Castor Meal and Ground Hydrothermalized Phonolite Optimize Sweet Potato Nutrition, Yield, and Quality
Abstract
1. Introduction
2. Materials and Methods
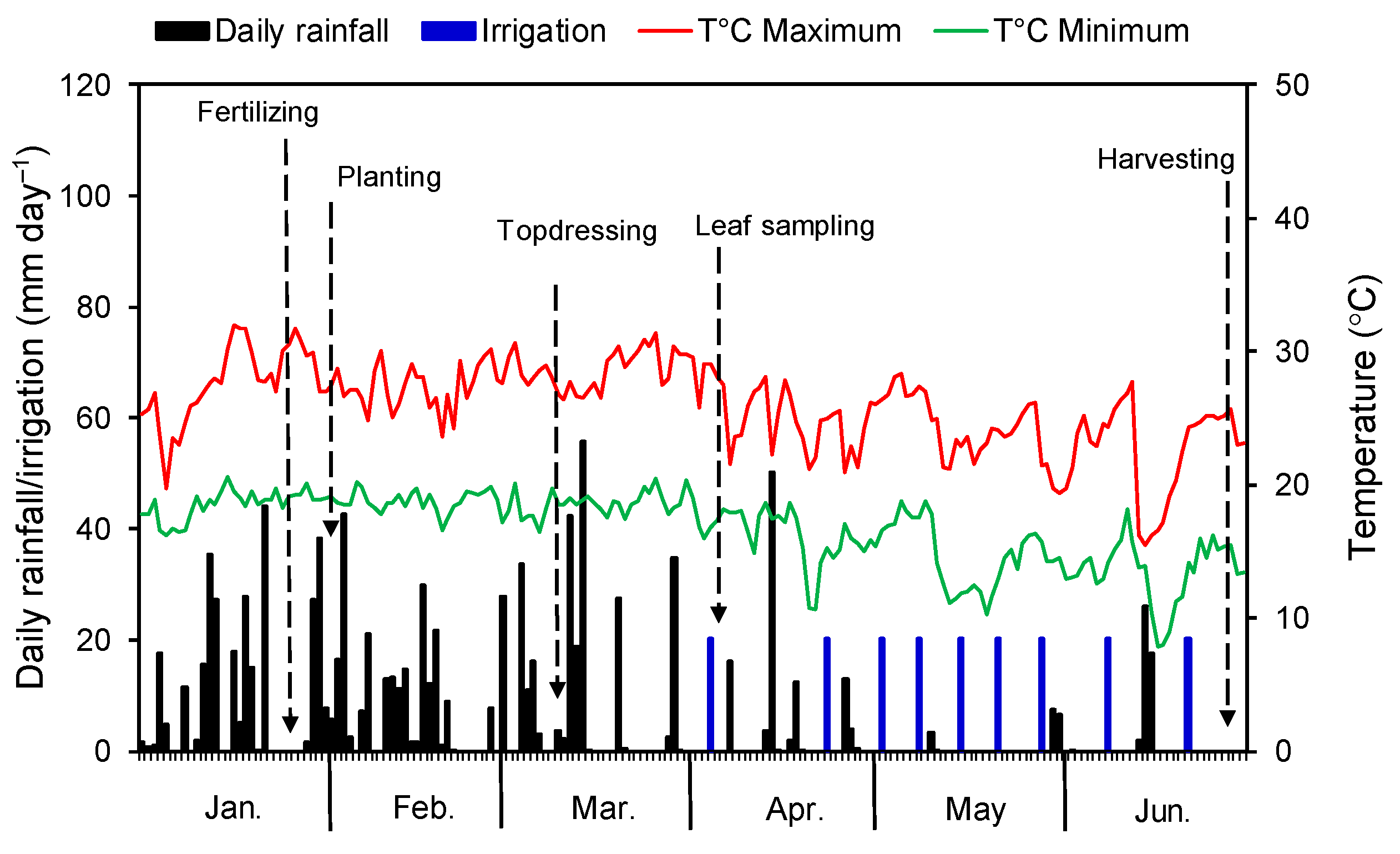
2.1. Site Characteristics and Climate
2.2. Experimental Design, Treatments, and Crop Management
2.3. Sampling and Analyses
2.3.1. Leaf Nutrient Concentrations
2.3.2. Soil Health Indices
2.3.3. Storage Root Yield and Sorting
2.3.4. Storage Root Dry Matter Content
2.3.5. Nutrient Concentration and Removal in the Storage Roots
2.3.6. Texture Properties and Soluble Solids of Storage Roots
2.3.7. Starch, Reducing Sugar, Total Sugar, and Crude Fiber Contents in the Storage Roots
2.4. Statistical Analyses
3. Results
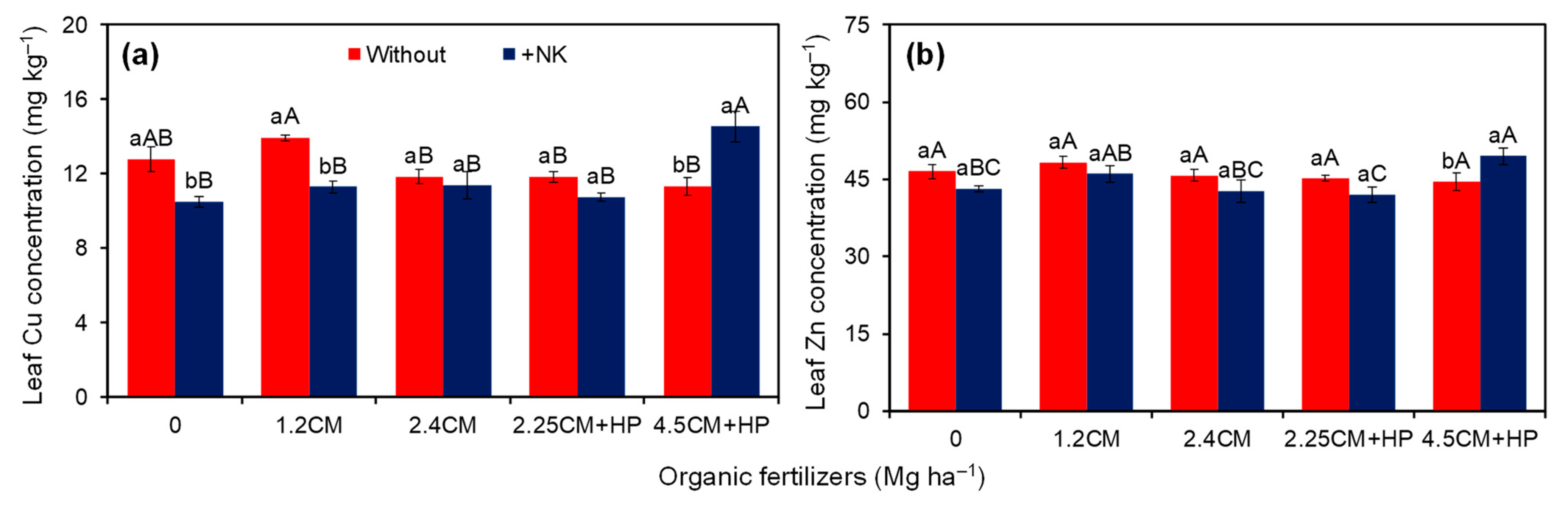
3.1. Leaf Nutrient Concentrations
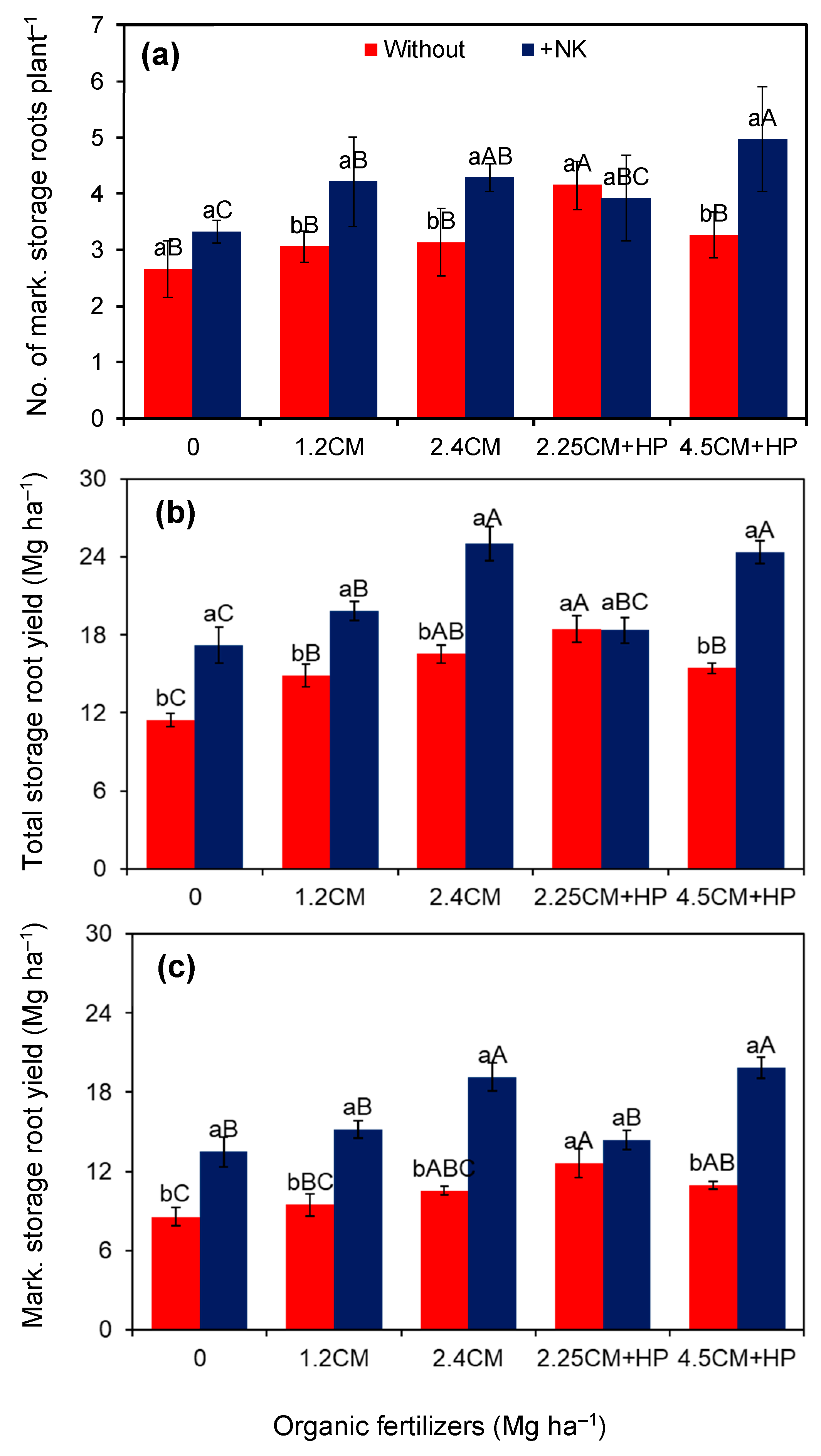
3.2. Storage Root Yield and Chemical Composition
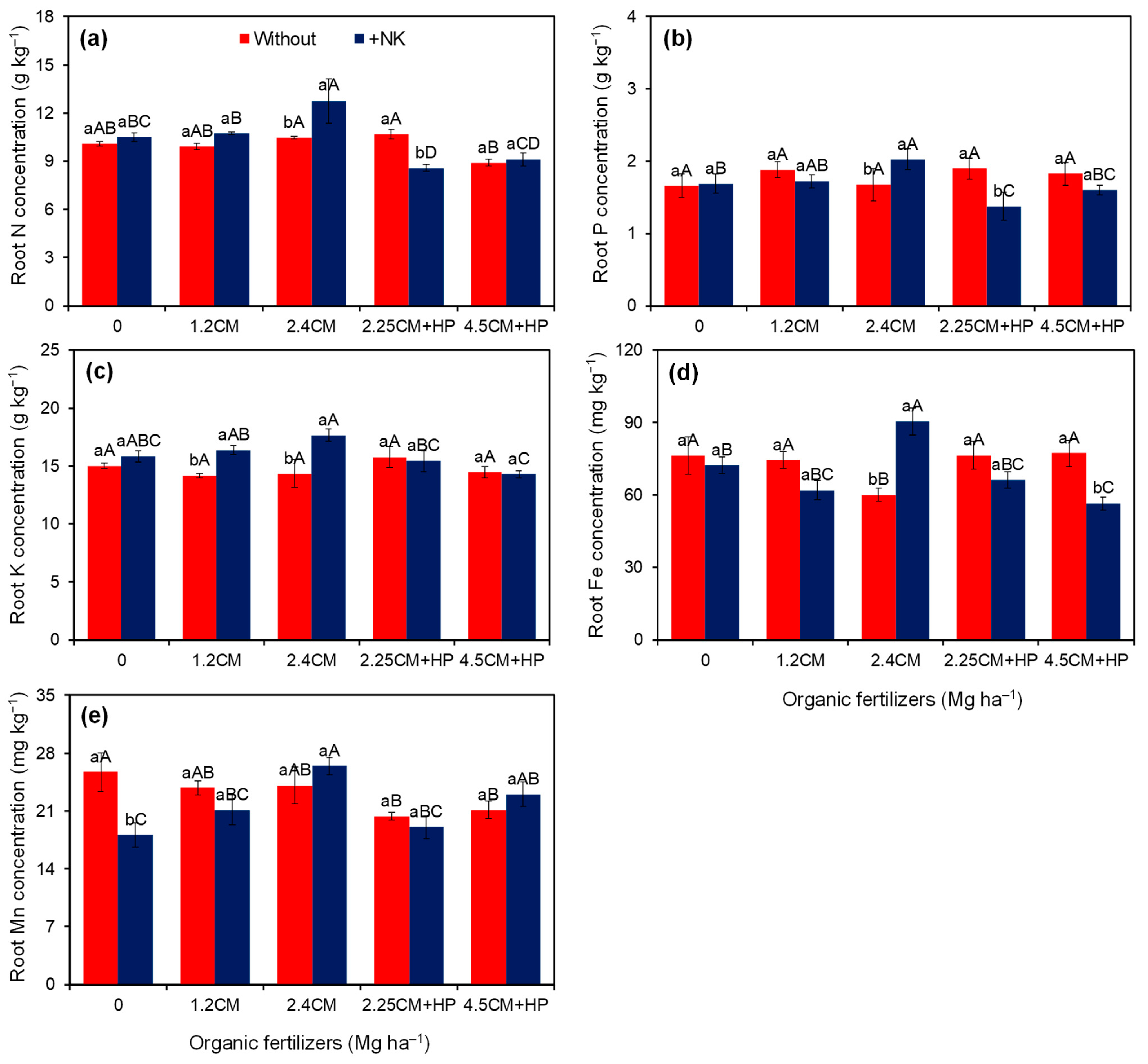
3.3. Concentration and Removal of Nutrients in the Storage Roots
3.4. Soil Health Indices
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Truong, V.D.; Avula, R.Y.; Pecota, K.V.; Yencho, G.C. Sweetpotato Production, Processing, and Nutritional Quality. In Handbook of Vegetables and Vegetable Processing, 2nd ed.; Siddiq, M., Uebersax, M.A., Eds.; Vol. II. John Wiley & Sons Ltd.: Hoboken, NJ, USA, 2018. [Google Scholar]
- Kakabouki, I.; Tataridas, A.; Mavroeidis, A.; Kousta, A.; Roussis, I.; Katsenios, N.; Efthimiadou, A.; Papastylianou, P. Introduction of alternative crops in the Mediterranean to satisfy EU Green Deal goals. A review. Agron. Sustain. Dev. 2021, 41, 71. [Google Scholar] [CrossRef]
- Bechoff, A.; Sindi, K.; Low, J.; Ndyetabula, D. Everything You Ever Wanted to Know about Sweetpotato: Reaching Agents of Change tot Manual; International potato Center: Nairobi, Kenya, 2013. [Google Scholar]
- CIP—International Potato Center. Facts and Figures about Sweetpotato. 2010. Available online: https://cipotato.org/crops/sweetpotato/ (accessed on 15 January 2024).
- Ahn, Y.O.; Sun, H.K.; Kim, C.Y.; Lee, J.S. Exogenous sucrose utilization and starch biosynthesis among sweetpotato cultivars. Carbohyd. Res. 2010, 345, 55–60. [Google Scholar] [CrossRef]
- Gonçalves Neto, A.C.; Maluf, W.R.; Gomes, L.A.A.; Gonçalves, R.J.S.; Silva, V.F.; Lasmar, A. Aptidões de genótipos de batata-doce para consumo humano, produção de etanol e alimentação animal. Pesqu. Agropec. Bras. 2011, 46, 1513–1520. [Google Scholar] [CrossRef][Green Version]
- Geng, J.; Zhao, Q.; Li, Z.; Yang, X.; Lei, S.; Zhang, Q.; Li, H.; Lang, Y.; Huo, X.; Liu, Q. Effects of various potassium fertilizer dosages on agronomic and economic assessment of sweet potato fields. Horticulturae 2024, 10, 44. [Google Scholar] [CrossRef]
- Thiele, G.; Khan, A.; Heider, B.; Kroschel, J.; Harahagazwe, D.; Andrade, M.; Bonierbale, M.; Friedmann, M.; Gemenet, D.; Cherinet, M.; et al. Roots, tubers and bananas: Planning and research for climate resilience. Open Agric. 2017, 2, 350–361. [Google Scholar] [CrossRef]
- FAO—Food and Agriculture Organization of the United Nations. Food and Agriculture Data. Production-Crops; FAO: Rome, Italy, 2024; Available online: https://www.fao.org/faostat/en/#data/QCL (accessed on 15 January 2024).
- Filgueira, F.A.R. Novo Manual de Olericultura: Agrotecnologia Moderna na Produção e Comercialização de Hortaliças; UFV: Viçosa, Brazil, 2008. [Google Scholar]
- Echer, F.R.; Dominato, J.C.; Creste, J.E. Absorção de nutrientes e distribuição da massa fresca e seca entre órgãos de batata-doce. Hortic. Bras. 2009, 27, 176–182. [Google Scholar] [CrossRef]
- Foloni, J.S.S.; Corte, A.J.; Corte, J.R.N.; Echer, F.R.; Tiritan, C.S. Adubação de cobertura na batata-doce com doses combinadas de nitrogênio e potássio. Semin.-Cienc. Agrar. 2013, 34, 117–126. [Google Scholar] [CrossRef][Green Version]
- Fernandes, A.M.; Campos, L.G.; Senna, M.S.; Silva, C.L.; Assunção, N.S. Yield and nitrogen use efficiency of sweet potato in response to cover crop and nitrogen management. Agron. J. 2018, 110, 2004–2015. [Google Scholar] [CrossRef]
- Minemba, D.; Gleeson, D.B.; Veneklaas, E.; Ryan, M.H. Variation in morphological and physiological root traits and organic acid exudation of three sweet potato (Ipomoea batatas) cultivars under seven phosphorus levels. Sci. Hort. 2019, 256, 108572. [Google Scholar] [CrossRef]
- Brito, C.H.D.; Oliveira, A.P.D.U.; Alves, A.U.; Dorneles, C.S.; Santos, J.F.; Nóbrega, J.P. Produtividade da batata-doce em função das doses de K2O em solo arenoso. Hort. Bras. 2006, 24, 320–323. [Google Scholar] [CrossRef]
- Thumé, M.A.; Dias, L.E.; Silveira, M.A.D.; Assis, I.R.D. Níveis críticos foliares de nutrientes de três cultivares de batata-doce, selecionados para a produção de etanol. Rev. Ceres 2013, 60, 863–875. [Google Scholar] [CrossRef]
- Fernandes, A.M.; Assunção, N.S.; Ribeiro, N.P.; Gazola, B.; Silva, R.M. Nutrient uptake and removal by sweet potato fertilized with green manure and nitrogen on sandy soil. Rev. Bras. Cienc. Solo 2020, 44, e0190127. [Google Scholar] [CrossRef]
- Silva, J.B.C.; Lopes, C.A.; Magalhães, J.S. Cultura da batata-doce. In Agricultura: Tuberosas amiláceas Latino Americanas; Cereda, M.P., Ed.; Fundação Cargill: São Paulo, Brazil, 2002; Volume 2, pp. 448–504. [Google Scholar]
- Duan, W.; Zhang, H.; Xie, B.; Wang, B.; Zhang, L. Impacts of nitrogen fertilization rate on the root yield, starch yield and starch physicochemical properties of the sweet potato cultivar Jishu 25. PLoS ONE 2019, 14, e0221351. [Google Scholar] [CrossRef]
- Dong, H.T.; Li, Y.; Henderson, C.; Brown, P.; Xu, C.-Y. Optimum nitrogen application promotes sweetpotato storage root initiation. Horticulturae 2022, 8, 710. [Google Scholar] [CrossRef]
- Cantarella, H. Nitrogênio. In Fertilidade do Solo, 1st ed.; Novais, F.R., Alvarez, V.V.H., Barros, N.F., Fontes, R.L.F., Cantarutti, R.B., Neves, J.C.L., Eds.; Sociedade Brasileira de Ciência do Solo: Viçosa, Brazil, 2007; pp. 375–470. [Google Scholar]
- Rao, B.R.R. Biomass and essential oil yields of rainfed palmarosa (Cymbopogon martinii (roxb.) Wats.Var. motia Burk.) supplied with different levels of organic manure and fertilizer nitrogen in semi-arid tropical climate. Ind. Crops Prod. 2001, 14, 171–178. [Google Scholar] [CrossRef]
- Rodrigues, A.C.; Cavalcante, L.F.; Oliveira, A.P.; Sousa, J.T.; Mesquita, F.O. Produção e nutrição mineral do maracujazeiro-amarelo em solo com biofertilizante supermagro e potássio. Rev. Bras. Eng. Agríc. Amb. 2009, 13, 117–124. [Google Scholar] [CrossRef]
- Silva, S.D.; Presotto, R.A.; Marota, H.B.; Zonta, E. Uso de torta de mamona como fertilizante orgânico. Pesqu. Agropec. Trop. 2012, 42, 19–27. [Google Scholar] [CrossRef][Green Version]
- Ming, L.C.; Ferreira, M.I.; Gonçalves, G.G. Pesquisas agronômicas das plantas medicinais da Mata Atlântica regulamentadas pela ANVISA. Rev. Bras. Plantas Med. 2012, 14, 131–137. [Google Scholar] [CrossRef]
- Gitari, H.I.; Gachene, C.K.K.; Karanja, N.N.; Kamau, S.; Nyawade, S.; Schulte-Geldermann, E. Potato-legume intercropping on a sloping terrain and its effects on soil physico-chemical properties. Plant Soil 2019, 438, 447–460. [Google Scholar] [CrossRef]
- Lima, R.L.S.; Severino, L.S.; Sampaio, L.R.; Sofiatti, V.; Gomes, J.A.; Beltrão, N.E.M. Blends of castor meal and castor husks for optimized use as organic fertilizer. Ind. Crops Prod. 2011, 33, 364–368. [Google Scholar] [CrossRef]
- Silva, P.N.L.; Lanna, N.B.L.; Cardoso, A.I.I. Produção de beterraba em função de doses de torta de mamona em cobertura. Hort. Bras. 2016, 34, 416–421. [Google Scholar] [CrossRef][Green Version]
- Ramesha, Y.M.; Manjunatha, B.; Krishnamurthy, D. Effect of castor de-oiled cake and inorganic fertilizers on growth, yield and economics of rice (Oryza Sativa L.). Acta Sci. Agric. 2017, 1, 11–15. [Google Scholar]
- Mello, G.A.B.; Carvalho, D.F.; Medici, L.O.; Silva, A.C.; Gomes, D.P.; Pinto, M.F. Organic cultivation of onion under castor cake fertilization and irrigation depths. Acta Sci. Agron. 2018, 40, e34993. [Google Scholar] [CrossRef]
- Severino, L.S.; Auld, D.L.; Baldanzi, M.; Cândido, M.J.D.; Chen, G.; Crosby, W.; Tan, D.; He, X.; Lakshmamma, P.; Lavanya, C.; et al. A review on the challenges for increased production of castor. Agron. J. 2012, 104, 853–880. [Google Scholar] [CrossRef]
- Zapata, N.; Vargas, M.; Reyes, J.F.; Belmar, G. Quality of biodiesel and press cake obtained from Euphorbia lathyris, Brassica napus and Ricinus communis. Ind. Crops Prod. 2012, 38, 1–5. [Google Scholar] [CrossRef]
- Nogueira, T.A.R.; Miranda, B.G.; Jalal, A.; Lessa, L.G.F.; Filho, M.C.M.T.; Marcante, N.C.; Abreu-Junior, C.H.; Jani, A.D.; Capra, G.F.; Moreira, A.; et al. Nepheline Syenite and Phonolite as Alternative Potassium Sources for Maize. Agronomy 2021, 11, 1385. [Google Scholar] [CrossRef]
- Soratto, R.P.; Crusciol, C.A.C.; Campos, M.; Costa, C.H.M.; Gilabel, A.P.; Castro, G.S.A.; Ferrari Neto, J. Silicate rocks as an alternative potassium fertilizer for upland rice and common bean crops. Pesqu. Agropec. Bras. 2021, 56, e01411. [Google Scholar] [CrossRef]
- Soratto, R.P.; Crusciol, C.A.C.; Campos, M.; Gilabel, A.P.; Costa, C.H.M.; Castro, G.S.A.; Ferrari Neto, J. Efficiency and residual effect of alternative potassium sources in grain crops. Pesqu. Agropec. Bras. 2021, 56, e02686. [Google Scholar] [CrossRef]
- Mancuso, M.A.C.; Soratto, R.P.; Crusciol, C.A.C.; Castro, G.S.A. Effect of potassium sources and rates on Arabica coffee yield, nutrition and macronutrient export. Rev. Bras. Cienc. Solo 2014, 38, 1448–1456. [Google Scholar] [CrossRef]
- Martins, V.; Silva, D.R.G.; Marchi, G.; Leite, M.C.A.; Martins, É.S.; Gonçalves, A.S.F.; Guilherme, L.R.G. Effect of alternative multinutrient sources on soil chemical properties. Rev. Bras. Cienc. Solo 2015, 39, 194–204. [Google Scholar] [CrossRef]
- Teixeira, A.M.S.; Garrido, F.M.S.; Medeiros, M.E.; Sampaio, J.A. Estudo do comportamento térmico da rocha fonolito com fins à produção de fertilizantes. Holos 2015, 31, 52–64. [Google Scholar] [CrossRef][Green Version]
- Teixeira, A.M.S.; Sampaio, J.A.; Garrido, F.M.S.; Medeiros, M.E. Avaliação da rocha fonolito como fertilizante alternativo de potássio. Holos 2012, 28, 21–33. [Google Scholar] [CrossRef]
- Tavares, L.F.; Carvalho, A.M.X.; Camargo, L.G.B.; Pereira, S.G.F.; Cardoso, I.M. Nutrients release from powder phonolite mediated by bioweathering actions. Inter. J. Recyc. Organic Waste Agric. 2018, 7, 89–98. [Google Scholar] [CrossRef]
- Ciceri, D.; Oliveira, M.; Allanore, A. Potassium fertilizer via hydrothermal alteration of K-feldspar ore. Green Chem. 2017, 19, 5187–5202. [Google Scholar] [CrossRef]
- Okpara, D.A.; Njoku, J.C.; Asiegbu, J.E. Responses of two sweet potato varieties to four green manure sources and inorganic fertilizer in a humid tropical Ultisol. Biol. Agric. Hortic. 2004, 22, 81–90. [Google Scholar] [CrossRef]
- Oliveira, A.P.; Santos, J.F.; Cavalcante, L.F.; Pereira, W.E.; Santos, M.C.C.; Oliveira, A.N.P.; Silva, N.V. Yield of sweet potato fertilized with cattle manure and biofertilizer. Hort. Bras. 2010, 28, 277–281. [Google Scholar] [CrossRef][Green Version]
- FAO—Food and Agriculture Organization of the United Nations. World Reference Base for Soil Resources 2014: International Soil Classification System for Naming Soils and Creating Legends for Soil Maps; FAO: Rome, Italy, 2015; Available online: http://www.fao.org/3/i3794en/I3794en.pdf (accessed on 24 September 2022).
- van Raij, B.; Andrade, J.C.; Cantarella, H.; Quaggio, J.A. Análise Química para Avaliação da Fertilidade de solos Tropicais; Instituto Agronômico: Campinas, Brazil, 2001. [Google Scholar]
- EMBRAPA—Empresa Brasileira de Pesquisa Agropecuária. Manual de Métodos de Análise de Solo; Embrapa-CNPS: Rio de Janeiro, Brasil, 1997; Available online: https://ainfo.cnptia.embrapa.br/digital/bitstream/item/169149/1/Manual-de-metodos-de-analise-de-solo-2-ed-1997.pdf (accessed on 24 September 2022).
- Feltran, J.C.; Peresin, V.A.; Granja, N.P.; Silva Filho, H.M.; Lorenzi, J.O.; Fernandes, A.M.; Soratto, R.P.; Factor, T.L.; Rós, A.B.; Aguiar, E.B. Raízes e tubérculos. In Boletim 100: Recomendações de Adubação e Calagem Para o Estado de São Paulo; Cantarella, H., Quaggio, J.A., Mattos, D., Jr., Boaretto, R.M., van Raij, B., Eds.; Instituto Agronômico: Campinas, Brazil; pp. 314–338.
- Malavolta, E.; Vitti, G.C.; Oliveira, S.A. Avaliação do Estado Nutricional das Plantas: Princípios e Aplicações, 2nd ed.; Associação Brasileira para Pesquisa da Potassa e do Fosfato: Piracicaba, Brazil, 1997. [Google Scholar]
- Tabatabai, M.A. Soil Enzymes. In METHODS of Soil analysis: Part 2: Microbiological and Biochemical Properties; SSSA Book Series: 5; SSSA: Madison WI, USA, 1994; pp. 775–833. [Google Scholar]
- Miranda, J.E.C.; França, F.H.; Carrijo, O.A.; Souza, A.F.; Pereira, W.; Lopes, C.A.; Silva, J.B.C. A Cultura da Batata-Doce; Coleção Plantar; EMBRAPA-CNPH: Brasília, Brazil, 1995. [Google Scholar]
- Nelson, N. A photometric adaptation of the Somogyi method for the determination of glucose. J. Biol. Chem. 1944, 153, 375–390. [Google Scholar] [CrossRef]
- AOAC—Association of Official Analytical Chemists. Official Methods of Analysis of AOAC International, 18th ed.; AOAC: Rockville, MD, USA, 2007. [Google Scholar]
- Figueiredo, R.T.; Fernandes, A.M.; Garreto, F.G.S.; Silva, J.A.; Nunes, J.G.S.; Vargas, P.F. Sweetpotato responses to potassium rate and timing in tropical sandy soils. Agron. J. 2023, 115, 2044–2057. [Google Scholar] [CrossRef]
- Yamaguchi, T.; Takei, T.; Yazawa, Y.; Wong, M.T.F.; Gilkes, R.J.; Swift, R.S. Effect of humic acid, sodium, and calcium additions on the formation of water-stable aggregates in Western Australian wheatbelt soils. Aust. J. Soil Res. 2004, 42, 435–439. [Google Scholar] [CrossRef]
- Canellas, L.P.; Zandonadi, D.B.; Médici, L.O.; Peres, L.E.P.; Olivares, F.L. Bioatividade de substâncias húmicas—Ação sobre o desenvolvimento e metabolismo das plantas. In Humosfera: Tratado Preliminar Sobre a Química das Substâncias Húmicas; Canellas, L.P., Santos, G.A., Eds.; Ed. do Autor: Campos dos Goytacazes, Brazil, 2005; pp. 224–243. [Google Scholar]
- Billingham, K.L. Humic Products-Potential or Presumption for Agriculture? Can Humic Products Improve My Soil? 27th Annual Conference; Grassland Society of NSW Inc.: Orange, NSW, USA, 2012. [Google Scholar]
- Ampong, K.; Thilakaranthna, M.S.; Gorim, L.Y. Understanding the role of humic acids on crop performance and soil health. Front. Agron. 2022, 4, 848621. [Google Scholar] [CrossRef]
- Canellas, L.P.; Olivares, F.L. Physiological responses to humic substances as plant growth promoter. Chem. Biol. Techn. Agric. 2014, 1, 1–11. [Google Scholar] [CrossRef]
- Maji, D.; Misra, P.; Singh, S.; Kalra, A. Humic acid rich vermicompost promotes plant growth by improving microbial community structure of soil as well as root nodulation and mycorrhizal colonization in the roots of Pisum sativum. App. Soil Ecol. 2017, 110, 97–108. [Google Scholar] [CrossRef]
- Li, Y.; Fang, F.; Wei, J.; Wu, X.; Cui, R.; Li, G.; Zheng, F.; Tan, D. Humic acid fertilizer improved soil properties and soil microbial diversity of continuous cropping peanut: A three-year experiment. Sci. Rep. 2019, 9, 12014. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, A.M.; Ribeiro, N.P.; Assunção, N.S.; Nunes, J.G.S.; Sorroche, C.P.; Leonel, M. Impact of nitrogen and green manure on yield and quality of sweet potato in sandy soil: A Brazilian case study. J. Agric. Food Res. 2021, 4, 100131. [Google Scholar] [CrossRef]
- Okpara, D.A.; Okon, O.E.; Ekeleme, F. Optimizing nitrogen fertilization for production of white and orange-fleshed sweet potato in southeast Nigeria. J. Plant Nutr. 2009, 32, 878–891. [Google Scholar] [CrossRef]
- Du, X.; Kong, L.; Xi, M.; Zhang, X. Split application improving sweetpotato yield by enhancing photosynthetic and sink capacity under reduced nitrogen condition. Field Crops Res. 2019, 238, 56–63. [Google Scholar]
- Byju, G.; George, J. Potassium nutrition of sweet potato. Adv. Hortic. Sci. 2005, 19, 221–239. [Google Scholar]
- Lv, Z.; Lu, G. New curve of critical leaf potassium concentration based on the maximum root dry matter for diagnosing k nutritional status of sweetpotato. Front. Plant Sci. 2021, 12, 714279. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, A.P.; Oliveira, M.R.T.; Barbosa, J.A.; Silva, G.G.; Nogueira, D.H.; Moura, M.F.; Braz, M.S.S. Yield and quality of sweet potato roots fertilized with urea. Hort. Bras. 2005, 23, 925–928, (In Portuguese, with English abstract). [Google Scholar] [CrossRef][Green Version]
- Santos Neto, A.R.; Silva, T.O.; Blank, A.F.; Silva, J.O.; Araújo Filho, R.N. Produtividade de clones de batata doce em função de doses de nitrogênio. Hortic. Bras. 2017, 35, 445–452. [Google Scholar] [CrossRef][Green Version]
- Fageria, N.K. Eficiência do uso de potássio pelos genótipos de arroz de terras altas. Pesqu. Agropec. Bras. 2000, 35, 2115–2120. [Google Scholar] [CrossRef]
- Rietra, R.P.; Heinen, M.; Dimkpa, C.O.; Bindraban, P.S. Effects of nutrient antagonism and synergism on yield and fertilizer use efficiency. Commun. Soil Sci. Plant Anal. 2017, 48, 1895–1920. [Google Scholar] [CrossRef]
- Baligar, V.C.; Fageria, N.K.; He, Z.L. Nutrient use efficiency in plants. Comm. Soil Sci. Plant Anal. 2001, 32, 921–950. [Google Scholar] [CrossRef]
- Bourke, R.M. Influence of nitrogen and potassium fertilizer on growth of sweet potato (Ipomoea batatas) in Papua New Guinea. Field Crops Res. 1985, 12, 363–375. [Google Scholar] [CrossRef]
- Mugo, N.J.; Karanja, N.N.; Gachene, C.K.; Dittert, K.; Ireri, G.H.; Schulte-Geldermann, E. Response of potato crop to selected nutrients in Central and Eastern highlands of Kenya. Cogent Food Agric. 2021, 7, 1898762. [Google Scholar] [CrossRef]
- Lopes, A.A.C.; Sousa, D.M.G.; Chaer, G.M.; Reis Junior, F.B.; Goedert, W.J.; Mendes, I.C. Interpretation of microbial soil indicators as a function of crop yield and organic carbon. Soil Sci. Soc. Am. 2013, 77, 461–472. [Google Scholar] [CrossRef]
- Lopes, A.A.C.; Sousa, D.M.G.; Reis Junior, F.B.; Figueiredo, C.C.; Malaquias, J.V.; Souza, L.M.; Mendes, I.C. Temporal variation and critical limits of microbial indicators in oxisols in the Cerrado, Brazil. Geoderma Reg. 2018, 12, 72–82. [Google Scholar] [CrossRef]
- Ganeshamurthy, A.N.; Nielsen, N.E. Arylsulphatase and the biochemical mineralization of soil organic sulphur. Soil Biol. Biochem. 1990, 22, 1163–1165. [Google Scholar] [CrossRef]
- Mendes, I.C.; Souza, L.M.D.; Sousa, D.M.G.; Lopes, A.A.D.C.; Reis Junior, F.B.D.; Lacerda, M.P.C.; Malaquias, J.V. Critical limits for microbial indicators in tropical Oxisols at post-harvest: The FERTBIO soil sample concept. App. Soil Eco. 2019, 139, 85–93. [Google Scholar] [CrossRef]
- Nogueira, M.A.; Melo, W.J. Enxofre disponível para a soja e atividade de arilsulfatase em solo tratado com gesso agrícola. Rev. Bras. Cienc. Solo 2003, 27, 655–663. [Google Scholar] [CrossRef]
- Nyawade, S.O.; Karanja, N.N.; Gachene, C.K.K.; Gitari, H.I.; Schulte-Geldermann, E.; Parker, M.L. Short-term dynamics of soil organic matter fractions and microbial activity in smallholder legume intercropping systems. Appl. Soil Ecol. 2019, 142, 123–135. [Google Scholar] [CrossRef]
- Shao, Z.; Mwakidoshi, E.R.; Muindi, E.M.; Soratto, R.P.; Ranjan, S.; Padhan, S.R.; Wamukota, A.W.; Sow, S.; Wasonga, D.O.; Nasar, J.; et al. Synthetic fertilizer application coupled with bioslurry optimizes potato (Solanum tuberosum) growth and yield. Agronomy 2023, 13, 2162. [Google Scholar] [CrossRef]
- Tabatabai, M.A.; Bremner, J.M. Factors affecting soil arylsulfatase activity. Soil Sci. Soc. Am. Proc. 1970, 34, 427–429. [Google Scholar] [CrossRef]

| Variable | Synthetic Fertilizers | Organic Fertilizers (Mg ha−1) | ANOVA (p > F) | CV (%) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Without | +NK | 0 | 1.2CM | 2.4CM | 2.25CM+HP | 4.5CM+HP | SF | OF | SF × OF | ||
| N (g kg−1) | 37.1 ± 0.6 a | 36.3 ± 0.5 a | 35.3 ± 0.4 b | 36.4 ± 0.6 b | 35.8 ± 0.9 b | 36.9 ± 0.7 ab | 38.9 ± 0.9 a | 0.22 | 0.02 | 0.36 | 5.6 |
| P (g kg−1) | 4.2 ± 0.10 a | 3.6 ± 0.08 b | 3.7 ± 0.15 a | 4.1 ± 0.23 a | 3.8 ± 0.18 a | 3.8 ± 0.17 a | 4.2 ± 0.17 a | <0.01 | 0.09 | 0.93 | 10.3 |
| K (g kg−1) | 38.5 ± 0.8 a | 38.4 ± 0.5 a | 37.8 ± 0.7 a | 38.3 ± 0.8 a | 39.4 ± 1.2 a | 37.8 ± 1.0 a | 39.0 ± 1.7 a | 0.87 | 0.82 | 0.77 | 8.8 |
| Ca (g kg−1) | 7.9 ± 0.14 a | 7.4 ± 0.11 b | 7.4 ± 0.21 a | 7.7 ± 0.17 a | 7.6 ± 0.16 a | 7.3 ± 0.18 a | 8.0 ± 0.29 a | <0.01 | 0.13 | 0.32 | 6.8 |
| Mg (g kg−1) | 4.1 ± 0.07 a | 3.7 ± 0.08 b | 3.8 ± 0.13 b | 3.9 ± 0.10 b | 3.8 ± 0.16 b | 3.8 ± 0.10 b | 4.3 ± 0.13 a | <0.01 | <0.01 | 0.12 | 6.7 |
| S (g kg−1) | 4.3 ± 0.05 a | 4.2 ± 0.07 a | 4.2 ± 0.09 a | 4.3 ± 0.08 a | 4.3 ± 0.14 a | 4.1 ± 0.06 a | 4.1 ± 0.08 a | 0.29 | 0.41 | 0.12 | 6.1 |
| B (mg kg−1) | 51.2 ± 1.2 a | 47.8 ± 1.2 b | 46.7 ± 1.6 b | 47.7 ± 1.5 b | 48.5 ± 0.9 b | 47.0 ± 1.8 b | 57.9 ± 1.1 a | <0.01 | <0.01 | 0.39 | 6.7 |
| Cu (mg kg−1) | 12.4 ± 0.3 a | 11.7 ± 0.4 b | 11.7 ± 0.6 bc | 12.6 ± 0.5 ab | 11.6 ± 0.4 bc | 11.3 ± 0.3 c | 13.0 ± 0.7 a | 0.04 | 0.01 | <0.01 | 8.4 |
| Fe (mg kg−1) | 147.2 ± 7.7 a | 154.7 ± 7.2 a | 165.9 ± 10.1 a | 151.4 ± 8.8 a | 128.1 ± 12.2 a | 165.5 ± 14.1 a | 143.9 ± 9.6 a | 0.42 | 0.08 | 0.19 | 19.2 |
| Mn (mg kg−1) | 119.0 ± 4.4 a | 107.9 ± 4.1 b | 134.0 ± 8.5 a | 111.8 ± 6.8 b | 101.6 ± 2.3 b | 105.3 ± 1.7 b | 114.5 ± 6.8 b | 0.04 | <0.01 | 0.80 | 14.5 |
| Zn (mg kg−1) | 46.1 ± 0.6 a | 44.7 ± 0.9 a | 44.9 ± 1.0 ab | 47.2 ± 1.0 a | 44.3 ± 1.3 b | 43.6 ± 0.9 b | 47.0 ± 1.4 a | 0.12 | 0.03 | 0.02 | 5.8 |
| Variable | Synthetic Fertilizers | Organic Fertilizers (Mg ha−1) | ANOVA (p > F) | CV (%) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Without | +NK | 0 | 1.2CM | 2.4CM | 2.25CM+HP | 4.5CM+HP | SF | OF | SF × OF | ||
| Roots plant−1 | 7.8 ± 0.30 a | 8.1 ± 0.36 a | 6.0 ± 0.26 b | 8.0 ± 0.39 a | 8.6 ± 0.30 a | 8.5 ± 0.42 a | 8.7 ± 0.56 a | 0.37 | <0.01 | 0.36 | 13.6 |
| Mark. roots plant−1 | 3.3 ± 0.15 b | 4.1 ± 0.17 a | 3.0 ± 0.19 b | 3.6 ± 0.30 a | 3.7 ± 0.24 a | 4.0 ± 0.19 a | 4.1 ± 0.37 a | <0.01 | <0.01 | 0.01 | 13.9 |
| Root mean weight (g) | 98.9 ± 1.8 b | 129.6 ± 3.8 a | 118.4 ± 8.9 a | 111.0 ± 8.1 a | 118.0 ± 8.3 a | 108.1 ± 3.0 a | 115.7 ± 7.3 a | <0.01 | 0.38 | 0.07 | 10.7 |
| Total yield (Mg ha−1) | 15.3 ± 0.60 b | 21.0± 0.85 a | 14.3 ± 1.19 d | 17.4 ± 1.10 c | 20.8 ± 1.74 a | 18.4 ± 0.69 bc | 19.9 ± 1.80 ab | <0.01 | <0.01 | <0.01 | 9.5 |
| Mark. yield (Mg ha−1) | 10.4 ± 0.40 b | 16.4 ± 0.72 a | 11.0 ± 1.06 d | 12.3 ± 0.20 cd | 14.8± 1.72 ab | 13.5 ± 0.67 bc | 15.4 ± 1.76 a | <0.01 | <0.01 | <0.01 | 11.9 |
| Root dry matter (%) | 22.2 ± 0.4 a | 22.2 ± 0.8 a | 21.5 ± 0.6 a | 21.8 ± 0.8 a | 21.9 ± 0.8 a | 21.6 ± 0.7 a | 24.3 ± 1.4 a | 0.98 | 0.26 | 0.84 | 12.7 |
| Firmness (N) | 30.1 ± 0.4 a | 30.0 ± 0.4 a | 29.0 ± 0.4 a | 29.8 ± 0.9 a | 30.6 ± 0.6 a | 29.9 ± 0.8 a | 30.9 ± 0.5 a | 0.87 | 0.27 | 0.28 | 5.9 |
| Total sugar (%) | 3.6 ± 0.16 a | 3.7 ± 0.26 a | 3.2 ± 0.15 a | 3.3 ± 0.22 a | 3.3 ± 0.12 a | 4.1 ± 0.43 a | 4.3 ± 0.44 a | 0.81 | 0.06 | 0.86 | 26.1 |
| Reducing sugars (%) | 2.3 ± 0.07 a | 2.3 ± 0.17 a | 2.2 ± 0.10 a | 2.4 ± 0.14 a | 2.5 ± 0.15 a | 2.0 ± 0.13 a | 2.6 ± 0.35 a | 0.99 | 0.24 | 0.13 | 23.1 |
| Starch (%) | 19.4 ± 0.6 a | 18.4 ± 0.9 a | 17.5 ± 0.8 a | 17.7 ± 1.1 a | 19.5 ± 1.2 a | 18.9 ± 1.0 a | 20.7 ± 1.4 a | 0.36 | 0.31 | 0.81 | 17.9 |
| Soluble solids (°Brix) | 5.7 ± 0.09 a | 5.4 ± 0.12 a | 5.2 ± 0.14 b | 5.5 ± 0.18 ab | 5.7 ± 0.16 a | 5.9 ± 0.15 a | 5.6 ± 0.17 ab | 0.07 | 0.04 | 0.14 | 7.9 |
| Crude fiber (%) | 7.0 ± 0.24 a | 7.4 ± 0.38 a | 7.3 ± 0.47 a | 7.4 ± 0.52 a | 6.9 ± 0.54 a | 6.6 ± 0.21 a | 7.7 ± 0.69 a | 0.38 | 0.65 | 0.78 | 21.2 |
| Variable | Synthetic Fertilizers | Organic Fertilizers (Mg ha−1) | ANOVA (p > F) | CV (%) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Without | +NK | 0 | 1.2CM | 2.4CM | 2.25CM+HP | 4.5CM+HP | SF | OF | SF × OF | ||
| N conc. (g kg−1) | 10.0 ± 0.2 a | 10.3 ± 0.4 a | 10.3 ± 0.2 b | 10.3 ± 0.2 b | 11.6 ± 0.8 a | 9.6 ± 0.4 bc | 9.0 ± 0.2 c | 0.29 | <0.01 | <0.01 | 9.5 |
| P conc. (g kg−1) | 1.8 ± 0.07 a | 1.7 ± 0.07 a | 1.7 ± 0.10 a | 1.8 ± 0.07 a | 1.9 ± 0.14 a | 1.6 ± 0.15 a | 1.7 ± 0.07 a | 0.13 | 0.24 | <0.01 | 12.1 |
| K conc. (g kg−1) | 14.8 ± 0.3 b | 15.9 ± 0.3 a | 15.4 ± 0.3 a | 15.3 ± 0.5 a | 16.0 ± 0.9 a | 15.6 ± 0.6 a | 14.4 ± 0.3 a | <0.01 | 0.17 | 0.04 | 8.4 |
| Ca conc. (g kg−1) | 1.5 ± 0.08 b | 1.8 ± 0.06 a | 1.6 ± 0.12 a | 1.6 ± 0.07 a | 1.5 ± 0.16 a | 1.6 ± 0.13 a | 1.8 ± 0.12 a | <0.01 | 0.24 | 0.56 | 17.5 |
| Mg conc. (g kg−1) | 0.6 ± 0.015 a | 0.7 ± 0.017 a | 0.6 ± 0.018 a | 0.6 ± 0.024 a | 0.7 ± 0.034 a | 0.7 ± 0.027 a | 0.7 ± 0.027 a | 0.11 | 0.76 | 0.15 | 9.5 |
| S conc. (g kg−1) | 0.7 ± 0.011 a | 0.7 ± 0.010 a | 0.7 ± 0.015 a | 0.7 ± 0.011 a | 0.7 ± 0.023 a | 0.7 ± 0.021 a | 0.7 ± 0.014 a | 0.94 | 0.67 | 0.08 | 6.2 |
| B conc. (mg kg−1) | 14.4 ± 0.25 a | 14.2 ± 0.27 a | 15.4 ± 0.17 a | 14.2 ± 0.40 b | 14.1 ± 0.33 b | 14.3 ± 0.38 b | 13.4 ± 0.42 b | 0.46 | <0.01 | 0.40 | 6.2 |
| Cu conc. (mg kg−1) | 4.6 ± 0.10 b | 5.0 ± 0.13 a | 4.4 ± 0.10 c | 4.6 ± 0.15 bc | 5.1 ± 0.31 a | 5.1 ± 0.16 a | 5.0 ± 0.10 ab | <0.01 | 0.02 | 0.50 | 9.7 |
| Fe conc. (mg kg−1) | 72.9 ± 2.6 a | 69.5 ± 3.1 a | 74.3 ± 4.0 a | 68.2 ± 3.4 a | 75.3 ± 6.5 a | 71.3 ± 3.7 a | 66.9 ± 4.9 a | 0.25 | 0.30 | <0.01 | 12.9 |
| Mn conc. (mg kg−1) | 23.0 ± 0.8 a | 21.6 ± 0.9 a | 21.9 ± 1.9 b | 22.5 ± 1.1 ab | 25.3 ± 1.2 a | 19.7 ± 0.7 b | 22.1 ± 0.9 b | 0.13 | 0.02 | 0.02 | 13.4 |
| Zn conc. (mg kg−1) | 6.2 ± 0.16 b | 7.2 ± 0.15 a | 6.7 ± 0.33 a | 7.1 ± 0.28 a | 6.7 ± 0.33 a | 6.7 ± 0.24 a | 6.4 ± 0.31 a | <0.01 | 0.44 | 0.57 | 10.8 |
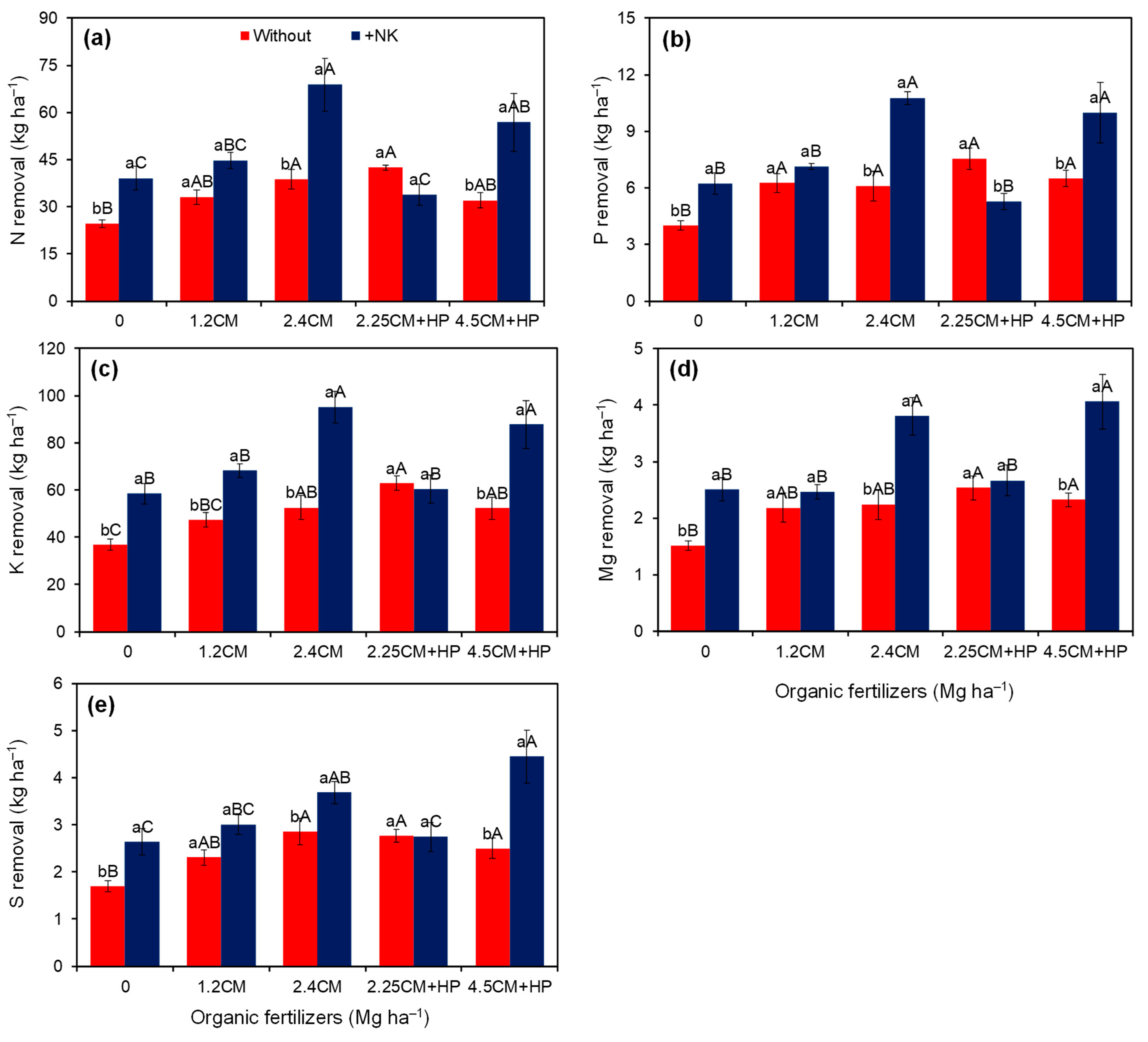
| N rem. (kg ha−1) | 34.2 ± 1.6 b | 48.7 ± 3.8 a | 31.9 ± 3.3 c | 38.9 ± 2.8 bc | 53.8 ± 7.1 a | 38.2 ± 2.3 bc | 44.5 ± 6.4 ab | <0.01 | <0.01 | <0.01 | 21.9 |
| P rem. (kg ha−1) | 6.1 ± 0.34 b | 7.9 ± 0.51 a | 5.1 ± 0.51 c | 6.7 ± 0.30 b | 8.4 ± 0.0.97 a | 6.4 ± 0.54 bc | 8.2 ± 1.01 a | <0.01 | <0.01 | <0.01 | 19.6 |
| K rem. (kg ha−1) | 50.3 ± 2.5 b | 74.0 ± 4.3 a | 47.6 ± 4.7 d | 57.7 ± 4.4 cd | 73.9 ± 9.0 a | 61.6 ± 3.2 bc | 70.0 ± 8.4 ab | <0.01 | <0.01 | <0.01 | 16.7 |
| Ca rem. (kg ha−1) | 5.1 ± 0.42 b | 8.4 ± 0.65 a | 5.1 ± 0.66 c | 5.9 ± 0.49 bc | 7.3 ± 1.22 ab | 6.3 ± 0.60 bc | 9.2 ± 1.41 a | <0.01 | <0.01 | 0.16 | 29.1 |
| Mg rem. (kg ha−1) | 2.2 ± 0.11 b | 3.1 ± 0.20 a | 2.0 ± 0.22 d | 2.3 ± 0.14 cd | 3.0 ± 0.36 ab | 2.6 ± 0.16 bc | 3.2 ± 0.40 a | <0.01 | <0.01 | 0.01 | 19.2 |
| S rem. (kg ha−1) | 2.4 ± 0.12 b | 3.3 ± 0.21 a | 2.2 ± 0.23 d | 2.7 ± 0.18 cd | 3.3 ± 0.23 ab | 2.8 ± 0.16 bc | 3.5 ± 0.46 a | <0.01 | <0.01 | 0.02 | 18.7 |
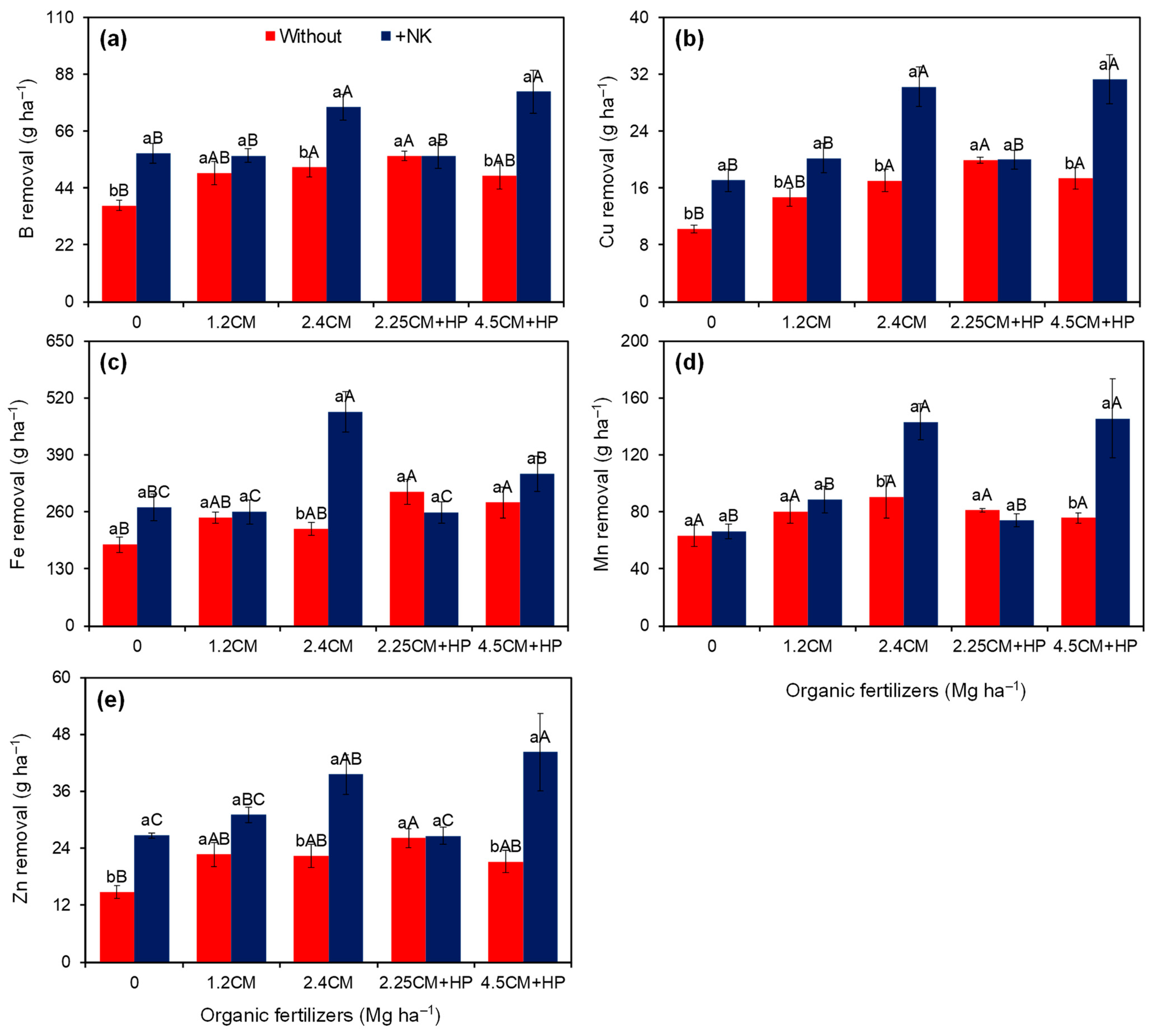
| B rem. (g ha−1) | 48.8 ± 2.0 b | 65.4 ± 3.2 a | 47.3 ± 4.3 b | 53.1 ± 2.7 b | 63.6 ± 5.2 a | 56.4 ± 2.4 ab | 64.9 ± 7.6 a | <0.01 | <0.01 | 0.01 | 16.0 |
| Cu rem. (g ha−1) | 15.9 ± 0.9 b | 23.7 ± 1.6 a | 13.7 ± 1.5 d | 17.4 ± 1.5 c | 23.6 ± 2.9 ab | 19.9 ± 0.7 bc | 24.3 ± 3.1 a | <0.01 | <0.01 | <0.01 | 18.5 |
| Fe rem. (g ha−1) | 247.8 ± 13.4 b | 324.7 ± 24.5 a | 227.4 ± 22.8 c | 253.3 ± 14.0 c | 354.3 ± 55.4 a | 282.2 ± 19.2 bc | 314.1 ± 27.8 ab | <0.01 | <0.01 | <0.01 | 20.7 |
| Mn rem. (g ha−1) | 78.1 ± 3.9 b | 103.5 ± 9.7 a | 64.8 ± 4.2 b | 84.2 ± 6.0 b | 116.8 ± 13.4 a | 77.5 ± 2.6 b | 110.5 ± 18.5 a | <0.01 | <0.01 | 0.01 | 26.9 |
| Zn rem. (g ha−1) | 21.4 ± 1.2 b | 33.6 ± 2.3 a | 20.7 ± 2.4 b | 26.9 ± 2.1 ab | 31.0 ± 3.9 a | 26.4 ± 1.2 ab | 32.7 ± 5.9 a | <0.01 | 0.02 | 0.04 | 25.5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Parecido, R.J.; Soratto, R.P.; Fernandes, A.M.; Blanes, M.C.; Fidelis, L.G.; Gitari, H.I.; Dutra, S.G. Castor Meal and Ground Hydrothermalized Phonolite Optimize Sweet Potato Nutrition, Yield, and Quality. Horticulturae 2024, 10, 775. https://doi.org/10.3390/horticulturae10080775
Parecido RJ, Soratto RP, Fernandes AM, Blanes MC, Fidelis LG, Gitari HI, Dutra SG. Castor Meal and Ground Hydrothermalized Phonolite Optimize Sweet Potato Nutrition, Yield, and Quality. Horticulturae. 2024; 10(8):775. https://doi.org/10.3390/horticulturae10080775
Chicago/Turabian StyleParecido, Renan J., Rogério P. Soratto, Adalton M. Fernandes, Mayara C. Blanes, Luis G. Fidelis, Harun I. Gitari, and Sérgio G. Dutra. 2024. "Castor Meal and Ground Hydrothermalized Phonolite Optimize Sweet Potato Nutrition, Yield, and Quality" Horticulturae 10, no. 8: 775. https://doi.org/10.3390/horticulturae10080775
APA StyleParecido, R. J., Soratto, R. P., Fernandes, A. M., Blanes, M. C., Fidelis, L. G., Gitari, H. I., & Dutra, S. G. (2024). Castor Meal and Ground Hydrothermalized Phonolite Optimize Sweet Potato Nutrition, Yield, and Quality. Horticulturae, 10(8), 775. https://doi.org/10.3390/horticulturae10080775









