Identifying Grapevine Rootstocks Tolerant to Copper Excess
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material and Growth Conditions
2.2. Height, Dry Matter Yield and Tissue Nutrient Concentration
2.3. Photosynthetic Activity
2.4. Chlorophyll a Fluorescence
2.5. Enzymatic Activity in Grapevine
2.6. Statistical Analysis
3. Results
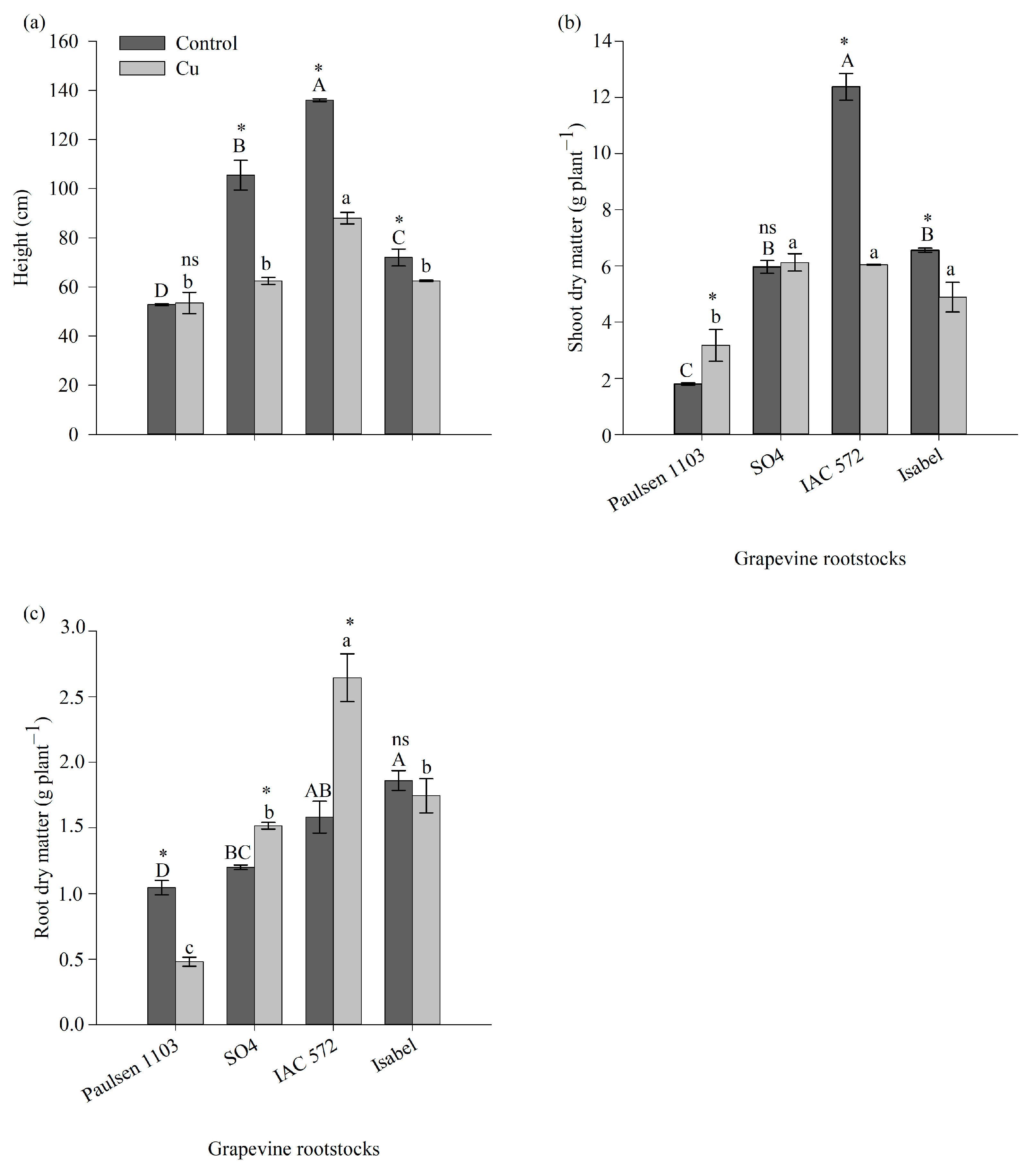
3.1. Growth of Different Grapevine Rootstock Genotypes
3.2. Nutrient Homeostasis
3.3. Photosynthetic Activity and Chlorophyll a Fluorescence
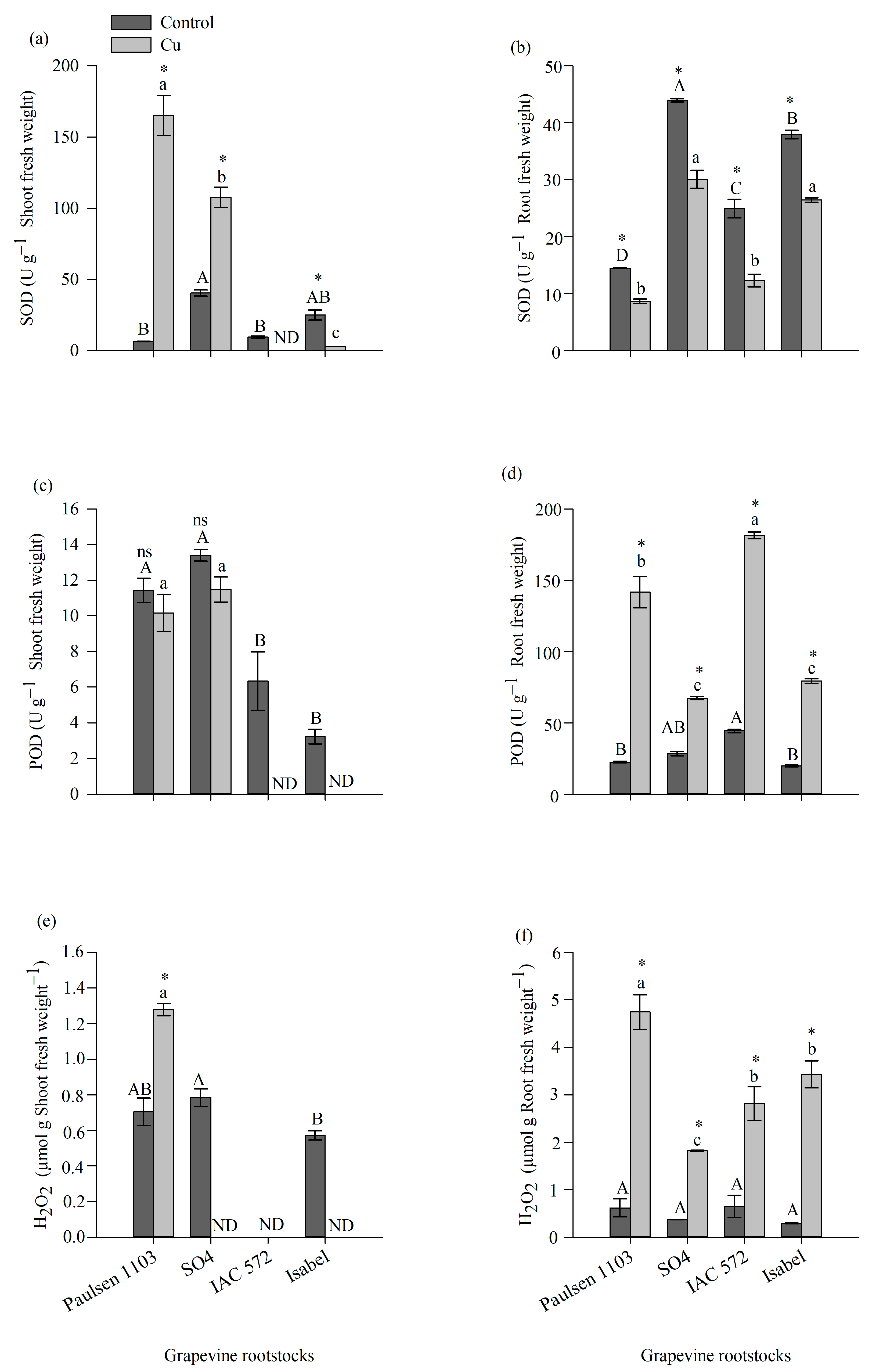
3.4. Enzymatic Activity
3.5. Principal Component Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Girotto, E.; Ceretta, C.A.; Brunetto, G.; Miotto, A.; Tiecher, T.L.; De Conti, L.; Lourenzi, C.R.; Lorensini, F.; Gubiani, P.I.; Silva, L.S.; et al. Copper availability assessment of Cu-contaminated vineyard soils using black oat cultivation and chemical extractants. Environ. Monit. Assess. 2014, 186, 9051–9063. [Google Scholar] [CrossRef]
- Miotto, A.; Ceretta, C.A.; Brunetto, G.; Nicoloso, F.T.; Girotto, E.; Farias, J.G.; Tiecher, T.L.; De Conti, L.; Trentin, G. Copper uptake, accumulation and physiological changes in adult grapevines in response to excess copper in soil. Plant Soil. 2014, 374, 593–610. [Google Scholar] [CrossRef]
- Brunetto, G.; Melo, J.W.B.; Terzano, R.; Del Buono, D.; Astolfi, S.; Tomasi, N.; Pii, Y.; Mimmo, T.; Cesco, S. Copper accumulation in vineyard soils: Rhizosphere processes and agronomic practices to limit its toxicity. Chemosphere 2016, 162, 293–307. [Google Scholar] [CrossRef]
- Tiecher, T.L.; Tiecher, T.; Ceretta, C.A.; Ferreira, P.A.A.; Nicoloso, F.T.; Soriani, H.H.; De Conti, L.; Kulmann, M.S.S.; Schneider, R.O.; Brunetto, G. Tolerance and translocation of heavy metals in young grapevine (Vitis vinifera) grown in sandy acidic soil with interaction of high doses of copper and zinc. Sci. Hortic. 2017, 222, 203–212. [Google Scholar] [CrossRef]
- Tiecher, T.L.; Soriani, H.H.; Tiecher, T.; Ceretta, C.A.; Nicoloso, F.T.; Tarouco, C.P.; Clasen, B.E.; De Conti, L.; Tassinari, A.; Melo, G.W.B.; et al. The interaction of high copper and zinc doses in acid soil changes the physiological state and development of the root system in young grapevines (Vitis vinifera). Ecotoxicol. Environ. Saf. 2018, 148, 985–994. [Google Scholar] [CrossRef]
- Baldi, E.; Miotto, A.; Ceretta, C.A.; Quartieri, M.; Sorrenti, G.; Brunetto, G.; Toselli, M. Soil-applied phosphorous is an effective tool to mitigate the toxicity of copper excess on grapevine grown in rhizobox. Sci. Hortic. 2018, 227, 102–111. [Google Scholar] [CrossRef]
- Yruela, I. Copper in plants: Acquisition, transport and interactions. Funct. Plant Biol. 2009, 36, 409–430. [Google Scholar] [CrossRef]
- Marschner, P. Mineral Nutrition of Higher Plants, 3rd ed.; Academic Press: London, UK, 2012. [Google Scholar]
- Adrees, M.; Ali, S.; Rizwan, M.; Ibrahim, M.; Abbas, F.; Farid, M.; Zia-Ur-Rehman, M.; Irshad, M.K.; Bharwana, S.A. The effect of excess copper on growth and physiology of important food crops: A review. Environ. Sci. Pollut. Res. Int. 2015, 22, 8148–8162. [Google Scholar] [CrossRef]
- Ollat, N.; Bordenave, L.; Tandonnet, J.P.; Marguerit, E.; Boursiquot, J.M. Grapevine rootstocks: Origins and perspectives. Acta Hortic. 2016, 1136, 11–22. [Google Scholar] [CrossRef]
- Warschefsky, E.; Klein, L.; Frank, M.; Chitwood, D. Rootstocks: Diversity, domestication, and impacts on shoot phenotypes. Trends Plant Sci. 2016, 21, 418–437. [Google Scholar] [CrossRef]
- Zhang, L.; Marguerit, E.; Rossdeutsch, L.; Ollat, N.; Gambetta, G.A. The influence of grapevine rootstocks on scion growth and drought resistance. Theor. Exp. Plant Physiol. 2016, 28, 143–157. [Google Scholar] [CrossRef]
- Ollat, N.; Peccoux, P.; Papura, D.; Esmenjaud, D.; Marguerit, E.; Tandonnet, J.P.; Bordenave, L.; Cookson, S.J.; Barrieu, F.; Rossdeutsch, L.; et al. Rootstocks as a component of adaptation to environment. In Grapevine in a Changing Environment: A Molecular and Ecophysiological Perspective, 1st ed.; Gerós, H., Chaves, M.M., Gil, H.M., Delrot, S., Eds.; Wiley: Hoboken, NJ, USA, 2016. [Google Scholar]
- EMBRAPA UVA E VINHO. Cultivares de Uva e Porta-Enxertos de Alta Sanidade. Available online: https://www.embrapa.br/uva-e-vinho/cultivares-e-porta-enxertos (accessed on 12 February 2018).
- Yruela, I. Copper in plants. Braz. J. Plant Physiol. 2005, 17, 145–156. [Google Scholar] [CrossRef]
- Fidalgo, F.; Azenha, M.; Silva, A.F.; Sousa, A.; Santiago, A.; Ferraz, P.; Teixeira, J. Copper-induced stress in Solanum nigrum L. and antioxidant defense system responses. Food Energy Secur. 2013, 2, 70–80. [Google Scholar] [CrossRef]
- Marastoni, L.; Sandri, M.; Pii, Y.; Valentinuzzi, F.; Brunetto, G.; Cesco, S.; Mimmo, T. Synergism and antagonisms between nutrients induced by copper toxicity in grapevine rootstocks: Monocropping vs. intercropping. Chemosphere 2019, 214, 563–578. [Google Scholar] [CrossRef]
- Hoagland, D.R.; Arnon, D.T. The Water Culture Method for Growth Plants without Soil; California Agriculture Experiment Station: Berkeley, CA, USA, 1950. [Google Scholar]
- Trentin, E.; Ferreira, P.A.A.; Ricachenevsky, F.K.; Facco, D.B.; Hammerschmitt, R.K.; Morsch, L.; Tarouco, C.P.; Nicoloso, F.T.; Araujo, M.M.; Berghetti, A.L.P.; et al. Growth, biochemical and physiological response of grapevine rootstocks to copper excess in nutrient solution. S. Afr. J. Bot. 2023, 162, 360–369. [Google Scholar]
- Murphy, J.; Riley, J.P. A Modified single solution method for determination of phosphate in natural waters. Anal. Chim. Acta 1962, 27, 31–36. [Google Scholar] [CrossRef]
- Bremner, J.M.; Mulvaney, C.S. Nitrogen total. In In Methods of Soil Analysis. Part 2, 2nd ed.; Page, A.L., Ed.; American Society of Agronomy: Madison, WI, USA, 1982; pp. 595–624. [Google Scholar]
- Chopin, E.I.B.; Marin, B.; Mkoungafoko, R.; Rigaux, A.; Hopgood, M.J.; Delannoy, E.; Cances, B.; Laurain, M. Factors affecting distribution and mobility of trace elements (Cu, Pb, Zn) in a perennial grapevine (Vitis vinifera L.) in the Champagne region of France. Environ. Pollut. 2008, 156, 1092–1098. [Google Scholar] [CrossRef]
- Busuioc, G.; Elekes, C.C.; Stihi, C.; Iordache, S.; Ciulei, S.C. The bioaccumulation and translocation of Fe, Zn, and Cu in species of mushrooms from Russula genus. Environ. Sci. Pollut. Res. Int. 2011, 18, 890–896. [Google Scholar] [CrossRef]
- Ferreira, P.A.A.; Ceretta, C.A.; Soriani, H.H.; Tiecher, T.L.; Soares, C.R.F.S.; Rossato, L.V.; Nicoloso, F.T.; Brunetto, G.; Paranhos, J.T.; Cornejo, P. Rhizophagus clarus and phosphate alter the physiological responses of Crotalaria juncea cultivated in soil with a high Cu level. Appl. Soil. Ecol. 2015, 91, 37–47. [Google Scholar] [CrossRef]
- Rascher, U.; Liebig, M.; Lüttge, U. Evaluation of instant light-response curves of chlorophyll fluorescence parameters obtained with a portable chlorophyll fluorometer on site in the field. Plant Cell Environ. 2000, 23, 1397–1405. [Google Scholar] [CrossRef]
- Loreto, F.; Velikova, V. Isoprene produced by leaves protects the photosynthetic apparatus against ozone damage, quences ozone products, and reduces lipid peroxidation of cellular membranes. Plant Physiol. 2001, 127, 1781–1787. [Google Scholar]
- Giannopolitis, C.N.; Ries, S.K. Superoxide dismutases: I. Occurrence in higher plants. Plant Physiol. 1977, 59, 309–314. [Google Scholar] [CrossRef]
- Zeraik, A.E.; Souza, F.S.; Fatibello-Filho, O. Desenvolvimento de um spot test para o monitoramento da atividade da peroxidase em um procedimento de purificação. Quím. Nova 2008, 31, 731–734. [Google Scholar] [CrossRef]
- Ferreira, D.F. Sisvar: Um programa para análises e ensino de estatística. Rev. Symp. 2008, 6, 36–41. [Google Scholar]
- Ter Braak, C.J.F.; Smilauer, P. Canoco Reference Manual and Cano Draw for Windows User’s Guide: Software for Canonical Community Ordination, version 4.5; Microcomputer Power: Ithaca, Greece, 2002; 500p. [Google Scholar]
- Pommer, C.V.; Terra, M.M.; Pires, E.J.P. Cultivares, melhoramento e fisiologia. In Uva: Tecnologia de Produção, Pós-Colheita, Mercado; Pommer, C.V., Ed.; Editora Cinco Continentes: Porto Alegre, Brazil, 2003; 778p. [Google Scholar]
- Marastoni, L.; Sandri, M.; Pii, Y.; Valentinuzzi, F.; Cesco, S.; Mimmo, T. Morphological root responses and molecular regulation of cation transporters are differently affected by copper toxicity and cropping system depending on the grapevine rootstock genotype. Front. Plant Sci. 2019, 10, 946. [Google Scholar] [CrossRef]
- Ambrosini, V.G.; Rosa, D.J.; Prado, J.P.C.; Borghezan, M.; Melo, G.W.B.; Soares, J.J.; Comin, C.R.F.S.; Simão, D.G.; Brunetto, G. Reduction of copper phytotoxicity by liming: A study of the root anatomy of young vines (Vitis labrusca L.). Plant Physiol. Biochem. 2015, 96, 270–280. [Google Scholar]
- Guimarães, P.R.; Ambrosini, V.G.; Miotto, A.; Ceretta, C.A.; Simão, D.G.; Brunetto, G. Black Oat (Avena strigosa Schreb.) Growth and Root Anatomical Changes in Sandy Soil with Different Copper and Phosphorus Concentrations. Water Air Soil Pollut. 2016, 227, 192. [Google Scholar] [CrossRef]
- Somavilla, L.M.; Simão, D.G.; Tiecher, T.L.; Hammerschimitt, R.K.; Oliveira, J.M.S.; Mayer, N.A.; Pavanello, E.P.; Trentin, E.; Belles, S.W.; Brunetto, G. Structural changes in roots of peach rootstock cultivars grown in soil with high zinc content. Sci. Hortic. 2018, 237, 1–10. [Google Scholar] [CrossRef]
- Ricachenevsky, F.K.; De Araújo, J.A.T.; Fett, J.P.; Sperotto, R.A. You shall not pass: Root vacuoles as a symplastic checkpoint for metal translocation to shoots and possible application to grain nutritional quality. Front. Plant Sci. 2018, 9, 412. [Google Scholar] [CrossRef]
- Kabata-Pendias, A. Trace Elements in Soils and Plants; CRC Press: Boca Ratón, FL, USA, 2011. [Google Scholar]
- De Freitas, T.A.; França, M.G.; De Almeida, A.A.; De Oliveira, S.J.; De Jesus, R.M.; Souza, V.L.; Dos Santos Silva, J.V.; Mangabeira, P.A. Morphology, ultrastructure and mineral uptake is affected by copper toxicity in young plants of Inga subnuda subs. luschnathiana (Benth.) T.D. Penn. Environ. Sci. Pollut. Res. Int. 2015, 22, 15479–15494. [Google Scholar] [CrossRef]
- Reichman, S.M. The Responses of Plants to Metal Toxicity: A Review Focusing on Copper, Manganese and Zinc; Environment, Australian Minerals & Energy Environment Foundation: Melbourne, Australia, 2002. [Google Scholar]
- Zanin, L.; Tomasi, M.; Rizzardo, C.; Gottardi, S.; Terzano, R.; Alfeld, M.; Janssens, K.; De Nobili, M.; Mimmo, T.; Cesco, S. Iron allocation in leaves of Fe-deficient cucumber plants fed with natural Fe complexes. Physiol. Plant. 2015, 154, 82–94. [Google Scholar] [CrossRef]
- Pii, Y.; Penn, A.; Terzano, R.; Crecchio, C.; Mimmo, T.; Cesco, S. Plant-microorganism-soil interactions influence the Fe availability in the rhizosphere of cucumber plants. Plant Physiol. Biochem. 2015, 87, 45–52. [Google Scholar] [CrossRef] [PubMed]
- Ambrosini, V.G.; Rosa, D.J.; Bastos, M.G.W.; Zalamena, J.; Cella, C.; Simão, D.G.; Silva, L.S.; Santos, H.P.; Toselli, M.; Tiecher, T.L.; et al. High copper content in vineyard soils promotes modifications in photosynthetic parameters and morphological changes in the root system of ‘Red Niagara’ plantlets. Plant Physiol. Biochem. 2018, 128, 89–98. [Google Scholar] [CrossRef] [PubMed]
- Cambrollé, J.; García, J.L.; Figueroa, M.E.; Cantos, M. Evaluating wild grapevine tolerance to copper toxicity. Chemosphere 2015, 120, 171–178. [Google Scholar] [CrossRef] [PubMed]
- Schwalbert, R.; Silva, L.O.S.; Schwalbert, R.A.; Tarouco, C.P.; Fernandes, G.S.; Marques, A.C.R.; Costa, C.C.; Hammerschmitt, R.K.; Brunetto, G.; Nicoloso, F.T. Physiological responses of soybean (Glycine max (L.) Merrill) cultivars to copper excess. An. Acad. Bras. Ciênc. 2019, 91, e20190121. [Google Scholar] [CrossRef] [PubMed]
- De Conti, L.; Melo, G.W.B.; Ceretta, C.A.; Tarouco, C.; Marques, A.; Nicoloso, F.; Tassinari, A.; Tiecher, T.; Cesco, S.; Mimmo, T.; et al. Photosynthesis and growth of young grapevines intercropped with native grasses in soils contaminated with copper. Acta Hortic. 2018, 1217, 179–184. [Google Scholar] [CrossRef]
- Trentin, E.; Facco, D.B.; Hammerschmitt, R.K.; Ferreira, P.A.A.; Morsch, L.; Belles, S.W.; Ricachenevsky, F.K.; Nicoloso, F.T.; Cerreta, C.A.; Tiecher, T.L.; et al. Potential of vermicompost and limestone in reducing copper toxicity in young grapevines grown in Cu-contaminated vineyard soil. Chemosphere 2019, 226, 421–430. [Google Scholar] [CrossRef] [PubMed]
- Maxwell, K.; Johnson, G.N. Chlorophyll fluorescence—A practical guide. J. Exp. Bot. 2000, 51, 659–668. [Google Scholar] [CrossRef] [PubMed]
- Cambrollé, J.; García, J.L.; Ocete, R.; Figueroa, M.E.; Cantos, M. Growth and photosynthetic responses to copper in wild grapevine. Chemosphere 2013, 93, 294–301. [Google Scholar] [CrossRef]
- Tiecher, T.L.; Tiecher, T.; Ceretta, C.A.; Ferreira, P.A.A.; Nicoloso, F.T.; Soriani, H.H.; Tassinari, A.; Paranhos, J.T.; De Conti, L.; Brunetto, G. Physiological and nutritional status of black oat (Avena strigosa Schreb.) grown in soil with interaction of high doses of copper and zinc. Plant Physiol. Biochem. 2016, 106, 253–263. [Google Scholar] [CrossRef]




| Rootstocks | Treatment | N | P | K | Ca | Mg |
|---|---|---|---|---|---|---|
| Shoots (g kg−1) | ||||||
| Paulsen 1103 | Control | 25.94 A ns(1) | 11.98 A * | 19.52 A * | 24.31 AB * | 6.30 A * |
| Cu | 23.64 a | 6.83 b | 14.29 a | 18.37 b | 4.90 ab | |
| SO4 | Control | 27.75 A * | 12.48 A * | 20.05 A * | 25.60 A * | 6.31 A * |
| Cu | 24.08 a | 9.55 a | 12.31 b | 19.92 a | 5.08 a | |
| IAC 572 | Control | 28.07 A * | 8.23 C * | 19.79 A * | 24.95 AB * | 5.08 B * |
| Cu | 22.88 a | 6.18 b | 14.77 a | 14.82 c | 4.28 b | |
| Isabel | Control | 29.25 A * | 10.36 B * | 17.76 B * | 23.96 B * | 4.85 B * |
| Cu | 25.36 a | 6.94 b | 14.77 a | 14.83 c | 3.52 c | |
| Roots (g kg−1) | ||||||
| Paulsen 1103 | Control | 14.35 B | 6.09 A * | 18.83 C * | 11.42 C ns | 5.11 B * |
| Cu | 20.50 b * | 2.49 b | 9.10 a | 11.40 b | 1.44 a | |
| SO4 | Control | 25.78 AB | 4.97 B * | 28.62 A * | 14.95 B ns | 6.48 A * |
| Cu | 28.40 a * | 3.61 a | 9.09 a | 14.11 a | 2.36 a | |
| IAC 572 | Control | 28.40 A * | 4.61 B * | 29.64 A * | 16.94 A * | 6.29 A * |
| Cu | 23.24 b | 3.11 ab | 9.70 a | 12.07 b | 2.28 a | |
| Isabel | Control | 23.54 B ns | 4.73 B * | 23.42 B * | 13.20 BC ns | 6.02AB * |
| Cu | 22.97 b | 2.90 b | 6.45 a | 11.83 b | 1.98 a | |
| Rootstocks | Treatment | Cu | Zn | Fe | Mn |
|---|---|---|---|---|---|
| Shoots (mg kg−1) | |||||
| Paulsen 1103 | Control | 5.50 B ns(1) | 27.70 A * | 174.83 B ns | 267.16 A * |
| Cu | 6.16 a | 16.28 b | 188.73 ab | 70.37 ab | |
| SO4 | Control | 4.66 B ns | 23.70 B * | 246.93 A * | 198.88 B * |
| Cu | 6.84 a | 11.84 c | 163.33 bc | 87.04 a | |
| IAC 572 | Control | 6.02 B ns | 24.34 AB * | 178.84 B ns | 121.74 D * |
| Cu | 5.46 a | 21.24 a | 199.92 a | 51.70 b | |
| Isabel | Control | 8.38 A * | 22.00 B ns | 132.48 C ns | 153.46 C * |
| Cu | 5.54 a | 22.29 a | 145.81 c | 78.86 a | |
| Roots (mg kg−1) | |||||
| Paulsen 1103 | Control | 51.46 A * | 19.54 C ns | 625.12 B * | 232.20 A ns |
| Cu | 4937.52 c | 24.40 b | 792.07 b | 200.08 b | |
| SO4 | Control | 24.66 A * | 50.26 A * | 664.80 AB * | 260.50 A * |
| Cu | 6377.28 b | 25.42 b | 931.87 a | 196.90 b | |
| IAC 572 | Control | 35.62 A * | 37.04 B ns | 725.52 A * | 250.78 A * |
| Cu | 8540.28 a | 35.90 a | 244.40 c | 344.20 a | |
| Isabel | Control | 31.28 A * | 38.98 B * | 677.36 AB * | 231.04 A ns |
| Cu | 8933.40 a | 30.04 ab | 294.32 c | 236.54 b | |
| Rootstocks | Treatment | TF |
|---|---|---|
| Paulsen 1103 | Control | 0.095 D *(1) |
| Cu | 0.001 a | |
| SO4 | Control | 0.207 B * |
| Cu | 0.001 a | |
| IAC 572 | Control | 0.175 C * |
| Cu | 0.000 a | |
| Isabel | Control | 0.226 A * |
| Cu | 0.01 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Trentin, E.; Ferreira, P.A.A.; Ricachenevsky, F.K.; Morsch, L.; Belles, S.W.; Hindersmann, J.; Tarouco, C.P.; Nicoloso, F.T.; Berghetti, Á.L.P.; Stefanello da Silva, L.O.; et al. Identifying Grapevine Rootstocks Tolerant to Copper Excess. Horticulturae 2024, 10, 883. https://doi.org/10.3390/horticulturae10080883
Trentin E, Ferreira PAA, Ricachenevsky FK, Morsch L, Belles SW, Hindersmann J, Tarouco CP, Nicoloso FT, Berghetti ÁLP, Stefanello da Silva LO, et al. Identifying Grapevine Rootstocks Tolerant to Copper Excess. Horticulturae. 2024; 10(8):883. https://doi.org/10.3390/horticulturae10080883
Chicago/Turabian StyleTrentin, Edicarla, Paulo Ademar Avelar Ferreira, Felipe Klein Ricachenevsky, Letícia Morsch, Simoni Weide Belles, Jacson Hindersmann, Camila Peligrinotti Tarouco, Fernando Teixeira Nicoloso, Álvaro Luís Pasquetti Berghetti, Lincon Oliveira Stefanello da Silva, and et al. 2024. "Identifying Grapevine Rootstocks Tolerant to Copper Excess" Horticulturae 10, no. 8: 883. https://doi.org/10.3390/horticulturae10080883
APA StyleTrentin, E., Ferreira, P. A. A., Ricachenevsky, F. K., Morsch, L., Belles, S. W., Hindersmann, J., Tarouco, C. P., Nicoloso, F. T., Berghetti, Á. L. P., Stefanello da Silva, L. O., Schwalbert, R., Santos, H. P. d., Melo, G. W. B. d., & Brunetto, G. (2024). Identifying Grapevine Rootstocks Tolerant to Copper Excess. Horticulturae, 10(8), 883. https://doi.org/10.3390/horticulturae10080883








