Cold Hardiness and Physio-Biochemical Responses of Annual Branches in Five Early-Fruiting Walnut Varieties (Juglans regia L.) Under Simulated Low-Temperature Stress
Abstract
1. Introduction
2. Materials and Methods
2.1. Orchards
2.2. Experimental Materials
2.3. Experimental Methods
2.3.1. Low-Temperature Stress Treatment
2.3.2. Measurement of Electrical Conductivity (EC)
2.3.3. Rehydration and Regrowth (RG) Test
2.3.4. Measurement of Osmoregulatory Substances
2.3.5. Measurement of Protective Enzyme System Activity
2.4. Data Analysis
3. Results
3.1. Effect of Low Temperature on Electrical Conductivity (EC) and Relative Electrical Conductivity (REC)
3.2. Logistic Equation Analysis and LT50 Estimation
3.3. Impact of Low Temperatures on Bud Germination and Regrowth (RG) Potential
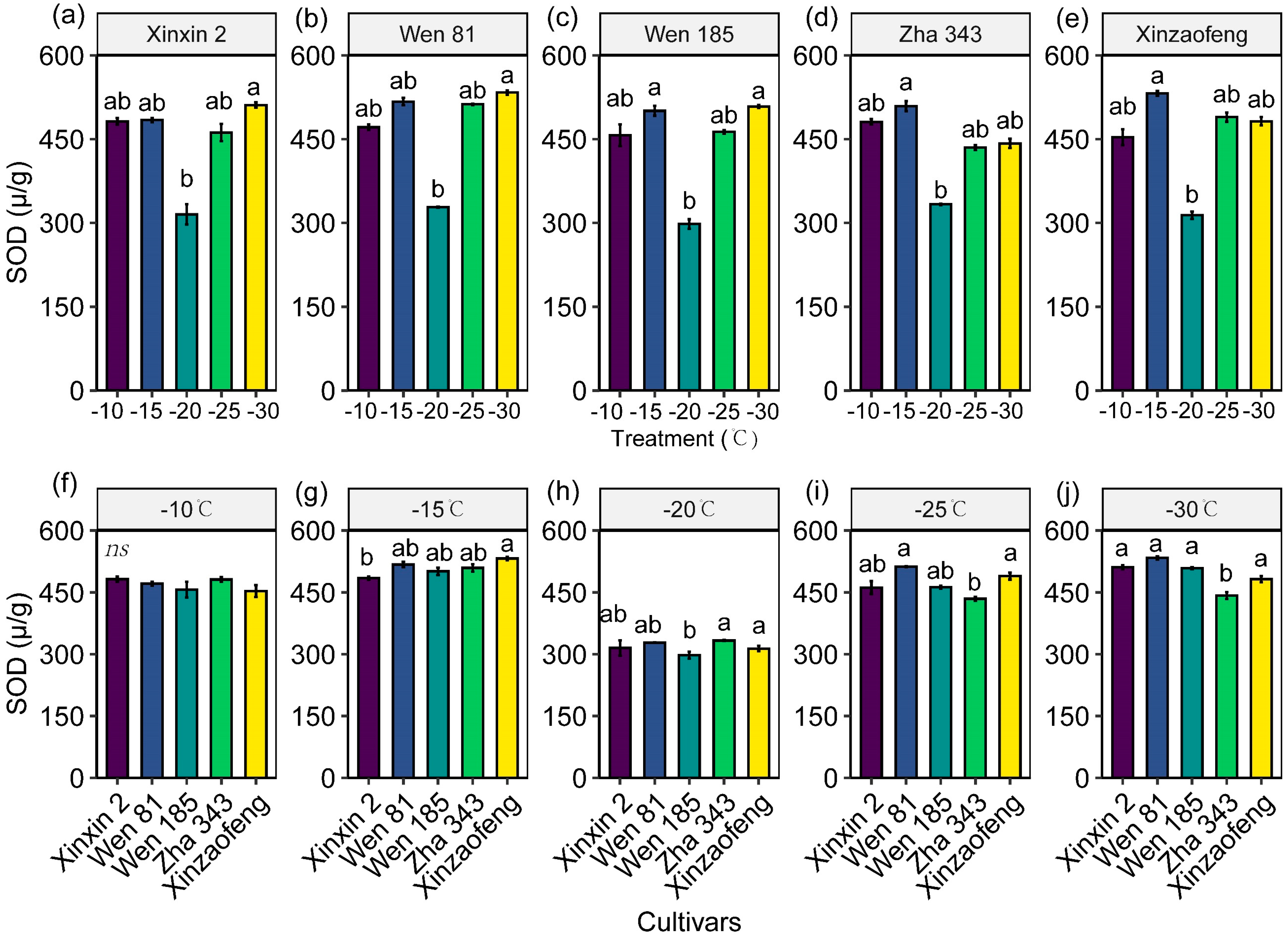
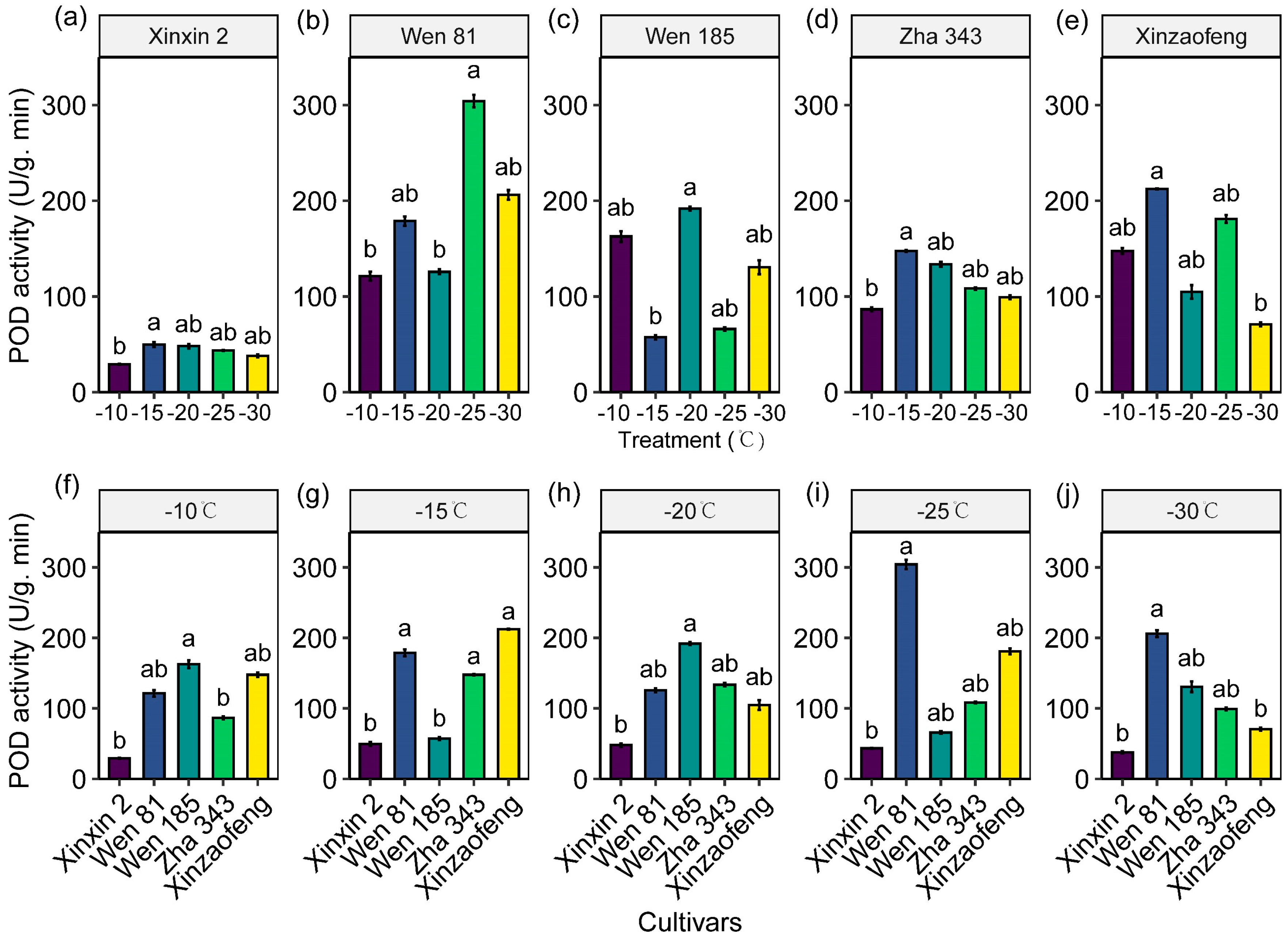
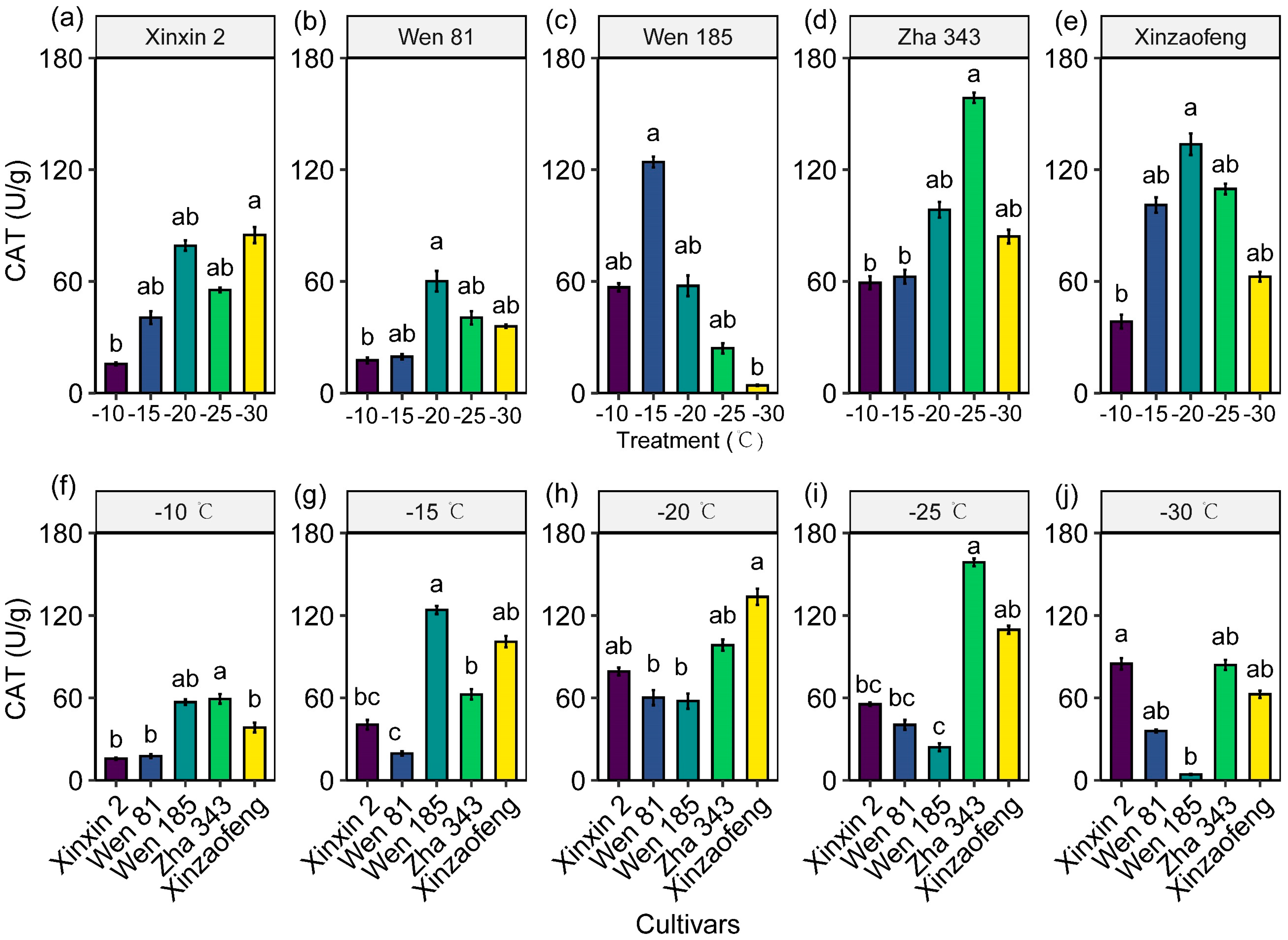
3.4. Variations in Antioxidant Enzyme Activities
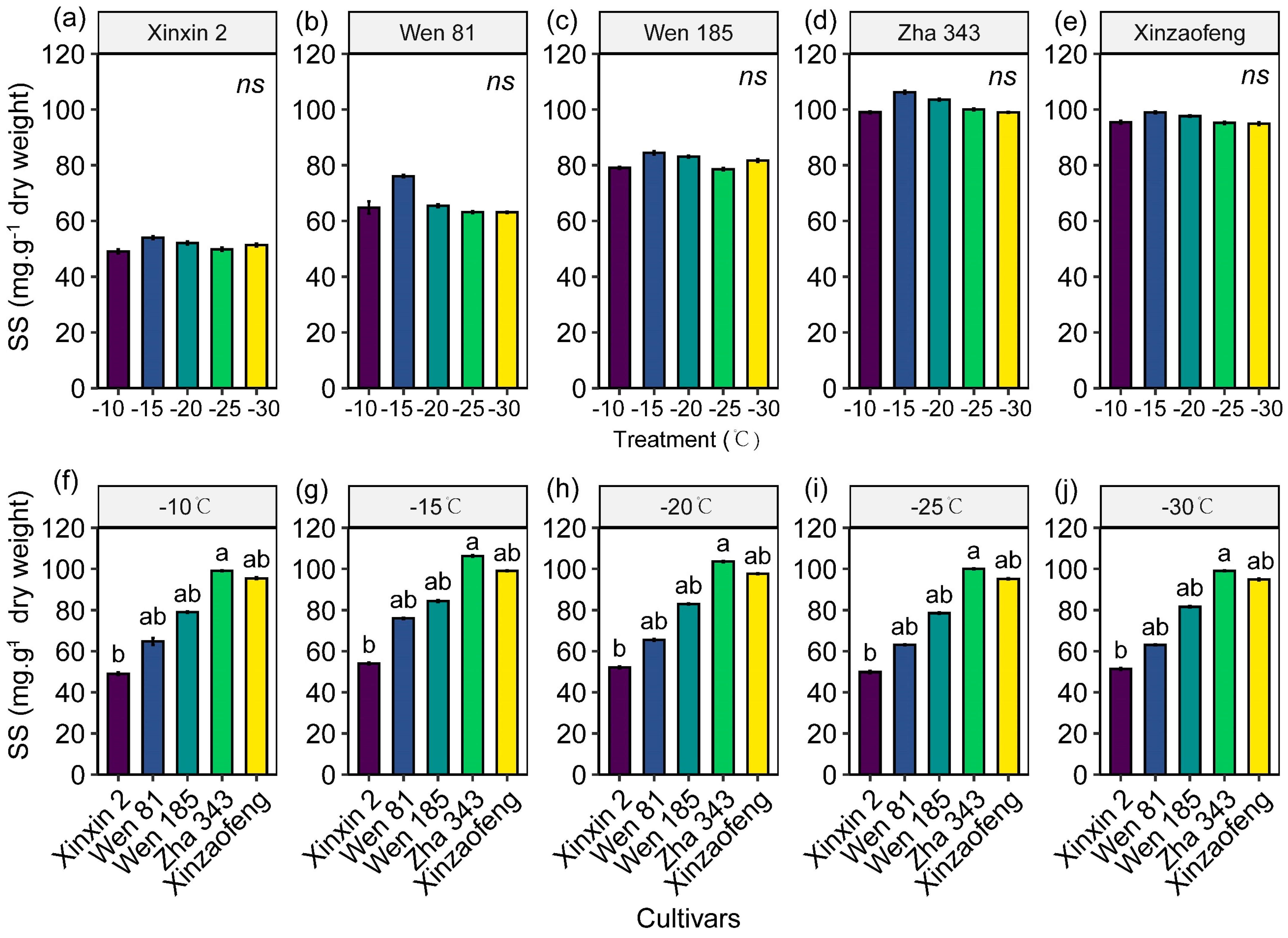
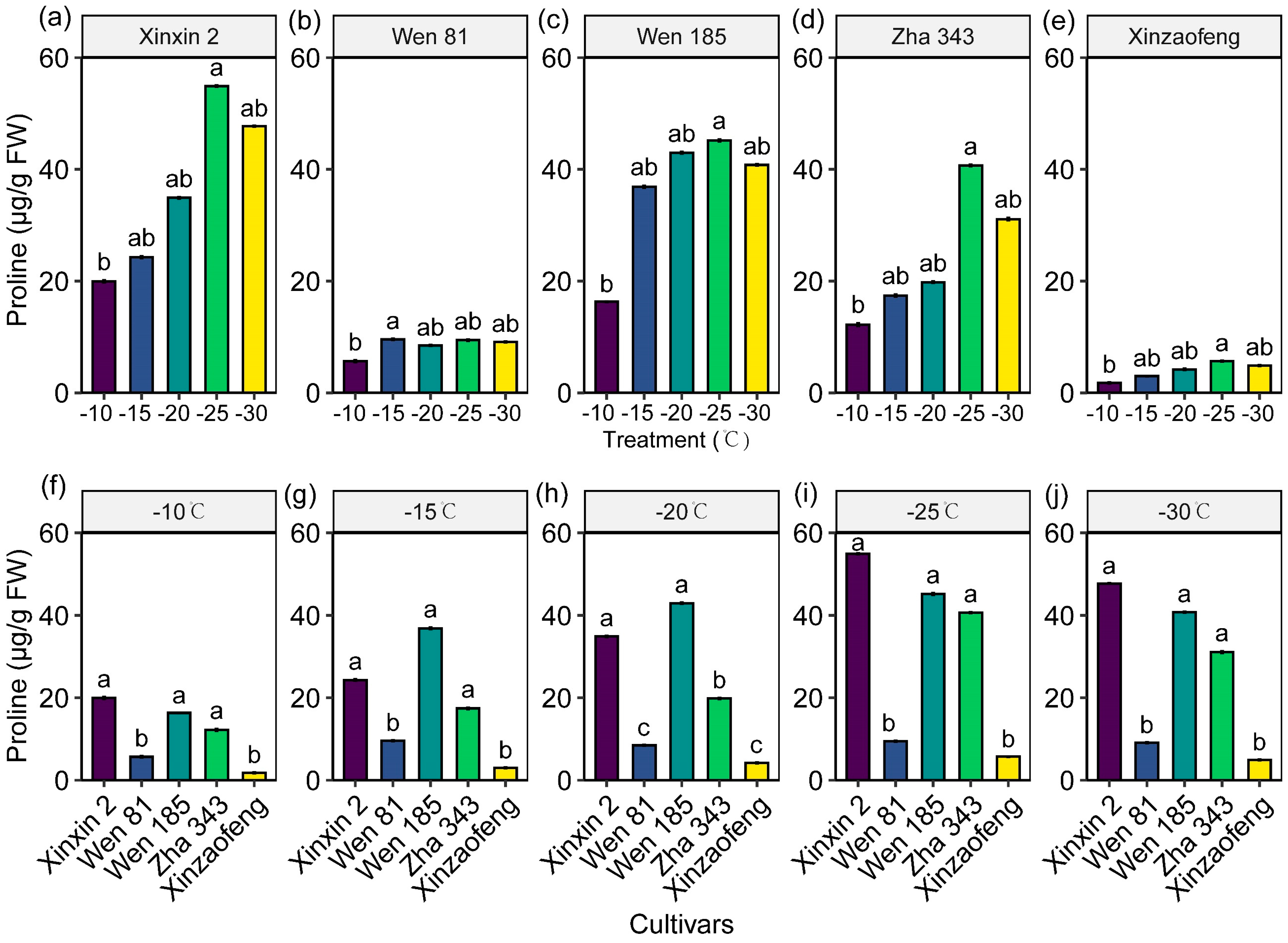
3.5. Accumulation of Osmoregulatory Substances
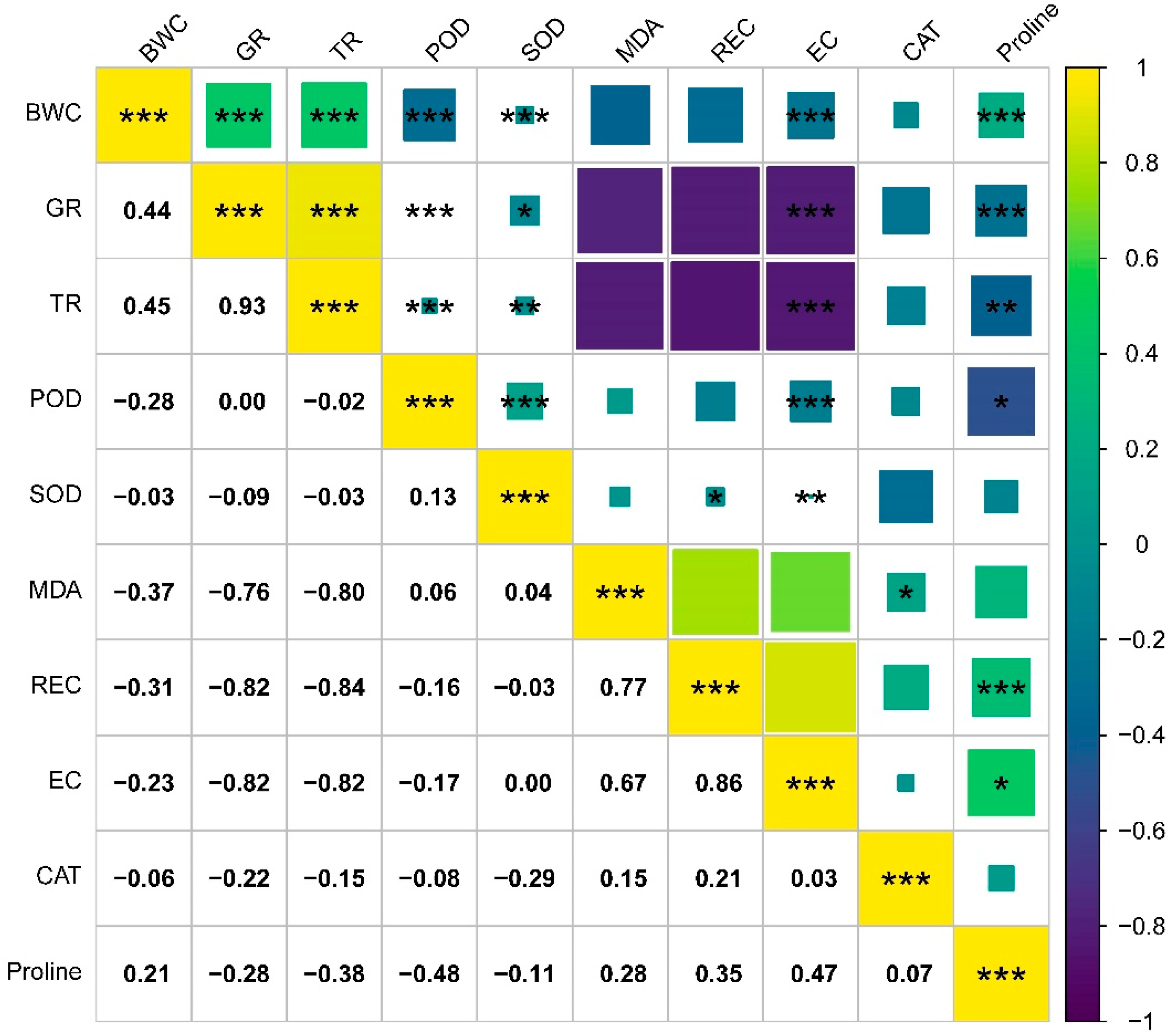
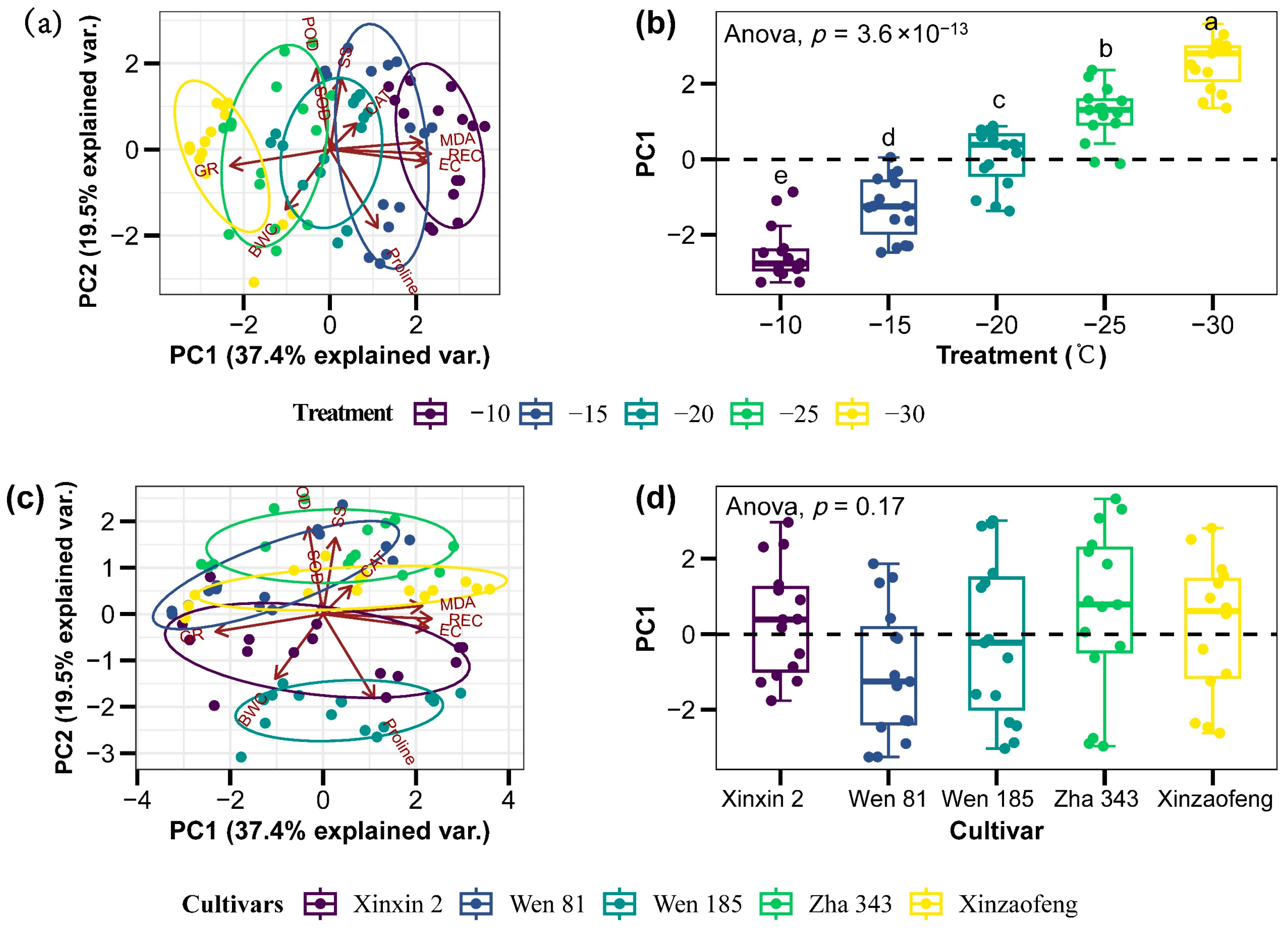
3.6. Correlation Analysis and PCA
3.7. Evaluating Cold Resistance Using Mean Membership Function
4. Discussion
4.1. Discrepancies in Cold Hardiness Assessments
4.2. The Protective Role of the Antioxidant System and Osmoregulatory Substances in Cold Injury
4.3. Climate Change and Breeding Implications
4.4. Limitations and Future Directions
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Traits | TR (df = 4) | Cultivars (df = 4) | TR × Cultivars (df = 16) | |||
|---|---|---|---|---|---|---|
| F | p | F | p | F | p | |
| EC | 697.43 | 0.000 *** | 152.63 | 0.000 *** | 33.31 | 0.000 *** |
| REC | 286.824 | 0.000 *** | 56.901 | 0.000 *** | 9.683 | 0.000 *** |
| GR | 593.856 | 0.000 *** | 37.273 | 0.000 *** | 4.144 | 0.000 *** |
| BWC | 4.878 | 0.002 ** | 5.560 | 0.000 *** | 0.162 | NS |
| SOD | 401.642 | 0.000 *** | 10.264 | 0.000 *** | 6.757 | 0.000 *** |
| POD | 69.83 | 0.000 *** | 1075.64 | 0.000 *** | 231.78 | 0.000 *** |
| MDA | 183.60 | 0.000 *** | 23.49 | 0.000 *** | 14.48 | 0.000 *** |
| CAT | 163.5 | 0.000 *** | 274.9 | 0.000 *** | 97.4 | 0.000 *** |
| Proline | 15,407 | 0.000 *** | 60,338 | 0.000 *** | 2574 | 0.000 *** |
| SS | 166.36 | 0.000 *** | 9249.63 | 0.000 *** | 14.97 | 0.000 *** |
| SP | 168.64 | 0.000 *** | 145.61 | 0.000 *** | 65.78 | 0.000 *** |
References
- Glenn, D.M.; Kim, S.-H.; Ramirez-Villegas, J.; Läderach, P. Response of Perennial Horticultural Crops to Climate Change. In Horticultural Reviews Volume 41; Wiley: Hoboken, NJ, USA, 2013; pp. 47–130. [Google Scholar]
- Bhattacharya, A. Effect of Low-Temperature Stress on Germination, Growth, and Phenology of Plants: A Review. In Physiological Processes in Plants Under Low Temperature Stress; Bhattacharya, A., Ed.; Springer: Singapore, 2022; pp. 1–106. [Google Scholar]
- Rehman, M.U.; Rather, G.H.; Gull, Y.; Mir, M.R.; Mir, M.M.; Waida, U.I.; Hakeem, K.R. Effect of Climate Change on Horticultural Crops. In Crop Production and Global Environmental Issues; Hakeem, K.R., Ed.; Springer International Publishing: Cham, Switzerland, 2015; pp. 211–239. [Google Scholar]
- Chamberlain, C.J.; Cook, B.I.; García de Cortázar-Atauri, I.; Wolkovich, E.M. Rethinking false spring risk. Glob. Chang. Biol. 2019, 25, 2209–2220. [Google Scholar] [CrossRef] [PubMed]
- Naqvi, R.Z.; Siddiqui, H.A.; Mahmood, M.A.; Najeebullah, S.; Ehsan, A.; Azhar, M.; Farooq, M.; Amin, I.; Asad, S.; Mukhtar, Z.; et al. Smart breeding approaches in post-genomics era for developing climate-resilient food crops. Front. Plant Sci. 2022, 13, 972164. [Google Scholar] [CrossRef] [PubMed]
- Kole, C.; Muthamilarasan, M.; Henry, R.; Edwards, D.; Sharma, R.; Abberton, M.; Batley, J.; Bentley, A.; Blakeney, M.; Bryant, J.; et al. Application of genomics-assisted breeding for generation of climate resilient crops: Progress and prospects. Front. Plant Sci. 2015, 6, 563. [Google Scholar] [CrossRef]
- Razzaq, A.; Kaur, P.; Akhter, N.; Wani, S.H.; Saleem, F. Next-Generation Breeding Strategies for Climate-Ready Crops. Front. Plant Sci. 2021, 12, 620420. [Google Scholar] [CrossRef] [PubMed]
- Muthuramalingam, P.; Shin, H.; Adarshan, S.; Jeyasri, R.; Priya, A.; Chen, J.-T.; Ramesh, M. Molecular Insights into Freezing Stress in Peach Based on Multi-Omics and Biotechnology: An Overview. Plants 2022, 11, 812. [Google Scholar] [CrossRef] [PubMed]
- Imani, A.; Barzegar, K.; Piripireivatlou, S. Relationship Between Frost Injury and Ion Leakage as an Indicator of Cold Hardiness in 60 Almond Selections. J. Nuts 2011, 2, 22–26. [Google Scholar]
- Wanjiku, J.; Bohne, H. Early frost reactions of different populations of hazelnut (Corylus avellana L.). Eur. J. Hortic. Sci. 2015, 80, 162–169. [Google Scholar] [CrossRef]
- Kaur, A.; Zhang, L.; Yang, M.; Maness, N.; Graham, C.J.; Kumari, R.; Sun, Y.; Panta, S.; Ferguson, L. Evaluation of Natural Spring Freeze Tolerance of Five Pecan Scion–Rootstock Combinations. HortScience 2023, 58, 1139–1148. [Google Scholar] [CrossRef]
- Jamshidi Goharrizi, K.; Meru, G.; Ghotbzadeh Kermani, S.; Heidarinezhad, A.; Salehi, F. Short-term cold stress affects physiological and biochemical traits of pistachio rootstocks. S. Afr. J. Bot. 2021, 141, 90–98. [Google Scholar] [CrossRef]
- Hajivand, S.; Kashanizadeh, S.; Javanshah, A. Effects of different antifreeze chemicals on late spring frost in pistachio. Protoplasma 2022, 259, 91–102. [Google Scholar] [CrossRef] [PubMed]
- Imani, A.; Ezaddost, M.; Asgari, F.; Masoumi, S.H.; Raeisi, I. Evaluation the Resistance of Almond to Frost in Controlled and Field Conditions. J. Nuts 2012, 3, 29–36. [Google Scholar]
- Hajivand, S. Assessment of Reaction of Some Iranian Hazelnut (Corylus avellana L.) Cultivars to Freezing Stress. J. Plant Prod. Res. 2021, 28, 67–84. [Google Scholar]
- Kaur, A.; Zhang, L.; Maness, N.O.; Ferguson, L.; Graham, C.J.; Sun, Y.; Panta, S.; Pokhrel, N.; Yang, M.; Moss, J.Q. Dormant carbohydrate reserves enhance pecan tree spring freeze tolerance: Controlled environment observations. Front. Plant Sci. 2024, 15, 1393305. [Google Scholar] [CrossRef] [PubMed]
- Kreyling, J. Winter climate change: A critical factor for temperate vegetation performance. Ecology 2010, 91, 1939–1948. [Google Scholar] [CrossRef] [PubMed]
- Körner, C.; Basler, D.; Hoch, G.; Kollas, C.; Lenz, A.; Randin, C.F.; Vitasse, Y.; Zimmermann, N.E. Where, why and how? Explaining the low-temperature range limits of temperate tree species. J. Ecol. 2016, 104, 1076–1088. [Google Scholar] [CrossRef]
- Vitasse, Y.; Lenz, A.; Körner, C. The interaction between freezing tolerance and phenology in temperate deciduous trees. Front. Plant Sci. 2014, 5, 541. [Google Scholar] [CrossRef]
- Zohner, C.M.; Mo, L.; Renner, S.S.; Svenning, J.-C.; Vitasse, Y.; Benito, B.M.; Ordonez, A.; Baumgarten, F.; Bastin, J.-F.; Sebald, V. Late-spring frost risk between 1959 and 2017 decreased in North America but increased in Europe and Asia. Proc. Natl. Acad. Sci. USA 2020, 117, 12192–12200. [Google Scholar] [CrossRef] [PubMed]
- Henry, H.A.L. Climate change and soil freezing dynamics: Historical trends and projected changes. Clim. Chang. 2008, 87, 421–434. [Google Scholar] [CrossRef]
- Augspurger, C.K. Reconstructing patterns of temperature, phenology, and frost damage over 124 years: Spring damage risk is increasing. Ecology 2013, 94, 41–50. [Google Scholar] [CrossRef]
- Lamichhane, J.R. Rising risks of late-spring frosts in a changing climate. Nat. Clim. Chang. 2021, 11, 554–555. [Google Scholar] [CrossRef]
- Vitasse, Y.; Schneider, L.; Rixen, C.; Christen, D.; Rebetez, M. Increase in the risk of exposure of forest and fruit trees to spring frosts at higher elevations in Switzerland over the last four decades. Agric. For. Meteorol. 2018, 248, 60–69. [Google Scholar] [CrossRef]
- McCulloh, K.A.; Augustine, S.P.; Goke, A.; Jordan, R.; Krieg, C.P.; O’Keefe, K.; Smith, D.D. At least it is a dry cold: The global distribution of freeze–thaw and drought stress and the traits that may impart poly-tolerance in conifers. Tree Physiol. 2023, 43, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Ebrahimi, A.; Lawson, S.S.; McKenna, J.R.; Jacobs, D.F. Morpho-Physiological and Genomic Evaluation of Juglans Species Reveals Regional Maladaptation to Cold Stress. Front. Plant Sci. 2020, 11, 229. [Google Scholar] [CrossRef] [PubMed]
- Gusta, L.V.; Wisniewski, M. Understanding plant cold hardiness: An opinion. Physiol. Plant. 2013, 147, 4–14. [Google Scholar] [CrossRef] [PubMed]
- Quamme, H.A. Mechanism of Supercooling in Overwintering Peach Flower Buds. J. Am. Soc. Hortic. Sci. 1978, 103, 57–61. [Google Scholar] [CrossRef]
- Wisniewski, M.; Bassett, C.; Gusta, L.V. An overview of cold hardiness in woody plants: Seeing the forest through the trees. HortScience 2003, 38, 952–959. [Google Scholar] [CrossRef]
- Wisniewski, M.; Nassuth, A.; Arora, R. Cold Hardiness in Trees: A Mini-Review. Front. Plant Sci. 2018, 9, 1394. [Google Scholar] [CrossRef] [PubMed]
- Kulan, E.G.; Kaya, M.D. Evaluation of Frost Tolerance in Sugar Beet Cultivars During Early Growth Stages. Sugar Tech. 2024, 26, 1274–1282. [Google Scholar] [CrossRef]
- Palonen, P.; Buszard, D. Current state of cold hardiness research on fruit crops. Can. J. Plant Sci. 1997, 77, 399–420. [Google Scholar] [CrossRef]
- Lodolini, E.M.; Alfei, B.; Santinelli, A.; Cioccolanti, T.; Polverigiani, S.; Neri, D. Frost tolerance of 24 olive cultivars and subsequent vegetative re-sprouting as indication of recovery ability. Sci. Hortic. 2016, 211, 152–157. [Google Scholar] [CrossRef]
- Uray, Y.; Köse, B. Performing of the Winter Buds and Phloem Tissue in Grape Cultivars After Cold Applications. Appl. Fruit Sci. 2024, 66, 997–1008. [Google Scholar] [CrossRef]
- Wang, Z.L.; Wu, D.; Hui, M.; Wang, Y.; Han, X.; Yao, F.; Cao, X.; Li, Y.H.; Li, H.; Wang, H. Screening of cold hardiness-related indexes and establishment of a comprehensive evaluation method for grapevines (V. vinifera). Front. Plant Sci. 2022, 13, 1014330. [Google Scholar] [CrossRef]
- Xu, G.; He, M.; Zhao, D.; Lyu, D.; Qin, S. Physiological and Structural Changes in Apple Tree Branches of Different Varieties during Dormancy. Horticulturae 2023, 9, 947. [Google Scholar] [CrossRef]
- Wisniewski, M.; Gusta, L.; Neuner, G. Adaptive mechanisms of freeze avoidance in plants: A brief update. Environ. Exp. Bot. 2014, 99, 133–140. [Google Scholar] [CrossRef]
- Niu, R.; Zhao, X.; Wang, C.; Wang, F. Physiochemical Responses and Ecological Adaptations of Peach to Low-Temperature Stress: Assessing the Cold Resistance of Local Peach Varieties from Gansu, China. Plants 2023, 12, 4183. [Google Scholar] [CrossRef] [PubMed]
- Mishra, N.; Jiang, C.; Chen, L.; Paul, A.; Chatterjee, A.; Shen, G. Achieving abiotic stress tolerance in plants through antioxidative defense mechanisms. Front. Plant Sci. 2023, 14, 1110622. [Google Scholar] [CrossRef] [PubMed]
- Rahemi, A.; Fisher, H.; Carter, K.; Taghavi, T. Mitigating grapevine winter damage in cold climate areas. Hortic. Sci. 2022, 49, 59–70. [Google Scholar] [CrossRef]
- Kwon, J.H.; Nam, E.Y.; Yun, S.K.; Kim, S.J.; Yu, D.J.; Lee, H.J. Comparative carbohydrate metabolism in the shoots of a cold-hardy and a cold-sensitive peach (Prunus persica) cultivar during cold acclimation and deacclimation. Hortic. Environ. Biotechnol. 2022, 63, 39–53. [Google Scholar] [CrossRef]
- Saadati, S.; Baninasab, B.; Mobli, M.; Gholami, M. Measurements of freezing tolerance and their relationship with some biochemical and physiological parameters in seven olive cultivars. Acta Physiol. Plant. 2019, 41, 51. [Google Scholar] [CrossRef]
- Kalberer, S.R.; Wisniewski, M.; Arora, R. Deacclimation and reacclimation of cold-hardy plants: Current understanding and emerging concepts. Plant Sci. 2006, 171, 3–16. [Google Scholar] [CrossRef]
- Zhang, W.; Zhou, J.; Feng, G.; Weindorf, D.C.; Hu, G.; Sheng, J. Characteristics of water erosion and conservation practice in arid regions of Central Asia: Xinjiang, China as an example. Int. Soil Water Conserv. Res. 2015, 3, 97–111. [Google Scholar] [CrossRef]
- Jiang, L.; Bao, A.; Jiapaer, G.; Guo, H.; Zheng, G.; Gafforov, K.; Kurban, A.; De Maeyer, P. Monitoring land sensitivity to desertification in Central Asia: Convergence or divergence? Sci. Total Environ. 2019, 658, 669–683. [Google Scholar] [CrossRef] [PubMed]
- Xu, G.; Li, J.; Hu, H.; Chen, T. Effect of deficit irrigation on physiological, morphological and fruit quality traits of six walnut tree cultivars in the inland area of Central Asia. Sci. Hortic. 2024, 329, 112951. [Google Scholar]
- Luedeling, E.; Girvetz, E.H.; Semenov, M.A.; Brown, P.H. Climate Change Affects Winter Chill for Temperate Fruit and Nut Trees. PLoS ONE 2011, 6, e20155. [Google Scholar] [CrossRef]
- Chen, R.; Wang, J.; Li, Y.; Bai, R.; Huang, M.; Zhang, Z.; Zhao, L.; Qu, Z.; Liu, L. Higher risk of spring frost under future climate change across China’s apple planting regions. Eur. J. Agron. 2024, 159, 127288. [Google Scholar] [CrossRef]
- Liu, B.; Liang, J.; Zhao, D.; Wang, K.; Jia, M.; Wang, J. Morphological and Compositional Analysis of Two Walnut (Juglans regia L.) Cultivars Growing in China. Plant Foods Hum. Nutr. 2020, 75, 116–123. [Google Scholar] [CrossRef]
- Vahdati, K.; Sarikhani Khorami, S.; Arab, M.M. Walnut: A potential multipurpose nut crop for reclaiming deteriorated lands and environment. Acta Hortic. 2018, 1190, 95–100. [Google Scholar] [CrossRef]
- Aslamarz, A.A.; Vahdati, K.; Hassani, D.; Rahemi, M.; Mohammadi, N.; Leslie, C. Cold hardiness and its relationship with proline content in Persian walnut. Eur. J. Hortic. Sci. 2011, 76, 84–90. [Google Scholar]
- Zhang, R.; Liu, B.; Xin, G.; Zhang, X.; Li, J.; Wang, Y. Evaluation of cold tolerance of seven walnut varieties. Cryoletters 2022, 43, 74–82. [Google Scholar] [CrossRef]
- Arora, R.; Wisniewski, M.E.; Scorza, R. Cold Acclimation in Genetically Related (Sibling) Deciduous and Evergreen Peach (Prunus persica L. Batsch): I. Seasonal Changes in Cold Hardiness and Polypeptides of Bark and Xylem Tissues. Plant Physiol. 1992, 99, 1562–1568. [Google Scholar] [CrossRef] [PubMed]
- Wisniewski, M.; Arora, R. Adaptation and response of fruit trees to freezing temperatures. In Cytology, Histology and Histochemistry of Fruit Tree Diseases; CRC Press: Boca Raton, FL, USA, 2019; pp. 299–320. [Google Scholar]
- Vafadar, M.; Rezaei, M.; Khadivi, A. Frost hardiness of 10 olive cultivars after natural and controlled freezing. Sci. Hortic. 2024, 325, 112687. [Google Scholar] [CrossRef]
- Malmqvist, C.; Wallertz, K.; Lindström, A. Storability and freezing tolerance of Douglas fir and Norway spruce seedlings grown in mid-Sweden. Scand. J. For. Res. 2017, 32, 30–38. [Google Scholar] [CrossRef]
- Chow, P.S.; Landhäusser, S.M.A. Method for routine measurements of total sugar and starch content in woody plant tissues. Tree Physiol. 2004, 24, 1129–1136. [Google Scholar] [CrossRef] [PubMed]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Bates, L.S.; Waldren, R.P.; Teare, I.D. Rapid determination of free proline for water-stress studies. Plant Soil 1973, 39, 205–207. [Google Scholar] [CrossRef]
- Dhindsa, R.S.; Plumb-Dhindsa, P.; Thorpe, T.A. Leaf Senescence: Correlated with Increased Levels of Membrane Permeability and Lipid Peroxidation, and Decreased Levels of Superoxide Dismutase and Catalase. J. Exp. Bot. 1981, 32, 93–101. [Google Scholar] [CrossRef]
- R Core Team. R: A language and Environment for Statistical Computing. R Foundation for Statistical Computing. 2024. Available online: https://www.R-project.org/ (accessed on 24 December 2024).
- Cui, P.; Li, Y.; Cui, C.; Huo, Y.; Lu, G.; Yang, H. Proteomic and metabolic profile analysis of low-temperature storage responses in Ipomoea batata Lam. tuberous roots. BMC Plant Biol. 2020, 20, 435. [Google Scholar] [CrossRef]
- Meng, D.; Li, S.; Feng, X.; Di, Q.; Zhou, M.; Yu, X.; He, C.; Yan, Y.; Wang, J.; Sun, M.; et al. CsBPC2 is essential for cucumber survival under cold stress. BMC Plant Biol. 2023, 23, 566. [Google Scholar] [CrossRef] [PubMed]
- Sutinen, M.L.; Palta, J.P.; Reich, P.B. Seasonal differences in freezing stress resistance of needles of Pinus nigra and Pinus resinosa: Evaluation of the electrolyte leakage method. Tree Physiol. 1992, 11, 241–254. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Dong, Z.; Li, X.; Tan, M.; Yang, R.; Yang, Z.; Tang, X. Analysis of cold resistance of wine grape based on recovery growth method. J. Fruit Resour. 2021, 2, 5–8. [Google Scholar]
- Chinnusamy, V.; Zhu, J.; Zhu, J.-K. Cold stress regulation of gene expression in plants. Trends Plant Sci. 2007, 12, 444–451. [Google Scholar] [CrossRef] [PubMed]
- Ding, Y.; Shi, Y.; Yang, S. Advances and challenges in uncovering cold tolerance regulatory mechanisms in plants. New Phytol. 2019, 222, 1690–1704. [Google Scholar] [CrossRef]
- Eom, S.H.; Ahn, M.-A.; Kim, E.; Lee, H.J.; Lee, J.H.; Wi, S.H.; Kim, S.K.; Lim, H.B.; Hyun, T.K. Plant Response to Cold Stress: Cold Stress Changes Antioxidant Metabolism in Heading Type Kimchi Cabbage (Brassica rapa L. ssp. Pekinensis). Antioxidants 2022, 11, 700. [Google Scholar] [CrossRef] [PubMed]
- Maruta, T.; Ishikawa, T. Ascorbate Peroxidase Functions in Higher Plants: The Control of the Balance Between Oxidative Damage and Signaling. In Antioxidants and Antioxidant Enzymes in Higher Plants; Gupta, D.K., Palma, J.M., Corpas, F.J., Eds.; Springer International Publishing: Cham, Switzerland, 2018; pp. 41–59. [Google Scholar]
- Charrier, G.; Poirier, M.; Bonhomme, M.; Lacointe, A.; Améglio, T. Frost hardiness in walnut trees (Juglans regia L.): How to link physiology and modelling? Tree Physiol. 2013, 33, 1229–1241. [Google Scholar] [CrossRef]
- Xiao, B.; Wang, M.; Liu, L. Effect of Naturally Low Temperature Stress on Cold Resistance of Fennel Varieties Resource. In Proceedings of the Advances in Computer Science, Environment, Ecoinformatics, and Education, Wuhan, China, 21–22 August 2011; Springer: Berlin/Heidelberg, Germany, 2011; pp. 370–374. [Google Scholar]
- Szügyi-Bartha, K.; Hajnal, V.; Szalay, L.; Bujdosó, G. Preliminary results of frost hardiness of Hungarian bred Persian walnut cultivars. Acta Hortic. 2016, 1139, 173–176. [Google Scholar] [CrossRef]
- Fallah, M.; Vahdati, K.; Hasani, D.; Rasouli, M.; Sarikhani, S. Breeding of Persian walnut: Aiming to introduce late-leafing and early-harvesting varieties by targeted hybridization. Sci. Hortic. 2022, 295, 110885. [Google Scholar] [CrossRef]
- Hajinia, Z.; Sarikhani, S.; Vahdati, K. Exploring low-chill genotypes of Persian walnut (Juglans regia L.) in west of Iran. Genet. Resour. Crop Evol. 2021, 68, 2325–2336. [Google Scholar] [CrossRef]
- Wambulwa, M.C.; Fan, P.-Z.; Milne, R.; Wu, Z.-Y.; Luo, Y.-H.; Wang, Y.-H.; Wang, H.; Gao, L.-M.; Xiahou, Z.-Y.; Jin, Y.-C.; et al. Genetic analysis of walnut cultivars from southwest China: Implications for germplasm improvement. Plant Divers. 2022, 44, 530–541. [Google Scholar] [CrossRef] [PubMed]
- Ji, F.; Ma, Q.; Zhang, W.; Liu, J.; Feng, Y.; Zhao, P.; Song, X.; Chen, J.; Zhang, J.; Wei, X.; et al. A genome variation map provides insights into the genetics of walnut adaptation and agronomic traits. Genome Biol. 2021, 22, 300. [Google Scholar] [CrossRef]
- Fallah, M.; Rasouli, M.; Hassani, D.; Lawson, S.S.; Sarikhani, S.; Vahdati, K. Tracing Superior Late-Leafing Genotypes of Persian Walnut for Managing Late-Spring Frost in Walnut Orchards. Horticulturae 2022, 8, 1003. [Google Scholar] [CrossRef]
- Shah, R.A.; Bakshi, P.; Jasrotia, A.; Wali, V.K.; Sharma, S.; Gupta, M.; Gupta, R.K.; Jamwal, M. Comparative morpho-molecular characterization of elite walnut variety Parbat (JWSP-06) with local selections of north-western Himalayan region of Jammu and Kashmir, India. Sci. Hortic. 2023, 319, 112176. [Google Scholar] [CrossRef]
- Dunne, J.C.; Tuong, T.D.; Livingston, D.P.; Reynolds, W.C.; Milla-Lewis, S.R. Field and Laboratory Evaluation of Bermudagrass Germplasm for Cold Hardiness and Freezing Tolerance. Crop Sci. 2019, 59, 392–399. [Google Scholar] [CrossRef]
- Svec, L.V.; Hodges, H.F. Cold-hardening and morphology of Barley seedlings in controlled and natural environments. Can. J. Plant Sci. 1972, 52, 955–963. [Google Scholar] [CrossRef]
- Loik, M.E.; Redar, S.P. Microclimate, freezing tolerance, and cold acclimation along an elevation gradient for seedlings of the Great Basin Desert shrub, Artemisia tridentata. J. Arid. Environ. 2003, 54, 769–782. [Google Scholar] [CrossRef]
- Eisenschmid, K.; Jabbusch, S.; Koch, M.A. Evolutionary footprints of cold adaptation in arctic-alpine Cochlearia (Brassicaceae)—Evidence from freezing experiments and electrolyte leakage. Perspect. Plant Ecol. Evol. Syst. 2023, 59, 125728. [Google Scholar] [CrossRef]
- Thomas, F.M.; Blank, R.; Hartmann, G. Abiotic and biotic factors and their interactions as causes of oak decline in Central Europe. For. Pathol. 2002, 32, 277–307. [Google Scholar] [CrossRef]
- Permann, C.; Stegner, M.; Roach, T.; Loacker, V.; Lewis, L.A.; Neuner, G.; Holzinger, A. Striking differences in frost hardiness and inability to cold acclimate in two Mougeotia species (Zygnematophyceae) from alpine and lowland habitats. Physiol. Plant. 2024, 176, e14167. [Google Scholar] [CrossRef]
- Girardin, M.P.; Guo, X.J.; Marchand, W.; Depardieu, C. Unravelling the biogeographic determinants of tree growth sensitivity to freeze and drought in Canada’s forests. J. Ecol. 2024, 112, 848–869. [Google Scholar] [CrossRef]
- Knight, M.R.; Knight, H. Low-temperature perception leading to gene expression and cold tolerance in higher plants. New Phytol. 2012, 195, 737–751. [Google Scholar] [CrossRef] [PubMed]




| Variety | Electrical Conductivity (μS·cm−1·g−1·mL−1) | ||||
|---|---|---|---|---|---|
| −10 °C | −15 °C | −20 °C | −25 °C | −30 °C | |
| Xinxin 2 | 221 ± 2.64 a | 233.33 ± 11.59 a | 234.67 ± 4.16 c | 253.67 ± 10.69 c | 324.00 ± 12.49 b |
| Wen 81 | 141.33 ± 5.03 d | 168.67 ± 3.21 c | 187.00 ± 10.53 d | 226.00 ± 11.53 d | 263.00 ± 9.16 c |
| Wen 185 | 176.33 ± 8.96 bc | 178.67 ± 4.16 bc | 263.00 ± 9.16 b | 343.67 ± 3.51 a | 443.67 ± 11.02 a |
| Zha 343 | 166.00 ± 6.24 c | 215.67 ± 20.79 a | 281.67 ± 7.02 a | 285.33 ± 11.23 b | 352.33 ± 9.02 b |
| Xinzaofeng | 187.00 ± 10.53 b | 193.00 ± 3.00 b | 260.33 ± 2.51 b | 287.00 ± 9.85 b | 341.33 ± 17.95 b |
| Cultivars | Logistics Equation | LT50/°C | R2 | Sequence of Cold Resistance |
|---|---|---|---|---|
| Xinxin 2 | Y = 100/(1 + 1.18 × 100.0253x) | −6.51 | 0.77 | 5 |
| Wen 81 | Y = 100/(1 + 2.72 × 100.0460x) | −21.73 | 0.97 | 1 |
| Wen 185 | Y = 100/(1 + 2.70 × 100.0559x) | −17.76 | 0.92 | 2 |
| Zha 343 | Y = 100/(1 + 2.65 × 100.0608x) | −16.00 | 0.87 | 3 |
| Xinzaofeng | Y = 100/(1 + 2.30 × 100.0580x) | −14.38 | 0.86 | 4 |
| Cultivars | GR (%) | ||||
|---|---|---|---|---|---|
| −10 °C | −15 °C | −20 °C | −25 °C | −30 °C | |
| Xinxin 2 | 82.14 ± 3.05 aA | 86.60 ± 4.38 abA | 47.51 ± 3.69 aB | 29.65 ± 3.70 aB | 0.56 ± 2.31 aC |
| Wen 81 | 90.19 ± 4.69 aA | 85.37 ± 0.89 aAB | 66.76 ± 2.40 aAB | 36.53 ± 5.16 aCB | 1.50 ± 4.16 aC |
| Wen 185 | 88.26 ± 5.33 aA | 88.59 ± 3.81 abA | 68.91 ± 2.27 aAB | 36.21 ± 4.45 aBC | 0.31 ± 1.88 aCD |
| Zha 343 | 77.27 ± 1.16 aA | 68.36 ± 3.22 abA | 41.70 ± 2.90 bB | 3.31 ± 1.60 bC | 0.27 ± 1.98 aC |
| Xinzaofeng | 73.41 ± 3.40 aA | 65.95 ± 1.86 bAB | 47.02 ± 1.45 bBC | 7.20 ± 1.00 bCD | 3.43 ± 4.85 aD |
| Cultivars | Xinxin 2 | Wen 81 | Wen 185 | Zha 343 | Xinzaofeng |
|---|---|---|---|---|---|
| EC | 0.235 | 0.000 | 1.000 | 0.504 | 0.518 |
| REC | 0.814 | 0.000 | 0.842 | 0.683 | 1.000 |
| BWC | 1.000 | 0.000 | 0.813 | 0.442 | 0.300 |
| GR | 0.687 | 0.917 | 1.000 | 0.000 | 0.000 |
| SP | 1.000 | 0.019 | 0.967 | 1.000 | 0.000 |
| SS | 0.000 | 0.265 | 0.573 | 1.000 | 0.905 |
| CAT | 0.233 | 0.122 | 0.000 | 1.000 | 0.637 |
| POD | 0.000 | 1.000 | 0.086 | 0.249 | 0.528 |
| SOD | 0.348 | 1.000 | 0.362 | 0.000 | 0.706 |
| MDA | 0.375 | 1.000 | 0.061 | 0.718 | 0.000 |
| Proline | 1.000 | 0.076 | 0.803 | 0.712 | 0.000 |
| Mean membership function value | 0.518 | 0.400 | 0.592 | 0.573 | 0.418 |
| Cold resistance level | MR | LR | HR | HR | LR |
| Trait Cultivars | Physiological Metrics | Biochemical Metrics | Comprehensive Indicator | ||||
|---|---|---|---|---|---|---|---|
| REC (%) | LT50 (°C) | GR (%) | SOD (mg/g) | POD (μg·min) | SS (mg/g) | Average Membership Function Values | |
| Xinxin 2 | 65.80 | −6.51 | 29.65 | 510.90 | 37.97 | 51.41 | 0.518 |
| Wen 81 | 60.36 | −21.73 | 36.53 | 533.33 | 206.22 | 63.10 | 0.40 |
| Wen 185 | 65.74 | −17.76 | 36.21 | 508.41 | 130.67 | 81.72 | 0.592 |
| Zha 343 | 72.47 | −16.00 | 3.31 | 442.51 | 99.22 | 98.99 | 0.573 |
| Xinzaofeng | 71.23 | −14.38 | 7.20 | 482.11 | 70.89 | 94.98 | 0.418 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ni, Z.; Hu, H.; Xu, G. Cold Hardiness and Physio-Biochemical Responses of Annual Branches in Five Early-Fruiting Walnut Varieties (Juglans regia L.) Under Simulated Low-Temperature Stress. Horticulturae 2025, 11, 72. https://doi.org/10.3390/horticulturae11010072
Ni Z, Hu H, Xu G. Cold Hardiness and Physio-Biochemical Responses of Annual Branches in Five Early-Fruiting Walnut Varieties (Juglans regia L.) Under Simulated Low-Temperature Stress. Horticulturae. 2025; 11(1):72. https://doi.org/10.3390/horticulturae11010072
Chicago/Turabian StyleNi, Zitong, Haifang Hu, and Guiqing Xu. 2025. "Cold Hardiness and Physio-Biochemical Responses of Annual Branches in Five Early-Fruiting Walnut Varieties (Juglans regia L.) Under Simulated Low-Temperature Stress" Horticulturae 11, no. 1: 72. https://doi.org/10.3390/horticulturae11010072
APA StyleNi, Z., Hu, H., & Xu, G. (2025). Cold Hardiness and Physio-Biochemical Responses of Annual Branches in Five Early-Fruiting Walnut Varieties (Juglans regia L.) Under Simulated Low-Temperature Stress. Horticulturae, 11(1), 72. https://doi.org/10.3390/horticulturae11010072






