Transcriptome–Metabolome Integration Reveals Mechanisms of Leaf Color Variation in Leafy Vegetable Sweet Potato
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Materials
2.2. Determination of Chlorophyll a, Chlorophyll b, Carotenoids and Anthocyanins Content
2.3. Metabolome Data Analysis
2.4. Transcriptome Sequencing
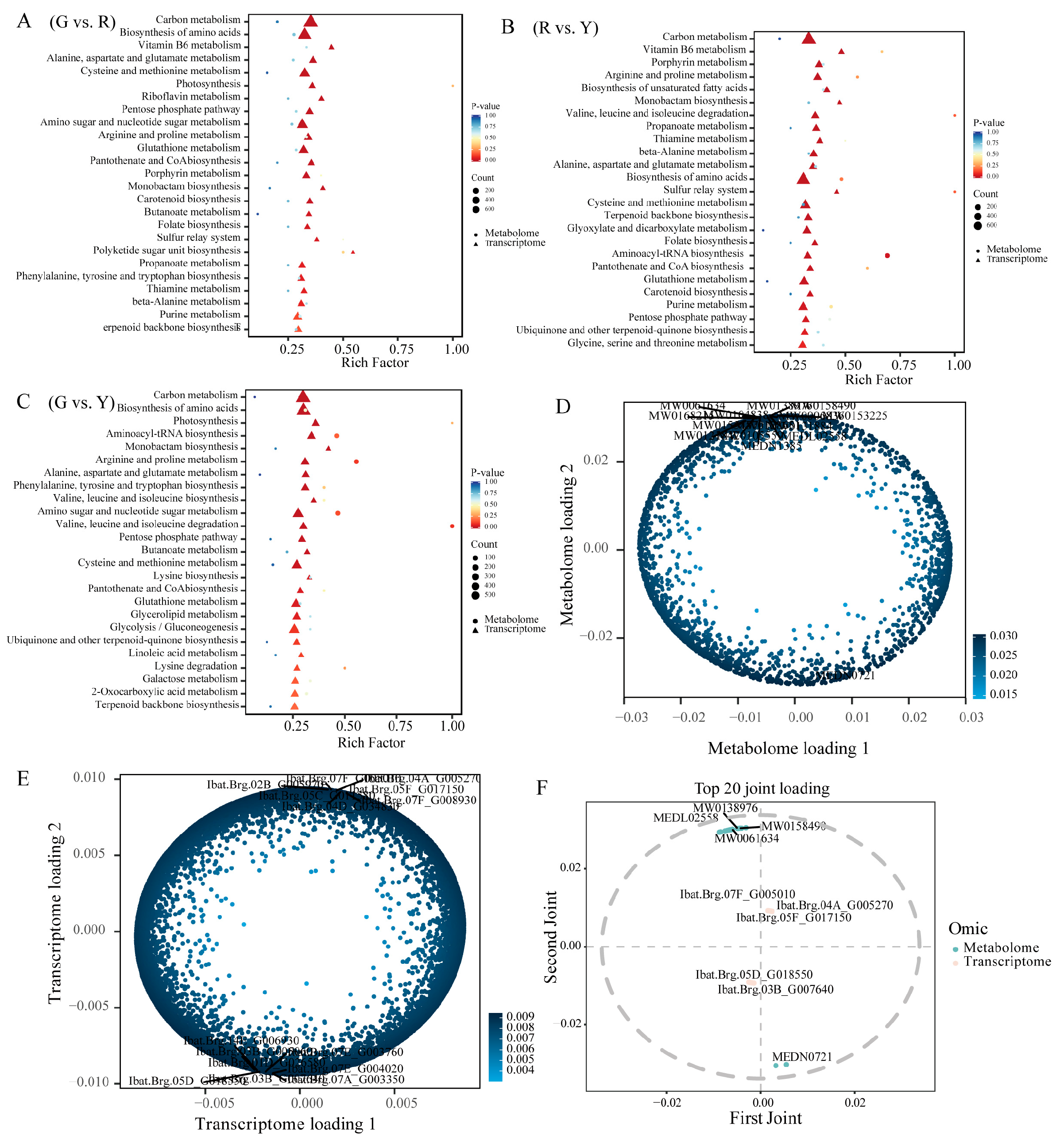
2.5. Transcriptome–Metabolome Joint Enrichment Analysis and O2PLS Analysis
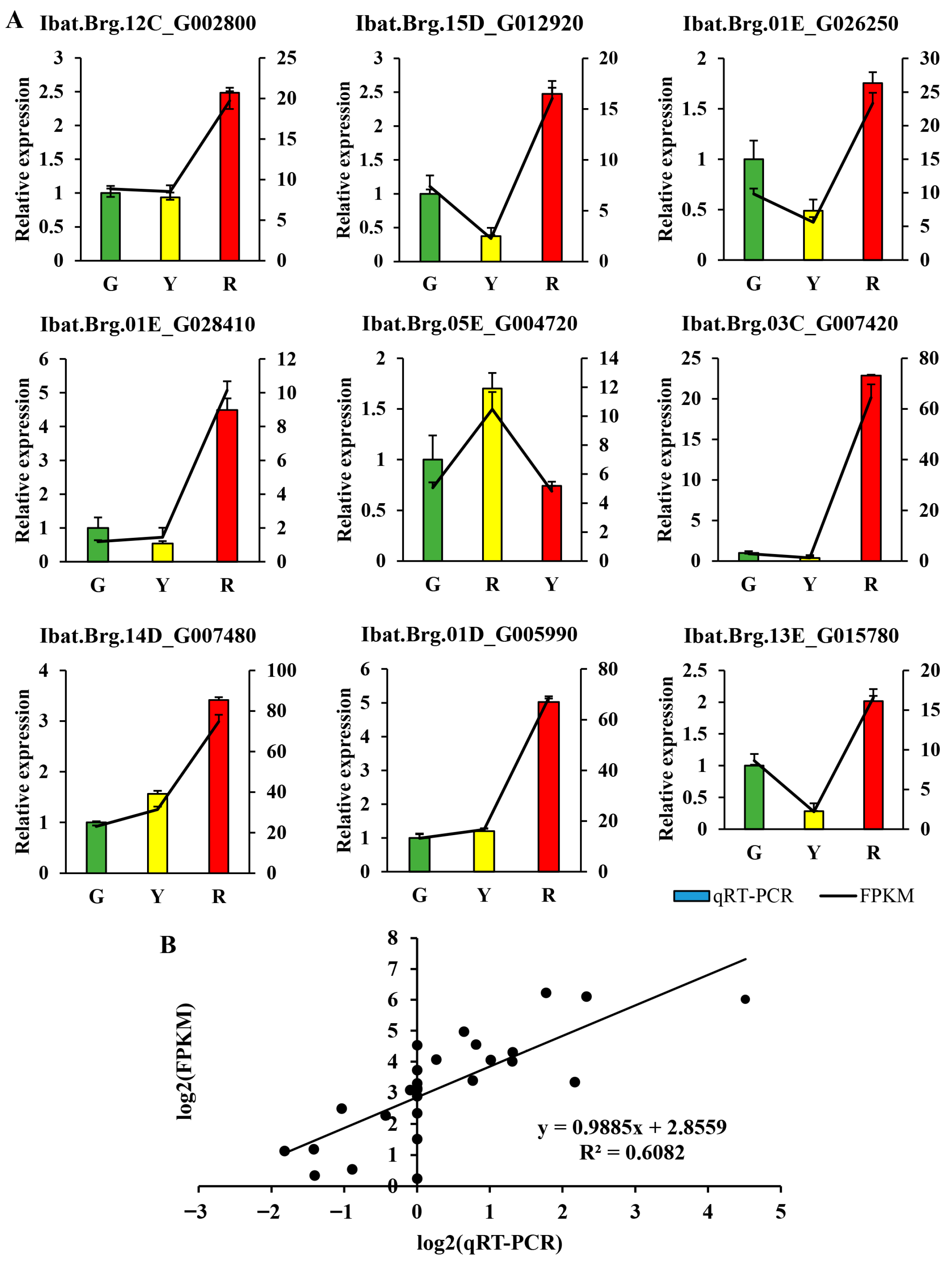
2.6. Quantitative Real-Time PCR (qRT-PCR) Validation
3. Results
3.1. Pigment Composition and Leaf Color Variation in Leafy Vegetable Sweet Potato
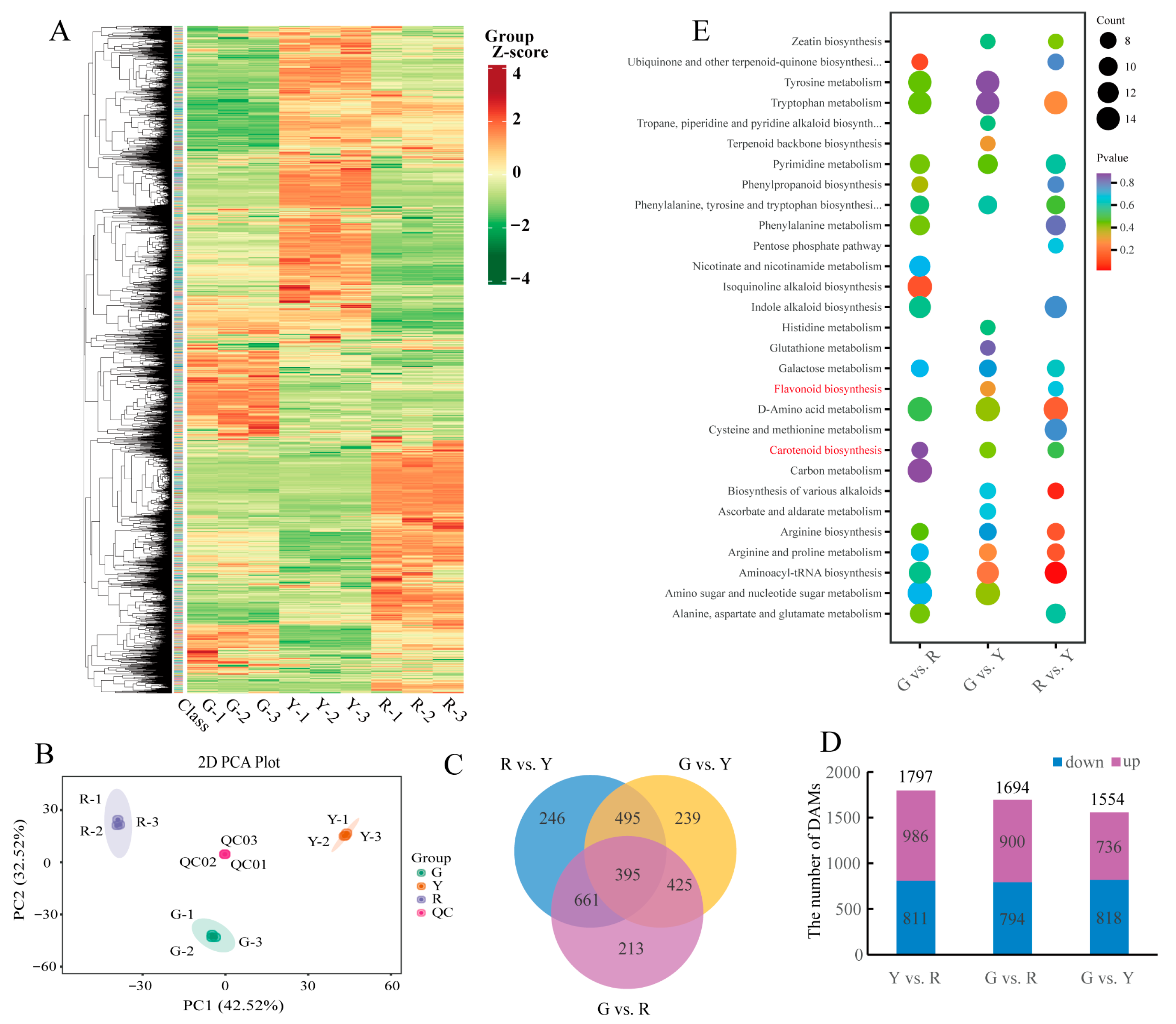
3.2. Overview of the Metabolic Data in Leafy Vegetable Sweet Potato
3.3. Transcriptome Sequencing and Gene Expression Profiles
3.4. DAMs and DEGs Were Enriched in Consistent Metabolic Pathways
3.5. Expression Profiles of Chlorophyll, Flavonoid, and Carotenoid Pathway-Related Genes in Leafy Vegetable Sweet Potato
3.6. Validation of the Transcriptome Data by qRT-PCR Analysis
4. Discussion
4.1. The Physiological Basis of Leaf Color Variation in Leafy Vegetable Sweet Potato
4.2. Differential Pigment Regulatory Mechanisms in Y and R Cultivars
4.3. Integrated Transcriptome-Metabolome Regulation of Leafy Vegetable Sweet Potato Leaf Pigmentation
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Tang, C.; Ameen, A.; Fang, B.; Liao, M.; Chen, J.; Huang, L.; Zou, H.; Wang, Z. Nutritional composition and health benefits of leaf-vegetable sweet potato in south china. J. Food Compos. Anal. 2021, 96, 103714. [Google Scholar] [CrossRef]
- Sun, H.; Mu, T.; Xi, L.; Zhang, M.; Chen, J. Sweet potato (Ipomoea batatas L.) Leaves as nutritional and functional foods. Food Chem. 2014, 156, 380–389. [Google Scholar] [CrossRef]
- Li, M.; Jang, G.Y.; Lee, S.H.; Kim, M.Y.; Hwang, S.G.; Sin, H.M.; Kim, H.S.; Lee, J.; Jeong, H.S. Comparison of functional components in various sweet potato leaves and stalks. Food Sci. Biotechnol. 2017, 26, 97–103. [Google Scholar] [CrossRef]
- Chen, S.P.; Wang, S.Y.; Huang, M.Y.; Lin, K.H.; Hua, S.M.; Lu, H.H.; Lai, Y.C.; Yang, C.M. Physiological and molecular analyses of chlorophyllase in sweet potatoes with different-colored leaves. S. Afr. J. Bot. 2018, 114, 272–279. [Google Scholar] [CrossRef]
- Shen, J.; Zou, Z.; Zhang, X.; Zhou, L.; Wang, Y.; Fang, W.; Zhu, X. Metabolic analyses reveal different mechanisms of leaf color change in two purple-leaf tea plant (Camellia sinensis L.) Cultivars. Hortic. Res. 2018, 5, 7. [Google Scholar] [CrossRef]
- Mattioli, R.; Francioso, A.; Mosca, L.; Silva, P. Anthocyanins: A comprehensive review of their chemical properties and health effects on cardiovascular and neurodegenerative diseases. Molecules 2020, 25, 3809. [Google Scholar] [CrossRef]
- Gude, K.; Talavera, M.; Sasse, A.M.; Rivard, C.L.; Pliakoni, E. Effect of light characteristics on the sensory properties of red lettuce (Lactuca sativa). Foods 2021, 10, 2660. [Google Scholar] [CrossRef]
- Sun, T.; Rao, S.; Zhou, X.; Li, L. Plant carotenoids: Recent advances and future perspectives. Mol. Hortic. 2022, 2, 3. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Qi, Y.; Chen, X.; He, H.; Liu, Z.; Zhang, Z.; Ren, Y.; Ren, X. Phenolic compounds and antioxidant activity in red-and in green-fleshed kiwifruits. Food Res. Int. 2019, 116, 291–301. [Google Scholar] [CrossRef] [PubMed]
- Jiao, B.; Meng, Q.; Lv, W. Roles of stay-green (SGR) homologs during chlorophyll degradation in green plants. Bot. Stud. 2020, 61, 25. [Google Scholar] [CrossRef] [PubMed]
- Zou, S.; Zhuo, M.; Abbas, F.; Hu, G.; Wang, H.; Huang, X. Transcription factor lcnac002 coregulates chlorophyll degradation and anthocyanin biosynthesis in litchi. Plant Physiol. 2023, 192, 1913–1927. [Google Scholar] [CrossRef] [PubMed]
- Fan, H.; Liao, H.; Shen, Y.; Sani, M.N.H.; Yong, J.W.H.; Song, J. Unravelling the physiological and molecular mechanisms of leaf color change in acer griseum through multi-omics analysis. Plant Physiol. Biochem. 2024, 216, 109198. [Google Scholar] [CrossRef]
- Li, Y.; Cao, T.; Guo, Y.; Grimm, B.; Li, X.; Duanmu, D.; Lin, R. Regulatory and retrograde signaling networks in the chlorophyll biosynthetic pathway. J. Integr. Plant Biol. 2025, 67, 887–911. [Google Scholar] [CrossRef] [PubMed]
- Nisar, N.; Li, L.; Lu, S.; Khin, N.C.; Pogson, B.J. Carotenoid metabolism in plants. Mol. Plant. 2015, 8, 68–82. [Google Scholar] [CrossRef]
- Sharma, H.; Sharma, P.; Kumar, A.; Chawla, N.; Dhatt, A.S. Multifaceted regulation of anthocyanin biosynthesis in plants: A comprehensive review. J. Plant Growth Regul. 2024, 43, 3048–3062. [Google Scholar] [CrossRef]
- Tan, W.; Guo, X.; Wang, Z.; Zhang, R.; Tang, C.; Jiang, B.; Jia, R.; Deng, Y.; Yang, S.; Chen, J. Metabolic profiles and morphological characteristics of leaf tips among different sweet potato (Ipomoea batatas Lam.) Varieties. J. Integr. Agric. 2024, 23, 494–510. [Google Scholar] [CrossRef]
- Deng, J.; Wu, D.; Shi, J.; Balfour, K.; Wang, H.; Zhu, G.; Liu, Y.; Wang, J.; Zhu, Z. Multiple myb activators and repressors collaboratively regulate the juvenile red fading in leaves of sweetpotato. Front. Plant Sci. 2020, 11, 941. [Google Scholar] [CrossRef]
- Jing, X.; Chen, P.; Jin, X.; Lei, J.; Wang, L.; Chai, S.; Yang, X. Physiological, photosynthetic, and transcriptomics insights into the influence of shading on leafy sweet potato. Genes 2023, 14, 2112. [Google Scholar] [CrossRef] [PubMed]
- Drapal, M.; Rossel, G.; Heider, B.; Fraser, P.D. Metabolic diversity in sweet potato (Ipomoea batatas, Lam.) Leaves and storage roots. Hortic. Res. 2019, 6, 2. [Google Scholar] [CrossRef]
- Shi, J.; Wu, Q.; Deng, J.; Balfour, K.; Chen, Z.; Liu, Y.; Kumar, S.; Chen, Y.; Zhu, Z.; Zhu, G. Metabolic profiling and antioxidant analysis for the juvenile red fading leaves of sweetpotato. Plants 2022, 11, 3014. [Google Scholar] [CrossRef]
- Zhang, R.; Li, M.; Tang, C.; Jiang, B.; Yao, Z.; Mo, X.; Wang, Z. Combining metabolomics and transcriptomics to reveal the mechanism of coloration in purple and cream mutant of sweet potato (Ipomoea batatas L.). Front. Plant Sci. 2022, 13, 877695. [Google Scholar] [CrossRef]
- Porra, R.J. The chequered history of the development and use of simultaneous equations for the accurate determination of chlorophylls a and b. Photosynth. Res. 2002, 73, 149–156. [Google Scholar] [CrossRef]
- Lichtenthaler, H.; Babani, F. Contents of photosynthetic pigments and ratios of chlorophyll a/b and chlorophylls to carotenoids (a+b)/(x+c) in c4 plants as compared to c3 plants. Photosynthetica 2022, 60, 3–9. [Google Scholar] [CrossRef]
- Jia, R.; Tang, C.; Chen, J.; Zhang, X.; Wang, Z. Total phenolics and anthocyanins contents and antioxidant activity in four different aerial parts of leafy sweet potato (Ipomoea batatas L.). Molecules 2022, 27, 3117. [Google Scholar] [CrossRef]
- Kim, D.; Paggi, J.M.; Park, C.; Bennett, C.; Salzberg, S.L. Graph-based genome alignment and genotyping with hisat2 and hisat-genotype. Nat. Biotechnol. 2019, 37, 907–915. [Google Scholar] [CrossRef]
- Liao, Y.; Smyth, G.K.; Shi, W. Featurecounts: An efficient general purpose program for assigning sequence reads to genomic features. Bioinformatics 2014, 30, 923–930. [Google Scholar] [CrossRef]
- Love, M.I.; Huber, W.; Anders, S. Moderated estimation of fold change and dispersion for rna-seq data with deseq2. Genome Biol. 2014, 15, 550. [Google Scholar] [CrossRef] [PubMed]
- Kanehisa, M. Kegg: Kyoto encyclopedia of genes and genomes. Nucleic. Acids. Res. 2000, 28, 27–30. [Google Scholar] [CrossRef] [PubMed]
- Ashburner, M.; Ball, C.A.; Blake, J.A.; Botstein, D.; Butler, H.; Cherry, J.M.; Davis, A.P.; Dolinski, K.; Dwight, S.S.; Eppig, J.T.; et al. Gene ontology: Tool for the unification of biology. Nat. Genet. 2000, 25, 25–29. [Google Scholar] [CrossRef] [PubMed]
- Trygg, J.; Wold, S. O2-PLS, a two-block (x − y) latent variable regression (LVR) method with an integral osc filter. J. Chemom. 2003, 17, 53–64. [Google Scholar] [CrossRef]
- Zhao, M.; Li, X.; Zhang, X.; Zhang, H.; Zhao, X. Mutation mechanism of leaf color in plants: A review. Forests 2020, 11, 851. [Google Scholar] [CrossRef]
- Gan, Y.; Kou, Y.; Yan, F.; Wang, X.; Wang, H.; Song, X.; Zhang, M.; Zhao, X.; Jia, R.; Ge, H.; et al. Comparative transcriptome profiling analysis reveals the adaptive molecular mechanism of yellow-green leaf in rosa beggeriana ‘aurea’. Front. Plant Sci. 2022, 13, 845662. [Google Scholar] [CrossRef]
- Tanaka, Y.; Sasaki, N.; Ohmiya, A. Biosynthesis of plant pigments: Anthocyanins, betalains and carotenoids. Plant J. 2008, 54, 733–749. [Google Scholar] [CrossRef] [PubMed]
- Huo, J.; Zhang, N.; Gong, Y.; Bao, Y.; Li, Y.; Zhang, L.; Nie, S. Effects of different light intensity on leaf color changes in a chinese cabbage yellow cotyledon mutant. Front. Plant Sci. 2024, 15, 1371451. [Google Scholar] [CrossRef]
- Shibaeva, T.G.; Sherudilo, E.G.; Rubaeva, A.A.; Titov, A.F. Continuous led lighting enhances yield and nutritional value of four genotypes of brassicaceae microgreens. Plants 2022, 11, 176. [Google Scholar] [CrossRef] [PubMed]
- Barickman, T.C.; Simpson, C.R.; Sams, C.E. Waterlogging causes early modification in the physiological performance, carotenoids, chlorophylls, proline, and soluble sugars of cucumber plants. Plants 2019, 8, 160. [Google Scholar] [CrossRef]
- Brychkova, G.; de Oliveira, C.L.; Gomes, L.A.A.; de Souza Gomes, M.; Fort, A.; Esteves-Ferreira, A.A.; Sulpice, R.; Mckeown, P.C.; Spillane, C. Regulation of carotenoid biosynthesis and degradation in lettuce (Lactuca sativa L.) From seedlings to harvest. Int. J. Mol. Sci. 2023, 24, 10310. [Google Scholar] [CrossRef]
- Xu, Z.; Rothstein, S.J. Ros-induced anthocyanin production provides feedback protection by scavenging ros and maintaining photosynthetic capacity in arabidopsis. Plant Signal. Behav. 2018, 13, e1451708. [Google Scholar] [CrossRef]
- Agati, G.; Guidi, L.; Landi, M.; Tattini, M. Anthocyanins in photoprotection: Knowing the actors in play to solve this complex ecophysiological issue. New Phytol. 2021, 232, 2228–2235. [Google Scholar] [CrossRef]
- Liu, X.; Zhang, B.; Wu, J.; Li, Z.; Han, F.; Fang, Z.; Yang, L.; Zhuang, M.; Lv, H.; Liu, Y.; et al. Pigment variation and transcriptional response of the pigment synthesis pathway in the s2309 triple-color ornamental kale (Brassica oleracea L. Var. Acephala) line. Genomics 2020, 112, 2658–2665. [Google Scholar] [CrossRef]
- Tavernier, F.; Savoi, S.; Torregrosa, L.; Hugueney, P.; Baltenweck, R.; Segura, V.; Romieu, C. The single-berry metabolomic clock paradigm reveals new stages and metabolic switches during grapevine berry development. J. Exp. Bot. 2025, 76, 3125–3140. [Google Scholar] [CrossRef]
- Ahmed, S.; Khan, M.S.S.; Xue, S.; Islam, F.; Ikram, A.U.; Abdullah, M.; Liu, S.; Tappiban, P.; Chen, J. A comprehensive overview of omics-based approaches to enhance biotic and abiotic stress tolerance in sweet potato. Hortic. Res. 2024, 11, uhae14. [Google Scholar] [CrossRef] [PubMed]
- Tian, Y.; Wang, H.; Zhang, Z.; Zhao, X.; Wang, Y.; Zhang, L. An rna-seq analysis reveals differential transcriptional responses to different light qualities in leaf color of Camellia sinensis cv. Huangjinya. J. Plant Growth Regul. 2022, 41, 612–627. [Google Scholar] [CrossRef]
- Wada, K.C.; Inagaki, N.; Sakai, H.; Yamashita, H.; Nakai, Y.; Fujimoto, Z.; Yonemaru, J.I.; Itoh, H. Genetic effects of red lettuce leaf genes on red coloration in leaf lettuce under artificial lighting conditions. Plant-Environ. Interact. 2022, 3, 179–192. [Google Scholar] [CrossRef] [PubMed]
- Renner, S.S.; Zohner, C.M. The occurrence of red and yellow autumn leaves explained by regional differences in insolation and temperature. New Phytol. 2019, 224, 1464–1471. [Google Scholar] [CrossRef]
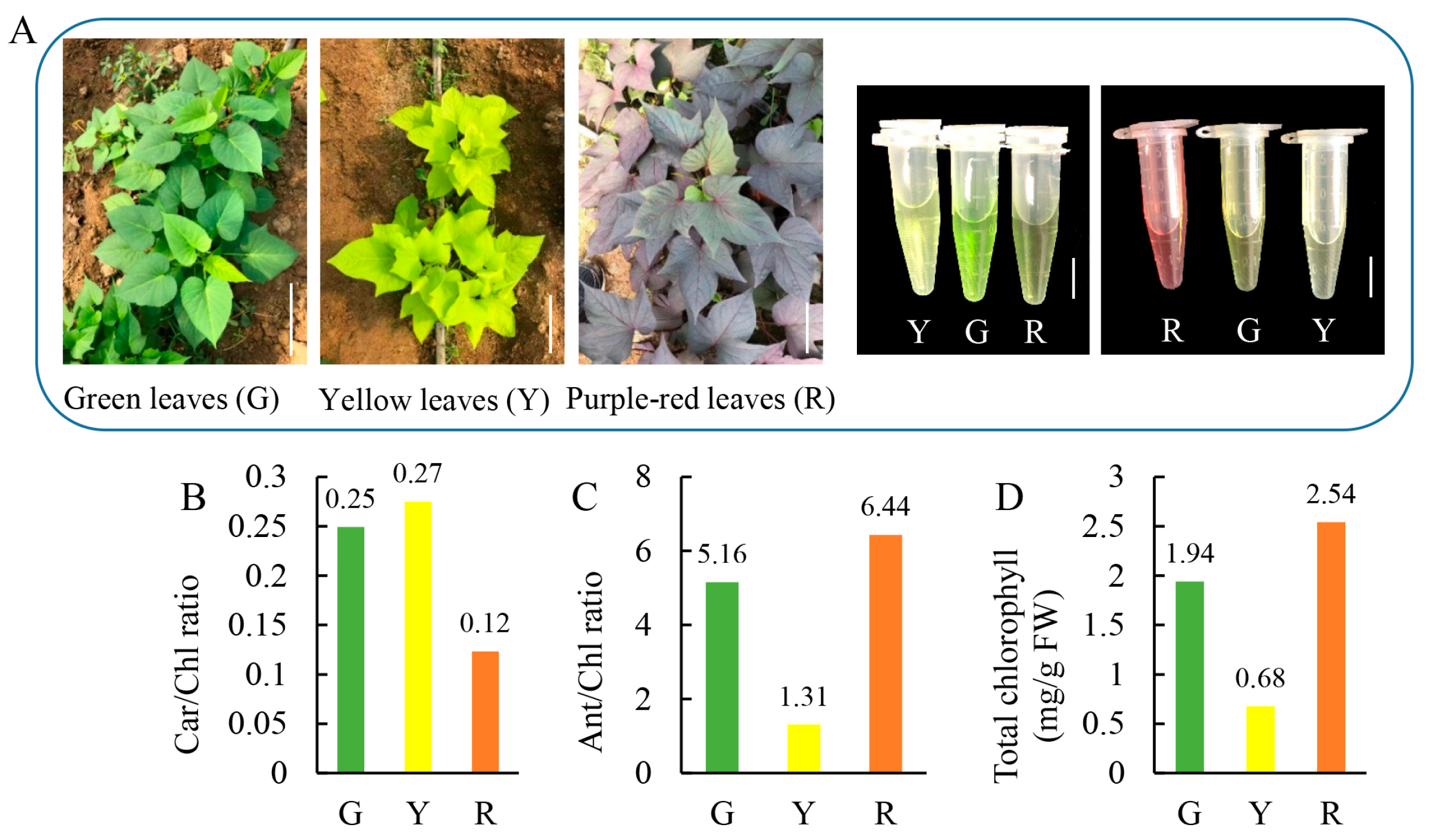


| Chlorophyll a (mg·g−1 FW) | Chlorophyll b (mg·g−1 FW) | Carotenoid (mg·g−1 FW) | Anthocyanin (mg·g−1 FW) | |
|---|---|---|---|---|
| G | 1.50 ± 0.13a | 0.44 ± 0.04b | 0.48 ± 0.03a | 10.02 ± 0.19b |
| Y | 0.57 ± 0.07b | 0.11 ± 0.01c | 0.19 ± 0.03c | 0.89 ± 0.64c |
| R | 1.51 ± 0.13b | 1.03 ± 0.07a | 0.31 ± 0.02b | 16.36 ± 0.67a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, S.; Chen, M.; Zhou, Q.; Huang, Y.; Zheng, W. Transcriptome–Metabolome Integration Reveals Mechanisms of Leaf Color Variation in Leafy Vegetable Sweet Potato. Horticulturae 2025, 11, 1317. https://doi.org/10.3390/horticulturae11111317
Wang S, Chen M, Zhou Q, Huang Y, Zheng W. Transcriptome–Metabolome Integration Reveals Mechanisms of Leaf Color Variation in Leafy Vegetable Sweet Potato. Horticulturae. 2025; 11(11):1317. https://doi.org/10.3390/horticulturae11111317
Chicago/Turabian StyleWang, Shenglin, Ming Chen, Qinghong Zhou, Yingjin Huang, and Wei Zheng. 2025. "Transcriptome–Metabolome Integration Reveals Mechanisms of Leaf Color Variation in Leafy Vegetable Sweet Potato" Horticulturae 11, no. 11: 1317. https://doi.org/10.3390/horticulturae11111317
APA StyleWang, S., Chen, M., Zhou, Q., Huang, Y., & Zheng, W. (2025). Transcriptome–Metabolome Integration Reveals Mechanisms of Leaf Color Variation in Leafy Vegetable Sweet Potato. Horticulturae, 11(11), 1317. https://doi.org/10.3390/horticulturae11111317





