Nitrous Oxide Treatment after Pollination Induces Ploidy Changes in Statice (Limonium sp.)
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material and Cultivation
2.2. Experimental Design
2.3. In Vitro Ovule Culture
2.4. Morphological Evaluation of L. sinuatum and L. perezii
2.4.1. Floral Characteristics
2.4.2. Vegetative Characteristics
2.5. Determination of Ploidy Level
2.6. Statistical Analysis
3. Results
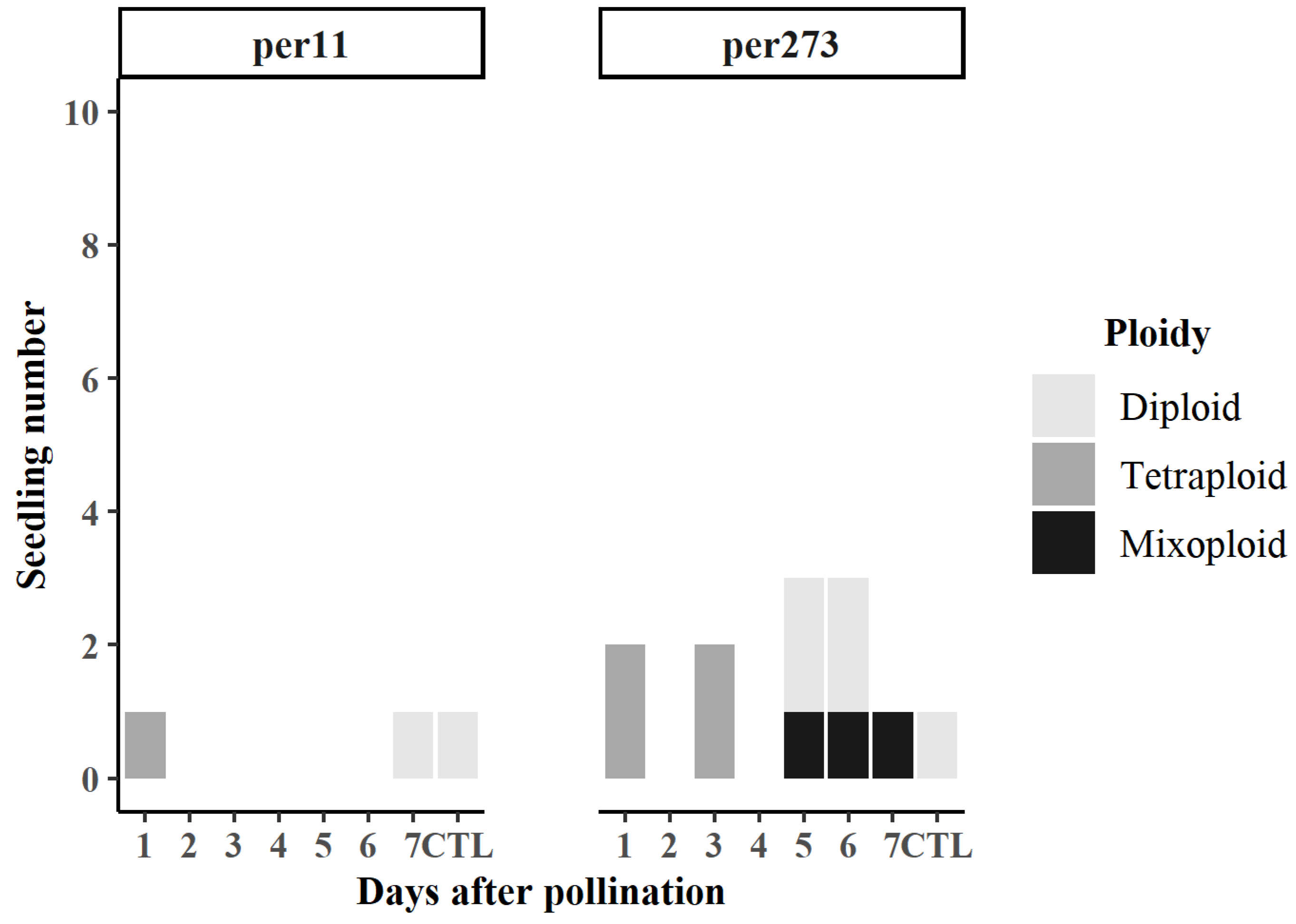
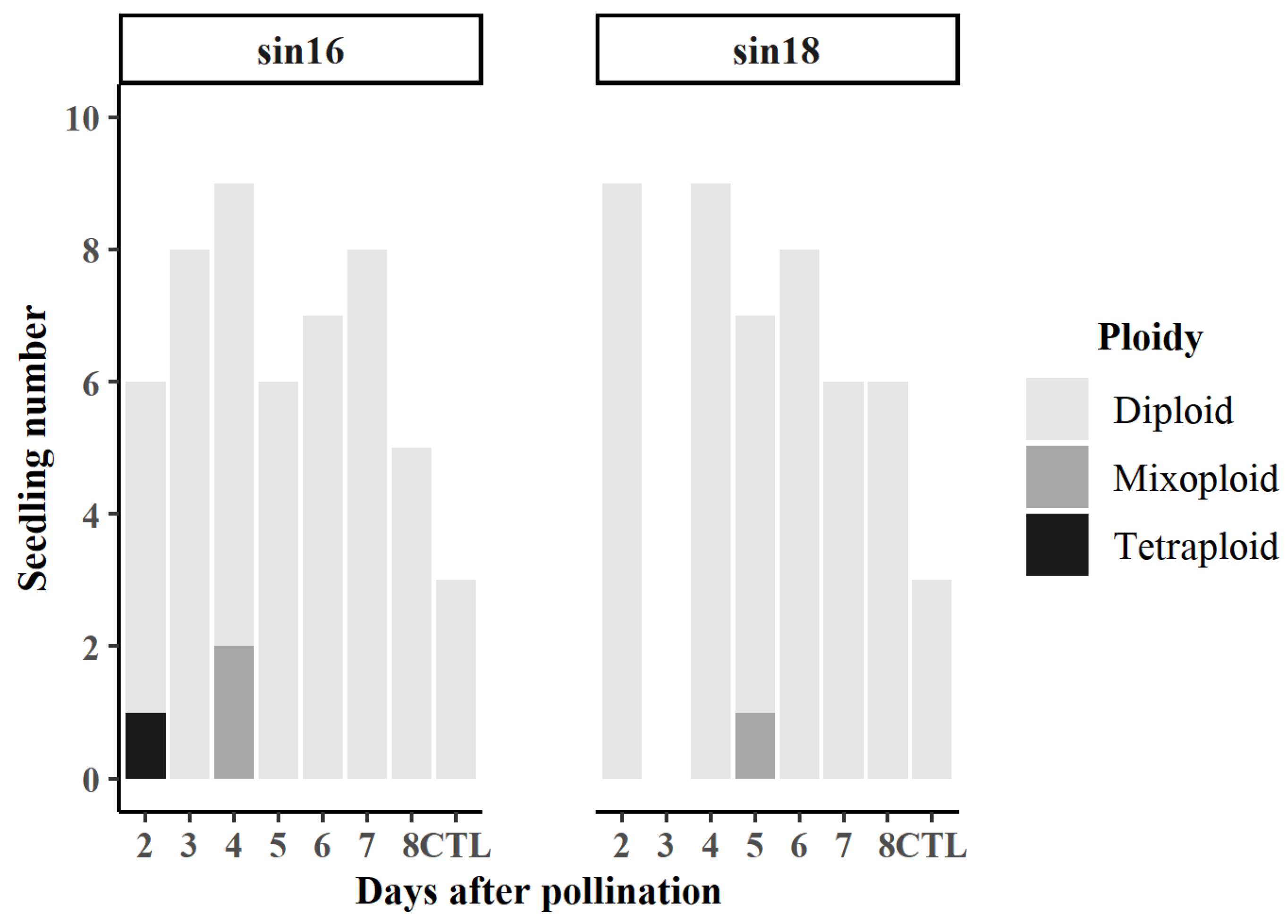
3.1. Seedlings Obtained
3.2. Effect of N2O Treatment on Ploidy
3.3. Morphological Evaluation
3.3.1. Floral Characteristics
3.3.2. Vegetative Characteristics
4. Discussion
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- International Association of Horticultural Producers (Ed.) International Statistics Flowers and Plants 2022; International Association of Horticultural Producers (AIPH): Hannover, Germany, 2022; Volume 70, p. 232. [Google Scholar]
- Sato, K.; Koreeda, K.; Takanashi, N. Variety of Limonium named ‘Ocean Blue’. U.S. Patent 19,930,072,716, 7 June 1993. [Google Scholar]
- RioRoses. Ball SB Introduces Limonium siNZii. Available online: https://www.rioroses.com/ball-sb-introduces-limonium-sinzii/ (accessed on 20 July 2024).
- Morgan, E.; Burge, G.; Seelye, J. Limonium breeding: New options for a well known genus. Acta Hortic. Sin. 2001, 552, 39–42. [Google Scholar] [CrossRef]
- Van de Peer, Y.; Mizrachi, E.; Marchal, K. The evolutionary significance of polyploidy. Nat. Rev. Genet. 2017, 18, 411–424. [Google Scholar] [CrossRef]
- Chansler, M.T.; Ferguson, C.J.; Fehlberg, S.D.; Prather, L.A. The role of polyploidy in shaping morphological diversity in natural populations of Phlox amabilis. Am. J. Bot. 2016, 103, 1546–1558. [Google Scholar] [CrossRef]
- Kumar, A.; Yadav, P. Induced polyploidization in Brassica fruticulosa—A wild relative of brassicas as potential source for mustard aphid tolerance. NEHU J. 2020, XVIII, 1–11. [Google Scholar]
- Bhattarai, K.; Kareem, A.; Deng, Z. In vivo induction and characterization of polyploids in gerbera daisy. Sci. Hortic. 2021, 282, 110054. [Google Scholar] [CrossRef]
- Birchler, J.; Veitia, R. Gene balance hypothesis: Connecting issues of dosage sensitivity across biological disciplines. Proc. Natl. Acad. Sci. USA 2012, 109, 14746–14753. [Google Scholar] [CrossRef] [PubMed]
- Róis, A.; Teixeira, G.; Sharbel, T.; Fuchs, J.; Martins, S.; Espírito-Santo, D.; Caperta, A. Male fertility versus sterility, cytotype, and DNA quantitative variation in seed production in diploid and tetraploid sea lavenders (Limonium sp., Plumbaginaceae) reveal diversity in reproduction modes. Plant Reprod. 2012, 25, 305–318. [Google Scholar] [CrossRef] [PubMed]
- Mori, S.; Yamane, T.; Yahata, M.; Shinoda, K.; Murata, N. Chromosome doubling in Limonium bellidifolium (Gouan) Dumort. by colchicine treatment of seeds. Hortic. J. 2016, 85, 366–371. [Google Scholar] [CrossRef]
- Gordej, I.S.; Lyusikov, O.M.; Gordej, I.A. Zygotic autopolyploidization of rye (Secale cereale L.). Cytol. Genet. 2019, 53, 357–362. [Google Scholar] [CrossRef]
- Mori, S.; Yahata, M.; Kuwahara, A.; Shirono, Y.; Ueno, Y.; Hatanaka, M.; Honda, Y.; Sugiyama, K.; Murata, N.; Okamoto, Y. Morphological characterization of tetraploids of Limonium sinuatum (L.) Mill. produced by oryzalin treatment of seeds. Horticulturae 2021, 7, 248. [Google Scholar] [CrossRef]
- Molenaar, W.S.; Schipprack, W.; Melchinger, A.E. Nitrous oxide-induced chromosome doubling of maize haploids. Crop Sci. 2018, 58, 650–659. [Google Scholar] [CrossRef]
- Zhang, L.; Wu, S.; Chang, X.; Wang, X.; Zhao, Y.; Xia, Y.; Trigiano, R.N.; Jiao, Y.; Chen, F. The ancient wave of polyploidization events in flowering plants and their facilitated adaptation to environmental stress. Plant Cell Environ. 2020, 43, 2847–2856. [Google Scholar] [CrossRef] [PubMed]
- Brullo, S.; Pavone, P. Chromosome numbers in the sicilian species of Limonium Miller (Plumbaginaceae). An. Jard. Bot. Madr. 1981, 37, 535–555. [Google Scholar]
- Hansen, F.L.; Andersen, S.B.; Due, I.K.; Olesen, A. Nitrous oxide as a possible alternative agent for chromosome doubling of wheat haploids. Plant Sci. 1988, 54, 219–222. [Google Scholar] [CrossRef]
- Ostergren, G. Production of polyploids and aneuploids of Phalaris by means of nitrous oxide. Hereditas 1957, 43, 512–516. [Google Scholar] [CrossRef]
- Sato, T.; Miyoshi, K.; Okazaki, K. Induction of 2n gametes and 4n embryo in Lilium (Lilium × formolongi hort.) by nitrous oxide gas treatment. Acta Hortic. 2010, 855, 243–248. [Google Scholar] [CrossRef]
- Siregar, A. Initial Exploration of Unreduced Gamete (2n Gamete) Pollen Development through Nitrous Oxide (N2O) Application in Limonium sinuatum. MSc Thesis, Massey University, Palmerston North, New Zealand, 2021. [Google Scholar]
- Kitamura, S.; Akutsu, M.; Okazaki, K. Mechanism of action of nitrous oxide gas applied as a polyploidizing agent during meiosis in lilies. Plant Reprod. 2009, 22, 9–14. [Google Scholar] [CrossRef] [PubMed]
- Dewitte, A.; Van Laere, K.; Van Huylenbroeck, J. Use of 2n gametes in plant breeding. Plant Breed. 2012, 11, 59–86. [Google Scholar] [CrossRef]
- Kato, A.; Geiger, H. Chromosome doubling of haploid maize seedling using nitrous oxide gas at the flower primordial stage. Plant Breed. 2002, 121, 370–377. [Google Scholar] [CrossRef]
- Nukui, S.; Kitamura, S.; Hioki, T.; Ootsuka, H.; Miyoshi, K.; Satou, T.; Takatori, Y.; Oomiya, T.; Okazaki, K. N2O induces mitotic polyploidization in anther somatic cells and restores fertility in sterile interspecific hybrid lilies. Breed. Sci. 2011, 61, 327–337. [Google Scholar] [CrossRef]
- Gooh, K.; Ueda, M.; Aruga, K.; Park, J.; Arata, H.; Higashiyama, T.; Kurihara, D. Live-cell imaging and optical manipulation of Arabidopsis early embryogenesis. Dev. Cell 2015, 34, 242–251. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Ye, T.; Han, C.; Ye, Z.; Zhang, J.; Xiao, S.; Yuan, D. Cytogenetic analysis of interspecific hybridization in oil-tea (Camellia oleifera). Euphytica 2021, 217, 28. [Google Scholar] [CrossRef]
- Deepo, D.M.; Islam, M.M.; Yesmin, R.; Lee, H.-M.; Kim, H.-Y.; Kim, C.-K.; Lim, K.-B. Karyotype and nuclear DNA content analyses of Korean native Hibiscus ‘Gangneung’ and ‘Baekryungdo’ using fluorescence in situ hybridization and flow cytometry. Hortic. Environ. Biotechnol. 2023, 64, 143–152. [Google Scholar] [CrossRef]
- Baker, H. Dimorphism and monomorphism in the plumbaginaceae: II. Pollen and stigmata in the genus Limonium. Ann. Bot. 1953, 17, 433–445. [Google Scholar] [CrossRef]
- Barba-Gonzalez, R.; Miller, C.T.; Ramanna, M.; van Tuyl, J.M. Nitrous oxide (N2O) induces 2n gametes in sterile F1 hybrids between Oriental × Asiatic lily (Lilium) hybrids and leads to intergenomic recombination. Euphytica 2006, 148, 303–309. [Google Scholar] [CrossRef]
- Karis, P.O. Taxonomy, phylogeny and biogeography of Limonium sect. Pteroclados (Plumbaginaceae), based on morphological data. Bot. J. Linn. Soc. 2004, 144, 461–482. [Google Scholar] [CrossRef]
- Murashige, T.; Skoog, F. A revised medium for rapid growth and bio assays with tobacco tissue cultures. Physiol. Plant. 1962, 15, 473–497. [Google Scholar] [CrossRef]
- Gamborg, O.L.; Miller, R.A.; Ojima, K. Nutrient requirements of suspension cultures of soybean root cells. Exp. Cell Res. 1968, 50, 151–158. [Google Scholar] [CrossRef]
- Morgan, E.; Burge, G.; Seelye, J.; Hopping, M.; Grant, J. Production of inter-specific hybrids between Limonium perezii (Stapf) Hubb. and Limonium sinuatum (L.) Mill. Euphytica 1998, 102, 109–115. [Google Scholar] [CrossRef]
- Hammer, N.; Löffler, S.; Feja, C.; Bechmann, I.; Steinke, H. Substitution of formaldehyde in cross anatomy is possible. J. Natl. Cancer Inst. 2011, 103, 610–611. [Google Scholar] [CrossRef]
- Hammer, N.; Löffler, S.; Bechmann, I.; Steinke, H.; Hädrich, C.; Feja, C. Comparison of modified thiel embalming and ethanol-glycerin fixation in an anatomy environment: Potentials and limitations of two complementary techniques. Anat. Sci. Educ. 2015, 8, 74–85. [Google Scholar] [CrossRef] [PubMed]
- Peterson, R.; Slovin, J.; Chen, C. A simplified method for differential staining of aborted and non-aborted pollen grains. Int. J. Plant Sci. 2010, 1, e13. [Google Scholar] [CrossRef]
- Carl Zeiss Microscopy GmbH Zen 2.6 (Blue Edition), Version 2.6.76.00000. Germany. 2018. Available online: https://www.micro-shop.zeiss.com/en/us/softwarefinder/software-categories/zen-blue/ (accessed on 20 July 2024).
- Schindelin, J.; Arganda-Carreras, I.; Frise, E.; Kaynig, V.; Longair, M.; Pietzsch, T.; Preibisch, S.; Rueden, C.; Saalfeld, S.; Schmid, B.; et al. Fiji: An open-source platform for biological-image analysis. Nat. Methods 2012, 9, 676–682. [Google Scholar] [CrossRef] [PubMed]
- Cohen, D.; Yao, J.-L. In vitro chromosome doubling of nine Zantedeschia cultivars. Plant Cell Tissue Organ Cult. 1996, 47, 43–49. [Google Scholar] [CrossRef]
- Otto, F. DAPI staining of fixed cells for high-resolution flow cytometry of nuclear DNA. In Methods in Cell Biology; Darzynkiewicz, Z., Crissman, H.A., Eds.; Academic Press: Cambridge, MA, USA, 1990; Volume 33, pp. 105–110. [Google Scholar]
- R Core Team. A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Viena, Austria, 2018. [Google Scholar]
- Wickham, H. Ggplot2: Elegant Graphics for Data Analysis, 1st ed.; Springer: New York, NY, USA, 2016; p. 213. [Google Scholar]
- Lyman, R.; Longnecker, M. An Introduction to Statistical Methods and Data Analysis, 5th ed.; Duxbury Press: Duxbury, MA, USA, 2001. [Google Scholar]
- Goedhart, P. Lrpair, 19th ed.; Wageningen University & Research: Wageningen, The Netherlands, 2018; pp. 97–98. [Google Scholar]
- Sebastien, L.; Josse, J.; Husson, F. Factominer: An R package for multivariate analysis. J. Stat. Softw. 2008, 25, 1–18. [Google Scholar] [CrossRef]
- Kassambara, A.; Fabian Mundt, F. Factoextra: Extract and Visualize the Results of Multivariate Data Analyses. 2020. Available online: http://www.sthda.com/english/rpkgs/factoextra (accessed on 20 July 2024).
- ten Hove, C.A.; Lu, K.-J.; Weijers, D. Building a plant: Cell fate specification in the early Arabidopsis embryo. Development 2015, 142, 420–430. [Google Scholar] [CrossRef]

| Genotype | Ploidy | Pollen Plan Area (µm2) z |
|---|---|---|
| sin15 (parent) | Diploid | 2200 ± 212 b |
| sin16 (parent) | Diploid | 1811 ± 274 c |
| sin191 (progeny) | Diploid | 1946 ± 180 c |
| sin192 (progeny) | Diploid | 1854 ± 363 c |
| sin193 (progeny) | Tetraploid | 2551 ± 407 a |
| sin194 (progeny) | Tetraploid | 2597 ± 353 a |
| sin201 (progeny) | Tetraploid | 2670 ± 329 a |
| sin202 (progeny) | Tetraploid | 2439 ± 336 a |
| sin203 (progeny) | Tetraploid | 2437 ± 276 a |
| sin204 (progeny) | Tetraploid | 2543 ± 528 a |
| Genotype | Ploidy | Guard Cell Length (µm) z | Stomatal Density x y |
|---|---|---|---|
| sin15 (parent) | Diploid | 23.8 ± 4.1 e | 2.2 ± 1.4 a |
| sin16 (parent) | Diploid | 24.0 ± 4.9 de | 2.3 ± 1.3 a |
| sin191 (progeny) | Diploid | 26.3 ± 3.2 e | 1.8 ± 1.0 b |
| sin192 (progeny) | Diploid | 30.3 ± 3.7 d | 1.3 ± 0.4 c |
| sin193 (progeny) | Tetraploid | 31.2 ± 4.6 c | 1.7 ± 1.6 c |
| sin194 (progeny) | Tetraploid | 33.7 ± 3.9 c | 1.4 ± 0.6 e |
| sin201 (progeny) | Tetraploid | 42.8 ± 5.8 b | 1.0 ± 0.6 f |
| sin202 (progeny) | Tetraploid | 38.2 ± 5.3 c | 1.3 ± 1.1 g |
| sin203 (progeny) | Tetraploid | 47.5 ± 5.6 a | 1.1 ± 1.0 h |
| sin204 (progeny) | Tetraploid | 45.1 ± 4.8 b | 1.0 ± 0.9 g |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cordoba-Sanchez, J.; Funnell, K.; Hedderley, D.; Roskruge, N.; Morgan, E. Nitrous Oxide Treatment after Pollination Induces Ploidy Changes in Statice (Limonium sp.). Horticulturae 2024, 10, 816. https://doi.org/10.3390/horticulturae10080816
Cordoba-Sanchez J, Funnell K, Hedderley D, Roskruge N, Morgan E. Nitrous Oxide Treatment after Pollination Induces Ploidy Changes in Statice (Limonium sp.). Horticulturae. 2024; 10(8):816. https://doi.org/10.3390/horticulturae10080816
Chicago/Turabian StyleCordoba-Sanchez, Juana, Keith Funnell, Duncan Hedderley, Nick Roskruge, and Ed Morgan. 2024. "Nitrous Oxide Treatment after Pollination Induces Ploidy Changes in Statice (Limonium sp.)" Horticulturae 10, no. 8: 816. https://doi.org/10.3390/horticulturae10080816
APA StyleCordoba-Sanchez, J., Funnell, K., Hedderley, D., Roskruge, N., & Morgan, E. (2024). Nitrous Oxide Treatment after Pollination Induces Ploidy Changes in Statice (Limonium sp.). Horticulturae, 10(8), 816. https://doi.org/10.3390/horticulturae10080816






