolerance and sodium accumulation. Sci. Hortic. 2013, 149, 47–54. [Google Scholar] [CrossRef]
- Razi, K.; Suresh, P.; Mahapatra, P.P.; Al Murad, M.; Venkat, A.; Notaguchi, M.; Bae, D.W.; Prakash, M.A.S.; Muneer, S. Exploring the role of grafting in abiotic stress management: Contemporary insights and automation trends. Plant Direct 2024, 8, e70021. [Google Scholar] [CrossRef]
- El-Ramady, H.; Prokisch, J.; Mansour, H.; Bayoumi, Y.A.; Shalaby, T.A.; Veres, S.; Brevik, E.C. Review of crop response to soil salinity stress: Possible approaches from leaching to nano-management. Soil Syst. 2024, 8, 11. [Google Scholar] [CrossRef]
- Ahmed, M.; Tóth, Z.; Decsi, K. The impact of salinity on crop yields and the confrontational behavior of transcriptional regulators, nanoparticles, and antioxidant defensive mechanisms under stressful conditions: A review. Int. J. Mol. Sci. 2024, 25, 2654. [Google Scholar] [CrossRef]
- Breś, W.; Kleiber, T.; Markiewicz, B.; Mieloszyk, E.; Mieloch, M. The effect of NaCl stress on the response of lettuce (Lactuca sativa L.). Agronomy 2022, 12, 244. [Google Scholar] [CrossRef]
- Kumari, S.; Chhillar, H.; Chopra, P.; Khanna, R.R.; Khan, M.I.R. Potassium: A track to develop salinity tolerant plants. Plant Physiol. Biochem. 2021, 167, 1011–1023. [Google Scholar] [CrossRef]
- Raddatz, N.; Morales de los Ríos, L.; Lindahl, M.; Quintero, F.J.; Pardo, J.M. Coordinated transport of nitrate, potassium, and sodium. Front. Plant Sci. 2020, 11, 247. [Google Scholar] [CrossRef] [PubMed]
- Marschner, H. Marschner’s Mineral Nutrition of Higher Plants; Academic Press: Cambridge, MA, USA, 2012. [Google Scholar]
- Malekzadeh, M.R.; Roosta, H.R.; Esmaeilzadeh, M. Employing complementary light spectra represents a novel approach for investigating the enhancement of plant resilience in stressful conditions. Greenh. Plant Prod. J. 2024, 1, 12–20. [Google Scholar] [CrossRef]
- Nieves-Cordones, M.; Al Shiblawi, F.R.; Sentenac, H. Roles and transport of sodium and potassium in plants. Alkali Met. Ions Their Role Life 2016, 16, 291–324. [Google Scholar]
- Keisham, M.; Mukherjee, S.; Bhatla, S.C. Mechanisms of sodium transport in plants—Progresses and challenges. Int. J. Mol. Sci. 2018, 19, 647. [Google Scholar] [CrossRef]
- Parida, A.K.; Das, A.B. Salt tolerance and salinity effects on plants: A review. Ecotoxicol. Environ. Saf. 2005, 60, 324–349. [Google Scholar] [CrossRef]
- Sarkar, B.; Hasanuzzaman, M.; Adak, M.K. Insights into the role of iron supplementation in conferring bicarbonate-mediated alkaline stress tolerance in maize. J. Soil Sci. Plant Nutr. 2022, 22, 2719–2734. [Google Scholar] [CrossRef]
- Cartmill, A.D.; Alarcón, A.; Valdez-Aguilar, L.A. Arbuscular mycorrhizal fungi enhance tolerance of Rosa multiflora cv. Burr to bicarbonate in irrigation water. J. Plant Nutr. 2007, 30, 1517–1540. [Google Scholar] [CrossRef]
- Guo, Z.; Qin, Y.; Lv, J.; Wang, X.; Dong, H.; Dong, X.; Zhang, T.; Du, N.; Piao, F. Luffa rootstock enhances salt tolerance and improves yield and quality of grafted cucumber plants by reducing sodium transport to the shoot. Environ. Pollut. 2023, 316, 120521. [Google Scholar] [CrossRef] [PubMed]
- Johnson, R.; Vishwakarma, K.; Hossen, M.S.; Kumar, V.; Shackira, A.; Puthur, J.T.; Abdi, G.; Sarraf, M.; Hasanuzzaman, M. Potassium in plants: Growth regulation, signaling, and environmental stress tolerance. Plant Physiol. Biochem. 2022, 172, 56–69. [Google Scholar] [CrossRef] [PubMed]
- Danchin, A.; Nikel, P.I. Why nature chose potassium. J. Mol. Evol. 2019, 87, 271–288. [Google Scholar] [CrossRef]
- Praveen, A.; Singh, S. The role of potassium under salinity stress in crop plants. Cereal Res. Commun. 2024, 52, 315–322. [Google Scholar] [CrossRef]
- Shelke, D.; Nikalje, G.; Nikam, T.; Maheshwari, P.; Punita, D.; Rao, K.; Kavi Kishor, P.; Suprasanna, P. Chloride (Cl−) uptake, transport, and regulation in plant salt tolerance. In Molecular Plant Abiotic Stress: Biology and Biotechnology; Wiley: Hoboken, NJ, USA, 2019; pp. 241–268. [Google Scholar]
- Behzadi Rad, P.; Roozban, M.R.; Karimi, S.; Ghahremani, R.; Vahdati, K. Osmolyte accumulation and sodium compartmentation has a key role in salinity tolerance of pistachios rootstocks. Agriculture 2021, 11, 708. [Google Scholar] [CrossRef]
- Stoeva, N.; Kaymakanova, M. Effect of salt stress on the growth and photosynthesis rate of bean plants (Phaseolus vulgaris L.). J. Cent. Eur. Agric. 2008, 9, 385–391. [Google Scholar]
- Sustr, M.; Soukup, A.; Tylova, E. Potassium in root growth and development. Plants 2019, 8, 435. [Google Scholar] [CrossRef] [PubMed]
- Ding, W.; Clode, P.L.; Lambers, H. Effects of pH and bicarbonate on the nutrient status and growth of three Lupinus species. Plant Soil 2020, 447, 9–28. [Google Scholar] [CrossRef]
- Penn, C.J.; Camberato, J.J. A critical review on soil chemical processes that control how soil pH affects phosphorus availability to plants. Agriculture 2019, 9, 120. [Google Scholar] [CrossRef]
- Bechtaoui, N.; Rabiu, M.K.; Raklami, A.; Oufdou, K.; Hafidi, M.; Jemo, M. Phosphate-dependent regulation of growth and stresses management in plants. Front. Plant Sci. 2021, 12, 679916. [Google Scholar] [CrossRef]
- Papadopoulos, I.; Rendig, V. Interactive effects of salinity and nitrogen on growth and yield of tomato plants. Plant Soil 1983, 73, 47–57. [Google Scholar] [CrossRef]
- Huang, J.; Jones, A.; Waite, T.D.; Chen, Y.; Huang, X.; Rosso, K.M.; Kappler, A.; Mansor, M.; Tratnyek, P.G.; Zhang, H. Fe (II) redox chemistry in the environment. Chem. Rev. 2021, 121, 8161–8233. [Google Scholar] [CrossRef]
- Galaris, D.; Barbouti, A.; Pantopoulos, K. Iron homeostasis and oxidative stress: An intimate relationship. Biochim. Biophys. Acta (BBA)-Mol. Cell Res. 2019, 1866, 118535. [Google Scholar] [CrossRef] [PubMed]
- Ogun, A.S.; Joy, N.V.; Valentine, M. Biochemistry, heme synthesis. In StatPearls [Internet]; StatPearls Publishing: Tampa, FL, USA, 2023. [Google Scholar]
- Lennarz, W.J.; Lane, M.D. Encyclopedia of Biological Chemistry; Academic Press: Cambridge, MA, USA, 2013. [Google Scholar]
- Molnár, Z.; Solomon, W.; Mutum, L.; Janda, T. Understanding the mechanisms of Fe deficiency in the rhizosphere to promote plant resilience. Plants 2023, 12, 1945. [Google Scholar] [CrossRef]
- Roosta, H.R.; Pourebrahimi, M.; Hamidpour, M. Effects of bicarbonate and different Fe sources on vegetative growth and physiological characteristics of bell pepper (Capsicum annuum L.) plants in hydroponic system. J. Plant Nutr. 2015, 38, 397–416. [Google Scholar] [CrossRef]
- Mohsenian, Y.; Roosta, H.; Karimi, H.; Esmaeilizade, M. Investigation of the ameliorating effects of eggplant, datura, orange nightshade, local Iranian tobacco, and field tomato as rootstocks on alkali stress in tomato plants. Photosynthetica 2012, 50, 411–421. [Google Scholar] [CrossRef]

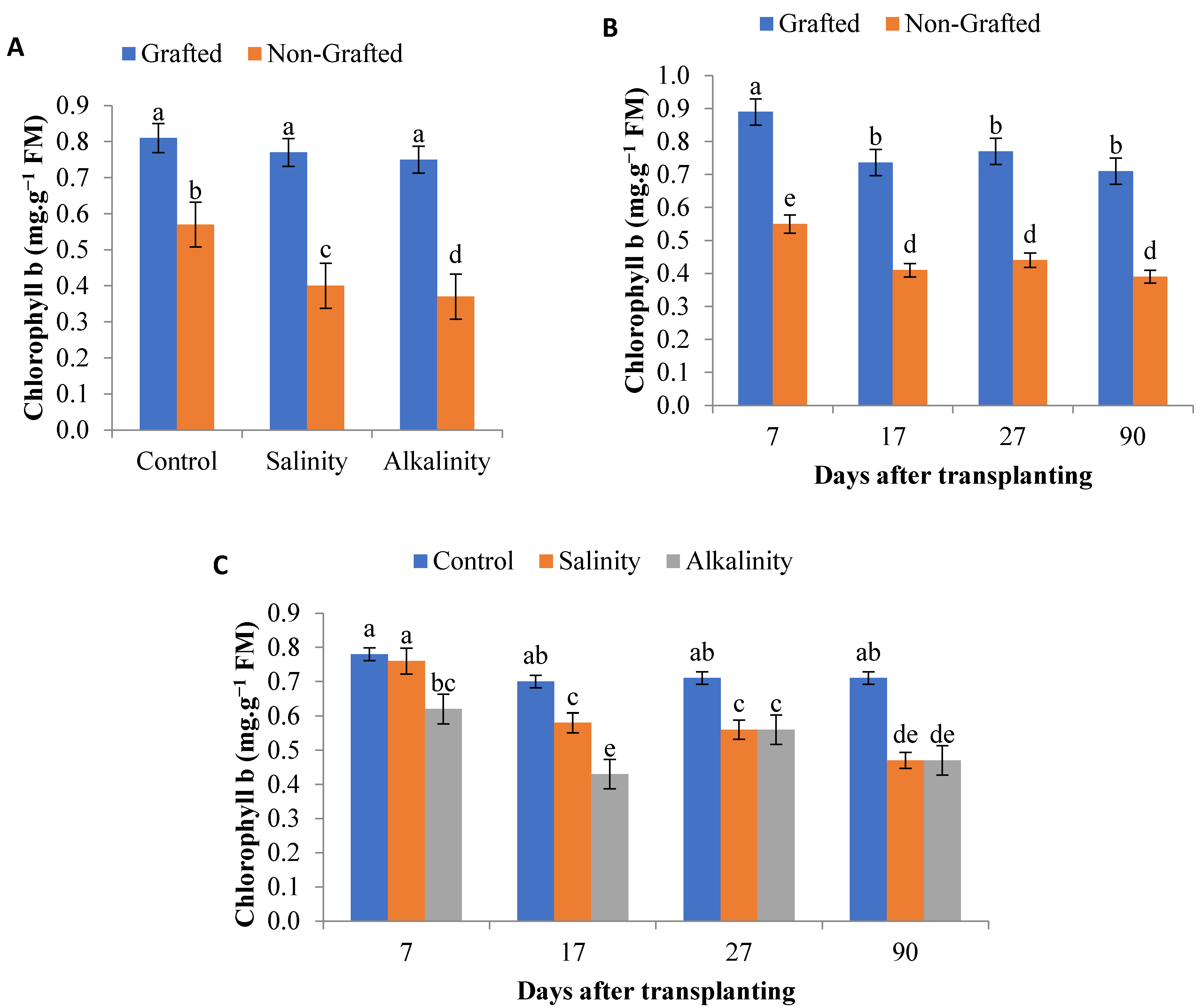
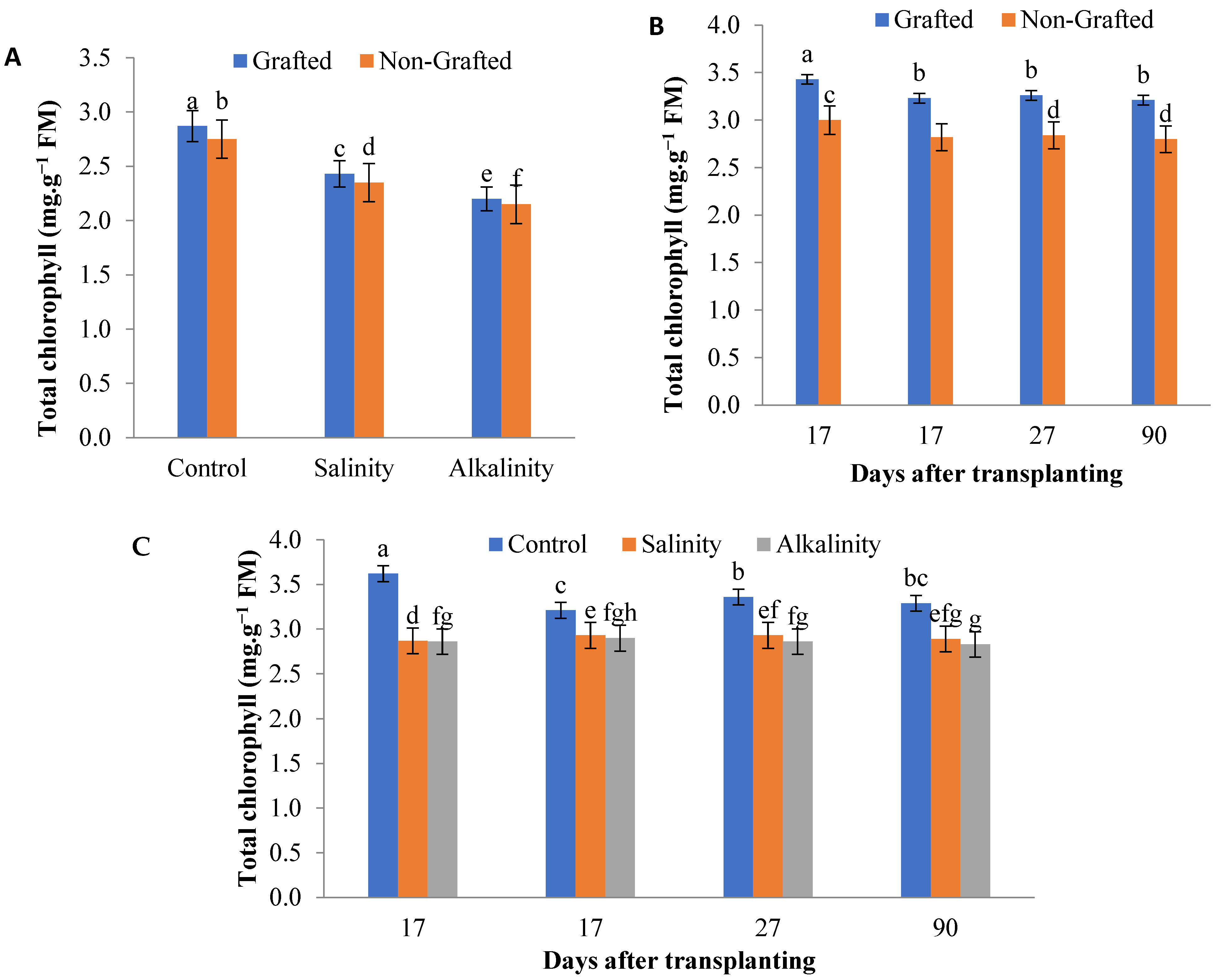


| Chemical Compounds | Chemical Formula | Final Concentration (mg L−1) (Seedling Stage to Fruit Set) | Final Concentration (mg L−1) (from Fruit Set to Harvest) |
|---|---|---|---|
| Macronutrients | |||
| Potassium Dihydrogen Phosphate | KH2PO4 | 270 | 270 |
| Potassium nitrate | KNO3 | 200 | 200 |
| Calcium nitrate | Ca(NO3)2 | 680 | 1357 |
| Magnesium sulfate | (MgSO4, 7H2O) | 500 | 500 |
| Micronutrients | |||
| Iron chelates | Fe-EDDHA | 25 | 25 |
| Boric acid | H3BO3 | 7.5 | 7.5 |
| Manganese sulfate | MnSO4, 7H2O | 8.4 | 8.4 |
| Copper sulfate | CuSO4, 5H2O | 0.54 | 0.54 |
| Molybdenum peroxide | MoO3 | 0.15 | 0.15 |
| Zinc sulfate | ZnSO4, 7H2O | 1.18 | 1.18 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Roosta, H.R.; Kazerani, S.; Raghami, M.R.; Soufi, H.R.; Gruda, N.S. Grafting Boosts Physiological Performance and Nutrient Acquisition of Cantaloupe Under Salt and Bicarbonate Stress in Soilless Culture. Horticulturae 2025, 11, 1389. https://doi.org/10.3390/horticulturae11111389
Roosta HR, Kazerani S, Raghami MR, Soufi HR, Gruda NS. Grafting Boosts Physiological Performance and Nutrient Acquisition of Cantaloupe Under Salt and Bicarbonate Stress in Soilless Culture. Horticulturae. 2025; 11(11):1389. https://doi.org/10.3390/horticulturae11111389
Chicago/Turabian StyleRoosta, Hamid Reza, Solmaz Kazerani, Mahmoud Reza Raghami, Hamid Reza Soufi, and Nazim S. Gruda. 2025. "Grafting Boosts Physiological Performance and Nutrient Acquisition of Cantaloupe Under Salt and Bicarbonate Stress in Soilless Culture" Horticulturae 11, no. 11: 1389. https://doi.org/10.3390/horticulturae11111389
APA StyleRoosta, H. R., Kazerani, S., Raghami, M. R., Soufi, H. R., & Gruda, N. S. (2025). Grafting Boosts Physiological Performance and Nutrient Acquisition of Cantaloupe Under Salt and Bicarbonate Stress in Soilless Culture. Horticulturae, 11(11), 1389. https://doi.org/10.3390/horticulturae11111389








