Role of Enzymes and Metabolites Produced by Bacillus spp. in the Suppression of Meloidogyne incognita in Tomato
Abstract
1. Introduction
2. Materials and Methods
2.1. Bacterial Strains, Media and Culture Conditions
2.2. Preparation of Meloidogyne incognita Inoculum
2.3. In Vitro Nematicidal Activity of Bacterial Strains Against Meloidogyne incognita
2.3.1. Meloidogyne incognita Eggs Hatching Test
2.3.2. Mortality Test on Second-Stage Juveniles
2.4. Bioassays In Planta
2.4.1. Soil Preparation and Inoculation with Meloidogyne incognita Eggs
2.4.2. Preparation of Bacterial Suspensions and Soil Treatment
2.4.3. Plant Material and Growing Conditions
2.4.4. Photosynthetic Measurements
2.4.5. Tomato Growth Promotion Assay
2.4.6. Quantification of M. incognita in Tomato Plants
2.5. Bacterial Metabolic and Enzymatic Profile
2.5.1. Analysis of Metabolites by GC-MS
2.5.2. Protein Quantification and Determination of Protease and Chitinase Activities
2.6. Molecular Identification of Bacterial Strains
2.6.1. PCR Amplification, Sequencing and Analysis of 16S rRNA Gene
2.6.2. 16S rRNA Gene Phylogenetic Analysis
2.7. Statistical Analysis
3. Results
3.1. Inhibition of M. incognita Eggs Hatching In Vitro
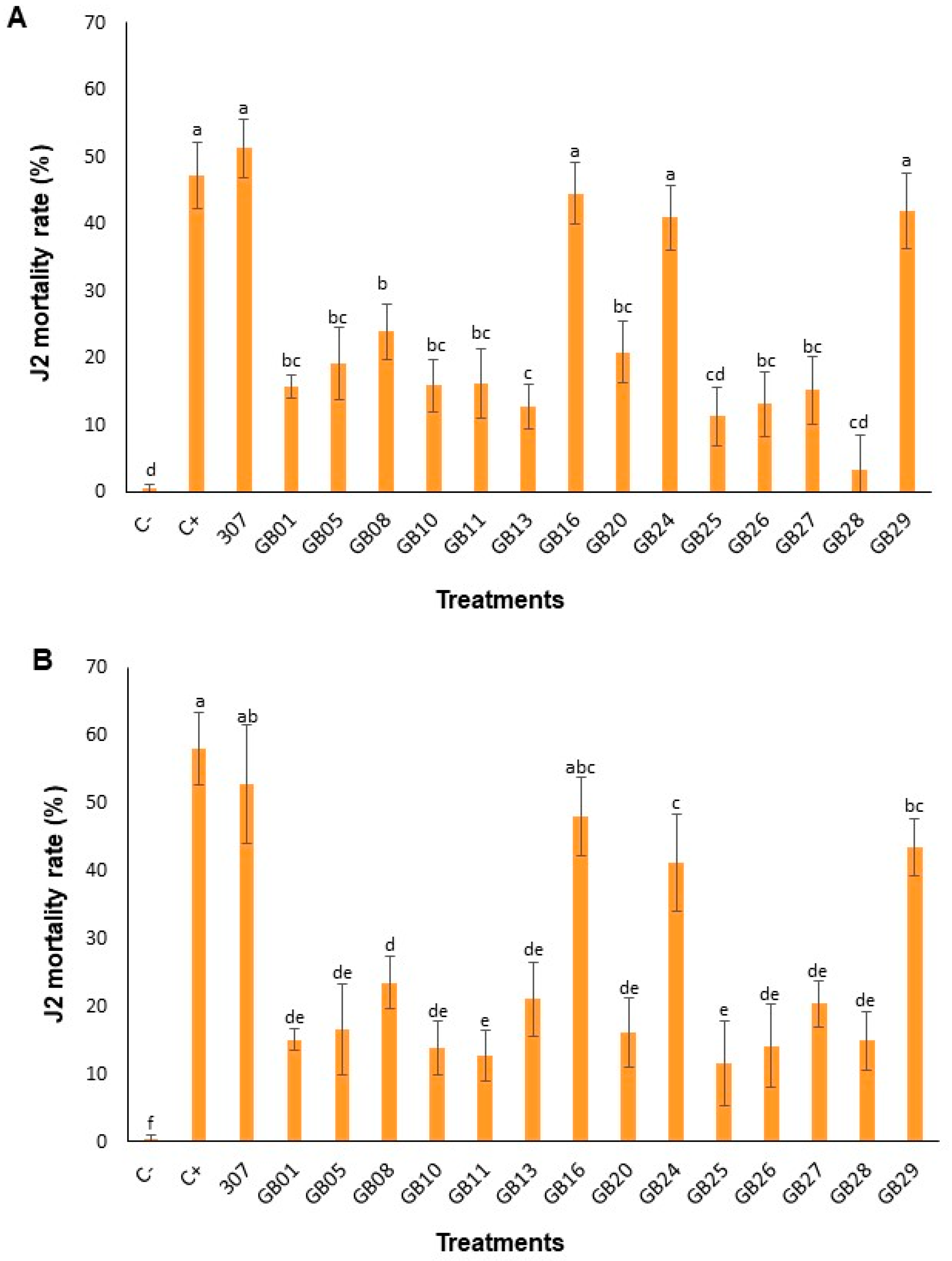
3.2. Mortality of Second-Stage Juveniles
3.3. Effect of Bacterial Treatments on the Photosynthetic Parameters of Tomato Plants Under M. incognita Infection
3.4. Effect of Bacterial Treatments on the Growth Promotion of Tomato Plants Under M. incognita Infection
3.5. Effect of Bacterial Treatments on the Control of M. incognita in Tomato Plants
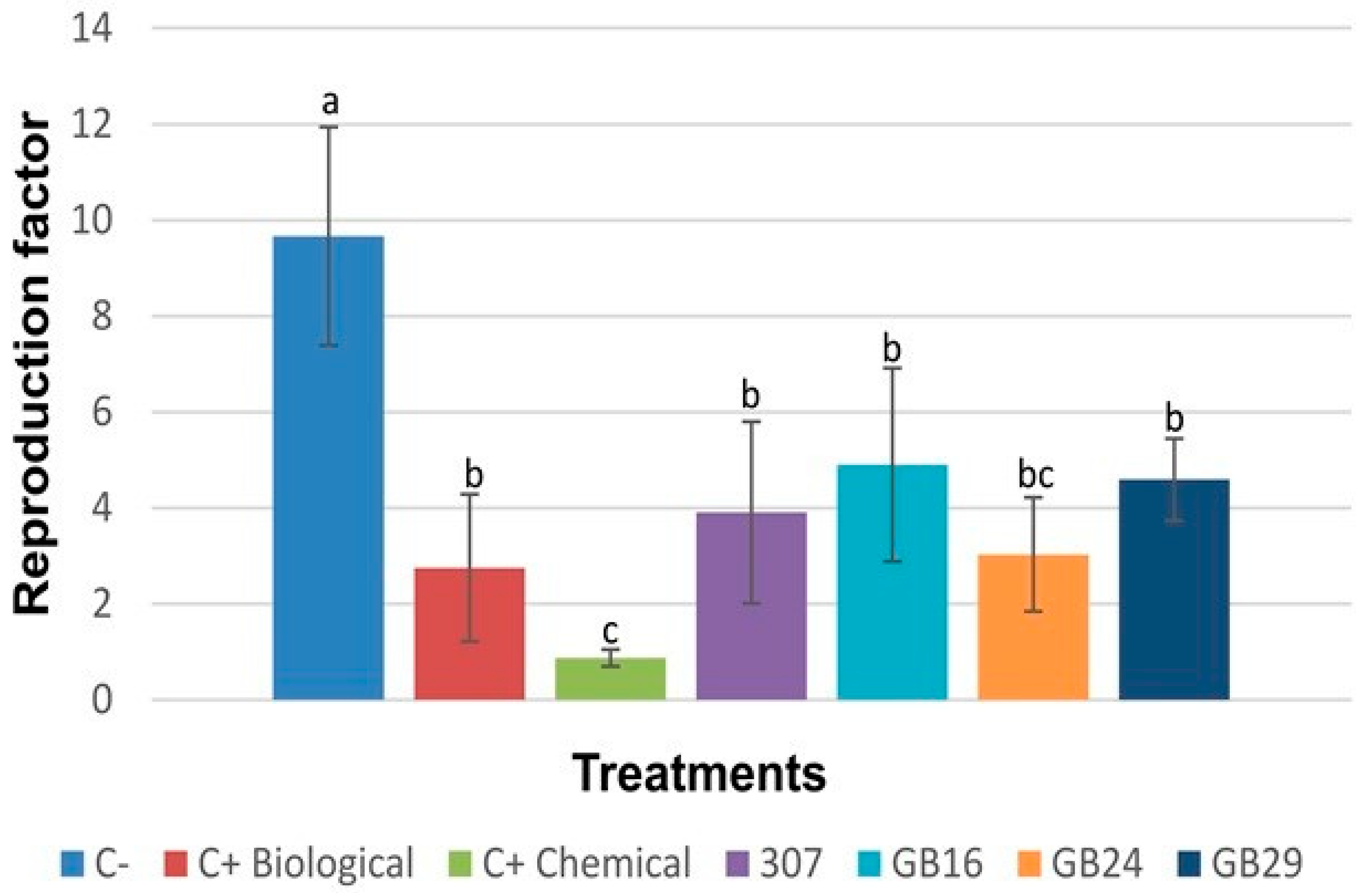
3.6. Bacterial Metabolic Profile and Enzyme Production
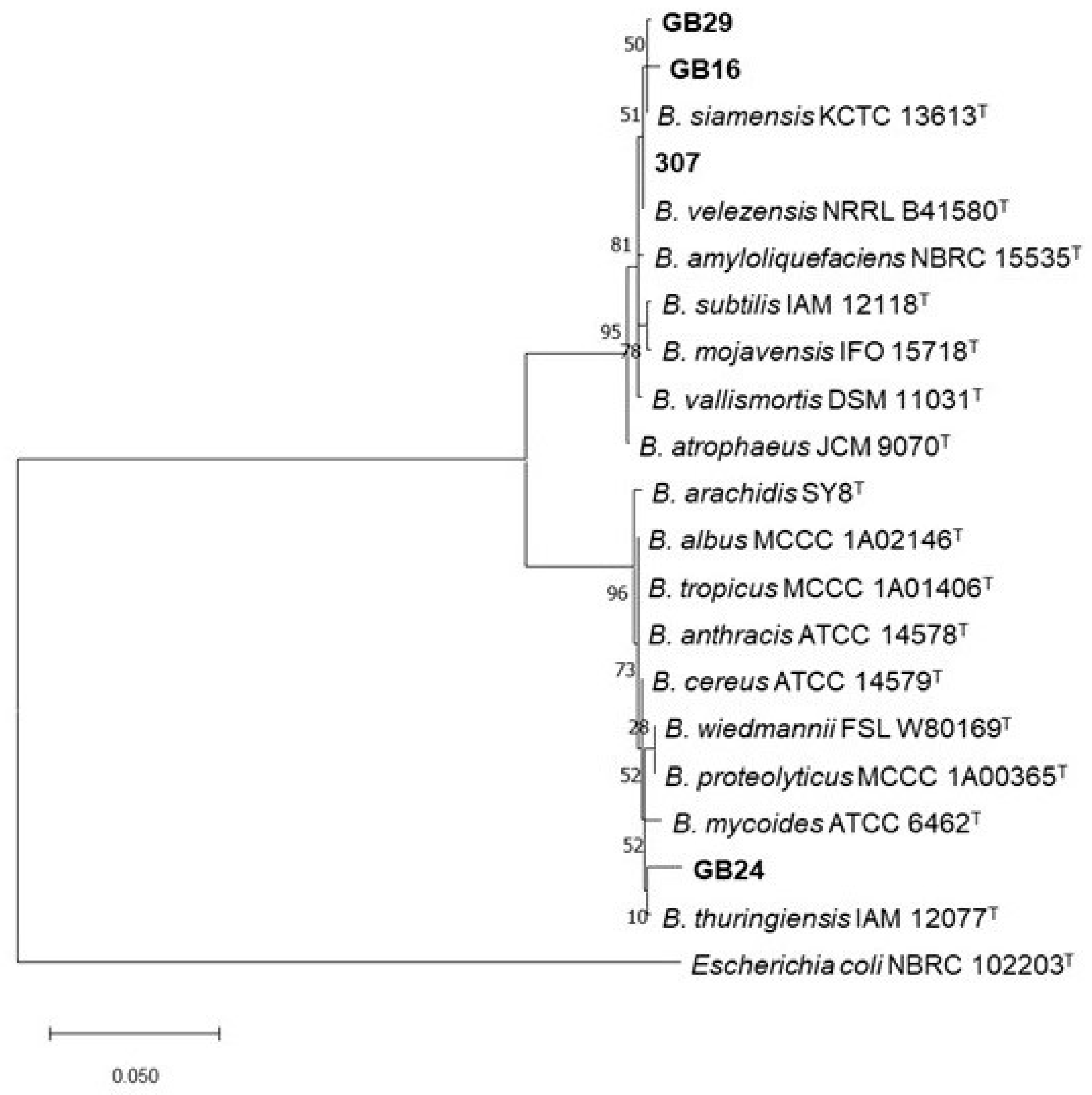
3.7. Molecular Identification and Phylogeny of Bacterial Strains
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sikandar, A.; Zhang, M.Y.; Wang, Y.Y.; Zhu, X.F.; Liu, X.Y.; Fan, H.Y.; Xuan, Y.H.; Chen, L.J.; Duan, Y.X. Meloidogyne incognita (Root-Knot Nematode) a Risk to Agriculture. Appl. Ecol. Environ. Res. 2020, 18, 1679–1690. [Google Scholar] [CrossRef]
- Nagachandrabose, S.; Shanthi, M.; Shanmugam, S.P.; Elaiyabharathi, T.; Sharmila, R.; Devrajan, K.; Manickam, R.; Srinivasan, R. Integrating Grafting and Bio-Inputs for Sustainable Management of Root Knot Nematode, Meloidogyne incognita, in Tomato Cultivation. Front. Plant Sci. 2025, 16, 1623444. [Google Scholar] [CrossRef] [PubMed]
- El-Sappah, A.H.; Islam, M.M.; El-awady, H.H.; Yan, S.; Qi, S.; Liu, J.; Cheng, G.; Liang, Y. Tomato Natural Resistance Genes in Controlling the Root-Knot Nematode. Genes 2019, 10, 925. [Google Scholar] [CrossRef] [PubMed]
- Fullana, A.M.; Expósito, A.; Escudero, N.; Cunquero, M.; Loza-Alvarez, P.; Giné, A.; Sorribas, F.J. Crop Rotation with Meloidogyne-Resistant Germplasm Is Useful to Manage and Revert the (a)Virulent Populations of Mi1.2 Gene and Reduce Yield Losses. Front. Plant Sci. 2023, 14, 1133095. [Google Scholar] [CrossRef] [PubMed]
- Grabau, Z.J.; Liu, C.; Sandoval-Ruiz, R. Meloidogyne incognita Management by Nematicides in Tomato Production. J. Nematol. 2021, 53, e2021-55. [Google Scholar] [CrossRef]
- Vasantha-Srinivasan, P.; Park, K.B.; Kim, K.Y.; Jung, W.J.; Han, Y.S. The Role of Bacillus Species in the Management of Plant-Parasitic Nematodes. Front. Microbiol. 2025, 15, 1510036. [Google Scholar] [CrossRef]
- Moreira, A.C.S.; Lopes, E.A.; Visôtto, L.E.; Soares, M.S.; Londe, M.L.A.; Ribeiro, L.B.; Terra, W.C.; Moreira, S.I.; Pedroso, M.P.; Pereira, L.F.; et al. Bacillus amyloliquefaciens Strain BaNCT02: An Antagonist with Multiple Mechanisms of Action against Meloidogyne incognita. Plant Pathol. 2025, 74, 320–329. [Google Scholar] [CrossRef]
- Sun, W.; Shahrajabian, M.H. Biostimulant and Beyond: Bacillus spp., the Important Plant Growth-Promoting Rhizobacteria (PGPR)—Based Biostimulant for Sustainable Agriculture. Earth Syst. Environ. 2025, 9, 1465–1498. [Google Scholar] [CrossRef]
- Deng, X.; Wang, X.; Li, G. Nematicidal Effects of Volatile Organic Compounds from Microorganisms and Plants on Plant-Parasitic Nematodes. Microorganisms 2022, 10, 1201. [Google Scholar] [CrossRef]
- Chavarria-Quicaño, E.; De La Torre-González, F.; González-Riojas, M.; Rodríguez-González, J.; Asaff-Torres, A. Nematicidal Lipopeptides from Bacillus paralicheniformis and Bacillus subtilis: A Comparative Study. Appl. Microbiol. Biotechnol. 2023, 107, 1537–1549. [Google Scholar] [CrossRef]
- Hu, H.; Gao, Y.; Li, X.; Chen, S.; Yan, S.; Tian, X. Identification and Nematicidal Characterization of Proteases Secreted by Endophytic Bacteria Bacillus cereus BCM2. Phytopathology 2020, 110, 336–344. [Google Scholar] [CrossRef] [PubMed]
- Pawar, P.; Doshi, J.; Patil, S.G.; Dandekar, P.; Poornima, K. The Characterization of Chitinolytic Soil Bacterial Isolates for their Antagonistic Activity against Root Knot Nematode Meloidogyne incognita: An Effort towards Developing ‘Green’ Nematicidal Agents. BioControl 2023, 68, 511–524. [Google Scholar] [CrossRef]
- Engelbrecht, G.; Horak, I.; Rensburg, P.J.J.; Claassens, S. Bacillus-Based Bionematicides: Development, Modes of Action and Commercialisation. Biocontrol Sci. Technol. 2018, 28, 629–653. [Google Scholar] [CrossRef]
- Horak, I.; Engelbrecht, G.; Rensburg, P.J.J.; Claassens, S. Microbial Metabolomics: Essential Definitions and the Importance of Cultivation Conditions for Utilizing Bacillus Species as Bionematicides. J. Appl. Microbiol. 2019, 127, 326–343. [Google Scholar] [CrossRef] [PubMed]
- Kačergius, A.; Sivojienė, D.; Gudiukaitė, R.; Bakšienė, E.; Masevičienė, A.; Žičkienė, L. Comparison of the Structure of Soil Microbial Communities of Different Ecosystems Using the Microbiome Sequencing Approach. Soil Syst. 2023, 7, 70. [Google Scholar] [CrossRef]
- Cao, X.; Yuan, Q.; Hu, C.; Zhang, H.; Sun, X.; Yan, B.; Ma, X.; Zhang, L.; Huang, L.; Li, S.; et al. Wild Wisdom Meets Cultivation: Comparative Rhizomicrobiome Analysis Unveils the Key Role of Paraburkholderia in Growth Promotion and Disease Suppression in Coptis chinensis. Microbiome 2025, 13, 150. [Google Scholar] [CrossRef]
- Valicente, F.H.; Barreto, M.R. Bacillus thuringiensis Survey in Brazil: Geographical Distribution and Insecticidal Activity against Spodoptera frugiperda (J.E. Smith) (Lepidoptera: Noctuidae). Neotrop. Entomol. 2003, 32, 639–644. [Google Scholar] [CrossRef]
- Logan, N.A.; Vos, P.D. Bacillus. In Bergey’s Manual of Systematics of Archaea and Bacteria; Whitman, W.B., Ed.; Wiley: Hoboken, NJ, USA, 2015; pp. 1–163. [Google Scholar] [CrossRef]
- Hussey, R.S.; Barker, K.R. A Comparison of Methods of Collecting Inocula of Meloidogyne spp., Including a New Technique. Plant Dis. Rep. 1973, 57, 1025–1028. [Google Scholar]
- Oostenbrink, M. Major Characteristics of the Relation between Nematodes and Plants. Mededelingen Landbouwhogeschool 1966, 66, 1–46. [Google Scholar]
- National Institute of Standards and Technology (NIST). NIST/EPA/NIH Mass Spectral Library with Search Program—SRD 1A; Version 2.2; U.S. Department of Commerce: Gaithersburg, MD, USA, 2014. Available online: https://chemdata.nist.gov/ (accessed on 11 July 2025).
- Bradford, M.M. A Rapid and Sensitive Method for the Quantitation of Microgram Quantities of Protein Utilizing the Principle of Protein-Dye Binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Charney, J.; Tomarelli, R.M. A Colorimetric Method for the Determination of the Proteolytic Activity of Duodenal Juice. J. Biol. Chem. 1947, 170, 501–505. [Google Scholar] [CrossRef]
- Miller, G.L. Use of DinitrosaIicyIic Acid Reagent for Determination of Reducing Sugar. Anal. Chem. 1959, 31, 426–428. [Google Scholar] [CrossRef]
- Weisburg, W.G.; Barns, S.M.; Pelletier, D.A.; Lane, D.J. 16S Ribosomal DNA Amplification for Phylogenetic Study. J. Bacteriol. 1991, 173, 697–703. [Google Scholar] [CrossRef]
- Schmitz, A.; Riesner, D. Purification of Nucleic Acids by Selective Precipitation with Polyethylene Glycol 6000. Anal. Biochem. 2006, 354, 311–313. [Google Scholar] [CrossRef]
- Altschul, S.F.; Gish, W.; Miller, W.; Myers, E.W.; Lipman, D.J. Basic Local Alignment Search Tool. J. Mol. Biol. 1990, 215, 403–410. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Stecher, G.; Li, M.; Knyaz, C.; Tamura, K. MEGA X: Molecular Evolutionary Genetics Analysis across Computing Platforms. Mol. Biol. Evol. 2018, 35, 1547–1549. [Google Scholar] [CrossRef]
- Tamura, K. Estimation of the Number of Nucleotide Substitutions when there are Strong Transition-Transversion and G+C-Content Biases. Mol. Biol. Evol. 1992, 9, 678–687. [Google Scholar] [CrossRef]
- Xiang, N.; Lawrence, K.S.; Kloepper, J.W.; Donald, P.A.; McInroy, J.A.; Lawrence, G.W. Biological Control of Meloidogyne incognita by Spore-Forming Plant Growth-Promoting Rhizobacteria on Cotton. Plant Dis. 2017, 101, 774–784. [Google Scholar] [CrossRef]
- Hu, H.J.; Chen, Y.L.; Wang, Y.F.; Tang, Y.Y.; Chen, S.L.; Yan, S.Z. Endophytic Bacillus cereus Effectively Controls Meloidogyne incognita on Tomato Plants Through Rapid Rhizosphere Occupation and Repellent Action. Plant Dis. 2017, 101, 448–455. [Google Scholar] [CrossRef]
- Yin, N.; Zhao, J.L.; Liu, R.; Li, Y.; Ling, J.; Yang, Y.H.; Xie, B.Y.; Mao, Z.C. Biocontrol Efficacy of Bacillus cereus Strain Bc-Cm103 Against Meloidogyne incognita. Plant Dis. 2021, 105, 2061–2070. [Google Scholar] [CrossRef] [PubMed]
- Cao, H.; Jiao, Y.; Yin, N.; Li, Y.; Ling, J.; Mao, Z.; Yang, Y.; Xie, B. Analysis of the Activity and Biological Control Efficacy of the Bacillus subtilis Strain Bs-1 against Meloidogyne incognita. Crop Prot. 2019, 122, 125–135. [Google Scholar] [CrossRef]
- Jamal, Q.; Cho, J.-Y.; Moon, J.-H.; Munir, S.; Anees, M.; Kim, K.Y. Identification for the First Time of Cyclo (d-Pro-l-Leu) Produced by Bacillus amyloliquefaciens Y1 as a Nematocide for Control of Meloidogyne incognita. Molecules 2017, 22, 1839. [Google Scholar] [CrossRef]
- Basyony, A.G.; Abo-Zaid, G.A. Biocontrol of the Root-Knot Nematode, Meloidogyne incognita, Using an Eco-Friendly Formulation from Bacillus subtilis, Lab. and Greenhouse Studies. Egypt. J. Biol. Pest Control 2018, 28, 87. [Google Scholar] [CrossRef]
- Nadeem, H.; Niazi, P.; Asif, M.; Kaskavalci, G.; Ahmad, F. Bacterial Strains Integrated with Surfactin Molecules of Bacillus subtilis MTCC441 Enrich Nematocidal Activity against Meloidogyne incognita. Plant Biol. 2021, 23, 1027–1036. [Google Scholar] [CrossRef] [PubMed]
- Peng, D.; Chai, L.; Wang, F.; Zhang, F.; Ruan, L.; Sun, M. Synergistic Activity between Bacillus thuringiensis Cry6Aa and Cry55Aa Toxins against Meloidogyne incognita. Microb. Biotechnol. 2011, 4, 794–798. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Z.; Zheng, J.; Zhang, Z.; Peng, D.; Sun, M. Nematicidal Spore-Forming Bacilli Share Similar Virulence Factors and Mechanisms. Sci. Rep. 2016, 6, 31341. [Google Scholar] [CrossRef]
- Lu, P.; Davis, R.F.; Kemerait, R.C.; Iersel, M.W.V.; Scherm, H. Physiological Effects of Meloidogyne incognita Infection on Cotton Genotypes with Differing Levels of Resistance in the Greenhouse. J. Nematol. 2014, 46, 352–359. [Google Scholar] [PubMed]
- Gámez, B.Y.S.; Garruña, R.; Suárez, J.M.T.; Valenzuela, O.A.M.; Ramírez, A.R.; Gough, R.E.V.; Catzim, C.E.A.; Palomar, L.T. Healthy Photosynthetic Mechanism Suggests ISR Elicited by Bacillus spp. in Capsicum chinense Plants Infected with PepGMV. Pathog. 2021, 10, 455. [Google Scholar] [CrossRef]
- Gámez, B.Y.S.; Garruña, R.; Suárez, J.M.T.; Can, J.K.; Ramírez, A.R.; Díaz, L.C. Bacillus spp. Inoculation Improves Photosystem II Efficiency and Enhances Photosynthesis in Pepper Plants. Chil. J. Agric. Res. 2016, 76, 409–416. [Google Scholar] [CrossRef]
- Magalhães, V.C.; Guimarães, R.A.; Silva, J.C.P.; Faria, A.F.; Pedroso, M.P.; Campos, V.P.; Marbach, P.A.S.; Medeiros, F.H.V.; Souza, J.T. The combination of two Bacillus strains supresses Meloidogyne incognita, but does not enhance plant growth. Pest Manag. Sci. 2021, 78, 722–732. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, N.; Bi, X.; Bi, T.; Baloch, F.B.; Miao, J.; Zeng, N.; Li, B.; An, Y. Growth Promotion on Maize and Whole-Genome Sequence Analysis of Bacillus velezensis D103. Microbiol. Spectr. 2024, 12, e01147-24. [Google Scholar] [CrossRef]
- Zou, Z.; Fan, Q.; Zhou, X.; Fu, X.; Jia, Y.; Li, H.; Liao, Y. Biochemical Pathways of Salicylic Acid Derived from l-Phenylalanine in Plants with Different Basal SA Levels. J. Agric. Food Chem. 2024, 72, 2898–2910. [Google Scholar] [CrossRef] [PubMed]
- Amdadul, A.K.M.; Bhuiyan, M.R.; Khan, M.A.I.; Mahmud, A.; Ahmad, M.U. Effect of Amino Acids on Root-Knot Nematode (Meloidogyne javanica) Infecting Tomato Plant. Arch. Phytopathol. Plant Prot. 2013, 47, 1921–1928. [Google Scholar] [CrossRef]
- Siddiqui, I.A.; Shaukat, S.S. Zinc and Glycerol Enhance the Production of Nematicidal Compounds in vitro and Improve the Biocontrol of a in Tomato by Fluorescent Pseudomonads. Lett. Appl. Microbiol. 2002, 35, 212–217. [Google Scholar] [CrossRef] [PubMed]
- Seo, H.J.; Park, A.R.; Kim, S.; Yeon, J.; Yu, N.H.; Ha, S.; Chang, J.Y.; Park, H.W.; Kim, J.-C. Biological Control of Root-Knot Nematodes by Organic Acid-Producing Lactobacillus brevis WiKim0069 Isolated from Kimchi. Plant Pathol. J. 2019, 35, 662–673. [Google Scholar] [CrossRef]
- Ye, L.; Wang, J.Y.; Liu, X.F.; Guan, Q.; Dou, N.X.; Li, J.; Zhang, Q.; Gao, Y.M.; Wang, M.; Li, J.S.; et al. Nematicidal Activity of Volatile Organic Compounds Produced by Bacillus altitudinis AMCC 1040 against Meloidogyne incognita. Arch. Microbiol. 2022, 204, 521. [Google Scholar] [CrossRef]
- Oliveira, D.F.; Santos Júnior, H.M.D.; Nunes, A.S.; Campos, V.P.; Pinho, R.S.C.D.; Gajo, G.C. Purification and Identification of Metabolites Produced by Bacillus cereus and B. subtilis Active against Meloidogyne exigua, and Their in Silico Interaction with a Putative Phosphoribosyltransferase from M. incognita. An. Acad. Bras. Ciênc. 2014, 86, 525–538. [Google Scholar] [CrossRef]
- Wang, J.Y.; Li, Q.Y.; Ren, L.; Guo, C.; Qu, J.P.; Gao, Z.; Wang, H.F.; Zhang, Q.; Zhou, B. Transcriptomic and Physiological Analysis of the Effect of Octanoic Acid on Meloidogyne incognita. Pestic. Biochem. Physiol. 2023, 193, 105432. [Google Scholar] [CrossRef]
- Soliman, G.M.; Ameen, H.H.; Abdel-Aziz, S.M.; El-Sayed, G.M. In Vitro Evaluation of Some Isolated Bacteria against the Plant Parasite Nematode Meloidogyne incognita. Bull. Natl. Res. Cent. 2019, 43, 171. [Google Scholar] [CrossRef]
- Wei, L.H.; Xue, Q.Y.; Wei, B.Q.; Wang, Y.M.; Li, S.M.; Chen, L.F.; Guo, J.H. Screening of Antagonistic Bacterial Strains against Meloidogyne incognita Using Protease AcTivity. Biocont. Sci. Technol. 2010, 20, 739–750. [Google Scholar] [CrossRef]

| Treatment | A (µmol m−2 s−1) | Gs (µmol H2O m−2 s−1) | Ci/Ca | Fv’/Fm’ |
|---|---|---|---|---|
| C- | 7.00 ± 1.70 b | 0.09 ± 0.02 a | 254.00 ± 31.30 b | 1.70 ± 0.10 b |
| C+ (Biological) | 8.60 ± 1.80 b | 0.10 ± 0.03 a | 271.90 ± 13.60 ab | 2.40 ± 0.30 ab |
| C+ (Chemical) | 10.90 ± 2.50 ab | 0.13 ± 0.06 a | 283.10 ± 26.90 ab | 2.70 ± 0.80 ab |
| 307 | 8.40 ± 2.50 b | 0.10 ± 0.05 a | 264.00 ± 26.70 ab | 2.70 ± 1.60 ab |
| GB16 | 15.20 ± 1.80 a | 0.15 ± 0.05 a | 251.10 ± 11.20 b | 4.20 ± 1.30 a |
| GB24 | 8.20 ± 2.00 b | 0.10 ± 0.06 a | 307.40 ± 27.20 a | 2.00 ± 0.70 ab |
| GB29 | 12.20 ± 4.50 ab | 0.12 ± 0.05 a | 234.40 ± 11.70 b | 3.40 ± 1.70 ab |
| CV% | 25.50 | 44.70 | 8.70 | 40.00 |
| Treatment | Aerial Part | Roots | |
|---|---|---|---|
| FMAP | DMAP | FMR | |
| C- | 76.10 ± 14.80 a | 10.70 ± 2.20 a | 27.90 ± 8.00 a |
| C+ (Biological) | 63.20 ± 19.60 a | 9.10 ± 2.90 a | 25.60 ± 12.80 a |
| C+ (Chemical) | 67.90 ± 24.20 a | 10.30 ± 3.80 a | 15.90 ± 6.80 a |
| 307 | 77.30 ± 10.60 a | 10.50 ± 1.70 a | 29.90 ± 9.40 a |
| GB16 | 81.30 ± 8.70 a | 11.20 ± 1.60 a | 25.40 ± 9.90 a |
| GB24 | 77.70 ± 11.90 a | 11.30 ± 2.40 a | 26.70 ± 16.40 a |
| GB29 | 74.60 ± 10.80 a | 10.90 ± 2.20 a | 23.00 ± 9.60 a |
| CV% | 20.90 | 24.60 | 41.70 |
| Treatments | Eggs/g of Root | J2/g of Root | Total Nematode (Eggs + J2)/g of Root | |||
|---|---|---|---|---|---|---|
| Nº | Red. (%) | Nº | Red. (%) | Nº | Red. (%) | |
| C- | 1128.43 ± 534.13 a | - | 617.62 ± 355.15 a | - | 1746.06 ± 771.13 a | - |
| C+ (Biological) | 498.46 ± 302.67 bc | 55.82 | 278.48 ± 141.32 bc | 54.90 | 776.94 ± 426.50 bc | 55.50 |
| C+ (Chemical) | 222.87 ± 123.31 c | 80.25 | 102.42 ± 75.87 c | 83.41 | 325.22 ± 288.57 c | 81.37 |
| 307 | 386.37 ± 253.05 bc | 65.76 | 287.81 ± 115.66 bc | 53.40 | 674.11 ± 298.82 bc | 61.39 |
| GB16 | 633.20 ± 352.75 bc | 43.88 | 343.81 ± 159.31 bc | 44.33 | 977.02 ± 424.22 b | 44.04 |
| GB24 | 500.74 ± 268.17 bc | 55.62 | 245.70 ± 154.67 bc | 60.21 | 746.45 ± 405.45 bc | 57.24 |
| GB29 | 704.56 ± 394.86 ab | 37.56 | 400.53 ± 221.60 ab | 35.14 | 1105.10 ± 444.43 b | 36.70 |
| CV% | 53.05 | 51.36 | 42.59 | |||
| Metabolite | Retention Time (min) | Similarity (%) | Area (%) | |||
|---|---|---|---|---|---|---|
| Proteic Amino Acids | 307 | GB16 | GB24 | GB29 | ||
| L-alanine | 4.54 | 97 | - | - | - | 2.90 |
| L-glycine | 7.74 | 94 | - | - | 5.12 | 9.85 |
| L-isoleucine | 6.98 | 94 | 4.98 | - | - | - |
| L-leucine | 8.35 | 95 | 10.02 | 20.22 | 6.67 | 8.42 |
| L-methionine | 12.79 | 92 | - | - | - | 5.10 |
| DL-phenylalanine | 13.09 | 97 | 9.27 | - | - | - |
| L-phenylalanine | 14.30 | 87 | 0.79 | - | - | - |
| L-proline | 13.80 | 90 | - | - | 12.21 | - |
| L-threonine | 10.89 | 92 | 3.28 | 16.13 | - | - |
| L-tyrosine | 19.67 | 87 | - | - | 7.71 | - |
| L-valine | 7.11 | 97 | 14.95 | 25.25 | 6.63 | 7.43 |
| Non-Proteic Amino Acids | ||||||
| L-Norvaline | 7.71 | 88 | 0.75 | - | - | - |
| L-5-oxoproline | 12.78 | 93 | 0.86 | - | - | - |
| Alcohols | ||||||
| Glycerol | 8.75 | 93 | 1.97 | - | - | - |
| Sylanol | 10.57 | 90 | 8.16 | - | - | 13.78 |
| Azoles | ||||||
| Pyrrolo | 18.34 | 95 | - | - | 5.27 | - |
| Nitrogenous Base | ||||||
| Uracil | 9.83 | 90 | 1.13 | - | - | - |
| Cyclic Polyalcohol | ||||||
| Inositol | 22.35 | 90 | - | 8.68 | 5.13 | 1.61 |
| Scyllo-inositol | 20.45 | 92 | 5.09 | - | - | - |
| Fatty Acids | ||||||
| Butanedioic acid | 9.35 | 95 | 10.29 | - | - | - |
| Butanoic acid | 4.24 | 96 | - | - | 3.43 | - |
| Hexadecanoic acid | 19.57 | 93 | 1.05 | - | - | - |
| Hexanedioic acid | 12.45 | 86 | 1.36 | - | - | - |
| Octadecanoic acid | 21.77 | 92 | 1.16 | - | - | - |
| Pentanoic acid | 9.86 | 95 | - | - | 7.11 | - |
| Propanoic acid | 6.67 | 95 | - | - | 4.47 | 3.98 |
| Monoacylglycerol | ||||||
| Glycerol monostearate | 27.06 | 86 | 1.30 | - | - | - |
| 1-monopalmitin | 25.32 | 88 | 0.88 | - | - | - |
| Organic Acids | ||||||
| Acetic acid | 10.88 | 87 | - | - | - | 16.22 |
| Fumaric acid | 11.71 | 89 | - | - | - | 7.16 |
| Lactic acid | 5.01 | 94 | 0.74 | - | - | - |
| Oxalic acid | 6.39 | 83 | 3.78 | - | - | - |
| Propionic acid | 15.55 | 89 | - | - | 9.78 | 5.33 |
| Pyroglutamic acid | 14.19 | 83 | - | - | 4.56 | 2.22 |
| Succinic acid | 11.17 | 95 | - | 12.51 | 13.09 | 2.26 |
| Sugar | ||||||
| Trehalose | 26.21 | 87 | 2.54 | - | - | - |
| Treatment | Specific Protease Activity (Abs440nm mg−1 Protein) | Specific Chitinase Activity (U mg−1 Protein) | ||
|---|---|---|---|---|
| 24 h | 48 h | 24 h | 48 h | |
| Control | 0.0000 ± 0.000 b | 0.0000 ± 0.000 c | 0.0000 ± 0.000 b | 0.0000 ± 0.000 c |
| 307 | 0.0018 ± 0.0004 a | 0.0029 ± 0.001 b | 10.429 ± 2.984 a | 0.8608 ± 0.059 b |
| GB16 | 0.0027 ± 0.0002 a | 0.0122 ± 0.002 a | 0.000 ± 0.000 b | 47.530 ± 1.750 a |
| GB24 | 0.0023 ± 0.0001 a | 0.0014 ± 0.000 b | 8.064 ± 3.500 a | 2.0690 ± 0.230 b |
| GB29 | 0.0022 ± 0.0001 a | 0.0026 ± 0.000 b | 0.000 ± 0.000 b | 1.6300 ± 0.100 b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Castro, M.V.; Santana, L.M.; Lopes, E.A.; da Cunha, W.V.; Catara, V.; Dimaria, G.; Visotto, L.E. Role of Enzymes and Metabolites Produced by Bacillus spp. in the Suppression of Meloidogyne incognita in Tomato. Horticulturae 2025, 11, 1189. https://doi.org/10.3390/horticulturae11101189
Castro MV, Santana LM, Lopes EA, da Cunha WV, Catara V, Dimaria G, Visotto LE. Role of Enzymes and Metabolites Produced by Bacillus spp. in the Suppression of Meloidogyne incognita in Tomato. Horticulturae. 2025; 11(10):1189. https://doi.org/10.3390/horticulturae11101189
Chicago/Turabian StyleCastro, Mariana Viana, Luanda Medeiros Santana, Everaldo Antônio Lopes, Walter Vieira da Cunha, Vittoria Catara, Giulio Dimaria, and Liliane Evangelista Visotto. 2025. "Role of Enzymes and Metabolites Produced by Bacillus spp. in the Suppression of Meloidogyne incognita in Tomato" Horticulturae 11, no. 10: 1189. https://doi.org/10.3390/horticulturae11101189
APA StyleCastro, M. V., Santana, L. M., Lopes, E. A., da Cunha, W. V., Catara, V., Dimaria, G., & Visotto, L. E. (2025). Role of Enzymes and Metabolites Produced by Bacillus spp. in the Suppression of Meloidogyne incognita in Tomato. Horticulturae, 11(10), 1189. https://doi.org/10.3390/horticulturae11101189







