Analysis of the Structures of Mating-Type A and B Loci in Stropharia rugosoannulata Based on Genomic Data and Development of SNP Molecular Markers
Abstract
1. Introduction
2. Materials and Methods
2.1. Fungal Strains
2.2. Methods for Acquisition of Monokaryotic Strains
2.3. Chromosome Number Determination and Morphological Observation
2.4. Mating Experiments and Identification of Mating Types
2.5. DNA/RNA Preparation
2.6. Genome Sequencing and Assembly
2.7. Chromosome-Level Genome Assembly
2.8. Mating-Type A and B Loci of Analyzed S. rugosoannulata Strain
2.9. SNP Primer Design and Validation
3. Results
3.1. Protoplasts and Basidiospores of Monokaryotic Cultures
3.2. Observation of Chromosome Number and Morphology
3.3. Mating Experiments and Mating-Type Identification
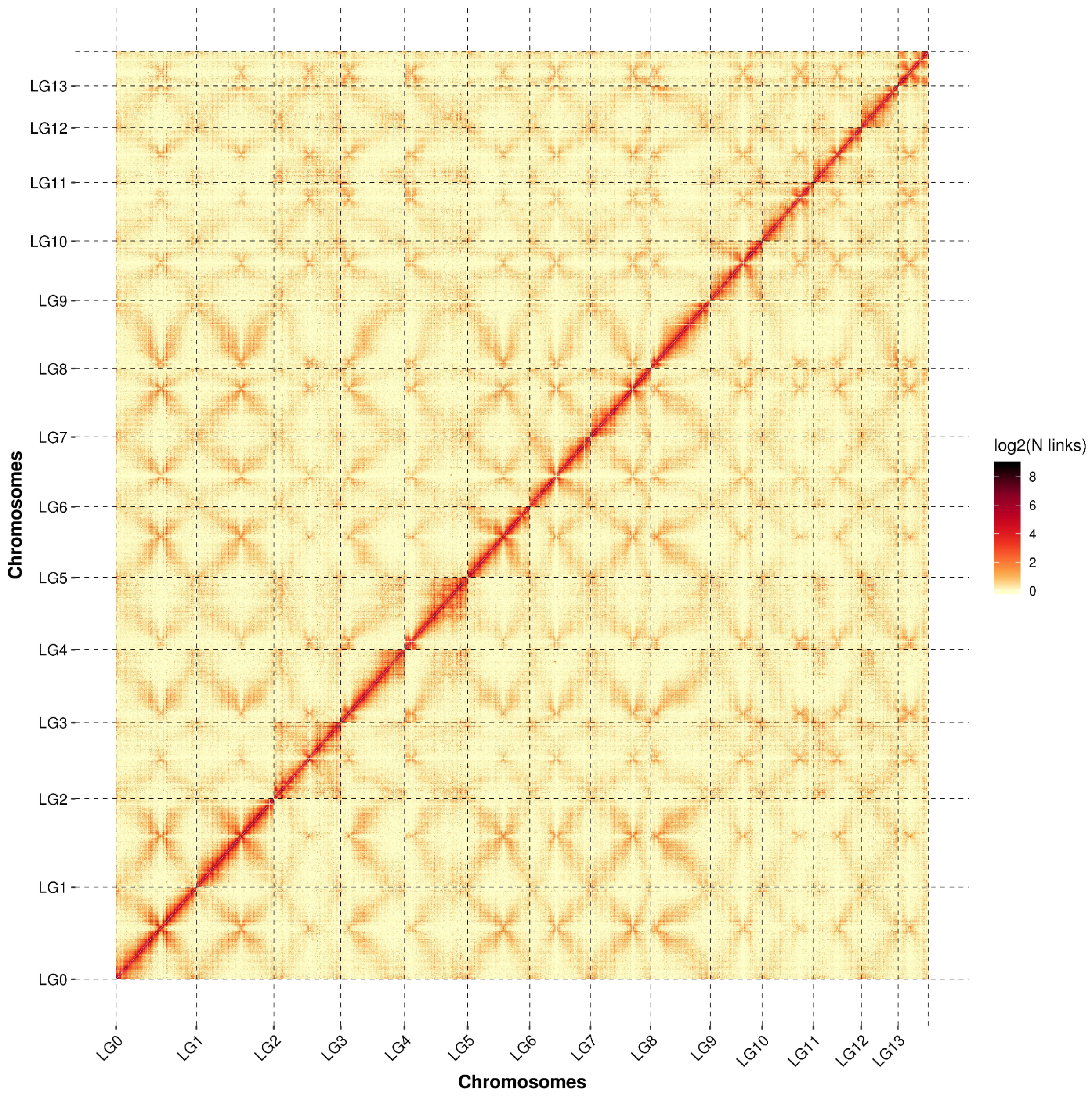
3.4. Fine Genomic Map of S. rugosoannulata Strain Q25 from S1
3.5. Mating-Type Loci in S. rugosoannulata
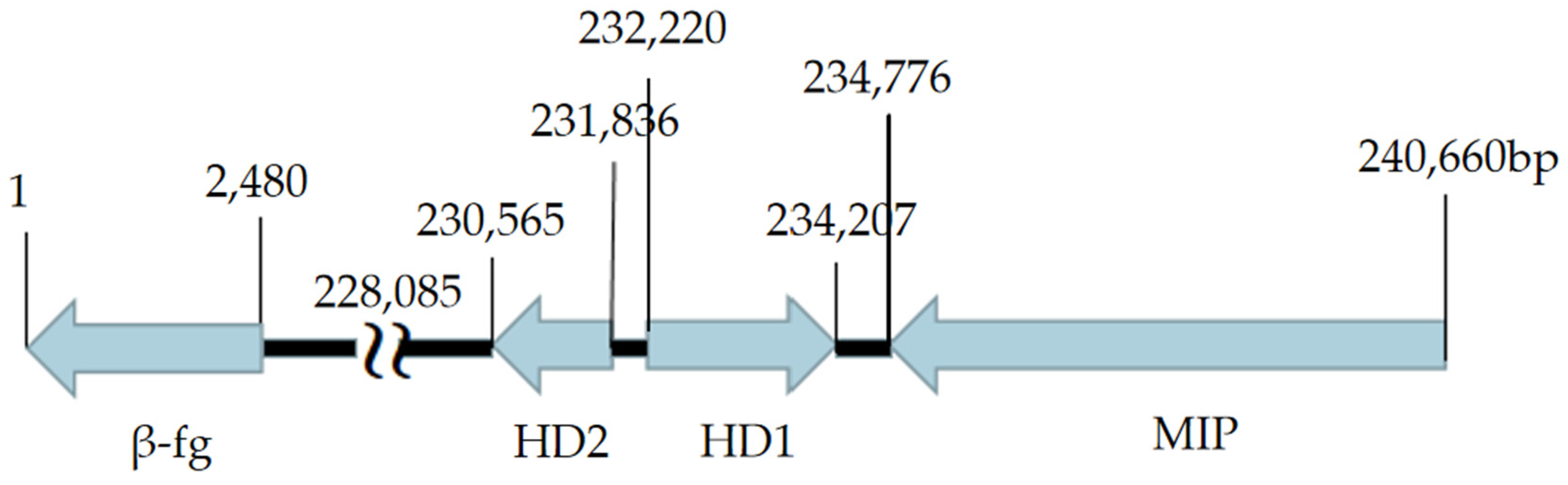
3.5.1. Mating-Type Locus A
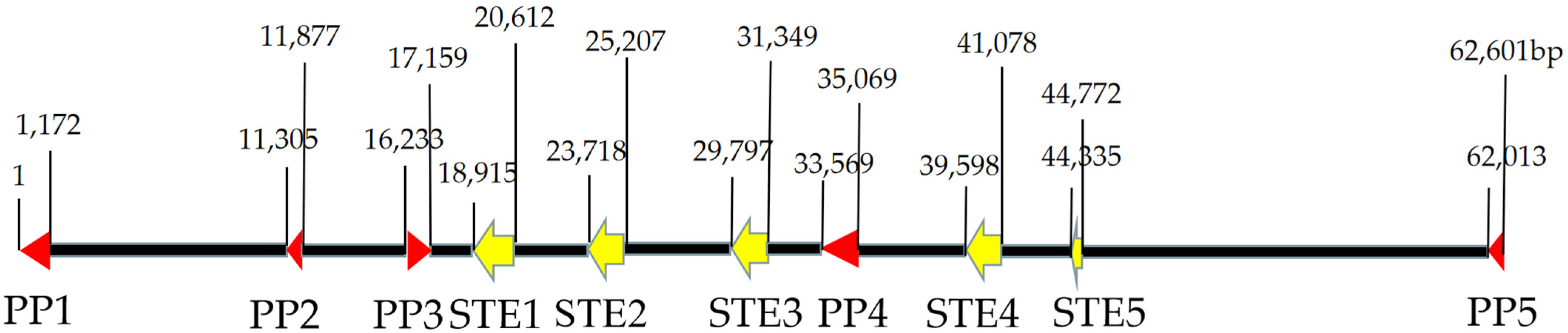
3.5.2. Mating-Type Locus B
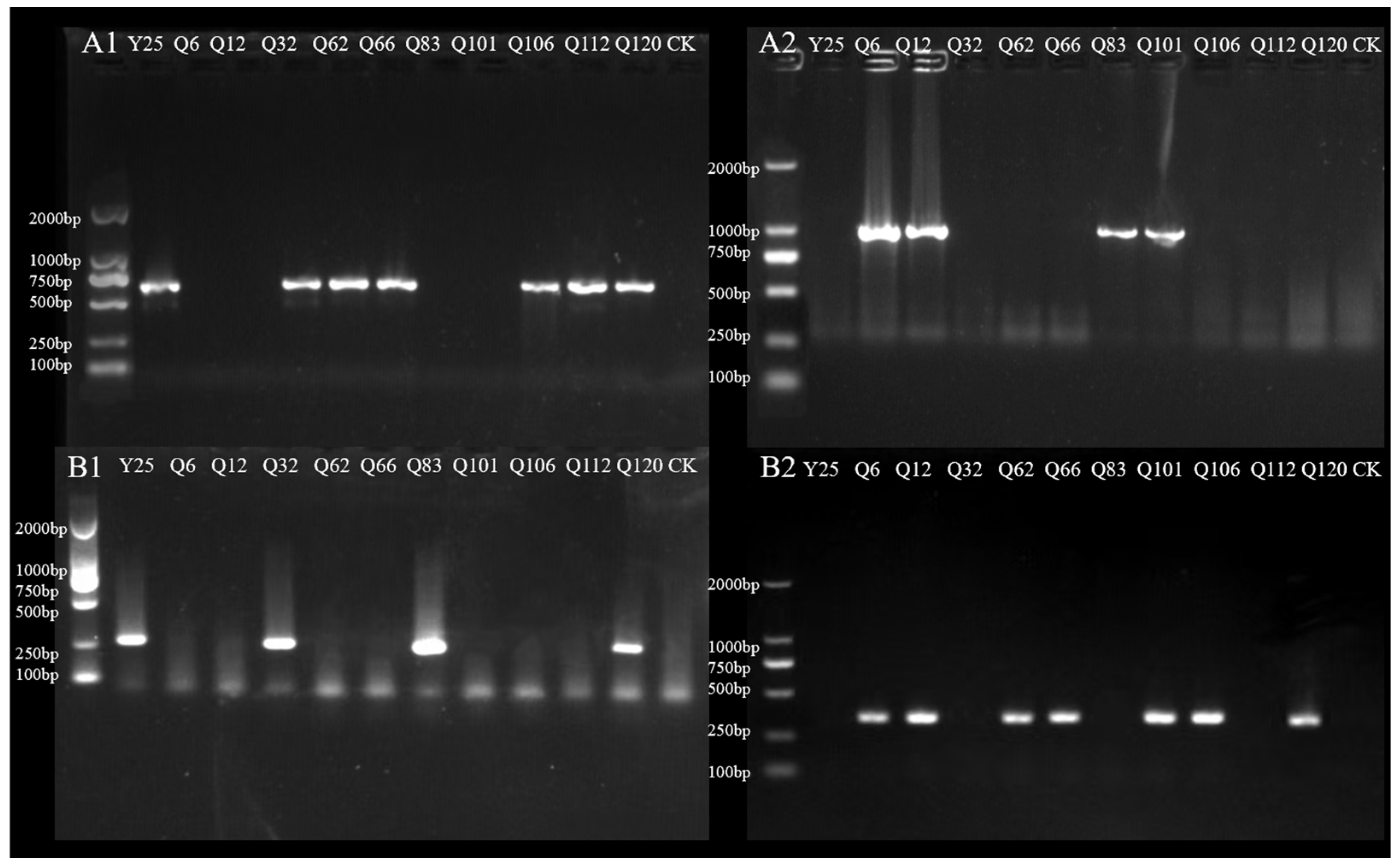
3.6. SNP Validation and Application
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ISSR | Inter-Simple Sequence Repeat |
| ITS | Internal Transcribed Spacer |
| MNP | Multi-Nucleotide Polymorphism |
| RAPD | Random Amplified Polymorphic DNA |
| SNP | Single-Nucleotide Polymorphism |
| SRAP | Sequence-Related Amplified Polymorphism |
| SSR | Simple Sequence Repeat |
References
- Huang, N. Classification and characterization of Strapharia rugoso-annulata. Edible Fungi 1995, 6, 11. [Google Scholar]
- Liu, D.; Chen, Y.Q.; Xiao, X.W.; Zhong, R.T.; Yang, C.F.; Liu, B.; Zhao, C. Nutrient properties and nuclear magnetic resonance-based metabonomic analysis of macrofungi. Foods 2019, 8, 397. [Google Scholar] [CrossRef] [PubMed]
- Shen, M.; Yan, J.; Wang, R.S.; Quan, X.H.; Yuan, Y.; Yao, X.T.; Jin, Q.L. Molecular mechanisms and horticultural implications of temperature-induced color variation in Stropharia rugosoannulata mushrooms. Food Chem. Mol. Sci. 2025, 11, 100276. [Google Scholar] [CrossRef]
- Hu, S.; Feng, X.; Huang, W.; Ibrahim, S.A.; Liu, Y. Effects of drying methods on non-volatile taste components of Stropharia rugoso-annulata mushrooms. LWT 2020, 127, 109428. [Google Scholar] [CrossRef]
- Wang, Q.; Zhao, Y.L.; Feng, X.; Ibrahim, S.A.; Huang, W.; Liu, Y. Effects of drying on the structural characteristics and antioxidant activities of polysaccharides from Stropharia rugosoannulata. J. Food Sci. Technol. 2021, 58, 3622–3631. [Google Scholar] [CrossRef]
- Wu, J.; Kobori, H.; Kawaide, M.; Suzuki, T.; Choi, J.H.; Yasuda, N.; Noguchi, K.; Matsumoto, T.; Hirai, H.; Kawagishi, H. Isolation of bioactive steroids from the Stropharia rugosoannulata mushroom and absolute configuration of strophasterol B. Biosci. Biotechnol. Biochem. 2013, 77, 1779–1781. [Google Scholar] [CrossRef]
- Zhou, B.; Jia, L.; Meng, F.Y.; Song, Z.; Liu, X.N.; Deng, P.; Fan, K.M. Statistical optimization of cultivation conditions for exopolysacchride production and mycelia growth by Stropharia rugosoannulata. Ann. Microbiol. 2010, 60, 89–96. [Google Scholar] [CrossRef]
- Wu, J.; Suzuki, T.; Choi, J.H.; Yasuda, N.; Noguchi, K.; Hirai, H.; Kawagishi, H. An unusual sterol from the mushroom Stropharia rugosoannulata. Tetrahedron Lett. 2013, 54, 4900–4902. [Google Scholar] [CrossRef]
- Otakar, R.; Jiri, M.; Tunde, J. Beta-glucans in higher fungi and their health effects. Nutr. Rev. 2009, 67, 624–631. [Google Scholar] [CrossRef]
- Liu, Y.; Hu, C.F.; Feng, X.; Cheng, L.; A Ibrahim, S.; Wang, C.T.; Huang, W. Isolation, characterization and antioxidant of polysaccharides from Stropharia rugosoannulata. Int. J. Biol. Macromol. 2020, 155, 883–889. [Google Scholar] [CrossRef]
- Zhang, W.W.; Tian, G.T.; Geng, X.R.; Zhao, Y.C.; Ng, T.; Zhao, L.Y.; Wang, H.X. Isolation and characterization of a novel lectin from the edible mushroom Stropharia rugosoannulata. Molecules 2014, 19, 19880–19891. [Google Scholar] [CrossRef]
- Hyun, J.W.; Kim, C.K.; Park, S.H.; Yoon, J.Y.; Shim, M.J.; Kang, C.Y.; Choi, E.C.; Kim, B.K.l. Antitumor components of Agrocybe cylindracea. Arch. Pharmacal Res. 1996, 19, 207–212. [Google Scholar] [CrossRef]
- Yang, Y.; Meng, G.L.; Ni, S.J.; Zhang, H.F.; Dong, C.H. Genomic analysis of Stropharia rugosoannulata reveals its nutritional strategy and application potential in bioremediation. J. Fungi 2022, 8, 162. [Google Scholar] [CrossRef]
- Liers, C.; Arnstadt, T.; Ullrich, R.; Hofrichter, M. Patterns of lignin degradation and oxidative enzyme secretion by different wood-and litter-colonizing basidiomycetes and ascomycetes grown on beech-wood. FEMS Microbiol. Ecol. 2011, 78, 91–102. [Google Scholar] [CrossRef] [PubMed]
- Shang, J.J.; Hou, D.; Li, Y.; Zhou, C.L.; Guo, T.; Tang, L.H.; Mao, W.J.; Chen, Q.; Bao, D.P.; Yang, R.H. Analyses of mating systems in Stropharia rugosoannulata based on genomic data. Mycosystema 2020, 39, 1152–1161. [Google Scholar] [CrossRef]
- Huang, L.; Si, C.; Shi, H.Y.; Huang, F.Q.; Duan, J.; He, C.M. Research progress on genetic breeding of Stropharia rugosoannulata. North. Hortic. 2024, 48, 118–125. (In Chinese) [Google Scholar] [CrossRef]
- Kumar, R.; Das, S.P.; Choudhury, B.U.; Kumar, A.; Prakash, N.R.; Verma, R.; Chakraborti, M.; Devi, A.G.; Bhattacharjee, B.; Das, R.; et al. Advances in genomic tools for plant breeding: Harnessing DNA molecular markers, genomic selection, and genome editing. Biol. Res. 2024, 57, 80. [Google Scholar] [CrossRef]
- Bao, D.P. Scientific Problems in Crossbreeding of Edible Fungi. Acta Edulis Fungi 2020, 27, 1–24. [Google Scholar] [CrossRef]
- Devi, E.L.; Devi, C.P.; Kumar, S.; Sharma, S.K.; Beemrote, A.; Chongtham, S.K.; Singh, C.H.; Tania, C.; Singh, T.B.; Ningombam, A.; et al. Marker assisted selection (MAS) towards generating stress tolerant crop plants. Plant Gene 2017, 11, 205–218. [Google Scholar] [CrossRef]
- Ke, L.N. Molecular Polymorphism in Germplasm Resource of Tricholoma giganteum Massee, Stropharia rugoso-annulata Farlow, Hercium erinaceus, Agrocybe cylindracea. Master’s Thesis, Fujian Agriculture and Forestry University, Fuzhou, China, 2008. [Google Scholar]
- Liu, F.; Cao, B.; Dai, H.M.; Li, G.J.; Li, S.M.; Gao, W.; Zhao, R.L. High-resolution core gene-associated multiple nucleotide polymorphism (cgMNP) markers for strain identification in the wine cap mushroom Stropharia rugosoannulata. Microorganisms 2025, 13, 1685. [Google Scholar] [CrossRef]
- Yan, P.S.; Jiang, J.H.; Cui, W.S. Characterization of protoplasts prepared from the edible fungus, Stropharia rugoso-annulata. World J. Microbiol. Biotechnol. 2004, 20, 173–177. [Google Scholar] [CrossRef]
- Kües, U. From two to many: Multiple mating types in Basidiomycetes. Fungal Biol. Rev. 2015, 29, 126–166. [Google Scholar] [CrossRef]
- Raper John, R. Genetics of sexuality in higher fungi. Bioscience 1966, 17, 846. [Google Scholar]
- Kothe, E. Tetrapolar fungal mating types: Sexes by the thousands. FEMS Microbiol. Rev. 1996, 18, 65–87. [Google Scholar] [CrossRef]
- Ha, B.; Moon, Y.J.; Song, Y.; Kim, S.; Kim, M.; Yoon, C.W.; Ro, H.S. Molecular analysis of B mating type diversity in Lentinula edodes. Sci. Hortic. 2019, 243, 55–63. [Google Scholar] [CrossRef]
- Casselton, L.A.; Olesnicky, N.S. Molecular genetics of mating recognition in basidiomycete fungi. Microbiol. Mol. Biol. Rev. 1998, 62, 55–70. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.Q.; Yang, Y.; Huang, X.H.; Huang, J.; Dong, C.H. Molecular and genetic evidence for a tetrapolar mating system in Sparassis latifolia. Fungal Biol. 2020, 124, 1004–1012. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.S.; Li, X.; Li, G.J.; Huang, Q.; Tian, J.H.; Wang, J.L.; Li, M.; Li, S.M. Genetic and molecular evidence of a tetrapolar mating system in the edible mushroom Grifola frondosa. J. Fungi 2023, 9, 959. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Chai, H.; Yang, W.; Zhang, X.; Chen, Y.; Zhao, Y. Characterization of non-coding regions in B mating loci of Agrocybe salicacola groups: Target sites for B mating type identification. Curr. Microbiol. 2017, 74, 772–778. [Google Scholar] [CrossRef]
- Wang, W.; Lian, L.D.; Xu, P.; Chou, T.S.; Irum, M.; Aron, O.; Muhammad, W.; Chen, B.Z.; Liu, X.R.; Liu, F.; et al. Advances in understanding mating type gene organization in the mushroom-forming fungus Flammulina velutipes. G3 Genes Genomes Genet. 2016, 6, 3635–3645. [Google Scholar] [CrossRef]
- Ha, B.; Kim, S.; Kim, M.; Moon, Y.J.; Song, Y.; Ryu, J.S.; Ryu, H.; Ro, H.S. Diversity of A mating type in Lentinula edodes and mating type preference in the cultivated strains. J. Microbiol. 2018, 56, 416–425. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Yang, J.J.; Zhang, L.K.; Luo, J.; Zhao, H.; Zhang, J.N.; Wen, C.L. A new SNP genotyping technology Target SNP-seq and its application in genetic analysis of cucumber varieties. Sci. Rep. 2020, 10, 5623. [Google Scholar] [CrossRef] [PubMed]
- Onda, Y.; Takahagi, K.; Shimizu, M.; Inoue, K.; Mochida, K. Multiplex PCR targeted amplicon sequencing (MTA-Seq): Simple, flexible, and versatile SNP genotyping by highly multiplexed PCR amplicon sequencing. Front. Plant Sci. 2018, 9, 201. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.; Zhou, Y.X.; Li, K.; Qi, L.X.; Zhang, Q.F.; Wang, M.C.; Xiao, J.H. A novel three-round multiplex PCR for SNP genotyping with next generation sequencing. Anal. Bioanal. Chem. 2016, 408, 4371–4377. [Google Scholar] [CrossRef]
- Meuwissen, T.H.; Hayes, B.J.; Goddard, M.E. Prediction of total genetic value using genome-wide dense marker maps. Genetics 2001, 157, 1819–1829. [Google Scholar] [CrossRef]
- Zhang, M.Y.; Song, C.Y.; Yu, H.L.; Tan, Q.; Xu, Z.; Wang, R.J.; Zhang, L.J.; Shang, X.D. AS-PCR-based identification of mating types in Lentinula edodes using SNP genotyping. Acta Edulis Fungi 2019, 26, 1–9. (In Chinese) [Google Scholar] [CrossRef]
- Campbell, N.R.; Harmon, S.A.; Narum, S.R. Genotyping-in-Thousands by sequencing (GT-seq): A cost effective SNP genotyping method based on custom amplicon sequencing. Mol. Ecol. Resour. 2014, 15, 855–867. [Google Scholar] [CrossRef]
- Song, H.Y.; Zhao, P. Research progress on molecular biological detection of edible mushroom strains. China Cucurbits Veg. 2024, 37, 9–17. [Google Scholar] [CrossRef]
- Gao, Y. Study on Key Technologies and Screening Strains of Stropharia rugosoannulata. Master’s Thesis, Northwest A&F University, Xianyang, China, 2014; p. 63. (In Chinese). [Google Scholar]
- Zhou, F.; Yang, H.L.; Zhao, Y.; Jiang, S.X.; Chen, M.J.; Li, Z.P.; Cha, L.; Li, Y. Characteristics of mononuclear properties and the protoplast monokaryogenesis of different varieties of Stropharia rugosoannulata. Acta Agric. Shanghai 2023, 39, 33–37. (In Chinese) [Google Scholar] [CrossRef]
- Guo, B.F. Karyotype Analysis and FISH Analysis of Infection Related Genes in Metarhizium Acridum. Master’s Thesis, Chongqing University of Technology, Chongqing, China, 2014; p. 63. (In Chinese). [Google Scholar]
- Du, X.H.; Zhao, Q.; Xia, E.H.; Gao, L.Z.; Richard, F.; Yang, Z.L. Mixed-reproductive strategies, competitive mating-type distribution and life cycle of fourteen black morel species. Sci. Rep. 2017, 7, 1493. [Google Scholar] [CrossRef]
- Cheng, H.; Concepcion, G.T.; Feng, X.; Zhang, H.; Li, H. Haplotype-resolved de novo assembly using phased assembly graphs with hifiasm. Nat. Methods 2021, 18, 170–175. [Google Scholar] [CrossRef]
- Walker, B.J.; Abeel, T.; Shea, T.; Priest, M.; Abouelliel, A.; Sakthikumar, S.; Cuomo, C.A.; Zeng, Q.; Wortman, J.; Young, S.K.; et al. An integrated tool for comprehensive microbial variant detection and genome assembly improvement. PLoS ONE 2014, 9, e112963. [Google Scholar] [CrossRef]
- Simão, F.A.; Waterhouse, R.M.; Ioannidis, P.; Kriventseva, E.V.; Zdobnov, E.M. BUSCO: Assessing genome assembly and annotation completeness with single-copy orthologs. Bioinformatics 2015, 31, 3210. [Google Scholar] [CrossRef]
- Li, H. Aligning sequence reads, clone sequences and assembly contigs with BWA-MEM. arXiv 2013, arXiv:1303.3997. [Google Scholar] [CrossRef]
- Burton, J.N.; Adey, A.; Patwardhan, R.P.; Qiu, R.; Kitzman, J.O.; Shendure, J. Chromosome-scale scaffolding of de novo genome assemblies based on chromatin interactions. Nat. Biotechnol. 2013, 31, 1119–1125. [Google Scholar] [CrossRef] [PubMed]
- Stanke, M.; Waack, S. Gene prediction with a hidden markov model and a new intron submodel. Bioinformatics 2003, 19, 1453–1454. [Google Scholar] [CrossRef] [PubMed]
- Majoros, W.H.; Pertea, M.; Salzberg, S.L. Tigrscan and glimmer HMM: Two open source ab initio eukaryotic gene-finders. Bioinformatics 2004, 20, 2878–2879. [Google Scholar] [CrossRef] [PubMed]
- Koressaar, T.; Lepamets, M.; Kaplinski, L.; Raime, K.; Andreson, R.; Remm, M. Primer3_masker: Integrating masking of template sequence with primer design software. Bioinformatics 2018, 34, 1937–1938. [Google Scholar] [CrossRef]
- Lu, M.; Li, J.; Sun, X.; Zhao, D.Q.; Zong, H.Z.; Tang, C.; Li, K.; Zhou, Y.X.; Xiao, J.H. Genotyping single nucleotide polymorphisms in homologous regions using multiplex kb level amplicon capture sequencing. Mol. Genet. Genom. 2024, 299, 99. [Google Scholar] [CrossRef]
- Choi, Y.J.; Jung, S.; Eom, H.; Hoang, T.; Han, H.G.; Kim, S.; Ro, H.S. Structural analysis of the A mating type locus and development of the mating type marker of Agaricus bisporus var. bisporus. J. Fungi 2023, 9, 284. [Google Scholar] [CrossRef]
- Fen, P.; Sun, Y.; Hao, L.B.; An, J.L.; Gai, Z.P.; Wang, Y.X.; Zheng, S.Y.; Wang, C.X. Study on isolation and mating type determination of monokaryotic spores of Pleurotus ostreatus ‘Jiping 16’. Edible Med. Mushrooms 2024, 32, 386–392. [Google Scholar]
- Larraya, L.M.; Pérez, G.; Iribarren, I.; Blanco, J.A.; Alfonso, M.; Pisabarro, A.G.; Ramírez, L. Relationship between monokaryotic growth rate and mating type in the edible basidiomycete Pleurotus ostreatus. Appl. Environ. Microbiol. 2001, 67, 3385–3390. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Cao, H.; Pan, Y.J.; Cao, J.Y. Study on mating system of Auricularia auricula. Mycosystema 2002, 21, 559–564. (In Chinese) [Google Scholar]
- Wang, Y.; Xiong, H.Y.; Yang, R.H.; Wu, Y.Y.; Liu, X.; Bao, D.P. Nuclear partial segregation occurred in protoplast monokaryotization and asexual spore monosporous isolation of wild strains of Flammulina filiformis. Mycosystema 2025, 44, 75. (In Chinese) [Google Scholar] [CrossRef]
- Ke, B.R.; Lu, Z.H.; Wu, X.P.; Guo, L.X.; Lan, Q.X. Determination and diversity analysis of mating types of Ganoderma lucidum basidispores. Chin. J. Trop. Crops 2018, 39, 145–150. [Google Scholar]
- Liu, X.Y. Preliminary Research on the Breeding of Excellent Hybrids of Stropharia rugosoannulata in this Mushroom and Rice Rotation. Master’s Thesis, Huazhong Agricultural University, Wuhan, China, 2023; p. 99. [Google Scholar] [CrossRef]
- Casselton, L.A. Fungal sex genes e searching for the ancestors. Bioassay 2008, 30, 711–714. [Google Scholar] [CrossRef]
- Raudaskoski, M.; Kothe, E. Basidiomycetes mating type gens and pheromone signaling. Eukaryot. Cell 2010, 9, 847–859. [Google Scholar] [CrossRef]
- Chen, L.; Gong, Y.; Cai, Y.; Liu, W.; Zhou, Y.; Xiao, Y.; Xu, Z.; Liu, Y.; Lei, X.; Wang, G.Z.; et al. Genome sequence of the edible cultivated mushroom Lentinula edodes (shiitake) reveals insights into lignocellulose degradation. PLoS ONE 2016, 11, e0160336. [Google Scholar] [CrossRef]
- Au, C.H.; Wong, M.C.; Bao, D.P.; Zhang, M.Y.; Song, C.Y.; Song, W.H.; Law, P.T.W.; Kües, U.; Kwan, H.S. The genetic structure of the A mating-type locus of Lentinula edodes. Gene 2014, 535, 184–190. [Google Scholar] [CrossRef]
- Ohm, R.A.; De Jong, J.F.; Lugones, L.G.; Aerts, A.; Kothe, E.; Stajich, J.E.; de Vries, R.P.; Record, E.; Levasseur, A.; Baker, S.E.; et al. Genome sequence of the model mushroom Schizophyllum commune. Nat. Biotechnol. 2010, 28, 957–963. [Google Scholar] [CrossRef]
- Nishimura, A.; Linder, M.E. Identification of a novel prenyl and palmitoyl modification at the CaaX Motif of Cdc42 that regulates RhoGDI binding. Mol. Cell. Biol. 2013, 33, 1417–1429. [Google Scholar] [CrossRef]
- Fowler, T.J.; Mitton, M.F.; Vaillancourt, L.J. Carlene a raper, changes in mate recognition through alterations of pheromones and receptors in the multisexual mushroom fungus Schizophyllum commune. Genetics 2001, 158, 1491–1503. [Google Scholar] [CrossRef] [PubMed]
- Meritxell, R.; Michael, P.C.; Lorna, A.C.; Andrew, J.B. The origin of multiple B mating specificities in Coprinus cinereus. Genetics 2005, 170, 1105–1119. [Google Scholar] [CrossRef] [PubMed]
- James, T.Y.; Kües, U.; Rehner, S.A.; Vilgalys, R. Evolution of the gene encoding mitochondrial intermediate peptidase and its cosegregation with the A mating-type locus of mushroom fungi. Fungal Genet. Biol. 2004, 41, 381–390. [Google Scholar] [CrossRef]
- Chen, B.S.; Fu, J.S.; Long, Y.; Zhang, L.; Zhang, X.Y.; Zhang, X.; Hu, F.L.; Jiang, Y.J.; Xie, B.G. Sexual life cycle of Volvariella volvacea revealed by investigation of the random migration of tetrad. Mycosystema 2017, 36, 466–472. [Google Scholar]
- Zhao, Y.N. Study on Mating Type of Pleurotus. Master’s Thesis, Anhui Agriclitural University, Hefei, China, 2023; p. 70. (In Chinese). [Google Scholar]
- Chen, B.Z.; Qiu, M.M.; Ye, L.Y.; Chen, T.C.; Lu, X.; Jiang, Y.; Wu, X.P. Genomic data reveal unique structures of the mating loci of the progeny of Pleurotus pulmonarius wild-type strain X1. Mycosystema 2019, 38, 2205–2213. (In Chinese) [Google Scholar] [CrossRef]




| Primer Name | Upstream Prime (5′-3′) | Primer Name | Downstream Primer (5′-3′) | Annealing Temperature |
|---|---|---|---|---|
| A1F8 | GCTCTTCAAACCCTCAATAATAGTC | A1R8-1 | TATTCTGGAATGAGGATCTCAGAGC | 55 °C |
| A2F1 | CATTGTTGCCAGCGAGGAC | A2R1-4 | ATGATGGTCCCAATACCCGA | 58 °C |
| B1F9 | TGTTGCGTAGGATCAGGGGAGT | B1R9-2 | CCATCTCTTCGCTCACCGATGT | 57 °C |
| B2F9 | TTGCGTAGGATCAGGGGAGG | B2F9-2 | ATCCACCTCTGGCAAGTTTAGGA | 58 °C |
| Selfed Strains | Hybrid Combinations | Mating Types | Fruiting Performance |
|---|---|---|---|
| D19 | Q6*Q62 | A2B2*A1B2 | − |
| D21 | Q101*Y25 | A2B2*A1B1 | + |
| D34 | Q83*Q66 | A2B1*A1B2 | + |
| D41 | Q12*Q120 | A2B2*A1B2 | − |
| D67 | Q83*Q32 | A2B1*A1B1 | − |
| D72 | Q101*Q112 | A2B2*A1B1 | + |
| D87 | Q83*Q106 | A2B1*A1B2 | + |
| D90 | Q101*Q62 | A2B2*A1B2 | − |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, P.; Shao, J.; Li, X.; Liu, H.; Xiao, S.; Ma, A.; Li, M.; Tian, J.; Wang, J.; Zhu, P.; et al. Analysis of the Structures of Mating-Type A and B Loci in Stropharia rugosoannulata Based on Genomic Data and Development of SNP Molecular Markers. Horticulturae 2025, 11, 1325. https://doi.org/10.3390/horticulturae11111325
Zhang P, Shao J, Li X, Liu H, Xiao S, Ma A, Li M, Tian J, Wang J, Zhu P, et al. Analysis of the Structures of Mating-Type A and B Loci in Stropharia rugosoannulata Based on Genomic Data and Development of SNP Molecular Markers. Horticulturae. 2025; 11(11):1325. https://doi.org/10.3390/horticulturae11111325
Chicago/Turabian StyleZhang, Panpan, Jiakun Shao, Xiao Li, Haodong Liu, Shangshang Xiao, Ao Ma, Ming Li, Jinghua Tian, Junling Wang, Peng Zhu, and et al. 2025. "Analysis of the Structures of Mating-Type A and B Loci in Stropharia rugosoannulata Based on Genomic Data and Development of SNP Molecular Markers" Horticulturae 11, no. 11: 1325. https://doi.org/10.3390/horticulturae11111325
APA StyleZhang, P., Shao, J., Li, X., Liu, H., Xiao, S., Ma, A., Li, M., Tian, J., Wang, J., Zhu, P., Shao, Y., Li, S., & Li, G. (2025). Analysis of the Structures of Mating-Type A and B Loci in Stropharia rugosoannulata Based on Genomic Data and Development of SNP Molecular Markers. Horticulturae, 11(11), 1325. https://doi.org/10.3390/horticulturae11111325






