Wounding Citrus Peel By-Products as Abiotic Stress to Induce the Synthesis of Phenolic Compounds?
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material
2.2. Initial Quality Parameters of Citrus Fruit
2.3. Wounding Treatment and Sample Preparation
2.4. Extraction of Bioactive Compounds
2.5. Chemical Reagents
2.6. Total Phenolic Content and Total Antioxidant Capacity
2.7. Quantification of Individual Phenolic Compounds by HPLC–ESI–QqQ–MS/MS
2.8. Statistical Analyses
3. Results
3.1. Initial Physicochemical Parameters of Citrus Fruits
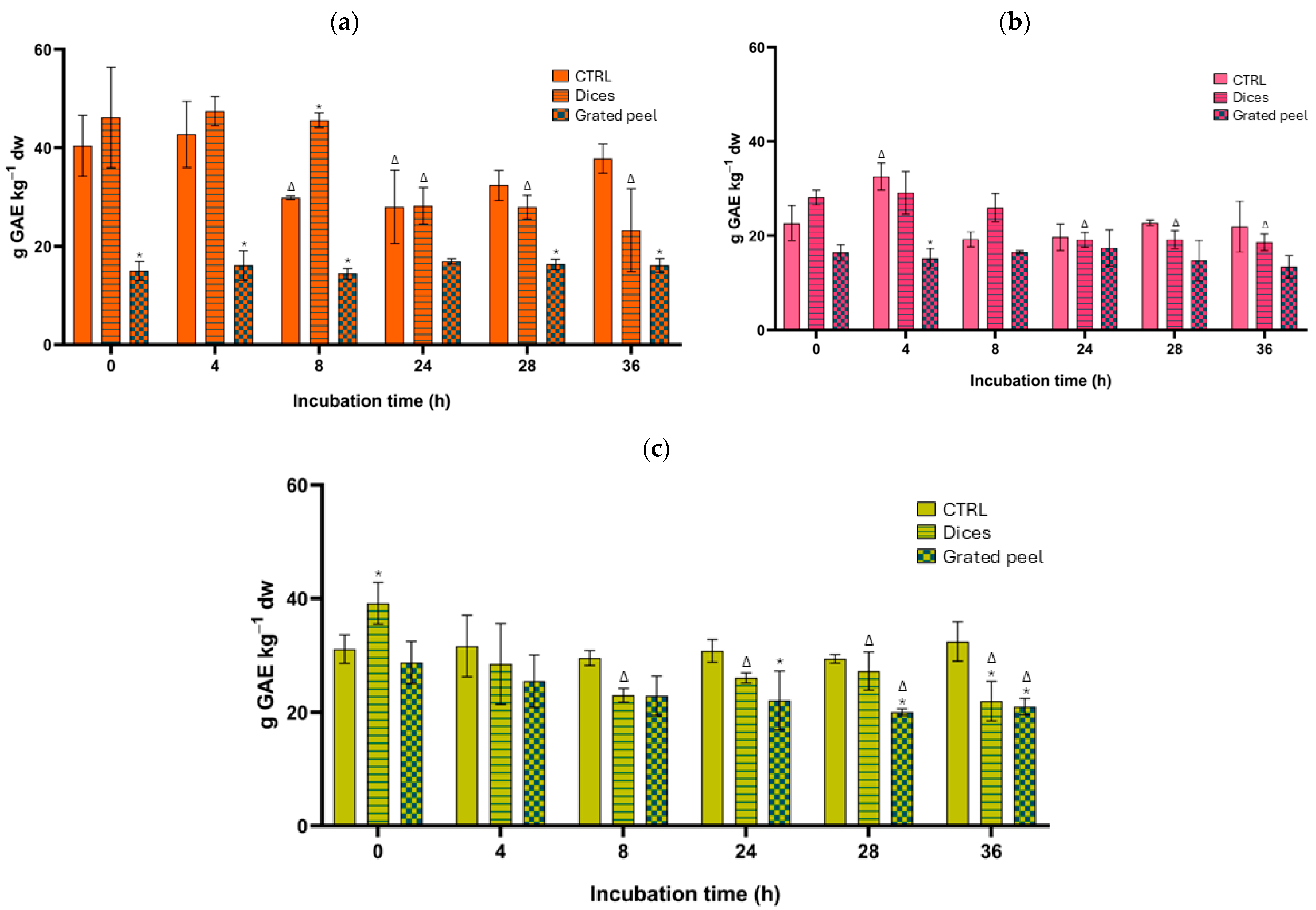
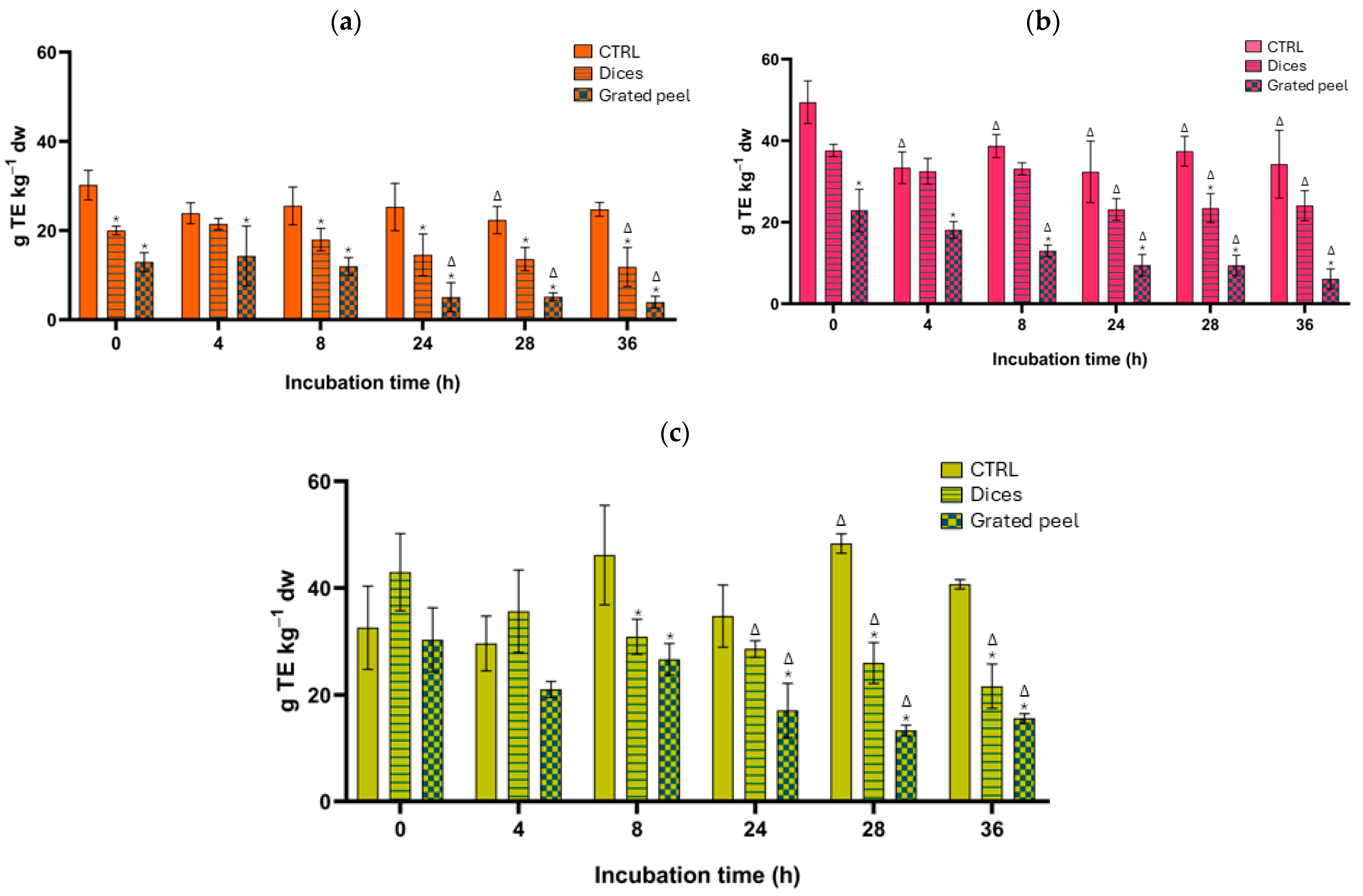
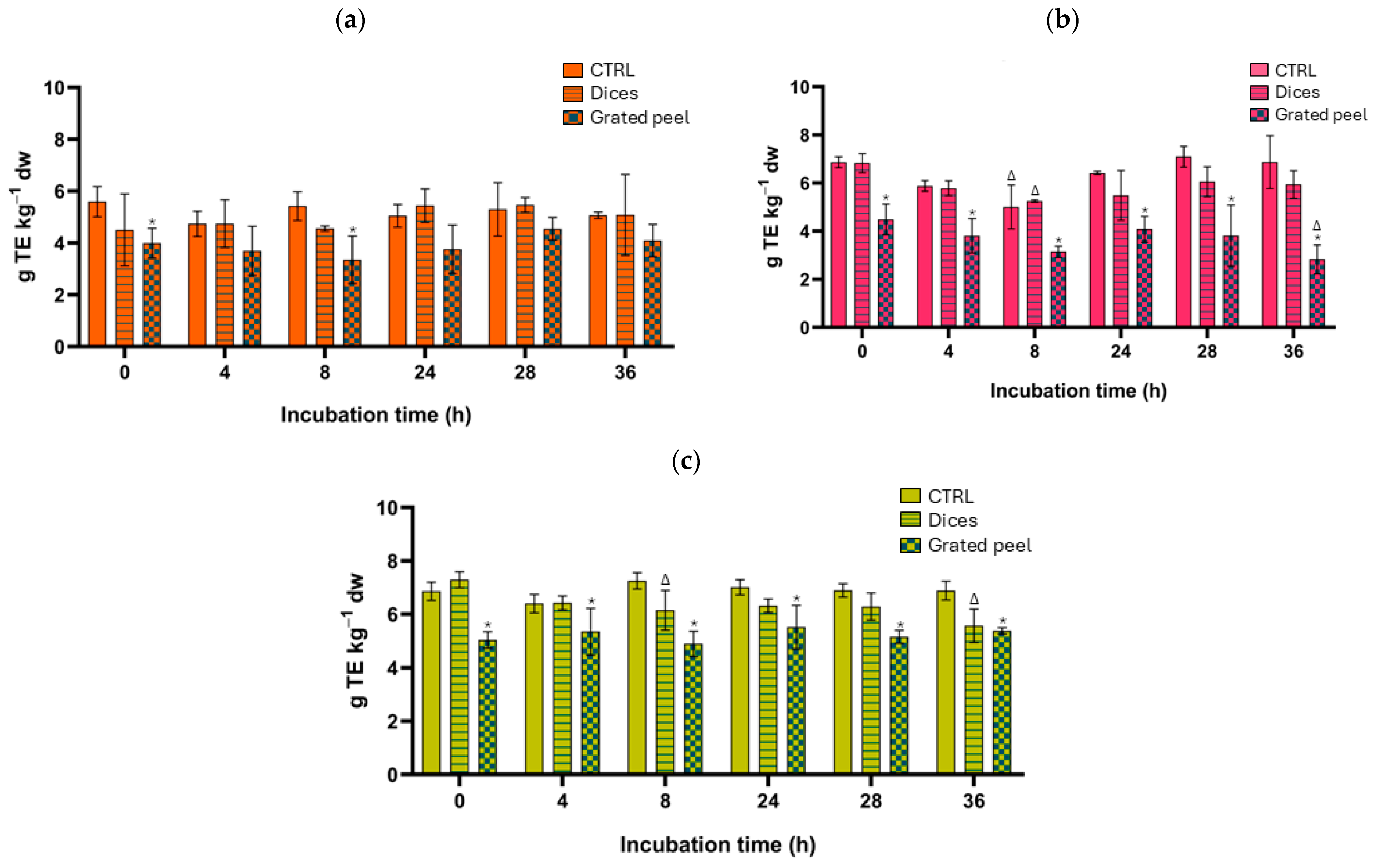
3.2. Total Phenolic Content and Total Antioxidant Capacity Results
3.3. Identification and Quantification of Individual Phenolic Compounds (HPLC–MS/MS)
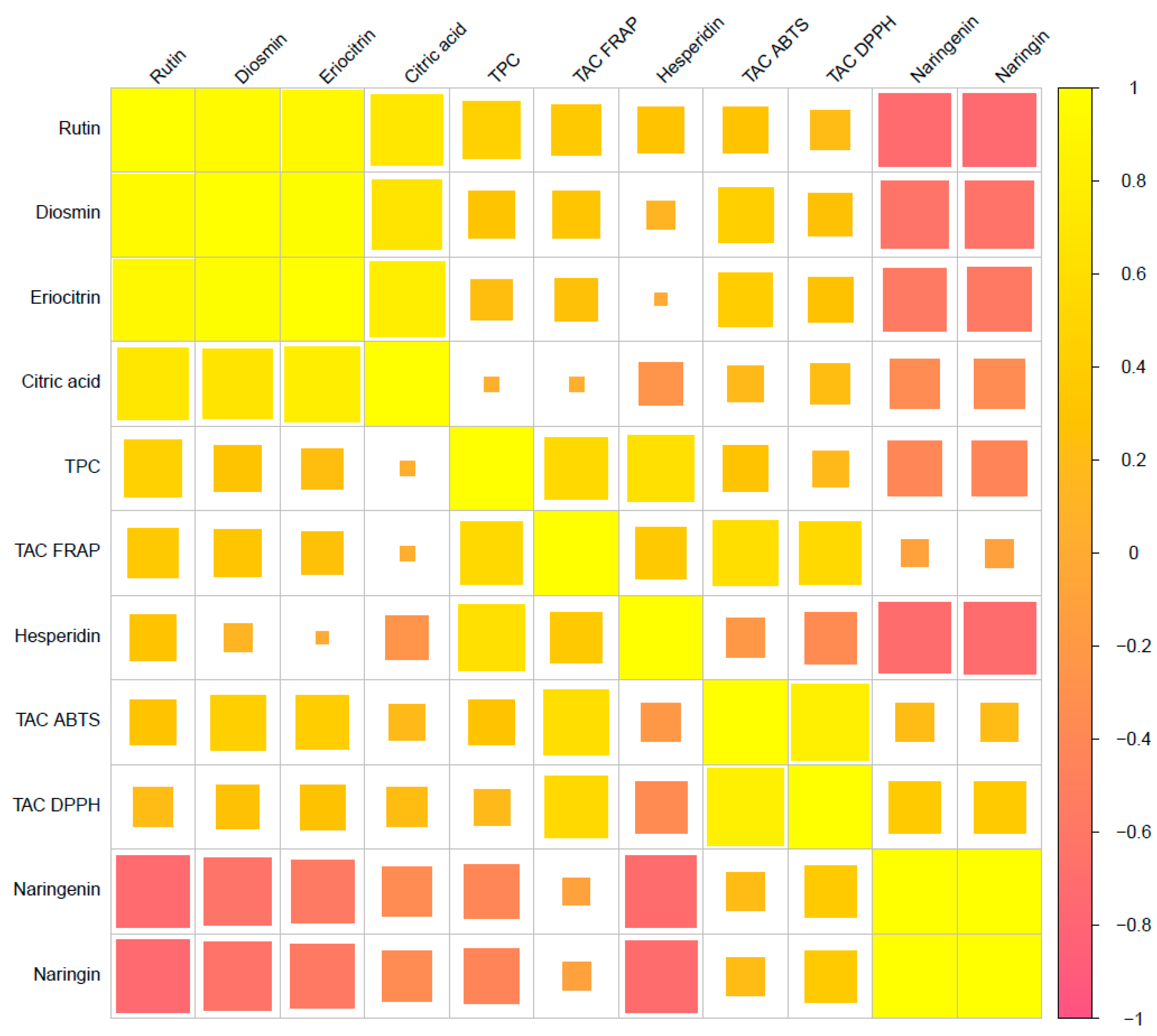
3.4. Correlation between the Studied Variables
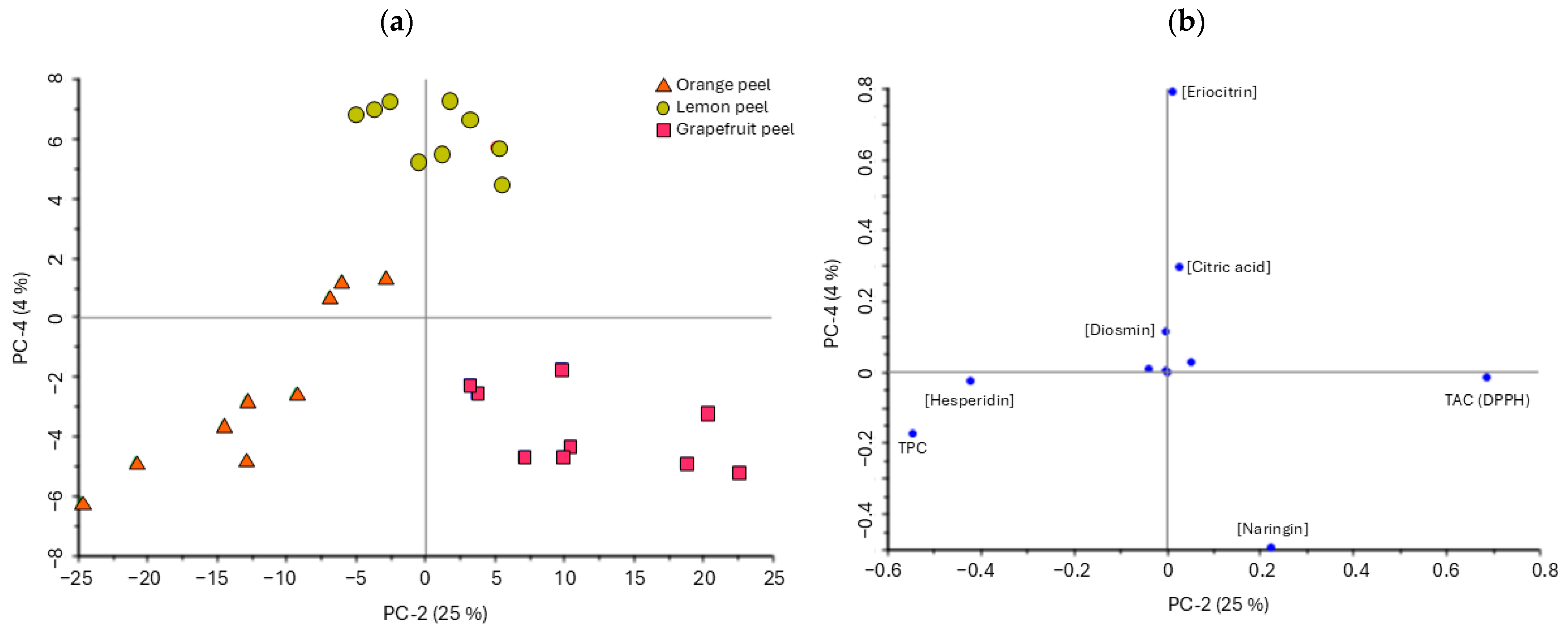
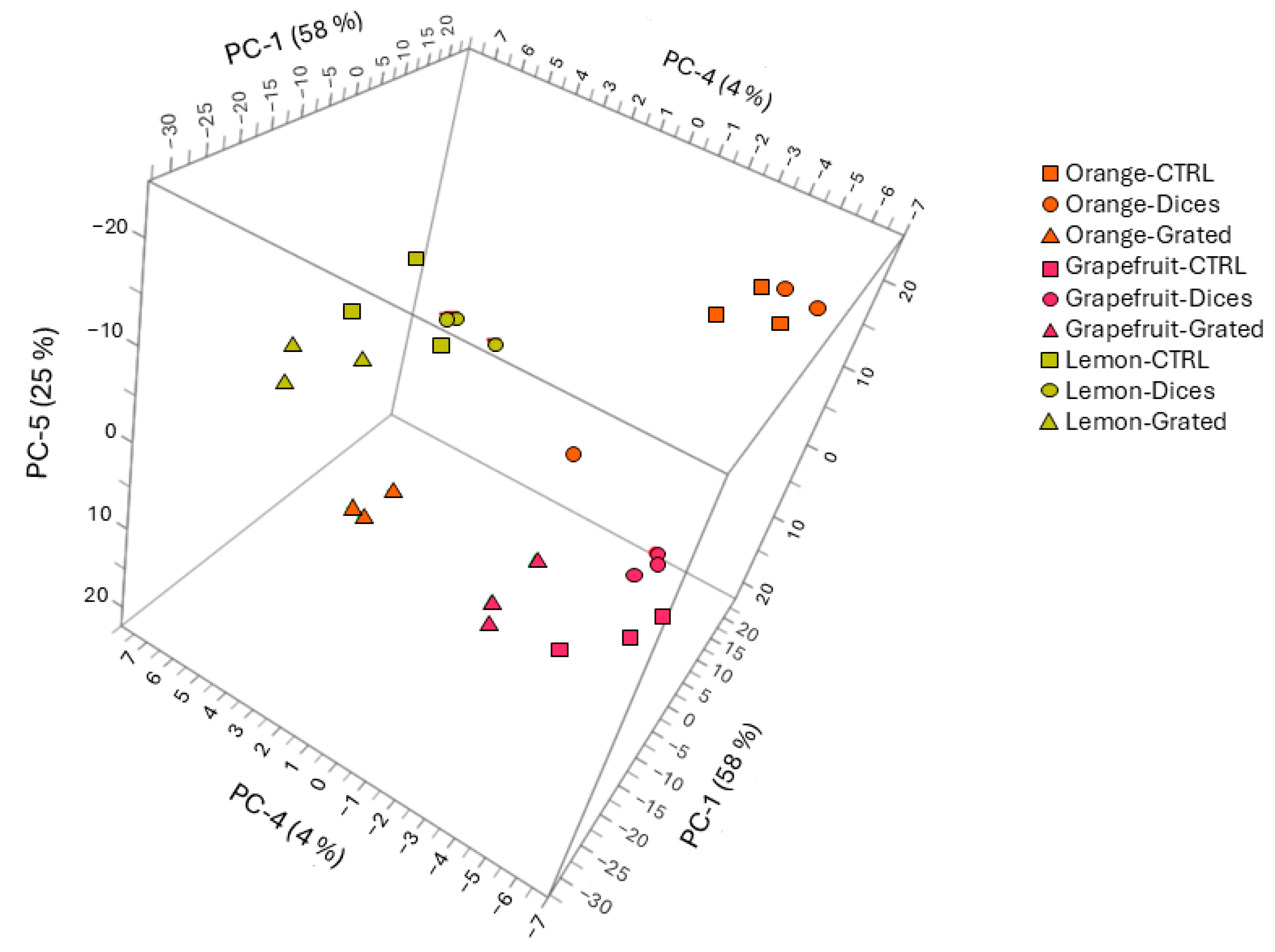
3.5. Principal Component Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cisneros-Zevallos, L. The Use of Controlled Postharvest Abiotic Stresses as a Tool for Enhancing the Nutraceutical Content and Adding-Value of Fresh Fruits and Vegetables. J. Food Sci. 2003, 68, 1560–1565. [Google Scholar]
- Toscano, S.; Trivellini, A.; Cocetta, G.; Bulgari, R.; Francini, A.; Romano, D.; Ferrante, A. Effect of Preharvest Abiotic Stresses on the Accumulation of Bioactive Compounds in Horticultural Produce. Front. Plant Sci. 2019, 10, 1212. [Google Scholar]
- Formica-Oliveira, A.C.; Martínez-Hernández, G.B.; Aguayo, E.; Gómez, P.A.; Artés, F.; Artés-Hernández, F. A Functional Smoothie from Carrots with Induced Enhanced Phenolic Content. Food Bioprocess. Technol. 2017, 10, 491–502. [Google Scholar]
- Oh, M.M.; Trick, H.N.; Rajashekar, C.B. Secondary Metabolism and Antioxidants Are Involved in Environmental Adaptation and Stress Tolerance in Lettuce. J. Plant Physiol. 2009, 166, 180–191. [Google Scholar] [CrossRef]
- Oh, M.M.; Carey, E.E.; Rajashekar, C.B. Environmental Stresses Induce Health-Promoting Phytochemicals in Lettuce. Plant Physiol. Biochem. 2009, 47, 578–583. [Google Scholar] [CrossRef]
- Castillejo, N.; Martínez-Zamora, L.; Artés-Hernández, F. Postharvest UV Radiation Enhanced Biosynthesis of Flavonoids and Carotenes in Bell Peppers. Postharvest Biol. Technol. 2022, 184, 111774. [Google Scholar] [CrossRef]
- Surjadinata, B.B.; Cisneros-Zevallos, L. Biosynthesis of Phenolic Antioxidants in Carrot Tissue Increases with Wounding Intensity. Food Chem. 2012, 134, 615–624. [Google Scholar] [CrossRef]
- Hodges, D.M.; Toivonen, P.M.A. Quality of Fresh-Cut Fruits and Vegetables as Affected by Exposure to Abiotic Stress. Postharvest Biol. Technol. 2008, 48, 155–162. [Google Scholar]
- Rivera-López, J.; Vázquez Ortiz, F.A.; Ayala-Zavala, F.; Sotelo-Mundo, R.R.; González-Aguilar, G.A. Cutting Shape and Storage Temperature Affect Overall Quality of Fresh-Cut Papaya Cv. “Maradol”. J. Food Sci. 2005, 70, s482–s489. [Google Scholar]
- Portela, S.I.; Cantwell, M.I. Cutting Blade Sharpness Affects Appearance and Other Quality Attributes of Fresh-Cut Cantaloupe Melon. J. Food Sci. 2001, 66, 1265–1270. [Google Scholar]
- Reyes, L.F.; Cisneros-Zevallos, L. Wounding Stress Increases the Phenolic Content and Antioxidant Capacity of Purple-Flesh Potatoes (Solanum tuberosum L.). J. Agric. Food Chem. 2003, 51, 5296–5300. [Google Scholar] [CrossRef]
- Torres-Contreras, A.M.; Nair, V.; Cisneros-Zevallos, L.; Jacobo-Velázquez, D.A. Plants as Biofactories: Stress-Induced Production of Chlorogenic Acid Isomers in Potato Tubers as Affected by Wounding Intensity and Storage Time. Ind. Crops Prod. 2014, 62, 61–66. [Google Scholar] [CrossRef]
- Hu, J.; Yang, L.; Wu, W.; Li, Y.; Zhan, L. Slicing Increases Antioxidant Capacity of Fresh-Cut Lotus Root (Nelumbo Nucifera G.) Slices by Accumulating Total Phenols. Int. J. Food Sci. Technol. 2014, 49, 2418–2424. [Google Scholar] [CrossRef]
- Villarreal-García, D.; Nair, V.; Cisneros-Zevallos, L.; Jacobo-Velázquez, D.A. Plants as Biofactories: Postharvest Stress-Induced Accumulation of Phenolic Compounds and Glucosinolates in Broccoli Subjected to Wounding Stress and Exogenous Phytohormones. Front. Plant Sci. 2016, 7, 45. [Google Scholar] [CrossRef]
- Gastélum-Estrada, A.; Rabadán-Chávez, G.; Reza-Zaldívar, E.E.; de la Cruz-López, J.L.; Fuentes-Palma, S.A.; Mojica, L.; Díaz de la Garza, R.I.; Jacobo-Velázquez, D.A. Biofortified Beverage with Chlorogenic Acid from Stressed Carrots: Anti-Obesogenic, Antioxidant, and Anti-Inflammatory Properties. Foods 2023, 12, 3959. [Google Scholar] [CrossRef]
- Jacobo-Velázquez, D.A.; González-Aguëro, M.; Cisneros-Zevallos, L. Cross-Talk between Signaling Pathways: The Link between Plant Secondary Metabolite Production and Wounding Stress Response. Sci. Rep. 2015, 5, 8608. [Google Scholar] [CrossRef]
- Candar, S. How Abiotic Stress Induced by Artificial Wounding Changes Maturity Levels and Berry Composition of Merlot (Vitis vinifera L.). Eur. Food Res. Technol. 2023, 249, 2611–2623. [Google Scholar] [CrossRef]
- Li, X.; Long, Q.; Gao, F.; Han, C.; Jin, P.; Zheng, Y. Effect of Cutting Styles on Quality and Antioxidant Activity in Fresh-Cut Pitaya Fruit. Postharvest Biol. Technol. 2017, 124, 1–7. [Google Scholar] [CrossRef]
- M’hiri, N.; Ioannou, I.; Ghoul, M.; Boudhrioua, N.M. Extraction Methods of Citrus Peel Phenolic Compounds. Food Rev. Int. 2014, 30, 265–290. [Google Scholar]
- Zayed, A.; Badawy, M.T.; Farag, M.A. Valorization and Extraction Optimization of Citrus Seeds for Food and Functional Food Applications. Food Chem. 2021, 355, 129609. [Google Scholar] [CrossRef] [PubMed]
- Mahato, N.; Sharma, K.; Sinha, M.; Baral, E.R.; Koteswararao, R.; Dhyani, A.; Hwan Cho, M.; Cho, S. Bio-Sorbents, Industrially Important Chemicals and Novel Materials from Citrus Processing Waste as a Sustainable and Renewable Bioresource: A Review. J. Adv. Res. 2020, 23, 61–82. [Google Scholar]
- Anoopkumar, A.N.; Aneesh, E.M.; Sirohi, R.; Tarafdar, A.; Kuriakose, L.L.; Surendhar, A.; Madhavan, A.; Kumar, V.; Awasthi, M.K.; Binod, P.; et al. Bioactives from Citrus Food Waste: Types, Extraction Technologies and Application. J. Food Sci. Technol. 2024, 61, 444–458. [Google Scholar]
- Cano-Lamadrid, M.; Artés-Hernández, F. By-Products Revalorization with Non-Thermal Treatments to Enhance Phytochemical Compounds of Fruit and Vegetables Derived Products: A Review. Foods 2022, 11, 59. [Google Scholar]
- Santos, D.I.; Martins, C.F.; Amaral, R.A.; Brito, L.; Saraiva, J.A.; Vicente, A.A.; Moldão-Martins, M. Pineapple (Ananas comosus L.) by-Products Valorization: Novel Bio Ingredients for Functional Foods. Molecules 2021, 26, 3216. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Rangel, J.C.; Benavides, J.; Jacobo-Velázquez, D.A. Production of Nutraceuticals in Carrot Bagasse Using Abiotic Stresses. In Proceedings of the VII International Postharvest Symposium 1012, Kuala Lumpur, Malaysia, 25–29 June 2012; pp. 1475–1479. [Google Scholar]
- Singleton, V.L.; Rossi, J.A. Colorimetry of Total Phenolics with Phosphomolybdic-Phosphotungstic Acid Reagents. Am. J. Enol. Vitic. 1965, 16, 144–158. [Google Scholar]
- Martínez-Zamora, L.; Castillejo, N.; Artés-Hernández, F. Postharvest UV-B and Photoperiod with Blue + Red LEDs as Strategies to Stimulate Carotenogenesis in Bell Peppers. Appl. Sci. 2021, 11, 3736. [Google Scholar] [CrossRef]
- Castillejo, N.; Martínez-Zamora, L.; Gómez, P.A.; Pennisi, G.; Crepaldi, A.; Fernández, J.A.; Orsini, F.; Artés-Hernández, F. Postharvest LED Lighting: Effect of Red, Blue and Far Red on Quality of Minimally Processed Broccoli Sprouts. J. Sci. Food Agric. 2021, 101, 44–53. [Google Scholar] [CrossRef]
- Castillejo, N.; Martínez-Hernández, G.B.; Artés-Hernández, F. Revalorized Broccoli By-Products and Mustard Improved Quality during Shelf Life of a Kale Pesto Sauce. Food Sci. Technol. Int. 2021, 27, 734–745. [Google Scholar] [CrossRef]
- Salas-Millán, J.Á.; Aznar, A.; Conesa, E.; Conesa-Bueno, A.; Aguayo, E. Functional Food Obtained from Fermentation of Broccoli By-Products (Stalk): Metagenomics Profile and Glucosinolate and Phenolic Compounds Characterization by LC-ESI-QqQ-MS/MS. LWT 2022, 169, 113915. [Google Scholar] [CrossRef]
- Ledesma-Escobar, C.A.; Priego-Capote, F.; Luque De Castro, M.D. Characterization of Lemon (Citrus Limon) Polar Extract by Liquid Chromatography-Tandem Mass Spectrometry in High Resolution Mode. J. Mass. Spectrom. 2015, 50, 1196–1205. [Google Scholar] [CrossRef]
- Aguilar-Hernández, M.G.; Núñez-Gómez, D.; Forner-Giner, M.Á.; Hernández, F.; Pastor-Pérez, J.J.; Legua, P. Quality Parameters of Spanish Lemons with Commercial Interest. Foods 2021, 10, 62. [Google Scholar] [CrossRef]
- Domínguez-Gento, A.; Di Giorgi, R.; García-Martínez, M.D.; Raigón, M.D. Effects of Organic and Conventional Cultivation on Composition and Characterization of Two Citrus Varieties ‘Navelina’ Orange and ‘Clemenules’ Mandarin Fruits in a Long-Term Study. Horticulturae 2023, 9, 721. [Google Scholar] [CrossRef]
- Srivastav, M. Fruit Quality, Antioxidant Enzymes Activity and Yield of Six Cultivars of Grapefruit (Citrus paradisi) Grown under Subtropical Conditions. Indian J. Agric. Sci. 2013, 83, 842–846. [Google Scholar]
- Jacobo-Velázquez, D.A.; Martínez-Hernández, G.B.; Del C. Rodríguez, S.; Cao, C.M.; Cisneros-Zevallos, L. Plants as Biofactories: Physiological Role of Reactive Oxygen Species on the Accumulation of Phenolic Antioxidants in Carrot Tissue under Wounding and Hyperoxia Stress. J. Agric. Food Chem. 2011, 59, 6583–6593. [Google Scholar] [CrossRef]
- Santana-Gálvez, J.; Santacruz, A.; Cisneros-Zevallos, L.; Jacobo-Velázquez, D.A. Postharvest Wounding Stress in Horticultural Crops as a Tool for Designing Novel Functional Foods and Beverages with Enhanced Nutraceutical Content: Carrot Juice as a Case Study. J. Food Sci. 2019, 84, 1151–1161. [Google Scholar] [CrossRef] [PubMed]
- Artés-Hernández, F.; Rivera-Cabrera, F.; Kader, A.A. Quality Retention and Potential Shelf-Life of Fresh-Cut Lemons as Affected by Cut Type and Temperature. Postharvest Biol. Technol. 2007, 43, 245–254. [Google Scholar] [CrossRef]
- Rahman, N.F.A.; Shamsudin, R.; Ismail, A.; Shah, N.N.A.K.; Varith, J. Effects of Drying Methods on Total Phenolic Contents and Antioxidant Capacity of the Pomelo (Citrus grandis (L.) Osbeck) Peels. Innov. Food Sci. Emerg. Technol. 2018, 50, 217–225. [Google Scholar] [CrossRef]
- Guimarães, R.; Barros, L.; Barreira, J.C.M.; Sousa, M.J.; Carvalho, A.M.; Ferreira, I.C.F.R. Targeting Excessive Free Radicals with Peels and Juices of Citrus Fruits: Grapefruit, Lemon, Lime and Orange. Food Chem. Toxicol. 2010, 48, 99–106. [Google Scholar] [CrossRef]
- Mcharek, N.; Hanchi, B. Maturational Effects on Phenolic Constituents, Antioxidant Activities and LC-MS/MS Profiles of Lemon (Citrus Limon) Peels. J. Appl. Bot. Food Qual. 2017, 90, 1–9. [Google Scholar] [CrossRef]
- Yerlikaya, P.; Gokoglu, N.; Topuz, O.K.; Gumus, B.; Aydan Yatmaz, H. Antioxidant Activities of Citrus Albedo and Flavedo Fragments against Fish Lipid Oxidation. J. Aquat. Food Prod. Technol. 2016, 25, 1339–1347. [Google Scholar] [CrossRef]
- Dixon, R.; Paiva, N. Stress-Induced Phenylpropanoid Metabolism. Plant Cell 1995, 7, 1085–1097. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.-S.; Lee, S.; Man Park, S.; Hyun Yun, S.; Gab, H.-S.; Suk Kim, S.; Kim, H.-J.; Comparative, H. Comparative Metabolomics Analysis of Citrus Varieties. Foods 2021, 10, 2826. [Google Scholar] [CrossRef]
- Zeng, J.; Chen, C.; Chen, M.; Chen, J. Comparative Transcriptomic and Metabolomic Analyses Reveal the Delaying Effect of Naringin on Postharvest Decay in Citrus Fruit. Front. Plant Sci. 2022, 13, 1045857. [Google Scholar] [CrossRef]
- Zhang, H.; Yang, Y.F.; Zhou, Z.Q. Phenolic and Flavonoid Contents of Mandarin (Citrus reticulata Blanco) Fruit Tissues and Their Antioxidant Capacity as Evaluated by DPPH and ABTS Methods. J. Integr. Agric. 2018, 17, 256–263. [Google Scholar] [CrossRef]
- Chang, S.Q.; Azrina, A. Antioxidant Content and Activity in Different Parts of Pomelo [Citrus grandis (L.) Osbeck] by-Products. In Proceedings of the III International Conference on Agricultural and Food Engineering 1152, Kuala Lumpur, Malaysia, 23–25 August 2017; Volume 1152, pp. 27–34. [Google Scholar]
- Tsai, H.L.; Chang, S.K.C.; Chang, S.J. Antioxidant Content and Free Radical Scavenging Ability of Fresh Red Pummelo [Citrus grandis (L.) Osbeck] Juice and Freeze-Dried Products. J. Agric. Food Chem. 2007, 55, 2867–2872. [Google Scholar] [CrossRef]
- Breksa, A.P.; Manners, G.D. Evaluation of the Antioxidant Capacity of Limonin, Nomilin, and Limonin Glucoside. J. Agric. Food Chem. 2006, 54, 3827–3831. [Google Scholar] [CrossRef]
- Zaporozhets, O.A.; Krushynska, O.A.; Lipkovska, N.A.; Barvinchenko, V.N. A New Test Method for the Evaluation of Total Antioxidant Activity of Herbal Products. J. Agric. Food Chem. 2004, 52, 21–25. [Google Scholar] [CrossRef]
- Corradini, C.; Borromei, C.; Cavazza, A.; Merusi, C.; De Rossi, A.; Nicoletti, I. Determination of Flavanones in Citrus Byproducts and Nutraceutical Products by a Validated RP-HPLC Method. J. Liq. Chromatogr. Relat. Technol. 2009, 32, 1448–1462. [Google Scholar] [CrossRef]
- Rosa, A.; Petretto, G.L.; Maldini, M.; Tirillini, B.; Chessa, M.; Pintore, G.; Sarais, G. Chemical Characterization, Antioxidant and Cytotoxic Activity of Hydroalcoholic Extract from the Albedo and Flavedo of Citrus limon Var. pompia Camarda. J. Food Meas. Charact. 2023, 17, 627–635. [Google Scholar] [CrossRef]


| Orange | Grapefruit | Lemon | |
|---|---|---|---|
| L* | 67.0 ± 0.9 | 74.2 ± 2.4 | 75.9 ± 1.9 |
| a* | 27.58 ± 1.95 | 14.81 ± 3.36 | 1.12 ± 2.12 |
| b* | 51.74 ± 1.40 | 46.15 ± 3.83 | 55.14 ± 2.86 |
| °h | 1.09 ± 0.04 | 1.27 ± 0.09 | 1.53 ± 0.02 |
| C | 58.8 ± 1.3 | 48.8 ± 2.7 | 55.2 ± 3.1 |
| Weight (g) | 214 ± 15 | 228 ± 8 | 159 ± 19 |
| Longitudinal diameter (mm) | 70.6 ± 3.9 | 74. ± 4.4 | 82.0 ± 9.4 |
| Equatorial diameter (mm) | 75.8 ± 3.4 | 83.3 ± 2.1 | 63.7 ± 1.4 |
| Deformation (%) | 2.5 ± 0.3 | 2.2 ± 0.4 | 3.2 ± 0.5 |
| Flavedo thickness (mm) | 2.0 ± 0.7 | 1.7 ± 0.4 | 1.2 ± 0.4 |
| Albedo thickness (mm) | 4.8 ± 0.9 | 4.8 ± 0.4 | 4.4 ± 1.2 |
| Albedo in the peel (%) | 71.0 ± 2.9 | 74.1 ± 4.8 | 79.1 ± 3.5 |
| Juice (%) | 46.6 ± 5.0 | 45.5 ± 3.4 | 42.3 ± 10.8 |
| TSS (°Brix) | 13.9 ± 0.5 | 12.3 ± 0.6 | 4.5 ± 0.7 |
| pH | 3.6 ± 0.4 | 2.83 ± 0.03 | 2.18 ± 0.09 |
| TA (g citric acid 100 mL−1) | 0.31 ± 0.07 | 0.98 ± 0.12 | 2.9 ± 0.5 |
| Maturity Index | 46.2 ± 10.9 | 12.8 ± 1.6 | 2.8 ± 0.4 |
| Compound (g kg−1 dw) | Naringin | Hesperidin | Diosmin | Rutin | Eriocitrin | Naringenin | Citric Acid | |
|---|---|---|---|---|---|---|---|---|
| Orange | CTRL | 1.14 ± 0.08 | 16.9 ± 0.7 a | 0.19 ± 0.08 | 0.577 ± 0.003 a | 1.194 ± 0.002 | 0.028 ± 0.001 | 0.50 ± 0.11 |
| D | 1.00 ± 0.05 | 13.0 ± 1.6 b | 0.12 ± 0.03 | 0.363 ± 0.002 ab | 1.140 ± 0.005 | 0.030 ± 0.001 | 0.30 ± 0.07 | |
| G | 0.64 ± 0.01 | 8.78 ± 0.10 c | 0.19 ± 0.01 | 0.213 ± 0.004 b | 0.704 ± 0.007 | 0.007 ± 0.001 | 0.47 ± 0.08 | |
| Grapefruit | CTRL | 9.1 ± 1.0 a | 0.14 ± 0.02 | nd | nd | 3.20 ± 0.01 | 0.25 ± 0.01 a | 0.93 ± 0.04 b |
| D | 8.2 ± 0.3 b | 0.24 ± 0.03 | nd | nd | 3.53 ± 0.03 | 0.24 ± 0.01 a | 1.48 ± 0.07 a | |
| G | 7.3 ± 0.4 c | 0.184 ± 0.004 | nd | nd | 2.66 ± 0.02 | 0.206 ± 0.001 b | 1.08 ± 0.14 b | |
| Lemon | CTRL | 0.125 ± 0.006 | 7.9 ± 0.5 a | 1.38 ± 0.14 b | 1.003 ± 0.007 b | 9.2 ± 1.0 a | nd | 2.75 ± 0.19 b |
| D | 0.099 ± 0.001 | 6.86 ± 0.10 a | 1.71 ± 0.10 a | 1.46 ± 0.01 a | 9.2 ± 0.3 a | nd | 2.93 ± 0.10 b | |
| G | 0.056 ± 0.002 | 3.98 ± 0.12 b | 1.02 ±0.02 c | 1.31 ± 0.01 a b | 8.1 ± 0.5 b | nd | 6.30 ± 0.11 a | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zapata, R.; Martínez-Zamora, L.; Cano-Lamadrid, M.; Artés-Hernández, F. Wounding Citrus Peel By-Products as Abiotic Stress to Induce the Synthesis of Phenolic Compounds? Horticulturae 2024, 10, 885. https://doi.org/10.3390/horticulturae10080885
Zapata R, Martínez-Zamora L, Cano-Lamadrid M, Artés-Hernández F. Wounding Citrus Peel By-Products as Abiotic Stress to Induce the Synthesis of Phenolic Compounds? Horticulturae. 2024; 10(8):885. https://doi.org/10.3390/horticulturae10080885
Chicago/Turabian StyleZapata, Rosa, Lorena Martínez-Zamora, Marina Cano-Lamadrid, and Francisco Artés-Hernández. 2024. "Wounding Citrus Peel By-Products as Abiotic Stress to Induce the Synthesis of Phenolic Compounds?" Horticulturae 10, no. 8: 885. https://doi.org/10.3390/horticulturae10080885
APA StyleZapata, R., Martínez-Zamora, L., Cano-Lamadrid, M., & Artés-Hernández, F. (2024). Wounding Citrus Peel By-Products as Abiotic Stress to Induce the Synthesis of Phenolic Compounds? Horticulturae, 10(8), 885. https://doi.org/10.3390/horticulturae10080885










