Abstract
During retail storage, potato tubers are exposed to light that results in tuber greening. Green tubers are toxic and rejected by consumers. In the present study, the effect of Citrashine® natural wax on the postharvest tuber greening of two potato cultivars (‘Mondial’ and ‘Sifra’) was studied. The tubers were irradiated with white light during a 12-day storage period at ambient temperature. During light exposure, tubers were evaluated for colour, pigmentation, chlorophyll fluorescence and starch granule distribution at 3-day intervals. The results showed that wax-treated tubers had significantly (p < 0.05) less green colour as represented by visual and objective colour parameters (a*, b*, C* and h°), compared to those treated with water (control). The pigmentation of the tubers was significantly influenced by the postharvest Citrashine® natural wax treatment. The total chlorophyll content was significantly lower in wax-treated tubers, while the carotenoid content was significantly higher in wax-treated tubers compared to their contents in control samples. Scanning electron microscopy showed that the starch granule size was normally distributed in wax-treated tubers compared to the untreated ones, which was negatively skewed. In conclusion, Citrashine® natural wax showed the potential to be a postharvest technology for controlling greening defects on potato tubers. The results provide a possible effective strategy for controlling the postharvest greening of potato tubers.
1. Introduction
Potato tubers (Solanum tuberosum) are widely consumed and economically important crops worldwide, as they are a staple food and a source of macro- and micronutrients such as carbohydrates and vitamins [1]. Belonging to the Solanaceae family, potatoes rank as the fourth most produced food crop and as the third most important food crop in the world after rice and wheat in terms of human consumption. The potato production system encounters difficulties in reaching the final consumer, as the quality of potato tubers is compromised throughout the postharvest value chain. Potato tubers are often illuminated with light in retail markets, for greater consumer awareness and purchase initiation [2]. However, light exposure often results in tuber greening. The postharvest greening of potato tubers is due to the accumulation of chlorophyll in the tuber periderm that is influenced by the conversion of amyloplasts into chloroplasts owing to light exposure [3]. During light exposure, due to stress that accelerates starch hydrolysis, potato starch granules are degraded into simpler sugars, leading to the generation of smaller starch granules [4]. Tuber greening is a common defect that affects the quality and market value of potatoes, as skin quality is the most important criterion for consumer purchase [3]. Furthermore, tuber greening is accompanied by the accumulation of toxic glycoalkaloids, which are harmful for human health [5]. Due to their toxic effects, such potatoes are often discarded, leading to great economic losses. Therefore, preventing the greening of potato tubers is of great significance. Various methods such as coatings, packaging materials and the manipulation of light regimes have been implemented to reduce the effect of light on tuber greening [6,7,8]. Coatings have been widely used to keep the quality of potato tubers during the postharvest. However, the use of coatings such as paraffin wax, copper gluconate and packaging materials, although reported to effectively reduce the greening of potato tubers [3], imposes a negative impact on human health and the environment [5]. Moreover, the manipulation of light regimes can be cost-ineffective for small retail stores to maintain [9]. Therefore, it is important to develop methods that are eco-friendly and cost-effective.
Edible coatings are eco-friendly and cost-effective methods that have the potential to be used for managing greening on potato tubers. These are thin layers of edible materials applied onto the surface of produce [10,11]. They are able to enhance appearance and provide a protective barrier that shields produce from external harm or keeps the quality of the produce by different means [12]. There is a diverse range of chemical-based or organic-based coatings that are used for keeping the postharvest quality of produce. Citrashine® is a commercially available wax, a composite made from the emulsion of carnauba and shellac waxes. Carnauba is a natural wax derived from the leaves of the carnauba palm tree [13]. Shellac wax is a natural resin produced by the female lac bug, which secretes it on trees [14]. These waxes are generally regarded as safe by the food and drug administration [15]. The two waxes are reported to prolong the shelf life of various fruits such as citrus, apples and pears [16,17]. However, to the best of our knowledge, they have never been used on potato tubers, especially to control defects such as greening. We hypothesize that since greening is the result of the skin and under-skin tissues receiving light, this wax can put a barrier on the skin of the potato and prevent the potato skin and tissue from receiving light. Therefore, the objective of this study was to determine the effect of Citrashine® natural wax on the postharvest greening of potato tubers.
2. Materials and Methods
2.1. Plant Material and Experimental Site
Fresh potato tubers (cultivars ‘Mondial’ and ‘Sifra’) of uniform size were bought from a commercial farm. The experimental units had the following quality variables: ‘Mondial’ tubers had 0% greening and 3.24% starch content. ‘Sifra’ tubers had 0% greening and 3.50% starch content. They were transported to the Plant Production Laboratory at University of Limpopo, South Africa (23°53′09.9″ S, 29°44′17.7″ E), for postharvest assessment.
2.2. Experimental Treatment
Edible wax (Citrashine® natural wax) at two levels (wax and no wax) and the duration of light exposure at ambient temperature (0, 3, 6, 9 and 12 days) were considered as independent factors. Two potato cultivars, ‘Mondial’ and ‘Sifra’, were used, as they are mostly susceptible to greening defects [18]. The tubers were thoroughly cleaned by rinsing them in water to remove any soil and debris. After rinsing the tubers, they were allowed to air-dry at room temperature, before treatment application. Twenty-two tubers per replicate were placed in a sack bag and dipped in a Citrashine® natural wax for 5 s, followed by air-drying. Control tubers were dipped in distilled water for 5 s then air-dried. After drying, tubers were exposed to Phillips fluorescent light of 58 watts with a relatively low colour rendering index of 33 that emits a cool white light with a colour temperature of 640 K with an intensity of 1800 ± 100 lux (lx 1108 lux meter, Lutron, Taipei, Taiwan) at a distance of 2.84 m. Tubers were exposed to continuous lighting for 12 days at ambient temperature and 85 ± 5% RH to simulate retail conditions and induce greening [5]. During 12-day light exposure, tubers were evaluated for colour, pigmentation, chlorophyll fluorescence and starch granule distribution at 3-day intervals. Five tubers were peeled and stored at −80 °C for destructive assays such as pigment determination. Later, the samples were freeze-dried for 48 h to obtain dry samples.
2.3. Colour Assessments
2.3.1. Subjective Colour Assessment
Tubers were graded on a greening scale proposed by Grunenfelder et al. [19] that ranges from 0 to 5 (0–1 none; 2—weak; 3—average; 4—severe; 5—very intensive) for subjective colour assessment. Tubers that ranged from the third to fifth value of the scale were considered to be green. Thereafter, the tuber greening percentage was calculated using the following equation:
2.3.2. Tuber Skin Colour Assessment
Peel colour was determined using a Chroma meter (Minolta CR-400, Ramsey, HJ, USA) with an 8 mm diameter light path aperture. Measurements were taken at three points on the light-exposed surface of each potato tuber [20]. The chroma meter was calibrated on a Minolta standard white tile each time before use on the white plate, and peel colour measurements were taken using L*, a* and b* values. The L* values were directly used to express lightness. Chroma and hue angle (h°) were calculated according to Equations (2) and (3), respectively.
2.4. Determination of Peel Pigmentation
Total chlorophyll and carotenoids were extracted according to Tilahun et al. [20]. Briefly, a 0.2 g powder of the potato peel was extracted with 10 mL of 80% acetone, kept on ice for one hour, and then centrifuged at 5000× g for 20 min. The analytical determination was performed with a UV–visible spectrophotometer (Jenway, Stones, Staffordshire UK) at the following wavelengths: 645 and 662 nm, for chlorophylls a and b, respectively. Thereafter, chlorophylls a, b and total chlorophyll were then quantified using Equations (4)–(6), respectively.
Chl a = 11.24 A662 − 2.04 A645
Chl b = 20.13 A645 − 4.19 A662
Total chlorophyll = chl a + chl b
2.5. Confocal Laser Scanning Microscope
The presence of chlorophyll in the tuber peel was observed using a confocal laser scanning microscope (CLSM) (Zeiss LSM 880, Carl Zeiss Microscopy GmbH, Jena, Germany) according to Zhang et al. [21]. A 1.0–1.5 mm thick sample of skin tissue was peeled from the tuber. The slices were washed with distilled water and placed on a glass slide, followed by the addition of one to two droplets of water. Then, the slide was covered with a coverslip and immediately observed under the confocal laser scanning microscope. The micrographs were taken under UV light at 40× and 200× magnification.
2.6. Scanning Electron Microscope
Peel surface granules were determined using a scanning electron microscope (FE-SEM Zeiss Ultra 540, Oberkochen, Germany). Potato peel samples were dehydrated with a series of ethanol (30%, 50% and 80%) for 2 h. Later, each sample was left in 100% ethanol for 30 min. The samples were then rid of ethanol, and a 50:50 mixture of hexamethylidulazane (HMDS) and 100% ethanol was added to the samples for 1 h. HMDS was then added to dry the samples. Dried samples were mounted onto aluminium stubs and coated with carbon. A micrograph of each sample was taken at 300× magnification, using an acceleration potential of 2 Kv.
2.7. Statistical Analysis
This experiment was conducted as a 2 × 5 factorial arranged in a completely randomised design with five replicates. Each replicate consisted of 22 tubers. An analysis of variance was performed using GenStat, version 21st, VSN International, Hemel Hempstead, Hertfordshire, UK. The means were separated through the least significant difference (LSD) at 5%. The granule size was measured using Image J software version 1.8.0. Graphs were produced using Excel 2016 (Microsoft) and Origin pro 8.1.
3. Results and Discussion
3.1. Peel Colour Changed as a Result of Coating during Postharvest Light Exposure in Both Cultivars
There was a significant interaction effect between wax treatment and storage duration (p < 0.05) on green colour development in potato peel (Figure 1). The application of wax delayed green colour development compared with the control samples (untreated). The green colour percentage, colour indices (greenness (a), lightness and hue) and Chroma decreased in wax-treated tubers. These effects were observed to be the same for both cultivars but with different magnitudes (Figure 2 and Figure 3). Wax-treated tubers showed 40% greening in ‘Mondial’ tubers compared with control tubers, which had 100% greening after 12-day storage (Figure 4). In ‘Sifra’ tubers, greening was 36% for wax-treated compared with control tubers (100%) (Figure 5). The colour index a*, where a negative value represents the green colour, decreased from 1.54 to 0.23 in wax-treated ‘Mondial’ tubers compared with control samples measured from 1.54 to −1.49 during 12-day storage. The decreasing trend of the a* value was similarly observed on wax-treated ‘Sifra’ tubers. There was a decrease in the yellowish tuber colour, as represented by hunter b*(+), as the day of light exposure increases with the control having a lower value as compared to the wax-treated tubers for both the ‘Mondial’ and ‘Sifra’ cultivars. There was an increase in Chroma (C*), which represents the intensity or saturation of a colour, in the wax-treated tubers as compared with the control which had a decreasing trend. In terms of hue angle, which represents greenness at 180° [22], wax-treated ‘Mondial’ tubers showed a hue angle of 89.41° compared to untreated samples with a hue angle of 95.06°, which is closer to 180° (Figure 4) during 12-day storage. The same trend was also observed on ‘Sifra’ tubers (Figure 5). These results suggest that the application of Citrashine® natural wax on potato tubers reduces green colour development on tuber peels and maintains the yellowish colour compared with untreated samples following exposure to light. Furthermore, ‘Sifra’ tubers showed less development of greening and colour change as a result of light exposure during postharvest assessment compared to the ‘Mondial’ cultivar. Coatings, including waxes, add a protective layer on produce skin [23]. As we hypothesized, coatings that prevent the penetration of light into produce can prevent greening due to their role as light shields. Therefore, Citrashine® wax might have reduced light transmission on/to the peel, which influences green colour development [3,5]. A study by [24] found that potato tubers packaged in material that reduces light transmission (black perforated and low-density polyethylene non-perforated bags) had less green colour development. Thus, by reducing light transmission on the tuber skin using Citrashine®, green colouration is reduced.
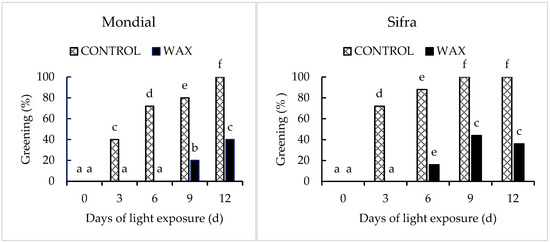
Figure 1.
Effect of Citrashine® natural wax on postharvest greening of ‘Mondial’ and ‘Sifra’ potato cultivars during 12 days of light exposure. Data were averages of five replicates ± standard error. Different letters represent statistical significance (p < 0.05).
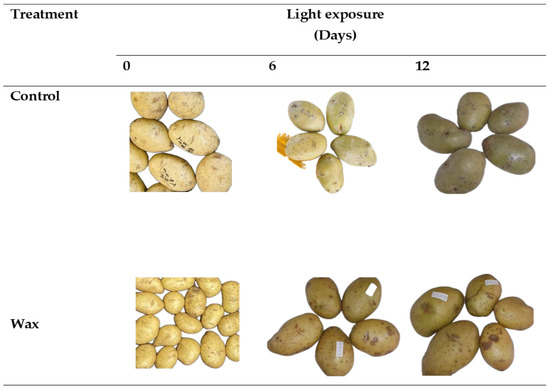
Figure 2.
Peel colour of ‘Mondial’ tubers treated with Citrashine® natural wax or untreated during 12-day light exposure.
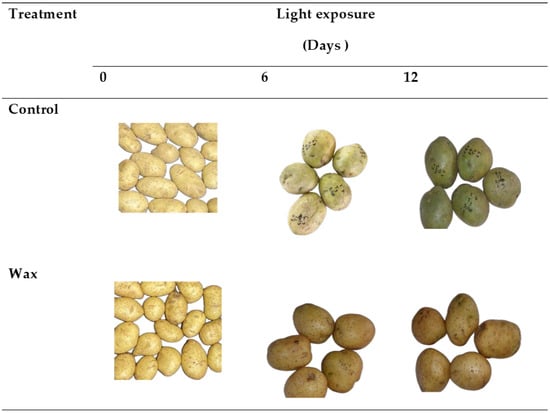
Figure 3.
Peel colour of ‘Sifra’ tubers treated with Citrashine® natural wax or untreated during 12-day light exposure.
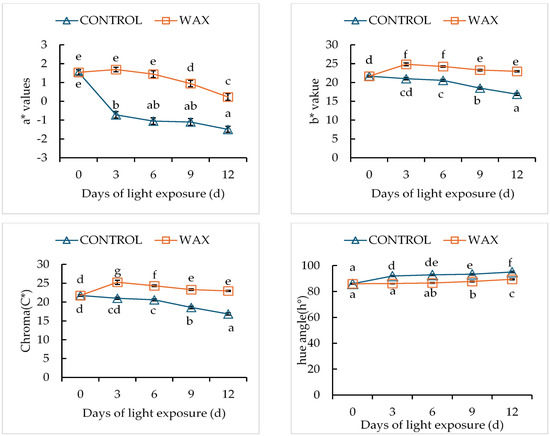
Figure 4.
Effect of Citrashine® natural wax on objective colour parameters of ‘Mondial’ potato cultivars during 12 days of light exposure. Data were averages of five replicates ± standard error. Different letters represent statistical significance (p < 0.05).
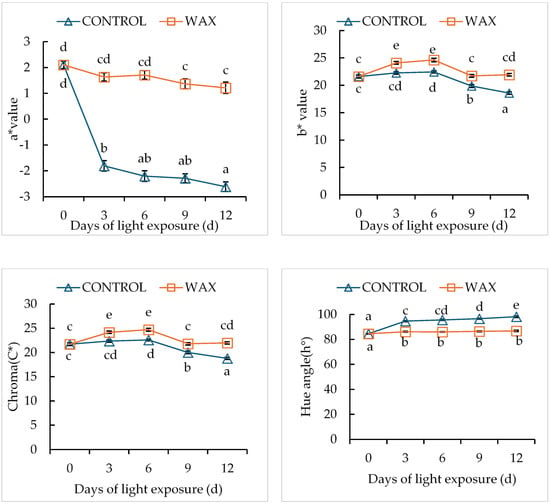
Figure 5.
Effect of Citrashine® natural wax on objective colour parameters of ‘Sifra’ potato cultivars during 12 days of light exposure. Data were averages of five replicates ± standard error. Different letters represent statistical significances (p < 0.05).
3.2. Pigmentation in Potato Tuber in Response to Coating during Postharvest Light Exposure
The green colour on potato skin is due to the formation of chlorophylls that is induced by exposure to light [3,5]. In the present study, wax treatment significantly (p < 0.05) reduced the chlorophyll content in the tuber skin when compared to the control samples during the 12-day light exposure. In both cultivars, the wax-treated tubers had a considerably lower chlorophyll (Figure 6) content than the control sample. However, the chlorophyll content in ‘Sifra’ tubers was higher than its content in ‘Mondial’ cultivars both with and without wax treatments. The results suggest that potato tubers treated with Citrashine® natural wax reduced the development of chlorophyll during light exposure. Chlorophyll develops when plant tissues are exposed to light. It also accumulates on potato peels when light penetrates into their tissues [25]. Therefore, the wax might have reduced light penetration due to the semi-permeable layer it forms on produce once applied [26,27]. Moreover, the transformation of amyloplasts into chloroplasts, a necessary physiological process for chlorophyll accumulation [3], might decrease in wax-treated tubers due to the reduced light penetration there. It has been shown that coatings such as paraffin wax and detergent solutions inhibit chlorophyll accumulation in potato tubers by impacting light penetration into tubers [28,29].
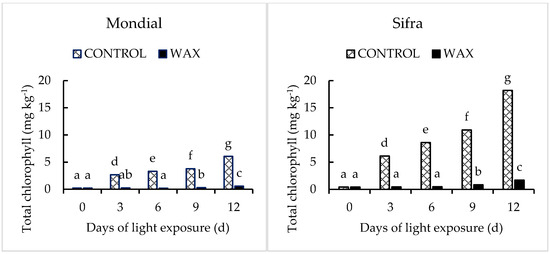
Figure 6.
Effect of Citrashine® natural wax on total chlorophyll content of ‘Mondial’ and ‘Sifra’ potato tubers peel during 12-day light exposure in ambient storage. Data were averages of five replicates ± standard error. Different letters represent statistical significance (p < 0.05).
3.3. Correlation of Evaluated Greening Parameter during Light Exposure
The correlation results between the greening percentage and the colour values (a*, b*, C* and L*) showed a significant negative relationship; however, the correlation between greening and h° and total chlorophyll showed a positive relationship in both the ‘Mondial’ and ‘Sifra’ potato cultivars (Table 1 and Table 2). In the ‘Mondial’ cultivar, greening shows a strong negative correlation with a* (r = −0.966; p < 0.01), b* (r = −0.852; p < 0.01), C* (r = −0.851; p < 0.01) and L* (r = −0.786; p < 0.01). This indicates that as the extent of greening increases, the redness (a*), yellowness (b*), chroma (C*) and lightness (L*) decrease [20]. Furthermore, greening is positively correlated with h° (r = 0.977; p < 0.01) and total chlorophyll (r = 0.942; p < 0.01), suggesting that as greening intensifies, the hue angle (h°) and total chlorophyll content increase. Meanwhile, in the ‘Sifra’ cultivar, there is a significant negative correlation with a* (r = −0.961; p < 0.01) and L* (r = −0.920; p < 0.01), meaning that as greening increases, redness and lightness decrease. It is also strongly positively correlated with h° (r = 0.959; p < 0.01) and total chlorophyll (r = 0.884; p < 0.01), which suggests a similar trend to that seen in ‘Mondial’: increased greening corresponds with a higher hue angle and greater chlorophyll content. The results are in agreement with Tilahun et al. [20], who showed a strong negative relationship between the a* and total chlorophyll content of the ‘Atlantic’ and ‘Trent’ potato cultivars. Furthermore, these findings are consistent with previous studies that have demonstrated the discolouration of potato tubers when exposed to light, which triggers chlorophyll biosynthesis [20,25]. As the chlorophyll content increases, the tubers exhibit a shift in colour from yellow hues to greener shades, which is reflected in the decrease in the a* and b* values and the increase in h°. The reduction in lightness (L*) further supports the notion that increased chlorophyll content results in darker tubers [19,20].

Table 1.
Pearson’s correlation between greening percentage, colour indices (lightness (L*), greenness (a*), yellowness (b*) and chromaticity (C*), hue angle (h°) and total chlorophyll content of ‘Mondial’ potato cultivar during 12 days of light exposure.

Table 2.
Pearson’s correlation between greening percentage, colour indices (lightness (L*), greenness (a*), yellowness (b*) and chromaticity (C*), hue angle (h°) and total chlorophyll content of ‘Sifra’ potato cultivar during 12 days of light exposure.
3.4. Chlorophyll Fluorescence in Tuber Peel Reduced by Coating during Postharvest Exposure in Both Potato Cultivars
Owing to light exposure, the conversion of amyloplasts to chloroplasts can be triggered, leading to the accumulation of chlorophyll, thus making tubers green [2,21]. Chlorophyll is excited by the energy received from the light environment. It emits fluorescent light promptly following the receiving of photons, which is widely used to study the fate of energy withinchlorophyll molecules [30,31]. In this study, the microscopic skin appearance of the control and wax-treated tubers were investigated (Figure 7). Chlorophyll auto-fluorescence was clearly observed around the cell wall in control tubers, whereas for the wax-treated tubers, no chlorophyll fluorescence was observed (Figure 7). This suggests that wax treatment inhibits chlorophyll synthesis and development in the tuber skin during 12-day light exposure. Coatings on the produce surface create a modified atmosphere in the tuber. A controlled atmosphere, with low oxygen and high carbon dioxide, reduces the various physiological and biochemical processes such as respiration and amyloplast degradation [32]. According to the results of chlorophyll content and fluorescence imaging, the effective reduction in tuber chlorophyll auto-fluorescence by Citrashine® natural wax might be attributed to reduction in amyloplastic degradation into chloroplasts, which is a physiological process involved in the appearance of chlorophyll during the greening of potato tubers or due to the inhibition of light penetration into the tissue. Thus, no chlorophyll auto-fluorescence was observed in the wax-treated tubers.
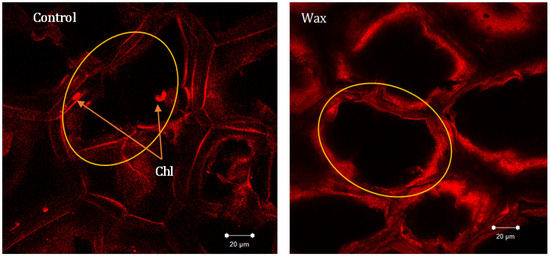
Figure 7.
Confocal laser scanning micrograph of potato tuber skin of ‘Sifra’ cultivar of wax-treated and untreated (control) tubers on day three of light exposure. Tubers were illuminated with Phillips fluorescent light in ambient storage. Chl indicates presence of chloroplasts.
3.5. Coating Maintained Starch Granule Size in Potato Tubers during Postharvest Light Exposure
During greening, starch is degraded to form chlorophyll [21]. In this study, the microscopic analysis showed that the control had smaller granules compared to the wax-treated tubers on the tuber skin surface (Figure 8A,B), and the distribution of the granules was determined. It was found that the highest granule size was observed in the wax-treated tubers with an average size of 23.35 µm (Figure 8D), whereas in the control, the average granule size was 17.94 µm (Figure 8C). Therefore, the granule size was more balanced in the wax-treated tubers than in the control tubers. Therefore, the application of Citrashine® natural wax reduces starch granule degradation during light exposure. The breakdown of amyloplasts involves the enzymatic degradation of starch molecules stored within them into fragments; thus, this supports the appearance of granules on the tuber surface [21]. Therefore, the normally distributed starch granule size on the wax-treated tubers is speculated to be due to slow amyloplast degradation, which results in fewer small starch granules on the peel surface [33]. It has been found that the combination of ethanol fumigation and modified atmosphere packaging prevents tuber greening by reducing the number of small starch granules [33]. Accordingly, the present study showed that reducing the number of small granules by using Citrashine® natural wax is an indication of slow amyloplast degradation and thus less greening on wax-treated tubers.
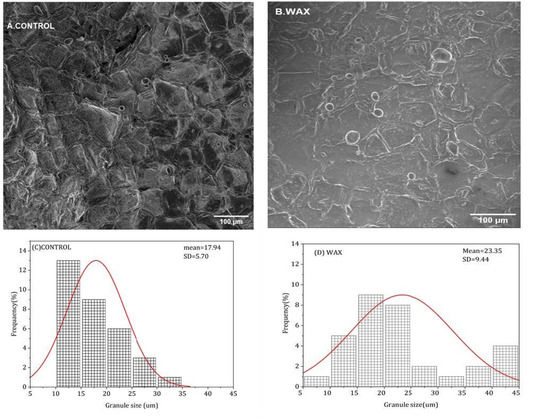
Figure 8.
Starch granule size distribution on surface of wax-treated and untreated ‘Sifra’ potato tubers following 12-day light exposure at ambient temperature.
4. Conclusions
This study investigated the effect of a plant-based wax, Citrashine® natural wax, on greening development on potato tubers when exposed to light. It was found that the application of Citrashine® natural wax minimized the green colour’s development on the tubers’ skin when they were exposed to light by improving attributes such as chromaticity (b*, C* and h° and visual colour), carotenoids and reducing chlorophyll accumulation in the tuber skin during light exposure. The application of Citrashine® natural wax resulted in a significant impact on reducing the greening development in potato tubers. This finding suggests that the use of Citrashine® natural wax can be an effective strategy for controlling the greening of potato tubers. Moreover, further research is needed to explore the long-term effects of Citrashine® natural wax application and its potential role in mitigating tuber greening and improving overall potato crop production and storage.
Author Contributions
Conceptualization, T.K.S. and T.P.M.; methodology, J.M.M. and T.K.S.; validation, J.M.M.; formal analysis, J.M.M.; investigation, J.M.M.; resources, T.P.M.; data curation, J.M.M.; writing—original draft preparation, J.M.M., writing—review and editing, J.M.M., T.K.S., T.P.M. and S.A.; visualization, J.M.M.; supervision, T.K.S. and T.P.M.; project administration, T.K.S.; funding acquisition, T.P.M. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors on request.
Acknowledgments
The authors acknowledge the T.K.S. Research Team, National Research Foundation and AgriSeta for funding J.M. in her postgraduate studies.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Zaheer, K.; Akhtar, M.H. Potato production, usage, and nutrition—A review. Crit. Rev. Food Sci. Nutr. 2016, 56, 711–721. [Google Scholar] [CrossRef]
- Olsen, N.L.; Brandt, T.; Price, W.J. The impact of retail light source on greening of russet Burbank potato tubers. Am. J. Potato Res. 2018, 95, 123–129. [Google Scholar] [CrossRef]
- Tanios, S.; Eyles, A.; Tegg, R.; Wilson, C. Potato tuber greening: A review of predisposing factors, management, and future challenges. Am. J. Potato Res. 2018, 95, 248–257. [Google Scholar] [CrossRef]
- Abbasi, K.S.; Masud, T.; Qayyum, A.; Ahmad, A.; Mehmood, A.; Bibi, Y.; Sher, A. Photo-induced changes in quality attributes of potato tubers during storage. J. Appl. Bot. Food Qual. 2016, 89, 315–321. [Google Scholar]
- Dhalsamant, K.; Singh, C.B.; Lankapalli, R. A review on greening and glycoalkaloids in potato tubers: Potential solutions. J. Agric. Food Chem. 2022, 70, 13819–13831. [Google Scholar] [CrossRef] [PubMed]
- Bang, Y.J.; Shankar, S.; Rhim, J.W. Preparation of polypropylene/poly (butylene adipate-co-terephthalate) composite films incorporated with melanin for prevention of greening of potatoes. Packag. Technol. Sci. 2020, 33, 433–441. [Google Scholar] [CrossRef]
- Emragi, E.; Kalita, D.; Jayanty, S.S. Effect of edible coating on physical and chemical properties of potato tubers under different storage conditions. LWT 2022, 153, 112580. [Google Scholar] [CrossRef]
- Lee, J.S.; Ahn, J.; Han, J. Enhancing effect on postharvest quality of potatoes through combined treatment of edible coating with UV-C irradiation. Food Sci. Biotechnol. 2024, 33, 1393–1405. [Google Scholar] [CrossRef] [PubMed]
- Wood, B.; Williams, O.; Nagarajan, V.; Sacks, G. Market strategies used by processed food manufacturers to increase and consolidate their power: A systematic review and document analysis. Glob. Health 2021, 17, 17. [Google Scholar] [CrossRef]
- Adhikary, T.; Singh, S.; Sinha, A.; Gill, P.P.S. Recent advances in packaging and edible coating for shelf-life enhancement in fruit crops. Curr. J. Appl. Sci. Technol. 2020, 39, 116–133. [Google Scholar] [CrossRef]
- Pham, T.T.; Nguyen, L.L.P.; Dam, M.S.; Baranyai, L. Application of edible coating in extension of fruit shelf life. AgriEngineering 2023, 5, 520–536. [Google Scholar] [CrossRef]
- Andriani, V.; Handayani, N.A. Recent technology of edible coating production: A review. Mater. Today Proc. 2023, 87, 200–206. [Google Scholar] [CrossRef]
- de Freitas, C.A.S.; de Sousa, P.H.M.; Soares, D.J.; da Silva, J.Y.G.; Benjamin, S.R.; Guedes, M.I.F. Carnauba wax uses in food—A review. Food Chem. 2019, 291, 38–48. [Google Scholar] [CrossRef]
- Yuan, Y.; He, N.; Xue, Q.; Guo, Q.; Dong, L.; Haruna, M.H.; Zhang, X.; Li, B.; Li, L. Shellac: A promising natural polymer in the food industry. Trends Food Sci. Technol. 2021, 109, 139–153. [Google Scholar] [CrossRef]
- Pashova, S. Application of plant waxes in edible coatings. Coatings 2023, 13, 911. [Google Scholar] [CrossRef]
- Bai, J.; Hagenmaier, R.D.; Baldwin, E.A. Coating selection for ‘Delicious’ and other apples. Postharvest Biol. Technol. 2003, 28, 381–390. [Google Scholar] [CrossRef]
- Miranda, M.; Sun, X.; Ference, C.; Plotto, A.; Bai, J.; Wood, D.; Assis, O.B.G.; Ferreira, M.D.; Baldwin, E. Nano-and micro-carnauba wax emulsions versus shellac protective coatings on postharvest citrus quality. J. Am. Soc. Hortic. Sci. 2021, 146, 40–49. [Google Scholar] [CrossRef]
- Potato, S.A. Limpopo cultivar trial under irrigation at tom burke in 2022. Chips, 28 July 2023; pp. 38–43. [Google Scholar]
- Grunenfelder, L.; Hiller, L.K.; Knowles, N.R. Colour indices for the assessment of chlorophyll development and greening of fresh market potatoes. Postharvest Biol. Technol. 2006, 40, 73–81. [Google Scholar] [CrossRef]
- Tilahun, S.; An, H.S.; Solomon, T.; Baek, M.W.; Choi, H.R.; Lee, H.C.; Jeong, C.S. Indices for the Assessment of Glycoalkaloids in Potato Tubers Based on Surface Colour and Chlorophyll Content. Horticulturae 2020, 6, 107. [Google Scholar] [CrossRef]
- Zhang, H.; Zhao, Z.; Song, B.; Du, P.; Liu, X. Light-induced ultrastructure changes of amyloplasts and effect of nitrogen fertilization on greening in potato tubers (Solanum tuberosum L.). Postharvest Biol. Technol. 2020, 168, 111275. [Google Scholar] [CrossRef]
- Pathare, P.B.; Opara, U.L.; Al-Said, F.A.J. Colour measurement and analysis in fresh and processed foods: A review. Food Bioprocess. Technol. 2013, 6, 36–60. [Google Scholar] [CrossRef]
- Blancas-Benitez, F.J.; Montaño-Leyva, B.; Aguirre-Güitrón, L.; Moreno-Hernández, C.L.; Fonseca-Cantabrana, Á.; del Carmen Romero-Islas, L.; González-Estrada, R.R. Impact of edible coatings on quality of fruits: A review. Food Control 2022, 139, 109063. [Google Scholar] [CrossRef]
- Nyankanga, R.O.; Murigi, W.W.; Shibairo, S.I. Effect of packaging material on shelf life and quality of ware potato tubers stored at ambient tropical temperatures. Potato Res. 2018, 61, 283–296. [Google Scholar] [CrossRef]
- Larsen, H.; Molteberg, E.L. Discolouration of potato tubers under retail light: Cultivar variations and effect of different packaging materials for folva potatoes stored at 20 and 6 C. Potato Res. 2023, 66, 507–523. [Google Scholar] [CrossRef]
- Maringgal, B.; Hashim, N.; Tawakkal, I.S.M.A.; Mohamed, M.T.M. Recent advance in edible coating and its effect on fresh/fresh-cut fruits quality. Trends Food Sci. Technol. 2020, 96, 253–267. [Google Scholar] [CrossRef]
- Tahir, H.E.; Xiaobo, Z.; Mahunu, G.K.; Arslan, M.; Abdalhai, M.; Zhihua, L. Recent developments in gum edible coating applications for fruits and vegetables preservation: A review. Carbohydr. Polym. 2019, 224, 115141. [Google Scholar] [CrossRef]
- Sinden, S.L. Control of potato greening with household detergents. Am. Potato J. 1971, 48, 53–56. [Google Scholar] [CrossRef]
- Wu, M.T.; Salunkhe, D.K. Control of chlorophyll and solanine syntheses and sprouting of potato tubers by hot paraffin wax. J. Food Sci. 1972, 37, 629–630. [Google Scholar] [CrossRef]
- Donaldson, L. Autofluorescence in plants. Molecules 2020, 25, 2393. [Google Scholar] [CrossRef]
- Kalaji, H.M.; Jajoo, A.; Oukarroum, A.; Brestic, M.; Zivcak, M.; Samborska, I.A.; Cetner, M.D.; Łukasik, I.; Goltsev, V.; Ladle, R.J. Chlorophyll a fluorescence as a tool to monitor physiological status of plants under abiotic stress conditions. Acta Physiol. Plant. 2016, 38, 102. [Google Scholar] [CrossRef]
- Maina, B.; Ambuko, J.; Hutchinson, M.; Owino, W. The effect of different waxing technologies on shelf life of ‘Apple’ Mango Fruits Stored under Different Storage Conditions. J. Biol. Agric. Healthc. 2018, 24, 24–30. [Google Scholar]
- Tang, J.; Cheng, J.; Li, Z.; Zhang, J.; Pan, Y. Ethanol fumigation combined with modified atmosphere packaging delays potato greening under light. Plant Physiol. Biochem. 2023, 202, 107962. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).