Host Genotype Shapes Fungal Symbiont-Mediated Nutrient and Growth Benefits in Citrus
Abstract
1. Introduction
2. Materials and Methods
2.1. Fungal Inoculum Preparation
2.2. Plant Culture
2.3. Experimental Design
2.4. Assessment of Root Colonization and Spring Shoot Growth
2.5. Determination of Leaf Gas Exchange Parameters
2.6. Analysis of Endogenous Phytohormones
2.7. Determination of Mineral Element Concentrations in Leaves
2.8. Analysis of Fe/Mg Transporter Gene Expression
2.9. Statistical Analysis and Data Visualization
3. Results
3.1. Effects of Fungal Inoculation on Root Colonization Rates of Citrus
3.2. Effects of Fungal Inoculation on Spring Shoot Growth and Growth Performance of Citrus
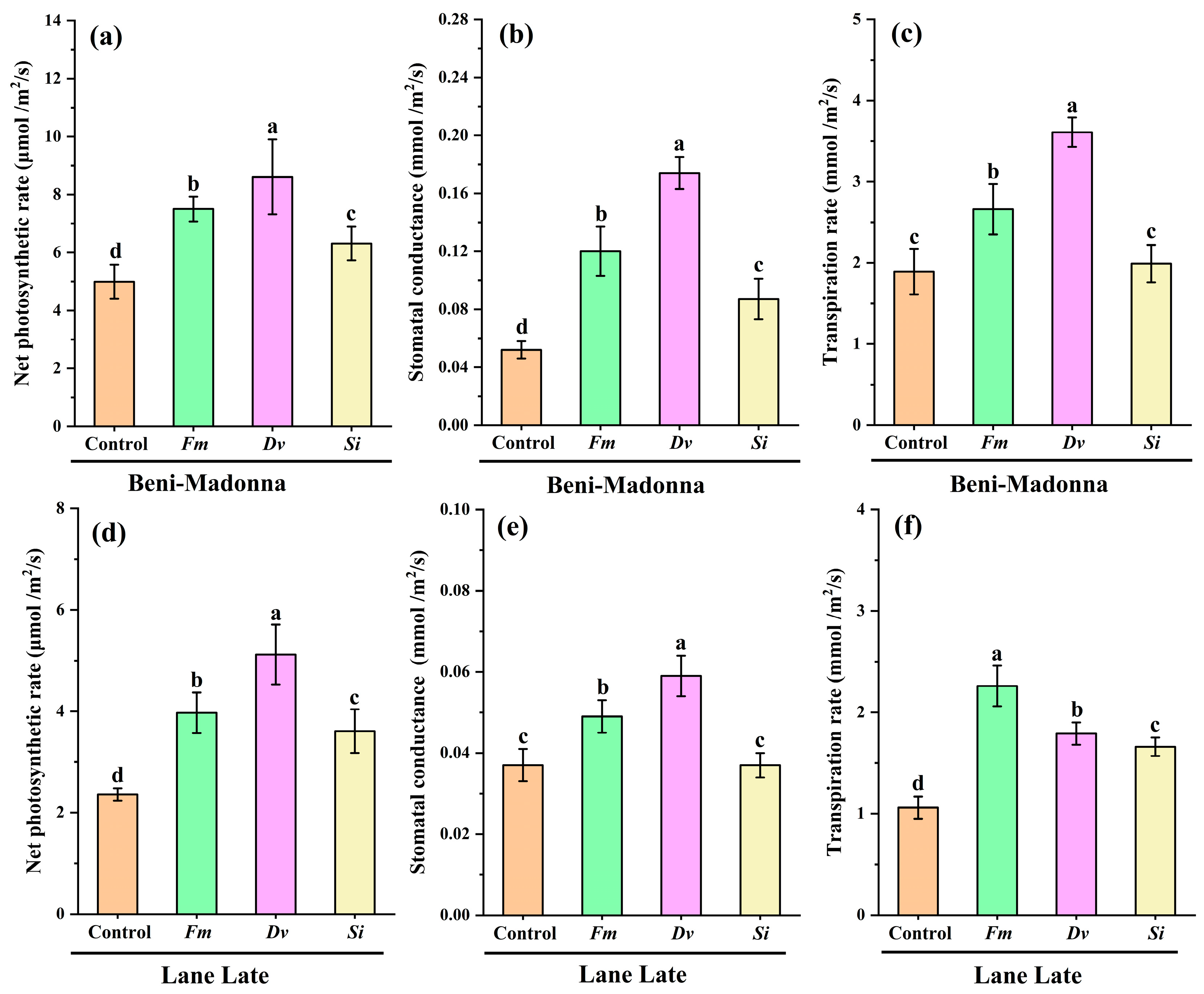
3.3. Effects of Fungal Inoculation on Leaf Gas Exchange Parameters of Citrus
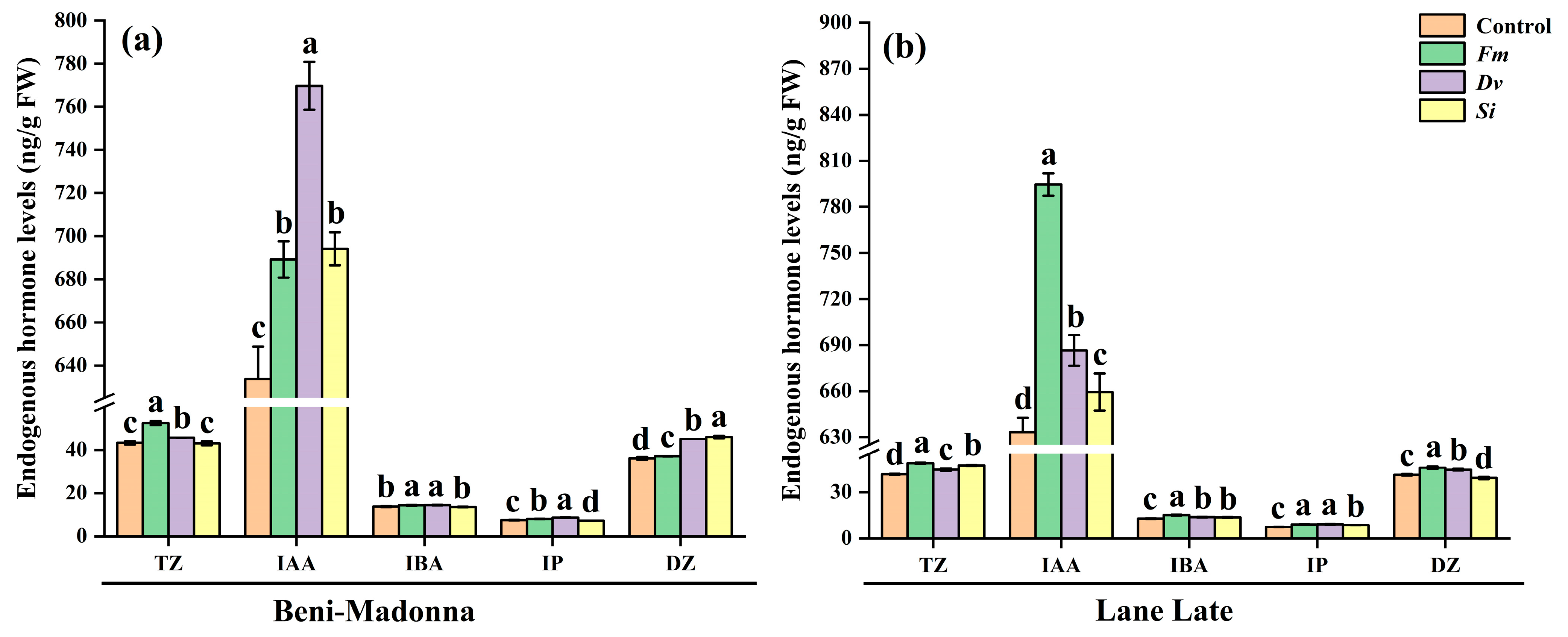
3.4. Effects of Fungal Inoculation on Leaf Endogenous Hormone Levels of Citrus
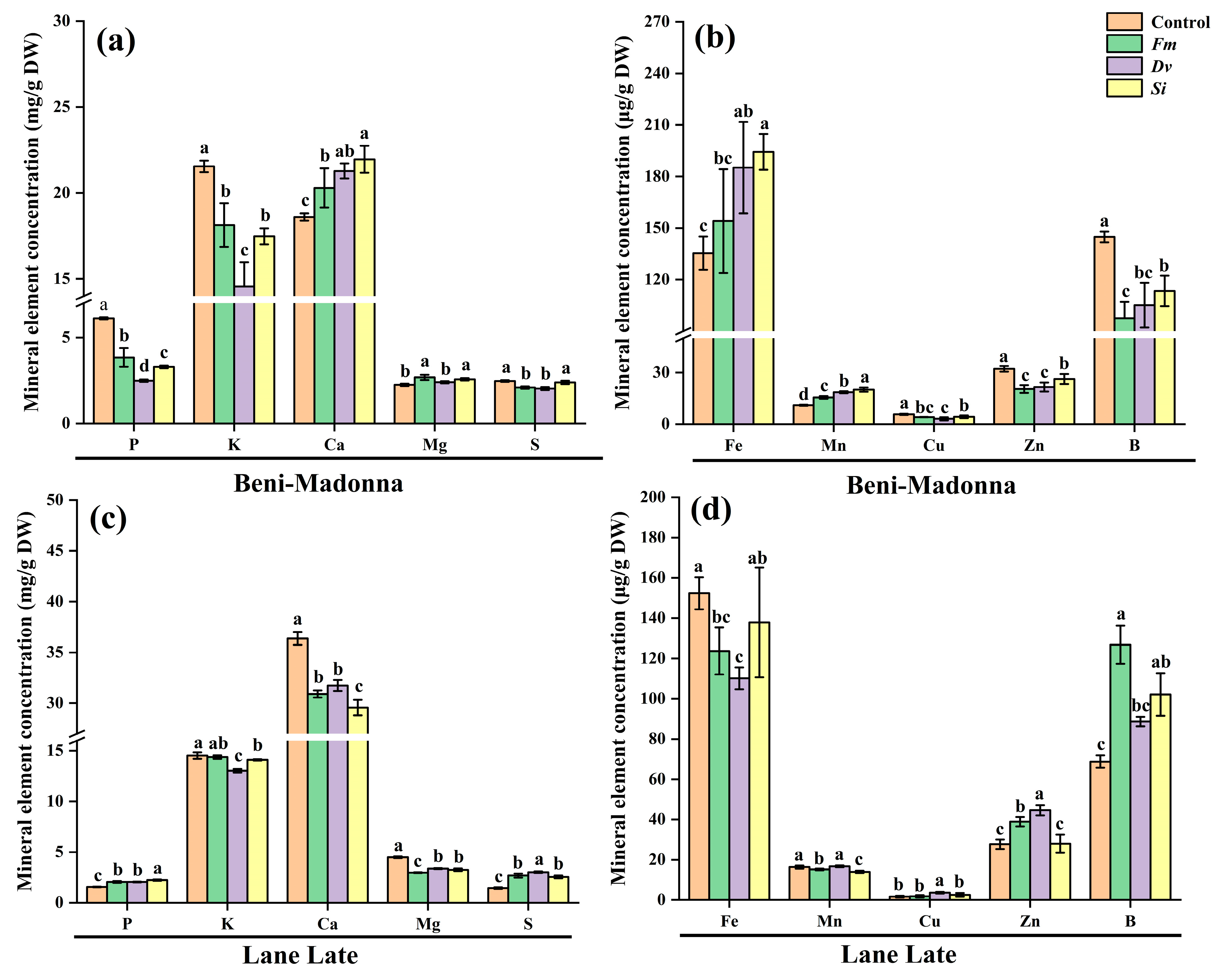
3.5. Effects of Fungal Inoculation on Leaf Mineral Element Concentrations of Citrus
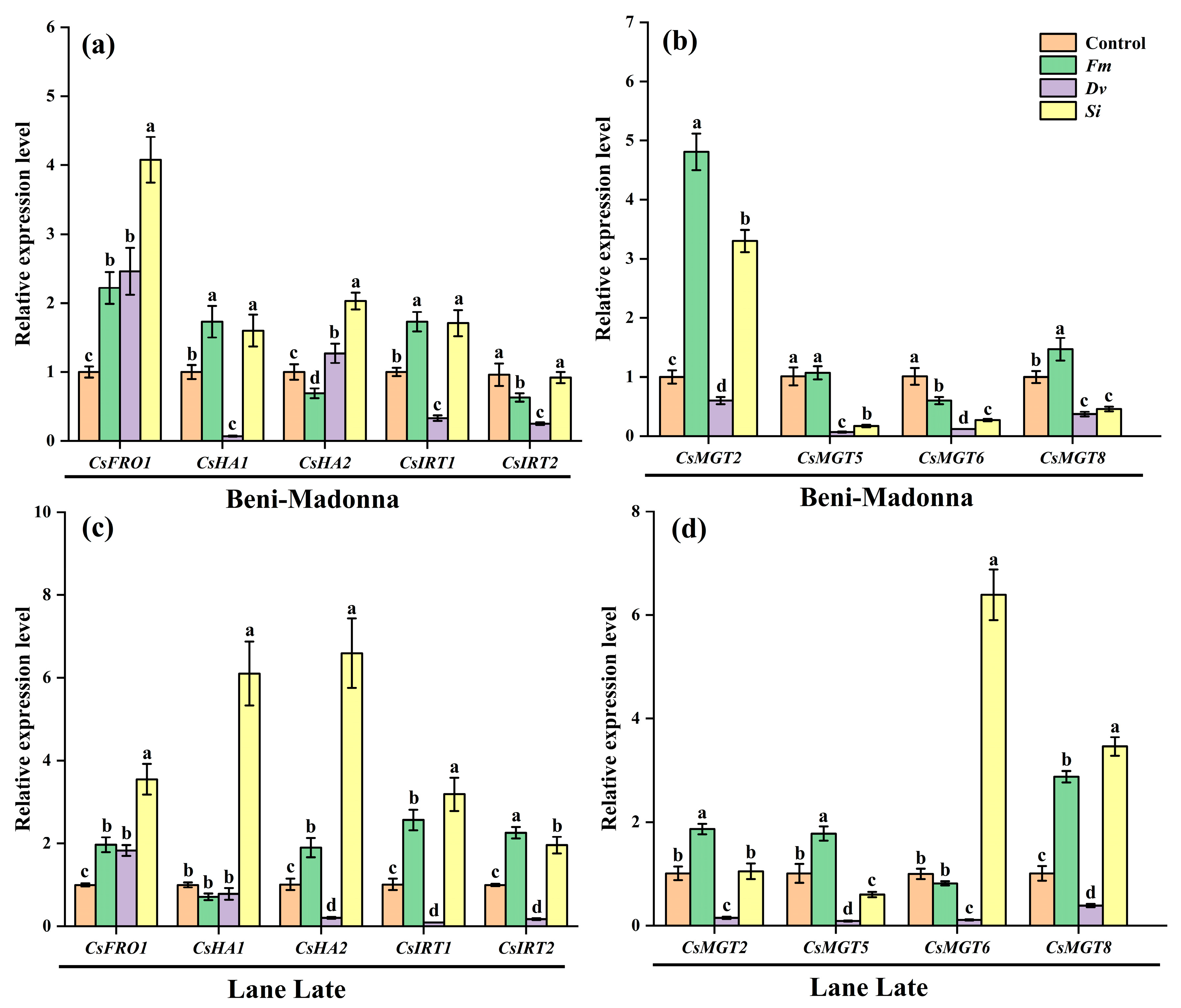
3.6. Effects of Fungal Inoculation on the Expression of Leaf Fe/Mg Transporter Genes of Citrus
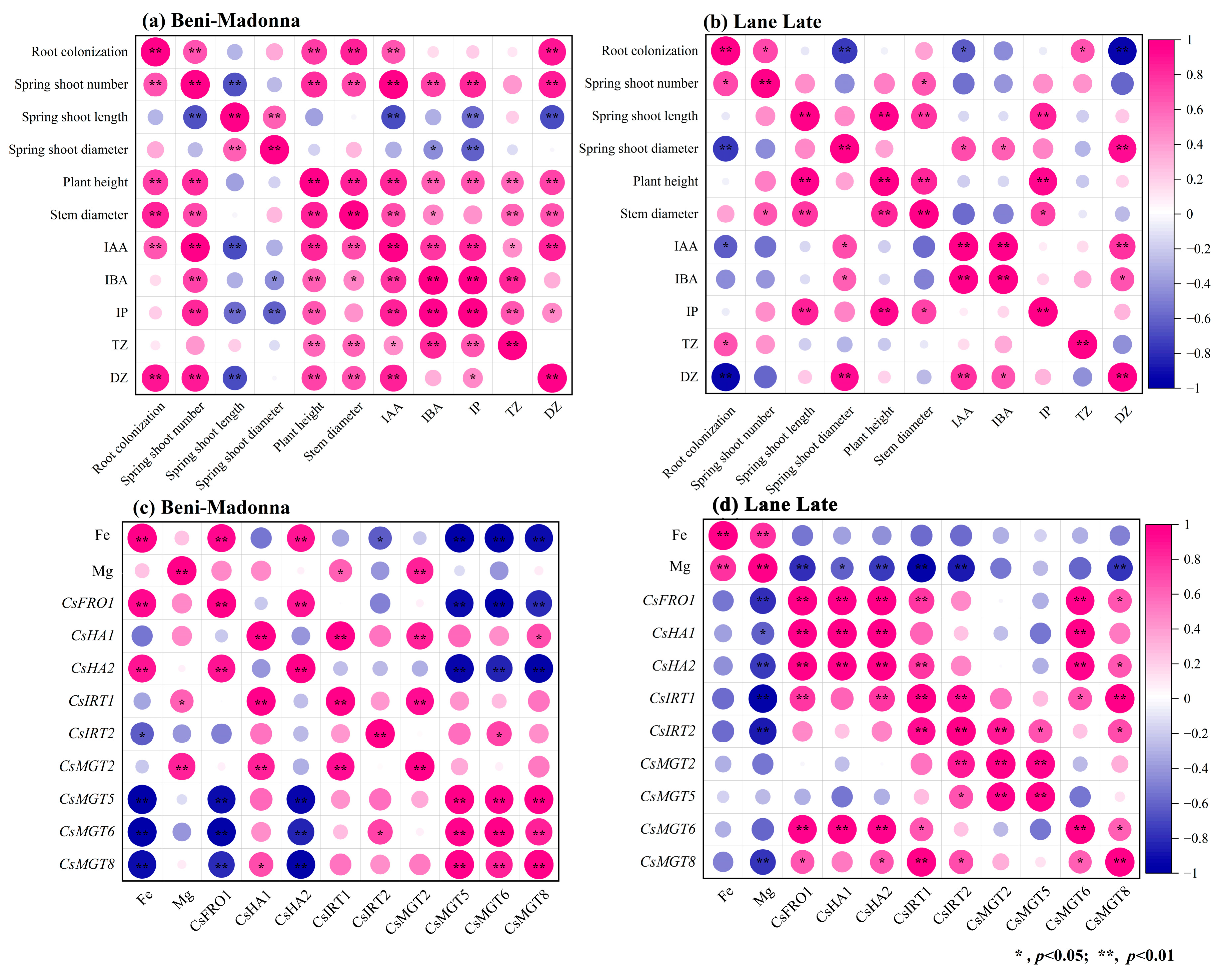
3.7. Correlation Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lu, X.M.; Zhao, C.Y.; Shi, H.; Liao, Y.C.; Xu, F.; Du, H.J.; Xiao, H.; Zheng, J.K. Nutrients and bioactives in citrus fruits: Different citrus varieties, fruit parts, and growth stages. Crit. Rev. Food Sci. 2023, 63, 2018–2041. [Google Scholar] [CrossRef]
- Liu, S.S.; Qian, M.F. Introduction and efficient cultivation techniques of the new citrus variety ‘Beni-Madonna’. Jiangxi Agric. 2020, 10, 11–12. [Google Scholar]
- Hu, Y.P. Comparison and Analysis of Fruit Quality of ‘Lant Late’ and ‘Newhall’ Navel Orange. Master’s Thesis, Huazhong Agricultural University, Wuhan, China, 2024. [Google Scholar]
- Singh, R.; Singh, G.S. Traditional agriculture: A climate-smart approach for sustainable food production. Energy Ecol. Environ. 2017, 2, 296–316. [Google Scholar] [CrossRef]
- Shafqat, W.; Naqvi, S.A.; Maqbool, R.; Haider, M.S.; Jaskani, M.J.; Khan, I.A. Climate change and citrus. In Citrus-Research, Development and Biotechnology; Khan, M.S., Khan, I.A., Eds.; IntechOpen: London, UK, 2021; pp. 147–164. [Google Scholar]
- Hyde, K.D.; Soytong, K. The fungal endophyte dilemma. Fungal Divers. 2008, 33, 173. [Google Scholar]
- Smith, S.E.; Jakobsen, I.; Grønlund, M.; Smith, F.A. Roles of arbuscular mycorrhizas in plant phosphorus nutrition: Interactions between pathways of phosphorus uptake in arbuscular mycorrhizal roots have important implications for understanding and manipulating plant phosphorus acquisition. Plant Physiol. 2011, 156, 1050–1057. [Google Scholar] [CrossRef]
- Basu, S.; Rabara, R.C.; Negi, S. AMF: The future prospect for sustainable agriculture. Physiol. Mol. Plant Pathol. 2018, 102, 36–45. [Google Scholar] [CrossRef]
- Khaliq, A.; Perveen, S.; Alamer, K.H.; Zia Ul Haq, M.; Rafique, Z.; Alsudays, I.M.; Althobaiti, A.T.; Saleh, M.A.; Hussain, S.; Attia, H. Arbuscular mycorrhizal fungi symbiosis to enhance plant-soil interaction. Sustainability 2022, 14, 7840. [Google Scholar] [CrossRef]
- Battini, F.; Grønlund, M.; Agnolucci, M.; Giovannetti, M.; Jakobsen, I. Facilitation of phosphorus uptake in maize plants by mycorrhizosphere bacteria. Sci. Rep. 2017, 7, 4686. [Google Scholar] [CrossRef]
- Wang, D.; Wu, W.J.; Tian, X.; Xiang, N.; Hashem, A.; Abd_Allah, E.F.; Wu, Q.S.; Zou, Y.N. AMF improves response to waterlogging stress in cucumber. Rhizosphere 2024, 30, 100891. [Google Scholar] [CrossRef]
- Cheng, H.Q.; Zou, Y.N.; Wu, Q.S.; Kuča, K. Arbuscular mycorrhizal fungi alleviate drought stress in trifoliate orange by regulating H+-ATPase activity and gene expression. Front. Plant Sci. 2021, 12, 659694. [Google Scholar] [CrossRef] [PubMed]
- Huang, G.M.; Zou, Y.N.; Wu, Q.S.; Xu, Y.J.; Kuča, K. Mycorrhizal roles in plant growth, gas exchange, root morphology, and nutrient uptake of walnuts. Plant Soil Environ. 2020, 66, 295–302. [Google Scholar] [CrossRef]
- Smith, S.E.; Dickson, S.; Smith, F.A. Nutrient transfer in arbuscular mycorrhizas: How are fungal and plant processes integrated? Funct. Plant Biol. 2001, 28, 685–696. [Google Scholar] [CrossRef]
- Lanfranco, L.; Fiorilli, V.; Gutjahr, C. Partner communication and role of nutrients in the arbuscular mycorrhizal symbiosis. New Phytol. 2018, 220, 1031–1046. [Google Scholar] [CrossRef]
- Zou, Y.N.; Xu, Y.J.; Liu, R.C.; Huang, G.M.; Kuča, K.; Srivastava, A.K.; Hashem, A.; Abd_Allah, E.F.; Wu, Q.S. Two different strategies of Diversispora spurca-inoculated walnut seedlings to improve leaf P acquisition at low and moderate P levels. Front. Plant Sci. 2023, 14, 1140467. [Google Scholar] [CrossRef] [PubMed]
- Weber, S.E.; Bascompte, J.; Kahmen, A.; Niklaus, P.A. Plant choice between arbuscular mycorrhizal fungal species results in increased plant P acquisition. PLoS ONE 2024, 19, e0292811. [Google Scholar] [CrossRef]
- Rahman, M.A.; Parvin, M.; Das, U.; Ela, E.J.; Lee, S.H.; Lee, K.W.; Kabir, A.H. Arbuscular mycorrhizal symbiosis mitigates iron (Fe)-deficiency retardation in Alfalfa (Medicago sativa L.) through the enhancement of Fe accumulation and sulfur-assisted antioxidant defense. Int. J. Mol. Sci. 2020, 21, 2219. [Google Scholar] [CrossRef]
- Liu, J.J.; Fang, L.; Pei, W.X.; Li, F.Y.; Zhao, J.R. Effects of magnesium application on the arbuscular mycorrhizal symbiosis in tomato. Symbiosis 2023, 89, 73–82. [Google Scholar] [CrossRef]
- Kandalgaonkar, K.N.; Barvkar, V.T. Intricate phytohormonal orchestration mediates mycorrhizal symbiosis and stress tolerance. Mycorrhiza 2025, 35, 1–20. [Google Scholar] [CrossRef]
- Liu, R.C.; Yang, L.; Zou, Y.N.; Wu, Q.S. Root-associated endophytic fungi modulate endogenous auxin and cytokinin levels to improve plant biomass and root morphology of trifoliate orange. Hortic. Plant J. 2022, 9, 463–472. [Google Scholar] [CrossRef]
- Varma, A.; Sree, K.S.; Arora, M.; Bajaj, R.; Prasad, R.; Kharkwal, A.C. Functions of novel symbiotic fungus-Piriformospora indica. Proc. Indian Natl. Sci. Acad. 2014, 80, 429–441. [Google Scholar] [CrossRef]
- Verma, S.; Varma, A.; Rexer, K.H.; Hassel, A.; Kost, G.; Sarbhoy, A.; Franken, P. Piriformospora indica, gen. et sp. nov., a new root-colonizing fungus. Mycologia 1998, 90, 896–903. [Google Scholar] [CrossRef]
- Wan, Y.X.; Kapoor, R.; da Silva, F.S.B.; Abd-Allah, E.F.; Kuča, K.; Hashem, A.; Wu, Q.S. Elucidating the mechanism regarding enhanced tolerance in plants to abiotic stress by Serendipita indica. Plant Growth Regul. 2024, 103, 271–281. [Google Scholar] [CrossRef]
- Saleem, S.; Sekara, A.; Pokluda, R. Serendipita indica—A review from agricultural point of view. Plants 2022, 11, 3417. [Google Scholar] [CrossRef] [PubMed]
- Shukla, J.; Mohd, S.; Kushwaha, A.S.; Narayan, S.; Saxena, P.N.; Bahadur, L.; Kumar, M. Endophytic fungus Serendipita indica reduces arsenic mobilization from root to fruit in colonized tomato plant. Environ. Pollut. 2022, 298, 118830. [Google Scholar] [CrossRef]
- Cao, J.L.; He, W.X.; Zou, Y.N.; Wu, Q.S. An endophytic fungus, Piriformospora indica, enhances drought tolerance of trifoliate orange by modulating the antioxidant defense system and composition of fatty acids. Tree Physiol. 2023, 43, 452–466. [Google Scholar] [CrossRef]
- Rong, Z.Y.; Lei, A.Q.; Wu, Q.S.; Srivastava, A.K.; Hashem, A.; Abd_Allah, E.F.; Yang, T. Serendipita indica promotes P acquisition and growth in tea seedlings under P deficit conditions by increasing cytokinins and indoleacetic acid and phosphate transporter gene expression. Front. Plant Sci. 2023, 14, 1146182. [Google Scholar] [CrossRef]
- Zheng, F.L.; Tan, Z.P.; Zhang, Y.; Xu, X.H.; Hashem, A.; Debnath, A.; Wu, Q.S. Enhancing walnut growth and drought tolerance through Serendipita indica: Focus on mitochondrial antioxidant defense. Plant Growth Regul. 2024, 104, 1697–1706. [Google Scholar] [CrossRef]
- Cheng, X.F.; Xie, M.M.; Li, Y.; Liu, B.Y.; Liu, C.Y.; Wu, Q.S.; Kuča, K. Effects of field inoculation with arbuscular mycorrhizal fungi and endophytic fungi on fruit quality and soil properties of Newhall navel orange. Appl. Soil Ecol. 2022, 170, 104308. [Google Scholar] [CrossRef]
- Yang, L.; Cao, J.L.; Zou, Y.N.; Wu, Q.S.; Kuča, K. Piriformospora indica: A root endophytic fungus and its roles in plants. Not. Bot. Horti Agrobot. 2020, 48, 1–13. [Google Scholar] [CrossRef]
- Phillips, J.M.; Hayman, W.D. Improved procedures for clearing roots and staining parasitic and vesicular-arbuscular mycorrhizal fungi for rapid assessment of infection. Trans. Br. Mycol. Soc. 1970, 55, 158–161. [Google Scholar] [CrossRef]
- Martínez-Cuenca, M.R.; Forner-Giner, M.Á.; Iglesias, D.J.; Primo-Millo, E.; Legaz, F. Strategy I responses to Fe-deficiency of two citrus rootstocks differing in their tolerance to iron chlorosis. Sci. Hortic. 2013, 153, 56–63. [Google Scholar] [CrossRef]
- Liu, X.; Guo, L.X.; Luo, L.J.; Liu, Y.Z.; Peng, S.A. Identifcation of the magnesium transport (MGT) family in Poncirus trifoliata and functional characterization of PtrMGT5 in magnesium deficiency stress. Plant Mol. Biol. 2019, 101, 551–560. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCt method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Heidarianpour, M.B.; Aliasgharzad, N.; Olsson, P.A. Positive effects of co-inoculation with Rhizophagus irregularis and Serendipita indica on tomato growth under saline conditions, and their individual colonization estimated by signature lipids. Mycorrhiza 2020, 30, 455–466. [Google Scholar] [CrossRef] [PubMed]
- Dumbrell, A.J. Arbuscular mycorrhizal fungi throughout the year: Using massively parallel pyrosequencing to quantify spatiotemporal seasonal dynamics. In Molecular Microbial Ecology of the Rhizosphere, 1st ed.; Buscot, F., Varma, A., Eds.; Wiley-Blackwell: Hoboken, NJ, USA, 2013; Volume 1, pp. 1113–1122. [Google Scholar]
- Xin, G.; Ye, S.P.; Wu, E.N.; Wang, Y.T.; Sugawara, K. Seasonal dynamics in arbuscular mycorrhizal fungal colonization and spore numbers in the rhizosphere of Dactylis glomerata L. and Trifolium repens L. Pak. J. Bot. 2012, 44, 2087–2092. [Google Scholar]
- Deng, X.X.; Cao, W.X.; Wang, S.L.; Wang, W.H.; Liu, W.T. Diversity and seasonal dynamics of culturable endophytic fungi from two Salix species roots in alpine shrub grassland. Chin. J. Ggrassl. 2023, 45, 89–98. [Google Scholar]
- Xu, L.; Wu, C.; Oelmüller, R.; Zhang, W. Role of phytohormones in Piriformospora indica-induced growth promotion and stress tolerance in plants: More questions than answers. Front. Microbiol. 2018, 9, 1646. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.S.; Lai, C.W.; Liu, L.; Zhou, Y.Y.; Xie, J. Research on biochemical defense strategies of plants in response to abiotic stress. J. Org. Chem. Res. 2023, 11, 346–355. [Google Scholar] [CrossRef]
- Lehmann, A.; Rillig, M.C. Arbuscular mycorrhizal contribution to copper, manganese and iron nutrient concentrations in crops-a meta-analysis. Soil Biol. Biochem. 2015, 81, 147–158. [Google Scholar] [CrossRef]
- Christian, N.; Perlin, M.H. Plant-endophyte communication: Scaling from molecular mechanisms to ecological outcomes. Mycologia 2024, 116, 227–250. [Google Scholar] [CrossRef]
- Smith, S.E.; Smith, F.A. Roles of arbuscular mycorrhizas in plant nutrition and growth: New paradigms from cellular to ecosystem scales. Annu. Rev. Plant Biol. 2011, 62, 227–250. [Google Scholar] [CrossRef]
- Wan, Y.X.; Liang, S.M.; Wu, Q.S.; Hashem, A.; Abd_Allah, E.F.; Zou, Y.N. Serendipita indica accelerates chlorophyll synthesis pathway and photosynthetic efficiency in trifoliate orange subjected to water deficit. Sci. Hortic. 2024, 338, 113667. [Google Scholar] [CrossRef]
- Mathur, S.; Tomar, R.S.; Jajoo, A. Arbuscular mycorrhizal fungi (AMF) protects photosynthetic apparatus of wheat under drought stress. Photosynth. Res. 2019, 139, 227–238. [Google Scholar] [CrossRef]
- Zheng, M.J.; Zhong, S.P.; Wang, W.J.; Tang, Z.Z.; Bu, T.L.; Li, Q.F. Serendipita indica promotes the growth of tartary buckwheat by stimulating hormone synthesis, metabolite production, and increasing systemic resistance. J. Fungi 2023, 9, 1114. [Google Scholar] [CrossRef] [PubMed]
- Oelmüller, R.; Sherameti, I.; Tripathi, S.; Varma, A. Piriformospora indica, a cultivable root endophyte with multiple biotechnological applications. Symbiosis 2009, 49, 1–17. [Google Scholar] [CrossRef]
- Rong, Z.Y.; Zhang, Z.Z.; Alqahtani, M.D.; Wu, Q.S.; Gao, X.B. Serendipita indica is a biostimulant that improves tea growth at low P levels by modulating P acquisition and hormone levels. Rhizosphere 2023, 28, 100796. [Google Scholar] [CrossRef]
- He, L.; Li, C.Y.; Liu, R.J. Indirect interactions between arbuscular mycorrhizal fungi and Spodoptera exigua alter photosynthesis and plant endogenous hormones. Mycorrhiza 2017, 27, 525–535. [Google Scholar] [CrossRef] [PubMed]
- Jakobsen, S.T. Interaction between plant nutrients: IV. Interaction between calcium and phosphate. Acta Agric. Scand. Sect. B Soil Plant Sci. 1993, 43, 6–10. [Google Scholar] [CrossRef]
- Lee, Y.J. Subcellular distribution of P and Ca in arbuscular mycorrhizal plant: An X-ray microanalytical study. J. Appl. Biol. Chem. 2004, 47, 189–193. [Google Scholar]
- Wu, F.; Zhang, H.Q.; Fang, F.R.; Wu, N.; Zhang, Y.X.; Tang, M. Effects of nitrogen and exogenous Rhizophagus irregularis on the nutrient status, photosynthesis and leaf anatomy of Populus × canadensis ‘Neva’. J. Plant Growth Regul. 2017, 36, 824–835. [Google Scholar] [CrossRef]
- Lü, X.T.; Huang, X.P.; Wang, Y.; Wang, C.S.; Cheng, F.X. Study on the symbiotic effect of Piriformis indica and hybrid orchid. Southeast Hortic. 2020, 8, 20–24. [Google Scholar]
- Amir, H.; Gensous, S.; Cavaloc, Y.; Wantiez, L. Phosphorus fertilization of an ultramafic soil reduced effects of arbuscular mycorrhizal fungi but not mycorrhizal colonization. J. Soil Sci. Plant Nutr. 2021, 21, 3544–3554. [Google Scholar] [CrossRef]
- Wang, J.H.; Sui, J.K.; Cui, L.J.; Shi, K.M. Effects of AMF on the growth and physiological characteristics of Phoebe zhennan under salt stress. J. Central South Univ. For. Technol. 2023, 43, 51–58. [Google Scholar]
- Upadhayay, V.K.; Singh, J.; Khan, A.; Lohani, S.; Singh, A.V. Mycorrhizal mediated micronutrients transportation in food-based plants: A biofortification strategy. In Mycorrhizosphere and Pedogenesis; Varma, A., Choudhary, D.K., Eds.; Springer: Singapore, 2019; pp. 1–24. [Google Scholar]
- Rui, W.; Mao, Z.; Li, Z. The roles of phosphorus and nitrogen nutrient transporters in the arbuscular mycorrhizal symbiosis. Int. J. Mol. Sci. 2022, 23, 11027. [Google Scholar] [CrossRef]
- Kabir, A.H.; Debnath, T.; Das, U.; Prity, S.A.; Haque, A.; Rahman, M.M.; Parvez, M.S. Arbuscular mycorrhizal fungi alleviate Fe-deficiency symptoms in sunflower by increasing iron uptake and its availability along with antioxidant defense. Plant Physiol. Biochem. 2020, 150, 254–262. [Google Scholar] [CrossRef]
- Pii, Y.; Borruso, L.; Brusetti, L.; Crecchio, C.; Cesco, S.; Mimmo, T. The interaction between iron nutrition, plant species and soil type shapes the rhizosphere microbiome. Plant Physiol. Biochem. 2016, 99, 39–48. [Google Scholar] [CrossRef]
- Zhou, C.; Guo, J.S.; Zhu, L.; Xiao, X.; Xie, Y.; Zhu, J.; Ma, Z.Y.; Wang, J.F. Paenibacillus polymyxa BFKC01 enhances plant iron absorption via improved root systems and activated iron acquisition mechanisms. Plant Physiol. Biochem. 2016, 105, 162–173. [Google Scholar] [CrossRef]
- Urwat, U.; Ahmad, S.M.; Masi, A.; Ganai, N.A.; Murtaza, I.; Khan, I.; Zargar, S.M. Fe and Zn stress induced gene expression analysis unraveled mechanisms of mineral homeostasis in common bean (Phaseolus vulgaris L.). Sci. Rep. 2021, 11, 24026. [Google Scholar] [CrossRef] [PubMed]
- Santi, S.; Cesco, S.; Varanini, Z.; Pinton, R. Two plasma membrane H+-ATPase genes are differentially expressed in iron-deficient cucumber plants. Plant Physiol. Biochem. 2005, 43, 287–292. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, W. Iron homeostasis in plants: Sensing and signaling pathways. J. Plant Nutr. 2003, 26, 2211–2230. [Google Scholar] [CrossRef]
- Zulfiqar, U.; Albadrani, G.M.; Hussain, S. Mechanisms of Iron uptake and homeostasis in plants: Implications for biofortification in cereal grains. In Mineral Biofortification in Crop Plants for Ensuring Food Security; Hasanuzzaman, M., Tahir, M.S., Tanveer, M., Shah, A.N., Eds.; Springer: Singapore, 2023; pp. 121–137. [Google Scholar]
- Li, S.Z.; Ma, S.A.; Song, Z.Z.; Li, Y.; Liu, X.Q.; Yang, W.Z.; Wang, T.Y.; Zhou, X.J.; Chen, R.M. Regulatory mechanisms of iron homeostasis in maize mediated by ZmFIT. Crop J. 2024, 12, 1426–1436. [Google Scholar] [CrossRef]
- Briat, J.F. Iron dynamics in plants. Adv. Bot. Res. 2007, 46, 137–180. [Google Scholar]
- Tang, L.; Xiao, L.D.; Huang, Y.F.; Xiao, B.; Gong, C.M. Cloning and magnesium transport function analysis of CsMGTs genes in tea plants (Camellia sinensis). J. Tea Sci. 2021, 41, 761–776. [Google Scholar]
- Betz, R.; Heidt, S.; Figueira-Galán, D.; Hartmann, M.; Langner, T.; Requena, N. Alternative splicing regulation in plants by SP7-like effectors from symbiotic arbuscular mycorrhizal fungi. Nat. Commun. 2024, 15, 7107. [Google Scholar] [CrossRef]

| Gene Name | Accession Number | Sequence of Forward Prime (5′→3′) | Sequence of Reverse Primer (5′→3′) |
|---|---|---|---|
| CsFRO1 | Cs3g01120 | GATCGATTTCTGCGGTTCTG | AACTCAGGGCATTGTATCGT |
| CsHA1 | Cs7g07300 | GATTGATCAGTCTGCCCTCA | CCACTGCCTCTATTTCACCT |
| CsHA2 | Cs5g04360 | ACCCTTGAGAAAACAGCTCA | AAGACCAACTTCTTGACCGT |
| CsIRT1 | Cs2g10720 | ACCCTTGAGAAAACAGCTCA | AAGACCAACTTCTTGACCGT |
| CsIRT2 | Cs_ont_8g000350 | CAGGCAGGATTCAACTTTGG | TCATAGCCCGTCACTGAAAA |
| CsMGT2 | Cs7g11510 | AACAAAGGCTGGACTCTTCA | GCAATTTCTGAGCTCCACTG |
| CsMGT5 | Cs3g06130 | ACGACAACGAAGATATGGCT | GATGGGATGCTTTAGGGACA |
| CsMGT6 | Cs9g08150 | ATGACTAGGTTGACTGCTCG | CCAATTAGCAGCACCTGAAC |
| CsMGT8 | Cs8g03920 | CAGTTCACAGCACAGTAAGC | GTCTACATACTCCCTCAGCG |
| β-Actin | Cs1g05000 | CCGACCGTATGAGCAAGGAAA | TTCCTGTGGACAATGGATGGA |
| Cultivars | Treatments | March | June | September | December |
|---|---|---|---|---|---|
| Beni-Madonna | Control | 3.1 ± 0.2 d | 3.2 ± 0. 6 c | 3.6 ± 0.6 c | 3.4 ± 0.7 c |
| Fm | 20.6 ± 1. 9 c | 23.3 ± 4.3 b | 26.3 ± 4.3 b | 21.4 ± 2.9 b | |
| Dv | 26.9 ± 5.5 b | 26.1 ± 2.8 b | 27. 8 ± 5.41 b | 26.8 ± 1.9 b | |
| Si | 51.5 ± 5.6 a | 56.6 ± 10.5 a | 61.3 ± 9.6 a | 52.8 ± 9.6 a | |
| Lane Late | Control | 2.8 ± 0.4 d | 3.2 ± 0.3 d | 3.5 ± 0.4 d | 3.1 ± 0.6 d |
| Fm | 24.9 ± 2.9 b | 26.9 ± 3.5 b | 32.0 ± 5.2 b | 23.1 ± 2.7 b | |
| Dv | 18.3 ± 2.4 c | 18.9 ± 2.1 c | 21.9 ± 2.6 c | 17.4 ± 2.2 c | |
| Si | 55.4 ± 7.4 a | 56.2 ± 8.7 a | 62.4 ± 12.0 a | 55.6 ± 7.9 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wan, Y.-X.; Lü, Y.; Rong, Z.-Y.; Zou, Y.-N.; Wu, Q.-S. Host Genotype Shapes Fungal Symbiont-Mediated Nutrient and Growth Benefits in Citrus. Horticulturae 2025, 11, 1321. https://doi.org/10.3390/horticulturae11111321
Wan Y-X, Lü Y, Rong Z-Y, Zou Y-N, Wu Q-S. Host Genotype Shapes Fungal Symbiont-Mediated Nutrient and Growth Benefits in Citrus. Horticulturae. 2025; 11(11):1321. https://doi.org/10.3390/horticulturae11111321
Chicago/Turabian StyleWan, Yu-Xi, Yang Lü, Zi-Yi Rong, Ying-Ning Zou, and Qiang-Sheng Wu. 2025. "Host Genotype Shapes Fungal Symbiont-Mediated Nutrient and Growth Benefits in Citrus" Horticulturae 11, no. 11: 1321. https://doi.org/10.3390/horticulturae11111321
APA StyleWan, Y.-X., Lü, Y., Rong, Z.-Y., Zou, Y.-N., & Wu, Q.-S. (2025). Host Genotype Shapes Fungal Symbiont-Mediated Nutrient and Growth Benefits in Citrus. Horticulturae, 11(11), 1321. https://doi.org/10.3390/horticulturae11111321








