Variations in Essential Oil Compositions and Changes in Oil Cells during Leaf Development of Citral Chemotype of Camphora officinarum Nees ex Wall.
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Determination of Leaf Size
2.3. Determination of Moisture Content
2.4. Acquisition of Oil Yield and GC-MS Analysis
2.5. Determination of Oil Cell Size
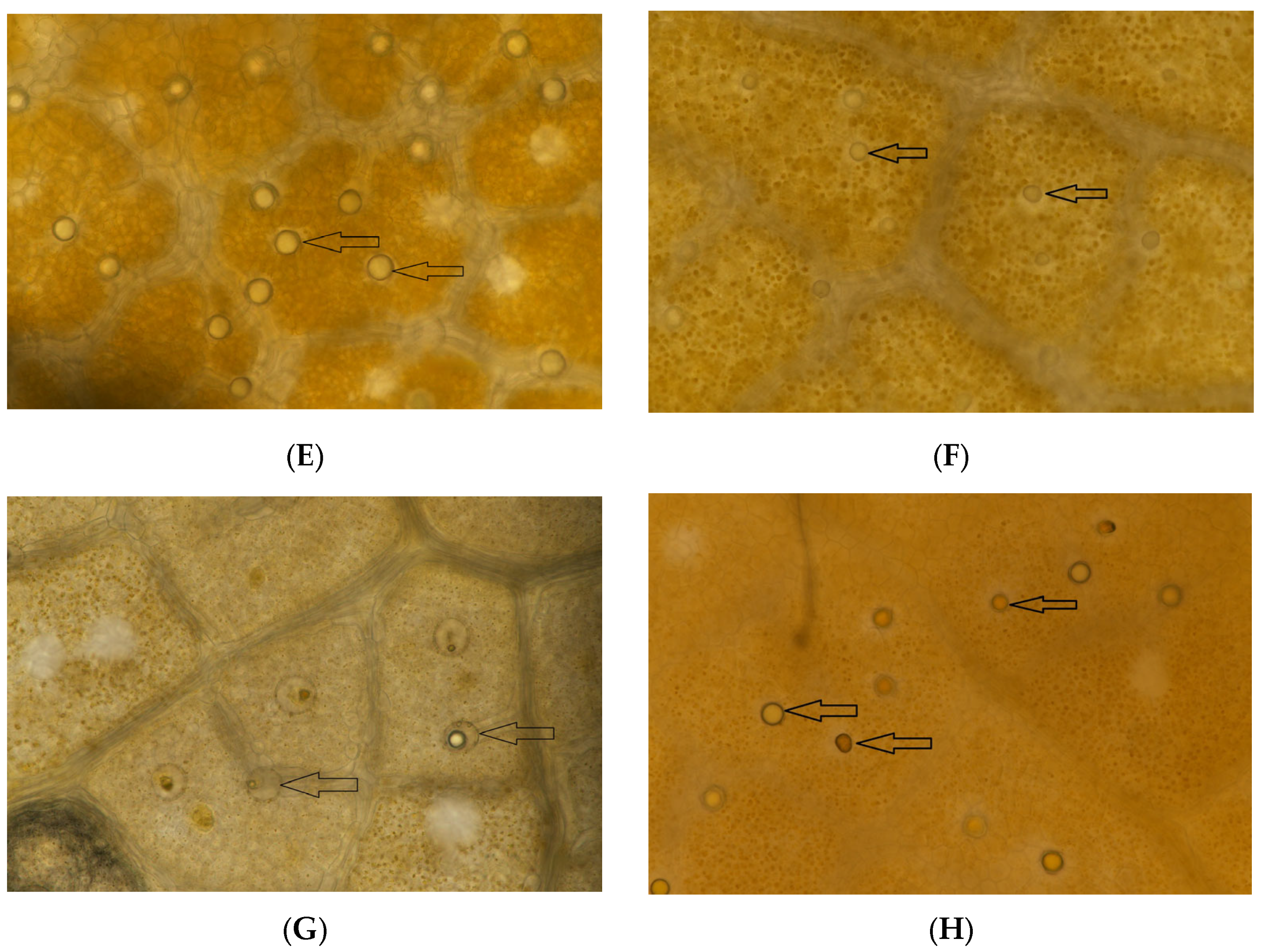
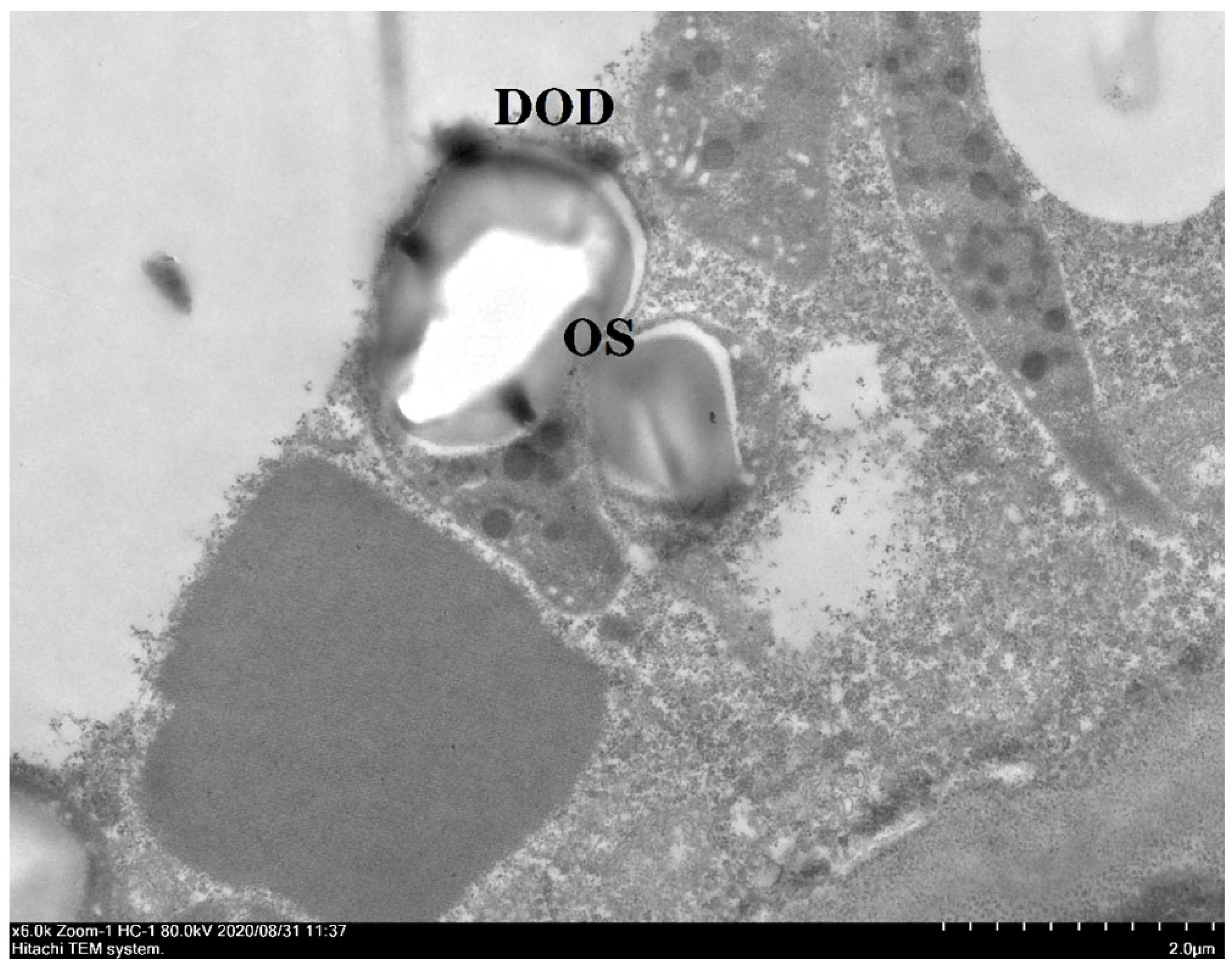
2.6. Microscopic Observation of Oil Cells
2.7. Statistical Analysis
3. Results
3.1. Dynamic Changes in Leaf Size
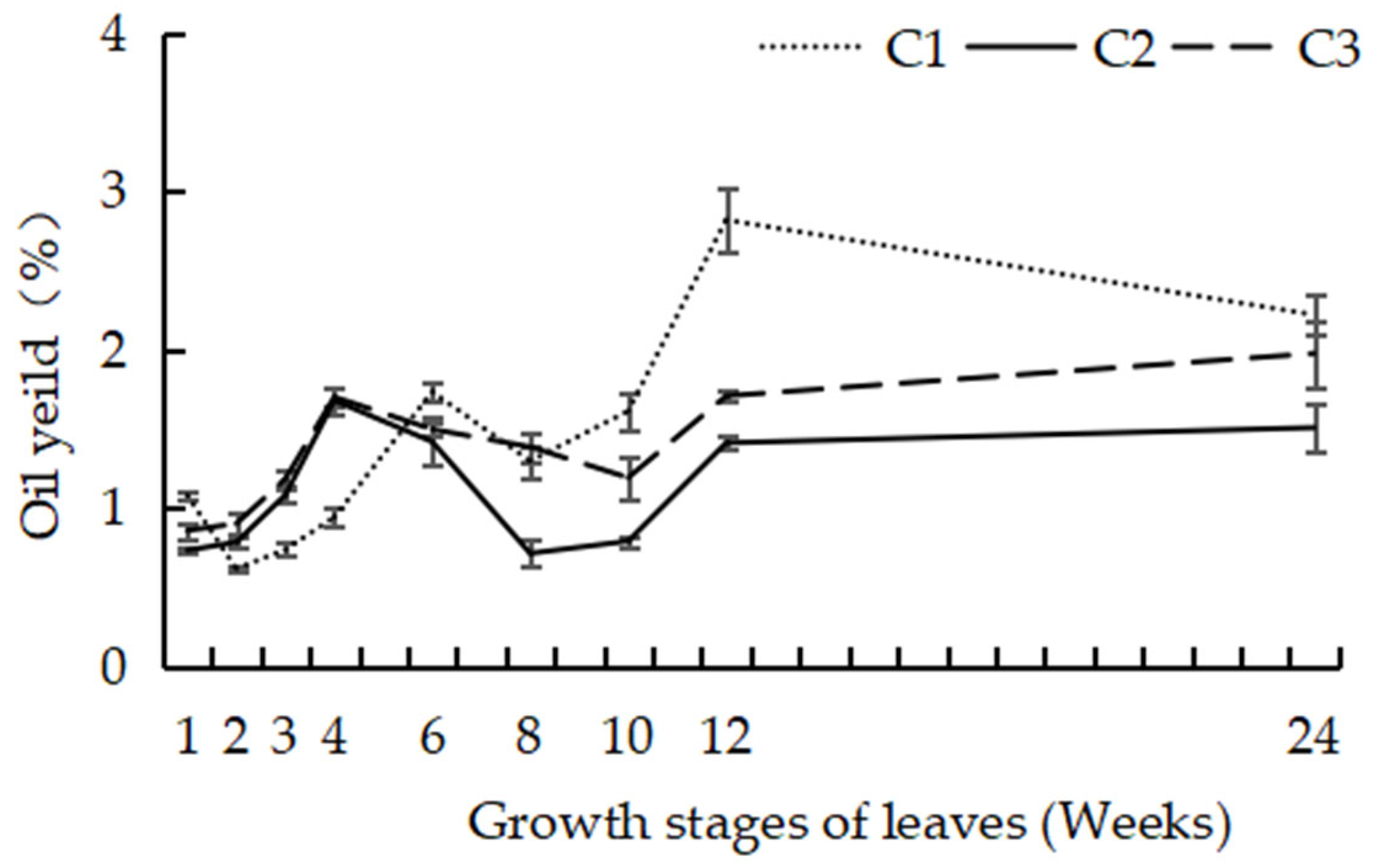
3.2. Dynamic Accumulation of EO
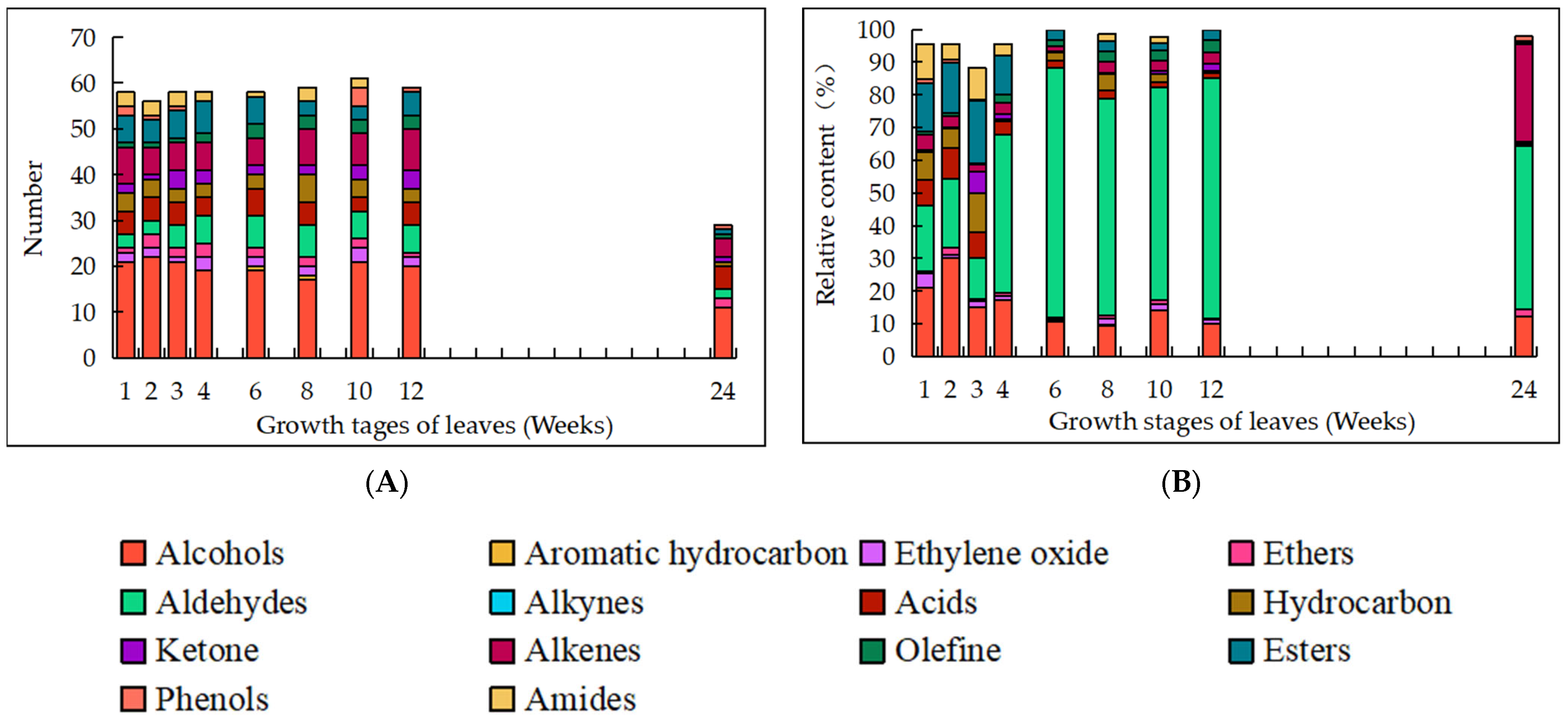
3.3. Dynamic Changes in the OCs of EO
3.4. Dynamic Changes in Oil Cells
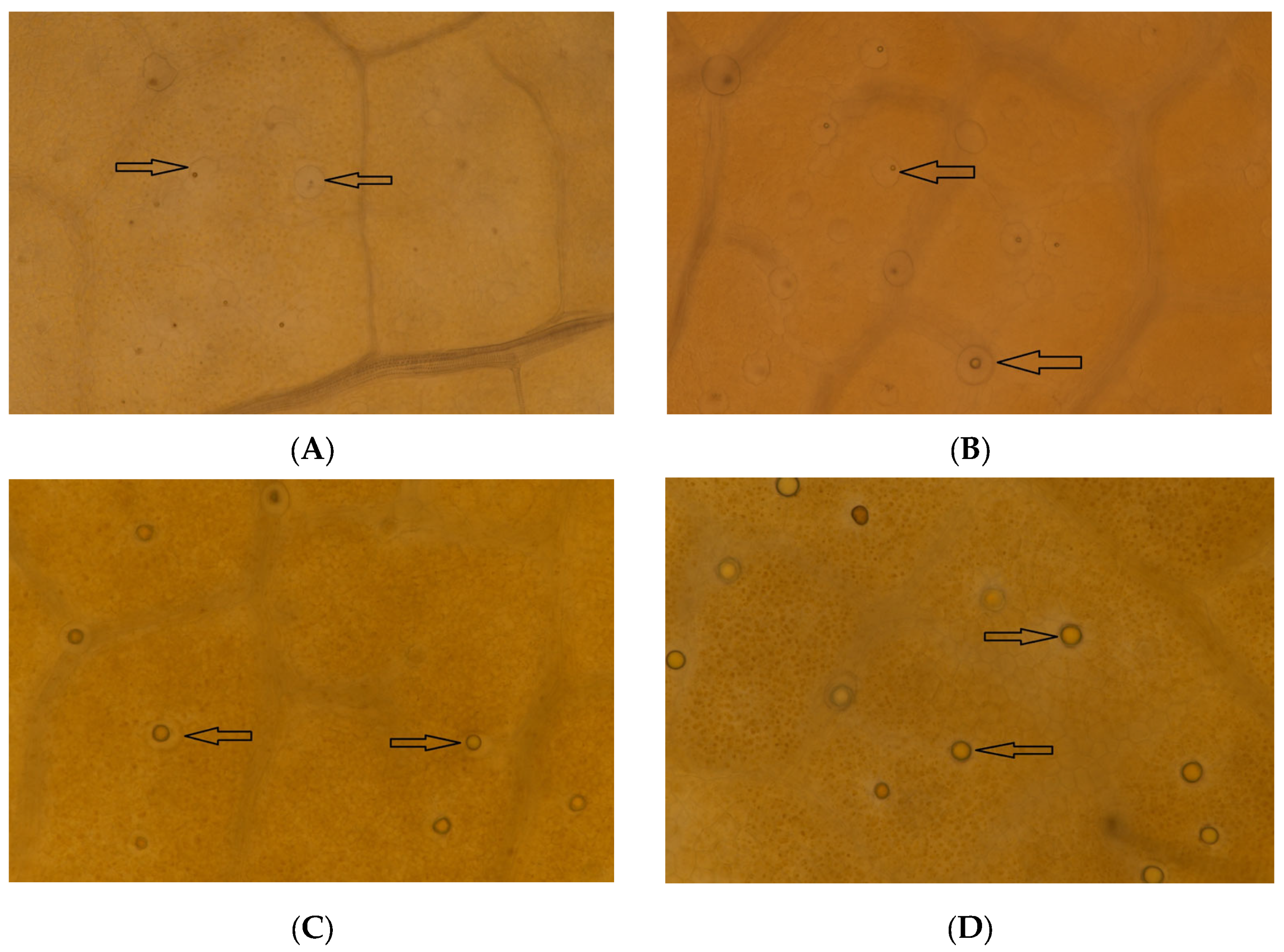
3.5. Microscopic Observation of Oil Cells
4. Discussion
4.1. Leaf Development, Oil Cells, and Essential Oil Quantity
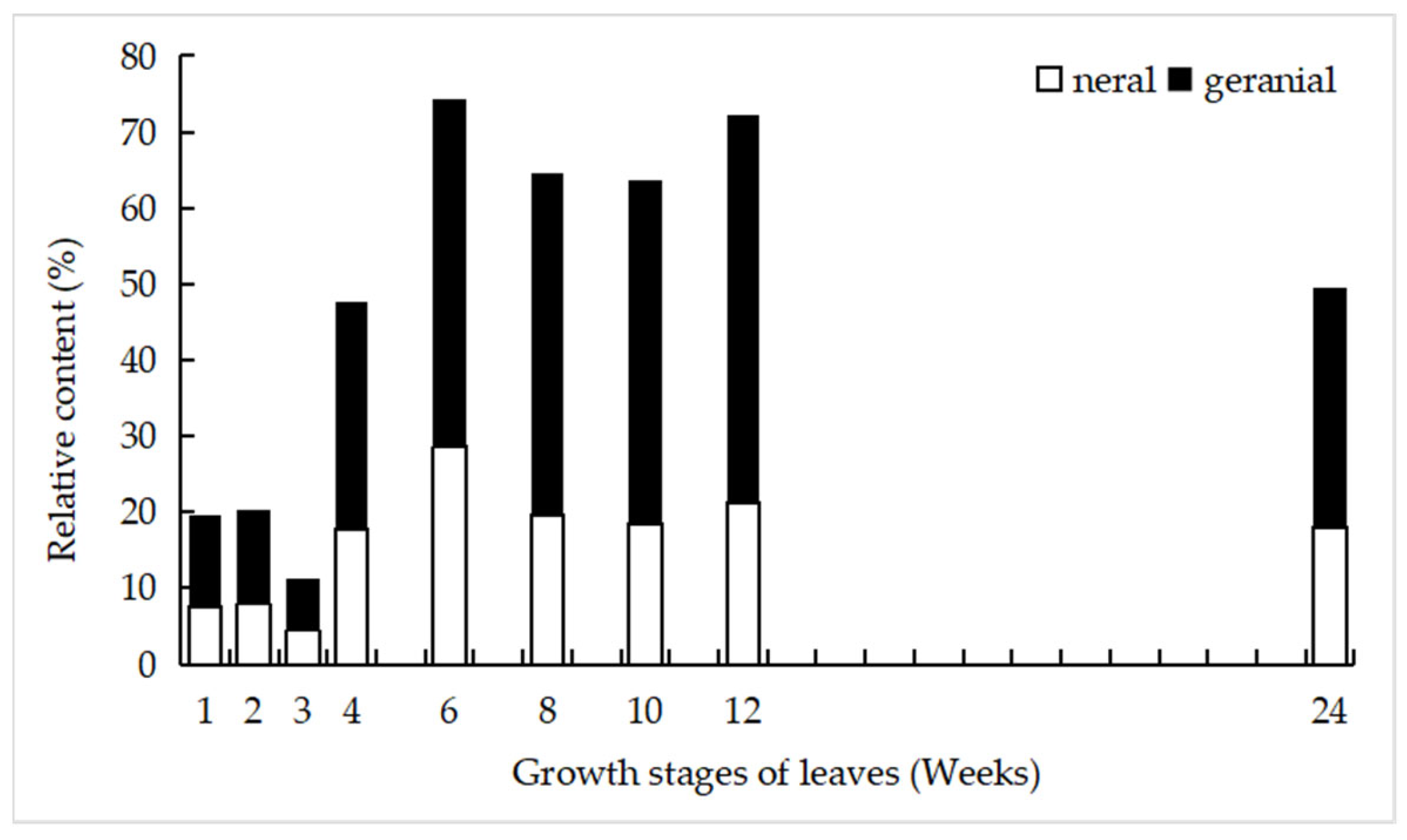
4.2. EO Quality, Leaf Development, and Oil Cells
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ling, Q.Y.; Zhang, B.H.; Wang, Y.B.; Xiao, Z.F.; Hou, J.X.; Xiao, C.L.; Liu, Y.Q.; Jin, Z.N. Chemical composition and antioxidant activity of the essential oils of citral-rich chemotype Cinnamomum camphora and Cinnamomum bodinieri. Molecules 2022, 27, 7356. [Google Scholar] [CrossRef] [PubMed]
- Capetti, F.; Cagliero, C.; Argenziano, M.; Cavalli, R.; Dianzani, C.; Pavarino, M.; Bicchi, C.; Rubiolo, P.; Sgorbini, B. A new blend of Litsea cubeba, Pinus mugo, and Cymbopogon winterianus essential oil active as an anti-tyrosinase ingredient in topical formulations. Planta Med. 2024, 90, 267–275. [Google Scholar] [CrossRef] [PubMed]
- Dangol, S.; Poudel, D.K.; Ojha, P.K.; Maharjan, S.; Poudel, A.; Satyal, R.; Rokaya, A.; Timsina, S.; Dosoky, N.S.; Satyal, P.; et al. Essential oil composition analysis of Cymbopogon Species from eastern Nepal by GC-MS and chiral GC-MS, and antimicrobial activity of some major compounds. Molecules 2023, 28, 543. [Google Scholar] [CrossRef] [PubMed]
- Lu, H.X.; Sun, Y.; Wang, X.D.; Lu, Z.; Zhu, J.L. Transcriptomics reveal the antibiofilm mechanism of NaCl combined with citral against Vibrio parahaemolyticus. Appl. Microbiol. Biot. 2022, 107, 313–326. [Google Scholar] [CrossRef] [PubMed]
- García, L.T.; Leal, A.F.; Moreno É, M.; Stashenko, E.E.; Arteaga, H.J. Differential anti-proliferative effect on K562 leukemia cells of Lippia alba (Verbenaceae) essential oils produced under diverse growing, collection and extraction conditions. Ind. Crops Prod. 2017, 96, 140–148. [Google Scholar] [CrossRef]
- Kaur, G.; Arya, S.K.; Singh, B.; Singh, S.; Sushmita; Saxena, G.; Verma, P.C.; Ganjewala, D. Comparative transcriptional analysis of metabolic pathways and mechanisms regulating essential oil biosynthesis in four elite Cymbopogon spp. Int. J. Biol. Macromol. 2023, 229, 943–951. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.X.; Wang, M.Y.; Chen, Y.C.; Gao, M.; Wu, L.W.; Wang, Y.D. LcERF134 increases the production of monoterpenes by activating the terpene biosynthesis pathway in Litsea cubeba. Int. J. Biol. Macromol. 2023, 232, 123378. [Google Scholar] [CrossRef] [PubMed]
- Yan, K.; Zheng, Y.; Hu, L. Effect of H2O2-mediated endophytic fungal elicitors on essential oil accumulation in suspension cells of Cinnamomum longepaniculatum. Open Access Libr. J. 2020, 7, 1–10. [Google Scholar] [CrossRef]
- Kharraf, S.E.; El-Guendouz, S.; Farah, A.; Mateus, M.C.; Hadrami, E.M.E.; Miguel, M.G. Impact of fifteen combinations of the main components of rosemary, lavender and citrus essential oils on in vitro biological activities. S. Afr. J. Bot. 2023, 156, 163–168. [Google Scholar] [CrossRef]
- Zhang, T.; Zheng, Y.J.; Fu, C.; Yang, H.K.; Liu, X.L.; Qiu, F.Y.; Wang, X.D.; Wang, Z.D. Chemical variation and environmental influence on essential oil of Cinnamomum camphora. Molecules 2023, 28, 973. [Google Scholar] [CrossRef]
- Ghosh, D.; Choudhary, N.; Kumari, K.U.; Singh, J.; Tripathi, P.; Meena, A.; Luqman, S.; Yadav, A.; Chanotiya, C.S.; Pandey, G.; et al. Diversity of essential oil-secretory cells and oil composition in flowers and buds of Magnolia sirindhorniae and its biological activities. Chem. Biodivers. 2020, 18, e2000750. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.B.; Quan, Q.G.; Duan, L.P.; Yang, M.; Yang, J.Z.; Li, C. Extraction and content variation of essential oils from Cinnamomum camphora (L.) leaf in Yongzhou. Nat. Prod. Res. Dev. 2016, 28, 83–89. [Google Scholar] [CrossRef]
- Zhang, B.H.; Wu, C.S.; Xiao, Z.F.; Zhang, H.Y.; Cao, M.; Liu, Y.Q.; Jin, Z.N. Chemical Constituents and Chemotypes of Fresh Leaf Essential Oil of Wild Species Belonging to Sect. Camphor (Trew.) Meissn. in Southeastern China. J. Essent. Oil Bear. Plant 2019, 4, 1115–1122. [Google Scholar] [CrossRef]
- Liu, N.; Wan, Y.H.; Han, J.C.; Li, H.; Bo, Z.D.; Bo, H.D.; Luo, D.F.; Li, Z.R. Design, synthesis and herbicidal activity of novel N-(5-(3,5-dinitrophenyl) thiazol-2-yl)-2-phenoxyacetamide compounds. Chin. J. Pestic. Sci. 2024, 3, 324–336. [Google Scholar] [CrossRef]
- Li, Y.Q.; Kong, D.X.; Huang, R.S.; Liang, H.L. Variations in essential oil yields and compositions of Cinnamomum cassia leaves at different developmental stages. Ind. Crops Prod. 2013, 47, 92–101. [Google Scholar] [CrossRef]
- Olaranont, Y.; Stewart, A.B.; Traiperm, P. Effects of crude oil on plant growth and leaf anatomical structures in a common coastal plant. Int. J. Phytoremediation 2021, 23, 162–170. [Google Scholar] [CrossRef] [PubMed]
- Abacar, C.; Cyrielle, G.; Christian, S.; Jérôme, M.; Marc, C. Effect of Flower Development Stages on the Dynamics of Volatile Compounds in Ylang-Ylang (Cananga odorata) Essential Oil. Horticulturae 2022, 8, 986. [Google Scholar] [CrossRef]
- Lv, T.T.; Qin, Z.; Liu, H.M.; Wang, X.D.; He, J.R. Chemical composition and antioxidant capacity of proanthocyanidins from Chinese quince (Chaenomeles sinensis) fruit at different growth stages. J. Food Meas. Charact. 2024, 18, 2318–2330. [Google Scholar] [CrossRef]
- Liu, H.F.; Specht, C.D.; Zhao, T.; Liao, J.P. Morphological Anatomy of Leaf and Rhizome in Zingiber officinale Roscoe, with Emphasis on Secretory Structures. HortScience 2020, 55, 204–207. [Google Scholar] [CrossRef]
- Si, M.Z.; Li, J.W.; Yang, Y.A.; Zhang, D.Q.; Li, L.; Zhang, C.Y. Oil cells distribution on different parts of Melaleuca alternifolia and its research by micro-raman spectroscopy. Spectrosc Spect. Anal. 2021, 41, 813–816. [Google Scholar]
- Luthra, R.; Singh, N.; Sharma, S. Changes in monoterpene content accompanying development of Cymbopogon winterianus Jowitt leaves. J. Essent. Oil Res. 1991, 3, 349–354. [Google Scholar] [CrossRef]
- Hsin-I, W.; Naohiro, A.; Ryu, O. Variation in essential oil content and compsition during leaf development and growth of Lemongrass. Trop. Agric. Dev. 2012, 56, 14–24. [Google Scholar] [CrossRef]
- Nermen, F.; Dalia, E.A.; Asmaa, O.; Sameh, A.Z. Studies on essential oil from rose-scented geranium, Pelargonium graveolens L’Hérit. (Geraniaceae). Nat. Prod. Res. 2021, 15, 2593–2597. [Google Scholar] [CrossRef] [PubMed]
- Southwell, I.A.; Russell, M.F. Volatile oil comparison of cotyledon leaves of chemotypes of Melaleuca alternifolia. Phytochemistry 2002, 59, 391–393. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.Q.; Kong, D.X.; Lin, X.M.; Xie, Z.H.; Bai, M.; Huang, S.S.; Nian, H.; Wu, H. Quality evaluation for essential oil of Cinnamomum verum leaves at different growth stages based on GC–MS, FTIR and Microscopy. Food Anal. Methods 2016, 9, 202–212. [Google Scholar] [CrossRef]
- Lan, G.Y.; Chen, J.Z.; Ma, Y.Z.; Liu, X.; Zhou, S.Y. Regulations of fruit development in Litsea cubeba and changes of its inclusions and essential oil. Non-Wood For. Res. 2020, 38, 201–208. [Google Scholar] [CrossRef]
- Janeiro, A.; Lima, A.; Arruda, F.; Wortham, T.; Rodrigues, T.; Baptista, J.; Lima, E. Essential oils from Azorean Cryptomeria japonica female cones at different developmental stages: Variations in the yields and chemical compositions. Separations 2024, 11, 62. [Google Scholar] [CrossRef]
- Yuan, Y.; Huang, M.; Pang, Y.X.; Yu, F.L.; Chen, C.; Liu, L.W.; Chen, Z.X.; Zhang, Y.B.; Chen, X.L.; Hu, X. Variations in essential oil yield, composition, and antioxidant activity of different plant organs from Blumea balsamifera (L.) DC. at different growth times. Molecules 2016, 21, 1024. [Google Scholar] [CrossRef] [PubMed]
- Ma, L.; Chen, Y.Z.; Zhong, H.Y.; Peng, S.F.; Li, Z.G.; Li, M.Q.; Xu, Y.M.; Zhang, Z. Accumulation dynamics of chemical constituents in Camellia oleifera seeds during maturation. J. Cent. S. Univ. For. Technol. 2019, 39, 47–50. [Google Scholar] [CrossRef]




| Weeks of Leaf Development | Leaf Area (mm2) | Leaf Length (mm) | Leaf Width (mm) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| C1 | C2 | C3 | C1 | C2 | C3 | C1 | C2 | C3 | |
| 1 | 147.77 ± 11.75 g | 324.97 ± 15.12 g | 394.60 ± 18.84 g | 31.17 ± 2.60 e | 39.83 ± 2.01 e | 46.00 ± 2.69 f | 9.47 ± 0.44 g | 16.00 ± 0.53 h | 17.35 ± 0.43 h |
| 2 | 529.05 ± 36.23 f | 1028.18 ± 27.03 f | 813.78 ± 37.71 f | 52.00 ± 2.32 d | 69.20 ± 3.04 d | 62.50 ± 2.63 e | 18.75 ± 1.05 f | 29.42 ± 0.53 g | 26.02 ± 0.49 g |
| 3 | 1434.37 ± 33.16 e | 1531.42 ± 65.64 e | 1702.52 ± 36.78 e | 84.33 ± 1.10 b | 80.33 ± 2.19 c | 88.83 ± 2.01 d | 32.22 ± 0.38 e | 35.82 ± 0.75 f | 40.65 ± 0.56 ef |
| 4 | 1528.35 ± 35.73 de | 1667.35 ± 61.86 e | 1845.63 ± 72.88 e | 89.17 ± 3.10 ab | 83.83 ± 4.05 c | 88.33 ± 1.37 d | 35.90 ± 0.86 cde | 38.68 ± 1.24 ef | 38.80 ± 1.17 f |
| 6 | 1582.5 ± 3.26 cde | 2057.40 ± 34.54 d | 2317.17 ± 110.27 d | 90.33 ± 0.70 ab | 100.17 ± 3.36 ab | 107.00 ± 2.98 c | 35.18 ± 0.70 de | 40.13 ± 1.07 de | 43.42 ± 1.61 de |
| 8 | 1641.52 ± 6.47 cd | 2200.83 ± 90.35 d | 2746.82 ± 62.54 c | 90.83 ± 1.21 ab | 99.00 ± 1.27 b | 114.83 ± 2.98 bc | 41.02 ± 1.21 ab | 43.30 ± 1.00 cd | 44.62 ± 1.02 d |
| 10 | 1769.28 ± 5.89 bc | 2508.54 ± 80.04 c | 3109.02 ± 120.57 c | 91.83 ± 1.47 ab | 103.86 ± 3.71 ab | 123.33 ± 3.04 ab | 41.17 ± 1.40 cd | 45.36 ± 1.27 c | 49.27 ± 1.91 c |
| 12 | 1944.77 ± 5.69 b | 3047.17 ± 155.99 b | 3748.85 ± 70.54 b | 91.33 ± 1.84 ab | 105.50 ± 2.57 ab | 121.67 ± 1.56 bc | 43.72 ± 1.84 a | 52.10 ± 1.50 b | 57.93 ± 0.49 b |
| 24 | 2295.58 ± 144.25 a | 3468.98 ± 94.55 a | 4862.22 ± 284.46 a | 100.33 ± 2.64 a | 110.00 ± 5.39 a | 132.50 ± 8.73 a | 39.55 ± 1.56 bc | 60.18 ± 2.10 a | 64.47 ± 0.95 a |
| F | 115.85 | 122.71 | 118.92 | 31.73 | 41.39 | 47.49 | 78.00 | 97.82 | 151.07 |
| P | 2.03 × 10−27 | 5.93 × 10−28 | 1.16 × 10−27 | 4.85 × 10−16 | 3.04 × 10−18 | 2.04 × 10−19 | 8.47 × 10−24 | 7.35 × 10−26 | 6.79 × 10−30 |
| Growth Stages of Leaves (Weeks) | OIL Yield (%) | ||
|---|---|---|---|
| C1 | C2 | C3 | |
| 1 | 1.08 ± 0.02 ef | 0.73 ± 0.02 c | 0.86 ± 0.05 d |
| 2 | 0.62 ± 0.02 g | 0.78 ± 0.04 bc | 0.90 ± 0.07 d |
| 3 | 0.74 ± 0.04 fg | 1.08 ± 0.04 b | 1.18 ± 0.05 cd |
| 4 | 0.94 ± 0.06 efg | 1.68 ± 0.08 a | 1.70 ± 0.06 ab |
| 6 | 1.73 ± 0.05 c | 1.42 ± 0.15 a | 1.50 ± 0.05 bc |
| 8 | 1.30 ± 0.10 de | 0.71 ± 0.08 c | 1.39 ± 0.09 bc |
| 10 | 1.61 ± 0.12 cd | 0.79 ± 0.03 bc | 1.19 ± 0.13 cd |
| 12 | 2.82 ± 0.20 a | 1.41 ± 0.04 a | 1.71 ± 0.04 ab |
| 24 | 2.22 ± 0.12 b | 1.51 ± 0.15 a | 1.98 ± 0.21 a |
| F | 35.32 | 13.83 | 9.92 |
| P | 1.85 × 10−9 | 3.0 × 10−6 | 3.2 × 10−5 |
| No. | Oil Compositions | Retention Time | Growth Stages of Leaves (Weeks)/Relative Content of Oil Compositions (%) | Category | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Week 1 | Week 2 | Week 3 | Week 4 | Week 6 | Week 8 | Week 10 | Week 12 | Week 24 | ||||
| 1 | 1,3,5,7-tetroxane | 3.31 | - | - | - | - | - | - | - | - | 0.28 | Ethers |
| 2 | spiro[2.4]hepta-4,6-diene | 3.77 | - | - | - | - | - | - | - | - | 26.45 | Alkenes |
| 3 | 2,2-dimethoxybutane | 4.40 | - | - | - | - | - | - | - | - | 0.62 | Hydrocarbon |
| 4 | α-pinene | 5.28 | - | - | - | - | - | - | - | - | 3.02 | Alkenes |
| 5 | 3-(2-methoxyethoxymethoxy)-2-methylpentan-1-ol | 5.40 | - | - | - | - | - | - | - | - | 1.52 | Alcohols |
| 6 | β-pinene | 5.95 | - | - | - | - | 0.16 | 0.18 | 0.14 | 0.09 | 0.11 | Alkenes |
| 7 | o-cymene | 6.85 | - | - | - | - | 0.08 | 0.10 | - | - | - | Aromatic hydrocarbon |
| 8 | eucalyptol | 6.99 | 0.37 | 0.64 | 0.17 | 0.23 | 0.71 | 1.15 | 0.69 | 0.30 | 2.07 | Ethers |
| 9 | 1,6-octadien-3-ol,3,7-dimethyl- | 8.17 | 0.54 | 1.15 | 0.57 | 0.48 | 0.88 | 1.84 | 0.89 | 0.65 | - | Alcohols |
| 10 | trans-3(10)-caren-2-ol | 8.34 | - | - | - | - | - | - | - | - | 0.25 | Alcohols |
| 11 | 1,7-nonadien-4-ol, 4,8-dimethyl- | 8.99 | - | - | - | - | 0.14 | 0.13 | 0.14 | 0.15 | 0.10 | Alcohols |
| 12 | (+)-2-bornanone | 9.10 | 0.43 | - | - | - | - | - | - | - | - | Ketone |
| 13 | 6-octenal,3,7-dimethyl-,(R)- | 9.15 | - | - | - | 0.35 | 0.71 | 0.63 | 0.54 | 0.77 | Aldehydes | |
| 14 | (S)-cis-verbenol | 9.35 | - | - | - | - | 0.22 | 0.12 | 0.15 | 0.22 | 0.13 | Alcohols |
| 15 | endo-borneol | 9.49 | 0.91 | 0.77 | 0.25 | 0.30 | 0.47 | 0.28 | 0.31 | 0.33 | 0.08 | Alcohols |
| 16 | limonene oxide | 9.67 | - | - | - | 0.22 | 0.34 | 0.22 | 0.22 | 0.28 | - | Alkenes |
| 17 | terpinen-4-ol | 9.68 | 0.40 | 0.45 | - | - | - | - | - | - | 0.10 | Alcohols |
| 18 | α-terpineol | 9.91 | 2.28 | 2.64 | 0.40 | 0.30 | 0.42 | 0.39 | 0.47 | 0.36 | 0.10 | Alcohols |
| 19 | exo-2-hydroxycineole | 10.29 | - | - | - | - | - | - | - | - | 2.15 | Alcohols |
| 20 | 2-oxabicyclo[2.2.2]octan-6-ol,1,3,3-trimethyl- | 10.48 | - | - | - | - | - | - | - | - | 1.99 | Alcohols |
| 21 | citronellol | 10.54 | 0.83 | 1.06 | 0.95 | 1.61 | 2.16 | 2.17 | 1.62 | 1.87 | - | Alcohols |
| 22 | neral | 10.75 | 7.65 | 7.95 | 4.46 | 17.88 | 28.63 | 19.70 | 18.48 | 21.18 | 18.09 | Aldehydes |
| 23 | geraniol | 10.95 | 3.24 | 4.30 | 2.61 | 4.29 | 4.48 | 2.16 | 2.66 | 3.89 | 5.48 | Alcohols |
| 24 | geranial | 11.26 | 11.99 | 12.35 | 6.90 | 29.79 | 45.70 | 44.94 | 45.16 | 51.12 | 31.33 | Aldehydes |
| 25 | pinane,2,3-epoxy- | 11.47 | - | 0.26 | - | 0.34 | - | - | - | - | - | Ethylene oxide |
| 26 | limonene 1,2-epoxide | 11.55 | - | - | - | - | 0.47 | 0.65 | 0.67 | 0.58 | 0.39 | Olefine |
| 27 | α-methyl-α-[4-methylpentyl]oxiranmethanol | 11.62 | - | - | - | - | - | - | 0.38 | 0.29 | - | Alcohols |
| 28 | bornyl acetate | 11.64 | - | - | - | 0.37 | 0.36 | 0.34 | - | - | - | Esters |
| 29 | 5-isopropyl-6-methyl-hepta-3,5-dien-2-ol | 11.95 | - | - | - | - | 0.08 | 0.08 | - | - | - | Alcohols |
| 30 | neric acid | 12.14 | - | - | - | - | 0.16 | 0.22 | 0.17 | 0.13 | 0.40 | Acids |
| 31 | oxiranemethanol, 3-methyl-3-(4-methyl-3-pentenyl)- | 12.37 | - | - | - | - | 0.34 | 0.07 | - | - | - | Aldehydes |
| 32 | 2,7-dimethyl-2,7-octanediol | 12.43 | - | - | - | - | - | 0.18 | 0.16 | 0.11 | - | Alcohols |
| 33 | citronellol acetate(6-octen-1-ol,3,7-dimethyl-,acetate) | 12.63 | - | - | - | 0.36 | 0.23 | 0.38 | 0.24 | 0.22 | - | Esters |
| 34 | geranic acid | 12.76 | 0.88 | 0.94 | 1.32 | 0.44 | 0.85 | 1.20 | 0.95 | 0.90 | Acids | |
| 35 | eugenol | 12.78 | 0.58 | - | - | - | - | - | - | - | 1.48 | Phenols |
| 36 | nerol acetate | 12.82 | - | - | - | - | 0.14 | - | - | - | Esters | |
| 37 | oxiranecarboxaldehyde,3-methyl-3-(4-methyl-3-pentenyl)- | 13.03 | - | - | - | - | 0.54 | 0.44 | 0.35 | 0.26 | - | Aldehydes |
| 38 | 2,6-octadien-1-ol,3,7-dimethyl-,acetate,(Z)- | 13.14 | 0.39 | 0.37 | 1.01 | 1.68 | 1.77 | 2.41 | 1.68 | 2.22 | 0.59 | Esters |
| 39 | 6-(3-hydroxy-but-1-enyl)-1,5,5-t rimethyl-7-oxabicyclo[4.1.0]hep tan-2-ol | 13.37 | - | - | - | - | - | - | - | - | 0.16 | Alcohols |
| 40 | bicyclo[5.3.0]decane,2-methylene-5-(1-methylvinyl)-8-methyl- | 13.42 | - | - | - | - | - | 0.16 | 0.16 | 0.19 | - | Olefine |
| 41 | S-(+)-5-(1-hydroxy-1-methyleth yl)-2-methyl-2-cyclohexen-1-one | 13.47 | - | - | - | 0.23 | 0.20 | 0.21 | 0.13 | 0.19 | - | Ketone |
| 42 | isocaryophyllene | 13.73 | - | - | - | 0.25 | 1.03 | - | - | - | - | Olefine |
| 43 | caryophyllene | 13.95 | 1.09 | 0.94 | 0.31 | 2.31 | 0.41 | 2.45 | 2.22 | 3.11 | - | Olefine |
| 44 | trans-p-mentha-2,8-dienol | 14.27 | - | - | - | - | 0.15 | 0.20 | 0.17 | 0.17 | - | Alcohols |
| 45 | 1H-cycloprop[e]azulene,decahydro-1,1,7-trimethyl-4-methylene- | 14.29 | 0.32 | 0.26 | 0.29 | 0.14 | - | - | - | - | - | Alkenes |
| 46 | humulene | 14.53 | 0.82 | 0.86 | 0.57 | 1.38 | 0.65 | 1.33 | 1.06 | 1.66 | 0.13 | Alkenes |
| 47 | 2-isopropenyl-4a,8-dimethyl-1,2,3,4,4a,5,6,7-octahydronaphthalene | 14.90 | - | - | - | - | - | 0.11 | - | 0.08 | - | Alkenes |
| 48 | 1H-benzocycloheptene,2,4a,5,6,7,8,9,9a-octahydro-3,5,5-trimethyl-9-methylene- | 14.95 | - | - | - | - | 0.09 | - | - | - | - | Alkenes |
| 49 | ylangene | 14.96 | 0.30 | 0.39 | - | - | - | - | - | - | - | Alkenes |
| 50 | germacrene D | 15.03 | 0.27 | - | - | 0.37 | - | 0.26 | 0.25 | 0.19 | - | Alkenes |
| 51 | eudesma-4(14),11-diene | 15.16 | 0.75 | 0.57 | 0.25 | - | - | - | - | 0.1 | - | Alkenes |
| 52 | guaia-1(10),11-diene | 15.31 | 1.41 | 1.23 | 0.83 | - | - | - | - | - | - | Alkenes |
| 53 | γ-elemene | 15.34 | - | - | - | 1.31 | 0.21 | 0.4 | 0.77 | 0.55 | - | Alkenes |
| 54 | 4-epi-cubedol | 15.70 | - | - | - | - | 0.06 | 0.09 | - | - | - | Alcohols |
| 55 | copaene | 15.83 | 0.36 | 0.32 | 0.27 | 0.15 | 0.1 | 0.24 | 0.17 | 0.15 | - | Alkenes |
| 56 | spiro[2.3]hexan-4-one, 5,5-diethyl-6-methyl- | 16.15 | - | - | - | - | - | 0.07 | - | - | - | Ketone |
| 57 | cyclohexanemethanol | 16.41 | 0.23 | - | - | 0.18 | - | 0.11 | - | 0.09 | - | Alcohols |
| 58 | nerolidol(1,6,10-dodecatrien-3-ol, 3,7,11-trimethyl-) | 16.61 | 1.81 | - | 0.22 | 0.55 | 0.18 | 0.3 | 0.12 | 0.28 | - | Alcohols |
| 59 | (-)-spathulenol | 17.21 | 1.1 | 0.4 | 0.36 | 0.21 | 0.12 | 0.18 | 0.66 | 0.22 | - | Alcohols |
| 60 | 4-(3,3-dimethyl-but-1-ynyl)-4-hydroxy-2,6,6-trimethylcyclohex-2-enone | 17.37 | - | - | - | - | - | - | - | - | 0.43 | Ketone |
| 61 | globulol | 17.40 | - | 0.62 | 0.63 | - | - | - | - | - | - | Alcohols |
| 62 | alloaromadendrene oxide- | 17.42 | 1.17 | - | - | 0.3 | 0.33 | 1.41 | 1.37 | 1.19 | - | Ethylene oxide |
| 63 | epiglobulol | 17.63 | 0.29 | 0.28 | - | - | - | - | - | - | - | Alcohols |
| 64 | guaiol | 17.68 | 0.28 | 0.37 | 0.71 | - | - | - | - | - | - | Alcohols |
| 65 | 12-oxabicyclo[9.1.0]dodeca-3,7-diene, 1,5,5,8-tetramethyl-,[1R-(1R*,3E,7E,11R*)]- | 18.13 | 0.43 | - | 0.18 | - | 0.22 | 0.5 | 0.45 | 0.42 | - | Alkenes |
| 66 | selina-6-en-4-ol | 18.37 | - | - | - | 0.63 | - | - | - | - | - | Alcohols |
| 67 | α-benzylphenethylamine | 18.4 | 1.87 | 2.35 | 1.03 | - | - | 0.16 | - | - | - | Amides |
| 68 | tricyclo[5.2.2.0(1,6)]undecan-3-ol,2-methylene-6,8,8-trimethyl- | 18.93 | 0.43 | 0.32 | 0.21 | - | - | - | 0.15 | 0.07 | - | Alcohols |
| 69 | tumulane-1,6-dien-3-ol | 19.52 | 1.96 | 1.48 | 1.94 | 0.64 | 0.62 | 0.52 | 0.34 | 0.4 | - | Alcohols |
| 70 | guai-1(10)-en-11-ol | 19.91 | - | - | 0.20 | - | - | - | - | - | - | Alcohols |
| 71 | cis, trans-farnesal | 21.60 | - | - | 0.23 | - | - | - | - | - | - | Aldehydes |
| 72 | trans-farnesol | 21.85 | 0.69 | 0.65 | 0.86 | 0.69 | 0.26 | 0.45 | 0.28 | 0.34 | - | Alcohols |
| 73 | isoaromadendrene epoxide | 21.94 | - | - | - | - | - | - | 0.24 | - | - | Ethylene oxide |
| 74 | 3-methylbut-2-enoic acid, 4-nitrophenyl ester | 22.24 | 0.83 | 1.02 | - | 2.29 | - | - | - | - | - | Esters |
| 75 | 2,6,10-dodecatrienal,3,7,11-trimethyl- | 22.68 | - | - | 0.42 | 0.23 | 0.07 | 0.16 | 0.18 | 0.11 | - | Aldehydes |
| 76 | phenol,p-(2-nitrovinyl)- | 25.21 | - | 0.27 | - | - | - | - | - | - | - | Alcohols |
| 77 | 2-propen-1-ol,3-(2,6,6-trimethyl-1-cyclohexen-1-yl)- | 25.54 | - | - | - | - | - | - | 0.11 | - | - | Alcohols |
| 78 | ledene oxide-(II) | 27.78 | - | - | - | - | 0.07 | - | - | - | - | Ethylene oxide |
| 79 | n-hexadecanoic acid | 28.08 | 1.9 | 2.46 | 1.69 | 0.87 | 0.36 | 0.37 | 0.2 | 0.2 | - | Acids |
| 80 | cedrane,8-propoxy- | 28.47 | - | 0.31 | 0.42 | - | 0.07 | - | - | - | - | Ethers |
| 81 | acetic acid,7-(1-hydroxymethyl-vinyl)-1,4a-dimethyl-3-oxo-2,3,4,4a,5,6,7,8-octahydronaphthalen-2-yl ester | 28.79 | - | 0.37 | 0.37 | 0.19 | 0.34 | - | - | 0.13 | - | Esters |
| 82 | bicyclo[3.3.1]nonan-9-one,2,4-dimethyl-3-nitro- (exo)- | 28.97 | - | 0.29 | - | - | 0.09 | - | - | - | - | Ketone |
| 83 | 2(3H)-oxazolone,3-[(3,4-dimethoxyphenyl)methyl]-4,5-diphenyl- | 29.32 | - | - | 0.63 | 0.22 | - | - | 0.16 | - | - | Ketone |
| 84 | limonen-6-ol,pivalate | 29.50 | 0.22 | - | - | - | - | - | - | - | - | Esters |
| 85 | 2,6,10-dodecatrien-1-ol,3,7,11-trimethyl-, (Z,E)- | 29.78 | - | - | - | 0.32 | 0.06 | - | 0.14 | - | - | Alcohols |
| 86 | 6,11-dimethyl-2,6,10-dodecatrien-1-ol | 29.95 | - | 0.54 | - | 0.24 | - | - | - | - | - | Alcohols |
| 87 | benzene, 4-butyl-1,2-dimethoxy- | 30.13 | - | 1.21 | - | 0.41 | - | 0.09 | 0.49 | - | - | Ethers |
| 88 | 6-isopropenyl-4,8a-dimethyl-1,2,3,5,6,7,8,8a-octahydro-naphtha len-2-ol | 30.26 | - | - | 0.47 | - | 0.07 | - | - | - | - | Alcohols |
| 89 | trans-geranylgeraniol | 30.45 | 0.23 | 3.15 | 0.21 | 0.86 | 0.25 | - | Alcohols | |||
| 90 | phytol | 30.49 | 0.76 | 6.37 | 0.68 | 0.81 | - | 0.27 | 3.2 | 0.12 | - | Alcohols |
| 91 | 1,6,10,14-hexadecatetraen-3-ol, 3,7,11,15-tetramethyl-, (E,E)- | 30.69 | - | - | - | 0.17 | - | - | - | - | - | Alcohols |
| 92 | 17-octadecynoic acid | 30.76 | - | - | - | - | - | - | - | 0.11 | - | Acids |
| 93 | 9,12-octadecadienoic acid (Z,Z)- | 30.78 | 1.29 | 1.58 | 1.4 | 1.79 | 0.24 | 0.3 | - | - | - | Acids |
| 94 | 12-methyl-E,E-2,13-octadecadien-1-ol | 30.85 | - | - | - | - | - | - | 0.17 | 0.16 | - | Alcohols |
| 95 | 9,12,15-octadecatrienoic acid, (Z,Z,Z)- | 30.87 | 3.23 | 4.02 | 3.03 | 0.89 | 0.54 | 0.59 | - | - | - | Acids |
| 96 | octadecan-4-one | 31.05 | 0.22 | - | 0.23 | - | - | - | - | 0.07 | - | Ketone |
| 97 | stearic acid | 31.15 | 0.46 | 0.58 | 0.45 | - | 0.06 | - | - | 0.08 | - | Acids |
| 98 | 2,6,10-undecatrien-8-ol,2,6-dimethyl- | 31.25 | 0.64 | 1.07 | - | - | - | - | - | - | - | Alcohols |
| 99 | (E)-3,7-dimethylocta-2,6-dien-1 -yl propyl carbonate | 31.28 | - | - | - | - | 0.12 | - | - | - | - | Esters |
| 100 | palmitic acid vinyl ester | 31.37 | - | - | 0.36 | 0.94 | - | - | 0.46 | 0.09 | - | Esters |
| 101 | propanoic acid, 2-methyl-, 1-methyl-1-(4-methyl-3-cyclohe xen-1-yl)ethyl ester | 31.39 | 0.55 | 0.96 | - | - | - | - | - | - | - | Esters |
| 102 | 2-heptadecanone | 31.48 | - | - | 3.6 | 1.12 | - | - | 0.78 | - | Ketone | |
| 103 | 16-hentriacontanone | 31.73 | - | - | 2.08 | - | - | - | - | 1.94 | Ketone | |
| 104 | 9-(3,3-dimethyloxiran-2-yl)-2,7-dimethylnona-2,6-dien-1-ol | 32.08 | 1.49 | 2.65 | 0.2 | - | - | - | - | - | Alcohols | |
| 105 | 1-cyclopentyleicosane | 32.31 | - | 0.44 | - | - | - | - | - | - | Hydrocarbon | |
| 106 | Z-5-methyl-6-heneicosen-11-one | 32.43 | - | - | - | - | - | - | - | 0.08 | Ketone | |
| 107 | 2-octyldecan-1-ol | 32.47 | - | - | - | 4.05 | 0.08 | - | 1.61 | - | Alcohols | |
| 108 | hexacosane | 32.52 | 7.43 | 4.84 | 10.46 | - | - | 2.52 | - | - | Hydrocarbon | |
| 109 | nonadecane | 32.87 | - | - | - | 0.18 | 0.13 | 0.16 | - | - | Hydrocarbon | |
| 110 | α-D-mannofuranoside, farnesyl- | 33.48 | - | - | 0.23 | - | - | - | - | - | Alcohols | |
| 111 | 1-heptacosanol | 33.85 | - | - | 1.96 | 0.71 | - | - | 0.33 | 0.25 | Alcohols | |
| 112 | 2-methylhexacosane | 34.00 | 0.39 | - | - | - | - | 0.15 | 0.38 | 0.1 | Hydrocarbon | |
| 113 | 1-heptatriacotanol | 34.16 | - | - | - | - | - | - | - | - | Alcohols | |
| 114 | methylenebis (methyltertbutylphenol | 34.33 | 0.76 | 0.95 | 0.29 | - | - | - | - | 0.07 | Phenols | |
| 115 | n-tetracosanol-1 | 35.03 | 0.27 | 0.3 | 0.33 | - | - | - | - | - | Alcohols | |
| 116 | tricosane | 35.11 | 0.41 | 0.27 | 1.00 | 0.25 | 0.17 | 0.28 | 0.23 | 0.08 | Hydrocarbon | |
| 117 | phthalic acid,di(2-propylpentyl) ester | 35.80 | - | - | 0.27 | - | - | - | - | - | Esters | |
| 118 | campesterol | 35.82 | 1.54 | - | - | - | - | - | - | - | Alcohols | |
| 119 | heneicosane,11-(1-ethylpropyl)- | 36.01 | - | 0.39 | 0.51 | 0.15 | - | - | - | - | Hydrocarbon | |
| 120 | 1-heneicosyl formate | 36.34 | 0.28 | 0.18 | - | - | - | - | - | Esters | ||
| 121 | eicosane | 36.40 | 0.26 | - | - | - | - | 0.08 | 0.12 | - | Hydrocarbon | |
| 122 | octadecanal | 36.91 | 0.62 | 0.55 | 0.43 | 0.27 | 0.12 | 0.12 | 0.41 | 0.07 | Aldehydes | |
| 123 | 1H,3H-furo[3,4-c]furan, 1,4-bis(3,4-dimethoxyphenyl)tetr ahydro- | 37.17 | - | - | - | 0.15 | - | - | - | - | Ethers | |
| 124 | oxirane, heptadecyl- | 37.50 | 3.51 | 0.74 | 1.90 | 0.69 | - | 0.46 | 0.46 | 0.09 | Ethylene oxide | |
| 125 | 2(3H)-furanone,3,4-bis(1,3-benzodioxol-5-ylmethyl)dihydro-,(3R-trans)- | 37.84 | 12.43 | 12.49 | 16.96 | 5.91 | - | - | - | 0.34 | Esters | |
| 126 | triacontane | 37.94 | - | - | - | - | 2.23 | 1.90 | 1.81 | 0.38 | Hydrocarbon | |
| 127 | 1,30-triacontanediol | 38.52 | 0.96 | 0.94 | 1.07 | 0.23 | - | - | - | 0.14 | Alcohols | |
| 128 | 13-docosenamide, (Z)-erucylamide | 39.48 | 2.87 | 1.21 | 4.81 | 1.61 | 0.07 | 0.68 | 0.59 | - | Amides | |
| 129 | [4-(2,4-dimethoxybenzyl)piperazin-1-yl]-(2-methoxyphenyl)methanone | 39.65 | 5.91 | 1.22 | 3.77 | 2.03 | - | 1.41 | 1.25 | - | Amides | |
| 130 | 1-dodecanol, 2-octyl- | 39.80 | - | 0.40 | - | - | 0.09 | - | - | - | Alcohols | |
| Growth Stages of Leaves (Weeks) | Oil Cell Diameter (μm) | ||
|---|---|---|---|
| C1 | C2 | C3 | |
| 1 | 30.38 ± 1.42 e | 33.91 ± 0.51 de | 27.85 ± 2.05 d |
| 2 | 34.12 ± 0.25 d | 37.6 ± 1.76 c | 28.79 ± 0.44 d |
| 3 | 33.23 ± 1.55 de | 38.6 ± 1.00 e | 33.59 ± 0.31 c |
| 4 | 35.57 ± 0.29 cd | 39.6 ± 0.75 cd | 35.76 ± 0.63 c |
| 6 | 38.02 ± 0.16 c | 40.6 ± 0.21 c | 35.77 ± 0.12 c |
| 8 | 41.26 ± 0.44 b | 41.6 ± 0.54 c | 41.03 ± 1.35 b |
| 10 | 47.66 ± 1.10 a | 42.6 ± 1.37 a | 48.73 ± 0.77 a |
| 12 | 44.16 ± 0.46 b | 43.6 ± 0.57 b | 45.8 ± 1.24 a |
| 24 | 48.67 ± 0.80 a | 44.6 ± 0.69 a | 44.92 ± 0.56 a |
| F | 37.72 | 23.53 | 37.51 |
| P | 1.67 × 10−9 | 5.06 × 10−8 | 1.12 × 10−9 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, B.; Ling, Q.; Xiao, Z.; Zhong, Q.; Zhao, R.; Jin, Z. Variations in Essential Oil Compositions and Changes in Oil Cells during Leaf Development of Citral Chemotype of Camphora officinarum Nees ex Wall. Horticulturae 2024, 10, 597. https://doi.org/10.3390/horticulturae10060597
Zhang B, Ling Q, Xiao Z, Zhong Q, Zhao R, Jin Z. Variations in Essential Oil Compositions and Changes in Oil Cells during Leaf Development of Citral Chemotype of Camphora officinarum Nees ex Wall. Horticulturae. 2024; 10(6):597. https://doi.org/10.3390/horticulturae10060597
Chicago/Turabian StyleZhang, Beihong, Qingyan Ling, Zufei Xiao, Qing Zhong, Ruiqi Zhao, and Zhinong Jin. 2024. "Variations in Essential Oil Compositions and Changes in Oil Cells during Leaf Development of Citral Chemotype of Camphora officinarum Nees ex Wall." Horticulturae 10, no. 6: 597. https://doi.org/10.3390/horticulturae10060597
APA StyleZhang, B., Ling, Q., Xiao, Z., Zhong, Q., Zhao, R., & Jin, Z. (2024). Variations in Essential Oil Compositions and Changes in Oil Cells during Leaf Development of Citral Chemotype of Camphora officinarum Nees ex Wall. Horticulturae, 10(6), 597. https://doi.org/10.3390/horticulturae10060597






