Wild Edible Plant Species in the ‘King’s Lagoon’ Coastal Wetland: Survey, Collection, Mapping and Ecological Characterization
Abstract
1. Introduction
- (1)
- The aim of the experimental work presented here was to survey and identify the alimurgical species present in the wetland at this stage of ecological succession, to map their spatial location according to a land cover composition, to verify their presence and abundance, to establish the most frequent correspondences between the various species and any systematic associations between them, and to define a monitoring plan that can be adopted in subsequent years to follow the ongoing succession of the vegetation (limited to alimurgical plant species in this particular study).
- (2)
- Another aim of the research is to select the most widespread, suitable and useful WEPs for the possible experimental start of a productive activity of cultivation, processing and marketing of these alimurgical plants, in order to plan an activity that, while maintaining the priority of safeguarding the precious and fragile natural context, also allows a certain rural sustainable development in the area of interest.
2. Materials and Methods
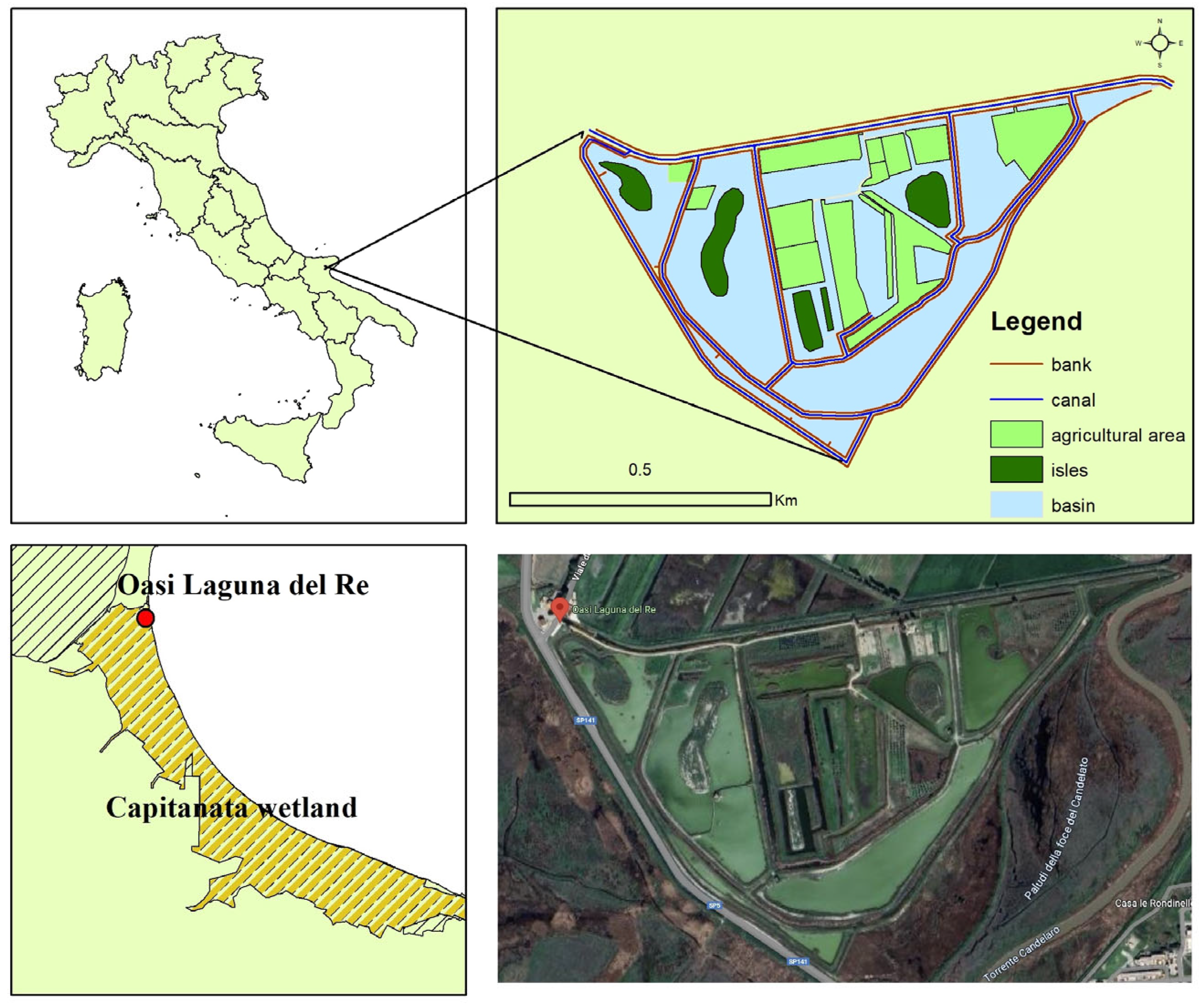
2.1. Study Area
2.2. Land Cover Mapping
- -
- Unique definition of each category;
- -
- Hierarchical organization of the classification structure, i.e., the higher-level categories are made up of a set of lower level categories;
- -
- Categories at the same level are mutually exclusive.
2.3. The Species under Study
2.4. Plant Species Detection and Survey Design
2.5. Data Analysis and Statistical Processing
[C;C]M = M11 + M00 − M10 − M01
N = M11 + M00 + M10 + M01
2.6. Seed Collection and Conservation
3. Results
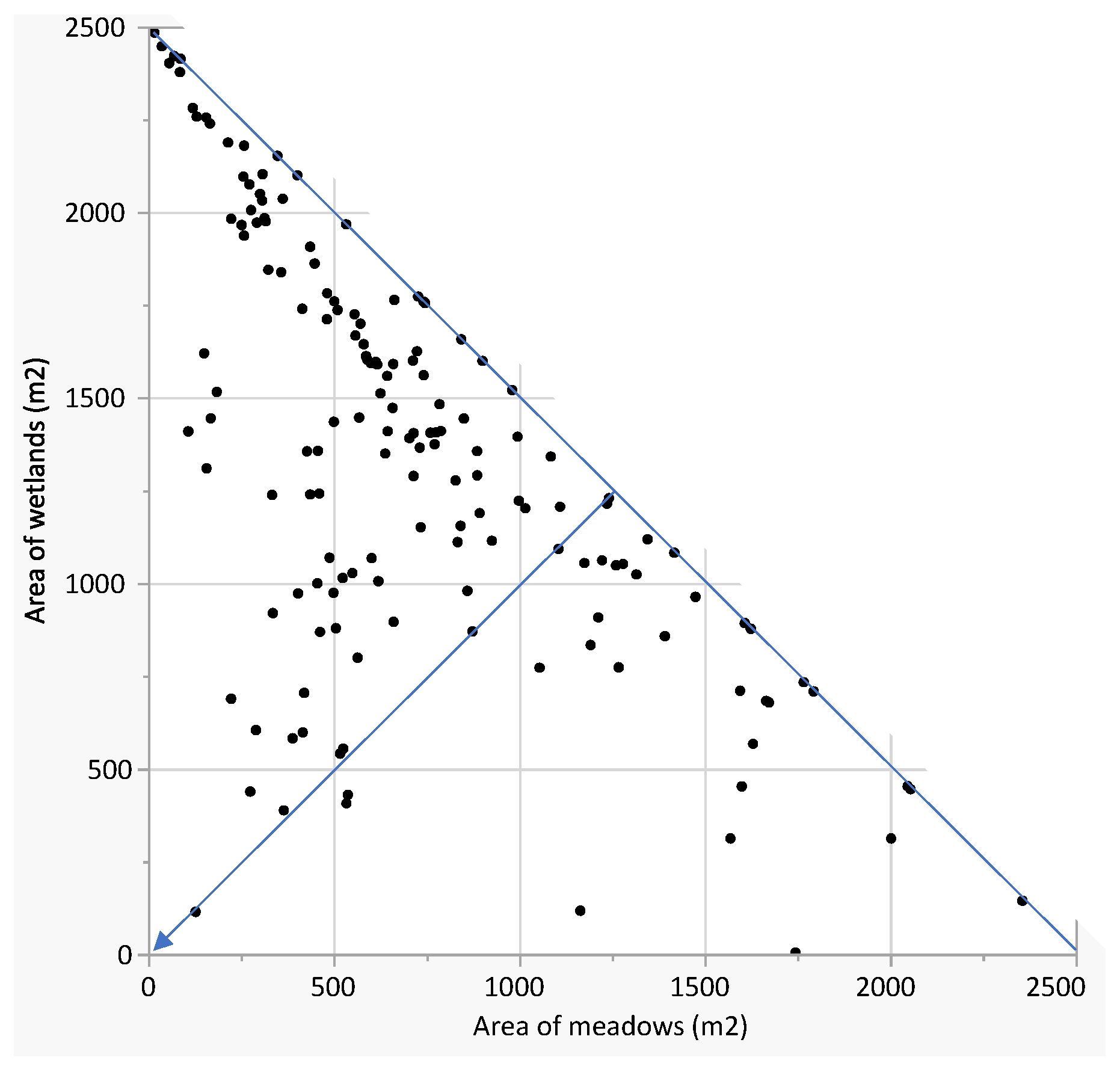
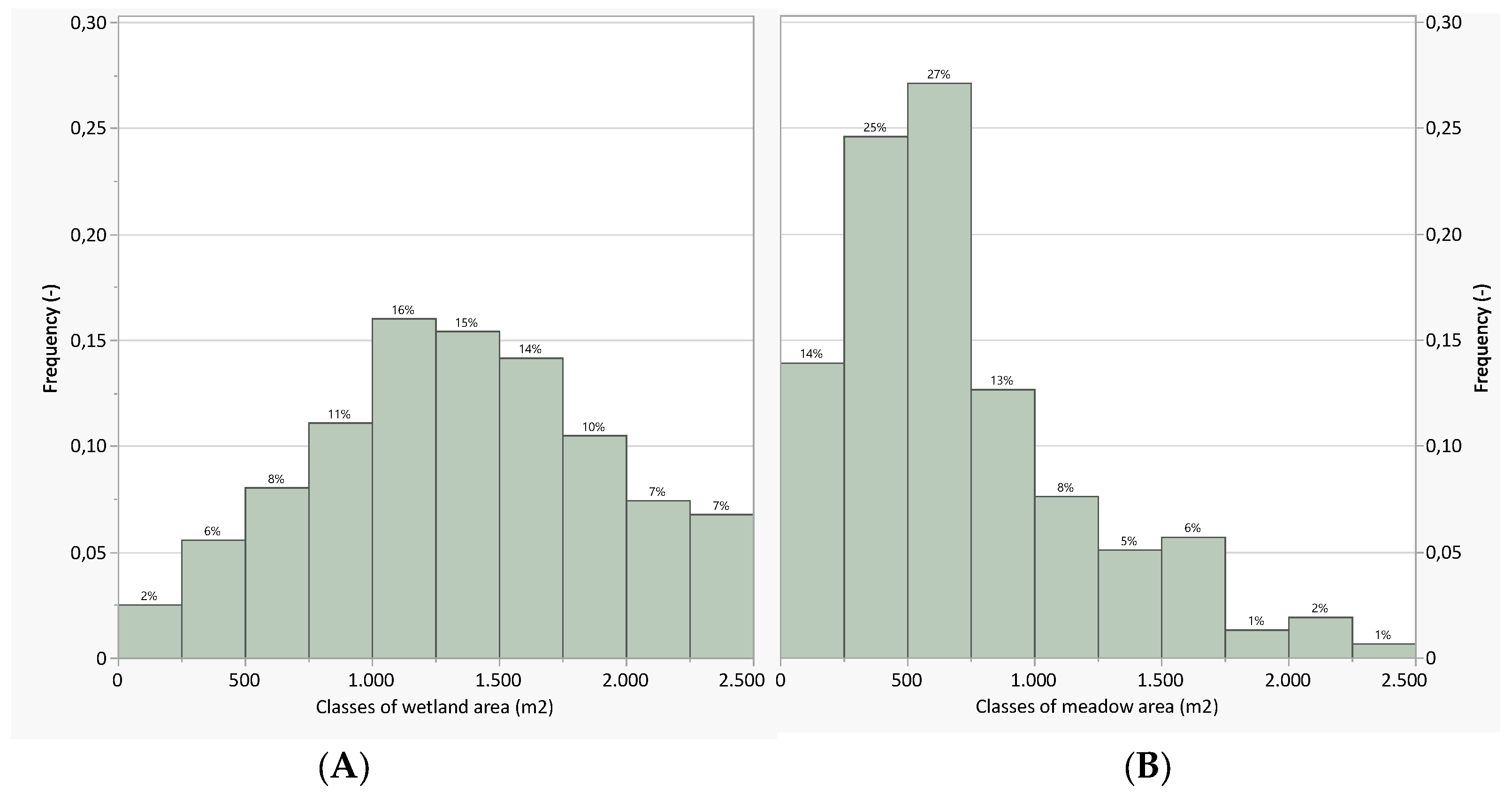
3.1. Land-Cover Analysis
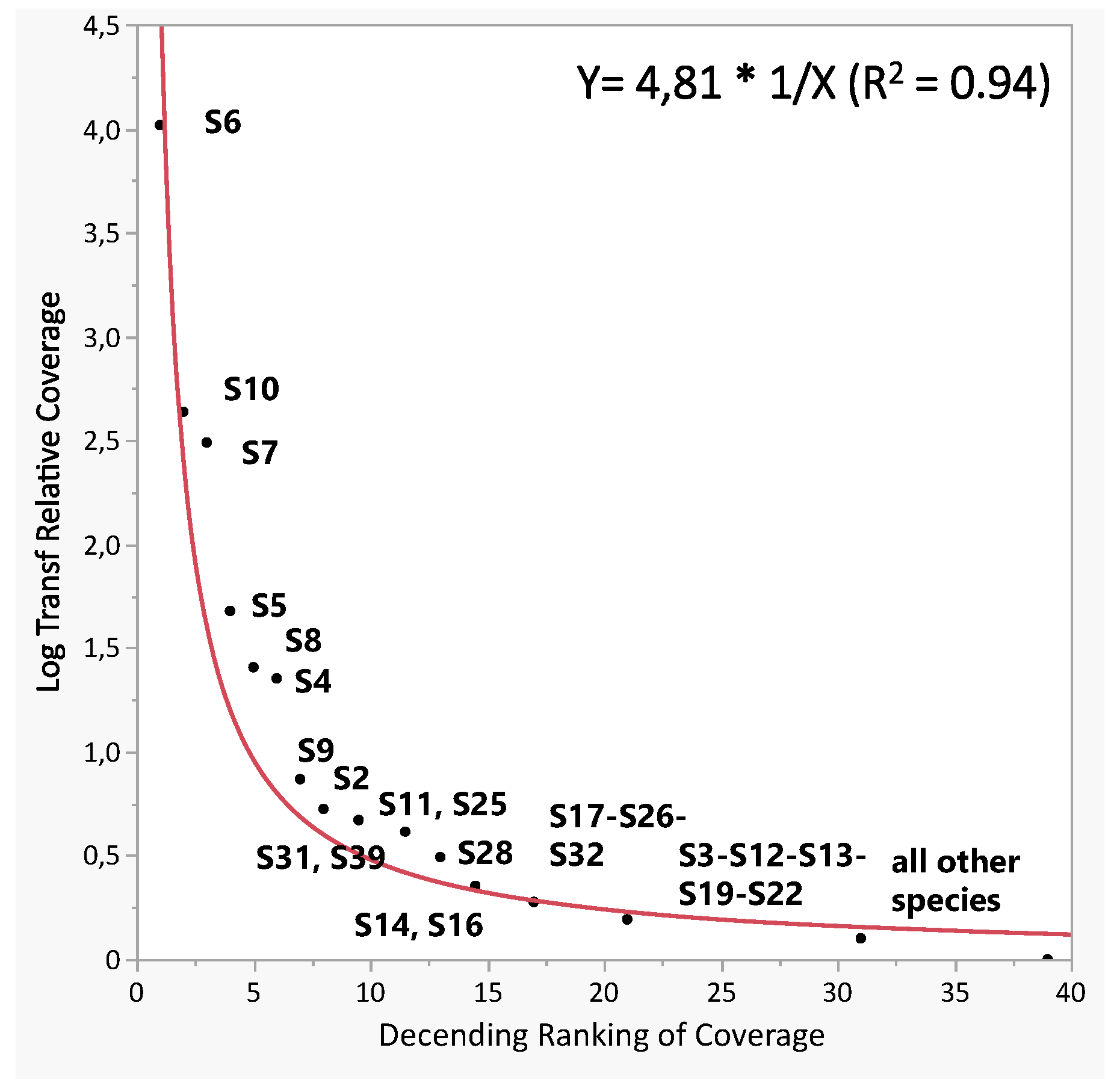
3.2. Species Analysis
3.3. Cells/Sites Analysis
4. Discussion
4.1. Plant Survey and Ecological Charactwrization
- -
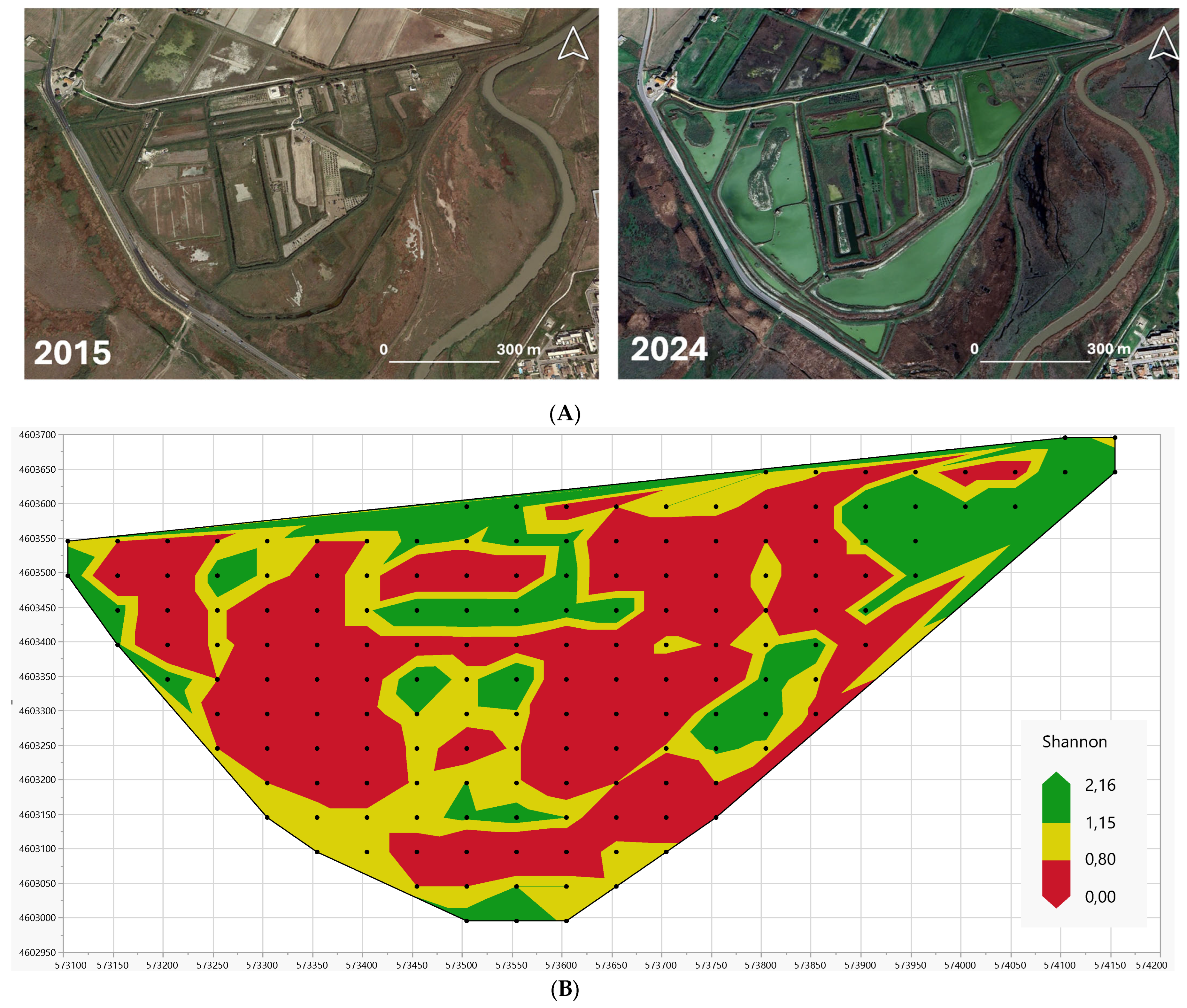
- Sites with the highest diversity (green areas in Figure 8B). These correspond with the forth (upper) quartile of the Shannon distribution (Figure 7A, cumulative frequency higher than 75%). Undisturbed sites are included in this category, i.e., sites not involved in the LIFE project earthworks aimed at wetland restoring; embankment areas, i.e., areas along the edge line between agriculture and reedbeds, which correspond with areas that existed prior to restoration, but which are adjacent to new wetlands created as a result of recent excavations; and “corridors” connecting new stretches of water (“valleys”) and halophilic meadows, which are periodically and partially flooded and act as “sources” of biodiversity. In all of these areas, the natural vegetation has developed freely, without any particular interference or disturbance from human activity.
- -
- Sites of higher diversity (yellow areas in Figure 8B). These correspond with the third quartile of the Shannon distribution (Figure 7A, cumulative frequency in the range 50–75%). These sites are located along the banks of streams with soils of different textures; from west to east the soil texture changes from clayey to sandy, which slightly affects plant diversity.
- -
- Sites with lower diversity (red areas in Figure 8B). These correspond with the first and second quartiles of the Shannon distribution (Figure 7A, cumulative frequency in the range 0–50%). These sites are characterized by high soil salinity and have therefore been occupied by pioneer Salicornia vegetation and other annuals colonizing mud and sand.
4.2. New Edible Crop Potential: From Wild to Cultivated Plant
- -
- Promoting low-input/low-impact farming practices;
- -
- Developing agronomic practices based on the ecosystem structure and functioning;
- -
- Applying innovative cropping models and agricultural practices related to permaculture (food forest) and agroforestry;
- -
- Cultivating traditional crops, old varieties, and phytoalimurgic species;
- -
- Cultivating halophytic species;
- -
- Creating permanent strips of spontaneous or cultivated vegetation;
- -
- Making proper use of brackish water irrigation techniques;
- -
- Applying alkaline soil remediation techniques;
- -
- Utilizing climatic adaptation to increase the resilience of farming systems (mixed- and inter-cropping, minimum soil mechanical disturbance, maintenance of a permanent soil cover, diversification of plant species, prevention of soil losses from water run-off and consequent erosion, improvement of the agricultural soil quality, etc.).
5. Conclusions
- To repeat the spring survey in the coming years, at least every two years, in order to reconstruct precisely the dynamics of the ecological succession in which this particular ecosystem is developing, once it has returned to its natural development.
- To integrate the information to be obtained from the surveys at a broader and more general level, in the sense of carrying out a comprehensive botanical analysis.
- To derive some indications and suggestions on the potential for establishing appropriate intercropping between alimurgical species, to be applied in the possible start of cultivation practices of these species for commercial purposes. Indeed, good insights can be gained by analyzing natural plant associations (i.e., the strong and balanced combination of some of the most representative plant species in the wetland).
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Clark, M.A.; Domingo, N.G.G.; Colgan, K.; Thakrar, S.K.; Tilman, D.; Lynch, J.; Azevedo, I.L.; Hill, J.D. Global Food System Emissions Could Preclude Achieving the 1.5° and 2°C Climate Change Targets. Science 2020, 370, 705–708. [Google Scholar] [CrossRef] [PubMed]
- Crane, P.R.; Ge, S.; Hong, D.; Huang, H.; Jiao, G.; Knapp, S.; Kress, W.J.; Mooney, H.; Raven, P.H.; Wen, J.; et al. The Shenzhen Declaration on Plant Sciences—Uniting Plant Sciences and Society to Build a Green, Sustainable Earth. Plants People Planet 2019, 1, 59–61. [Google Scholar] [CrossRef]
- FAO (Ed.) Transforming Food Systems for Food Security, Improved Nutrition and Affordabel Healthy Diets for All; The State of Food Security and Nutrition in the World; FAO: Rome, Italy, 2021; ISBN 978-92-5-134325-8. [Google Scholar]
- Mariutti, L.R.B.; Rebelo, K.S.; Bisconsin-Junior, A.; De Morais, J.S.; Magnani, M.; Maldonade, I.R.; Madeira, N.R.; Tiengo, A.; Maróstica, M.R.; Cazarin, C.B.B. The Use of Alternative Food Sources to Improve Health and Guarantee Access and Food Intake. Food Res. Int. 2021, 149, 110709. [Google Scholar] [CrossRef] [PubMed]
- Tardío, J. CHAPTER 10 Spring Is Coming the Gathering and Consumption of Wild Vegetables in Spain. In Ethnobotany in the New Europe; Pardo-de-Santayana, M., Pieroni, A., Puri, R.K., Eds.; Berghahn Books: New York, NY, USA, 2022; pp. 211–238. ISBN 978-1-84545-814-0. [Google Scholar]
- Biscotti, N. Peregrinazioni Fitoalimurgiche-Dal Gargano Alle Puglie; Centro Grafico Francescano: Foggia, Italy, 2012. [Google Scholar]
- Aliotta, G. Edible Wild Plants in Italy. Inform. Bot. Ital. 1987, 19, 17–30. [Google Scholar]
- Guarrera, P.M.; Savo, V. Perceived Health Properties of Wild and Cultivated Food Plants in Local and Popular Traditions of Italy: A Review. J. Ethnopharmacol. 2013, 146, 659–680. [Google Scholar] [CrossRef] [PubMed]
- Luciano, R.; Gatti, C. Erbe Spontanee Commestibili; Nuova ed. riv. e integrata.; Araba Fenice: Boves, Italy, 2014; ISBN 978-88-95853-10-9. [Google Scholar]
- Dempewolf, H.; Eastwood, R.J.; Guarino, L.; Khoury, C.K.; Müller, J.V.; Toll, J. Adapting Agriculture to Climate Change: A Global Initiative to Collect, Conserve, and Use Crop Wild Relatives. Agroecol. Sustain. Food Syst. 2014, 38, 369–377. [Google Scholar] [CrossRef]
- Reyes-García, V.; Menendez-Baceta, G.; Aceituno-Mata, L.; Acosta-Naranjo, R.; Calvet-Mir, L.; Domínguez, P.; Garnatje, T.; Gómez-Baggethun, E.; Molina-Bustamante, M.; Molina, M.; et al. From Famine Foods to Delicatessen: Interpreting Trends in the Use of Wild Edible Plants through Cultural Ecosystem Services. Ecol. Econ. 2015, 120, 303–311. [Google Scholar] [CrossRef]
- Schulp, C.J.E.; Thuiller, W.; Verburg, P.H. Wild Food in Europe: A Synthesis of Knowledge and Data of Terrestrial Wild Food as an Ecosystem Service. Ecol. Econ. 2014, 105, 292–305. [Google Scholar] [CrossRef]
- Łuczaj, Ł.; Pieroni, A.; Tardío, J.; Pardo-de-Santayana, M.; Sõukand, R.; Svanberg, I.; Kalle, R. Wild Food Plant Use in 21st Century Europe: The Disappearance of Old Traditions and the Search for New Cuisines Involving Wild Edibles. Acta Soc. Bot. Pol. 2012, 81, 359–370. [Google Scholar] [CrossRef]
- De Groot, R.S.; Fisher, B.; Christie, M.; Aronson, J.; Braat, L.; Haines-Young, R.; Gowdy, J.; Maltby, E.; Neuville, A.; Polasky, S. Integrating the Ecological and Economic Dimensions in Biodiversity and Ecosystem Service Valuation. In The Economics of Ecosystems and Biodiversity: Ecological and Economic Foundations; Kumar, P., Ed.; Earthscan: London, UK; Washington, DC, USA, 2012; pp. 9–40. [Google Scholar]
- Hassan, R.M.; Scholes, R.J.; Ash, N.; Millennium Ecosystem Assessment (Program) (Eds.) Ecosystems and Human Well-Being: Current State and Trends: Findings of the Condition and Trends Working Group of the Millennium Ecosystem Assessment; The Millennium Ecosystem Assessment Series; Island Press: Washington, DC, USA, 2005; ISBN 978-1-55963-227-0. [Google Scholar]
- Ghirardini, M.P.; Carli, M.; Del Vecchio, N.; Rovati, A.; Cova, O.; Valigi, F.; Agnetti, G.; Macconi, M.; Adamo, D.; Traina, M.; et al. The Importance of a Taste. A Comparative Study on Wild Food Plant Consumption in Twenty-One Local Communities in Italy. J. Ethnobiol. Ethnomed. 2007, 3, 22. [Google Scholar] [CrossRef]
- THE 17 GOALS|Sustainable Development. Available online: https://sdgs.un.org/goals (accessed on 2 May 2024).
- Heinrich, M.; Kufer, J.; Leonti, M.; Pardo-de-Santayana, M. Ethnobotany and Ethnopharmacology—Interdisciplinary Links with the Historical Sciences. J. Ethnopharmacol. 2006, 107, 157–160. [Google Scholar] [CrossRef] [PubMed]
- Heinrich, M.; Nebel, S.; Leonti, M.; Rivera, D.; Obón, C. ‘Local Food-Nutraceuticals’: Bridging the Gap between Local Knowledge and Global Needs. In Forum of Nutrition; Heinrich, M., Müller, W.E., Galli, C., Eds.; S. Karger AG: Basel, Switzerland, 2006; Volume 59, pp. 1–17. ISBN 978-3-8055-8124-0. [Google Scholar]
- Aberoumand, A.; Deokule, S.S. Determination of Elements Profile of Some Wild Edible Plants. Food Anal. Methods 2009, 2, 116–119. [Google Scholar] [CrossRef]
- Amirul Alam, M.; Juraimi, A.S.; Rafii, M.Y.; Hamid, A.A.; Kamal Uddin, M.; Alam, M.Z.; Latif, M.A. Genetic Improvement of Purslane (Portulaca oleracea L.) and Its Future Prospects. Mol. Biol. Rep. 2014, 41, 7395–7411. [Google Scholar] [CrossRef]
- Guil Guerrero, J.L.; Giménez Martínez, J.J.; Torija Isasa, M.E. Mineral Nutrient Composition of Edible Wild Plants. J. Food Compos. Anal. 1998, 11, 322–328. [Google Scholar] [CrossRef]
- Visioli, F.; Galli, C. The Role of Antioxidants in the Mediterranean Diet. Lipids 2001, 36, S49–S52. [Google Scholar] [CrossRef]
- Visioli, F.; Grande, S.; Bogani, P.; Galli, C. The Role of Antioxidants in the Mediterranean Diets: Focus on Cancer. Eur. J. Cancer Prev. 2004, 13, 337–343. [Google Scholar] [CrossRef]
- Goulet, O. Potential Role of the Intestinal Microbiota in Programming Health and Disease: Figure 1. Nutr. Rev. 2015, 73, 32–40. [Google Scholar] [CrossRef] [PubMed]
- Bondonno, C.P.; Croft, K.D.; Ward, N.; Considine, M.J.; Hodgson, J.M. Dietary Flavonoids and Nitrate: Effects on Nitric Oxide and Vascular Function. Nutr. Rev. 2015, 73, 216–235. [Google Scholar] [CrossRef] [PubMed]
- El Hafid, R.; Blade, S.F.; Hoyano, Y. Seeding Date and Nitrogen Fertilization Effects on the Performance of Borage (Borago officinalis L.). Ind. Crops Prod. 2002, 16, 193–199. [Google Scholar] [CrossRef]
- Uddin, M.K.; Juraimi, A.S.; Ali, M.E.; Ismail, M.R. Evaluation of Antioxidant Properties and Mineral Composition of Purslane (Portulaca oleracea L.) at Different Growth Stages. Int. J. Mol. Sci. 2012, 13, 10257–10267. [Google Scholar] [CrossRef]
- De Lisi, A.; Montesano, V.; Negro, D.; Sarli, G.; Blanco, E.; Sonnante, G.; Laghetti, G. Genetic Diversity in Borago Officinalis Germplasm as Revealed by Seed Oils and AFLP Polymorphism. Genet. Resour. Crop. Evol. 2014, 61, 853–859. [Google Scholar] [CrossRef]
- Grieve, C.M. Purslane (Portulaca oleracea L.): A Halophytic Crop for Drainage Water Reuse Systems. Plant Soil 1997, 192, 277–283. [Google Scholar] [CrossRef]
- Biscotti, N.; Bonsanto, D.; Del Viscio, G. The Traditional Food Use of Wild Vegetables in Apulia (Italy) in the Light of Italian Ethnobotanical Literature. Ital. Bot. 2018, 5, 1–24. [Google Scholar] [CrossRef]
- Paura, B.; Di Marzio, P. Making a Virtue of Necessity: The Use of Wild Edible Plant Species (Also Toxic) in Bread Making in Times of Famine According to Giovanni Targioni Tozzetti (1766). Biology 2022, 11, 285. [Google Scholar] [CrossRef]
- Garn, S.M.; Leonard, W.R. What Did Our Ancestors Eat? Nutr. Rev. 2009, 47, 337–345. [Google Scholar] [CrossRef]
- Chivenge, P.; Mabhaudhi, T.; Modi, A.; Mafongoya, P. The Potential Role of Neglected and Underutilised Crop Species as Future Crops under Water Scarce Conditions in Sub-Saharan Africa. Int. J. Environ. Res. Public Health 2015, 12, 5685–5711. [Google Scholar] [CrossRef]
- Collins, W.W.; Qualset, C.O. (Eds.) Biodiversity in Agroecosystems; CRC Press: Boca Raton, FL, USA, 1998; ISBN 978-1-00-304066-8. [Google Scholar]
- Prescott-Allen, R.; Prescott-Allen, C. How Many Plants Feed the World? Conserv. Biol. 1990, 4, 365–374. [Google Scholar] [CrossRef]
- Schunko, C.; Vogl, C.R. Organic Farmers Use of Wild Food Plants and Fungi in a Hilly Area in Styria (Austria). J. Ethnobiol. Ethnomed. 2010, 6, 17. [Google Scholar] [CrossRef]
- Tardío, J.; Pardo-De-Santayana, M.; Morales, R. Ethnobotanical Review of Wild Edible Plants in Spain. Bot. J. Linn. Soc. 2006, 152, 27–71. [Google Scholar] [CrossRef]
- Bonet, M.À.; Vallès, J. Use of Non-Crop Food Vascular Plants in Montseny Biosphere Reserve (Catalonia, Iberian Peninsula). Int. J. Food Sci. Nutr. 2002, 53, 225–248. [Google Scholar] [CrossRef]
- Cornara, L.; La Rocca, A.; Marsili, S.; Mariotti, M.G. Traditional Uses of Plants in the Eastern Riviera (Liguria, Italy). J. Ethnopharmacol. 2009, 125, 16–30. [Google Scholar] [CrossRef]
- Della, A.; Paraskeva-Hadjichambi, D.; Hadjichambis, A.C. An Ethnobotanical Survey of Wild Edible Plants of Paphos and Larnaca Countryside of Cyprus. J. Ethnobiol. Ethnomed. 2006, 2, 34. [Google Scholar] [CrossRef]
- González, J.A.; García-Barriuso, M.; Amich, F. The Consumption of Wild and Semi-Domesticated Edible Plants in the Arribes Del Duero (Salamanca-Zamora, Spain): An Analysis of Traditional Knowledge. Genet. Resour. Crop. Evol. 2011, 58, 991–1006. [Google Scholar] [CrossRef]
- Idolo, M.; Motti, R.; Mazzoleni, S. Ethnobotanical and Phytomedicinal Knowledge in a Long-History Protected Area, the Abruzzo, Lazio and Molise National Park (Italian Apennines). J. Ethnopharmacol. 2010, 127, 379–395. [Google Scholar] [CrossRef]
- Mattalia, G.; Quave, C.L.; Pieroni, A. Traditional Uses of Wild Food and Medicinal Plants among Brigasc, Kyé, and Provençal Communities on the Western Italian Alps. Genet. Resour. Crop. Evol. 2013, 60, 587–603. [Google Scholar] [CrossRef]
- Nebel, S.; Heinrich, M. Ta Chòrta: A Comparative Ethnobotanical-Linguistic Study of Wild Food Plants in a Graecanic Area in Calabria, Southern Italy. Econ. Bot. 2009, 63, 78–92. [Google Scholar] [CrossRef]
- Rigat, M.; Bonet, M.A.; Garcia, S.; Garnatje, T.; Valles, J. Ethnobotany of Food Plants in the High River Ter Valley (Pyrenees, Catalonia, Iberian Peninsula): Non-Crop Food Vascular Plants and Crop Food Plants with Medicinal Properties. Ecol. Food Nutr. 2009, 48, 303–326. [Google Scholar] [CrossRef]
- Łuczaj, Ł.; Szymański, W.M. Wild Vascular Plants Gathered for Consumption in the Polish Countryside: A Review. J. Ethnobiol. Ethnomed. 2007, 3, 17. [Google Scholar] [CrossRef]
- Pardo-de-Santayana, M.; Tardío, J.; Blanco, E.; Carvalho, A.M.; Lastra, J.J.; San Miguel, E.; Morales, R. Traditional Knowledge of Wild Edible Plants Used in the Northwest of the Iberian Peninsula (Spain and Portugal): A Comparative Study. J. Ethnobiol. Ethnomed. 2007, 3, 27. [Google Scholar] [CrossRef]
- Pouta, E.; Sievänen, T.; Neuvonen, M. Recreational Wild Berry Picking in Finland—Reflection of a Rural Lifestyle. Soc. Nat. Resour. 2006, 19, 285–304. [Google Scholar] [CrossRef]
- Pieroni, A.; Nebel, S.; Quave, C.; Münz, H.; Heinrich, M. Ethnopharmacology of Liakra: Traditional Weedy Vegetables of the Arbëreshë of the Vulture Area in Southern Italy. J. Ethnopharmacol. 2002, 81, 165–185. [Google Scholar] [CrossRef]
- Stryamets, N.; Elbakidze, M.; Angelstam, P. Role of Non-Wood Forest Products for Local Livelihoods in Countries with Transition and Market Economies: Case Studies in Ukraine and Sweden. Scand. J. For. Res. 2012, 27, 74–87. [Google Scholar] [CrossRef][Green Version]
- Menendez-Baceta, G.; Aceituno-Mata, L.; Tardío, J.; Reyes-García, V.; Pardo-de-Santayana, M. Wild Edible Plants Traditionally Gathered in Gorbeialdea (Biscay, Basque Country). Genet. Resour. Crop. Evol. 2012, 59, 1329–1347. [Google Scholar] [CrossRef]
- Seeland, K.; Staniszewski, P. Indicators for a European Cross-Country State-of-the-Art Assessment of Non-Timber Forest Products and Services. Small-Scale For. 2007, 6, 411–422. [Google Scholar] [CrossRef]
- Bacchetta, L.; Visioli, F.; Cappelli, G.; Caruso, E.; Martin, G.; Nemeth, E.; Bacchetta, G.; Bedini, G.; Wezel, A.; Van Asseldonk, T.; et al. A Manifesto for the Valorization of Wild Edible Plants. J. Ethnopharmacol. 2016, 191, 180–187. [Google Scholar] [CrossRef]
- LIFE 3.0-LIFE Project Public Page. Available online: https://webgate.ec.europa.eu/life/publicWebsite/project/LIFE09-NAT-IT-000150/conservation-actions-of-habitats-in-the-coastal-wetlands-of-sci-wetlands-of-capitanata (accessed on 2 May 2024).
- Cammerino, A.R.B.; Ingaramo, M.; Monteleone, M. Complementary Approaches to Planning a Restored Coastal Wetland and Assessing the Role of Agriculture and Biodiversity: An Applied Case Study in Southern Italy. Water 2023, 16, 153. [Google Scholar] [CrossRef]
- Uso del Suolo-S.I.T.-SIT Puglia. Available online: https://pugliacon.regione.puglia.it/web/sit-puglia-sit/uso-del-suolo (accessed on 2 May 2024).
- Puglia. Available online: https://www.isprambiente.gov.it/it/servizi/sistema-carta-della-natura/cartografia/carta-della-natura-alla-scala-1-50.000/puglia (accessed on 2 May 2024).
- Pignatti, S.; Bianco, P.M.; Fanelli, G.; Paglia, S.; Pitrosanti, S.; Tescarollo, P. Le Piante Come Indicatori Ambientali; Manuale tecnico-scientifico; ANPA RTI CTN_CON 1/2001; Agenzia Nazionale Protezione Ambiente: Roma, Italy, 2001. [Google Scholar]
- Lombardi, T.; Bertacchi, A.; Pistelli, L.; Pardossi, A.; Pecchia, S.; Toffanin, A.; Sanmartin, C. Biological and Agronomic Traits of the Main Halophytes Widespread in the Mediterranean Region as Potential New Vegetable Crops. Horticulturae 2022, 8, 195. [Google Scholar] [CrossRef]
- Lombardi, T.; Ventura, I.; Bertacchi, A. Floristic Inventory of Ethnobotanically Important Halophytes of North-Western Mediterranean Coastal Brackish Areas, Tuscany, Italy. Agronomy 2023, 13, 615. [Google Scholar] [CrossRef]
- Wang, X.; Shao, X.; Zhang, W.; Sun, T.; Ding, Y.; Lin, Z.; Li, Y. Genus Suaeda: Advances in Phytology, Chemistry, Pharmacology and Clinical Application (1895–2021). Pharmacol. Res. 2022, 179, 106203. [Google Scholar] [CrossRef]
- Mohammed, H.A. The Valuable Impacts of Halophytic Genus Suaeda; Nutritional, Chemical, and Biological Values. Med. Chem. 2020, 16, 1044–1057. [Google Scholar] [CrossRef]
- Ayaz, A.; Jamil, Q.; Hussain, M.; Anjum, F.; Sarfraz, A.; Alqahtani, T.; Hussain, N.; Gahtani, R.M.; Dera, A.A.; Alharbi, H.M.; et al. Antioxidant and Gastroprotective Activity of Suaeda Fruticosa Forssk. Ex J.F.Gmel. Molecules 2022, 27, 4368. [Google Scholar] [CrossRef]
- Stanković, M.; Stojanović-Radić, Z.; Jakovljević, D.; Zlatić, N.; Luković, M.; Dajić-Stevanović, Z. Coastal Halophytes: Potent Source of Bioactive Molecules from Saline Environment. Plants 2023, 12, 1857. [Google Scholar] [CrossRef]
- Custodio, L.; Garcia-Caparros, P.; Pereira, C.G.; Castelo-Branco, P. Halophyte Plants as Potential Sources of Anticancer Agents: A Comprehensive Review. Pharmaceutics 2022, 14, 2406. [Google Scholar] [CrossRef] [PubMed]
- Ksouri, R.; Ksouri, W.M.; Jallali, I.; Debez, A.; Magné, C.; Hiroko, I.; Abdelly, C. Medicinal Halophytes: Potent Source of Health Promoting Biomolecules with Medical, Nutraceutical and Food Applications. Crit. Rev. Biotechnol. 2012, 32, 289–326. [Google Scholar] [CrossRef]
- Braun-Blanquet, J. Pflanzensoziologie; Springer: Vienna, Austria, 1964; ISBN 978-3-7091-8111-9. [Google Scholar]
- Pignatti, S. Flora d’Italia; Seconda Edizione in 4 Volumi.; Edagricole: Milano, Italy, 2017; Volume 2, ISBN 978-88-506-5243-3. [Google Scholar]
- Pignatti, S. New species of Limonium from Italy and Tunesia. Webbia 1982, 36, 47–56. [Google Scholar] [CrossRef]
- Bartolucci, F.; Peruzzi, L.; Galasso, G.; Albano, A.; Alessandrini, A.; Ardenghi, N.M.G.; Astuti, G.; Bacchetta, G.; Ballelli, S.; Banfi, E.; et al. An Updated Checklist of the Vascular Flora Native to Italy. Plant Biosyst.-Int. J. Deal. All Asp. Plant Biol. 2018, 152, 179–303. [Google Scholar] [CrossRef]
- Galasso, G.; Conti, F.; Peruzzi, L.; Ardenghi, N.M.G.; Banfi, E.; Celesti-Grapow, L.; Albano, A.; Alessandrini, A.; Bacchetta, G.; Ballelli, S.; et al. An Updated Checklist of the Vascular Flora Alien to Italy. Plant Biosyst.-Int. J. Deal. All Asp. Plant Biol. 2018, 152, 556–592. [Google Scholar] [CrossRef]
- Westhoff, V.; Van der Maarel, E. The Braun-Blanquet Approach. In Classificationof Plant Communities; Whittaker, R.H., Ed.; Springer: Hague, The Netherlands, 1973; pp. 617–726. [Google Scholar]
- Chmura, D.; Salachna, A. The Errors in Visual Estimation of Plants Cover in the Context of Education of Phytosociology. Chem.-Didact.-Ecol. -Metrol. 2016, 21, 75–82. [Google Scholar] [CrossRef]
- Acta Plantarum. 2023. Available online: https://www.actaplantarum.org/ (accessed on 2 May 2024).
- Whittaker, R.H. Evolution and Measurement of Species Diversity. Taxon 1972, 21, 213–251. [Google Scholar] [CrossRef]
- Whittaker, R.H. Dominance and Diversity in Land Plant Communities: Numerical Relations of Species Express the Importance of Competition in Community Function and Evolution. Science 1965, 147, 250–260. [Google Scholar] [CrossRef]
- Spellerberg, I.F.; Fedor, P.J. A Tribute to Claude Shannon (1916–2001) and a Plea for More Rigorous Use of Species Richness, Species Diversity and the ‘Shannon–Wiener’ Index. Glob. Ecol. Biogeogr. 2003, 12, 177–179. [Google Scholar] [CrossRef]
- Shannon, C.E. A Mathematical Theory of Communication. Bell Syst. Tech. J. 1948, 27, 379–423. [Google Scholar] [CrossRef]
- Jaccard, P. Contribution Au Problème de l’immigration Post-Glaciaire de La Flore Alpine: Étude Comparative de La Flore Alpine Du Massif de Wildhorn, Du Haut Bassin Du Trient et de La Haute Vallée de Bagnes. Bull. Soc. Vaudoise Sci. Nat. 1900, 36, 87–130. [Google Scholar] [CrossRef]
- Peet, R.K. The Measurement of Species Diversity. Annu. Rev. Ecol. Syst. 1974, 5, 285–307. [Google Scholar] [CrossRef]
- Whittaker, R.H. Vegetation of the Siskiyou Mountains, Oregon and California. Ecol. Monogr. 1960, 30, 279–338. [Google Scholar] [CrossRef]
- Anderson, M.J.; Ellingsen, K.E.; McArdle, B.H. Multivariate Dispersion as a Measure of Beta Diversity. Ecol. Lett. 2006, 9, 683–693. [Google Scholar] [CrossRef]
- Chao, A.; Chazdon, R.L.; Colwell, R.K.; Shen, T. A New Statistical Approach for Assessing Similarity of Species Composition with Incidence and Abundance Data. Ecol. Lett. 2005, 8, 148–159. [Google Scholar] [CrossRef]
- Sorensen, T. A Method of Establishing Groups of Equal Amplitude in Plant Sociology Based on Similarity of Species Content and Its Application to Analyses of the Vegetation on Danish Commons. Biol. Skr. /K. Dan. Vidensk. Selsk. 1948, 5, 1–34. [Google Scholar]
- Laurance, W.F.; Yensen, E. Predicting the Impacts of Edge Effects in Fragmented Habitats. Biol. Conserv. 1991, 55, 77–92. [Google Scholar] [CrossRef]
- Schonewald-Cox, C.; Buechner, M. Park Protection and Public Roads. In Conservation Biology; Fiedler, P.L., Jain, S.K., Eds.; Springer: Boston, MA, USA, 1992; pp. 373–395. ISBN 978-1-4684-6428-3. [Google Scholar]
- Naz, N.; Fatima, S.; Hameed, M.; Ashraf, M.; Ahmad, M.S.A.; Ahmad, F.; Shah, S.M.R.; Islam, F.; Ahmad, I.; Ejaz, F.; et al. Contribution of Structural and Functional Adaptations of Hyper-Accumulator Suaeda Vera Forssk. Ex J.F. Gmel. for Adaptability across Salinity Gradients in Hot Desert. Environ. Sci. Pollut. Res. 2022, 29, 64077–64095. [Google Scholar] [CrossRef]
- Sordes, F.; Pellequer, E.; Sahli, S.; Sarzynski, T.; Denes, M.; Techer, I. Phytoremediation of Chloride from Marine Dredged Sediments: A New Model Based on a Natural Vegetation Recolonization. J. Environ. Manag. 2023, 344, 118508. [Google Scholar] [CrossRef] [PubMed]
- Salonikioti, A.; Petropoulos, S.; Antoniadis, V.; Levizou, E.; Alexopoulos, A. Wild Edible Species with Phytoremediation Properties. Procedia Environ. Sci. 2015, 29, 98–99. [Google Scholar] [CrossRef]
- Adkins, S.W.; Wills, D.; Boersma, M.; Walker, S.R.; Robinson, G.; Mcleod, R.J.; Einam, J.P. Weeds Resistant to Chlorsulfuron and Atrazine from the North-east Grain Region of Australia. Weed Res. 1997, 37, 343–349. [Google Scholar] [CrossRef]
- Chauhan, B.S.; Johnson, D.E. Responses of Rice Flatsedge (Cyperus Iria) and Barnyardgrass (Echinochloa Crus-Galli) to Rice Interference. Weed Sci. 2010, 58, 204–208. [Google Scholar] [CrossRef]
- Manalil, S.; Haider Ali, H.; Chauhan, B.S. Germination Ecology of Turnip Weed (Rapistrum rugosum (L.) All.) in the Northern Regions of Australia. PLoS ONE 2018, 13, e0201023. [Google Scholar] [CrossRef] [PubMed]
- Skorupa, M.; Gołębiewski, M.; Kurnik, K.; Niedojadło, J.; Kęsy, J.; Klamkowski, K.; Wójcik, K.; Treder, W.; Tretyn, A.; Tyburski, J. Salt Stress vs. Salt Shock-the Case of Sugar Beet and Its Halophytic Ancestor. BMC Plant Biol. 2019, 19, 57. [Google Scholar] [CrossRef] [PubMed]
- Yolcu, S.; Alavilli, H.; Ganesh, P.; Panigrahy, M.; Song, K. Salt and Drought Stress Responses in Cultivated Beets (Beta vulgaris L.) and Wild Beet (Beta maritima L.). Plants 2021, 10, 1843. [Google Scholar] [CrossRef]
- Davy, A.J. Development of Eco-Hydrological Guidelines for Dune Habitats–Phase 1; English Nature: Peterborough, UK, 2006. [Google Scholar]
- Ventura, Y.; Eshel, A.; Pasternak, D.; Sagi, M. The Development of Halophyte-Based Agriculture: Past and Present. Ann. Bot. 2015, 115, 529–540. [Google Scholar] [CrossRef] [PubMed]
- Mishra, A.; Tanna, B. Halophytes: Potential Resources for Salt Stress Tolerance Genes and Promoters. Front. Plant Sci. 2017, 8, 829. [Google Scholar] [CrossRef]
- Davy, A.J.; Bishop, G.F.; Costa, C.S.B. Salicornia L. (Salicornia pusilla J. Woods, S. ramosissima J. Woods, S. europaea L., S. obscura P.W. Ball & Tutin, S. nitens P.W. Ball & Tutin, S. fragilis P.W. Ball & Tutin and S. dolichostachya Moss). J. Ecol. 2001, 89, 681–707. [Google Scholar] [CrossRef]
- He, F.; Tang, D. Estimating the Niche Preemption Parameter of the Geometric Series. Acta Oecologica 2008, 33, 105–107. [Google Scholar] [CrossRef]
- Caçador, I.; Neto, J.M.; Duarte, B.; Barroso, D.V.; Pinto, M.; Marques, J.C. Development of an Angiosperm Quality Assessment Index (AQuA-Index) for Ecological Quality Evaluation of Portuguese Water Bodies—A Multi-Metric Approach. Ecol. Indic. 2013, 25, 141–148. [Google Scholar] [CrossRef]





| First-Order Land Cover Categories | Second-Order Land Cover Categories |
|---|---|
| WET Wetlands and aquatic/riparian ecosystems |
|
| MEAD Semi-natural vegetation areas (meadows) |
|
| BUILT Built-up areas |
|
| AGR Agricultural areas |
|
| Land Cover Code or Score | Land Cover Range (Relative Abundance) (%) | Corresponding Average Land Cover (%) | |
|---|---|---|---|
| 0 (A = absent) | 0 | (Unseen or unobserved species) | - |
| 1 (R = rare or occasional species) | <1 | (Just one or few individuals) | 0.5 |
| 2 (U = uncommon) | 1–25 | (Slightly low land cover) | 12.0 |
| 3 (C = quite common) | 25–50 | (Medium land cover) | 37.5 |
| 4 (CC = common) | 50–75 | (Slightly high land cover) | 62.5 |
| 5 (CCC = very common) | 75–100 | (High land cover) | 87.5 |
| Land Cover Category § | Absolute Occurrence (N) | Relative Occurrence (%) | Area (m2) | Area Partitioning (%) |
|---|---|---|---|---|
| WET | 163 | 98.8 | 225.411 | 56.7 |
| MEAD | 160 | 97.0 | 112.043 | 28.2 |
| AGR | 46 | 27.9 | 31.774 | 8.0 |
| BUILT | 125 | 75.8 | 28.541 | 7.2 |
| Total | 165 | 397.768 | 100.0 |
| Code | Species | Code | Species | Code | Species |
|---|---|---|---|---|---|
| S1 | Glycyrrhiza glabra L. | S14 | Nigella damascena L. | S27 | Plantago argentea Chax |
| S2 | Portulaca oleracea L. | S15 | Plantago coronopus L. | S28 | Dittrichia viscosa (L.) Greuter |
| S3 | Cichorium intybus L. | S16 | Verbascum sinuatum L. | S29 | Juncus sp. |
| S4 | Beta vulgaris L. | S17 | Scabiosa columbaria L. | S30 | Allium roseum L. |
| S5 | Salicornia spp. | S18 | Echium vulgare L. | S31 | Ferula communis L. |
| S6 | Suaeda vera J.F.Gmel. | S19 | Scolymus hispanicus L. | S32 | Ficus carica L. |
| S7 | Sonchus oleraceus L. | S20 | Diplotaxis erucoides (L.) DC. | S33 | Cidonia oblonga Mill. |
| S8 | Daucus carota L. | S21 | Solanum nigrum L. | S34 | Punica granatum L. |
| S9 | Malva sylvestris L. | S22 | Borago officinalis L. | S35 | Prunus domestica L. |
| S10 | Picris hieracioides L. | S23 | Plantago lanceolata L. | S36 | Malus domestica (Suckow) Borkh |
| S11 | Limonium bellidifolium (Gouan) Dumort. | S24 | Rumex conglomeratus Murray | S37 | Morus nigra L. |
| S12 | Cirsium arvense (L.) Scop. | S25 | Rumex acetosa L. | S38 | Pistacia lentiscus L. |
| S13 | Carlina gummifera (L.) Less | S26 | Dipsacus follonum L. | S39 | Tamarix gallica L. |
| Species Groups | Species | Relative Species Occurrence | Relative Coverage | Area of Coverage | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| A (%) | R (%) | U (%) | C (%) | CC (%) | CCC (%) | Total § (%) | ||||
| (%) | (m2) | |||||||||
| 1 | S6 | 27.27 | 3.64 | 6.67 | 4.85 | 8.48 | 49.09 | 72.73 | 54.52 | 59,200 |
| 2 | S7 | 41.21 | 57.58 | - | - | 0.61 | 0.61 | 58.79 | 11.05 | 12,002 |
| S10 | 52.73 | 26.67 | 16.97 | 1.82 | 1.21 | 0.61 | 47.27 | 12.96 | 14,079 | |
| 3 | S8 | 84.24 | 13.94 | 1.82 | - | - | - | 15.76 | 3.08 | 3347 |
| S4 | 84.85 | 13.94 | 1.21 | - | - | - | 15.15 | 2.87 | 3116 | |
| S5 | 86.06 | 6.67 | 5.45 | - | 1.82 | - | 13.94 | 4.36 | 4731 | |
| 4 | S9 | 92.12 | 7.88 | - | - | - | - | 7.88 | 1.38 | 1500 |
| S25 | 94.55 | 5.45 | - | - | - | - | 5.45 | 0.96 | 1039 | |
| S11 | 95.15 | 4.24 | 0.61 | - | - | - | 4.85 | 0.96 | 1039 | |
| S31–S39 | 95.15 | 4.85 | - | - | - | - | 4.85 | 1.70 | 1846 | |
| 5 | S2 | 97.58 | 1.21 | - | - | 1.21 | - | 2.42 | 1.06 | 1154 |
| S28 | 97.58 | 1.21 | 1.21 | - | - | - | 2.42 | 0.64 | 692 | |
| S14, S16 | 97.58 | 2.42 | - | - | - | - | 2.42 | 0.85 | 923 | |
| S17, S26, S32 | 98.18 | 1.82 | - | - | - | - | 1.82 | 0.96 | 1039 | |
| S3, S13, S19, S22 | 98.79 | 1.21 | - | - | - | - | 1.21 | 0.85 | 923 | |
| S12 | 99.39 | - | 0.61 | - | - | - | 0.61 | 0.21 | 231 | |
| remaining species | 99.39 | 0.61 | - | - | - | - | 0.61 | 1.59 | 1731 | |
| (A) Species | S6 | S7 | S10 | S8 | S4 | S5 | |
|---|---|---|---|---|---|---|---|
| S6 | 72.73 | ||||||
| S7 | 55.15 | 58.79 | |||||
| S10 | 44.24 | 42.42 | 47.27 | ||||
| S8 | 15.15 | 13.33 | 10.91 | 15.76 | |||
| S4 | 15.15 | 15.15 | 9.70 | 1.82 | 15.15 | ||
| S5 | 13.94 | 11.52 | 7.27 | 4.24 | 2.42 | 13.94 | |
| S9 | 7.27 | 6.67 | 6.67 | 2.42 | 1.82 | ||
| S25 | 3.64 | 4.85 | 4.85 | 1.82 | |||
| S11 | 4.85 | 4.85 | 3.03 | 1.21 | |||
| S31 | 4.85 | 3.03 | 2.42 | 1.82 | 1.82 | ||
| S39 | 4.85 | ||||||
| Percentages below the value of 1 have been deleted | |||||||
| Values > 40% | |||||||
| Values = 10–16% | |||||||
| (B) Species | S6 | S7 | S10 | S8 | S4 | S5 | |
| S6 | 4.34 | ||||||
| S7 | 1.73 | 1.04 | |||||
| S10 | 1.83 | 0.94 | 1.38 | ||||
| S8 | 0.96 | 0.44 | 0.50 | 0.53 | |||
| S4 | 0.93 | 0.46 | 0.48 | 0.18 | 0.50 | ||
| S5 | 1.19 | 0.47 | 0.45 | 0.30 | 0.20 | 0.87 | |
| S9 | 0.57 | 0.30 | 0.38 | 0.20 | 0.15 | ||
| S25 | 0.42 | 0.25 | 0.41 | 0.15 | |||
| S11 | 0.49 | 0.27 | 0.22 | 0.13 | 0.15 | ||
| S31 | 0.47 | 0.20 | 0.22 | 0.13 | 0.15 | 0.27 | |
| S39 | 0.56 | ||||||
| Link strengths below the value of 0.10 have been deleted | |||||||
| Values > 1.00 | |||||||
| Values = 0.50–1.00 | |||||||
| Richness | Abundance | Shannon | Simpson | |
|---|---|---|---|---|
| Richness | 1.00 | 0.90 | 0.98 | 0.91 |
| Abundance | 0.90 | 1.00 | 0.87 | 0.85 |
| Shannon | 0.98 | 0.87 | 1.00 | 0.97 |
| Simpson | 0.91 | 0.85 | 0.97 | 1.00 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cammerino, A.R.B.; Piacquadio, L.; Ingaramo, M.; Gioiosa, M.; Monteleone, M. Wild Edible Plant Species in the ‘King’s Lagoon’ Coastal Wetland: Survey, Collection, Mapping and Ecological Characterization. Horticulturae 2024, 10, 632. https://doi.org/10.3390/horticulturae10060632
Cammerino ARB, Piacquadio L, Ingaramo M, Gioiosa M, Monteleone M. Wild Edible Plant Species in the ‘King’s Lagoon’ Coastal Wetland: Survey, Collection, Mapping and Ecological Characterization. Horticulturae. 2024; 10(6):632. https://doi.org/10.3390/horticulturae10060632
Chicago/Turabian StyleCammerino, Anna Rita Bernadette, Lorenzo Piacquadio, Michela Ingaramo, Maurizio Gioiosa, and Massimo Monteleone. 2024. "Wild Edible Plant Species in the ‘King’s Lagoon’ Coastal Wetland: Survey, Collection, Mapping and Ecological Characterization" Horticulturae 10, no. 6: 632. https://doi.org/10.3390/horticulturae10060632
APA StyleCammerino, A. R. B., Piacquadio, L., Ingaramo, M., Gioiosa, M., & Monteleone, M. (2024). Wild Edible Plant Species in the ‘King’s Lagoon’ Coastal Wetland: Survey, Collection, Mapping and Ecological Characterization. Horticulturae, 10(6), 632. https://doi.org/10.3390/horticulturae10060632












