Abstract
Banana pericarp is highly susceptible to chilling injury (CI), negatively affecting its quality and shelf life. Melatonin (MT), a plant tryptophan derivative, has shown promising effects in mitigating CI and related physiological disorders in tropical and subtropical fruits. This study investigated the efficacy of MT at different concentrations (0, 50, and 100 µM/L) in the coating of banana pericarp to control CI and physicochemical degradation during storage at 7 °C for five days, and on each day, fruits were assessed for quality. The MT100 treatment significantly (p < 0.05) mitigated the severity of the CI index, electrolytic leakage (EL), and malondialdehyde (MDA) levels. Reactive oxygen species (ROS) levels were substantially higher in control samples, whereas MT treatments notably suppressed their increase. Glutathione (GSH) and ascorbic acid (AA) levels were elevated in those banana pericarps treated with higher MT concentrations. Although total phenolic content (TPC) and total carotenoid contents (TCC) were increased in MT-treated samples, the MT concentrations did not significantly affect them. The level of phenolic compounds, such as gallic acid (GA), chlorogenic acid (CA), quinic acid (QA), protocatechuic acid (PA), and catechin (CC), exhibited continuous growth during the storage period, with the highest levels found in MT100-treated samples. Activities of enzymes such as lipoxygenase (LOX), phospholipase D (PLD), phenylalanine ammonia-lyase (PAL), and polyphenol oxidase (PPO) were significantly (p < 0.05) higher in control samples and rose continuously over time, yet were effectively reduced in MT-treated pericarps. This study shows that applying a higher MT coating (100 µM/L) to bananas is an effective post-harvest strategy to considerably lower the incidence of CI and associated losses.
1. Introduction
Banana (Musa acuminata ‘Gros Michel’) is a prominent tropical fruit commonly found in countries worldwide, particularly in Southeast Asia [1]. Its cultivation and consumption are incredibly high in Thailand, and these continue to increase due to the ready adaptability of the banana to grow in a range of tropical and subtropical regions, as well as a continuous increase in consumer demand [2]. The banana is used widely in Thai cuisine; they can be consumed directly as fresh and whole fruit or used in preparing snacks and desserts [3]. Apart from its delicious taste, suitability for consumption at all ages, and affordability, this fruit is rich in nutritional benefits. It is a rich source of macro- and micronutrients, mainly native and resistant starch, fibers, oligosaccharides, particularly fructooligosaccharides, inulin, essential amino acids, and potassium. Furthermore, it also contains a rich source of polyphenols, particularly GA and CC [4,5]. Bananas also exhibit various health benefits, including muscle relaxation, relief from gastrointestinal issues, blood pressure management, and the prevention of diseases such as colon cancer and diabetes [6]. It is a climacteric fruit and undergoes various physiological and biochemical changes upon ripening, including higher respiration rate and ethylene emission, as well changing in fruit pericarp color, taste, and textural changes followed by severe detrimental changes upon prolonged storage under warm conditions that impact its post-harvest quality and shelf life [7]. Although the fruit offers many benefits, it is naturally susceptible to rapid deterioration after harvest, causing a short shelf life due to its pericarp color changes and vulnerability to environmental conditions [8]. The storage temperature significantly affects the banana’s shelf life, with both room and cold temperatures playing a crucial role in diminishing the quality of the fruit [9,10]. Storing bananas at low temperatures can prevent microbial growth but may adversely cause CI, leading to taste, texture, and overall ripeness changes. On the other hand, exposure to room temperature can increase ethylene gas production and respiration, affecting their quality and safety [11]. Bananas stored in refrigerated conditions could suffer from CI, particularly affecting the pericarp more than the flesh. When transferred from cold storage to ambient conditions, CI worsens rapidly. The pericarp gradually browns, spreading into the fruit and rendering it overripe [12]. Bananas at 75–85% maturity are optimal for chilling storage as this aids their ripening [13].
The efficacious management of post-harvest processes significantly prolongs shelf life and preserves the quality of bananas [14,15]. Recent advancements have identified MT treatment as a promising post-harvest technique for mitigating deterioration in tropical and subtropical fruits under chilling stress conditions [16,17,18]. MT is also known as N-acetyl-5-methoxytryptamine, an indoleamine derivative of tryptophan [19], widely present in fruits, especially in cherries [20] and tomatoes [21]. Within the plant kingdom, MT serves as a pivotal biological signal orchestrating a myriad of physiological processes, including but not limited to photosynthesis, development, defense mechanisms, germination, flowering, budding, leaf senescence, the integrity of cell membranes, root proliferation, and osmoregulation [22]. Furthermore, it is instrumental in fortifying plants against biotic and abiotic stresses, notably CI, thereby underscoring its relevance in post-harvest applications. The MT protection effect from fruit deterioration varies among species and varieties. MT could extend the cold tolerance in CI-sensitive plants, scavenging ROS and enhancing the overall antioxidant activities [23]. Studies have shown that exogenous application of MT enhances chilling tolerance and preserves the qualities and phytonutrients from loss during cold storage across various tropical and subtropical fruits [17,24,25]. Application of MT on plants stored under chilling sensitive temperatures activated the expression of genes associated with a cold response and antioxidant activities [26]. MT treatment on tea plants exhibits reduced ROS and MDA levels, alongside diminished lipid peroxidation, and this is mainly achieved by the enhanced level of AA and GSH, which helps contribute to the overall antioxidant systems [27]. MT treatments have demonstrated efficacy in mitigating CI symptoms, decreasing EL, MDA content, hydrogen peroxide (H2O2), and superoxide anion (O2•−) radical levels while enhancing proline content, antioxidant enzyme activities, and concurrently reducing the cell membrane degrading enzyme activities in sapodilla fruit under prolonged cold storage [28]. Moreover, MT has been shown to enhance post-harvest fruit quality under chilling stress by regulating ethylene production and maintaining quality in tomatoes at a concentration of 50 µM [29]. In yellow-fleshed peaches stored at 4 °C and 90% RH, MT significantly preserves firmness, soluble solids content, and color while also reducing oxidative stress markers and boosting antioxidant enzyme levels, further augmenting gamma-aminobutyric acid (GABA) synthesis [30]. Additionally, a 100 µM MT method that coated strawberries by immersion for 15 min effectively delayed discoloration, maintained acidity and firmness, and diminished microbial growth, thereby preserving their market value under cold storage conditions [31].
Though various fruits have been tested using MT to alleviate CI and improve post-harvest quality, the studies on bananas treated with MT with a view to extending the quality and storage period under chilling conditions still need to be improved. The current study investigates the influence of MT coating on the different enzyme activities in the banana pericarp. The main aim is to assess the efficacy of MT treatment in extending shelf life, preserving fruit quality, and analyzing a spectrum of physicochemical and phytochemical parameters during storage under ambient conditions. The quality assessment includes measurements of the CI index, EL, MDA, ROS, GSH, AA, TPC, TCC, phenolic acids, and enzyme activities (LOX, PLD, PAL, and POD). Consequently, this study takes the opportunity to examine the effectiveness and efficiency of MT treatment on bananas during cold storage, focusing on various physicochemical, phytochemical, and enzymatic analyses.
2. Materials and Methods
2.1. Chemicals and Reagents
All the chemicals and reagents (hypochlorite and Sporgon) used in Section 2.2 were of food grade, whereas the chemicals and reagents (acetone, ammonium tetrarhodanatodiammonchromate, calcium chloride, choline chloride, dichlorophenol-indophenol, dihydroethidium, dithiobis-(2-nitrobenzoic acid), ethanol, Folin–Ciocalteu reagent, glutathione reductase, L-phenylalanine, nicotinamide adenine dinucleotide phosphate, petroleum ether, phosphatidylcholine, polyvinylpolypyrrolidone, scopoletin, sodium bicarbonate, sulfosalicylic acid, thiobarbituric acid, titanium sulfate, trichloroacetic acid, and 4-methyl catechol) used in Section 2.3 were of analytical grade. They were obtained from Sigma Aldrich, Bangkok, Thailand.
2.2. Plant Materials and Treatments
The raw materials for this study were procured by selecting mature bananas (Musa acuminata ‘Gros Michel’) of commercial quality (at breaker stage 5, such as half green and half yellow) from an orchard in Southern Thailand. The selection process was based on the uniform color, size, and maturity level of approximately 75–80%. Upon arrival at the laboratory, the bananas underwent rigorous quality inspection to ensure their freedom from visible defects. They were then carefully separated from their bunches, with the ends kept intact, and washed with sterile distilled water to remove any surface dirt or debris. After the washing process, surface moisture was removed using sterile paper towels. The bananas were then treated by immersing them in a solution containing 1% hypochlorite and 0.05% Sporgon, which contains 46% Prochloraz-Mn, for 5 min. This treatment was administered to target any potential invisible fungal growth. The bananas were then categorized into three groups: Control, MT50, and MT100. The control group was immersed in sterile distilled water, while the MT50 and MT100 groups were treated with MT solutions at concentrations of 50 µM/L and 100 µM/L, respectively. All groups were submerged in their respective solutions for 30 min. After treatment, the bananas were air-dried using an electric fan to remove any residual moisture at ambient temperatures with 60–70% relative humidity. After the fruit surface dried, the fruits were packaged and stored under controlled chilling conditions (7 °C and at 85% relative humidity) for five days. For each treatment group, nine bananas were collected, packed in perforated polyethylene bags, and stored under the above-mentioned chilling conditions (Figure 1). A range of analyses were conducted to assess the bananas’ quality, detailed in Section 2.3.
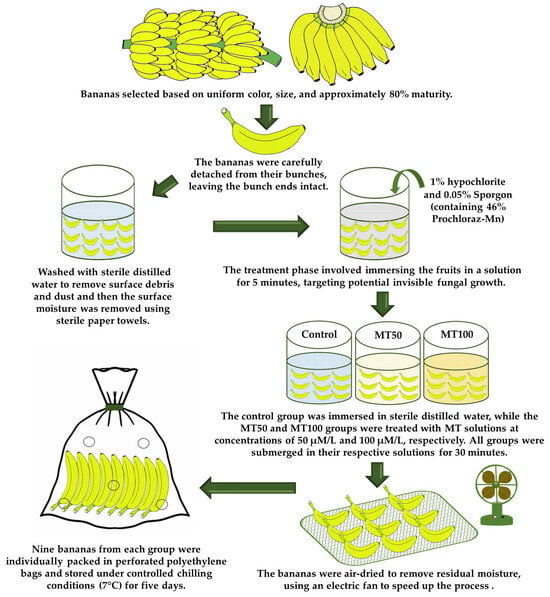
Figure 1.
Infographic presentation of raw material preparation, and coating formulation and application on banana fruit surface.
2.3. Quality Analysis
2.3.1. Determination of CI index
The CI index was determined on the surface of the banana by following the method of Chen et al. [32]. The CI index of banana fruits was assessed based on the degree of browning on the surface. A rating system was utilized where a score of 1 indicates minimal to no browning presence; a score of 2 signifies browning covering 1% to 25% of the surface; a score of 3 is for 25% to 50% surface browning; a score of 4 corresponds to 50% to 75% browning; and a score of 5 is used when browning affects 75% to 100% of the surface area. The following formula was used to calculate the CI index:
2.3.2. Determination of EL
Banana pericarp samples were utilized for measuring the EL in accordance with the method of Wang et al. [33]. A total of 20 discs were extracted from the banana pericarp with a 0.8 cm diameter stainless steel borer. These discs were then rigorously rinsed with distilled water, gently blotted to remove surface moisture with a paper towel, and subsequently arranged in a test tube. Distilled water was added to the tube and placed in a water bath shaker for 30 min under room temperature conditions. Initial electrical conductivity (L0) was recorded using a conductivity meter. The test tube was then heated to 100 °C for 20 min, after which the electrical conductivity was measured again (L1). The EL was determined using the following formula:
2.3.3. Determination of MDA
The MDA levels in the banana pericarps were measured in accordance with the method of Chen et al. [34]. An amount of 2 g of banana pericarp tissues was homogenized in 8 mL of 50 mM phosphate buffer (pH 7.8). Following homogenization, the mixture was centrifuged at 12,000× g for 20 min. The supernatant was carefully transferred to a clean test tube for MDA analysis. For the analysis, 1 mL of the supernatant was combined with 3 mL of thiobarbituric acid (5 g/L) dissolved in trichloroacetic acid (100 g/L).
This mixture was then heated in a boiling water bath for 15 min, and after that, the mixture was rapidly cooled and centrifuged at 12,000× g for 10 min. Then, the supernatant was collected and absorbance was measured at different wavelengths, such as 532 nm, 600 nm, and 450 nm.
Vt, Vr, and Vs represent the total volume of the extraction solution, the total volume of the reaction mixture solution, and the volume of the extract solution contained in the reaction mixture solution, respectively. m represents the mass of the samples. FW represents fresh weight.
2.3.4. ROS Determination
Determination of O2•− Radicals
The O2•− radical levels were determined following the protocol described by Yang et al. [35] with some modifications. A mass of 1 g of banana pericarp samples was homogenized with 3 mL of potassium phosphate buffer (20 mM, pH 6.0) for 30 min in a mortar and pestle. After that, the homogenate was centrifuged for 10 min at 1900× g. Thereafter, the supernatant was collected and incubated in the dark with 2 mL of dihydroethidium (10 µM) and CaCl2 (100 µM) at pH 4.75 for 1 h. After incubation, the reaction mixture was measured spectrophotometrically at 510 nm. The results were calculated and expressed in nmol kg−1 FW.
Determination of H2O2 Radicals
The H2O2 radical levels were determined following the protocol described by Schopfer et al. [36] with some modifications. An amount of 1 g of banana pericarp samples was homogenized with 3 mL of 0.02 M phosphate buffer at pH 6.0 for half an hour. After homogenization, the mixture was centrifuged at 1900× g for 10 min to obtain the supernatant. The collected supernatant (1 mL) was then incubated in the dark at 25 °C on a shaker. This incubation solution included 3 mL of 0.02 M phosphate buffer (pH 6.0), adding 5 μM scopoletin and 3 mg/mL horseradish peroxidase. The incubation aimed to monitor the reduction in scopoletin; observation was measured using a spectrometer at an excitation wavelength of 346 nm and an emission wavelength of 455 nm against a reagent blank. The absorbance values were then converted into H2O2 molar concentrations using a pre-established linear calibration curve. The results were calculated and expressed in nmol kg−1 FW.
Determination of •OH Radicals
The determination of •OH radicals was carried out in accordance with the methodology described by Pongprasert et al. [37], with some modifications. A 3 g banana pericarp sample was homogenized in a solution that comprised 100 mL of 5% trichloroacetic acid (TCA) and 0.5 g of polyvinylpolypyrrolidone (PVPP). The mixture was centrifugated for 25 min at 7000× g after homogenization. Afterward, 1 mL of the clear supernatant was combined with an equal volume of 0.1% titanium sulfate, prepared in 20% sulfuric acid (v/v). This solution was then centrifuged at 7000× g for 15 min. The absorbance of the resulting yellow-colored supernatant was measured at a wavelength of 410 nm using a spectrophotometer. The results were calculated and expressed in nmol kg−1 FW.
2.3.5. Determination of GSH
The GSH content in the banana pericarp was measured in accordance with the method of Chotikaham et al. [38] with some modifications. A total of 2 g of banana pericarp was homogenized for 5 min in a cold condition (4 °C) with 10 mL of 5% of 5-sulfosalicylic acid. Then, the homogenate was centrifuged for 20 min at 11,000× g in a cold condition (4 °C). After that, 10 µL of the supernatant was added in a test tube that contained 1.1 mL of the reaction mixture, which included 54.5 mM sodium phosphate buffer set to pH 7.0, 0.14% of 5,5′-dithiobis-(2-nitrobenzoic acid), 0.18% nicotinamide adenine dinucleotide phosphate and glutathione reductase (0.09 unit). After that, the reaction mixture was thoroughly vortexed for 1 min and kept at room temperature for 30 min, and the absorbance was measured at 412 nm. The results were calculated and expressed in µg g−1 FW.
2.3.6. Determination of AA
The AA content was quantified using a titration method adapted from Nielsen et al. [39] with some modifications. Approximately 10 g of banana pericarp tissue was homogenized with 90 mL of 3% metaphosphoric acid solution using a blender. Then, the homogenate was filtered and titrated against a 2,6-dichlorophenol-indophenol dye solution, and the titration was terminated when the titrant changed color to pink persisting for more than 15 s. The AA standard was used to calculate the level in the banana pericarp, and the results were calculated and expressed in µg g−1 FW.
2.3.7. Determination of TPC
The TPC level in the banana pericarp was measured in accordance with the method of Singleton et al. [40]. A 5 g banana pericarp tissue was homogenized in cold 80% ethanol and centrifuged at 8000× g for 10 min. Then, the supernatant was collected, and 0.1 mL was mixed with a freshly prepared working reagent containing 0.5 mL Folin–Ciocalteu reagent and 2.9 mL distilled water and followed by the addition of 2 mL of 20% sodium bicarbonate solution. The mixture was thoroughly vortexed and incubated for 90 min before measuring its absorbance at 760 nm using a UV-Vis spectrophotometer against a blank. The absorbance values were calculated using the GA standard curve, and the results were calculated and expressed in µg GAE g−1 FW.
2.3.8. Determination of TCC
The TCC in the banana samples was measured in accordance with the method of Liu et al. [41] with some modifications. Banana pericarp (1 g) was pulverized using liquid nitrogen and then homogenized with 2 mL acetone (80%), and after that, the homogenate was centrifuged for 10 min at 10,000× g. Then, the supernatant was collected, and the absorption was measured at 663, 646, and 470 nm using a spectrophotometer. The following formula was used to calculate the total carotenoid content in the banana samples:
2.3.9. Determination of Phenolic Acids
Phenolic compounds were extracted from banana pericarps using a modified method from Liu et al. [42]. The process involved homogenizing 10 g of banana pericarp tissue with 50 mL of 80% acetone solution using a homogenizer, to which 0.2% formic acid by volume was added to enhance the extraction of phenolic compounds. After extraction, the resultant homogenate was concentrated using a rotary evaporator to reduce volume and increase phenolic concentration. The concentrated extract was then filtered through Supelclean LC-18 Cartridges to remove non-phenolic materials and concentrate the phenolic constituents. A high-performance liquid chromatography (HPLC) analysis was employed to quantitatively determine the phenolic content, utilizing a Waters Alliance E2695 system equipped with a C18 column. The mobile phase consisted of eluent A (1% (v/v) acetic acid in water) and eluent B (methanol). The elution gradient was set in the following manner: a progression from 12% to 25% of solvent B within the initial 15 min, increasing to 35% of B from 15 to 25 min, further rising to 55% B from 25 to 50 min, then to 65% B between 50 and 60 min, and finally, reverting to 12% B in the time frame from 60 to 70 min. The chromatographic conditions were maintained by a column thermostat at 35 °C, an injection quantity of 10 µL, and phenolic detection achieved through a UV-diode array at a wavelength of 280 nm. The determination of phenolic compounds in the analyzed samples was achieved by comparing their relative retention times and UV spectra against known external standards. The concentrations of the phenolic compounds were quantified and reported in µg g−1 FW.
2.3.10. Determination of Enzyme Activities
LOX Activity
LOX activity in the banana pericarp was measured in accordance with the method of Lin et al. [43]. Banana pericarp (5 g) was homogenized at 4 °C with 10 mL of 50 mM potassium phosphate buffer (pH 7). Then, the homogenate was centrifugated at 15,000× g for 15 min at 4 °C; then, the supernatant was collected and served as the crude enzyme extract. LOX activity was assessed by mixing 0.2 mL of the extract with 2.8 mL of the same buffer containing 25 mM sodium linoleate as the substrate. Changes in absorbance at 234 nm, reflecting linoleic acid hydroperoxide production, were monitored using a UV-Vis spectrophotometer. One unit of LOX activity was defined as the amount of enzyme that caused a 0.01 increase in absorbance per minute under these conditions. Finally, LOX activity was calculated and expressed as units per gram of FW (unit g−1 FW).
PLD Activity
The measurement of PLD in the pericarp banana was done in accordance with the method of Yi et al. [44]. Pericarp tissue (0.5 g) was homogenized in 2.5 mL of 0.1 M Tris-HCl buffer (pH 7.0) and centrifuged at 4 °C for 30 min at 13,000× g. After that, the supernatant was collected and used for the PLD assay. A substrate containing 0.05 g of phosphatidylcholine, 3 mL of chloroform, and 3 mL of water was prepared and evaporated at 40 °C using a rotary evaporator. Then, rehydrated in 250 mL of 100 mM acetate buffer (pH 5.5, containing 5 mM dithiothreitol and 25 mM CaCl2), the substrate was mixed with 1 mL of supernatant and stirred vigorously for 1 h at room temperature and then treated with petroleum ether thus separating the aqueous phase. Next, in the aqueous phase, 2 g of ammonium tetrarhodanatodiammonchromate in 100 mL of methanol was added, forming a precipitate. This precipitate was centrifuged at 26,000× g for 15 min at 4 °C and dissolved in 3 mL of acetone. The absorbance of the clear supernatant was measured at 520 nm using a UV-visible spectrophotometer. Choline chloride (20 mg) was diluted in 100 mL of 100 mM acetate buffer (pH 5.6) to generate a calibration curve. One unit of PLD activity was defined as the micromoles of choline produced per minute per gram of fresh-weight tissue (FW), and the results were calculated and reported in unit g−1 FW.
PAL Activity
PAL activity in the pericarp of bananas was measured using the method of Chen et al. [45]. Banana pericarp (5 g) was homogenized at 4 °C with a chilled buffer containing 0.1 M sodium borate (pH 8.0), 0.5 g polyvinylpyrrolidone, 5 mM β-mercaptoethanol, and 2 mM EDTA. The resulting homogenate was centrifuged at 19,000× g to separate the liquid fraction (supernatant), which was then mixed with the same buffer supplemented with 3 mM l-phenylalanine for an enzyme assay. Following a one-hour incubation at room temperature, the increase in absorbance at 290 nm, reflecting the trans-cinnamate formation, was measured using a UV-Vis spectrophotometer. One unit of enzyme activity was defined as the amount that catalyzes a 0.01 absorbance increase per hour under these conditions, and the results were calculated and reported in unit g−1 FW.
PPO Activity
To assess the PPO activity as detailed in Jiang [46], 5 g of banana pericarp samples were homogenized with 20 mL of chilled phosphate buffer (0.05 M, pH 7.0), which included 0.5 g of insoluble polyvinylpyrrolidone. Subsequently, the homogenate was filtered and centrifugated at 19,000× g for 20 min at a temperature of 4 °C. The supernatant obtained post-centrifugation was then utilized for the PPO activity assessment. In a test tube, 0.1 mL of the supernatant was introduced, followed by 2.9 mL of 10 mM 4-methyl catechol. After thorough mixing, the solution was transferred into a cuvette for absorbance measurement at 420 nm with a spectrophotometer, recorded at one-minute intervals. Enzymatic activity was defined as the change in absorbance of 0.001 per minute, and the results were calculated and reported in unit g−1 FW.
2.4. Statistical Analysis
Each experiment was conducted in three replicates, with results shown as the average value ± standard deviation. The mean values were compared for significant discrepancies using a one-way analysis of variance (ANOVA), with a significance threshold set at p < 0.05. Statistical analyses were performed utilizing the SPSS software for Windows (Version 6, SPSS Inc., Chicago, IL, USA). Correlation and association of coating conditions and physicochemical parameters were excluded from the principal component analysis (PCA) input matrix using Minitab statistical software (Version 17, Minitab Inc., Boston, MA, USA) to explore and quantify the variability in the data set. The PCA plots of PC1 versus PC2 provide a way to visualize the relationships between samples based on multiple characteristics.
3. Results and Discussion
3.1. CI, Membrane Permeability, and MDA Levels
Generally, the tropical and subtropical fruit varieties that are stored at cold temperatures but above their freezing point can develop CI. CI can severely impact the overall quality of bananas when subjected to prolonged storage at low temperatures [47]. Figure 2A shows the effect of MT on controlling CI in banana fruit during storage at low temperatures. CI began to occur on the surface of the bananas in the control samples from day 1 and tended to increase continuously with storage time. Throughout the storage, the results showed that control samples had a higher CI incidence than the MT-treated samples. In MT-coated samples, CI symptoms occurred on day 2 of storage and gradually increased, which is consistent with the results of Wang et al. [48]. After 3 days of storage at a low temperature, noticeable changes in CI formation were found in the MT-treated banana samples; however, the incidence was far lower compared to the control. Furthermore, bananas treated with MT100 had a slightly lower increase in CI than MT50. In general, the CI incidence in climacteric tropical fruits is predominantly high due to the severity of membrane degradation, which is rapid in the presence of ROS. As fruits are stored in an unsuitable environment, they undergo physiological changes that induce stress. This stress leads to the production of reactive oxygen species (ROS) and the degradation of the cell membrane. Consequently, these changes initiate a chain reaction of lipid oxidation, leading to oxidative damage and membrane dysfunction in cells [49,50]. MT is an endogenous indole molecule with numerous biological functions in plants, particularly inducing stress tolerance [41]. MT helps control CI by maintaining a higher proportion of unsaturated fatty acids in the cell membrane, enhancing membrane stability and resistance to chilling stress. This effect of MT on membrane lipid desaturation has been observed in other fruits and is a key mechanism in protecting fruits against CI during low-temperature storage [18]. The extent of CI is directly linked to the degree of membrane damage and permeability. Tropical and subtropical fruits susceptible to chilling exhibit more significant alterations in membrane properties, resulting in quicker and more severe development of CI. Moreover, the transition of cell membranes from a flexible, liquid-crystalline phase to a rigid, gel-like structure at low temperatures increases the risk of losing the controlled semi-permeability of the cell membranes [51]. In addition, this study showed that banana fruits’ prolonged exposure to CI significantly (p < 0.05) increased the EL levels (Figure 2B). The EL levels of the initial day did not differ much from each other; however, when the storage period was prolonged, the severity of the membrane degradation was well established in the banana fruits, particularly in the control samples, and continuously increased throughout storage. Whereas the MT-treated samples showed a better-regulating capacity against the increase in EL levels over control samples, the performance between MT treatments showed that fruits treated with MT100 had better capacity for controlling the EL than those treated with MT50. MT is a potent antioxidant that scavenges ROS and preserves cell membrane integrity to prevent ionic leakage and cold-induced disruptions [52,53,54]. MT regulates membrane permeability through its effects on lipid composition and channel interactions and enhances stress signaling, leading to protective molecule production like proline for membrane stabilization [55]. Additionally, MT’s direct interaction with membrane phospholipids strengthens their structure against temperature-induced damage and thus reduces the EL, highlighting its crucial role in protecting cells under cold stress [56]. Furthermore, our study observed an increasing trend in MDA levels in banana fruits during prolonged cold storage, as illustrated in Figure 2C. The control group showed a significant (p < 0.05) and continuous accumulation of MDA from the initial day of storage, indicating heightened oxidative stress. In contrast, MT effectively controlled MDA levels. Throughout the storage period, MT managed to maintain MDA levels below 1 nmol kg−1 for both MT-treated groups. Notably, the MT100 treatment demonstrated a more significant (p < 0.05) suppression of the increment of MDA content in the banana pericarp than the MT50 treatment. This finding suggests that MT plays a crucial role in combating CI in bananas by mitigating MDA, a key marker of oxidative stress. The potential underlying mechanisms may involve MT’s antioxidant activity, which helps maintain the integrity of banana cell membranes. Additionally, MT might promote the biosynthesis of flavonoids, thereby enhancing the fruit’s antioxidant defenses [57]. These flavonoids likely play a significant role in enhancing the pericarps’ ability to eliminate ROS, which is associated with further observed reductions in MDA content [58,59].
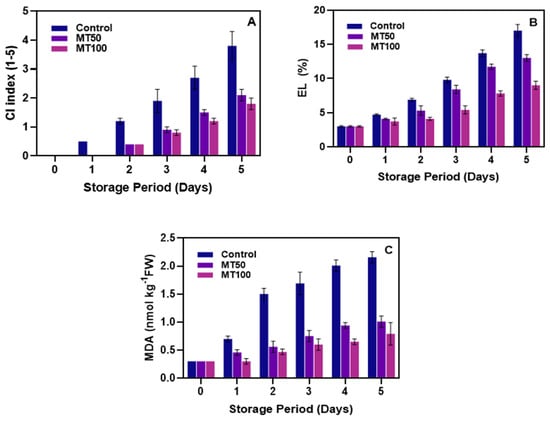
Figure 2.
Effect of melatonin treatment on controlling CI index (A), EL (B), and MDA (C) levels in banana fruits during cold storage (n = 3). Note: The CI index, EL, and MDA content correspond to the chilling injury index, electrolyte leakage, and malondialdehyde content, respectively.
3.2. Production of ROS
Cold temperatures directly affect membrane structure, exacerbating the damage through oxidative stress in plants [60]. Elevated levels of ROS, such as O2•−, H2O2, and •OH radicals, are observed under cold stress, contributing to cell membrane damage via lipid peroxidation [61]. This stress also leads to the oxidation of vital cellular components, evidenced by loss of membrane integrity, protein oxidation, enzymatic activity inhibition, and DNA and RNA damage [35]. Figure 3 elucidates the impacts of MT on the production and accumulation of ROS in the banana fruit during low-temperature storage. Overall, the study showed that the production of ROS in the banana pericarp continuously increased during storage despite the sample variance. The O2•− radical production in the banana pericarp was the most accumulated ROS during storage compared to the others (Figure 3A). Control samples had the highest level of O2•− radical followed by the MT treatments, and among the MTs, there were not many differences observed in the O2•− production till the 3rd day of storage, after which the differences in the O2•− was more noticeable. A similar trend was observed in the H2O2 radical, in which the control samples were predominantly high in accumulating H2O2 radicals in banana pericarp during storage. In contrast, the MT treatments were highly significant (p < 0.05) in controlling the level of H2O2 compared with the control (Figure 3B), although, at the beginning of storage, the control samples and the MT treatments did not differ much. However, when the storage period was prolonged, the differences between control and MT samples were clearly established, and they continued to increase with the storage period. Similarly, the •OH radical levels in the banana pericarp continuously increased in the control fruits throughout the storage period. At the beginning of storage, no significant differences in the •OH radical levels were observed among all the samples. However, the difference in the •OH radical levels became clearly established as the storage period extended. Among the MT-treated samples, the differences in controlling the •OH radical levels were minimal at the beginning of storage but later significantly differed throughout the storage period (Figure 3C). Overall, the ROS results showed that the differences in MT concentrations were not much involved when the ROS accumulation was low in the banana fruits; however, when the level increased, the higher concentration (MT100) outperformed the MT50 and control samples. This is in accordance with Song et al. [62], whose study found that increased concentrations of MT could be effective at controlling the severity of CI in eggplants. Wang et al. [63] studied the effect of MT on controlling CI in banana fruit, and their findings reported that MT treatment effectively enhanced the chilling tolerance and induced the accumulation of phospholipids and unsaturated fatty acids, which reduced the membrane degradation and consequently lowered ROS production. Several studies have tested and reported that the exogenous MT treatment could be able to improve the chilling tolerances in tropical fruits and vegetables by enhancing the antioxidant activities [64], upregulating the gene expression related to endogenous MT biosynthesis [62], increasing the cold stress response [52] and cold defense enzyme activities [65], as well as modulating the critical components in the senescence process [66].
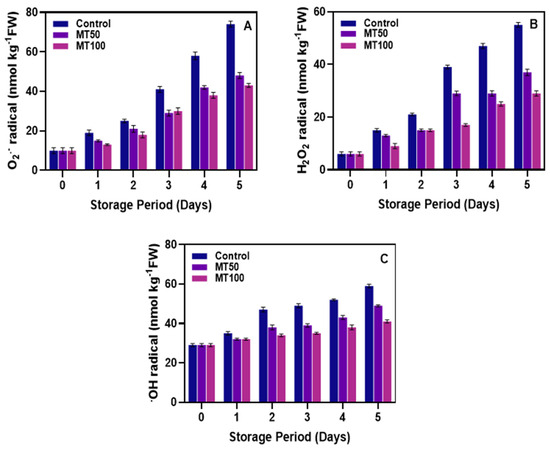
Figure 3.
Effect of melatonin treatment on alleviating the reactive oxygen species including O2•− radical (A), H2O2 radical (B), and •OH radical (C) levels in banana fruits during cold storage. Note: O2•−, H2O2, and •OH radicals correspond to the superoxide anion, hydrogen peroxide and hydroxyl radicals, respectively.
3.3. Changes in GSH, AA, TPC, and TCC Levels
Plants possess a complex non-enzymatic antioxidative system composed of secondary metabolites such as GSH, AA, TPC, and TCC, which have the potential to combat or eliminate ROS and the stress generated by them [67]. Figure 4A shows that the prolonged cold storage gradually increased the GSH content in all sample variants. However, the control samples had the lowest level of GSH in comparison with the MT-treated samples. In the initial days of storage, there were few differences observed among the samples; however, with the prolonged exposure to chilling conditions, the MT-treated samples outperformed the control. Furthermore, increased MT concentration significantly (p < 0.05) increased the GSH levels. On the other hand, the AA content in the banana fruits continuously decreased in all the sample variants during the storage (Figure 4B). Generally, a decline in the AA level in fruit peel samples could be the result of temperature-dependent degradation [68] and oxidative stress [69] under prolonged exposure to cold storage, which accelerates the degradation, such as peel necrosis [70]. Chen et al. [32] observed similar results when banana fruit stored under prolonged storage exhibited a decline. MT-treated banana fruits were slightly higher in AA than in the control group; however, MT could not alter the trend against the decline in AA. At the end of storage, samples treated with MT100 were able to retain a higher AA level than the other treatments. Jiao et al. [71] found that the exogenous application of MT on kiwifruit enhanced the antioxidant activities by improving the GSH and controlling the loss of AA, thus significantly reducing the CI in the fruit. Furthermore, the application of MT on fruits and vegetables could increase the protective function of AA and GSH as antioxidants and reduce the severity of ROS production [72]. Phenolic compounds, which include flavonoids and phenolic acids, are major secondary metabolites abundant in fruits and are highly sensitive to enzymatic oxidation, leading to their significant depletion [73]. TPC in the banana peel of the control sample gradually increased till the 3rd day of storage, and afterward, it tended to decrease as the storage extended (Figure 4C). Control samples showed a high TPC level as compared to the MT-treated samples at the beginning of storage, and then it tended to decrease dramatically. The differences between the MT samples were insignificant (p < 0.05), and the TPC level in the MT samples was steadily maintained. At the end of storage, a slight drop in the TPC level was observed. Damaged plant cells undergo enzymatic browning, a process where internal chemicals, including phenolics and PPO, react, similar to the browning of a banana peel during ripening [74,75]. MT treatments substantially control these changes by decreasing the expression of PLD, LOX, and PPO genes, thus minimizing the activity of these enzymes and, consequently, reducing the incidence of phenolic oxidation in the banana pericarp. This is in accordance with the study of Wang et al. [53]. On the other hand, a continuous decrease in TCC levels in the banana pericarp was observed throughout the storage period (Figure 4D). In the initial days of storage, there were insignificant differences in the TCC levels in all the samples, and when the cold exposure was prolonged, the control samples, from the 2nd day of storage, lost the enormous level of TCC content. Whereas the MT treatments were retained, the TCC level was substantially higher, and the changes in the TCC level during storage were minimal despite its overall decreasing trend. Among the MT samples, there were slight fluctuations; however, at the end of storage, MT100 exhibited higher TCC levels. Kan et al. [76] reported that TCC has a weak and highly variable stability; in some fresh plants, degradation can occur within a short period. This is in accordance with the present study, in which the degradation of TCC was observed from the 1st day of storage. Calvo-Brenes et al. [77] observed that TCC loss in plants during cold storage is directly related to their antioxidant potential and the accumulation of ROS. This implies that TCCs in plants are utilized in neutralizing free radicals, with their depletion possibly impacting the plants’ defense against oxidative stress. Manafi et al. [78] reported that exogenous application of MT on strawberry plants maintained their TCC level better than the control.
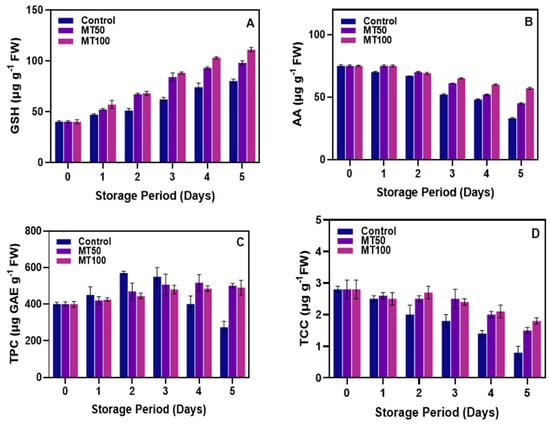
Figure 4.
Effect of melatonin treatment on GSH (A), AA (B), TPC (C) and TCC (D) levels in banana fruits during cold storage. Note: GSH, AA, TPC and TCC correspond to the glutathione, ascorbic acid, total phenolic content, and total carotenoid content, respectively.
3.4. Changes in Phenolic Acids
Bananas are a rich source of phenolic compounds, which have strong natural antioxidant properties. Specifically, GA, CA, QA, PA, CC, and FA are the major phenolic compounds found in bananas [79,80]. The levels of phenolic acid in banana fruit pericarp stored at chilling temperatures are shown in Figure 5. Phenolic acid results indicate that QA and CA are the predominant phenolic compounds present in the pericarp of banana fruit, and GA, CC, FA, and PA were found in minimal amounts. This is in accordance with the study of Zhang et al. [81]. Several studies indicate that derivatives of QA, such as CA and GA, possess beneficial health effects, including antioxidant, antimicrobial, anti-inflammatory, and neuroprotective properties. Additionally, these compounds are absorbed and metabolized within the human gastrointestinal system [82,83]. Saravanan and Aradhya [84] reported that CC, ferulic, and PA help prevent cardiovascular, cancer, neurodegenerative diseases, oxidative stress, inflammation, DNA damage, and displayed anticancer, antidiabetic, and antihypertensive properties. Our study found that the storage duration was observed to markedly elevate the phenolic acid concentrations in the banana pericarp. Siqueira et al. [85] similarly reported that bananas subjected to stress conditions during storage displayed an increase in overall phenolic content, albeit with a slight reduction in PA levels. The changes in GA levels in banana pericarp during cold storage are shown in Figure 5A. The results show that the MT100-coated bananas had a significantly (p < 0.05) higher level of GA in the fruit pericarp compared to the MT50 and control samples. This improvement was sustained throughout the storage period. In contrast, the control samples showed a decline in GA levels starting from the 3rd day of storage. The MT-treated fruits were able to maintain higher levels of GA throughout the storage period. The significance of these changes was evident at the end of the storage period. CA levels were not significantly different between variables in the initial days of storage, but they gradually changed from the 2nd day of storage, and the changes were highly significant (p < 0.05) when comparing control with treated samples. The levels of CA in the MT100 samples were significantly (p < 0.05) higher compared to the other samples (Figure 5B). QA levels in all treated samples increased steadily until the 3rd day of storage, and minimal changes were observed thereafter (Figure 5C). There were no significant differences in QA levels among the samples. The levels of PA in the banana pericarps stored under prolonged cold storage are shown in Figure 5D. Initially, there were no differences in PA levels among the samples, but as the storage period increased, the levels in the MT100 treated samples increased significantly (p < 0.05) compared to the other samples. The highest levels of PA were observed on the 3rd day of storage and then gradually decreased. However, the levels at the end of storage were still higher than the initial days of storage. A higher concentration of MT retained more PA than the others. CC levels in the banana pericarp increased significantly (p < 0.05) with storage time, but the differences between control and treated samples were not significant at the beginning of storage, especially for the MT50 samples (Figure 5E). However, as the storage period increased, the differences became more significant (p < 0.05), and the MT100 samples had the highest CC levels compared to the other samples. On the other hand, FA levels were elevated at the beginning of storage (Figure 5F) and then remained stable with minimal changes in the MT-treated samples, while the control samples showed a continuous decrease in FA levels towards the end of storage. This study demonstrated that prolonged storage periods resulted in more significant declines in phenolic compounds, specifically GA, CA, and QA, in the control samples. Studies have suggested that the decrease in the phenolic acids in banana pericarp during cold storage could be due to enzymatic oxidation induced by PPO activities [86]; this is in accordance with the present study (see Figure 6D). At the same time, the MT treatment substantially controlled the loss of phenolics by suppressing the membrane degradation induced by ROS and various stressors. Evidence suggests that MT application leads to an increase in plant phenolic compounds, often connected with greater tolerance to stress and bolstered secondary metabolism [87,88,89,90]. Gao et al. [18] discovered that the efficacy of MT in mitigating the depletion of phenolic acids in fruits is dependent on the dosage. This aligns with the findings of the current study.
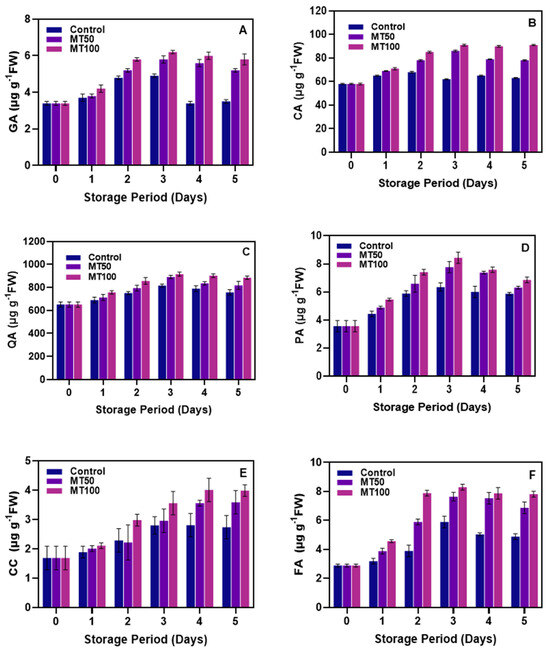
Figure 5.
Effect of melatonin treatment on GA (A), CA (B), QA (C), PA (D), CC (E) and FA (F) levels in banana fruits during cold storage. Note: GA, CA, QA, PA, CC, and FA correspond to gallic acid, chlorogenic acid, quinic acid, protocatechuic acid, catechin, and ferulic acid, respectively.
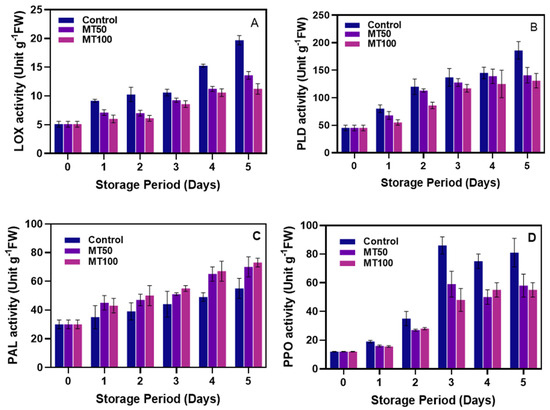
Figure 6.
Effect of melatonin treatment on controlling the LOX (A), PLD (B), PAL (C) and PPO (D) enzyme activities in banana fruits during cold storage (n = 3). Note: LOX, PLD, PAL and PPO correspond to the lipoxygenase, phospholipase, phenylalanine ammonia-lyase, and polyphenol oxidase activities, respectively.
3.5. Enzyme Activities
The LOX, PLD, PAL, and POD enzymes are crucial in enhancing and reacting to plant resistance through their roles in antimicrobial activity, cell wall biosynthesis, and wound healing processes, and in general, these enzyme activities are always high in extreme stress conditions [91]. Changes in LOX, PLD, PAL, and PPO activity in the MT-treated banana fruit pericarp from extended storage under cold temperatures are shown in Figure 6. Overall, the results show that despite the treatment variations, the extended cold storage continuously induces the banana pericarp’s enzyme activities. The highest activities were noticed mainly from the enzymes PLD and PPO, whereas those from LOX and PAL were found to be minimal. Overall, the MT-treated fruits exhibited better control against these enzymes. In general, plants include a number of proactive defensive mechanisms that aid in fending off infections and environmental stress by reacting to unusual alterations in their surroundings, and this causes adverse effects, particularly on their biochemical qualities [92]. However, the exogenous application of MT on the banana fruit might effectively control the biomolecular signaling and biological stimulants [93]. This is in accordance with the present study. The LOX activity in pericarp banana was extremely high in the control samples, followed by the treated ones (Figure 6A). In the MT samples, the higher MT dose showed a significant (p < 0.05) reduction in the LOX activity.
Similarly, the PLD activity in the cold-stored banana samples also showed a continuous increase, and it extended with the increased storage period. In the initial days, the PLD activity was somewhat insignificant, but when the storage increased, the differences between the control and MT-treated samples became clearly established (Figure 6B). Among the samples, the PLD activity was relatively low in the MT100-treated samples. In general, extreme cold stress in plants decreases ATP production due to reduced activities of enzymes like cytochrome c oxidase, succinate dehydrogenase, hydrogen-ion-transporting ATPase, and calcium-ion-transporting ATPase (Ca2+-ATPase) while increasing PLD and LOX activity. This leads to higher cytosolic Ca2+ levels due to decreased Ca2+-ATPase activity and a less effective ROS system, causing a buildup of O2•− and H2O2. The resulting electron leakage from NADH dehydrogenase and cytochrome b/c1 complex inefficiency promotes lipid peroxidation, impairing membrane integrity as indicated by elevated electrolyte leakage and MDA levels [94]. The application of MT might reduce the incidence of cold stress in plants by controlling the expression of genes that are involved in adverse biochemical alterations and thus controlling the membrane-degrading enzymes [95]. Generally, the PAL enzymes possess no adverse effect on plants; they only tend to increase their activity under stressful circumstances to reduce the impact of the adverse activities by phenolics, which are potent antioxidants and highly efficient in controlling ROS [96]. The PAL activity in the control samples’ pericarp was extremely low during all the storage, whereas the MT-treated samples significantly (p < 0.05) increased the PAL activity during storage (Figure 6C). This is in accordance with the study of Gao et al. [18]. However, the present study found an insignificant difference in the PAL activity despite the variations in the MT concentrations. Similarly, the PPO activity in the banana pericarp was found to be in continuous increments. In comparison with the other tested enzymes, the PPO initial activity was very low, and no rapid increment was found, indicating the freshness and the cellular integrity of the fruit pericarp. However, as the storage period extended, the severity of the PPO activity became extreme, and the predominant level of activity was observed in the control fruit as compared to treated samples. Similar to PAL activity, the differences between the MT samples in controlling the PPO activity were also insignificant during all the storage (Figure 6D). However, they very effectively suppressed the PPO activity in the banana fruit during cold storage. This indicates that MT-treatment of the banana fruit strengthens the cellular compartment, which suppresses the enzyme and substrate interactions; consequently, less PPO activity is observed. This aligns with the findings of Venkatachalam et al. [97], who discovered that longkong fruit treated with MT exhibited significantly reduced activity of enzymes (PPO and POD) related to browning.
3.6. Correlation and Association of Coating Conditions and Physicochemical Parameters
The biplot of the first and second principal components (PC) was plotted using the PCA, and the results were employed to examine the relationship between the qualitative assessment of banana fruits during cold storage and the implications of MT treatment (Figure 7). The differences between the control and the MT-coated fruits were identified using the PCA biplot, and together with PC1 and PC2, they accounted for 99.82%. The PC1 encapsulates an impressive 96.83% of the total variance, denoting its primary role in delineating the physicochemical responses of the treated samples. Conversely, PC2 accounts for a mere 2.99% of the variance, offering additional but distinctly minor differentiation. The clustering of the control group near the origin of the PCA plot signifies the baseline of the tested physicochemical parameters. Meanwhile, the MT50 and MT100 groups are observed to progressively diverge along PC1, demonstrating a dose-dependent influence of MT on the physicochemical parameters. The marked divergence of the MT50 and MT100 groups, found predominantly along the PC1 plot, signifies a notable shift in biochemical states, possibly reflecting enhanced antioxidant activity, as inferred from the positive correlations with compounds like AA and TPC. This observed pattern aligns with the postulated protective function of MT against oxidative stress, exhibiting an intensified reaction at elevated doses. Nonetheless, oxidative stress indicators such as MDA and H2O2 are also displaced in the MT-treated groups, suggesting that higher concentrations of MT might have a sophisticated modulatory impact. These findings from the PCA underscore the capacity of MT to modulate oxidative stress and antioxidant defenses in a dose-dependent manner. The PCA analysis also exposed how PAL and PPO activity and phenolic acids, particularly GA, were positively correlated to each other, and similarly, CI, MDA, and LOX were also positively correlated. Overall, the PCA analysis showed that major key parameters that adversely affect banana fruits were positively correlated with the control groups, indicating that control groups were severely affected. In contrast, the MT-treated fruits lacked those adversely affecting parameters, and instead, they were occupied with vitamin and phenolic activities.
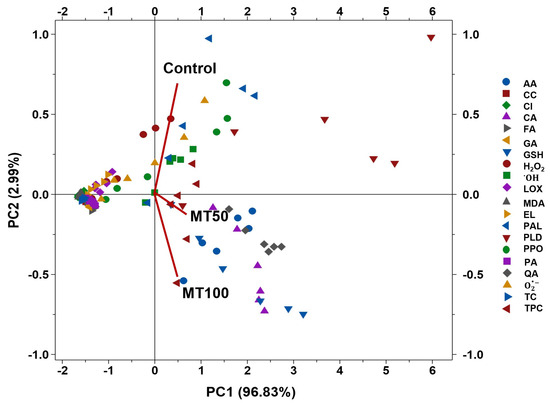
Figure 7.
PCA bi-plot for control and MT-treated banana fruit stored at cold temperatures. Note: CI, EL, MDA, O2•−, H2O2, OH, GSH, AA, TPC, TCC, GA, CA, QA, PA, CC, FA, LOX, PLD, PAL, and PPO correspond to the chilling injury index, electrolytic leakage, superoxide anion radical, hydrogen peroxide radical, hydroxyl radical, glutathione, ascorbic acid, total phenolic content, total carotenoid content, gallic acid, chlorogenic acid, quinic acid, protocatechuic acid, catechin, ferulic acid, lipoxygenase, phospholipase D, phenylalanine ammonia lyase and polyphenol oxidase, respectively.
4. Conclusions
The current study demonstrates that melatonin significantly reduces chilling injury symptoms in bananas, enhancing their resistance to cold stress. The melatonin application preserves cellular integrity by limiting lipid peroxidation and decreasing reactive oxygen species levels, which is crucial for maintaining fruit quality during refrigerated storage. Elevated melatonin concentrations effectively maintain membrane fluidity and reduce oxidative stress markers, such as malondialdehyde. Moreover, MT treatment boosts the antioxidant defense mechanisms in banana fruits, evidenced by increased levels of glutathione, ascorbic acid, total phenolic content, and total carotenoids. It also regulates the lipoxygenase, phospholipase D, phenylalanine ammonia-lyase, and polyphenol oxidase activities, which are crucial for prolonging storage life. Collectively, these findings endorse MT100 treatment as an effective post-harvest strategy to extend shelf life and improve the quality of bananas and potentially other fruits susceptible to cold-induced stress.
Author Contributions
Conceptualization, N.C. and K.V.; methodology, N.C., K.V. and P.C.; software, N.C. and B.P.; validation, N.C., B.P. and K.V.; formal analysis, N.C., K.V. and P.C.; investigation, N.C. and K.V.; resources, N.C. and K.V.; data curation, B.P. and P.C.; writing—original draft preparation, N.C. and K.V.; writing—review and editing, N.C., P.C., B.P. and K.V.; visualization, N.C. and K.V.; supervision, K.V.; project administration, K.V.; funding acquisition, K.V. All authors have read and agreed to the published version of the manuscript.
Funding
This research was financially supported by Prince of Songkla University, Surat Thani Campus, 2024.
Data Availability Statement
All data are contained within this article.
Acknowledgments
The authors would like to thank Prince of Songkla University, Surat Thani campus, and Burapha University Chanthaburi Campus for providing the resources and facilities to complete this research. Furthermore, the authors gratefully acknowledge the Research Office of Prince of Songkla University, Surat Thani campus, for funding this research in 2024.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Maseko, K.H.; Regnier, T.; Meiring, B.; Wokadala, O.C.; Anyasi, T.A. Musa Species Variation, Production, and the Application of Its Processed Flour: A Review. Sci. Hortic. 2024, 325, 112688. [Google Scholar] [CrossRef]
- Aurore, G.; Parfait, B.; Fahrasmane, L. Bananas, Raw Materials for Making Processed Food Products. Trends Food Sci. Technol. 2009, 20, 78–91. [Google Scholar] [CrossRef]
- Hettiaratchi, U.P.K.; Ekanayake, S.; Welihinda, J. Chemical Compositions and Glycemic Responses to Banana Varieties. Int. J. Food Sci. Nutr. 2011, 62, 307–309. [Google Scholar] [CrossRef] [PubMed]
- Happi Emaga, T.; Andrianaivo, R.H.; Wathelet, B.; Tchango, J.T.; Paquot, M. Effects of the Stage of Maturation and Varieties on the Chemical Composition of Banana and Plantain Peels. Food Chem. 2007, 103, 590–600. [Google Scholar] [CrossRef]
- Anyasi, T.A.; Jideani, A.I.O.; Mchau, G.R.A. Functional Properties and Post-harvest Utilization of Commercial and Noncommercial Banana Cultivars. Compr. Rev. Food Sci. Food Saf. 2013, 12, 509–522. [Google Scholar] [CrossRef] [PubMed]
- Sarma, P.P.; Gurumayum, N.; Verma, A.K.; Devi, R. A Pharmacological Perspective of Banana: Implications Relating to Therapeutic Benefits and Molecular Docking. Food Funct. 2021, 12, 4749–4767. [Google Scholar] [CrossRef] [PubMed]
- Jin, Z.-Q.; Xu, B.-Y.; Liu, J.-H.; Su, W.; Zhang, J.-B.; Yang, X.-L.; Jia, C.-H.; Li, M.-Y. Identification of Genes Differentially Expressed at the Onset of the Ethylene Climacteric in Banana. Postharvest Biol. Technol. 2009, 52, 307–309. [Google Scholar] [CrossRef]
- Bapat, V.A.; Trivedi, P.K.; Ghosh, A.; Sane, V.A.; Ganapathi, T.R.; Nath, P. Ripening of Fleshy Fruit: Molecular Insight and the Role of Ethylene. Biotechnol. Adv. 2010, 28, 94–107. [Google Scholar] [CrossRef] [PubMed]
- Der Agopian, R.G.; Peroni-Okita, F.H.G.; Soares, C.A.; Mainardi, J.A.; do Nascimento, J.R.O.; Cordenunsi, B.R.; Lajolo, F.M.; Purgatto, E. Low Temperature Induced Changes in Activity and Protein Levels of the Enzymes Associated to Conversion of Starch to Sucrose in Banana Fruit. Postharvest Biol. Technol. 2011, 62, 133–140. [Google Scholar] [CrossRef]
- Yang, X.; Zhang, Z.; Joyce, D.; Huang, X.; Xu, L.; Pang, X. Characterization of Chlorophyll Degradation in Banana and Plantain during Ripening at High Temperature. Food Chem. 2009, 114, 383–390. [Google Scholar] [CrossRef]
- Du, L.; Song, J.; Forney, C.; Palmer, L.C.; Fillmore, S.; Zhang, Z. Proteome Changes in Banana Fruit Peel Tissue in Response to Ethylene and High-Temperature Treatments. Hortic. Res. 2016, 3, 16012. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Joyce, D.C.; Jiang, W.; Lu, W. Effects of Chilling Temperatures on Ethylene Binding by Banana Fruit. Plant Growth Regul. 2004, 43, 109–115. [Google Scholar] [CrossRef]
- Li, W.; Shao, Y.; Chen, W.; Jia, W. The Effects of Harvest Maturity on Storage Quality and Sucrose-Metabolizing Enzymes During Banana Ripening. Food Bioprocess. Technol. 2011, 4, 1273–1280. [Google Scholar] [CrossRef]
- Jiang, Y.; Joyce, D.C.; Macnish, A.J. Extension of the Shelf Life of Banana Fruit by 1-Methylcyclopropene in Combination with Polyethylene Bags. Postharvest Biol. Technol. 1999, 16, 187–193. [Google Scholar] [CrossRef]
- Mulagund, J.; Porika, H.; Soorianathasundaram, K.; Deepika, C. Influence of Growth Regulators Combined with Chemicals to Improve Post-harvest Fruit Quality in Banana Cv. Nendran (Musa AAB). J. Food Process Technol. 2015, 6, 1000428. [Google Scholar] [CrossRef]
- Liu, J.; Sun, J.; Pan, Y.; Yun, Z.; Zhang, Z.; Jiang, G.; Jiang, Y. Endogenous Melatonin Generation Plays a Positive Role in Chilling Tolerance in Relation to Redox Homeostasis in Litchi Fruit during Refrigeration. Postharvest Biol. Technol. 2021, 178, 111554. [Google Scholar] [CrossRef]
- Jannatizadeh, A. Exogenous Melatonin Applying Confers Chilling Tolerance in Pomegranate Fruit during Cold Storage. Sci. Hortic. 2019, 246, 544–549. [Google Scholar] [CrossRef]
- Gao, H.; Lu, Z.; Yang, Y.; Wang, D.; Yang, T.; Cao, M.; Cao, W. Melatonin Treatment Reduces Chilling Injury in Peach Fruit through Its Regulation of Membrane Fatty Acid Contents and Phenolic Metabolism. Food Chem. 2018, 245, 659–666. [Google Scholar] [CrossRef]
- Dreux, C. Biochemical studies of methoxy indoles. II—Determination of methoxy-5 tryptamine by spectrofluorometry. Clin. Chim. Acta 1970, 30, 519–526. [Google Scholar] [CrossRef]
- Gonzalez-Gomez, D.; Lozano, M.; Fernandez-Leon, M.F.; Ayuso, M.C.; Bernalte, M.J.; Rodriguez, A.B. Detection and Quantification of Melatonin and Serotonin in Eight Sweet Cherry Cultivars (Prunus Avium L.). Eur. Food Res. Technol. 2009, 229, 223–229. [Google Scholar] [CrossRef]
- Sturtz, M.; Cerezo, A.B.; Cantos-Villar, E.; Garcia-Parrilla, M.C. Determination of the Melatonin Content of Different Varieties of Tomatoes (Lycopersicon Esculentum) and Strawberries (Fragariaananassa). Food Chem. 2011, 127, 1329–1334. [Google Scholar] [CrossRef] [PubMed]
- Kumari, A.; Singh, S.K.; Mathpal, B.; Verma, K.K.; Garg, V.K.; Bhattacharyya, M.; Bhatt, R. The Biosynthesis, Mechanism of Action, and Physiological Functions of Melatonin in Horticultural Plants: A Review. Horticulturae 2023, 9, 913. [Google Scholar] [CrossRef]
- Turk, H.; Erdal, S.; Genisel, M.; Atici, O.; Demir, Y.; Yanmis, D. The Regulatory Effect of Melatonin on Physiological, Biochemical and Molecular Parameters in Cold-Stressed Wheat Seedlings. Plant Growth Regul. 2014, 2, 139–152. [Google Scholar] [CrossRef]
- Liu, G.; Zhang, Y.; Yun, Z.; Hu, M.; Liu, J.; Jiang, Y.; Zhang, Z. Melatonin Enhances Cold Tolerance by Regulating Energy and Proline Metabolism in Litchi Fruit. Foods 2020, 9, 454. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Randhawa, M.S.; Azam, M.; Liu, H.; Ejaz, S.; Ilahy, R.; Qadri, R.; Khan, M.I.; Umer, M.A.; Khan, M.A.; et al. Exogenous Melatonin Treatment Reduces Post-harvest Senescence and Maintains the Quality of Papaya Fruit during Cold Storage. Front. Plant Sci. 2022, 13, 1039373. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Chang, J.; Zheng, J.; Dong, Y.; Liu, Q.; Yang, X.; Wei, C.; Zhang, Y.; Ma, J.; Zhang, X. Local Melatonin Application Induces Cold Tolerance in Distant Organs of Citrullus Lanatus L. via Long Distance Transport. Sci. Rep. 2017, 7, 40858. [Google Scholar] [CrossRef] [PubMed]
- Yang, N.; Han, M.-H.; Teng, R.-M.; Yang, Y.-Z.; Wang, Y.-H.; Xiong, A.-S.; Zhuang, J. Exogenous Melatonin Enhances Photosynthetic Capacity and Related Gene Expression in A Dose-Dependent Manner in the Tea Plant (Camellia Sinensis (L.) Kuntze). Int. J. Mol. Sci. 2022, 23, 6694. [Google Scholar] [CrossRef] [PubMed]
- Mirshekari, A.; Madani, B.; Yahia, E.M.; Golding, J.B.; Vand, S.H. Post-harvest Melatonin Treatment Reduces Chilling Injury in Sapota Fruit. J. Sci. Food Agric. 2020, 100, 1897–1903. [Google Scholar] [CrossRef] [PubMed]
- Xu, T.; Chen, Y.; Kang, H. Melatonin Is a Potential Target for Improving Post-Harvest Preservation of Fruits and Vegetables. Front. Plant Sci. 2019, 10, 1388. [Google Scholar] [CrossRef]
- Gao, H.; Zhang, Z.K.; Chai, H.K.; Cheng, N.; Yang, Y.; Wang, D.N.; Yang, T.; Cao, W. Melatonin Treatment Delays Post-harvest Senescence and Regulates Reactive Oxygen Species Metabolism in Peach Fruit. Postharvest Biol. Technol. 2016, 118, 103–110. [Google Scholar] [CrossRef]
- Promyou, S.; Raruang, Y.; Chen, Z.-Y. Melatonin Treatment of Strawberry Fruit during Storage Extends Its Post-Harvest Quality and Reduces Infection Caused by Botrytis Cinerea. Foods 2023, 12, 1445. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.L.; Shan, W.; Cai, D.L.; Chen, J.Y.; Lu, W.J.; Su, X.G.; Kuang, J.F. Post-harvest Application of Glycine Betaine Ameliorates Chilling Injury in Cold-Stored Banana Fruit by Enhancing Antioxidant System. Sci. Hortic. 2021, 287, 110264. [Google Scholar] [CrossRef]
- Wang, Y.; Luo, Z.; Huang, X.; Yang, K.; Gao, S.; Du, R. Effect of Exogenous γ-Aminobutyric Acid (GABA) Treatment on Chilling Injury and Antioxidant Capacity in Banana Peel. Sci. Hortic. 2014, 168, 132–137. [Google Scholar] [CrossRef]
- Chen, J.; He, L.; Jiang, Y.; Wang, Y.; Joyce, D.C.; Ji, Z.; Lu, W. Role of Phenylalanine Ammonia-lyase in Heat Pretreatment-induced Chilling Tolerance in Banana Fruit. Physiol. Plant. 2008, 132, 318–328. [Google Scholar] [CrossRef]
- Yang, S.; Su, X.; Yang, B.; Cheng, G.; Chen, Y.; Yang, E.; Jiang, Y. Oxidation and Peroxidation of Post-harvest Banana Fruit during Softening. Pak. J. Bot. 2008, 40, 2023–2202. [Google Scholar]
- Schopfer, P.; Plachy, C.; Frahry, G. Release of Reactive Oxygen Intermediates (Superoxide Radicals, Hydrogen Peroxide, and Hydroxyl Radicals) and Peroxidase in Germinating Radish Seeds Controlled by Light, Gibberellin, and Abscisic Acid. Plant Physiol. 2001, 125, 1591–1602. [Google Scholar] [CrossRef]
- Pongprasert, N.; Sekozawa, Y.; Sugaya, S.; Gemma, H. The role and mode of action of UV-C hormesis in reducing cellular oxidative stress and the consequential chilling injury of banana fruit peel. Int. Food Res. J. 2011, 18, 741–749. [Google Scholar]
- Chotikakham, S.; Faiyue, B.; Uthaibutra, J.; Saengnil, K. Exogenous Methyl Salicylate Alleviates Senescent Spotting by Enhancing the Activity of Antioxidative Ascorbate-Glutathione Cycle in Harvested’ Sucrier’ Bananas. Sci. Hortic. 2020, 267, 109324. [Google Scholar] [CrossRef]
- Nielsen, S.S. Vitamin C Determination by Indophenol Method. In Food Analysis Laboratory Manual; Food Science Text Series; Springer: Cham, Switzerland, 2017. [Google Scholar] [CrossRef]
- Alberti, A.; Zielinski, A.A.F.; Zardo, D.M.; Demiate, I.M.; Nogueira, A.; Mafra, L.I. Optimisation of the Extraction of Phenolic Compounds from Apples Using Response Surface Methodology. Food Chem. 2014, 149, 151–158. [Google Scholar] [CrossRef]
- Liu, J.; Li, F.; Liang, L.; Jiang, Y.; Chen, J. Fibroin Delays Chilling Injury of Post-harvest Banana Fruit via Enhanced Antioxidant Capability during Cold Storage. Metabolites 2019, 9, 152. [Google Scholar] [CrossRef]
- Liu, H.; Cao, J.; Jiang, W. Changes in Phenolics and Antioxidant Property of Peach Fruit during Ripening and Responses to 1-Methylcyclopropene. Postharvest Biol. Technol. 2015, 108, 111–118. [Google Scholar] [CrossRef]
- Lin, Y.; Lin, Y.; Lin, H.; Zhang, S.; Chen, Y.; Shi, J. Inhibitory Effects of Propyl Gallate on Browning and Its Relationship to Active Oxygen Metabolism in Pericarp of Harvested Longan Fruit. LWT Food Sci. Technol. 2015, 60, 1122–1128. [Google Scholar] [CrossRef]
- Yi, C.; Qu, H.X.; Jiang, Y.M.; Shi, J.; Duan, X.W.; Joyce, D.C.; Li, Y.B. ATP-Induced Changes in Energy Status and Membrane Integrity of Harvested Litchi Fruit and Its Relation to Pathogen Resistance. J. Phytopathol. 2008, 156, 365–371. [Google Scholar] [CrossRef]
- Chen, X.; Ren, L.; Li, M.; Qian, J.; Fan, J.; Du, B. Effects of Clove Essential Oil and Eugenol on Quality and Browning Control of Fresh-Cut Lettuce. Food Chem. 2017, 214, 432–439. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y. Role of Anthocyanins, Polyphenol Oxidase and Phenols in Lychee Pericarp Browning. J. Sci. Food Agric. 2000, 80, 305–310. [Google Scholar] [CrossRef]
- Cainelli, N.; Ruperti, B. Biochemistry and Molecular Biology in Fruits during Cold Storage. In Annual Plant Reviews Online; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2019; pp. 659–688. [Google Scholar] [CrossRef]
- Lukatkin, A.; Brazaityte, A.; Bobinas, C.; Duchovskis, P. Chilling Injury in Chilling-Sensitive Plants: A Review. Zemdirb. Agric. 2012, 99, 111–124. [Google Scholar]
- Chang, L.-Y.; Sargent, S.A.; Kim, J.; Brecht, J.K. Delaying Ripening Using 1-MCP Reveals Chilling Injury Symptom Development at the Putative Chilling Threshold Temperature for Mature Green Banana. Front. Plant Sci. 2022, 13, 966789. [Google Scholar] [CrossRef] [PubMed]
- Tian, S.; Qin, G.; Li, B. Reactive Oxygen Species Involved in Regulating Fruit Senescence and Fungal Pathogenicity. Plant Mol. Biol. 2013, 82, 593–602. [Google Scholar] [CrossRef] [PubMed]
- Patel, B.; Tandel, Y.; Patel, A.H.; Patel, B. Chilling Injury in Tropical and Subtropical Fruit: A Cola Storage Problem and Its Remedies: A Review. Int. J. Sci. Env. Technol. 2016, 5, 1882–1887. [Google Scholar]
- Liu, J.; Wu, H.; Wang, B.; Zhang, Y.; Wang, J.; Cheng, C.; Huang, Y. Exogenous Melatonin Enhances Cold Resistance by Improving Antioxidant Defense and Cold-Responsive Genes Expression in Banana. Horticulturae 2022, 8, 260. [Google Scholar] [CrossRef]
- Wang, Z.; Pu, H.; Shan, S.; Zhang, P.; Li, J.; Song, H.; Xu, X. Melatonin Enhanced Chilling Tolerance and Alleviated Peel Browning of Banana Fruit under Low Temperature Storage. Postharvest Biol. Technol. 2021, 179, 111571. [Google Scholar] [CrossRef]
- Qari, S.H.; Hassan, M.U.; Chattha, M.U.; Mahmood, A.; Naqve, M.; Nawaz, M.; Barbanti, L.; Alahdal, M.A.; Aljabri, M. Melatonin Induced Cold Tolerance in Plants: Physiological and Molecular Responses. Front. Plant Sci. 2022, 13, 843071. [Google Scholar] [CrossRef] [PubMed]
- Kong, X.; Ge, W.; Wei, B.; Zhou, Q.; Zhou, X.; Zhao, Y.; Ji, S. Melatonin Ameliorates Chilling Injury in Green Bell Peppers during Storage by Regulating Membrane Lipid Metabolism and Antioxidant Capacity. Postharvest Biol. Technol. 2020, 170, 111315. [Google Scholar] [CrossRef]
- Raza, A.; Charagh, S.; Garcia-Caparros, P.; Rahman, M.A.; Ogwugwa, V.H.; Saeed, F.; Jin, W. Melatonin-Mediated Temperature Stress Tolerance in Plants. GM Crops Food 2022, 13, 196–217. [Google Scholar] [CrossRef]
- Liang, D.; Shen, Y.; Ni, Z.; Wang, Q.; Lei, Z.; Xu, N.; Deng, Q.; Lin, L.; Wang, J.; Lv, X.; et al. Exogenous Melatonin Application Delays Senescence of Kiwifruit Leaves by Regulating the Antioxidant Capacity and Biosynthesis of Flavonoids. Front. Plant Sci. 2018, 9, 426. [Google Scholar] [CrossRef]
- Sharma, P.; Jha, A.B.; Dubey, R.S.; Pessarakli, M. Reactive Oxygen Species, Oxidative Damage, and Antioxidative Defense Mechanism in Plants under Stressful Conditions. J. Bot. 2012, 2012, e217037. [Google Scholar] [CrossRef]
- Samiasih, A.; Subagio, W.H.; Dharmana; Susanto, H.; Sadhana, U.; Sunoko, H.R.; Santosa, B. Banana Peels Extract (Musa Paradisiaca Var Kepok) Decreased MDA in New Zealand White Rabbit with DM Hyperlipidemia. IOP Conf. Ser. Earth Environ. Sci. 2019, 292, 012008. [Google Scholar] [CrossRef]
- Liu, X.; Zhou, Y.; Xiao, J.; Bao, F. Effects of Chilling on the Structure, Function and Development of Chloroplasts. Front. Plant Sci. 2018, 9, 1715. [Google Scholar] [CrossRef] [PubMed]
- Miura, K.; Furumoto, T. Cold Signaling and Cold Response in Plants. Int. J. Mol. Sci. 2013, 14, 5312–5337. [Google Scholar] [CrossRef]
- Song, L.; Tan, Z.; Zhang, W.; Li, Q.; Jiang, Z.; Shen, S.; Luo, S.; Chen, X. Exogenous Melatonin Improves the Chilling Tolerance and Preharvest Fruit Shelf Life in Eggplant by Affecting ROS- and Senescence-Related Processes. Hortic. Plant J. 2023, 9, 523–540. [Google Scholar] [CrossRef]
- Wang, Z.; Zhang, L.; Duan, W.; Li, W.; Wang, Q.; Li, J.; Song, H.; Xu, X. Melatonin Maintained Higher Contents of Unsaturated Fatty Acid and Cell Membrane Structure Integrity in Banana Peel and Alleviated Post-harvest Chilling Injury. Food Chem. 2022, 397, 133836. [Google Scholar] [CrossRef] [PubMed]
- Ali, S.; Nawaz, A.; Naz, S.; Ali, M.; Ejaz, S.; Azam, M.; Razzaq, K. Exogenous Melatonin Mitigates Chilling Injury in Zucchini Fruit by Enhancing Antioxidant System Activity, Promoting Endogenous Proline and GABA Accumulation, and Preserving Cell Wall Stability. Postharvest Biol. Technol. 2023, 204, 112445. [Google Scholar] [CrossRef]
- Wang, L.; Shen, X.; Chen, X.; Ouyang, Q.; Tan, X.; Tao, N. Exogenous Application of Melatonin to Green Horn Pepper Fruit Reduces Chilling Injury during Postharvest Cold Storage by Regulating Enzymatic Activities in the Antioxidant System. Plants 2022, 11, 2367. [Google Scholar] [CrossRef] [PubMed]
- Madebo, M.P.; Hu, S.; Zheng, Y.; Jin, P. Mechanisms of Chilling Tolerance in Melatonin Treated Post-harvest Fruits and Vegetables: A Review. J. Future Foods 2021, 1, 156–167. [Google Scholar] [CrossRef]
- Das, K.; Roychoudhury, A. Reactive Oxygen Species (ROS) and Response of Antioxidants as ROS-Scavengers during Environmental Stress in Plants. Front. Environ. Sci. 2014, 2, 53. [Google Scholar] [CrossRef]
- Kan, C.; Gao, Y.; Wan, C.; Chen, M.; Zhao, X.; Liu, S.; Chen, J. Influence of Different Cold Storage Times on Quality of “Cuiguan” Pear Fruits during Shelf Life. J. Food Process. Preserv. 2019, 43, e14245. [Google Scholar] [CrossRef]
- Buccheri, M.; Picchi, V.; Grassi, M.; Gandin, D.; Bianchi, G.; Scalzo, R.L. Dynamic Changes of Antioxidants and Fermentative Metabolites in Apple Peel in Relation to Storage, Controlled Atmosphere, and Initial Low Oxygen Stress. Sci. Hortic. 2021, 288, 110312. [Google Scholar] [CrossRef]
- Gapper, N.E.; Hertog, M.L.A.T.M.; Lee, J.; Buchanan, D.A.; Leisso, R.S.; Fei, Z.; Qu, G.; Giovannoni, J.J.; Johnston, J.W.; Schaffer, R.J.; et al. Delayed Response to Cold Stress Is Characterized by Successive Metabolic Shifts Culminating in Apple Fruit Peel Necrosis. BMC Plant Biol. 2017, 17, 77. [Google Scholar] [CrossRef]
- Jiao, J.; Jin, M.; Liu, H.; Suo, J.; Yin, X.; Zhu, Q.; Rao, J. Application of Melatonin in Kiwifruit (Actinidia Chinensis) Alleviated Chilling Injury during Cold Storage. Sci. Hortic. 2022, 296, 110876. [Google Scholar] [CrossRef]
- Galano, A.; Castaneda-Arriaga, R.; Perez-Gonzalez, A.; Tan, D.-X.; Reiter, R.J. Phenolic Melatonin-Related Compounds: Their Role as Chemical Protectors against Oxidative Stress. Molecules 2016, 21, 1442. [Google Scholar] [CrossRef]
- Haminiuk, C.W.I.; Maciel, G.M.; Plata-Oviedo, M.S.V.; Peralta, R.M. Phenolic Compounds in Fruits—An Overview. Int. J. Food Sci. Technol. 2012, 47, 2023–2044. [Google Scholar] [CrossRef]
- Khademi, O.; Ashtari, M.; Razavi, F. Effects of Salicylic Acid and Ultrasound Treatments on Chilling Injury Control and Quality Preservation in Banana Fruit during Cold Storage. Sci. Hortic. 2019, 249, 334–339. [Google Scholar] [CrossRef]
- Navina, B.; Keshav Huthaash, K.; Velmurugan, N.K.; Korumilli, T. Insights into Recent Innovations in Anti Browning Strategies for Fruit and Vegetable Preservation. Trends Food Sci. Technol. 2023, 139, 104128. [Google Scholar] [CrossRef]
- Kan, E.; Sargent, S.; Simonne, A.; Shaw, N.; Cantliffe, D. Changes in the Post-harvest Quality of Datil Hot Peppers as Affected by Storage Temperature. Proc. Fla. State Hort. Soc. 2007, 120, 246–250. [Google Scholar]
- Calvo-Brenes, P.; O’Hare, T. Effect of Freezing and Cool Storage on Carotenoid Content and Quality of Zeaxanthin-Biofortified and Standard Yellow Sweet-Corn (Zea Mays L.). J. Food Compos. Anal. 2020, 86, 103353. [Google Scholar] [CrossRef]
- Manafi, H.; Baninasab, B.; Gholami, M.; Talebi, M.; Khanizadeh, S. Exogenous Melatonin Alleviates Heat-Induced Oxidative Damage in Strawberry (Fragaria × Ananassa Duch. Cv. Ventana) Plant. J. Plant Growth Regul. 2022, 41, 52–64. [Google Scholar] [CrossRef]
- Aludatt, M.H.; Rababah, T.; Alhamad, M.N.; Al-Mahasneh, M.A.; Almajwal, A.; Gammoh, S.; Ereifej, K.; Johargy, A.; Alli, I. A Review of Phenolic Compounds in Oil-Bearing Plants: Distribution, Identification and Occurrence of Phenolic Compounds. Food Chem. 2017, 218, 99–106. [Google Scholar] [CrossRef]
- Ramirez-Bolanos, S.; Perez-Jimenez, J.; Diaz, S.; Robaina, L. A Potential of Banana Flower and Pseudo-Stem as Novel Ingredients Rich in Phenolic Compounds. Int. J. Food Sci. Technol. 2021, 56, 5601–5608. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, Y.; Yang, B.; Li, Y.; Liu, L.; Zhou, W.; Zheng, S.-J. Profiling of Phenolic Compounds of Fruit Peels of Different Ecotype Bananas Derived from Domestic and Imported Cultivars with Different Maturity. Horticulturae 2022, 8, 70. [Google Scholar] [CrossRef]
- Clifford, M.N.; Jaganath, I.B.; Ludwig, I.A.; Crozier, A. Chlorogenic Acids and the Acyl-Quinic Acids: Discovery, Biosynthesis, Bioavailability and Bioactivity. Nat. Prod. Rep. 2017, 34, 1391–1421. [Google Scholar] [CrossRef]
- Valanciene, E.; Malys, N. Advances in Production of Hydroxycinnamoyl-Quinic Acids: From Natural Sources to Biotechnology. Antioxidants 2022, 11, 2427. [Google Scholar] [CrossRef] [PubMed]
- Saravanan, K.; Aradhya, S.M. Polyphenols of Pseudostem of Different Banana Cultivars and Their Antioxidant Activities. J. Agric. Food Chem. 2011, 59, 3613–3623. [Google Scholar] [CrossRef]
- Siqueira, M.D.S.B.; Barbosa, N.C.; Cordenunsi, B.R.; Hassimotto, N.M.A.; de Resende, E.D. Chemical Changes in Prata-Ana Banana Stored under Reduced O2 and Increased CO2 Levels. Acta Sci. Technol. 2018, 40, 20219951313. [Google Scholar] [CrossRef]
- Ali, S.; Nawaz, A.; Naz, S.; Ejaz, S.; Maqbool, M.; Siddiqui, M.H.; Kalaji, H.M.; Wróbel, J.; Telesiński, A.; Auriga, A. Hydrogen Sulfide Mitigates Chilling Injury of Post-harvest Banana Fruits by Regulating γ-Aminobutyric Acid Shunt Pathway and Ascorbate–Glutathione Cycle. Front. Plant Sci. 2022, 13, 941246. [Google Scholar] [CrossRef]
- Ahammed, G.; Wu, M.; Wang, Y.; Yan, Y.; Mao, Q.; Ren, J.; Ma, R.; Liu, A.; Chen, S. Melatonin Alleviates Iron Stress by Improving Iron Homeostasis, Antioxidant Defense and Secondary Metabolism in Cucumber. Sci. Hortic. 2020, 265, 109205. [Google Scholar] [CrossRef]
- Li, S.; Xu, Y.; Bi, Y.; Zhang, B.; Shen, S.; Jiang, T.; Zheng, X. Melatonin Treatment Inhibits Gray Mold and Induces Disease Resistance in Cherry Tomato Fruit during Post-harvest. Postharvest Biol. Technol. 2019, 157, 110962. [Google Scholar] [CrossRef]
- Jahan, M.S.; Guo, S.; Baloch, A.R.; Sun, J.; Shu, S.; Wang, Y.; Ahammed, G.J.; Kabir, K.; Roy, R. Melatonin Alleviates Nickel Phytotoxicity by Improving Photosynthesis, Secondary Metabolism and Oxidative Stress Tolerance in Tomato Seedlings. Ecotoxicol. Env. Saf. 2020, 197, 110593. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Huber, D.J.; Hu, M.; Jiang, G.; Gao, Z.; Xu, X.; Jiang, Y.; Zhang, Z. Delay of Post-harvest Browning in Litchi Fruit by Melatonin via the Enhancing of Antioxidative Processes and Oxidation Repair. J. Agric. Food Chem. 2018, 66, 7475–7484. [Google Scholar] [CrossRef] [PubMed]
- Jayanna, S.K.; Umesha, S. Enhancement of the Expression of Defense Genes in Tomato against Ralstonia Solanacearum by N-Octanoyl-L-Homoserine Lactone. Afr. J. Microbiol. Res. 2017, 11, 194–203. [Google Scholar] [CrossRef]
- Kaur, S.; Samota, M.K.; Choudhary, M.; Choudhary, M.; Pandey, A.K.; Sharma, A.; Thakur, J. How Do Plants Defend Themselves against Pathogens-Biochemical Mechanisms and Genetic Interventions. Physiol. Mol. Biol. Plants 2022, 28, 485–504. [Google Scholar] [CrossRef]
- Hu, W.; Yang, H.; Tie, W.; Yan, Y.; Ding, Z.; Liu, Y.; Wu, C.; Wang, J.; Reiter, R.J.; Tan, D.-X.; et al. Natural Variation in Banana Varieties Highlights the Role of Melatonin in Post-harvest Ripening and Quality. J. Agric. Food Chem. 2017, 65, 9987–9994. [Google Scholar] [CrossRef] [PubMed]
- Aghdam, M.S.; Jannatizadeh, A.; Luo, Z.; Paliyath, G. Ensuring Sufficient Intracellular ATP Supplying and Friendly Extracellular ATP Signaling Attenuates Stresses, Delays Senescence and Maintains Quality in Horticultural Crops during Post-harvest Life. Trends Food Sci. Technol. 2018, 76, 67–81. [Google Scholar] [CrossRef]
- Wang, Y.; Reiter, R.J.; Chan, Z. Phytomelatonin: A Universal Abiotic Stress Regulator. J. Exp. Bot. 2018, 69, 963–974. [Google Scholar] [CrossRef] [PubMed]
- Bartwal, A.; Mall, R.; Lohani, P.; Guru, S.K.; Arora, S. Role of Secondary Metabolites and Brassinosteroids in Plant Defense Against Environmental Stresses. J. Plant Growth Regul. 2013, 32, 216–232. [Google Scholar] [CrossRef]
- Venkatachalam, K.; Charoenphun, N.; Lekjing, S.; Noonim, P. Investigation of Melatonin Incorporated CMC-Gelatin Based Edible Coating on the Alleviation of Chilling Injury Induced Pericarp Browning in Longkong. Foods 2024, 13, 72. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).