Using Machine Learning Algorithms to Investigate the Impact of Temperature Treatment and Salt Stress on Four Forage Peas (Pisum sativum var. arvense L.)
Abstract
1. Introduction
2. Materials and Methods
2.1. Material
2.2. Analysis of Variance
2.3. Machine Learning (ML) Analysis
3. Results
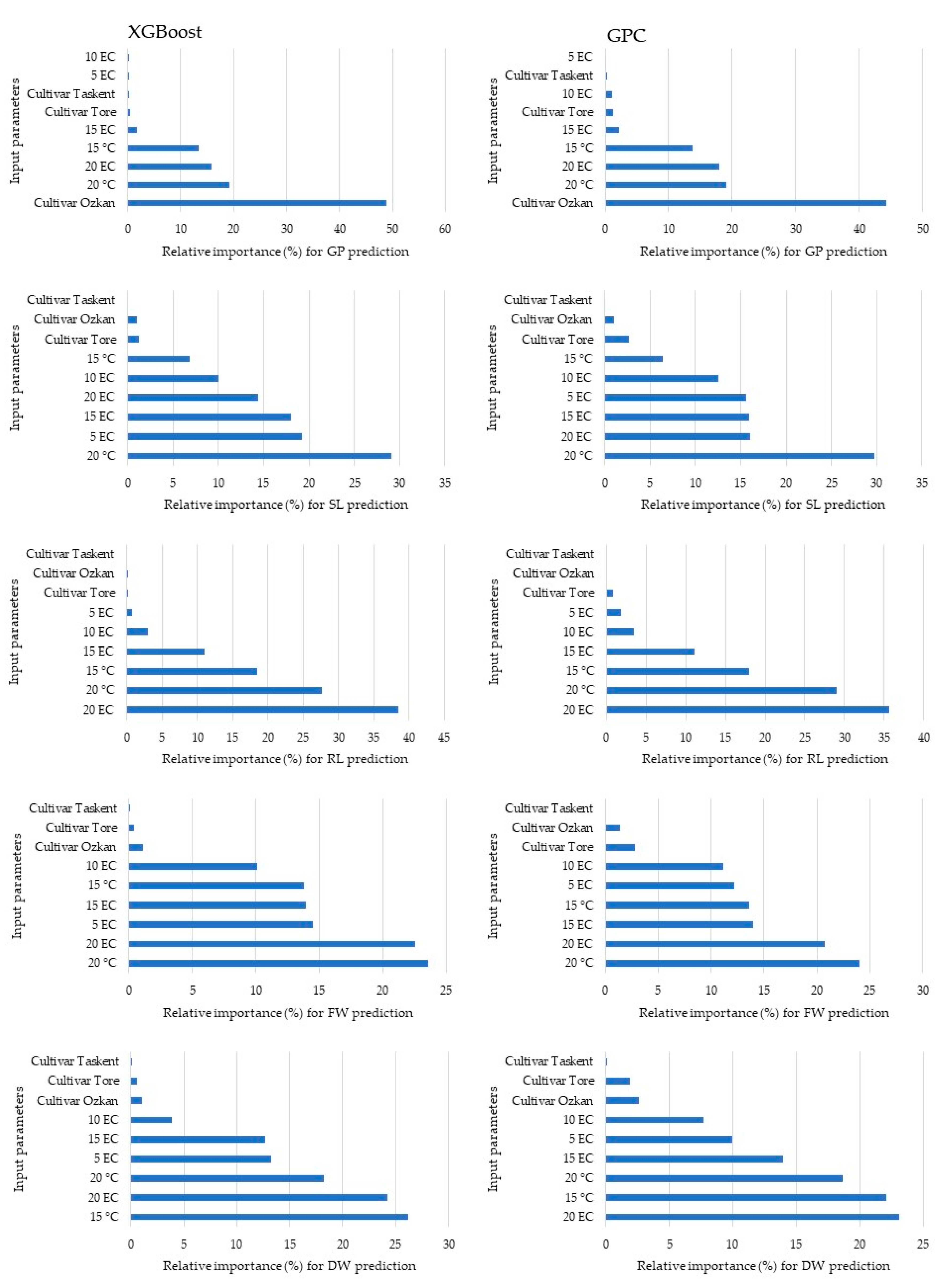
ML Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Kopecká, R.; Kameniarová, M.; Černý, M.; Brzobohatý, B.; Novák, J. Abiotic stress in crop production. Int. J. Mol. Sci. 2023, 24, 6603. [Google Scholar] [CrossRef]
- Prasad, P.V.V.; Staggenborg, S.A.; Ristic, Z. Impacts of drought and/or heat stress on physiological, developmental, growth, and yield processes of crop plants. In Response of Crops to Limited Water; Ahuja, L.R., Reddy, V.R., Saseendran, S.A., Yu, Q., Eds.; ASA-CSSA-SSSA: Madison, WI, USA, 2008; Volume 1, pp. 301–355. [Google Scholar]
- Küçüközcü, G.; Avcı, S. Tolerance of forage pea cultivars to salinity and drought stress during germination and seedling growth. Int. J. Agric. Environ. Food Sci. 2020, 4, 368–375. [Google Scholar] [CrossRef]
- Lamichaney, A.; Parihar, A.K.; Hazra, K.K.; Dixit, G.P.; Katiyar, P.K.; Singh, D.; Singh, N.P. Untangling the influence of heat stress on crop phenology, seed set, seed weight, and germination in field pea (Pisum sativum L.). Front. Plant Sci. 2021, 12, 635868. [Google Scholar] [CrossRef]
- Bayuelo-Jiménez, J.S.; Craig, R.; Lynch, J.P. Salinity tolerance of Phaseolus species during germination and early seedling growth. Crop Sci. 2002, 42, 1584–1594. [Google Scholar] [CrossRef]
- Wahid, A.; Gelani, S.; Ashraf, M.; Foolad, M.R. Heat tolerance in plants: An overview. Environ. Exp. Bot. 2007, 61, 199–223. [Google Scholar] [CrossRef]
- Munns, R.; Passioura, J.B.; Colmer, T.D.; Byrt, C.S. Osmotic adjustment and energy limitations to plant growth in saline soil. New Phytol. 2020, 225, 1091–1096. [Google Scholar] [CrossRef]
- Desoky, E.M.; Merwad, A.M.; Elrys, A.S. Response of pea plants to natural bio-stimulants under soil salinity stress. Am. J. Plant Physiol. 2017, 12, 28–37. [Google Scholar]
- El Sabagh, A.; Islam, M.S.; Skalicky, M.; Ali, R.M.; Singh, K.; Anwar, H.M.; Hossain, A.; Mahboob, W.; Aamir, I.M.; Ratnasekera, D.; et al. Salinity stress in wheat (Triticum aestivum L.) in the changing climate: Adaptation and management strategies. Front. Agron. 2021, 3, 661932. [Google Scholar] [CrossRef]
- Hasanuzzaman, M.; Raihan, M.R.H.; Masud, A.A.C.; Rahman, K.; Nowroz, F.; Rahman, M.; Nahar, K.; Fujita, M. Regulation of reactive oxygen species and antioxidant defense in plants under salinity. Int. J. Mol. Sci. 2021, 22, 9326. [Google Scholar] [CrossRef]
- Kataria, S.; Baghel, L.; Guruprasad, K.N. Pre-treatment of Seeds with Static Magnetic Field Improves Germination and Early Growth Characteristics under Salt Stress in Maize and Soybean. Biocatal. Agric. Biotechnol. 2017, 10, 83–90. [Google Scholar] [CrossRef]
- Cirka, M.; Kaya, A.R.; Eryigit, T. Influence of Temperature and Salinity Stress on Seed Germination and Seedling Growth of Soybean (Glycine max L.). Legume Res. Int. J. 2021, 44, 1053–1059. [Google Scholar]
- George, T.S.; Taylor, M.A.; Dodd, I.C.; White, P.J. Climate Change and Consequences for Potato Production: A Review of Tolerance to Emerging Abiotic Stress. Potato Res. 2017, 60, 239–268. [Google Scholar] [CrossRef]
- Khan, Z.; Shahwar, D. Role of Heat Shock Proteins (HSPs) and Heat Stress Tolerance in Crop Plants. In Sustainable Agriculture in the Era of Climate Change; Springer: Cham, Switzerland, 2020; pp. 211–234. [Google Scholar]
- Jahan, M.S.; Shu, S.; Wang, Y.; Chen, Z.; He, M.; Tao, M.; Guo, S. Melatonin Alleviates Heat-Induced Damage of Tomato Seedlings by Balancing Redox Homeostasis and Modulating Polyamine and Nitric Oxide Biosynthesis. BMC Plant Biol. 2019, 19, 414. [Google Scholar] [CrossRef]
- Maryum, Z.; Luqman, T.; Nadeem, S.; Khan, S.M.U.D.; Wang, B.; Ditta, A.; Khan, M.K.R. An Overview of Salinity Stress, Mechanism of Salinity Tolerance and Strategies for Its Management in Cotton. Front. Plant Sci. 2022, 13, 907937. [Google Scholar] [CrossRef]
- Altieri, M.A.; Nicholls, C.I.; Henao, A.; Lana, M.A. Agroecology and the design of climate change-resilient farming systems. Agron. Sustain. Dev. 2015, 35, 869–890. [Google Scholar] [CrossRef]
- Tan, M.; Yolcu, H. Current status of forage crops cultivation and strategies for the future in Turkey: A review. J. Agric. Sci. 2021, 27, 114–121. [Google Scholar] [CrossRef]
- Kara, E.; Sürmen, M. Yield and quality characteristics of forage pea varieties at different phenological stages. Adnan Menderes Univ. J. Fac. Agric. 2023, 20, 295–301. [Google Scholar] [CrossRef]
- Avci, S. Potential impact of annual forage legumes on sustainable cropping systems in Turkey. In Sustainable Agriculture Reviews 51. Legume Agriculture and Biotechnology; Lichtfouse, E., Ed.; Springer: Cham, Switzerland, 2021; Volume 2, pp. 97–118. [Google Scholar]
- Sarıkaya, M.F.; İleri, O.; Erkovan, Ş.; Erkovan, H.; Koç, A. Growing forage pea (Pisum arvense L.) for hay: Different sowing dates and plant densities in Central Anatolia. J. Atatürk Univ. Fac. Agric. 2023, 54, 75–80. [Google Scholar] [CrossRef]
- Yasam, S.; Nair, S.A.H.; Kumar, K.S. Machine learning based robust model for seed germination detection and classification. Int. J. Intell. Syst. Appl. Eng. 2023, 11, 116–124. [Google Scholar]
- Benlioğlu, B.; Demirel, F.; Türkoğlu, A.; Haliloğlu, K.; Özaktan, H.; Kujawa, S.; Niedbała, G. Insights into drought tolerance of tetraploid wheat genotypes in the germination stage using machine learning algorithms. Agriculture 2024, 14, 206. [Google Scholar] [CrossRef]
- Cetin, N.; Ozaktan, H.; Uzun, S.; Uzun, O.; Ciftci, C.Y. Machine learning based mass prediction and discrimination of chickpea (Cicer arietinum L.) cultivars. Euphytica 2023, 219, 20. [Google Scholar] [CrossRef]
- Türkoğlu, A.; Bolouri, P.; Haliloğlu, K.; Eren, B.; Demirel, F.; Işık, M.I.; Niedbała, G. Modeling callus induction and regeneration in hypocotyl explant of fodder pea (Pisum sativum var. arvense L.) using machine learning algorithm method. Agronomy 2023, 13, 2835. [Google Scholar] [CrossRef]
- Türkoğlu, A.; Haliloğlu, K.; Demirel, F.; Aydin, M.; Çiçek, S.; Yiğider, E.; Niedbała, G. Machine Learning Analysis of the Impact of Silver Nitrate and Silver Nanoparticles on Wheat (Triticum aestivum L.): Callus Induction, Plant Regeneration, and DNA Methylation. Plants 2023, 12, 4151. [Google Scholar] [CrossRef]
- Demirel, F.; Eren, B.; Yilmaz, A.; Türkoğlu, A.; Haliloğlu, K.; Niedbała, G.; Nowosad, K. Prediction of grain yield in wheat by CHAID and MARS algorithms analyses. Agronomy 2023, 13, 1438. [Google Scholar] [CrossRef]
- Chen, T.; Guestrin, C. Xgboost: A scalable tree boosting system. In Proceedings of the 22nd ACM SIGKDD International Conference on Knowledge Discovery and Data Mining, San Francisco, CA, USA, 13–17 August 2016; pp. 785–794. [Google Scholar]
- Rasmussen, C.E. Gaussian processes in machine learning. In Summer School on Machine Learning; MIT Press: Cambridge, MA, USA, 2003; pp. 63–71. [Google Scholar]
- Demirel, F.; Uğur, R.; Popescu, G.C.; Demirel, S.; Popescu, M. Usage of Machine learning algorithms for establishing an effective protocol for the in vitro micropropagation ability of black chokeberry (Aronia melanocarpa (Michx.) Elliott). Horticulturae 2023, 9, 1112. [Google Scholar] [CrossRef]
- Camacho-Pérez, E.; Lugo-Quintal, J.M.; Tirink, C.; Aguilar-Quiñonez, J.A.; Gastelum-Delgado, M.A.; Lee-Rangel, H.A.; Chay-Canul, A.J. Predicting carcass tissue composition in Blackbelly sheep using ultrasound measurements and machine learning methods. Trop. Anim. Health Prod. 2023, 55, 300. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria. Available online: https://www.R-project.org/ (accessed on 1 December 2023).
- Wolde, G.; Adamu, C. Impact of salinity on seed germination and biomass yields of field pea (Pisum sativum L.). Asian J. Sci. Technol. 2018, 9, 7565–7569. [Google Scholar]
- Özaktan, H.; Çiftçi, C.Y.; Kaya, M.D.; Uzun, S.; Akdogan, G. Chloride salts inhibit emergence and seedling growth of chickpea rather than germination. Legum. Res. Int. J. 2017, 40, 60–66. [Google Scholar] [CrossRef]
- Majid, A.; Mohsen, S.; Mandana, A.; Saeid, J.H.; Ezatollah, E.; Fariborz, S. The effects of different levels of salinity and indole-3-acetic acid (IAA) on early growth and germination of wheat seedling. J. Stress Physiol. Biochem. 2013, 9, 206–212. [Google Scholar]
- Esechie, H.A. Partitioning of chloride ion in the germinating seed of two forage legumes under varied salinity and temperature regimes. Commun. Soil Sci. Plant Anal. 1995, 26, 3357–3370. [Google Scholar] [CrossRef]
- Morais, M.C.; Panuccio, M.R.; Muscolo, A.; Freitas, H. Does salt stress increase the ability of the exotic legume Acacia longifolia to compete with native legumes in sand dune ecosystems? Environ. Exp. Bot. 2012, 82, 74–79. [Google Scholar] [CrossRef]
- Piwowarczyk, B.; Tokarz, K.; Kamińska, I. Responses of grass pea seedlings to salinity stress in in vitro culture conditions. Plant Cell Tissue Organ Cult. 2016, 124, 227–240. [Google Scholar] [CrossRef]
- Ibrahim, E.A. Seed priming to alleviate salinity stress in germinating seeds. J. Plant Physiol. 2016, 192, 38–46. [Google Scholar] [CrossRef]
- Hasegawa, P.M.; Bressan, R.A.; Zhu, J.K.; Bohnert, H.J. Plant cellular and molecular responses to high salinity. Annu. Rev. Plant Biol. 2020, 51, 463–499. [Google Scholar] [CrossRef]
- Aslam, M.; Fakher, B.; Ashraf, M.A.; Cheng, Y.; Wang, B.; Qin, Y. Plant low-temperature stress: Signaling and response. Agronomy 2022, 12, 702. [Google Scholar] [CrossRef]
- Jankovska-Bortkevič, E.; Katerova, Z.; Todorova, D.; Jankauskienė, J.; Mockevičiūtė, R.; Sergiev, I.; Jurkonienė, S. Effects of auxin-type plant growth regulators and cold stress on the endogenous polyamines in pea plants. Horticulturae 2023, 9, 244. [Google Scholar] [CrossRef]
- Çaçan, E.; Özbay, N.; Kökten, K. Determination of germination and emergence performances of some forage pea lines and varieties at different temperatures. Nevsehir J. Sci. Technol. 2016, 5, 62–68. [Google Scholar]
- Sivritepe, H.Ö. Assessment of Seed Viability. Alatarım J. 2011, 10, 94–105. [Google Scholar]
- Brar, G.S.; Gomez, J.F.; McMichael, B.L.; Matches, A.G.; Taylor, H.M. Germination of twenty forage legumes as influenced by temperature. Agron. J. 1991, 83, 173–175. [Google Scholar] [CrossRef]
- Aasim, M.; Katırcı, R.; Akgur, O.; Yildirim, B.; Mustafa, Z.; Nadeem, M.A.; Baloch, F.S.; Karakoy, T.; Yılmaz, G. Machine learning (ML) algorithms and artificial neural network for optimizing in vitro germination and growth indices of industrial hemp (Cannabis sativa L.). Ind. Crops Prod. 2022, 181, 114801. [Google Scholar] [CrossRef]
- Gökmen, F.; Uygur, V.; Sukuşu, E. Extreme gradient boosting regression model for soil available boron. Eurasian Soil Sci. 2023, 56, 738–746. [Google Scholar] [CrossRef]
- Tırınk, C.; Piwczyński, D.; Kolenda, M.; Önder, H. Estimation of body weight based on biometric measurements by using random forest regression, support vector regression and cart algorithms. Animals 2023, 13, 798. [Google Scholar] [CrossRef] [PubMed]
- Şimşek, Ö.; Dalda Şekerci, A.; Isak, M.A.; Bulut, F.; İzgü, T.; Tütüncü, M.; Dönmez, D. Optimizing micropropagation and rooting protocols for diverse lavender genotypes: A Synergistic Approach Integrating Machine Learning Techniques. Horticulturae 2024, 10, 52. [Google Scholar] [CrossRef]
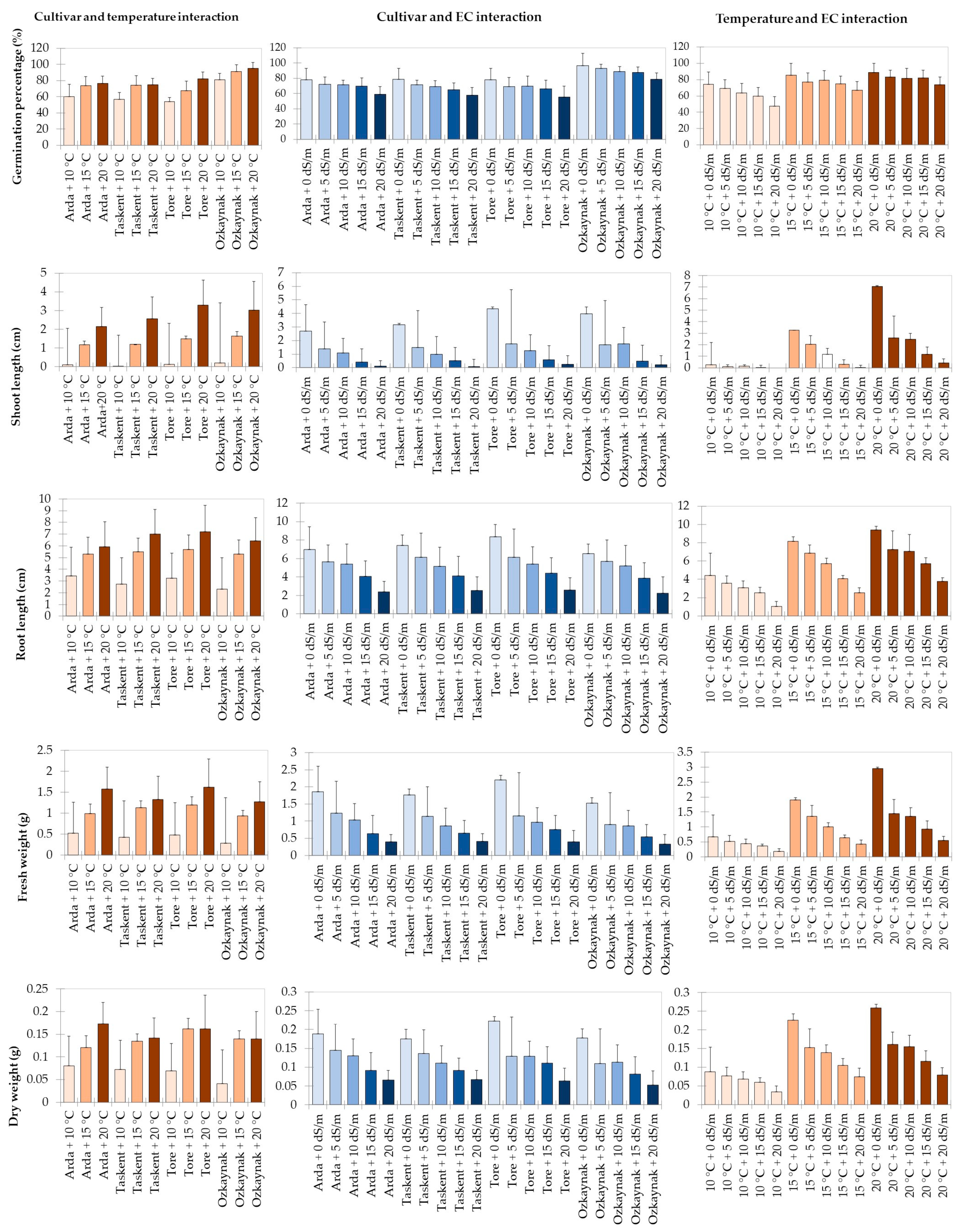


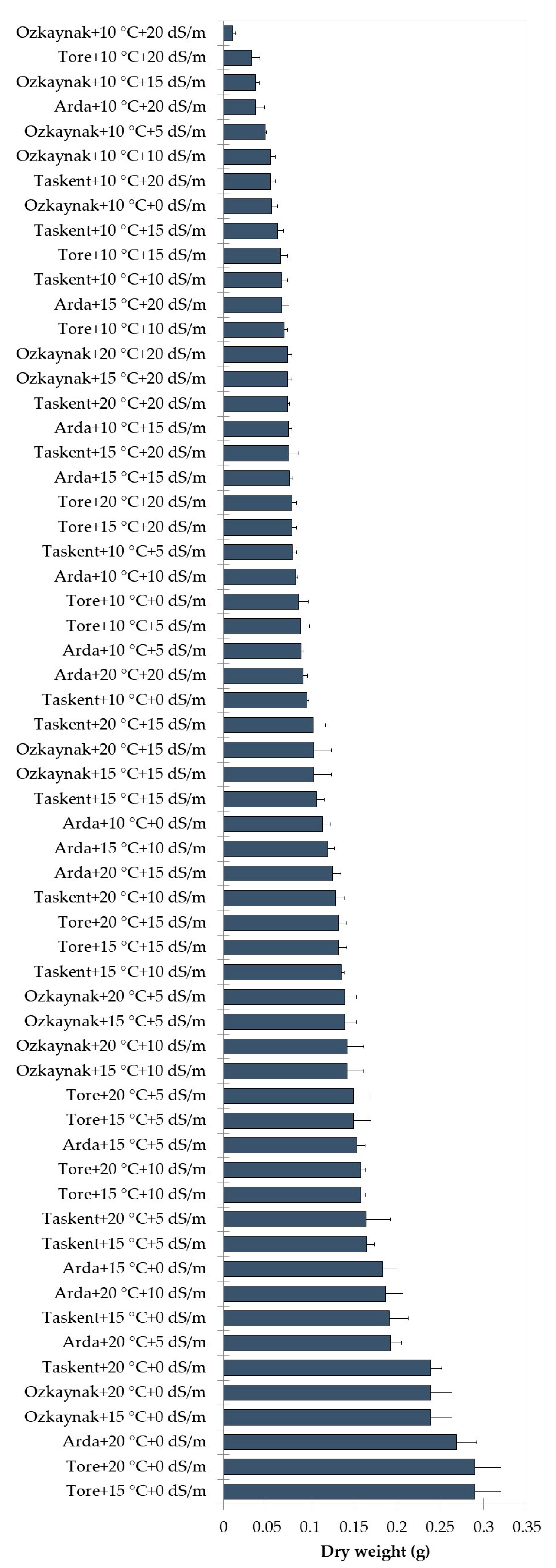
| Germination Percentage (%) | Shoot Length (cm) | Root Length (cm) | Fresh Weight (g) | Dry Weight (g) | |
|---|---|---|---|---|---|
| Cultivar | |||||
| Arda | 70.13 ± 11.79 b * | 1.14 ± 1.40 b | 4.89 ± 2.22 bc | 1.03 ± 0.73 b | 0.12 ± 0.06 b |
| Ozkaynak | 89.13 ± 9.20 a | 1.62 ± 2.09 a | 4.69 ± 2.39 c | 0.83 ± 0.64 d | 0.11 ± 0.06 d |
| Taskent | 68.53 ± 12.10 bc | 1.25 ± 1.75 b | 5.07 ± 2.54 b | 0.97 ± 0.66 c | 0.12 ± 0.05 c |
| Tore | 67.80 ± 14.86 c | 1.63 ± 2.37 a | 5.38 ± 2.67 a | 1.10 ± 0.87 a | 0.13 ± 0.07 a |
| Temperature (°C) | |||||
| 10 | 62.95 ± 15.04 c * | 0.10 ± 0.17 c | 2.93 ± 1.30 c | 0.43 ± 0.20 c | 0.07 ± 0.02 c |
| 15 | 76.75 ± 12.01 b | 1.38 ± 1.26 b | 5.46 ± 2.08 b | 1.06 ± 0.56 b | 0.14 ± 0.05 b |
| 20 | 82.00 ± 10.55 a | 2.75 ± 2.49 a | 6.63 ± 2.24 a | 1.45 ± 0.86 a | 0.15 ± 0.06 a |
| EC (dS m−1) | |||||
| 0 | 82.92 ± 11.57 a * | 3.54 ± 3.04 a | 7.31 ± 2.51 a | 1.84 ± 1.01 a | 0.19 ± 0.08 a |
| 5 | 76.58 ± 12.96 b | 1.58 ± 1.16 b | 5.90 ± 2.02 b | 1.11 ± 0.46 b | 0.13 ± 0.04 b |
| 10 | 74.92 ± 12.61 b | 1.27 ± 1.04 c | 5.29 ± 1.75 c | 0.93 ± 0.41 c | 0.12 ± 0.04 c |
| 15 | 72.25 ± 14.01 c | 0.51 ± 0.55 d | 4.10 ± 1.39 d | 0.64 ± 0.26 d | 0.09 ± 0.03 d |
| 20 | 62.83 ± 16.21 d | 0.16 ± 0.31 e | 2.43 ± 1.22 e | 0.38 ± 0.16 e | 0.06 ± 0.02 e |
| Cultivar | 6245.066 *** | 3.882 *** | 5.149 *** | 0.765 *** | 0.006 *** |
| Temperature | 7745.400 *** | 140.465 *** | 287.179 *** | 21.107 *** | 0.180 *** |
| EC | 2576.733 *** | 83.294 *** | 163.564 *** | 14.668 *** | 0.110 *** |
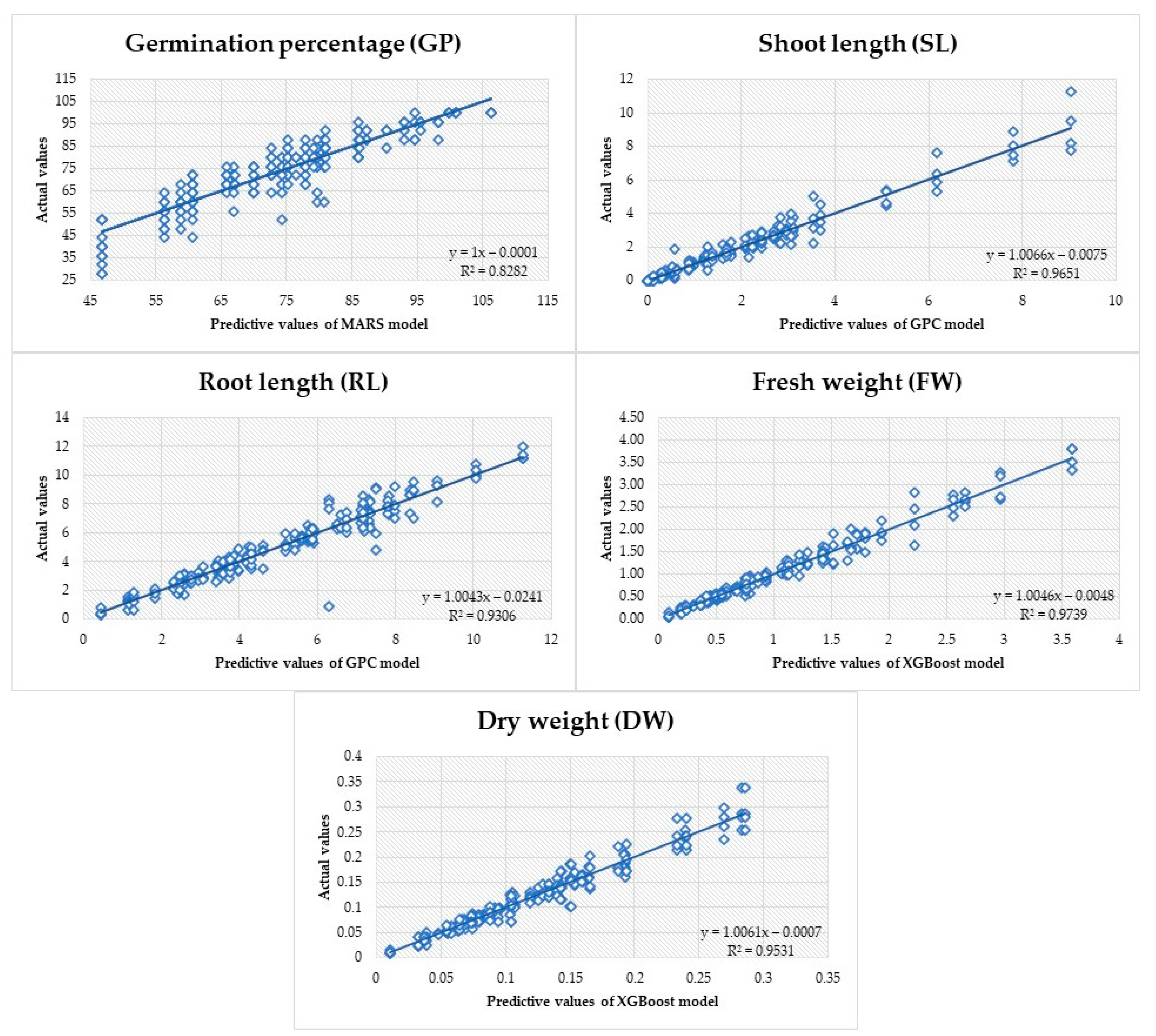
| Variables | ML Criterion | MARS | XGboost | GPC | |||
|---|---|---|---|---|---|---|---|
| Train | Test | Train | Test | Train | Test | ||
| GP | R2 | 0.863 | 0.899 | 0.926 | 0.823 | 0.916 | 0.849 |
| RMSE | 5.530 | 5.344 | 3.990 | 6.560 | 4.263 | 6.083 | |
| MAPE | 6.515 | 6.953 | 4.360 | 7.541 | 4.908 | 7.675 | |
| MAD | 4.176 | 4.125 | 2.948 | 4.533 | 3.308 | 4.699 | |
| SD ratio | 0.370 | 0.317 | 0.926 | 0.420 | 0.291 | 0.389 | |
| SL | R2 | 0.891 | 0.869 | 0.980 | 0.897 | 0.974 | 0.922 |
| RMSE | 0.641 | 0.781 | 0.264 | 0.690 | 0.298 | 0.602 | |
| MAPE | 20.896 | 15.077 | 13.319 | 12.970 | 13.438 | 11.850 | |
| MAD | 0.364 | 0.427 | 0.155 | 0.383 | 0.167 | 0.326 | |
| SD ratio | 0.330 | 0.357 | 0.143 | 0.319 | 0.162 | 0.280 | |
| RL | R2 | 0.854 | 0.891 | 0.933 | 0.875 | 0.926 | 0.900 |
| RMSE | 0.939 | 0.791 | 0.657 | 0.805 | 0.689 | 0.719 | |
| MAPE | 20.276 | 15.429 | 12.643 | 13.333 | 13.947 | 12.673 | |
| MAD | 0.653 | 0.615 | 0.401 | 0.560 | 0.433 | 0.554 | |
| SD ratio | 0.382 | 0.328 | 0.259 | 0.353 | 0.272 | 0.315 | |
| FW | R2 | 0.880 | 0.903 | 0.973 | 0.966 | 0.970 | 0.956 |
| RMSE | 0.255 | 0.273 | 0.123 | 0.130 | 0.129 | 0.149 | |
| MAPE | 29.992 | 32.514 | 9.694 | 11.635 | 9.719 | 12.947 | |
| MAD | 0.186 | 0.196 | 0.078 | 0.090 | 0.082 | 0.107 | |
| SD ratio | 0.346 | 0.306 | 0.165 | 0.183 | 0.173 | 0.206 | |
| DW | R2 | 0.828 | 0.827 | 0.960 | 0.895 | 0.957 | 0.896 |
| RMSE | 0.027 | 0.027 | 0.013 | 0.021 | 0.014 | 0.021 | |
| MAPE | 22.138 | 21.838 | 8.294 | 12.395 | 9.244 | 14.362 | |
| MAD | 0.020 | 0.020 | 0.009 | 0.013 | 0.010 | 0.014 | |
| SD ratio | 0.415 | 0.416 | 0.200 | 0.324 | 0.208 | 0.321 | |
| GP | SL | RL | FW | DW | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Terms | Model | Coefficient | Model | Coefficient | Model | Coefficient | Model | Coefficient | Model | Coefficient |
| 1 | Intercept | 68.800 | Intercept | 0.103 | Intercept | 5.107 | Intercept | 0.963 | Intercept | 0.134 |
| 2 | Ozkan | 19.800 | 15 °C | 2.557 | Tore | 0.496 | 15 °C | 0.633 | Ozkan | −0.014 |
| 3 | Tore | −5.600 | 20 °C | 6.962 | 15 °C | 2.533 | 20 °C | 1.988 | 15 °C | 0.076 |
| 4 | 15 °C | 13.800 | 15 °C × 10 EC | −1.468 | 20 °C | 3.707 | 5 EC | −0.346 | 20 °C | 0.099 |
| 5 | 20 °C | 14.850 | 15 °C × 15 EC | −2.335 | 5 EC | −1.410 | 10 EC | −0.560 | 5 EC | −0.061 |
| 6 | 5 EC | −6.333 | 15 °C × 20 EC | −2.611 | 10 EC | −2.025 | 15 EC | −0.781 | 10 EC | −0.070 |
| 7 | 10 EC | −8.000 | 20 °C × 5 EC | −4.464 | 15 EC | −3.213 | 20 EC | −0.980 | 15 EC | −0.096 |
| 8 | 15 EC | −10.667 | 20 °C × 10 EC | −4.597 | 20 EC | −4.877 | 20 °C × 5 EC | −1.159 | 20 EC | −0.097 |
| 9 | 20 EC | −22.000 | 20 °C × 15 EC | −5.876 | 20 °C × 10 EC | −1.034 | Tore × 15 °C | 0.026 | ||
| 10 | Tore × 20 °C | 12.200 | 20 °C × 20 EC | −6.628 | 20 °C × 15 EC | −1.237 | 15 °C × 20 EC | −0.042 | ||
| 11 | 20 °C × 20 EC | 5.750 | 20 °C × 20 EC | −1.422 | 20 °C ×20 EC | −0.054 | ||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Okumuş, O.; Say, A.; Eren, B.; Demirel, F.; Uzun, S.; Yaman, M.; Aydın, A. Using Machine Learning Algorithms to Investigate the Impact of Temperature Treatment and Salt Stress on Four Forage Peas (Pisum sativum var. arvense L.). Horticulturae 2024, 10, 656. https://doi.org/10.3390/horticulturae10060656
Okumuş O, Say A, Eren B, Demirel F, Uzun S, Yaman M, Aydın A. Using Machine Learning Algorithms to Investigate the Impact of Temperature Treatment and Salt Stress on Four Forage Peas (Pisum sativum var. arvense L.). Horticulturae. 2024; 10(6):656. https://doi.org/10.3390/horticulturae10060656
Chicago/Turabian StyleOkumuş, Onur, Ahmet Say, Barış Eren, Fatih Demirel, Satı Uzun, Mehmet Yaman, and Adnan Aydın. 2024. "Using Machine Learning Algorithms to Investigate the Impact of Temperature Treatment and Salt Stress on Four Forage Peas (Pisum sativum var. arvense L.)" Horticulturae 10, no. 6: 656. https://doi.org/10.3390/horticulturae10060656
APA StyleOkumuş, O., Say, A., Eren, B., Demirel, F., Uzun, S., Yaman, M., & Aydın, A. (2024). Using Machine Learning Algorithms to Investigate the Impact of Temperature Treatment and Salt Stress on Four Forage Peas (Pisum sativum var. arvense L.). Horticulturae, 10(6), 656. https://doi.org/10.3390/horticulturae10060656









