Abstract
Blue light is an important light wavelength in regulating plant flowering. In a controlled environment (CE) plant production systems, blue light can be manipulated easily and even precisely through electric lighting, especially with the advancement of light-emitted diode (LED) technologies. However, the results of previous studies in the literature about blue-light-mediated flowering are inconsistent, which would limit its practical application in CE plant production while implying that an in-depth study of the relevant physiological mechanism is necessary in the future. This review consolidates and analyzes the diverse findings from previous studies on blue light-mediated plant flowering in varying high-value crops from ornamental plants to fruits, vegetables, and specialty crops. By synthesizing the contrasting results, we proposed the possible explanations and even the underlying mechanisms related to blue light intensity and exposure duration, its co-action with other light wavelengths, background environment conditions, and the involved photoreceptors. We have also identified the knowledge gaps based on these studies and outlined future directions for research and potential application in this promising field. This review provides valuable insights into the important and diverse role of blue light in plant flowering and offers a foundation for further investigations to optimize plant flowering through lighting technologies.
1. Introduction
Flowering is a prerequisite physiological process for plants to produce blooms, fruits, and seeds; regulation (promotion or inhibition) of this process is necessary for change of harvesting time according to different production purposes [1]. The flowering process can be divided into two seemingly disparate phases: floral differentiation and floral development, and this process can be affected by environmental factors such as light [2].
Among the different light wavelengths, blue light (BL; 400–500 nm) has been shown to have a significant role in regulating various aspects of plant flowering. BL as an important photosynthetically active radiation (PAR) component can contribute to plant photosynthesis and thus provide a necessary carbon source for plant flowering. Also, BL can regulate the flowering process through its photoreceptors such as cryptochromes, through crosstalk with other photoreceptors such as phytochromes.
In controlled environments such as a greenhouse and vertical farm, BL can be more precisely manipulated through electric lighting, especially with the development of LED technology. Unlike traditional lighting, blue LEDs can provide narrow-band BL without containing other light wavelengths (i.e., pure BL), which can affect photoreceptors’ activity differently from impure BL (i.e., normally including low-levels other wavelengths) from non-LED lighting [3]. Also, blue LEDs have become more and more efficient and inexpensive for commercial applications [4]. Consequently, LED technology makes it possible to apply BL alone or in combination with other wavelengths to mediate plant growth and development (including flowering).
Like diverse plant elongation responses to BL as presented in our recent review [3], inconsistent results have also been reported on plant flowering response to the BL manipulation through electric lighting in the literature. This would affect its application in commercial plant production. Based on the existing literature as well as our research, this review summarizes and compares the relevant results, analyzes and explains the possible reasons, and identifies and suggests the knowledge gaps and future research directions. Also, this review focuses on the relevant application research rather than mechanistic research, in the controlled environment production of different crops, especially flowering plants.
2. Application of Electric Lighting to Manipulate BL in Ornamental Plant Flowering
Electric lighting has been increasingly used for controlled environment production of ornamental plants [5,6]. There are different ways to apply electric lighting in production such as night interruption lighting, day extension lighting, supplemental lighting, and sole-source lighting, for different production purposes.
2.1. Night Interruption Lighting
Under short-day conditions, night interruption (NI) uses low-intensity (3–5 µmol m−2 s−1) lights from incandescent bulbs traditionally to break up a lengthy period of darkness, resulting in a long-day condition [7,8]. During short-day seasons, NI normally expedites the flowering of long-day plants, while inhibiting the flowering of short-day plants [7,8]. When BL is used for NI lighting, its effectiveness is dependent on lighting spectral (i.e., BL alone or its combination with other wavelengths), intensity, duration, as well as other factors such as daytime light conditions and plant genotype (Table 1).

Table 1.
Effects of blue light manipulation through night interruption (NI) lighting on flowering and other traits of ornamental plants grown in a controlled environment.
2.1.1. BL Alone
In some plant genotypes, low-intensity BL is not an effective NI light signal to regulate plant flowering. Under short-day conditions in the greenhouse, 4 h NI lighting with blue LED at 1.5 µmol m−2 s−1 was not perceived as a long day by the tested short-day plants including chrysanthemum, cosmos, dahlia, and marigold, and long-day plants such as dianthus and rudbeckia due to failing to regulate flowering in these plants [16]. Similarly, a 4 h NI lighting provided by blue LEDs at 3.3 µmol m−2 s−1 was not perceived as a long-day signal by the long-day plants such as petunia, rudbeckia, and tickseed in a greenhouse under natural short-day conditions because of no promotional effects on their flowering [22]. Also, a long-day treatment with 4 h NI lighting from blue LED at 10 µmol m−2 s−1 did not inhibit flowering in chrysanthemum, an obligate short-day plant, grown in a chamber [9,10]. Furthermore, in some short-day plants, chrysanthemum, dahlia, and African marigold, BL was not needed to interrupt the long night to inhibit flowering, but a moderate to high red/far-red (R: FR) ratio of light was most effective at interrupting the night [23].
In some other plant genotypes, but not for all, low-intensity blue LED light, like an incandescent light, can also be effective as an NI lighting source for regulating flowering. For example, a 4 h NI lighting provided by blue LEDs at 4 µmol m−2 s−1 promoted flowering in cyclamen, a quantitative long-day genotype, compared with the short-day treatment without NI lighting [18]. Another long-day plant, lisianthus flowered earlier under a 5 h NI lighting with blue LEDs at 5 µmol m−2 s−1 compared with ambient short-day conditions without an NI lighting [21]. However, among three long-day plant genotypes and five short-day genotypes growing in a greenhouse under natural short-day conditions, only perilla, a short-day plant, showed inhibited flowering by a 3 h NI lighting with blue LEDs at 8–10 µmol m−2 s−1 [20]. Possibly, the effective BL intensities for NI lighting varied with plant genotype, which has been supported by a study on some long-day plant genotypes [24].
It has been found that, unlike red light, BL can influence flowering only when delivered with sufficiently high intensities as NI lighting but is ineffective in regulating flowering at low intensities [6]. A 4 h NI lighting provided by blue LEDs at 0.8 or 3.3 µmol m−2 s−1 was not perceived as a long-day signal by chrysanthemum plants because of no inhibitory effects on the flowering [11]. Also, a 4 h NI lighting with blue LEDs at 10 µmol m−2 s−1 failed to inhibit the flowering of chrysanthemums [9,10]. However, 4 h NI lighting with blue LED at 30 µmol m−2 s−1 rather than a lower intensity (1 or 15 µmol m−2 s−1) delayed flowering in chrysanthemum as well as marigold in a greenhouse under short-day conditions [15].
In addition to NI lighting intensity, under short-day conditions, the effectiveness of BL for NI is also affected by daytime light quality. For chrysanthemum growing in a chamber, a 4 h NI lighting with blue LEDs did not inhibit flowering when daytime lighting was delivered by white light [12]. However, a 4 h NI lighting with blue LEDs strongly inhibited flowering, when daytime lighting was provided by blue LEDs rather than RB-LED [12]. Blue LED had a lower phytochrome photostationary state (PPS) than white light and RB-LED. It appeared that, as an NI lighting source, blue LED was not effective when daytime lighting created a high phytochrome activity in the plant. This suggested that light quality during the daytime affected the sensitivity to NI lighting at midnight. Possibly, at least two distinct phytochrome-mediated flowering regulation systems might exist during daytime and nighttime [25].
Besides NI lighting intensity and daytime light quality, flowering mediated by NI lighting with BL is also affected by daytime light duration (i.e., main photoperiod), showing an interaction between NI light intensity and daytime light duration. For chrysanthemum in a growth chamber, the flowering was completely inhibited by 4 h NI lighting with blue LED at 40 µmol m−2 s−1 under 13 h main photoperiod, but not by that at 10−30 µmol m−2 s−1 under 13 h main photoperiod nor by that at 10−40 µmol m−2 s−1 under 10 h main photoperiod [13]. However, for the lower-intensity (<40 µmol m−2 s−1) NI lighting, the flowering was gradually promoted with increasing lighting intensities from 10 to 30 µmol m−2 s−1 regardless of the main photoperiod, [13]. For NI lighting in this genotype, it appeared that there was a complicated interaction between BL intensity and daytime duration to act on flowering.
Whether the effectiveness of BL for NI is affected by daytime light duration is also dependent upon plant genotypes even if they have the same photoperiodism. In kalanchoe, a qualitative short-day plant grown indoors, plants under 4 h NI lighting with blue LED flowered only under short day (10 h) rather than under long day (13 h) conditions [7]. However, in another qualitative short-day plant chrysanthemum grown under 4 h NI lighting with blue LED flowered in all plants under either short-day (10 h) or long-day (13 h) conditions [10].
Limited information is available on the effects of blue LED for NI on day-neutral plants. For geranium grown in a chamber under 10 h short-day conditions, a 4 h NI lighting with blue LEDs showed more pronounced effects on morphogenesis than on flowering [19]. Compared with short-day treatment, NI lighting with blue LED promoted plant flower initiation, and increased flower stalk length while increasing plant height and enlarging total leaf area, but did not affect flowering plant percentage nor flower number per plant [19].
2.1.2. BL in Combination with Other Light Wavelength(s)
In addition to BL alone, a combination of BL with other light wavelength(s) can also be used for NI lighting, and its effectiveness at flowering regulation varies with NI light spectral quality and plant genotypes. For example, a 4 h NI lighting with a mixture of blue and far-red LEDs (51% blue) was perceived as a long-day signal by short-day plants, marigold and dahlia ‘Leanne’, but not by chrysanthemum or dahlia ‘Gallery Pablo’ and by a long-day plant, rudbeckia, under short-day conditions [16,17]. However, a 4 h NI lighting with a mixture of blue and red LEDs (RB-LED; 48% blue) promoted the flowering of most long-day plants tested [17]. Similarly, under short-day conditions, a 4 h NI lighting with RB-LED (50% Blue) was effective at promoting the flowering of cyclamen, a quantitative long-day genotype [18]. Also, 4 h NI with RB-LED (48% blue) was effective at inhibiting flowering by creating a long day for all short-day plants except chrysanthemum, suggesting varying sensitivity among plant genotypes [16].
When BL is applied with red light sequentially for NI lighting under short-day conditions, the effectiveness of BL for NI lighting is affected by its application time frame. For example, chrysanthemum plants grown indoors under short-day conditions were provided a total of 4 h NI lighting, using 2 h blue LEDs first and then 2 h red LEDs (NI-B-R), or 2 h red LEDs first and then 2 h blue LEDs (NI-R-B). Flowering was observed in the NI-R-B rather than NI-B-R. However, when blue LED is applied with far-red LED sequentially for NI lighting, both NI treatments (NI-B-FR and NI-FR-B) promoted flowering to a greater degree than NI-R-B [14]. Both blue and far-red LED had lower PPS values (<0.5) than red LED (>0.8), and could potentially cause inactive phytochrome responses in plants and reverse the effects of red LEDs [26,27]. It appeared that the effects of NI lighting using the combination of BL with other wavelengths on plant flowering might be related to the final phytochrome activity after NI lighting.
2.2. Day Extension Lighting
In addition to NI lighting, day extension (DE) lighting can also create long day conditions through prolonging photoperiod to regulate plant flowering. Differing from NI lighting delivered in the middle of the night (from 10:00 pm to 2:00 am), DE lighting is normally started before the sun sets and kept on until the desired day length is achieved. In short-day conditions, BL alone or its combination with other wavelengths can be used for DE lighting (Table 2).

Table 2.
Effects of blue light manipulation through day extension (DE) lighting on flowering and other traits of ornamental plants grown in a controlled environment.
2.2.1. BL Alone
Delivering BL as DE lighting or NI lighting may result in different flowering responses in some genotypes. For example, for okra, a short-day plant, grown indoors under short-day treatment, 4 µmol m−2 s−1 of blue LEDs delayed flowering when delivered as 12 h DE lighting but did not when delivered as 4 h NI lighting [36]. Possibly, in this genotype, the sensitivity of flowering response to BL was related to lighting amount (lighting duration × lighting intensity) rather than the ways of prolonged-photoperiod lighting (i.e., DE lighting or NI lighting). A similar dependence of BL effectiveness for flowering on the lighting amount was also found in another short-day plant under DE lighting. For chrysanthemums growing in high tunnels covered with shading nets, overnight DE lighting with blue LEDs at about 20 µmol m−2 s−1 inhibited flowering, but failed to prevent flowering when DE lighting time was shortened to 14 h d−1 [28].
The dose–response relationship between prolonged-photoperiod lighting with BL and its effectiveness at flowering regulation has been also identified in some long-day plants. For coreopsis, snapdragon, petunia, and rudbeckia grown in a greenhouse under natural short-day conditions, at a sufficiently high intensity, blue LED delivered as a 7 h DE lighting or 4 h NI lighting promoted the flowering of all four genotypes compared to no DE or NI lighting [24]. The threshold (minimum) intensity of BL was 5 µmol m−2 s−1 for coreopsis and snapdragon, and 15 µmol m−2 s−1 for petunia and rudbeckia, which was higher than that of red + far-red light (2–3 µmol m−2 s−1) when delivered as prolonged-photoperiod lighting [24]. Possibly, BL receptors’ function on flowering induction depends more on light intensity, compared with the photoreceptors of red + far-red light.
Differently, for chrysanthemums growing indoors under short-day daytime lighting, the flowering response to DE lighting with BL seems to be independent of BL amount (either light intensity or light duration), since DE lighting with blue LED that extends total photoperiod to longer than critical day length cannot inhibit flowering while promoting stem elongation, when delivered at a light intensity varying from 10 to 100 µmol m−2 s−1. For example, a 4 h DE lighting treatment with blue LED of 10 µmol m−2 s−1 after 9 h daytime lighting did not delay flowering time and reduce flower number compared with no DE lighting, despite increased plant height [10]. Also, a 4 h DE lighting with BL at about 70 µmol m−2 s−1 did not inhibit the flowering of chrysanthemum growing under 12 h daytime lighting [29]. Furthermore, a 4 h DE lighting or 13 h overnight lighting with blue LED at 100 µmol m−2 s−1 did not inhibit flowering but did promote stem elongation for plants growing under 11 h daytime lighting [30,31].
It appears that blue LED can potentially be used as a DE lighting source during short-day conditions for controlled environment production of cut chrysanthemum flowers to promote stem elongation with no inhibition of flowering. Chrysanthemums are quantitative short-day plants that flower uniformly when the critical day-length photoperiod is ≈13.5 h or less but fail to flower under longer photoperiods than the critical day length [31]. The grading standards for cut chrysanthemum on the world market require an elongated and unbranched plant shape and large-sized flowers [5]. Due to the required stem length specification in cut chrysanthemum production, artificially long days are maintained routinely for 2–3 weeks before the onset of short days [31]. Although the long-day treatment guarantees a sufficient stem length, it delays the transition to flowering in cut chrysanthemum [31]. In this case, DE lighting with blue LED may address this issue.
It is worthwhile to note that different flowering responses were found in chrysanthemums growing under electrical lighting and solar light during the daytime. Under short-day conditions, 4 h DE lighting with blue LED inhibited chrysanthemum flowering in a greenhouse with daytime solar light, but not in a growth chamber with daytime lighting from RB-LEDs (40% B), despite an increased stem length in both greenhouse and chamber [32]. This contrasting flowering response to DE lighting from blue LED might have resulted from different daytime spectral qualities, which was also observed for NI lighting mentioned before [12]. A recent indoor study indicated that the inclusion of FR light, but not a green light, in the daytime lighting was necessary for 4 h DE lighting with blue LED to inhibit flowering in chrysanthemums [33]. It appeared that a high daytime phytochrome activity might have prevented chrysanthemum perception of subsequent BL as a long-day signal [33].
The flowering response in chrysanthemums to DE lighting with BL is not universal but varies with genotype even if they are short-day plants. For some short-day genotypes growing indoors under short-day daytime lighting with RB-LED (40% B), 4 h DE lighting with blue LED inhibited flowering for kalanchoe, perilla, and stevia, but not for artemisia, chrysanthemum, cosmos, or poinsettia compared to short day treatment despite a flowering delay in cosmos, and poinsettia [35]. Also, the plants under 4 h DE lighting with blue LED resulted in a 4% to 36% increase in total dry weight. It appeared that increasing growth rate under short-day conditions through DE lighting with BL, without compromising flowering and quality, was possible for some, but not all short-day genotypes.
2.2.2. BL in Combination with Other Light Wavelengths
When delivered as DE lighting, a combination of BL with high-level (e.g., 80%) red light (i.e., RB-light) differs from BL alone in its effect on plant flowering and other traits. In a study on impatiens, a day-neutral plant, compared with blue LED alone, RB-LED delayed flowering when delivered as 2 h or 4 h DE lighting, despite an increased number of flower buds and open flowers when delivered as 4 h DE lighting [34]. Also, compared with blue LED alone, RB-LED reduced plant height, leaf area, and aboveground biomass, especially when delivered as 2 h DE lighting [34]. It appeared that DE lighting with RB-light and BL were perceived by plants as different photoperiod signals, which may be related to different involved key photoreceptors as well as their different activities.
When delivered as DE lighting, a combination of BL with low levels of other wavelength(s) (i.e., impure BL) and BL alone (i.e., pure BL) can affect plant flowering differently, despite varying sensitivity among plant genotypes. In one of our recent unpublished studies, we treated chrysanthemum (obligate short-day plant), geranium (day-neutral plant), calibrachoa (facultative long-day plant), and gerbera (facultative short-day plant) plants in a greenhouse using 4 h DE lighting with pure BL, i.e., blue LED (PPS = 0.47) or impure BL, i.e., a combination of blue, red, and FR LEDs (94% B; PPS = 0.66) during winter. In chrysanthemums, pure BL slightly delayed flowering time and increased flower bud number, and impure BL inhibited flower initiation, compared with no DE lighting. In calibrachoa, both DE lighting treatments promoted earlier flowering compared with no DE lighting, but impure BL had a greater promotion effect on the number of flower buds than pure BL. In geranium, both DE lighting treatments did not affect flowering initiation time, but increased flower bud size compared with no DE lighting treatment. In gerbera, both DE lighting treatments did not affect flowering initiation time or flower size compared with no DE lighting. It appeared that BL in combination with low levels of other light wavelengths might have changed phytochrome activity based on PPS values and thus shows different flowering responses for the same plant genotype.
2.3. Supplemental Lighting
When the daytime light level is low, supplemental lighting (SL) is normally used for increasing light amount to compensate for insufficient photo-assimilates, while changing spectral quality and extending photoperiod if applied at nighttime. BL can be applied alone or combined with other light wavelength(s) as an SL source during daytime or nighttime in a greenhouse or indoor environment to regulate flowering as well as morphology in ornamental plants, based on production purposes (Table 3).

Table 3.
Effects of blue light manipulation through supplemental lighting (SL) on flowering and other traits of ornamental plants grown in a controlled environment.
2.3.1. BL Alone
The effect of daytime SL with low-intensity BL on the flowering of long-day ornamental plants in a greenhouse is dependent on the natural light level and the presence of FR light. For example, for petunia grown in a greenhouse under plastic film transmitting or not transmitting FR light, daytime SL with blue LED at 50 µmol m−2 s−1 promoted flowering, compared to high-pressure sodium (HPS) light, during early spring when the natural irradiance was low; however, in late spring, when the natural irradiance was higher, the effect of blue LED in an FR-deficient environment was not different from red LED on plant flowering [49].
For some short-day plants grown indoors under long-day conditions, whether daytime SL with BL can promote flowering depends on plant genotypes, which may differ in dominant photoreceptors for controlling flowering. For orchids grown in growth chambers under white fluorescent lamps, daytime SL with blue LED did not differ in flowering time from no SL treatment and delayed flowering compared to SL with red LED [48]. In addition to a lower spiking ratio, SL with blue LED also reduced spike length, compared to SL with red LED [48]. It appeared that whether the spiking of orchid was improved by daytime SL might be dependent on the relative amount of active phytochrome. However, for seedlings of morning glory, grown indoors under white lighting, daytime SL with blue LEDs of 450 nm was the most effective at promoting flowering among various peak wavelengths from 400−495 nm, and the flowering responses to different peak wavelengths corresponded to the absorption spectrum of cryptochromes [47]. Possibly, in morning glory, the activated cryptochrome by the daytime SL with BL might have promoted flowering as a strong stimulus over the critical day length.
Daytime SL with BL for several hours before dark (pre-dark SL) can promote flowering and improve the aesthetic value of some short-day ornamental plants grown indoors. For kalanchoe, a 4 h pre-dark SL with blue LED promoted flowering and enhanced plant growth compared with no SL, with a greater promotional effect on flowering in the long- vs. short day (13 h vs. 10 h) condition [7]. In chrysanthemum grown indoors under 13 h photoperiod, a 4 h pre-dark SL with blue LED promoted flowering with increased plant height, compared with no SL [10]. It appeared that it was possible to apply pre-dark SL with blue LED as an alternative method to using blackout curtains in long-day seasons to induce flowering of short-day plants.
It is worthwhile to note that the effect of pre-dark SL with BL on the flowering of short-day plants is affected by daytime duration (or main photoperiod), SL intensity, as well as SL duration. For chrysanthemums grown indoors, a 4 h pre-dark SL with blue LED at 10−30 µmol m−2 s−1 did not affect flowering compared with no SL treatment under 10 h main photoperiod but promoted flowering compared with no SL treatment under 13 h main photoperiod [13]. Also, under the blue LED lighting treatments, the flowering was gradually promoted when the PPFD increased from 10 to 30 µmol m−2 s−1 but inhibited when the PPFD increased up to 40 µmol m−2 s−1 regardless of the photoperiod [13]. In addition, when blue LED light of 30 µmol m−2 s−1 was applied as 4 h pre-dark SL under 13 h main photoperiod at different intervals (from once every 7 days to once every day), the number of flowers increased and the flower buds appeared earlier as the proportion of SL days increased [37].
In short-day plants, daytime and nighttime SL with modest-intensity BL have different effects on flowering. For kalanchoe, grown in a greenhouse under short-day natural light conditions, daytime SL with blue LED at 100 µmol m−2 s−1 accelerated flowering compared with SL with red or green LED (no flowering), with an equal or greater promotional effect relative to no SL treatment, depending on cultivar [45]. However, when these LEDs were used as nighttime SL at the same intensity, the blue LED completely inhibited flowering similar to other LEDs, compared with no SL [45]. Possibly, nighttime SL with modest-intensity BL could be sensed as a photoperiod signal.
Like NI or DE lighting, nighttime SL with low-intensity BL is not sensed as a photoperiod signal to affect flowering in some short-day plants such as chrysanthemum. For chrysanthemum in a greenhouse under short-day natural light conditions, 15 h nighttime SL with BL at 3.6 µmol m−2 s−1 did not affect flower initiation, but the BL at 7.0 µmol m−2 s−1 delayed flowering compared with no nighttime SL [38]. Also, the nighttime SL with BL at both intensities reduced flower size, and flower dry mass compared to no SL, with a greater decrease in flower dry mass at a higher BL intensity, [38]. In another trial on the genotype from the same group under the same daytime conditions, 6 h nighttime SL with BL at 0.4−3.5 µmol m−2 s−1 did not affect flower dry mass compared with no SL treatment. This indicated that for chrysanthemum long-day treatment through SL with BL at intensities < 3.5 µmol m−2 s−1 did not adversely affect the flowering process [38].
For some long-day ornamental plants grown under short-day conditions, nighttime SL with BL cannot necessarily promote flowering relative to no SL, and its promotional effect is less than far-red light. For Eustoma grandiflorum, grown in a phytotron under 8 h sunlight, flower budding was promoted by overnight SL with blue LED at 8.8 µmol m−2 s−1, compared with no SL, but the flower budding with blue LED was later than those with far-red LED at 10 µmol m−2 s−1 [40]. For Gypsophila paniculata under 9 h natural light conditions, 15 h nighttime SL with blue LED at 20−30 µmol m−2 s−1 did not promote flowering, but far-red LED did, compared with no SL [44]. Possibly, for these long-day plants, their flowering responses to nighttime SL with BL might require different threshold BL amount (intensity × duration), as mentioned before in prolonged-photoperiod lighting.
For some perennial ornamental plants insensitive to photoperiod, nighttime SL with BL can promote flowering relative to no SL, and its promotional effect relative to other wavelengths differs among plant genotypes. For tulips grown in a greenhouse under short-day season, 4 h nighttime SL with BL rather than white light from colored fluorescent lights induced earlier flowering and improved flowering uniformity compared with no SL, despite no effects on flower morphology [53]. For Delphinium grown indoors under 8 h short-day conditions, 12 h nighttime SL with blue LED light promoted flowering compared with no SL but reduced the number of spikes, and total florets without affecting cut flower length [39]. Although flowering was promoted by blue LED relative to no SL, its promotional effect relative to other LEDs varied among cultivars. Compared with red LED, the promotional effect of blue LED was less in ‘Aurora Light Blue’ and was greater in ‘Super Grand Blue’. Compared with far-red LED, the promotional effect of blue LED was less in both cultivars [39].
2.3.2. BL in Combination with Other Light Wavelengths
As an SL source in the greenhouse, a mixture of blue with red light (RB-light) with 15−20% BL has equivalent or better effects on flowering and other metrics compared with HPS (high-pressure sodium; normally 5% B). For potted poinsettia, with strict requirement of plant height in production, daytime SL with RB-LED (20% B) did not delay bract color formation, visible cyathia or flowering, but produced shorter plants, and decreased leaf and bract area, chlorophyll content, and total dry matter accumulation, compared with HPS lamps [51]. Similarly, for roses, SL with RB-LED (20% B) did not affect the time to open flowers and the total dry mass production, despite causing floral initiation at a higher leaf number and enhancing more compact plants, compared with HPS [52]. For gerbera, plants under daytime SL with RB-LED (15% B) had a larger flower diameter in ‘Acapulco’, compared to those in HPS treatment, despite no differences in total or marketable flower numbers [42]. Also, the SL with the RB-LED changed stem length and improved fresh biomass in some cultivars [42].
In greenhouse production, SL from RB light with higher BL proportions (>20%) can cause different effects on flowering and other metrics among different plant genotypes. For marigolds grown under SL, low-blue RB-LED (25% B) caused more blooms than high-blue RB-LED (50% B), or HPS, although the latter two SL treatments also produced more blooms than no SL treatment [46]. Also, the low-blue RB-LED increased shoot growth compared with the HPS treatment, but the high-blue RB-LED was not different from the HPS treatment. Plants in both RB-LED light treatments had greater petal pigment content than plants in the no-SL treatment [46]. However, for potted geranium plants, SL with blue-rich RB-LED (45% B) promoted early flowering and flower number, as well as canopy compactness, compared with HPS [41]. In the future, it is necessary to explore the optimal BL percentages in RB light for SL in greenhouse production for some commercially important ornamental plants in terms of flowering as well as other metrics.
Unlike long-time (≥10 h daily) SL, short-time (2−4 h daily) pre-dark SL using RB-LED with varying BL percentages seems to have minimal effects on plant flowering and other traits. In a study conducted in our lab on four potted flowers, petunia, marigold, calibrachoa, and geranium grown indoors under 14 h daytime lighting from broad-spectrum LED, 2 h pre-dark SL treatments with RB-LED (10−75% B) did not affect flowering nor plant elongation compared with pre-dark SL treatment with daytime-spectrum LED, except for a small decrease in plant height for petunia [50]. Also, plant responses did not differ among RB-LEDs with different BL proportions. Possibly, the effects of BL proportions in RB-LED could also be related to SL duration.
In addition to RB-LED, a mixture of RB-LED with far-red (RBFR-LED), or both far-red and green LED (RBGFR-LED) can also be used as an SL source in greenhouse conditions. When the blue proportion in these LED combinations is optimal, they can cause a similar or better effect on plant flowering compared with blue LED or HPS. For three long-day specialty cut flowers, godetia, snapdragon, and stock, grown in a greenhouse under SL, RBFR-LED (17% B) resulted in earlier flowering by 10−15 days depending on genotypes compared with red LED and showed no difference from the blue LED and HPS [43]. However, RBFR-LED, as well as blue LED and HPS, caused shorter cut flowers than red LED. RBGFR-LED (17% B) slightly delayed flowering time relative to RBFR-LED and promoted earlier flowering than red LED. Stem caliper was thinner for stock grown under SL with blue LED compared to the other treatments. Flower petal color was not commercially different between SL treatments. It was recommended to utilize an SL fixture providing a spectrum similar to the RBFR-LED or RBGFR-LED [43].
2.4. Daytime Sole-Source Lighting
For ornamental plants grown in an indoor environment excluding natural light, BL alone or a mixture with other light wavelength(s) can be used for daytime sole-source lighting to mediate the flowering initiation and development (Table 4). In addition to pre-harvest lighting, BL alone or a mixture with other light wavelength(s) can also be used as sole-source lighting for postharvest lighting to mediate flower development such as senescence.

Table 4.
Effects of blue light manipulation through daytime sole-source lighting on flowering and other traits of ornamental plants grown in a controlled environment.
2.4.1. Pre-Harvest Lighting
- (1)
- BL alone
For some short-day plants grown indoors, daytime sole-source lighting with BL for short-day hours (below critical day length) is less effective in inducing flowering than red, white, or green light. For duckweed, sole-source lighting with BL at as well as far-red light failed to induce flowering under a short-day photoperiod (<13 h), although these plants responded as a typical short-day plant in red and white light [62]. For kalanchoe, sole-source lighting with blue LED for 8 h d−1 delayed flowering, compared with white, red, and green LEDs, with the earliest flowering occurring under red LED [45]. Possibly, the inhibitory action of BL or far-red light might be due to the lowering of phytochrome activity below those required to start the dark reactions that lead to flowering. This has been supported by the reverse flowering response to R/FR or R/BL in a study on duckweed [62].
In contrast, for some short-day plants grown indoors, daytime sole-source lighting with BL for long-day hours (above critical day length) is more effective in inducing flowering than red, white, or green light. When morning glory plants were exposed to sole-source lighting with blue, green, red, or white light at 120 µmol m−2 s−1 for 16 h daily, flowering occurred in BL, but not at all under green, red, or white light [68]. For morning glory plants under long-day conditions, active phytochromes seemed to have little effect on flower induction, but BL receptors, such as cryptochromes, might have played a key role in flowering initiation. It appeared that the duration of sole-source lighting with BL at low to modest intensities was not sensed by short-day plants as a day-length signal in terms of flowering initiation response.
In addition to short-day plants, the flowering of long-day plants and day-neutral plants grown indoors can also be promoted by daytime sole-source lighting with BL for long-day hours relative to red light. In our studies on petunia, calibrachoa, geranium, and marigold, daytime lighting for either 24 h or 16 h daily with blue LED promoted plant flowering (earlier flowering, a greater flowering index, and more visible flower buds and open flowers) compared with red LED [26,72]. Also, even when blue or red LED was applied only at the transplant stage and switched to other spectral lights at the post-transplanting stage, the promotional effect of blue LED relative to red LED was still observed in the afterward flowering of mature plants [73]. Through a series of studies, we have concluded that the promotional effect of blue LED on flowering relative to red LED is related to its low PPS, which can induce lower activity of phytochromes and CRY1, but higher activity of CRY2 and phototropins in plants [26,75,76,77]. Furthermore, the promotional effect of blue LED relative to red LED was greater under 24 h than 16 h lighting in many cases [72]. It appeared that plant flowering response to daytime sole-source lighting with BL could be positively affected by photoperiod (i.e., lighting duration) under long-day conditions.
Besides photoperiod, different plant genotypes can also contribute to the varying effect of daytime sole-source lighting with BL relative to red light on plant flowering, which has been observed in some perennial ornamental plants. For Jasminum sambac, sole-source lighting with blue LED delayed flowering time and decreased the number of flower buds, compared with red light [65]. However, for tulips grown indoors under sole-source lighting, blue LED did not affect flowering time nor flower diameter, compared with red LED, despite increased flower length [74]. Also, for narcissus bulbs forced under sole-source lighting, BL did not differ from red light in its effects on flowering date or the number of flowers [69].
For the same plant genotype(s), the effect of daytime sole-source lighting with BL on flowering varies with reference light wavelength or growth temperature. In marigold and salvia, daytime lighting with blue LED reduced the number of flowers compared with fluorescent light; however, blue LED resulted in similar flower numbers as red LED [67]. For morning glory exposed to daytime sole-source lighting at temperatures of 18, 23, or 28 °C, BL did not differ from red or white light in its effects on flowering at 18 °C and promoted flowering at 23 °C or 28 °C, with a greater promotional effect at 23 °C [68].
- (2)
- BL in combination with other wavelength(s)
BL can also be applied with red light (RB light) as daytime sole-source lighting in controlled environment production of ornamental plants. However, the effect of RB light (20−50% B) on flowering relative to BL alone depends on plant genotypes and photoperiod. In cyclamen under sole-source lighting for 12 or 10 h d−1, RB-LED (50% B) improved flower induction, with the highest number of flower buds and open flowers under 10 h photoperiod, compared with blue LED [60]. For chrysanthemums, sole-source lighting with RB-LED (20% B) delayed flower initiation by 30 d at a photoperiod of 15 h, but not at a photoperiod of 11 h compared with blue LED for 11 h daily [30]. It appeared that BL-mediated plant flowering could be affected by its co-action with red light, due to a cross-talk between cryptochrome and phytochromes.
For some ornamental plants, a higher BL percentage in RB-lighting as sole-source lighting benefits plant flowering. In Hippeastrum hybrid under sole-source lighting for 14 h d−1, compared with white LED, RB-LED with a higher B% (90%) promoted early flowering initiation and extended flowering time, while RB-LED with a lower B% (10%) delayed flowering [64]. For morning glory under sole-source lighting from RB-light for 13 h d−1, with BL proportions in the RB-lighting increasing from 20% to 85%, plant flowering was promoted, showing a sensitive response to BL proportions [47]. It appeared that at least for these genotypes under the normal photoperiod, BL could effectively promote flowering with the co-action of red light.
It is worthwhile to note that for a certain plant genotype under RB-light as sole-source lighting, the optimal BL proportion for flowering is not necessarily also optimal for other plant traits. When sole-source lighting with RB-LEDs with varying BL percentages was applied to indoor cultivation of Lilium, plants were the tallest and had the largest flower under 20% BL, the longest vase life under 40% BL, the shortest growing cycle under 60% BL, and the largest leaf area and the most intense petal color under 80% BL [66]. So, the optimization of BL proportion in RB-LED lighting could be based on specific production purposes.
When BL percentages in sole-source lighting vary within a smaller range (e.g., 5−33%), it may have little effect on plant flowering for many ornamental plant genotypes; however, in this case, light spectral quality change may play a more important role in mediating flowering. In roses, compared to HPS lamps (5% B), sole-source lighting with RB-LED (20% B) did not affect flowering s [52]. For different types of ornamental transplants under sole-source lighting with RB-LEDs, increased BL proportions from 25% to 33% did not affect the subsequent flowering of annual bedding plants [61]. Differently, the addition of FR at ≥20 µmol m−2 s−1 to RB-LED accelerated the flowering of at least some long-day plants such as snapdragons, but did not affect the flowering of day-neutral or short-day plants [61]. However, in our recent study on gerbera transplants under sole-source lighting, when adding low-level UVB, UVA, green, or far-red light to RB-LED (15% B) and keeping the same total PPFD for all treatments, the flowering time after transplanting was not different among the different lighting treatments [63]. When the low levels of other light wavelengths were added to RB-LED, and the phytochrome activity was changed little (with the PPS values varying 0.84−0.88). In this case, possibly, BL percentage still played a key role in flowering mediation.
When maintaining high BL proportions in sole-source lighting, mixtures of BL with different other light wavelengths even at low levels may trigger contrasting flowering responses. In our recent studies on petunia, calibrachoa, geranium, and marigold under sole-source lighting, adding a low level of red to blue LED light (94% B) delayed plant flowering time compared with blue LED alone [26,73]. However, further adding low levels of far-red LED light gradually increased its promotional effects on flower initiation and eventually showed a similar effect to blue LED. Similar promotional effects were found on flower size for calibrachoa [26,73]. It appeared that impure and pure BL might have contrasting effects on plant flowering, depending on the contaminated light wavelengths that cause varying phytochrome activity. BL associated with lower phytochrome activity could promote flowering, although there was a varying level of sensitivity among plant genotypes.
For plant genotypes sensitive to variations of BL proportions, it is possible to apply dynamic lighting with varying BL proportions during different growth stages to mediate flowering and morphology. For petunia seedlings grown under sole-source lighting, dynamic lighting using RB-LEDs with a gradual increase in BL proportion from 0 to 100% resulted in the earliest flowering time despite causing less flowers, compared with HPS lamp [70]. Also, the dynamic lighting enhanced shoot dry weight, shoot length, and leaf area, compared to RB-LEDs with constant BL proportions, or HPS lamps. It appeared that modifying BL percentages in RB-LED lighting during the seedling stage could be an efficacious alternative to standard lighting systems [70]. However, in our recent study on campanula under sole-source lighting, dynamic lighting with red LED, blue LED, and RB-LED sequentially at three stages did not differ in its effect on flowering time from concurrent lighting using RB-LED with the same total DLI through the whole growth period, despite modified plant morphology such as promoted plant elongation [55]. Possibly, flowering response to BL percentage was less sensitive for campanula than petunia.
2.4.2. Post-Harvest Lighting
Improving the marketability of pot flowers and extending the vase life of cut flowers have practical significance for the development of the flower industry. In addition to controlling the other storage environment factors and post-harvest use of floral preservatives, recently, it has been found that post-harvest sole-source lighting with BL is an effective environmental controlling method in improving the postharvest quality and extending the vase life of some floral crops [56]. Although sole-source lighting with BL can be introduced as a physical factor to improve some traits of post-harvested flowers, the response can vary with crop types, light duration, flower traits, or light spectra.
Among different light wavelengths, low-intensity BL seems to be the most effective one for sole-source lighting during post-harvest storage of potted flowers in terms of flowering responses. For example, in potted chrysanthemums stored indoors, sole-source lighting at 30 µmol m−2 s−1 with BL resulted in the greatest number of developed flower heads, the earliest opening and coloring of inflorescence buds, and the longest post-harvest preservation among all lights (white, blue, green, yellow and red light) [59]. Also, BL led to bigger flower heads than white and green light [59].
For cut flowers, postharvest sole-source lighting with low- to modest-intensity BL can delay flower senescence and thus improve vase life relative to other light wavelengths or no lighting, but its improving effects vary with the photoperiod of the lighting. In cut flowers of carnation, postharvest LED lighting with BL at 150 µmol m−2 s−1 for 12 h d−1 delayed senescence and improved vase life compared to red light and white light [56,57]. BL also delayed the decline in petal carotenoid contents in comparison to red or white light [56,57]. In Alstroemeria cut flowers under postharvest lighting with BL at 15 µmol m−2 s−1, a lighting duration of 12 h within the tested photoperiod ranges (6−24 h daily) was most effective at improving vase life relative to no lighting treatment [54]. The highest water uptake and total chlorophyll and the lowest ethylene were also obtained from the flowers exposed to 12 h of BL [54].
Effects of postharvest sole-source lighting with BL on cut flowers vary with flower traits and background light. In cut flowers of petunia, postharvest lighting with BL had little to no effect on volatile emission, but slightly decreased benzaldehyde emission relative to white light [71]. Unlike red and FR light, BL treatments did not affect the emission of phenylacetaldehyde, 2-phenylethanol, benzyl alcohol, and benzyl benzoate [71]. However, postharvest lighting with BL was found to affect flower color effectively associated with increased anthocyanin, the main pigment of cherry blossoms, especially with a coaction of red light. In cherry blossom, when cut shoots bearing flowers in bloom were exposed to postharvest lighting for five days, both the blue and red LEDs were involved in cherry blossom coloration, but the blue LED of 450 nm was the most effective [58]. Also, irradiation with both blue and red LED induced greater anthocyanin production than irradiation with blue LED alone, indicating an association between the BL and red light receptors [58].
3. Application of Electric Lighting to Manipulate BL in Other Plants
For plants other than floral crops, such as tree fruits and fruit vegetables, although flowers are not harvested organs for the crops, flowering is a prerequisite physiological process for their production. For some vegetables (e.g., broccoli head, Chinese kale) and some medicinal crops (e.g., saffron, cannabis), flowers are also the main harvested organs. Even when vegetative parts are the main harvested organs, promotion or inhibition of flowering is necessary for shortening or prolonging the time of the cropping cycle according to different production purposes. In this case, the studies about BL manipulation through electric lighting to mediate the flowering of some fruit crops, vegetable crops, and specialty crops are also included in this review (Table 5).

Table 5.
Effects of blue light manipulation through electric lighting on flowering and other traits of fruit crops, vegetable crops, and specialty crops grown in a controlled environment.
3.1. Fruit Crops
Most fruit crops, especially tree fruits, are rarely grown in controlled environments except for some berry fruits such as strawberries. Recently, there has been a worldwide rise in controlled environment production of berry fruits in greenhouses or vertical farms, where the use of electrical lighting is a common approach for promoting flower initiation and improved fruit yield [111]. Strawberry plants are one of the candidate berry fruit crops for controlled environment production because they are smaller in size and need lower necessary light intensity (100–300 µmol m−2 s−1) for cultivation [105]. In this case, it is feasible to manipulate the light environment to mediate flowering and fruiting for strawberry production in controlled environments. However, for fruit-bearing crops such as strawberries, their flowering response to light varies with plant genotype and growth environment [111]. Therefore, it is necessary to consider these factors during the manipulation of BL to optimize light spectral composition to control flowering.
For strawberries grown indoors under sole-source lighting, flowering, and fruit response to BL varies with plant variety, photoperiod, and reference light. In everbearing strawberry ‘HS138’, sole-source lighting with blue LEDs for either 16 h d−1 or 24 h d−1 during the nursery period resulted in earlier flowering compared to red LEDs, with a greater effect under longer photoperiods [104]. Despite a shorter vegetative growth period, plants grown under 24 h lighting with blue LEDs had a higher fruit yield compared to those grown under white fluorescent lamps at a 16 h photoperiod [104]. In another study by the same research group, 24 h sole-source lighting was applied to the same genotype of strawberry plants during the nursery period [105]. The number of days to anthesis did not differ among blue LEDs with three different peak wavelengths, and all blue LED types stimulated more flowering compared to red LEDs with three different peak wavelengths except 685 nm. Although there was no difference in daily fruit production relative to red light, BL accelerated harvesting due to the advancement in flowering [105]. However, in June-bearing strawberry ‘Elsanta’, 14 h sole-source lighting with blue LED did not affect flowering time or flower number per plant, but enhanced fruit set and increased final yield, as compared to fluorescence light or red LED [103]. Moreover, blue LED increased flower stem length for easy harvesting but did not affect main fruit quality traits [103].
For strawberries grown under supplemental lighting (SL), flowering response to BL varies with plant variety, and environmental conditions, especially background light. In the everbearing strawberry ‘Pechka’ inside a greenhouse, nighttime SL with blue LED produced the largest number of flower clusters among blue, green, and red LEDs [106]. The blue LED increased the total weight of flower clusters (including developed fruits) per plant than the red LED in June because the BL promoted the flowering and fruiting of each floret [106]. For the day-neutral strawberry ‘Yellow Wonder’ and ‘Hawaii-4’ grown inside a growth chamber under daytime lighting with sunlight-mimicking LED, flower induction was most successful under nighttime SL with blue LED and under 24 h SL with far-red light [107]. Both light treatments overruled the photoperiodic control of flowering, and boosted flowering, leading to a higher fruit yield [107]. For the strawberry ‘Festival’ inside a net house in Bangladesh during the winter season, 3 h end-of-day SL with blue LED was less effective than red LED at promoting reproductive growth in terms of days to first flower bud, flowering, fruit setting, and fruit harvesting [100]. However, RB-LED treatment showed the best in all the parameters and produced the highest yield [100].
When the combination of BL and other wavelength(s) is used for strawberry production either as sole-source lighting, SL, or NI lighting, its effect on flowering varies with BL proportions in the lighting. For example, sole-source lighting from RB-LED lighting with 5% B (within the range from 5% to 17% B) was most effective in enhancing the number of inflorescences and t fruit yield per plant, while increasing flowering stem length compared with HPS light [101]. For transplants grown in a greenhouse, SL using blue + far-red (BFR) LEDs with a higher blue percentage (83% B) (within the range from 17% to 83% B) increased flower bud induction, with a greater promotional effect under 24 h vs. 10 h photoperiod [102]. Furthermore, transplants grown under BFR-LED with 83% B supplied as 2 h NI lighting produced the highest flower buds per plant among all treatments and promoted flower development outside the crown [102].
Limited information is available on other berry fruit crops. For blueberries grown under sole-source lighting, plant flowering, and growth response to BL varies with plant variety and light spectral. In two blueberry varieties grown under sole-source lighting with blue LED, RB-LED (50% B) or red LED, ‘Misty’ had the highest cumulative flower number under blue LED light, while ‘Sharpblue’ had the highest flower number under RB-LED light [80]. Blue LED light caused growth delay in ‘Sharpblue’ plants, but RB-LED light promoted desirable vegetative growth and continuous flowering. Fruits grown under blue LED and RB-LED lights had higher soluble solids and fruits under RB-LED lights had greater fresh weight, compared with red LED [80].
For tree fruits, the relevant information is nearly unavailable since tree fruits are mainly produced in open fields rather than greenhouse. However, greenhouse production of tree fruits has developed rapidly in China since the 1990s, which triggered the research interests in the application of lighting such as nighttime SL to address low natural light issues during early-spring production. For example, the researchers from Beijing University of Agriculture studied the effect of SL with different colored fluorescent lights on greenhouse peaches, “Ruiguang 27” and “Jing Yu” (Figure 1) [89]. Daily 4 h SL at 30 µmol m−2 s−1 with BL did not affect flower density of 1-year-stem for both peach varieties in the next year, but red or white light increased this trait in “Ruiguang 27” and “Jing Yu”, respectively, compared with no SL. Also, BL is less effective at inhibiting new shoot elongation growth than white light. However, similarly to white light, BL improved fruit size, compared to no SL [89].

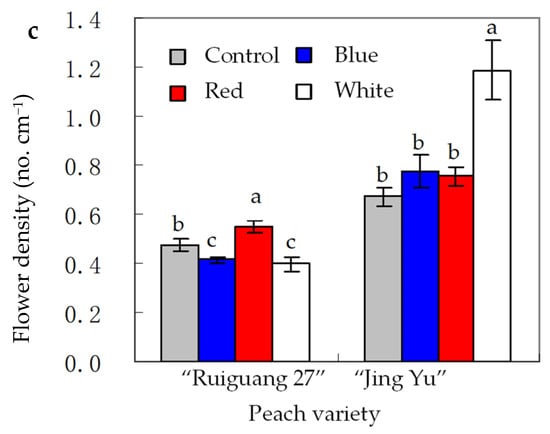
Figure 1.
An example of the application of supplemental lighting with colored fluorescent light to greenhouse peach production in China [89]. (a) Plants from blue light treatment during fruit growth period; (b) Plants from blue light treatment during flowering period; (c) Flower density under supplement lighting with blue, red or white light, and no lighting as control. Flower density = flower number on one-year stem/length of one-year stem.
3.2. Vegetable Crops
Flowering regulation plays an important role in the controlled environment production of vegetables, especially fruit vegetables. In fruit vegetables, strong and dwarf transplants with earlier flowers would not only benefit plant growth after transplanting but also promote early yield. For example, during the production of high-quality tomato seedlings in Japan, in addition to controlling the stem length, the node position of the first flower truss is also crucial due to the requirement to suppress stem elongation and promote flowering simultaneously [108]. Besides fruit vegetables, the crop yield and quality of vegetables with flowers as the main edible organs (such as broccoli and Chinese kale) are directly determined by their flowering process. For the above vegetables, BL manipulation can affect plant flowering (such as sex expression and flower initiation) and thus crop yield and quality.
Sole-source lighting with BL or its combination with red light can affect flower sex expression by increasing female flower ratios in some fruit vegetables depending on plant genotypes. For bitter gourd seedlings, sole-source lighting with blue LED increased the total female flower number and female flower nod ratio on main stems, compared with red LED [79]. For squash seedlings, sole-source lighting with blue LED did not differ from the red LED in female flower numbers [97]. However, RB-LED with a lower blue proportion (56% B) induced more female flowers and produced more fruits compared with blue LED as well as red LED and RB-LED with a higher blue proportion (71% B) [97].
For indoor production of fruit vegetable transplants under sole-source lighting, plant flowering response to BL relative to red light varies with plant genotype, and RB-LED is a more reliable light source than blue LED alone. In pepper, sole-source lighting with blue LED promoted early flowering compared with green or red LED [90]. Another study on tomato and pepper indicated that sole-source lighting with blue LED delayed time to flowering compared with red LED [110]. However, RB-LED (33−67% B) resulted in earlier first flower and fruit formation and higher fruit yield compared with blue LED [110]. In tomatoes, sole-source lighting using RB-LED with less than 50% B (with BL intensity of 75 µmol m−2 s−1) was recommended to promote flowering while suppressing spindly growth during seedling growth [108].
In addition to RB-LED, RBG-LED can also be applied to the indoor production of fruit vegetables under sole-source lighting; however, the optimal blue proportions for flowering differ from those for growth. For tomatoes grown indoors under sole-source lighting with RBG- LED at varying light intensities, the optimal BL proportion was 15−20% for earlier flowering and around 15% for the growth of young plants [109]. However, the optimal value shifted to a higher blue proportion if the growth light intensity was low, suggesting the dependence of the optimal BL proportion on the light intensity as well [109].
During greenhouse production of fruit vegetables, supplemental lighting (SL) with low- to modest-intensity BL alone or its combination with red light has minimal effect on flowering, despite with significant effect on other metrics. For cucumber, tomato, and sweet pepper transplants growing in greenhouses under HPS lamps, SL with blue LED at 15 µmol m−2 s−1 did not affect flower bud formation of tomato and sweet pepper transplants, compared with no LED SL, although the SL with blue LED slightly enhanced flower bud formation of cucumber transplant and increased leaf area, fresh and dry weight, and photosynthetic pigment content of all vegetable transplants [88]. In contrast, for cucumber grown in a greenhouse under SL at 30 µmol m−2 s−1, BL did not affect flower initiation time compared with no SL, with a similar effect as red light [87]. Differing from red light, BL relative to no SL reduced fruit number, which was increased by red light [87]. In our study on podded peas grown in a greenhouse under SL at a PPFD of 50−140 µmol m−2 s−1, RB-LED (20% B) did not affect flower initiation regardless of lighting intensities, compared with no SL, despite increased pod yield, promoted plant growth, and improved pod quality [96]. Possibly, the effect of low- to modest-intensity BL from SL on the flowering of these plants was covered by background lighting or natural light.
In Chinese kale, a vegetable with its flower stalk as the main edible part, it was found that short-term pre-harvest SL with BL could be a promising strategy to enhance growth, yield, and both at-harvest and post-harvest quality of the flower stalks of this vegetable [86]. A 10-day pre-harvest SL with blue LED for 12 h daily improved the growth, yield, and quality of Chinese kale in the greenhouse relative to no SL, and 50 µmol m−2 s−1 was recommended as an optimal SL intensity [85]. In another study from the same research group, at the optimized intensity, short-term pre-harvest SL with blue LED reduced the weight loss of the flower stalk and maintained a higher antioxidant activity during storage compared with no SL [86]. Meanwhile, SL with blue LED resulted in higher contents of vitamin C, soluble protein, free amino acids, and chlorophyll at harvest.
Broccoli head, a tight cluster of unopened flower buds, despite having high nutritional value, can senesce quickly after harvest causing product quality deterioration, with a visible decreasing green color in sepals. It has been found that continuous sole-source lighting with blue LED in combination with white LED at a low intensity (20 µmol m−2 s−1) extended the shelf life of broccoli heads, demonstrated by greater greenness and higher chlorophyll levels at 5 °C or 22 °C, and slower sugar loss rate and higher carotenoid content at 22 °C, compared with darkness [81]. The LED treatment would be a feasible and low-cost technology to extend the postharvest storage of whole broccoli heads [81].
3.3. Specialty Crops
Some medicinal plants such as saffron, cannabis, and St. John’s wort have their flowers as the primary harvested organs. For these medicinal plants, regulation of the flowering process through lighting technology can directly affect their harvesting time and crop yield. For example, saffron flower number and stigma yield are negatively affected by low light conditions such as cloudy or rainy weather [95]. Also, the level of secondary metabolites in saffron is affected by environmental factors such as light [92]. For indoor cultivation of saffron, it is possible to improve flower quality and yield through the manipulation of light, including BL [94].
For other medicinal plants, although flowers are not the main harvested organs, earlier flowering through light adjustment can shorten the cropping cycle and increase total biomass unit area and time, thus potentially increasing the yield of biologically active compounds for medicinal use. The accumulation of medicinal compounds can be also affected by light stimuli [99]. To meet the high demand for superior quality medicinal plants, controlled environment production systems with electrical lighting systems can be advantageous. However, before implementing such systems, it is essential to determine the specific light spectrum, such as the proportion of BL required for each plant genotype [99].
Saffron is a flowering specialty plant renowned for its expensive dried stigma, and this highly valued product holds significant importance in culinary, industrial, and medical applications [92,94]. Saffron quality is determined by the content of three apocarotenoids, including crocin, picrocrocin, and safranal [92]. The effects of BL on saffron flowering and other metrics vary with lighting amount. Sole-source lighting with blue LED at 150 µmol m−2 s−1 for 11 h daily accelerated saffron flowering, increased flower number and stigma weight, and improved biomass partitioning to flowers, compared to red LED or RB-LED [92,93]. Besides improved flowering properties, the highest content of phytochemicals such as crocin and picrocrocin was detected in plants grown with blue LED. Therefore, in this study, blue LED performed the best in terms of the quantity and quality of saffron products under controlled environments [92]. On the contrary, for saffron plants grown under sole-source lighting at 50 µmol m−2 s−1 for 12 h daily, blue LED performed worse than red LED, demonstrated by delayed flowering, reduced flower size and number, and decreased yield of dry stigma [94]. Possibly, the contrasting response to BL was due to an interaction of light spectral with light amount, which was proven to be an important affecting factor. A study under sole-source lighting with RBFR-LED (20% B) indicated that when total daily light integral (TDLI; TDLI = DLI × days = PPFD × photoperiod × days) varied from 79 to 166 mol m−2 due to different lighting days, flower number, daily flowering proportion, stigma dry weight, and crocetin esters content were significantly correlated with TDLI, and the 150 mol m−2 of TDLI was the most favorable condition for flower number and stigma quality of saffron [95].
Cannabis is a medicinal plant treasured for its secondary metabolites, especially cannabinoids, the unique biologically active compounds in the plant that are considered to be affected by light spectra [83]. For cannabis grown indoors under sole-source lighting, a combination of BL with other wavelength(s) performs better than BL alone in terms of inflorescence yield, but the optimal BL proportions vary with the combined light wavelength(s) with BL. For example, when delivered as sole-source lighting, the blue LED had lower fresh inflorescence mass than HPS, as well as other LEDs such as red LED or RB-LEDs (9% B or 67% B) despite increased ∆9-tetrahydrocannabinol (THC) concentration in terms of dry mass, and cannabigerol (CBG) and terpene concentration [82]. Differently, in another study on cannabis under sole-source lighting, RB-LED with 47% B or 18% B increased inflorescence yields compared with HPS (5% B), or white LED (26% B) [83]. Also, blue-rich light such as RB-LED with 47% B and white LED increased the accumulation of cannabigerolic acid (CBGA), the primary cannabinoid and a precursor for most other cannabinoids, but reduced plant height, compared with HPS light [83]. It appeared that full-spectrum light did not necessarily improve inflorescence yield compared with RB-LED light, and manipulating BL proportions in LED lighting could fine-tune cannabis and cannabinoid production. However, contrasting results were achieved in another study on cannabis under sole-source lighting with different BL proportions (the lowest was 4% from HPS, and increased to 9.8%, 10.4%, 16%, and 20% from white or white + red LEDs) [84]. As the BL proportions increased from 4% to 20%, dry flower yield decreased linearly by 12%, but there was no effect on CBD or THC concentration. Although the white + red LED fixture (10.4% B) caused a lower yield than the HPS, it was more economically efficient due to the higher efficacy of the lighting fixtures [84].
Hypericum perforatum, commonly known as St John’s wort, is a medicinal plant with flowers as the main source for the accumulation of secondary metabolites [98,99]. The three important active compounds in St. John’s wort, including hypericin, pseudohypericin, and hyperforin, are effective in treating some diseases, especially mild to moderate depression. For Hypericum perforatum plants grown indoors under sole-source lighting, BL or RB-light performs worse than red light in terms of plant flowering and other metrics. For example, when plantlets were grown under sole-source lighting, blue LED or increased B% from 20% to 80% in RB-LEDs had a deterrent effect on flowering, demonstrated by decreased flowering shoot number, flower number, flower diameter, fresh and dry weight of flowers, and percentage of hypericin, pseudohypericin, and hyperforin in flowers, compared with red LED [98,99]. Also, blue LED reduced plant growth relative to red LED. On the contrary, without the presence of BL, i.e., red LED had the greatest promotional effects not only on flowering but also on plant growth, as well as secondary metabolite accumulation [98,99].
Scutellaria lateriflora (American skullcap) and Scutellaria baicalensis Georgi (Baikal skullcap) are two ethnobotanical medicinal plants used to treat gastrointestinal, respiratory, and inflammatory disorders, in addition to demonstrated anti-cancer properties [78]. The predominant bioactive compounds produced in these genotypes are unique 4-deoxyflavones, in the roots of S. baicalensis and leaves of S. lateriflora [78]. For these skullcap plants grown indoors under sole-source lighting with a broad spectrum, higher BL proportions benefit flowering and morphology, but its effects on other metrics vary with plant genotype. For example, sole-source lighting with higher-BL broad-spectrum LED (84% B) resulted in earlier flowering, caused more compact plants, and increased flavone accumulation in leaves for S. lateriflora but decreased it in roots for S. baicalensis Georgi, compared with lower-BL broad-spectrum LED (18% B) [78].
Polygonum tinctorium is not only a medicinal plant, but also an important industrial crop producing indigo blue dyes, and indican is an important substance as a precursor of indigo [91]. The indigo plant has been a useful material for traditional herbal medicine in Japan to exhibit anti-oxidant, anti-allergic effect, anti-cancer, and anti-inflammatory activities [91]. For indigo plants grown indoors under sole-source lighting, the blue LED appears to be a good light source in terms of both flowering response and other metrics. For example, sole-source lighting with blue LED increased the flowering rate of indigo plants by six-fold, compared with fluorescent light, while none of the plants flowered with red LED treatment [91]. Indican content was greatly enhanced by blue LED light under 24 h continuous irradiation. It appeared that blue LED light could provide a feasible strategy for artificially regulating plant flowering and indican synthesis in P. tinctorium.
4. Future Direction
4.1. Strengthing the Relevant Foundation Research
There is a common opinion that BL can promote the flowering of long-day plants and inhibit the flowering of short-day plants [4]. However, from the aforementioned research, it is easy to find that BL-mediated flowering is affected by many factors. This brings challenges in the application of BL in practical production while also indicating that BL-mediated flowering is a complicated physiological process. It is necessary to conduct the relevant basic research for optimization and development of effective ways of manipulating BL through electric lighting to mediate plant flowering. Despite many studies on Arabidopsis in this field, limited information is available on horticultural plants. For example, in chrysanthemums under short-day conditions, what is the mechanism behind the phenomena observed in recent studies that prolonged-photoperiod lighting with pure BL (e.g., blue LED light) cannot inhibit flower initiation [9,10,29,30,31,35]? Also, differing from a complete flowering inhibition for chrysanthemum plants under long-day conditions, why can pre-dark SL with pure BL for hours promote flowering of these plants in LD conditions? It appears that pure BL is not sensed by chrysanthemum plants as a photoperiodism light signal. A recent study by a Korean group has revealed a co-regulation of photosynthetic carbon assimilation and differential photoreceptors in this process, supported by a high expression of CRY1 and PHYA, a low expression of PHYB, and high carbohydrate accumulation [13]. The high expression of CRY1 under pure BL contrasts with our observation on flowering response in Arabidopsis cry mutant and overexpressing plants, where pure BL decreased CRY1 activity and increased CRY2 activity [77]. If there is a high expression of CRY1 in chrysanthemum plants, it is difficult to understand why, unlike pure BL, the high-PPS impure BL, created in our recent study by adding low-level R and FR LED to blue LED (R: FR= 3:1; PPS = 0.66) completely inhibit the flower initiation. Based on our studies on Arabidopsis, the high-PPS impure BL was supposed to increase CRY1 activity [77].
4.2. Broadening the Field of Application Research
The research about the application of electric lighting to manipulate BL for flowering regulation has been conducted on controlled-environment production of more and more types of crops (from ornamental plants to fruits, vegetables, and specialty crops), especially with the rise of vertical farming. Despite the limited relevant research on controlled environment production of field crops such as soybean and rice, increasing efforts have been started to put on speed breeding of some field crops in this way. Plant breeding plays a key role in agricultural production, but the process of developing suitable cultivars is time-consuming, partly because of the long generation times of crops. Recently, using a controlled environment for speed breeding has been introduced for long-day crops, but a similar protocol for short-day crops is lacking to date. In this case, German researchers developed a speed breeding protocol through the application of LED lighting with optimized spectral quality to promote flowering and thus increase breeding efficiency in some short-day crops, soybean, rice, and amaranth [112]. In their research, using LED lighting with a BL-enriched, and FR-deprived light spectrum at a 10 h photoperiod facilitated the growth of short and sturdy soybean plants that flowered about 23 days after sowing and matured within 77 days, thus allowing up to five generations per year. In rice and amaranth, flowering was achieved about 60 and 35 days after sowing, respectively. The developed protocol enables several generations per year using crop-specific LED-based lighting regimes, without the need for tissue culture tools such as embryo rescue. Moreover, this approach can be readily applied to a multi-story 96-cell tray-based system to integrate speed breeding with genomics toward a higher improvement rate in breeding [112].
5. Conclusions
Recent developments in LED technologies have made it easier to manipulate BL in a controlled environment. In this review, we have summarized the feasible ways to manipulate BL to mediate plant flowering through electric lighting such as night interruption lighting, day extension, supplemental lighting, and daytime sole-source lighting. Also, we have compared the effects of BL alone or its combination with other light wavelengths on plant flowering to those of no lighting treatment or other lighting sources. Furthermore, we have identified the possible factors resulting in inconsistent results of previous studies, including BL intensities and durations, background or previous environment conditions, and plant genotypes. This would not only provide useful information for growers to accurately and effectively manipulate BL to control flowering in production but also to identify the knowledge gaps for researchers to conduct relevant fundamental studies due to a complicated signal network involved in BL-mediated plant flowering response. In this review, we have included varying high-value crops from ornamental plants to fruits, vegetables, and specialty crops during either the pre-harvest or post-harvest stage, which would be potentially helpful for different crop growers.
Author Contributions
Conceptualization, Y.K. and Y.Z.; methodology, Y.K. and Y.Z.; investigation, Y.K.; writing—original draft preparation, Y.K.; writing—review and editing, Y.K. and Y.Z.; funding acquisition, Y.Z. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Weston Family Foundation, grant number SA-10721.
Data Availability Statement
Data are contained within the article.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Jung, C.; Pillen, K.; Staiger, D.; Coupland, G.; Von Korff, M. Recent advances in flowering time control. Front. Plant Sci. 2017, 7, 2011. [Google Scholar] [CrossRef] [PubMed]
- Weigel, D. The genetics of flower development: From floral induction to ovule morphogenesis. Annu. Rev. Genet. 1995, 29, 19–39. [Google Scholar] [CrossRef] [PubMed]
- Kong, Y.; Zheng, Y. Magic Blue Light: A Versatile Mediator of Plant Elongation. Plants 2023, 13, 115. [Google Scholar] [CrossRef] [PubMed]
- Runkle, E. Effects of Blue Light on Plants. Michigan State University, Extension Floriculture Team. 2017. Available online: https://www.canr.msu.edu/floriculture/uploads/files/blue-light.pdf (accessed on 30 May 2024).
- Trivellini, A.; Toscano, S.; Romano, D.; Ferrante, A. LED Lighting to Produce High-Quality Ornamental Plants. Plants 2023, 12, 1667. [Google Scholar] [CrossRef] [PubMed]
- Kharshiing, E.V.; Mawphlang, O.I.L.; Lama, V.; Bhattacharjee, R.; Sahoo, L. Manipulation of light environment for optimising photoreceptor activity towards enhancing plant traits of agronomic and horticultural importance in crops. J. Hortic. Sci. Biotechnol. 2022, 97, 535–551. [Google Scholar] [CrossRef]
- Yang, J.; Song, J.; Jeong, B.R. Low-intensity blue light supplemented during photoperiod in controlled environment induces flowering and antioxidant production in kalanchoe. Antioxidants 2022, 11, 811. [Google Scholar] [CrossRef] [PubMed]
- Chandel, A.; Thakur, M.; Singh, G.; Dogra, R.; Bajad, A.; Soni, V.; Bhargava, B. Flower regulation in floriculture: An agronomic concept and commercial use. J. Plant Growth Regul. 2023, 42, 2136–2161. [Google Scholar] [CrossRef]
- Park, Y.G.; Jeong, B.R. Night interruption light quality changes morphogenesis, flowering, and gene expression in Dendranthema grandiflorum. Hortic. Environ. Biotechnol. 2019, 60, 167–173. [Google Scholar] [CrossRef]
- Park, Y.G.; Jeong, B.R. How Supplementary or Night-Interrupting Low-Intensity Blue Light Affects the Flower Induction in Chrysanthemum, A Qualitative Short-Day Plant. Plants 2020, 9, 1694. [Google Scholar] [CrossRef]
- Ho, C.; Yang, C.; Hsiao, C. Effects of nighttime lighting with specific wavebands on flowering and flower quality of Chrysanthemum. Crop Environ. Bioinform. 2012, 9, 265–277. [Google Scholar]
- Higuchi, Y.; Sumitomo, K.; Oda, A.; Shimizu, H.; Hisamatsu, T. Day light quality affects the night-break response in the short-day plant chrysanthemum, suggesting differential phytochrome-mediated regulation of flowering. J. Plant Physiol. 2012, 169, 1789–1796. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Song, J.; Jeong, B.R. The flowering of SDP chrysanthemum in response to intensity of supplemental or night-interruptional blue light is modulated by both photosynthetic carbon assimilation and photoreceptor-mediated regulation. Front. Plant Sci. 2022, 13, 981143. [Google Scholar] [CrossRef] [PubMed]
- Park, Y.G.; Muneer, S.; Jeong, B.R. Morphogenesis, flowering, and gene expression of Dendranthema grandiflorum in response to shift in light quality of night interruption. Int. J. Mol. Sci. 2015, 16, 16497–16513. [Google Scholar] [CrossRef] [PubMed]
- Meng, Q.; Runkle, E.S. Moderate-intensity blue radiation can regulate flowering, but not extension growth, of several photoperiodic ornamental crops. Environ. Exp. Bot. 2017, 134, 12–20. [Google Scholar] [CrossRef]
- Meng, Q.; Runkle, E.S. Low-intensity blue light in night-interruption lighting does not influence flowering of herbaceous ornamentals. Sci. Hortic. 2015, 186, 230–238. [Google Scholar] [CrossRef]
- Meng, Q. Investigating Use of Blue, Red, and Far-Red Light from Light-Emitting Diodes to Regulate Flowering of Photoperiodic Ornamental Crops. Master’s Thesis, Michigan State University, East Lansing, MI, USA, 2014. [Google Scholar]
- Shin, J.H.; Jung, H.H.; Kim, K.S. Night interruption using light emitting diodes (LEDs) promotes flowering of Cyclamen persicum in winter cultivation. Hortic. Environ. Biotechnol. 2010, 51, 391–395. [Google Scholar]
- Park, Y.G.; Muneer, S.; Soundararajan, P.; Manivnnan, A.; Jeong, B.R. Light quality during night interruption affects morphogenesis and flowering in geranium. Hortic. Environ. Biotechnol. 2017, 58, 212–217. [Google Scholar] [CrossRef]
- Hamamoto, H. Budding and bolting responses of horticultural plants to night-break treatments with LEDs of various colors. J. Agric. Meteorol. 2003, 59, 103–110. [Google Scholar] [CrossRef]
- Yamada, A.; Tanigawa, T.; Suyama, T.; Matsuno, T.; Kunitake, T. Effects of red: Far-red light ratio of night-break treatments on growth and flowering of Eustoma grandiflorum (Raf.) Shinn. Acta Hortic. 2011, 907, 313–317. [Google Scholar] [CrossRef]
- Craig, D.S. Determining Effective Ratios of Red and Far-Red Light from Light-Emitting Diodes That Control Flowering of Photoperiodic Ornamental Crops. Master’s Thesis, Michigan State University, East Lansing, MI, USA, 2012. [Google Scholar]
- Craig, D.S.; Runkle, E.S. A moderate to high red to far-red light ratio from light-emitting diodes controls flowering of short-day plants. J. Am. Soc. Hortic. Sci. 2013, 138, 167–172. [Google Scholar] [CrossRef]
- Lopez, R.G.; Meng, Q.; Runkle, E.S. Blue radiation signals and saturates photoperiodic flowering of several long-day plants at crop-specific photon flux densities. Sci. Hortic. 2020, 271, 109470. [Google Scholar] [CrossRef]
- Higuchi, Y. Florigen and anti-florigen: Flowering regulation in horticultural crops. Breed. Sci. 2018, 68, 109–118. [Google Scholar] [CrossRef]
- Kong, Y.; Schiestel, K.; Zheng, Y. Blue light associated with low phytochrome activity can promote flowering: A comparison with red light in four bedding plant species. Acta Hortic. 2020, 1296, 433–440. [Google Scholar] [CrossRef]
- Kong, Y.; Stasiak, M.; Dixon, M.A.; Zheng, Y. Blue light associated with low phytochrome activity can promote elongation growth as shade-avoidance response: A comparison with red light in four bedding plant species. Environ. Exp. Bot. 2018, 155, 345–359. [Google Scholar] [CrossRef]
- Nissim-Levi, A.; Kitron, M.; Nishri, Y.; Ovadia, R.; Forer, I.; Oren-Shamir, M. Effects of blue and red LED lights on growth and flowering of Chrysanthemum morifolium. Sci. Hortic. 2019, 254, 77–83. [Google Scholar] [CrossRef]
- Jeong, S.W.; Park, S.; Jin, J.S.; Seo, O.N.; Kim, G.-S.; Kim, Y.-H.; Bae, H.; Lee, G.; Kim, S.T.; Lee, W.S. Influences of four different light-emitting diode lights on flowering and polyphenol variations in the leaves of chrysanthemum (Chrysanthemum morifolium). J. Agric. Food Chem. 2012, 60, 9793–9800. [Google Scholar] [CrossRef] [PubMed]
- Singh, M.C.; van Ieperen, W.; Heuvelink, E.P. Effect of LEDs on flower bud induction in Chrysanthemum morifolium cv. Zembla. HortFlora Res. Spectr. 2013, 2, 185–188. [Google Scholar]
- Jeong, S.W.; Hogewoning, S.W.; van Ieperen, W. Responses of supplemental blue light on flowering and stem extension growth of cut chrysanthemum. Sci. Hortic. 2014, 165, 69–74. [Google Scholar] [CrossRef]
- SharathKumar, M.; Heuvelink, E.; Marcelis, L.F.; Van Ieperen, W. Floral induction in the short-day plant chrysanthemum under blue and red extended long-days. Front. Plant Sci. 2021, 11, 610041. [Google Scholar] [CrossRef]
- Kohler, A.E.; Birtell, E.M.; Runkle, E.S.; Meng, Q. Day-extension Blue Light Inhibits Flowering of Chrysanthemum When the Short Main Photoperiod Includes Far-red Light. J. Am. Soc. Hortic. Sci. 2023, 148, 89–98. [Google Scholar] [CrossRef]
- Kim, S.H.; Heo, Y.; Rhee, H.C.; Kang, J.S. Effect of LED light quality and supplemental time on the growth and flowering of impatiens. J. Bio-Environ. Control 2013, 22, 214–219. [Google Scholar] [CrossRef]
- SharathKumar, M.; Luo, J.; Xi, Y.; van Ieperen, W.; Marcelis, L.F.; Heuvelink, E. Several short-day species can flower under blue-extended long days, but this response is not universal. Sci. Hortic. 2024, 325, 112657. [Google Scholar] [CrossRef]
- Hamamoto, H.; Yamazaki, K. Reproductive response of okra and native rosella to long-day treatment with red, blue, and green light-emitting diode lights. HortScience 2009, 44, 1494–1497. [Google Scholar] [CrossRef]
- Yang, J.; Song, J.; Jeong, B.R. Blue light supplemented at intervals in long-day conditions intervenes in photoperiodic flowering, photosynthesis, and antioxidant properties in chrysanthemums. Antioxidants 2022, 11, 2310. [Google Scholar] [CrossRef] [PubMed]
- Stack, P.A.; Drummond, F.A.; Stack, L.B. Chrysanthemum flowering in a blue light-supplemented long day maintained for biocontrol of thrips. Hortscience 1998, 33, 710–715. [Google Scholar] [CrossRef]
- Sugawara, N.; Numazawa, M.; Abe, R.; Nishiyama, M.; Kato, K.; Kanayama, Y. Effect of light quality of long-day treatments on flowering in Delphinium. J. Agric. Meteorol. 2023, 79, 85–94. [Google Scholar] [CrossRef]
- Shibuya, T.; Takahashi, T.; Hashimoto, S.; Nishiyama, M.; Kanayama, Y. Effects of overnight radiation with monochromatic far-red and blue light on flower budding and expression of flowering-related and light quality-responsive genes in Eustoma grandiflorum. J. Agric. Meteorol. 2019, 75, 160–165. [Google Scholar] [CrossRef]
- Poel, B.R.; Runkle, E.S. Spectral effects of supplemental greenhouse radiation on growth and flowering of annual bedding plants and vegetable transplants. HortScience 2017, 52, 1221–1228. [Google Scholar] [CrossRef]
- Llewellyn, D.; Schiestel, K.; Zheng, Y. Light-emitting diodes can replace high-pressure sodium lighting for cut gerbera production. HortScience 2019, 54, 95–99. [Google Scholar] [CrossRef]
- Spall, C.E.; Lopez, R.G. Supplemental Lighting Quality Influences Time to Flower and Finished Quality of Three Long-Day Specialty Cut Flowers. Horticulturae 2023, 9, 73. [Google Scholar] [CrossRef]
- Hori, Y.; Nishidate, K.; Nishiyama, M.; Kanahama, K.; Kanayama, Y. Flowering and expression of flowering-related genes under long-day conditions with light-emitting diodes. Planta 2011, 234, 321–330. [Google Scholar] [CrossRef] [PubMed]
- Amaki, W.; Kunii, M. Effects of light quality on the flowering responses in Kalanchoe blossfeldiana. Acta Hortic. 2015, 1107, 279–284. [Google Scholar] [CrossRef]
- Sams, C.; Kopsell, D.; Morrow, R. Light quality impacts on growth, flowering, mineral uptake and petal pigmentation of marigold. Acta Hortic. 2016, 1134, 139–146. [Google Scholar] [CrossRef]
- Yamazaki, K.; Ishii, Y.; Tanaka, I. Spectral sensitivity of the promotion and inhibition of flowering in morning glory (Pharbitis nil). Environ. Control Biol. 2007, 45, 75–83. [Google Scholar] [CrossRef]
- Lu, C.H.; Liu, Y.C.; Hsu, Y.T.; Wang, H.L.; Der Chung, J. Enhancing flower stalk emergence in Phalaenopsis by red light supplementation. Am. J. Plant Sci. 2016, 7, 639–648. [Google Scholar]
- Gautam, P.; Terfa, M.T.; Olsen, J.E.; Torre, S. Red and blue light effects on morphology and flowering of Petunia × hybrida. Sci. Hortic. 2015, 184, 171–178. [Google Scholar] [CrossRef]
- Mah, J.J. Exploring Light for Growth Control in Ornamental Plant Production Using LEDs in Controlled Environments. Master’s Thesis, University of Guelph, Guelph, ON, Canada, 2019. [Google Scholar]
- Islam, M.A.; Kuwar, G.; Clarke, J.L.; Blystad, D.-R.; Gislerød, H.R.; Olsen, J.E.; Torre, S. Artificial light from light emitting diodes (LEDs) with a high portion of blue light results in shorter poinsettias compared to high pressure sodium (HPS) lamps. Sci. Hortic. 2012, 147, 136–143. [Google Scholar] [CrossRef]
- Terfa, M.T.; Solhaug, K.A.; Gislerød, H.R.; Olsen, J.E.; Torre, S. A high proportion of blue light increases the photosynthesis capacity and leaf formation rate of Rosa× hybrida but does not affect time to flower opening. Physiol. Plant. 2013, 148, 146–159. [Google Scholar] [CrossRef] [PubMed]
- Shen, H.; Shen, M.; Cheng, J.; Nana, L.; Zhang, J. Effect of supplemental lighting with different light quality on growth and bloom of tulip. J. Beijing Agric. Coll. 2007, 22, 16–18. [Google Scholar]
- Anvari, M.; Hashemabadi, D.; Asadpour, L.; Kaviani, B. Effect of blue light and nanosilver on vase life, antioxidant enzymes and some other physiologic parameters of Alstroemeria ‘Napoli’ cut flowers. Acta Sci. Pol. Hortorum Cultus 2022, 21, 111–122. [Google Scholar] [CrossRef]
- Kamath, D.; Kong, Y.; Dayboll, C.; Zheng, Y. Dynamic versus concurrent lighting with red and blue light-emitting diodes as the sole light source can potentially improve campanula stock plant morphology for cutting production. HortScience 2021, 56, 1439–1445. [Google Scholar] [CrossRef]
- Aalifar, M.; Aliniaeifard, S.; Arab, M.; Zare Mehrjerdi, M.; Dianati Daylami, S.; Serek, M.; Woltering, E.; Li, T. Blue light improves vase life of carnation cut flowers through its effect on the antioxidant defense system. Front. Plant Sci. 2020, 11, 511. [Google Scholar] [CrossRef] [PubMed]
- Aalifar, M.; Aliniaeifard, S.; Arab, M.; Mehrjerdi, M.Z.; Serek, M. Blue light postpones senescence of carnation flowers through regulation of ethylene and abscisic acid pathway-related genes. Plant Physiol. Biochem. 2020, 151, 103–112. [Google Scholar] [CrossRef] [PubMed]
- An, S.; Arakawa, O.; Tanaka, N.; Zhang, S.; Kobayashi, M. Effects of blue and red light irradiations on flower colouration in cherry blossom (Prunus × yedoensis ‘Somei-yoshino’). Sci. Hortic. 2020, 263, 109093. [Google Scholar] [CrossRef]
- Jerzy, M.; Zakrzewski, P.; Schroeter-Zakrzewska, A. Effect of colour of light on the opening of inflorescence buds and post-harvest longevity of pot chrysanthemums (Chrysanthemum × grandiflorum (Ramat.) Kitam). Acta Agrobot. 2011, 64, 13–18. [Google Scholar] [CrossRef]
- Heo, J.W.; Lee, C.W.; Murthy, H.; Paek, K.Y. Influence of light quality and photoperiod on flowering of Cyclamen persicum Mill. cv. ‘Dixie White’. Plant Growth Regul. 2003, 40, 7–10. [Google Scholar] [CrossRef]
- Zhang, M.; Park, Y.; Runkle, E.S. Regulation of extension growth and flowering of seedlings by blue radiation and the red to far-red ratio of sole-source lighting. Sci. Hortic. 2020, 272, 109478. [Google Scholar] [CrossRef]
- Ohtani, T.; Ishiguri, Y. Inhibitory Action of Blue and Far-Red Light in the Flowering of Lemna paucicostata. Physiol. Plant. 1979, 47, 255–259. [Google Scholar] [CrossRef]
- Kamath, D.; Kong, Y.; Dayboll, C.; Blom, T.; Zheng, Y. Growth and morphological responses of gerbera seedlings to narrow-band lights with different light spectral combinations as sole-source lighting in a controlled environment. Can. J. Plant Sci. 2021, 101, 943–953. [Google Scholar] [CrossRef]
- Wang, S.; Liu, X.; Liu, X.; Xue, J.; Ren, X.; Zhai, Y.; Zhang, X. The red/blue light ratios from light-emitting diodes affect growth and flower quality of hippeastrum hybridum ‘red lion’. Front. Plant Sci. 2022, 13, 1048770. [Google Scholar] [CrossRef]
- Chen, D.; Guo, Y.; Chen, X.; Wang, P.; Chen, G.; Ye, N. Transcriptome analysis of flowering regulated by red and blue light in Jasminum sambac. Chin. J. Biotechnol. 2020, 36, 1869–1886. [Google Scholar]
- Flores-Pérez, S.; Castillo-González, A.M.; Valdez-Aguilar, L.A.; Avítia-García, E. Use of different proportions of red and blue LEDs to improve the growth of Lilium spp. Rev. Mex. Cienc. Agríc. 2021, 12, 835–847. [Google Scholar]
- Heo, J.; Lee, C.; Chakrabarty, D.; Paek, K. Growth responses of marigold and salvia bedding plants as affected by monochromic or mixture radiation provided by a light-emitting diode (LED). Plant Growth Regul. 2002, 38, 225–230. [Google Scholar] [CrossRef]
- Yamazaki, K.; Ishii, Y.; Matsui, S.; Tanaka, I. Effects of light quality, daylength and growing temperature on flowering in morning glory (Pharbitis nil Choisy). Environ. Control Biol. 2003, 41, 211–219. [Google Scholar] [CrossRef]
- Woźny, A.; Jerzy, M. Effect of light wavelength on growth and flowering of narcissi forced under short-day and low quantum irradiance conditions. J. Hortic. Sci. Biotechnol. 2007, 82, 924–928. [Google Scholar] [CrossRef]
- Rashidi, A.; Tehranifar, A.; Samiei, L. Modifying spectral distributions during the seedling stage influences the flowering and branching of Petunia × hybrida. Sci. Hortic. 2023, 309, 111664. [Google Scholar] [CrossRef]
- Colquhoun, T.A.; Schwieterman, M.L.; Gilbert, J.L.; Jaworski, E.A.; Langer, K.M.; Jones, C.R.; Rushing, G.V.; Hunter, T.M.; Olmstead, J.; Clark, D.G. Light modulation of volatile organic compounds from petunia flowers and select fruits. Postharvest Biol. Technol. 2013, 86, 37–44. [Google Scholar] [CrossRef]
- Kong, Y.; Kamath, D.; Zheng, Y. Blue-light-promoted elongation and flowering are not artifacts from 24-h lighting: A comparison with red light in four bedding plant species. Acta Hortic. 2020, 1296, 659–666. [Google Scholar] [CrossRef]
- Kong, Y.; Vinson, K.; Kamath, D.; Llewellyn, D.; Zheng, Y. Pure Blue Light can Promote Flowering: A Comparison with Red Light in Bedding Plants. 2019. Available online: https://www.researchgate.net/publication/344675523_Pure_blue_light_can_promote_flowering_a_comparison_with_red_light_in_bedding_plants (accessed on 30 May 2024).
- Roh, Y.S.; Yoo, Y.K. Light quality of light emitting diodes affects growth, chlorophyll fluorescence and phytohormones of Tulip ‘Lasergame’. Hortic. Environ. Biotechnol. 2023, 64, 245–255. [Google Scholar] [CrossRef]
- Kong, Y.; Zheng, Y. Phototropin is partly involved in blue-light-mediated stem elongation, flower initiation, and leaf expansion: A comparison of phenotypic responses between wild Arabidopsis and its phototropin mutants. Environ. Exp. Bot. 2020, 171, 103967. [Google Scholar] [CrossRef]
- Kong, Y.; Zheng, Y. Phytochrome contributes to blue-light-mediated stem elongation and flower initiation in mature Arabidopsis thaliana plants. Can. J. Plant Sci. 2021, 102, 449–458. [Google Scholar] [CrossRef]
- Kong, Y.; Zheng, Y. Low-activity cryptochrome 1 plays a role in promoting stem elongation and flower initiation of mature Arabidopsis under blue light associated with low phytochrome activity. Can. J. Plant Sci. 2022, 102, 755–759. [Google Scholar] [CrossRef]
- Costine, B.; Zhang, M.; Pearson, B.; Nadakuduti, S.S. Impact of Blue Light on Plant Growth, Flowering and Accumulation of Medicinal Flavones in Scutellaria baicalensis and S. lateriflora. Horticulturae 2022, 8, 1141. [Google Scholar] [CrossRef]
- Wang, G.; Chen, Y.; Fan, H.; Huang, P. Effects of Light-Emitting Diode (LED) Red and Blue Light on the Growth and Photosynthetic Characteristics of Momordica charantia L. J. Agric. Chem. Environ. 2020, 10, 1–15. [Google Scholar]
- Cho, H.; Kadowaki, M.; Che, J.; Takahashi, S.; Horiuchi, N.; Ogiwara, I. Influence of light quality on flowering characteristics, potential for year-round fruit production and fruit quality of blueberry in a plant factory. Fruits 2019, 74, 3–10. [Google Scholar] [CrossRef]
- Hasperué, J.H.; Guardianelli, L.; Rodoni, L.M.; Chaves, A.R.; Martínez, G.A. Continuous white–blue LED light exposition delays postharvest senescence of broccoli. LWT-Food Sci. Technol. 2016, 65, 495–502. [Google Scholar] [CrossRef]
- Morello, V.; Brousseau, V.D.; Wu, N.; Wu, B.S.; MacPherson, S.; Lefsrud, M. Light quality impacts vertical growth rate, phytochemical yield and cannabinoid production efficiency in Cannabis sativa. Plants 2022, 11, 2982. [Google Scholar] [CrossRef]
- Danziger, N.; Bernstein, N. Light matters: Effect of light spectra on cannabinoid profile and plant development of medical cannabis (Cannabis sativa L.). Ind. Crops Prod. 2021, 164, 113351. [Google Scholar] [CrossRef]
- Westmoreland, F.M.; Kusuma, P.; Bugbee, B. Cannabis lighting: Decreasing blue photon fraction increases yield but efficacy is more important for cost effective production of cannabinoids. PLoS ONE 2021, 16, e0248988. [Google Scholar] [CrossRef]
- Li, Y.; Zheng, Y.; Liu, H.; Zhang, Y.; Hao, Y.; Song, S.; Lei, B. Effect of supplemental blue light intensity on the growth and quality of Chinese kale. Hortic. Environ. Biotechnol. 2019, 60, 49–57. [Google Scholar] [CrossRef]
- Jiang, H.; Li, X.; Tian, J.; Liu, H. Pre-harvest supplemental blue light enhanced antioxidant activity of flower stalk in chinese kale during storage. Plants 2021, 10, 1177. [Google Scholar] [CrossRef]
- Wang, S.; Fan, S.; Kong, Y.; Qingjun, C. Effect of light quality on the growth and photosynthetic characteristics of cucumber Cucumis sativus L. under solar greenhouse. Acta Hortic. 2007, 731, 243–251. [Google Scholar] [CrossRef]
- Samuolienė, G.; Brazaitytė, A.; Duchovskis, P.; Viršilė, A.; Jankauskienė, J.; Sirtautas, R.; Novičkovas, A.; Sakalauskienė, S.; Sakalauskaitė, J. Cultivation of vegetable transplants using solid-state lamps for the short-wavelength supplementary lighting in greenhouses. Acta Hortic. 2012, 952, 885–892. [Google Scholar] [CrossRef]
- Kong, Y. Utilization Pattern and Optimal Management of Photosynthetic Active Radiation within Canopies of Peach Trees Grown inside Chinese Lean-To Greenhouses; Beijing Education Committee: Beijing, China, 2012. [Google Scholar]
- Nie, W.; Li, Y.; Chen, Y.; Zhou, Y.; Yu, T.; Zhou, Y.; Yang, Y. Spectral light quality regulates the morphogenesis, architecture, and flowering in pepper (Capsicum annuum L.). J. Photochem. Photobiol. B Biol. 2023, 241, 112673. [Google Scholar] [CrossRef]
- Nakai, A.; Tanaka, A.; Yoshihara, H.; Murai, K.; Watanabe, T.; Miyawaki, K. Blue LED light promotes indican accumulation and flowering in indigo plant, Polygonum tinctorium. Ind. Crops Prod. 2020, 155, 112774. [Google Scholar] [CrossRef]
- Moradi, S.; Kafi, M.; Aliniaeifard, S.; Moosavi-Nezhad, M.; Pedersen, C.; Gruda, N.S.; Salami, S.A. Monochromatic blue light enhances crocin and picrocrocin content by upregulating the expression of underlying biosynthetic pathway genes in saffron (Crocus sativus L.). Front. Hortic. 2022, 1, 960423. [Google Scholar] [CrossRef]
- Moradi, S.; Kafi, M.; Aliniaeifard, S.; Salami, S.A.; Shokrpour, M.; Pedersen, C.; Moosavi-Nezhad, M.; Wróbel, J.; Kalaji, H.M. Blue light improves photosynthetic performance and biomass partitioning toward harvestable organs in saffron (Crocus sativus L.). Cells 2021, 10, 1994. [Google Scholar] [CrossRef]
- Zhu, J.; Zhang, Y.; Zhou, L.; Yang, L. Growth and flowering of saffron (Crocus sativus L.) with three corm weights under different led light qualities. Sci. Hortic. 2022, 303, 111202. [Google Scholar] [CrossRef]
- Gao, D.; Ji, X.; Yuan, Q.; Pei, W.; Zhang, X.; Li, F.; Han, Q.; Zhang, S. Effects of total daily light integral from blue and broad-band red LEDs on flowering of saffron (Crocus sativus L.). Sci. Rep. 2023, 13, 7175. [Google Scholar] [CrossRef]
- Kong, Y.; Zheng, Y. Response of growth, yield, and quality of edible-podded snow peas to supplemental LED lighting during winter greenhouse production. Can. J. Plant Sci. 2019, 99, 676–687. [Google Scholar] [CrossRef]
- Lin, K.H.; Chen, Y.C.; Wu, Q.E.; Lin, H.H. Effects of red and blue light ratio on the morphological traits and flower sex expression in Cucurbita moschata Duch. Not. Bot. Horti Agrobot. Cluj-Napoca 2023, 51, 13123. [Google Scholar] [CrossRef]
- Karimi, M.; Ahmadi, N.; Ebrahimi, M. Red LED light promotes biomass, flowering and secondary metabolites accumulation in hydroponically grown Hypericum perforatum L. (cv. Topas). Ind. Crops Prod. 2022, 175, 114239. [Google Scholar] [CrossRef]
- Karimi, M.; Ahmadi, N.; Ebrahimi, M. Photoreceptor regulation of Hypericum perforatum L. (cv. Topas) flowering under different light spectrums in the controlled environment system. Environ. Exp. Bot. 2022, 196, 104797. [Google Scholar] [CrossRef]
- Uddin, A.; Hoq, M.; Rini, S.; Urme, F.; Ahmad, H. Influence of supplement LED spectrum on growth and yield of Strawberry. J. Biosci. Agric. Res. 2018, 16, 1348–1355. [Google Scholar] [CrossRef]
- Naznin, M.; Lefsrud, M.; Gravel, V.; Hao, X. Using different ratios of red and blue LEDs to improve the growth of strawberry plants. Acta Hortic. 2016, 1134, 125–130. [Google Scholar] [CrossRef]
- Sidhu, V.; Bernier-English, V.; Lamontagne-Drolet, M.; Gravel, V. Effect of light quality and extended photoperiod on flower bud induction during transplant production of day-neutral strawberry cultivars. Can. J. Plant Sci. 2021, 102, 356–367. [Google Scholar] [CrossRef]
- Nadalini, S.; Zucchi, P.; Andreotti, C. Effects of blue and red LED lights on soilless cultivated strawberry growth performances and fruit quality. Eur. J. Hortic. Sci. 2017, 82, 12–20. [Google Scholar] [CrossRef]
- Yoshida, H.; Hikosaka, S.; Goto, E.; Takasuna, H.; Kudou, T. Effects of light quality and light period on flowering of everbearing strawberry in a closed plant production system. Acta Hortic. 2012, 956, 107–112. [Google Scholar] [CrossRef]
- Yoshida, H.; Mizuta, D.; Fukuda, N.; Hikosaka, S.; Goto, E. Effects of varying light quality from single-peak blue and red light-emitting diodes during nursery period on flowering, photosynthesis, growth, and fruit yield of everbearing strawberry. Plant Biotechnol. 2016, 33, 267–276. [Google Scholar] [CrossRef]
- Magar, Y.; Ohyama, K.; Noguchi, A.; Amaki, W.; Furufuji, S. Effects of light quality during supplemental lighting on the flowering in an everbearing strawberry. Acta Hortic. 2018, 1206, 279–284. [Google Scholar] [CrossRef]
- Prisca, M.; Maarten, V.; Bart, N.; Wouter, S.; Timo, H. Blue and far-red light control flowering time of woodland strawberry (Fragaria vesca) distinctively via CONSTANS (CO) and FLOWERING LOCUS T1 (FT1) in the background of sunlight mimicking radiation. Environ. Exp. Bot. 2022, 198, 104866. [Google Scholar] [CrossRef]
- Nanya, K.; Ishigami, Y.; Hikosaka, S.; Goto, E. Effects of blue and red light on stem elongation and flowering of tomato seedlings. Acta Hortic. 2012, 956, 261–266. [Google Scholar] [CrossRef]
- Utasi, L.; Kovács, V.; Gulyás, Z.; Marcek, T.; Janda, T.; Darko, E. Threshold or not: Spectral composition and light-intensity dependence of growth and metabolism in tomato seedlings. Sci. Hortic. 2023, 313, 111946. [Google Scholar] [CrossRef]
- Javanmardi, J.; Emami, S. Response of tomato and pepper transplants to light spectra provided by light emitting diodes. Int. J. Veg. Sci. 2013, 19, 138–149. [Google Scholar] [CrossRef]
- Warner, R.; Wu, B.-S.; MacPherson, S.; Lefsrud, M. A review of strawberry photobiology and fruit flavonoids in controlled environments. Front. Plant Sci. 2021, 12, 611893. [Google Scholar] [CrossRef]
- Jähne, F.; Hahn, V.; Würschum, T.; Leiser, W.L. Speed breeding short-day crops by LED-controlled light schemes. Theor. Appl. Genet. 2020, 133, 2335–2342. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).