Elucidation of Shoot and Root Growth, Physiological Responses, and Quality Traits of Tomato (Solanum lycopersicon L.) Exposed to Elevated Calcium Carbonate Concentrations
Abstract
1. Introduction
2. Materials and Methods
2.1. Measurement of Soil pH and Soil Electrical Conductivity (EC)
2.2. Measurement of Shoot Growth and Physiology
2.3. Measurement of Root Growth and Physiology
2.4. Measurement of Yield and Fruit Characteristics
2.5. Measurement of Fruit Quality
2.6. Data Statistical Analysis
3. Results
3.1. Soil pH and Soil EC
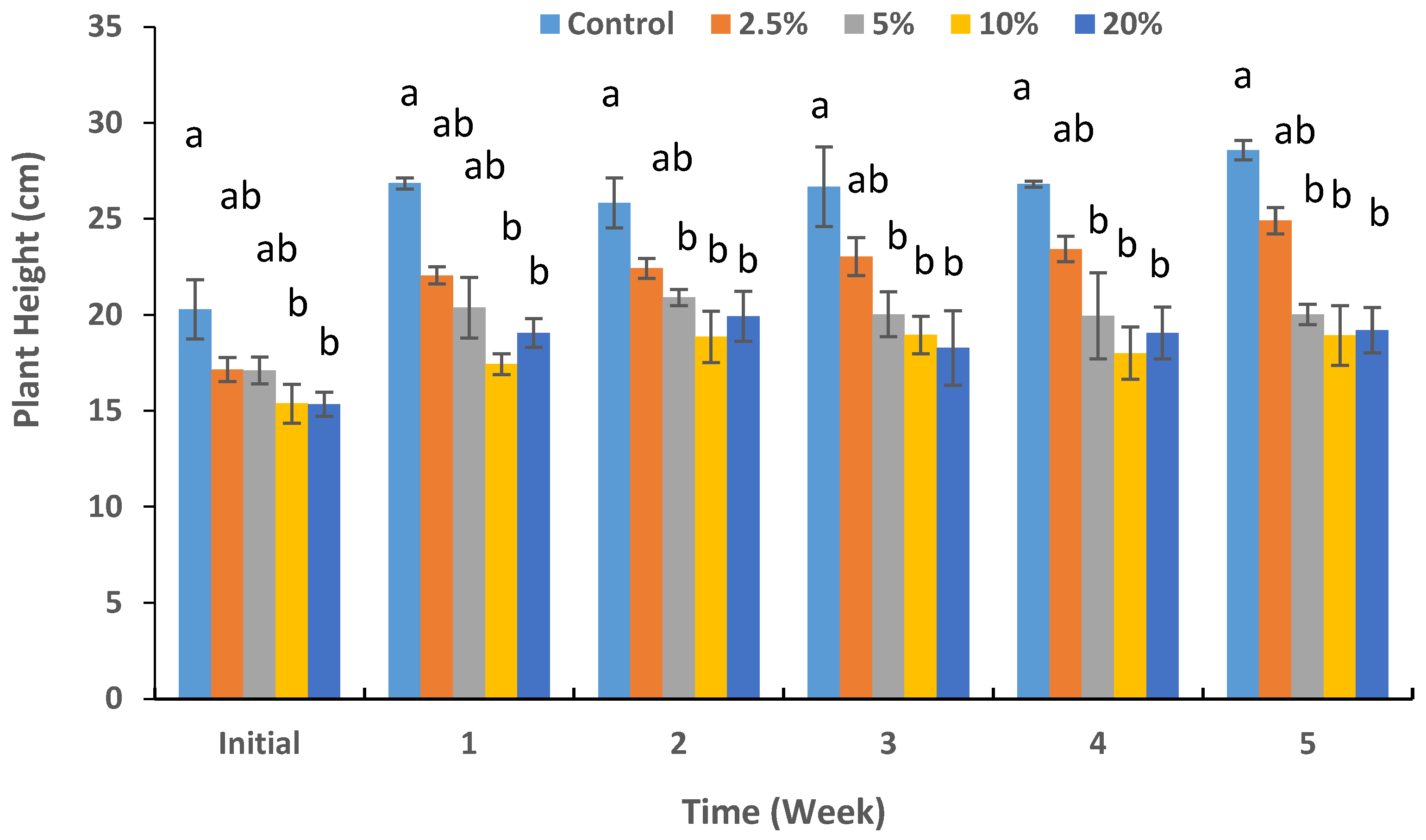
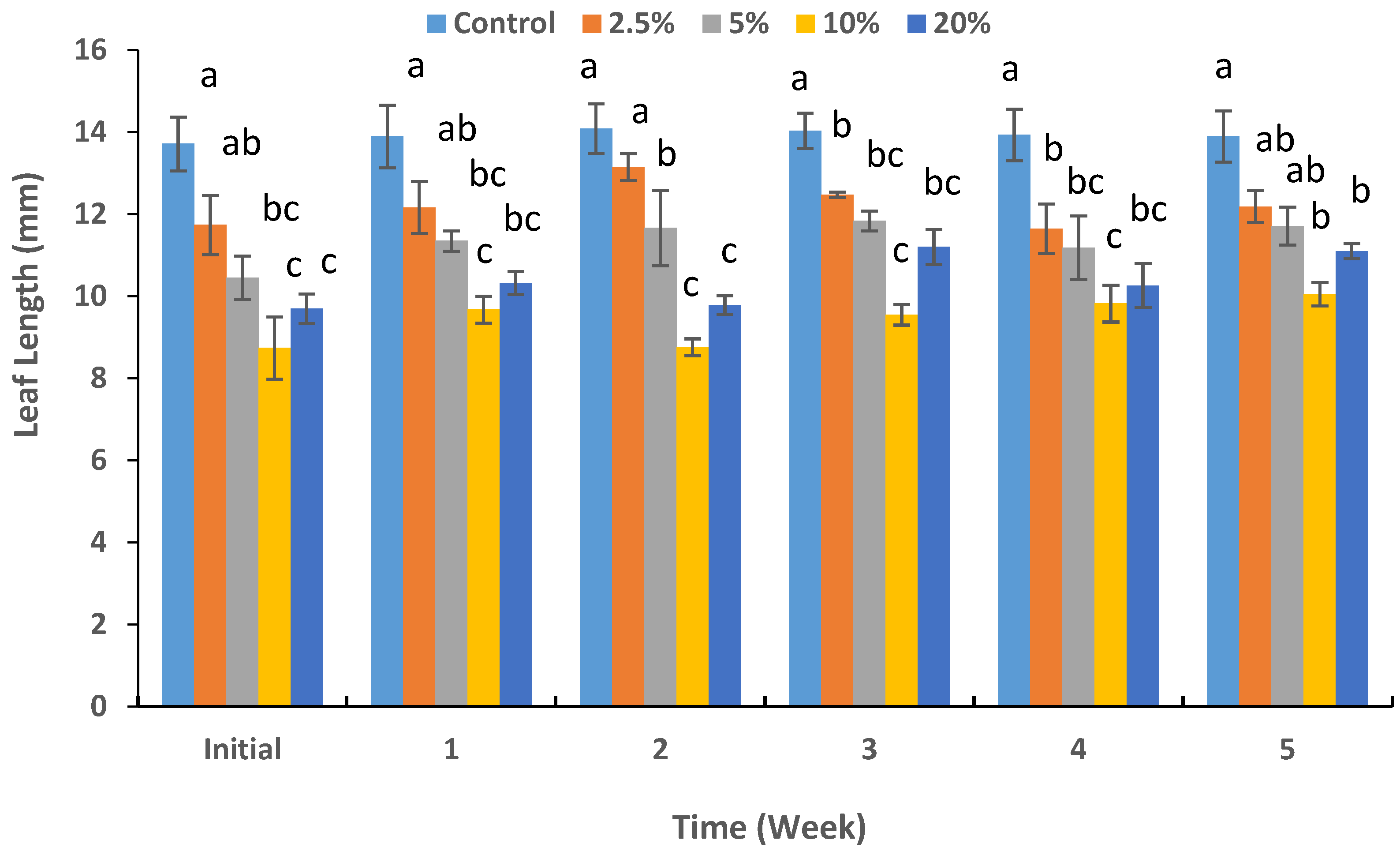
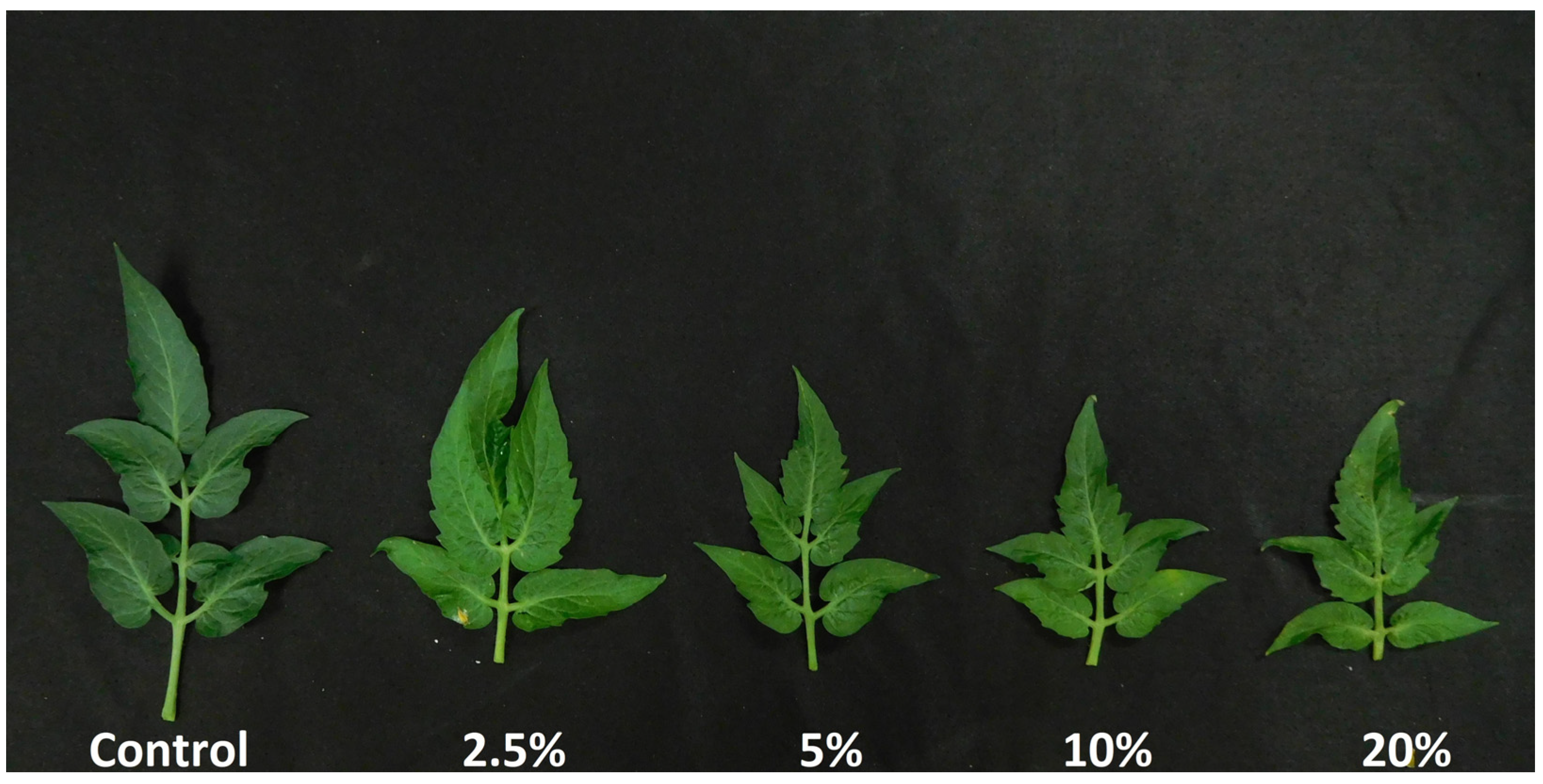
3.2. Shoot Growth and Physiology
3.3. Root Growth and Physiology
3.4. Number of Flowers and Fruits per Plant−1, Yield, and Its Components
3.5. Fruit Quality Characteristics
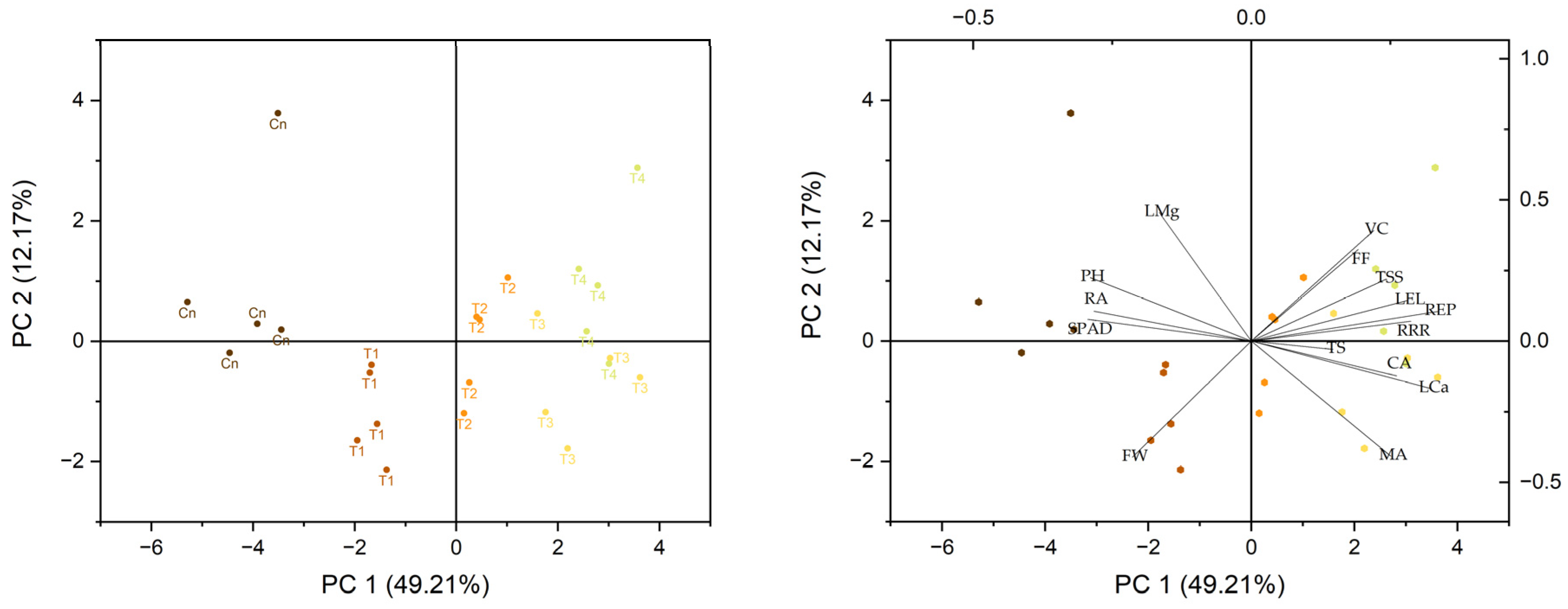
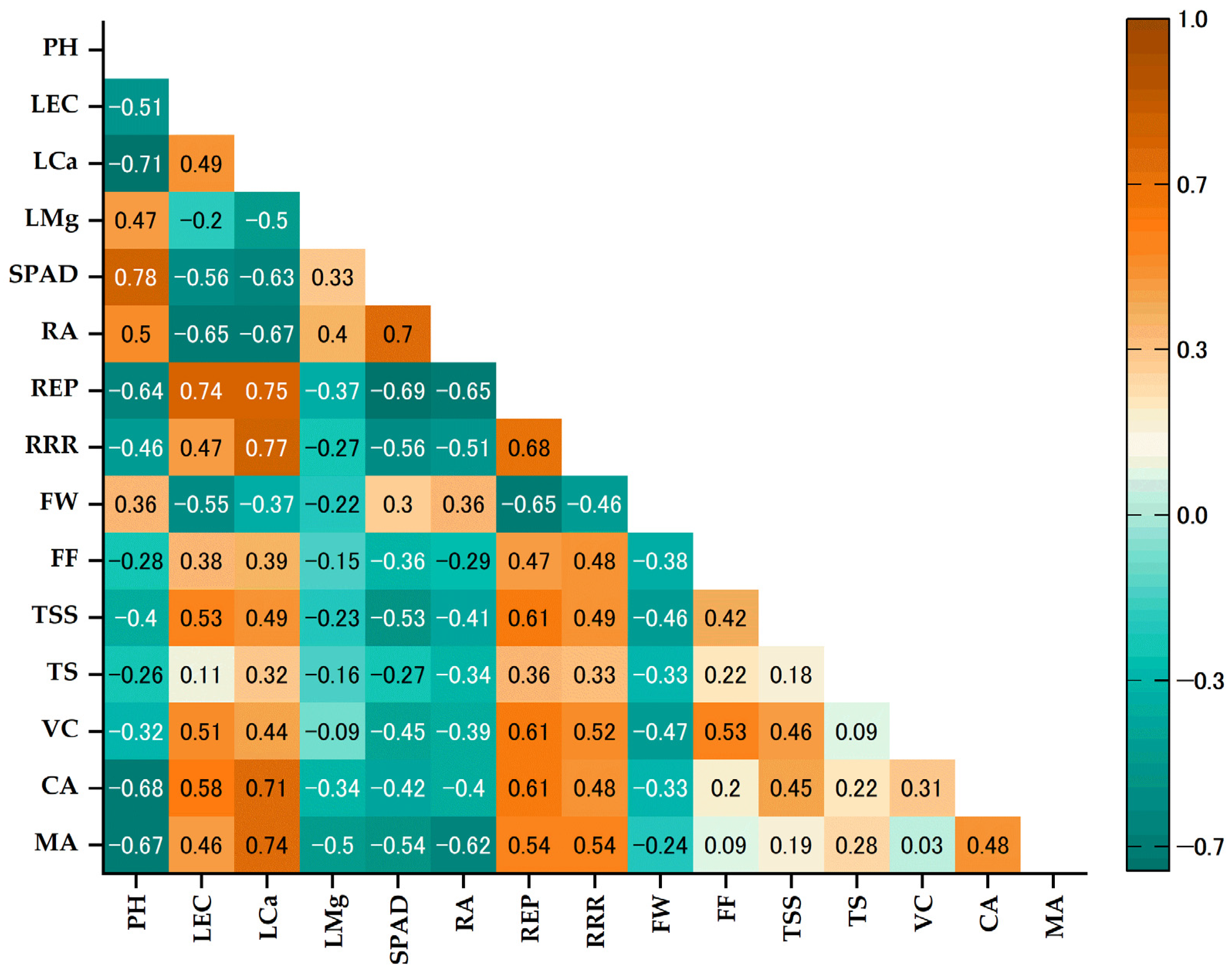
3.6. Prinicipal Component Analysis and Correlation Coeficient (Matrix)
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Delgado, A.; Gómez, J.A. The soil. Physical, chemical and biological properties. In Principles of Agronomy for Sustainable Agriculture; Villalobos, F., Fereres, E., Eds.; Springer: Cham, Switzerland, 2016; pp. 15–26. [Google Scholar] [CrossRef]
- Kowalska, J.B.; Zaleski, T.; Józefowska, A.; Mazurek, R. Soil formation on calcium carbonate-rich parent material in the outer Carpathian Mountains–A case study. Catena 2019, 174, 436–451. [Google Scholar] [CrossRef]
- Wahba, M.M.; Labib, F.; Zaghloul, A. Management of calcareous soils in arid region. Int. J. Environ. Pollut. Environ. Model 2019, 2, 248–258. [Google Scholar]
- Chesworth, W. Calcareous soils. In Encyclopedia of Soil Science; Chesworth, W., Ed.; Springer: Dordrecht, The Netherlands, 2008; pp. 76–79. [Google Scholar]
- Elhusieny, A.H.; Nada, W.; Elgezery, M. Influence of sulphur compost application on some chemical properties of calcareous soil and consequent responses of Hordeum Vulgare L. plants. Egypt. J. Soil. Sci. 2020, 60, 67–82. [Google Scholar]
- Taalab, A.S.; Ageeb, G.W.; Siam, H.S.; Mahmoud, S.A. Some characteristics of calcareous soils. A review AS Taalab1, GW Ageeb2, Hanan S. Siam1 and Safaa A. Middle East J. 2019, 8, 96–105. [Google Scholar]
- Management of calcareous soils. FAO Soils Portal. 2023. Available online: https://www.fao.org/soils-portal/soil-management/management-of-some-problem-soils/calcareous-soils/en/ (accessed on 19 September 2023).
- Pal, D.K.; Dasog, G.S.; Vadivelu, S.; Ahuja, R.L.; Bhattacharyya, T. Secondary calcium carbonate in soils of arid and semi-arid regions of India. Glob. Clim. Chang. Pedog. Carbonates 2000, 149–185. [Google Scholar]
- Lehto, T. Effects of soil pH and calcium on mycorrhizas of Picea abies. Plant Soil. 1994, 163, 69–75. [Google Scholar] [CrossRef]
- Marschner, H. Marschner’s Mineral Nutrition of Higher Plants, 3rd ed.; Academic Press: San Diego, CA, USA, 2011; pp. 444–455. [Google Scholar]
- Shaaban, M.M.; El-Fouly, M.M.; Abdel-Maguid, A.A. Zinc-Boron Relationship in wheat plants grown under low or high levels of calcium carbonate in the Soil. Pak. J. Biol. Sci. 2004, 7, 633–639. [Google Scholar]
- Dzida, K.; Jarosz, Z. Effect of calcium carbonate and differentiated nitrogen fertilization upon the yield and chemical composition of spinach beet. Acta Scientiarum. Polonorum. Hortorum. Cultus 2010, 9, 201–210. [Google Scholar]
- Saidajan, A. Effects of war on biodiversity and sustainable agricultural development in Afghanistan. J. Dev. Sustain. Agric. 2012, 7, 9–13. [Google Scholar]
- Average Precipitation in Depth (mm per Year)–Afghanistan. World Bank. 2023. Available online: https://data.worldbank.org/indicator/AG.LND.PRCP.MM?locations=AF (accessed on 19 September 2023).
- Ahmadi, J. Afghanistan geography: Mountain regions and region-specific soil type. Int. J. Multidiscipl. Res. Growth Eval. 2021, 2, 326–328. [Google Scholar]
- Food and Agriculture Organization of the United Nations/ United Nations Development Program (FAO/UNDP). Country Reports. In Calcareous Soils. Report of the FAO/UNDP Regional Seminar on Reclamation and Management of Calcareous Soils; FAO: Cairo, Egypt, 1973; pp. 140–154. [Google Scholar]
- Hashimi, R.; Matsuura, E.; Komatsuzaki, M. Effects of cultivating rice and wheat with and without organic fertilizer application on greenhouse gas emissions and soil quality in Khost, Afghanistan. Sustainability 2012, 12, 6508. [Google Scholar] [CrossRef]
- Ayubi, A.G. Principle of Irrigation and Drainage; Aazem Publ.: Kabul, Afghanistan, 2016; Volume 31, p. 174. [Google Scholar]
- United Nations Office for the Coordination of Humanitarian Affairs (OCHA). Needs Assessment on Soil and Water in Afghanistan: Future Harvest Consortium to Rebuild Agriculture in Afghanistan. 2023. Available online: https://reliefweb.int/report/afghanistan/needs-assessment-soil-and-water-afghanistan (accessed on 19 September 2023).
- Masunaga, T.; Kamidohzono, A.; Nezam, A.W.; Sadat, S.A. Paddy soil properties in Nangarhar Province, East Afghanistan. Jpn. Agric. Res. Q. JARQ 2014, 48, 299–306. [Google Scholar] [CrossRef]
- Comprehensive Agricultural and Rural Development Facility (Card-F). Midline 1 Report. Upperquartile and Altai Consulting 2017. pp. 39–40. Available online: https://assets.publishing.service.gov.uk/media/5afad49440f0b622e060e2cf/Comprehensive-Agriculture-Rural-Development.pdf (accessed on 19 September 2023).
- Afghanistan National Horticulture Survey 2003. Available online: http://www.fao.org/docrep/007/y5163e/y5163e03.htm#bm03 (accessed on 19 September 2023).
- Saito, T.; Ariizumi, T.; Okabe, Y.; Asamizu, E.; Hiwasa-Tanase, K.; Fukuda, N.; Mizoguchi, T.; Yamazaki, Y.; Aoki, K.; Ezura, H. TOMATOMA: A novel tomato mutant database distributing Micro-Tom mutant collections. Plant Cell Physiol. 2011, 52, 283–296. [Google Scholar] [CrossRef] [PubMed]
- Marti, E.; Gisbert, C.; Bishop, G.J.; Dixon, M.S.; García-Martínez, J.L. Genetic and physiological characterization of tomato cv. Micro-Tom. J. Exp. Bot. 2006, 57, 2037–2047. [Google Scholar] [CrossRef] [PubMed]
- Supanjani, S.; Tawaha, A.R.M.; YangMinSuk, Y.M.; Lee KyungDong, L.K. Role of calcium in yield and medicinal quality of Chrysanthemum coronarium L. J. Agron. 2005, 4, 186–190. [Google Scholar]
- Lee, G.J.; Kim, T.J. Effects of the use of additional calcium sources on growth and fruit characteristics in tomato hydroponics. Hort. Environ. Biotechnol. 2010, 51, 360–366. [Google Scholar]
- Babalar, M.; Mumivand, H.; Hadian, J.; Tabatabaei, S.M.F. Effects of nitrogen and calcium carbonate on growth, rosmarinic acid content and yield of Satureja hortensis L. J. Agric. Sci. 2010, 2, 92–98. [Google Scholar] [CrossRef]
- Obreza, T.A. Soil CaCO3 concentration affects growth of young grapefruit trees on Swingle citrumelo rootstock. In Proceedings of the Florida State Horticultural Society; Florida State Horticultural Society: Gainesville, FL, USA, 1995; Volume 108, pp. 147–150. [Google Scholar]
- Bessrour, M.; Chelbi, N.; Moreno, D.A.; Chibani, F.; Abdelly, C.; Carvajal, M. Interaction of salinity and CaCO3 affects the physiology and fatty acid metabolism in Portulaca oleracea. J. Agric. Food Chem. 2018, 66, 6683–6691. [Google Scholar] [CrossRef] [PubMed]
- Rayment, G.E.; Lyons, D.J. Soil Chemical Methods: Australasia; CSIRO Publ.: Collingwood, Australia, 2011; Volume 3, pp. 38–39. [Google Scholar]
- Wang, Y.S.; Tian, S.P.; Xu, Y. Effects of high oxygen concentration on pro-and anti-oxidant enzymes in peach fruits during postharvest periods. Food Chem. 2005, 91, 99–104. [Google Scholar] [CrossRef]
- Soil Plant General Analyzer SFP-3 4i. Available online: https://www.fujihira.co.jp/english/category/soi/pdf/SFP-4i.pdf (accessed on 19 September 2023).
- Kusunoki, M. Measurement of activity of roots of browning root rot-infected barley by triphenyl tetrazolium chloride (TTC) method. Proc. Assoc. Plant Protec. Shikoku. 1995, 30, 17–19. [Google Scholar]
- Castro-Concha, L.A.; Escobedo, R.M.; Miranda-Ham, M.L. Measurement of cell viability. Plant Cell Cult. Protoc. 2012, 877, 49–56. [Google Scholar] [CrossRef]
- Haris, M.M.; Silva, T.M.; Gulub, G.; Terada, N.; Shinohara, T.; Sanada, A.; Gemma, H.; Koshio, K. Growth, quality and capsaicin concentration of hot pepper (Capsicum annuum) under drought conditions. J. Int. Soc. Southeast Asian Agric. Sci. 2020, 26, 100–110. [Google Scholar]
- The Effect of Soil pH on Plants. Available online: https://www.southlandorganics.com/blogs/news/the-effect-of-soil-ph-on-plants (accessed on 19 September 2023).
- Ding, X.; Jiang, Y.; Zhao, H.; Guo, D.; He, L.; Liu, F.; Zhou, Q.; Nandwani, D.; Hui, D.; Yu, J. Electrical conductivity of nutrient solution influenced photosynthesis, quality, and antioxidant enzyme activity of pakchoi (Brassica campestris L. ssp. Chinensis) in a hydroponic system. PLoS ONE 2018, 13, e0202090. [Google Scholar] [CrossRef]
- Szczepaniak, S.; Henschke, M.; Czuchaj, P.; Kozik, E. Growth and flowering of lenten rose (Helleborus orientalis Lam.) depending on the dose of calcium carbonate and top dressing with Peters Professional Special. Acta Scien. Pol. Hort. Cult. 2008, 7, 13–22. [Google Scholar]
- Henschke, M.; Szczepaniak, S.; Czuchaj, P.; Kozik, E. The effect of calcium carbonate and top dressing with Peters Professional Special on the growth and flowering of Helleborus lividus Aiton. Folia Horticulturae. 2009, 21, 105–117. [Google Scholar] [CrossRef]
- Jessop, R.S.; Roth, G.; Sale, P. Effects of increased levels of soil CaCO3 on lupin (Lupinus angustifolius) growth and nutrition. Soil Res. 1990, 28, 955–962. [Google Scholar] [CrossRef]
- Mumivand, H.; Babalar, M.; Hadian, J.; Fakhr-Tabatabaei, M. Plant growth and essential oil content and composition of Satureja hortensis L. cv. Saturn in response to calcium carbonate and nitrogen application rates. J. Med. Plants Res. 2011, 5, 1859–1866. [Google Scholar]
- Chao, Y.Y.; Wang, W.J.; Liu, Y.T. Effect of calcium on the growth of djulis (Chenopodium formosanum Koidz.) sprouts. Agronomy 2021, 11, 82. [Google Scholar] [CrossRef]
- Rolny, N.; Costa, L.; Carrión, C.; Guiamet, J.J. Is the electrolyte leakage assay an unequivocal test of membrane deterioration during leaf senescence? Plant Physiol. Biochem. 2011, 49, 1220–1227. [Google Scholar] [CrossRef]
- Giel, P.; Bojarczuk, K. Effects of high concentrations of calcium salts in the substrate and its pH on the growth of selected rhododendron cultivars. Acta Soc. Bot. Pol. 2011, 80, 2. [Google Scholar] [CrossRef]
- Ota, Y. Diagnostic method for measurement of root activity in rice plant. Jpn. Agric. Res. Q. 1970, 5, 1–6. [Google Scholar]
- Li, J.; Xu, H.H.; Liu, W.C.; Zhang, X.W.; Lu, Y.T. Ethylene inhibits root elongation during alkaline stress through AUXIN1 and associated changes in auxin accumulation. Plant Physiol. 2015, 168, 1777–1791. [Google Scholar] [CrossRef]
- Růžička, K.; Ljung, K.; Vanneste, S.; Podhorská, R.; Beeckman, T.; Friml, J.; Benková, E. Ethylene regulates root growth through effects on auxin biosynthesis and transport-dependent auxin distribution. Plant Cell 2007, 19, 2197–2212. [Google Scholar] [CrossRef]
- Kinhal, V. Root Respiration: Importance and Applications. CID Bio-Science. Available online: https://cid-inc.com/blog/2023-guide-to-plant-and-crop-stress/ (accessed on 19 September 2023).
- Zaman, L.; Shafqat, W.; Sharief, N.; Raza, K.; ud Din, S.; Qureshi, M.A.; Ahmad, S.; Jiskani, M.J. Effect of foliar spray of calcium carbonate and zinc sulphate on fruit quality of kinnow mandarin. J. Global Innov. Agric. Soc. Sci. 2020, 8, 5–9. [Google Scholar] [CrossRef]
- Anthon, G.E.; LeStrange, M.; Barrett, D.M. Changes in pH, acids, sugars and other quality parameters during extended vine holding of ripe processing tomatoes. J. Sci. Food Agric. 2011, 91, 1175–1181. [Google Scholar] [CrossRef]

| Parameters | Treatment | ||||
|---|---|---|---|---|---|
| Control | 2.5% | 5% | 10% | 20% | |
| Soil pH | 6.74 ± 0.13 b | 7.69 ± 0.06 a | 7.8 ± 0.04 a | 7.72 ± 0.02 a | 7.6 ± 0.12 a |
| Soil EC | 1.05 ± 0.01 b | 0.94 ± 0.06 b | 1.0 ± 0.12 b | 1.43 ± 0.11 a | 1.55 ± 0.04 a |
| Parameter | Treatment | ||||
|---|---|---|---|---|---|
| Control | 2.5% | 5% | 10% | 20% | |
| No. leaves per plant | 172.25 ± 23.92 a | 156.75 ± 34.08 ab | 146.25 ± 9.6 abc | 115.25 ± 16.46 bc | 105.0 ± 4.76 c |
| Leaf Ca content (%) | 2.24 ± 0.12 c | 2.91 ± 0.06 b | 2.84 ± 0.15 b | 3.59 ± 0.24 a | 3.44 ± 0.14 a |
| Leaf Mg content (%) | 0.83 ± 0.09 a | 0.66 ± 0.06 a | 0.65 ± 0.03 a | 0.66 ± 0.02 a | 0.72 ± 0.04 a |
| Leaf EC (%) | 15.11 ± 4.05 b | 15.87 ± 1.91 b | 29.26 ± 8.56 ab | 33.27 ± 11.68 a | 37.9 ± 12.64 a |
| Leaf chlorophyll content (SPAD value) | 46.2 ± 3.95 a | 43.68 ± 2.06 ab | 42.14 ± 0.58 b | 41.06 ± 0.32 b | 41.1 ± 1.52 b |
| Parameter | Treatment | ||||
|---|---|---|---|---|---|
| Control | 2.5% | 5% | 10% | 20% | |
| Root length (cm) | 29.94 ± 4.9 a | 29.96 ± 2.43 a | 25.7 ± 4.89 ab | 23.69 ± 2.75 b | 21.1 ± 3.85 b |
| Root weight (g) | 5.23 ± 1.12 a | 4.55 ± 1.28 ab | 4.0 ± 1.21 ab | 3.13 ± 0.25 b | 3.1 ± 0.18 b |
| Root activity (mg FW g−1 h−1) | 0.3 ± 0.05 a | 0.28 ± 0.12 ab | 0.16 ± 0.02 abc | 0.06 ± 0.04 c | 0.13 ± 0.07 c |
| Root ethylene production (nL g−1 h−1) | 2.7 ± 0.4 c | 3.37 ± 0.22 bc | 3.89 ± 0.51 abc | 4.29 ± 0.5 ab | 5.01 ± 0.52 a |
| Root respiration rate [CO2 emission (µL g−1 h−1)] | 108.32 ± 24.84 b | 134.76 ± 5.73 ab | 151.6 ± 19.3 ab | 161.95 ± 35.32 a | 167.41 ± 15.95 a |
| Parameter | Treatment | ||||
|---|---|---|---|---|---|
| Control | 2.5% | 5% | 10% | 20% | |
| No. flower per plant | 86.25 ± 5.27 a | 59.0 ± 3.2 ab | 40.25 ± 3.0 bc | 34.75 ± 2.6 c | 35.5 ± 3.4 c |
| No. fruits per plant | 36.25 ± 6.18 a | 31.0 ± 2.94 ab | 20.25 ± 5.5 b | 21.25 ± 6.6 b | 20.0 ± 1.83 b |
| Fruit weight (g) | 2.22 ± 0.4 a | 2.85 ± 0.58 a | 2.1 ± 0.27 a | 2.05 ± 0.31 ab | 1.1 ± 0.53 b |
| Fruit diameter (mm) | 1.39 ± 0.15 ab | 1.47 ± 0.2 a | 1.49 ± 0.22 a | 1.43 ± 0.07 ab | 1.14 ± 0.14 b |
| Yield per plant (g) | 43.5 ± 1.25 a | 39.62 ± 12.22 ab | 38.68 ± 8.97 ab | 27.43 ± 4.15 bc | 24.37 ± 5.8 c |
| Parameter | Treatment | ||||
|---|---|---|---|---|---|
| Control | 2.5% | 5% | 10% | 20% | |
| Fruit firmness (N) | 1.63 ± 0.63 c | 2.17 ± 0.39 bc | 2.42 ± 0.8 ab | 2.71 ± 0.51 ab | 2.9 ± 0.79 a |
| Total soluble solids (%) | 6.3 ± 0.07 b | 6.28 ± 0.54 b | 7.02 ± 0.08 ab | 6.98 ± 0.04 ab | 7.2 ± 0.69 a |
| Total sugars (mg g−1 FW) | 17.83 ± 1.93 a | 18.5 ± 2.01 a | 19.22 ± 1.08 a | 19.66 ± 2.49 a | 19.65 ± 1.81 a |
| Ascorbic acid (mg 100 g−1 FW) | 20.22 ± 2.44 b | 22.2 ± 2.07 ab | 28.81 ± 2.26 a | 28.44 ± 4.69 a | 29.9 ± 1.96 a |
| Citric acid content (mg g−1 FW) | 11.35 ± 0.69 b | 11.96 ± 0.5 ab | 11.99 ± 0.68 ab | 13.23 ± 1.48 a | 13.41 ± 0.28 a |
| Malic acid content (mg g−1 FW) | 0.76 ± 0.06 b | 0.89 ± 0.06 ab | 0.91 ± 0.09 ab | 0.99 ± 0.02 a | 0.93 ± 0.09 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sediqui, N.; Amin, M.W.; Dawlatzai, N.; Gulab, G.; Poyesh, D.S.; Terada, N.; Sanada, A.; Kamata, A.; Koshio, K. Elucidation of Shoot and Root Growth, Physiological Responses, and Quality Traits of Tomato (Solanum lycopersicon L.) Exposed to Elevated Calcium Carbonate Concentrations. Horticulturae 2024, 10, 573. https://doi.org/10.3390/horticulturae10060573
Sediqui N, Amin MW, Dawlatzai N, Gulab G, Poyesh DS, Terada N, Sanada A, Kamata A, Koshio K. Elucidation of Shoot and Root Growth, Physiological Responses, and Quality Traits of Tomato (Solanum lycopersicon L.) Exposed to Elevated Calcium Carbonate Concentrations. Horticulturae. 2024; 10(6):573. https://doi.org/10.3390/horticulturae10060573
Chicago/Turabian StyleSediqui, Naveedullah, Mohammad Wasif Amin, Niamatullah Dawlatzai, Gulbuddin Gulab, Dawlat Shah Poyesh, Naoki Terada, Atsushi Sanada, Atsushi Kamata, and Kaihei Koshio. 2024. "Elucidation of Shoot and Root Growth, Physiological Responses, and Quality Traits of Tomato (Solanum lycopersicon L.) Exposed to Elevated Calcium Carbonate Concentrations" Horticulturae 10, no. 6: 573. https://doi.org/10.3390/horticulturae10060573
APA StyleSediqui, N., Amin, M. W., Dawlatzai, N., Gulab, G., Poyesh, D. S., Terada, N., Sanada, A., Kamata, A., & Koshio, K. (2024). Elucidation of Shoot and Root Growth, Physiological Responses, and Quality Traits of Tomato (Solanum lycopersicon L.) Exposed to Elevated Calcium Carbonate Concentrations. Horticulturae, 10(6), 573. https://doi.org/10.3390/horticulturae10060573







