Metabolism of Fluorinated Topolin Cytokinins in Micropropagated Phalaenopsis amabilis
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material and Growth Conditions
2.2. Cytokinin Analyses
2.3. Data Analysis
3. Results
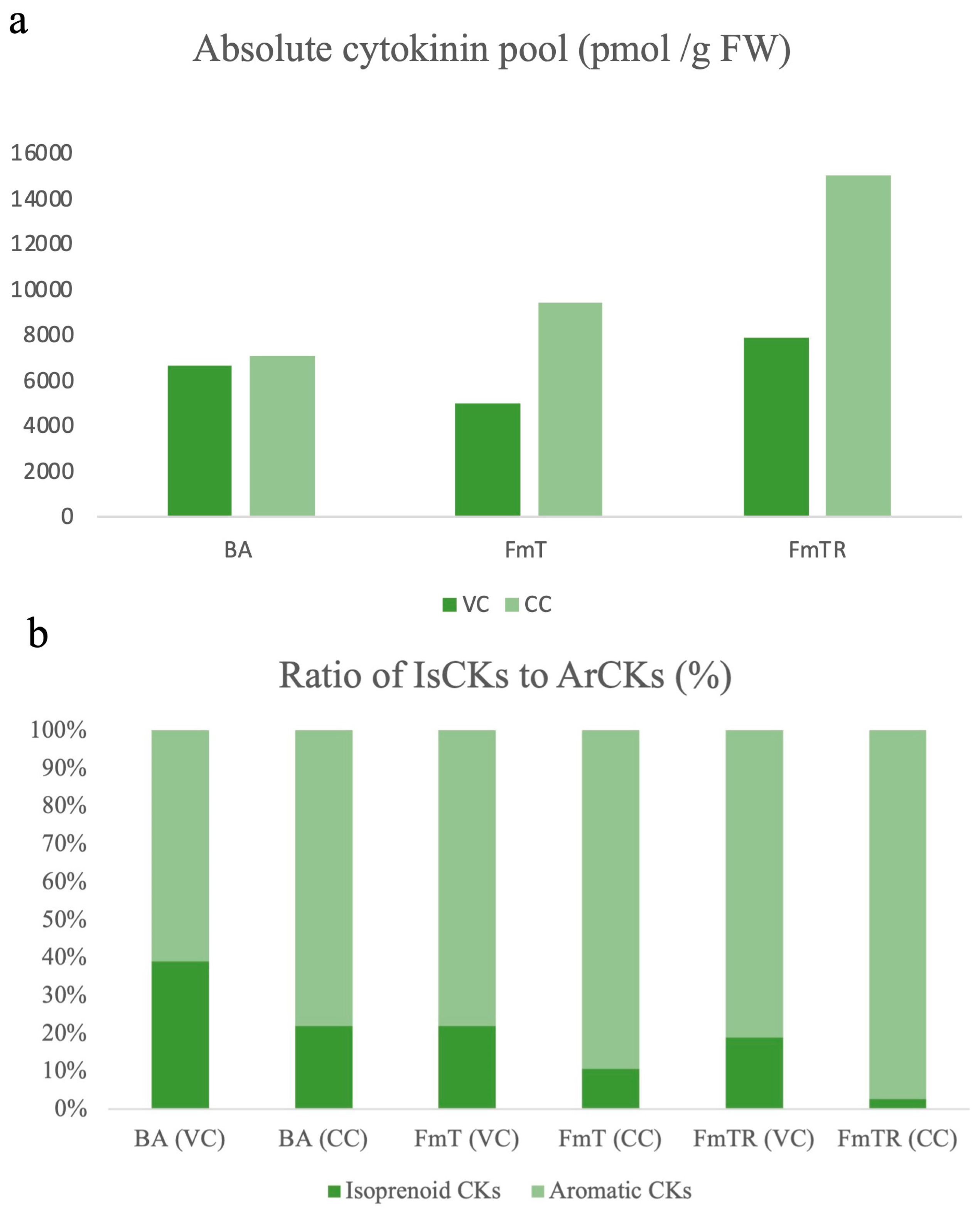
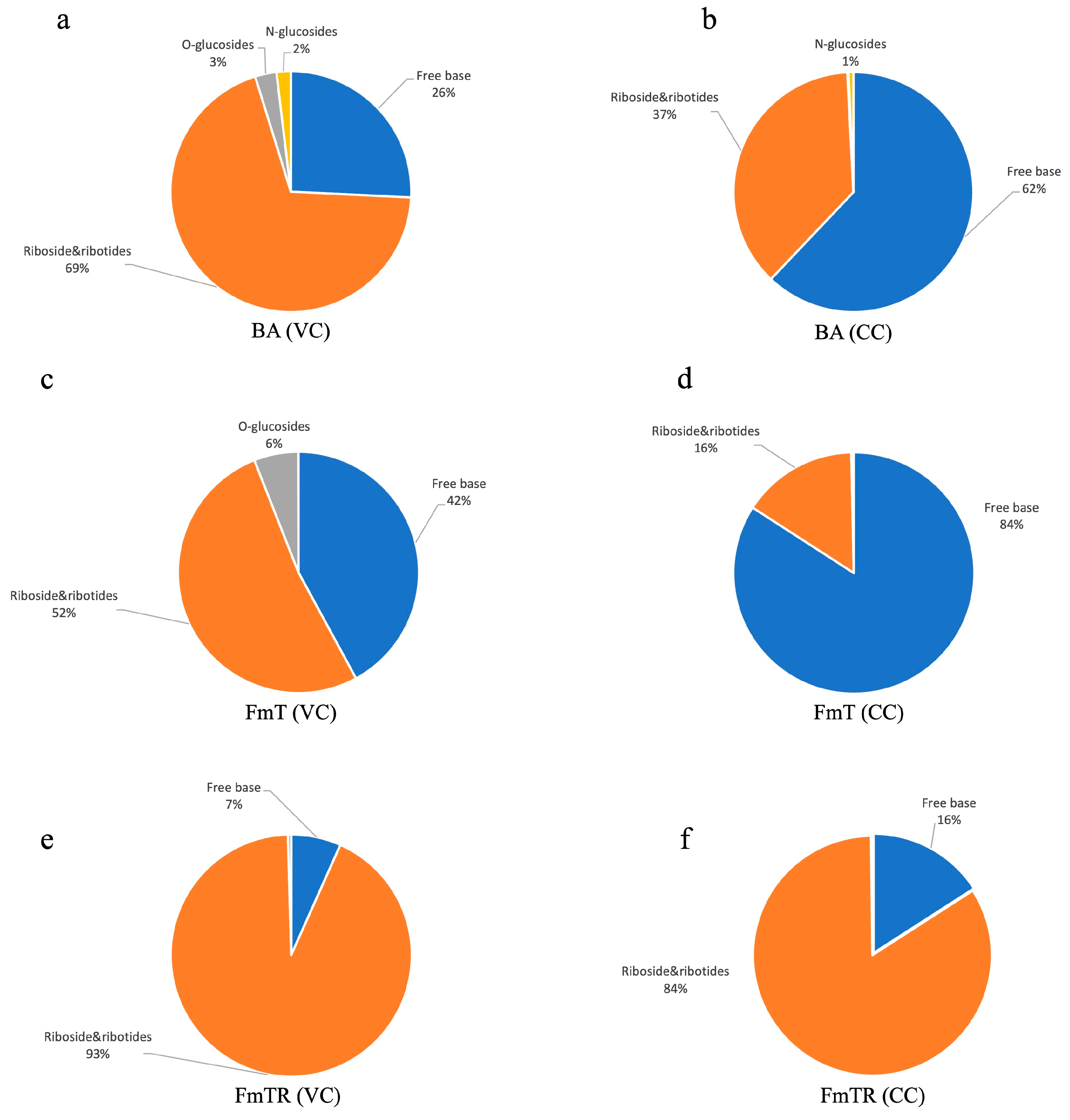
Effect of Exogenously Administered CKs on Total Quantified Cytokinin Levels and Their Metabolic Profile
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Kieber, J.J.; Schaller, G.E. Cytokinin signaling in plant development. Development 2018, 145, dev149344. [Google Scholar] [CrossRef] [PubMed]
- Singh, J.; Sengar, R.S.; Prasad, M.; Kumar, A. Effect of Different Plant Growth Regulators on In-vitro Micropropagation of Banana Cultivar Grand Naine (Musa spp.). J. Adv. Biol. Biotechnol. 2024, 27, 90–98. [Google Scholar] [CrossRef]
- Mazri, M.A. Role of CKs and physical state of the culture medium to improve in vitro shoot multiplication, rooting and acclimatization of date palm (Phoenix dactylifera L.) cv. Boufeggous. J. Plant Biochem. Biotechnol. 2015, 24, 268–275. [Google Scholar] [CrossRef]
- Zürcher, E.; Müller, B. Cytokinin synthesis, signaling, and function—Advances and new insights. Int. Rev. Cell Mol. Biol. 2016, 324, 1–38. [Google Scholar] [PubMed]
- Aremu, A.O.; Bairu, M.W.; Szüčová, L.; Doležal, K.; Finnie, J.F.; Van Staden, J. Genetic fidelity in tissue-cultured ‘Williams’ bananas–The effect of high concentration of topolins and benzyladenine. Sci. Hortic. 2013, 161, 324–327. [Google Scholar] [CrossRef]
- Ahmad, M.Z.; Hussain, I.; Roomi, S.; Zia, M.A.; Zaman, M.S.; Abbas, Z.; Shah, S.H. In vitro response of cytokinin and auxin to multiple shoot regeneration in Solanum tuberosum L. Am. Eurasian J. Agric. Environ. Sci. 2012, 12, 1522–1526. [Google Scholar]
- Lal, N.; Madhulika, S. Prospects of plant tissue culture in orchid propagation: A review. Indian J. Biol. 2020, 7, 103–110. [Google Scholar]
- Khatun, K.; Nath, U.K.; Rahman, M.S. Tissue culture of Phalaenopsis: Present status and future prospects. J. Adv. Biotechnol. Exp. Ther. 2020, 3, 273–285. [Google Scholar] [CrossRef]
- Parthibhan, S.; Rao, M.V.; Kumar, T.S. In vitro regeneration from protocorms in Dendrobium aqueum Lindley–An imperiled orchid. J. Genet. Eng. Biotechnol. 2015, 13, 227–233. [Google Scholar] [CrossRef]
- Zanello, C.A.; Duarte, W.N.; Gomes, D.M.; Cardoso, J.C. Micropropagation from inflorescence nodal segments of Phalaenopsis and acclimatization of plantlets using different substrates. Horticulturae 2022, 8, 340. [Google Scholar] [CrossRef]
- Murvanidze, N.; Doležal, K.; Werbrouck, S.P. Fluorine containing topolin CKs for Phalaenopsis Amabilis (L.) blume micropropagation. Propag. Ornam. Plants 2019, 19, 48–51. [Google Scholar]
- Plíhalová, L. Synthesis and chemistry of meta-Topolin and related compounds. In Meta-topolin: A Growth Regulator for Plant Biotechnology and Agriculture; Springer: Singapore, 2021; pp. 11–22. [Google Scholar]
- Mok, M.C. CKs and plant development—An overview. In Cytokinins—Chemistry, Activity, and Function; Mok, D.W.S., Mok, M.C., Eds.; CRC Press: Boca Raton, FL, USA, 1994; pp. 155–166. [Google Scholar]
- Zhao, J.; Wang, J.; Liu, J.; Zhang, P.; Kudoyarova, G.; Liu, C.-J.; Zhang, K. Spatially Distributed CKs: Metabolism, Signaling, and Transport. Plant Commun. 2024. [Google Scholar]
- Mok, D.W.S.; Mok, M.C. Cytokinin metabolism and action. Annu. Rev. Plant Biol. 2001, 52, 89–118. [Google Scholar] [CrossRef] [PubMed]
- Klemš, M.; Plačková, L.; Doležal, K.; Bettaieb, T.; Werbrouck, S.P. Changes in cytokinin levels and metabolism in tobacco (Nicotiana tabacum L.) explants during in vitro shoot organogenesis induced by trans-zeatin and dihydrozeatin. Plant Growth Regul. 2011, 65, 427–437. [Google Scholar] [CrossRef]
- Abdouli, D.; Plačková, L.; Doležal, K.; Bettaieb, T.; Werbrouck, S.P. Topolin CKs enhanced shoot proliferation, reduced hyperhydricity and altered cytokinin metabolism in Pistacia vera L. seedling explants. Plant Sci. 2022, 322, 111360. [Google Scholar] [CrossRef] [PubMed]
- Aremu, A.O.; Bairu, M.W.; Szüčová, L.; Doležal, K.; Finnie, J.F.; Van Staden, J. Assessment of the role of meta-topolins on in vitro produced phenolics and acclimatization competence of micropropagated ‘Williams’ banana. Acta Physiol. Plant. 2012, 34, 2265–2273. [Google Scholar] [CrossRef]
- Smykalova, I.; Plačková, L.; Doležal, K.; Bettaieb, T.; Werbrouck, S.P. The role of cytokinin regulation in micropropagation. Plant Cell Rep. 2019, 38, 123–132. [Google Scholar]
- Novák, O.; Hauserová, E.; Amakorová, P.; Doležal, K.; Strnad, M. Cytokinin profiling in plant tissues using ultra-performance liquid chromatography–electrospray tandem mass spectrometry. Phytochemistry 2008, 69, 2214–2224. [Google Scholar] [CrossRef]
- Plačková, L.; Hrdlička, J.; Smýkalová, I.; Cvečková, M.; Novák, O.; Griga, M.; Doležal, K. Cytokinin profiling of long-term in vitro pea (Pisum sativum L.) shoot cultures. Plant Growth Regul. 2015, 77, 125–132. [Google Scholar] [CrossRef]
- Kamada-Nobusada, T.; Sakakibara, H. Molecular basis for cytokinin biosynthesis. Phytochemistry 2009, 70, 444–449. [Google Scholar] [CrossRef]
- Werbrouck, S.P.O.; van der Jeugt, B.; Dewitte, W.; Prinsen, E.; Van Onckelen, H.A.; Debergh, P.C. The metabolism of benzyladenine in Spathiphyllum floribundum Schott ‘Petite’ in relation to acclimatisation problems. Plant Cell Rep. 1995, 14, 662–665. [Google Scholar] [CrossRef] [PubMed]
- Jameson, P.E. Cytokinin Translocation to, and Biosynthesis and Metabolism within, Cereal and Legume Seeds: Looking Back to Inform the Future. Metabolites 2023, 13, 1076. [Google Scholar] [CrossRef] [PubMed]
- Schäfer, M.; Plačková, L.; Doležal, K.; Bettaieb, T.; Werbrouck, S.P. The role of cis-zeatin-type CKs in plant growth regulation and mediating responses to environmental interactions. J. Exp. Bot. 2015, 66, 4873–4884. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.-Y.; Hao, Z.-G.; Miao, S.; Zhang, X.; Li, J.-Q.; Guo, S.-X.; Lee, Y.-I. Profiles of CKs Metabolic Genes and Endogenous CKs Dynamics during Shoot Multiplication In Vitro of Phalaenopsis. Int. J. Mol. Sci. 2022, 23, 3755. [Google Scholar] [CrossRef] [PubMed]
- Werbrouck, S.P.; Doležal, K.; Strnad, M.; Van Staden, J. Meta-topolin, an alternative to benzyladenine in tissue culture? Physiol. Plant. 1996, 98, 291–297. [Google Scholar] [CrossRef]
- Van Staden, J.; Drewes, F.E. The stability and metabolism of benzyladenine glucosides in soybean callus. J. Plant Physiol. 1992, 140, 92–95. [Google Scholar] [CrossRef]
- Holub, J.; Plačková, L.; Doležal, K.; Strnad, M. Biological activity of CKs derived from ortho-and meta-hydroxybenzyladenine. Plant Growth Regul. 1998, 26, 109–115. [Google Scholar] [CrossRef]
- Galuszka, P.; Popelková, H.; Werner, T.; Frébortová, J.; Pospíšilová, H.; Mik, V.; Köllmer, I.; Schmülling, T.; Frébort, I. Biochemical characterization of cytokinin oxidases/dehydrogenases from Arabidopsis thaliana expressed in Nicotiana tabacum L. J. Plant Growth Regul. 2007, 26, 255–267. [Google Scholar] [CrossRef]
- Grira, M.; Prinsen, E.; Werbrouck, S. New Understanding of Meta-Topolin Riboside Metabolism in Micropropagated Woody Plants. Plants 2024, 13, 1281. [Google Scholar] [CrossRef]
| Cytokinin Treatment | ||||||
|---|---|---|---|---|---|---|
| BA | FmT | FmTR | ||||
| Cytokinin Metabolites | Container Type | |||||
| Ventilated | Closed | Ventilated | Closed | Ventilated | Closed | |
| tZ | <LOD | <LOD | <LOD | <LOD | <LOD | <LOD |
| tZOG | <LOD | <LOD | <LOD | <LOD | <LOD | <LOD |
| tZR | <LOD | <LOD | <LOD | <LOD | <LOD | <LOD |
| tZROG | <LOD | <LOD | <LOD | <LOD | <LOD | <LOD |
| tZ7G | 20.2 ± 2.07 a | 4.9 ± 0.2 b | 1.6 ± 0.05 c | <LOD | <LOD | <LOD |
| tZ9G | <LOD | <LOD | <LOD | <LOD | <LOD | <LOD |
| tZR5’MP | <LOD | <LOD | <LOD | <LOD | <LOD | <LOD |
| cZ | 20.8 ± 2.5 a | 11.09 ± 1.4 b | <LOD | <LOD | <LOD | <LOD |
| cZOG | <LOD | <LOD | <LOD | <LOD | <LOD | <LOD |
| cZR | 2059.5 ± 96.3 a | 1296.1 ± 9.8 b | 743.5 ± 56.5 c | 893.7 ± 77.0 d | 1362.8 ±129.3 b | 270.5 ± 21.8 e |
| cZROG | 26.1 ± 1.7 a | 11.3 ± 0.5 b | <LOD | 3.85 ± 0.2 d | <LOD | 4.1 ± 0.1 c |
| cZ7G | <LOD | <LOD | <LOD | <LOD | <LOD | <LOD |
| cZ9G | <LOD | <LOD | <LOD | 8.6 ± 0.2 a | <LOD | 6.1 ± 0.4 b |
| cZR5’MP | <LOD | 74.6 ± 5.3 a | <LOD | 40.7 ± 3.6 c | <LOD | 66.1 ± 3.9 b |
| DHZ | <LOD | <LOD | <LOD | <LOD | <LOD | <LOD |
| DHZOG | <LOD | <LOD | <LOD | <LOD | <LOD | <LOD |
| DHZR | 5.7 ± 0.8 a | 3.8 ± 0.3 b | 3.4 ± 0.06 b | 1.5 ± 0.2 c | <LOD | <LOD |
| DHZROG | 164.1 ± 4.8 b | <LOD | 296.6 ± 20.1 a | <LOD | 34.9 ± 1.1 c | <LOD |
| DHZ7G | <LOD | 3.3 ± 0.07 b | <LOD | 2.4 ± 0.2 c | <LOD | 3.8 ± 0.1 a |
| DHZ9G | <LOD | <LOD | <LOD | <LOD | <LOD | <LOD |
| DHZR5’MP | <LOD | <LOD | <LOD | <LOD | <LOD | <LOD |
| iP | 8.6 ± 0.1 a | 5.2 ± 0.1 b | 1.8 ± 0.1 d | 3.1 ± 0.2 c | 3.1 ± 0.3 c | 2.5 ± 0.2 c |
| iPR | 272.9 ± 4.7 a | 125.8 ± 5.3 b | 53.2 ± 1.1 d | 22.1 ± 0.4 f | 87.07 ± 3.8 c | 23.8 ±1.2 e |
| iP7G | <LOD | <LOD | <LOD | <LOD | <LOD | <LOD |
| iP9G | <LOD | <LOD | <LOD | <LOD | <LOD | <LOD |
| iPR5’MP | <LOD | 21.5 ± 2.03 a | <LOD | 14.4 ± 1.3 b | <LOD | 20.01 ± 0.8 a |
| BA | 1398.3 ± 151.1 b | 4159.68 ± 497.2 a | 9.03 ± 0.6 d | 15.4 ± 3.1 c | <LOD | <LOD |
| BAR | 84.04 ± 2.9 a | 61.4 ± 1.3 b | 0.5 ± 0.09 e | <LOD | 1.1 ± 0.08 d | 3.1 ± 0.3 c |
| BA7G | <LOD | <LOD | <LOD | <LOD | <LOD | <LOD |
| BA9G | 106.5 ± 3.4 a | 37.3 ± 1.3 b | <LOD | <LOD | <LOD | <LOD |
| BAR5’MP | <LOD | 19.1 ± 0.4 | <LOD | <LOD | <LOD | <LOD |
| mT | 30.8 ± 1.9 a | 16.1 ± 0.3 b | <LOD | 9.1 ± 1.0 d | <LOD | 19.4 ± 1.3 c |
| mTR | 67.2 ± 6.3 a | 17.5 ± 0.9 e | 27.1 ± 1.3 c | 7.8 ± 0. 2 f | 32.2 ± 2.0 b | 26.6 ± 1.8 d |
| mT7G | <LOD | <LOD | <LOD | <LOD | <LOD | <LOD |
| mT9G | <LOD | <LOD | <LOD | 16.8 ± 0,1 a | <LOD | 15.1 ± 1.3 b |
| oT | <LOD | <LOD | <LOD | <LOD | <LOD | <LOD |
| oTR | 17.1 ± 1.5 a | 4.7 ± 0.4 b | <LOD | <LOD | <LOD | <LOD |
| oT7G | <LOD | <LOD | <LOD | <LOD | <LOD | <LOD |
| oT9G | <LOD | <LOD | <LOD | <LOD | <LOD | <LOD |
| pT | 147.6 ± 9.4 b | 27.8 ± 2.3 c | 140.7 ± 10.6 b | <LOD | 196.5 ± 6.1 a | <LOD |
| pTR | 1242.2 ± 27.8 b | 251.9 ± 17.8 d | 1142.1 ± 24.9 c | 21.6 ± 0.8 f | 1346.1 ± 74.4 a | 25.8 ±1.3 e |
| pT7G | <LOD | <LOD | <LOD | <LOD | <LOD | <LOD |
| pT9G | <LOD | <LOD | <LOD | <LOD | <LOD | <LOD |
| K | <LOD | <LOD | <LOD | <LOD | <LOD | <LOD |
| KR | <LOD | <LOD | <LOD | <LOD | <LOD | <LOD |
| K9G | <LOD | <LOD | <LOD | <LOD | <LOD | <LOD |
| FmT | 99.2 ± 11.2 f | 140.8 ± 11.4 e | 1952.3 ± 220.9 c | 7836.4 ± 713.3 a | 326.1 ± 25.4 d | 2350.7 ± 360.5 b |
| FmTR | 844.4 ± 107.2 c | 804.2 ± 88.6 d | 627.4 ± 44.5 e | 490.7 ± 41.03 f | 4478.6 ± 150.7 b | 12,156.5 ± 1712.8 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Murvanidze, N.; Doležal, K.; Plačková, L.; Werbrouck, S.P.O. Metabolism of Fluorinated Topolin Cytokinins in Micropropagated Phalaenopsis amabilis. Horticulturae 2024, 10, 727. https://doi.org/10.3390/horticulturae10070727
Murvanidze N, Doležal K, Plačková L, Werbrouck SPO. Metabolism of Fluorinated Topolin Cytokinins in Micropropagated Phalaenopsis amabilis. Horticulturae. 2024; 10(7):727. https://doi.org/10.3390/horticulturae10070727
Chicago/Turabian StyleMurvanidze, Nino, Karel Doležal, Lenka Plačková, and Stefaan P. O. Werbrouck. 2024. "Metabolism of Fluorinated Topolin Cytokinins in Micropropagated Phalaenopsis amabilis" Horticulturae 10, no. 7: 727. https://doi.org/10.3390/horticulturae10070727
APA StyleMurvanidze, N., Doležal, K., Plačková, L., & Werbrouck, S. P. O. (2024). Metabolism of Fluorinated Topolin Cytokinins in Micropropagated Phalaenopsis amabilis. Horticulturae, 10(7), 727. https://doi.org/10.3390/horticulturae10070727







