Abstract
Understanding the key roles of nutrient elements in soil–plant systems are essential for herbal medicine production and sustainable development. However, the ecological relationships between soil quality and nutrient elements, yield, saponins, or other active compounds in American ginseng remain unclear. In this study, 20 soil indicators, 10 root nutrient indicators, 9 quality indicators, and yields were investigated. The minimum dataset was constructed by principal component analysis, key factors were screened by correlation analysis and PLS-PM analysis, and the prediction model was constructed using linear fitting and tested by a validation test. The minimum dataset, constructed based on principal component analysis, comprised five indicators: SOM, TP, AK, AMg, and ACa. Correlation analysis, PLS-PM analysis, and linear fitting showed that K and Mg were the key factors relating soil quality to the yield and quality of American ginseng and that when AMg was 0.21 g/kg and AK was 0.30 g/kg, soil organic matter was 27%, total phosphorus was 1.19 g/kg in soil, K content in roots was 15.63 g/kg, Mg content was 1.91 g/kg, and the K/Mg of 8.85 could balance American ginseng yield and quality. In predicting and validating the model, predicting the DW, total ginsenoside, Rb1, Rb2, Rc, and Rd of American ginseng using K/Mg were reliable. This study provides a scientific basis for nutrient regulation, selecting planting sites, assessing soil quality, and predicting and evaluating American ginseng quality.
1. Introduction
American ginseng (Panax quifolium L.), the root of which is used in medicine, is a crucial natural remedy. Native to North America, it was successfully introduced to China in 1975 and planted in Jilin, Shandong, Shaanxi, and Sichuan Provinces [1]. Currently, China is the largest consumer of American ginseng and the third-largest producer worldwide [2]. Ginsenosides are the most well-known active ingredients of American ginseng and have various pharmacological activities, namely, improving immunity, preventing certain tumours, exerting anti-inflammatory effects, and delaying aging [3,4,5].
Soil quality is the ability of the soil to sustain the productivity of organisms in an ecosystem, maintain the quality of the environment, and promote the health of plants and animals. It is a comprehensive reflection of the physical, chemical, and biological properties of the soil, the quality of which is closely related to the nutritional and growth status of crops [6]. After the publication of the Land Capability Classification System by the Soil Conservation Service of the United States Department of Agriculture in 1961, several methods for assessing soil quality were established; among them, the soil quality index method can integrate the soil’s physical, chemical, and biological properties and has the advantages of, for example, flexibility in indicator selection and applicability to different soil types [7], and is widely used to assess soil spatial heterogeneity [8] and the impact of crop production on soil [9].
The soil quality index (SQI) is a combination of several soil physical, chemical, and biological properties into a complex index, and it is currently the most widely used method to assess soil quality [10]. SQIs are often used to assess changes in soil properties under different land use types or different agricultural management practices [11]. There are also studies assessing soil quality by measuring the quality of herbal medicines [12]. The first step in calculating the SQI is to create a minimum dataset (MDS) using low-cost, accurate, and easy-to-measure soil indicators as the total dataset [13]. Each indicator is then standardised using scoring functions (linear or non-linear) and integration methods (additive or weighted additive), which are usually built into the SQI. Principal component analysis (PCA), factor analysis (FA), discriminant analysis (DA), partial least squares regression (PLSR), and other multivariate techniques were used to select representative indicators from the total dataset (TDS) [14]. PCA and FA are the most commonly used methods to reduce the number of variables in soil quality assessment [14]. However, there is evidence that the use of PCA and FA may fail to include some important indicators in MDS [10]. This reduces the sensitivity to changes in soil quality and leads to inaccurate results. Multivariate analyses, DA, and PLSR perform better in eliminating data redundancy and covariance [11] and have now been applied to different management practices [11] and soil types [15] to categorise important soil variables with a more scientific approach to evaluation.
The material basis of herbal medicines is their active ingredients, and in the cultivation of herbal medicines, their growing areas are influenced by various ecological factors [16]. In a given environment, only certain active compounds can be synthesised and significantly increased [17]. Soil quality directly affects the nutrient content of roots, directly affecting the synthesis of primary and secondary plant metabolites [18]. Nutrients act as intermediates linking soil quality to crop yield and quality [19,20]. Therefore, studying the interactions among crop quality, yield, nutrient elements, and soil quality is essential [21]. Unfortunately, most studies evaluated soil quality and stopped after completing a correlation analysis on the effect of soil quality on quality [22,23], and a few studies have considered the relationship between soil quality, nutrient elements, yield, and herbal quality.
In the soil–crop system, plants absorb soil nutrients through the root system to ensure primary and secondary metabolism, and the nutrient elements in the roots relate soil quality to yield and quality. In view of this, it provides a reference for improving the quality of American ginseng, the soil quality evaluation system, and the precise nutrient regulation technology. In this study, by evaluating the soil in the root zone of American ginseng in four provinces, it was hypothesised that (1) SOM, AK and AMg are indicators of soil quality; (2) there are key factors to monitor the yield, quality, and soil quality of American ginseng, such as K and Mg in American ginseng roots; and (3) through the potassium and magnesium fertiliser efficiency test, it is assumed that potassium and magnesium fertiliser can improve the yield and quality of American ginseng.
2. Materials and Methods
2.1. Site Description and Experimental Design
2.1.1. Sampling Sites
The sampling sites were Jilin Province (JL), Shandong Province (SD), Shaanxi Province (SX), and Sichuan Province (SC); Sichuan Province is not the main production area, but it has been successfully introduced in recent years and has great potential for development. Nine sampling points were selected for each sampling site, and three-year-old American ginseng and rhizosphere soil were selected for collection (American ginseng is harvested for three years in the direct seeding field). Three sample plots (1 m × 1 m) were randomly selected from each sampling site and five healthy, disease-free, uniform plants, and 500 g of rhizosphere soil were selected from each sample plot and pooled as a composite sample. Composite soil samples were taken to soil depths of 0–20 cm, as close as possible to plants, resulting in 36 samples. Information regarding the sampling sites is provided in Table S1.
2.1.2. Validation Trial Design
Different K/Mg concentrations were constructed by controlling the exogenous application of potassium and magnesium. A field trial was set up at the experimental field of the Institute of Special Wild Economic Animal and Plant Sciences, Chinese Academy of Agricultural Sciences (125°25′8″ E, 43°46′31″ N) from 20 April to 30 September 2022 and used 3-year-old American ginseng as the test material. The background values for soil are given in Table S3. Urea (N ≥ 46.4%) was applied at a rate of 75 kg/ha, procalcium (P2O5 ≥ 16%) was applied at a rate of 400 kg/ha, potassium sulphate (K2O ≥ 54%) was applied at a rate of 0, 225, and 450 kg/ha, which were recorded as K0, K1, and K2; magnesium (MgO ≥ 16%) was applied at a rate of 0, 175, and 350 kg/ha, which were recorded as Mg0, Mg1, and Mg2; fertiliser application rates were calculated from the experimental predictions in Section 2.1.1. Urea, potassium sulphate, and magnesium sulphate were analytically pure reagents produced by the Beijing Chemical Industry Factory and calcium superphosphate by Tianjin Damao Chemical Reagent Factory. The experiment was conducted in a completely randomised block design with three replications and nine treatments (two-factor, three-level test), with a total of 27 experimental plots; the area of each experimental plot was 1.2 m × 3 m. At harvest, one square sample (1 m × 1 m) was randomly selected from each plot to collect five healthy, disease-free, uniformly growing plants and 500 g soil from the root zone, which were mixed to serve as a single mixed sample.
2.2. Sample Processing
After sampling, the root samples were washed with tap water and then with deionised water, air-dried in the laboratory, and crushed through a sieve (0.25 mm); next, the dry weight was recorded. All soil samples were homogenised by removing stones and plant roots, air-dried naturally in the laboratory, and then passed through 1 mm and 0.15 mm sieves.
2.3. Determination of Active Constituents of American Ginseng
The content of total ginsenoside (TS) in dried American ginseng roots was determined by the single point external standard method (Ginsenoside Re) in the Chinese Pharmacopeia, and the optical density was recorded at 544 nm [24].
High-performance liquid chromatography (model: H-Class, Waters, Milford, MA, USA) was used for determining the concentrations of Ginsenoside Rb1 (Rb1), Ginsenoside Rb2 (Rb2), Ginsenoside Rb3 (Rb3), Ginsenoside Rc (Rc), Ginsenoside Rd (Rd), Ginsenoside Re (Re), Ginsenoside Rg1 (Rg1), and Ginsenoside Rg2 (Rg2). A total of nine indices were determined [24]. Methanol extraction and high-performance liquid chromatography were used for determination. Preparation of the control solution: 10.00 mg of the 8 standards were weighed and the volume was fixed at 100 mL. The mother liquor of the standard solution was obtained by dissolution with methanol solution and then diluted with methanol to the gradient saponin mixed standard solution of 0.030–8.300 μg/L. Preparation of the test sample solution: 0.050 mg of American ginseng root powder was weighed through a 60 mesh sieve into a 50 mL volumetric flask and the volume was adjusted. The ultrasonic machine was operated at 25 °C, 250 W, and 40 kHz for 2 h. After ultrasonication, it was filtered through a 0.22 μm microporous filter membrane to obtain the test sample of American ginseng monosaponin. Chromatographic conditions: the chromatographic column was a Waters BEH C18 (1.7 μm, 2.1 × 100 mm); the column temperature was 45 °C; the mobile phases were A-acetonitrile and B-water at a flow rate of 0.5 mL/min; the injection volume was 3 μL; and the gradient elution mode was 0 to 4.5 min: 19% to 19%, 4.5 to 8.8 min: A19% to 28%, 8.8 to 17 min: A28%~37%; mass spectrometry conditions were ionisation mode: electrospray anion (ES-); capillary voltage: 2.80 kv; desolubilisation was nitrogen ≥95%; flow rate was 1000L/h; and temperature was 450 °C.
2.4. Plant Nutrient Elements and Soil Physicochemical Analysis
HNO3-HClO4 digestion: Weigh 0.1000 g of air-dried samples of American ginseng root/soil in 100 mL triangular flasks, add 10mL of concentrated nitric acid, place a small funnel at the top, place the triangular flasks on a heating plate at 80 °C for 30 min, and then gradually raise the temperature to 160 °C, and when the brown-red gas disappeared from the mouth of the flasks, add 2.5 mL of perchloric acid, and at this point raise the temperature to 180 °C and boil the liquid in the flasks until transparent. And cook until the liquid in the bottle was transparent; after cooling, it was fixed with distilled water into a 50 mL volumetric flask and then filtered into a 50 mL triangular flask.
Determination of nutrients in ginseng: carbon (RC), nitrogen (RN): elemental analyser (Vario EL III, Frankfurt, Germany); phosphorus (RP): concentrated nitric acid—perchloric acid—concentrated molybdenum antimony; antimony colourimetric method; potassium (RK): concentrated nitric acid—perchloric acid—elimination flame photometer method; calcium (RCa), magnesium (RMg), iron (RFe), manganese (RMn), copper (RCu), zinc (RZn): concentrated nitric acid—perchloric acid digestion—inductively coupled plasma spectrometer (ICP-OES) determination.
Soil physicochemical analyses: pH (pH): soil–water ratio of 1:2.5, determined by Mettler SK 220 pH meter; available nitrogen (AN): potassium chloride leaching, determined by AA-3 flow analyser (Nordrhein-Westfalen, Germany); available phosphorus (AP): sodium bicarbonate—molybdenum antimony colorimetric assay; available potassium (AK): ammonium acetate leaching-flame photometric assay; available calcium (ACa), available magnesium (AMg): ammonium acetate leaching, inductively coupled plasma spectrometry (ICP-OES) determination; available iron (AFe), available manganese (AMn), available copper (ACu), available zinc (AZn): DTPA leaching, inductively coupled plasma spectrometry (ICP-OES) determination; soil organic matter (SOM), total nitrogen (TN) (SOM), total nitrogen (TN), total phosphorus (TP), total potassium (TK), total calcium (TCa), total magnesium (TMg), total iron (TFe), total manganese (TMn), total copper (TCu), and total zinc (TZn) were determined in the same way as that of nutrient elements of American.
2.5. American Ginseng Quality Assessment Methods
The quality evaluation of the nine efficacy components of American ginseng was conducted using principal component analysis (PCA), and the principal components (PCs) were extracted based on the principle of eigenvalues > 1.0.
The comprehensive American ginseng quality index was the comprehensive index score of the quality of American ginseng (single sampling point), and the higher the score, the richer the active ingredients [12]. The calculation formula is as follows:
where is the number of indicators with an eigenvalue > 1, is the indicator weight of the th principal component, and is the composite score of each quality indicator of the th principal component. The indicator weights are the variance contributions of the PCs.
2.6. Soil Quality Assessment Methods
2.6.1. Determination of the Minimum Dataset (MDS)
The basis for establishing the MDS was PCA. The 20 candidate indicators used in this study comprehensively covered the major, intermediate, and trace elements required by plants. The soil indicators with the highest loadings on a particular PC were selected to be divided into a group. The Norm values of each indicator in each group were calculated separately, and the indicators with Norm values within 10% of the highest total score in each group were selected. The correlation coefficients between the selected indicators in each group were further analysed. The indicator with the highest Norm score was determined to be included in the MDS if |r| > 0.6. All of them are included in the MDS if |r| < 0.6 to obtain the final MDS [25]. MDS was performed to obtain the final MDS, where a higher value indicates a greater ability to interpret synthesised information. The formula is as follows:
where is the combined loading of the th variable on the first PCs with eigenvalues > 1, is the loading of the th variable on the th PCs, and is the eigenvalue of the th PCs.
2.6.2. Quantification of Soil Quality
The higher the soil quality composite index (SQI) score, the richer the soil nutrients [25]. The formula is as follows:
where is the number of indicators after screening, is the indicator weight and is the value of each soil indicator. is the ratio of the variance of the common factor of an indicator in the MDS to the total variance of the common factor.
The membership value was determined by the membership function to which the evaluation indicator belonged. Membership functions are generally divided into ascending and descending types.
The formula for the ascending membership function is
The formula for the descending membership function is
2.7. Calculating Prediction Accuracy
Prediction accuracy refers to the degree of agreement between the measured value and the true value under specific experimental conditions and is used to indicate the magnitude of the systematic error. The formula is as follows:
2.8. Data Analysis and Statistics
Data were statistically analysed using Microsoft Excel 2020 and IBM SPSS Statistics 22.0. Graphs were generated using Origin Pro 2019 and R Studio. The box plots compare nutrient indicators in American ginseng roots from different regions (n = 27). The lower part of the box represents the lower quartile (Q1), the horizontal line in the box is the median (Q2), the upper part of the box represents the upper quartile (Q3), and the height of the box represents the interquartile range (IQR = Q3 − Q1), with the lower limit = Q1 − 1.5 × IQR and the upper limit = Q3 + 1.5 × IQR. The scatter points on the right side of the box plot represent the raw data, and the long red line is the mean. The different letters indicate significant differences between groups at the p < 0.05 level in the analysis of significant differences.
3. Results
3.1. Screening of Key Factors
3.1.1. Nutrient Content of American Ginseng Roots
In this study, 10 nutrient indicators were determined in the roots of American ginseng from the four provinces (Figure 1), namely, essential major elements (C, N, P, K, Ca, and Mg) and trace elements (Fe, Mn, Cu, and Zn). The nutrient content varied among provinces: the RK content in JL was significantly higher than that of the other three provinces (p < 0.05); the RCa content in SD was significantly higher than that of the other three provinces (p < 0.05); the RP, RMn, RCu, and RZn contents in SX were significantly higher than that of the other three provinces (p < 0.05); the RC, RN, and RMg in SC were significantly higher than that of the other three provinces (p < 0.05).
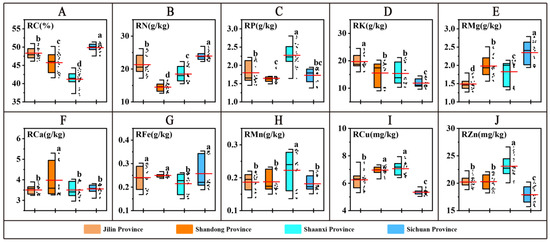
Figure 1.
Nutrient content of American ginseng roots. (A–J) denotes the comparison of nutrient element contents in the roots of American ginseng in the four provinces. The long red line is the mean. The different letters indicate significant differences between groups at the p < 0.05 level in the analysis of significant differences.
3.1.2. Active Ingredient Content and American Ginseng Quality Assessment
Active Ingredient Content of American Ginseng Roots
The nine ginsenosides in the roots of American ginseng were examined. The TS content was highest in JL but not significantly different from that of SC (p < 0.05); the content of Rb1 was highest in SD, which was not significantly different from that of JL and SC but was significantly higher than that of SX (p < 0). The Rb3, Rd, Re, and Rg1 contents of SX (p < 0.05) were significantly higher than that of the other provinces. SC had the highest Rb2, Rc, and Rg2 contents, but the Rb2 content was not significantly different from that of SD and SX; the Rc content was not significantly different from that of SD; and the Rg2 content was significantly higher than that of the other provinces (p < 0.05).
American Ginseng Quality Assessment
For the nine active ingredients, PCA was performed, and three principal components were extracted using the extraction principle of eigenvalues greater than 1.0. The contribution rates were PC1 33.06%, PC2 24.85%, and PC3 16.88%. The cumulative contribution rate of the first three principal factors was 74.78%, representing most of the information of the original data. The integrated quality index (AQI) values of American ginseng at different time points were calculated using equation (1) (Figure 2J). The results showed that SC (1.64) > SX (1.02) > SD (−0.35) > JL (−2.30) (p < 0.05).
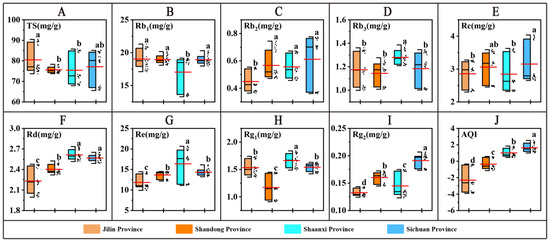
Figure 2.
Active ingredient content and AQI. (A–I) denotes the comparison of quality indicators of American ginseng roots in the four provinces. (J) denotes AQI. The long red line is the mean. The different letters indicate significant differences between groups at the p < 0.05 level in the analysis of significant differences.
3.1.3. Soil Quality Assessment
MDS Indicators
The content of each soil indicator is shown in Figure 3. PCA was performed on 20 soil indicators, and the principal components and loading matrices are shown in Table 1. The eigenvalues of the first four PCs were greater than 1.0, and the cumulative contribution rate was 88.854%. The Norm values were calculated using Equation (2) and grouped according to the method in Section 2.6.1 (Table 1). Further analysis of the correlation coefficients between the maximum Norm metrics and the remaining metrics in each group showed that |r| < 0.6 (−0.291) only for AK and TP in group 2 (Table S2); therefore, the final MDS was determined: SOM (Figure 3B), TP (Figure 3D), AK (Figure 3N), AMg (Figure 3O), and ACa (Figure 3P).
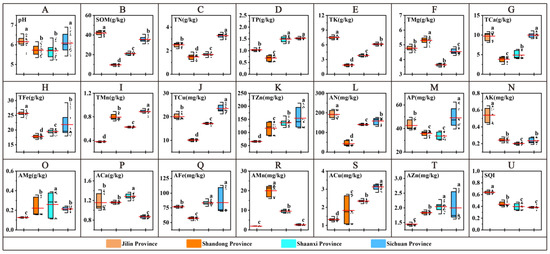
Figure 3.
Soil nutrient content and SQI. (A–T) are comparisons of soil quality indicators in the root zone of western ginseng in four provinces. (U) SQI.The long red line is the mean. The different letters indicate significant differences between groups at the p < 0.05 level in the analysis of significant differences.

Table 1.
Principal component analysis and normalised Norm value for soil factors.
The SOM content of the four provinces varied significantly (p < 0.05) from 7.84 to 44.71 g/kg, with the highest average SOM content of 41.98 g/kg in JL; the TP content of the four provinces varied from 0.52 to 1.65 g/kg, with the highest average TP content of 1.53 g/kg in SC, which was not significantly different from that of SX but significantly higher than that of SD and SC (p < 0.05); the variation range of the AK content in the four provinces was 0.18–0.65 g/kg, for which the average AK content in JL was the highest at 0.54 g/kg, significantly higher than that of the other provinces (p < 0.05), and there was no significant difference between SD and SC (p < 0.05); the variation range of the AMg content in the four provinces was 0.11~0.39 g/kg, for which the average AMg content in SX was the highest at 0.26 g/kg, which was significantly higher than that of the other provinces (p < 0.05), and there was no significant difference between that of SD and SC (p < 0.05); the variation in ACa content of the four provinces ranged from 0.82 to 1.42 g/kg, for which the average content in SX was the highest at 1.28 g/kg, which was significantly higher than that of the other provinces (p < 0.05), and there was no significant difference between that of JL and SD (p < 0.05).
Calculation of SQI
According to the method in Section 2.6.2, the weights of each soil index were calculated: 25.00% (SOM), 21.62% (TP), 21.78% (AK), 18.59% (AMg), and 13.01% (ACa). To determine the rise and fall of the affiliation function, we used yield (DW) and quality (AQI) as the productivity index of American ginseng. For determining the affiliation function, the yield (DW) and quality (AQI) of American ginseng were used as the productivity index (PI), PLS-PM analysis was conducted using the five indices in MDS, and the positive and negative effect coefficients were used to determine the type of affiliation functions. TP was observed to be a descending type of affiliation function (Figure S1). The affiliation values were determined by substituting the raw data into Equations (4) and (5). Based on Equation (3), the SQI values (Figure 3U) were calculated for each sampling point. The provincial rankings were as follows: JL (0.639), SD (0.437), SX (0.396), and SC (0.383) (p < 0.05).
Interaction of Soil Quality with Nutrient Elements, Yield and Quality of American Ginseng
To further investigate the interaction between soil quality and nutrient elements, dry weight, and the quality of American ginseng, this study performed a Pearson correlation analysis (Figure 4B) on the nutrient elements DW, AQI, and SQI in the roots of American ginseng. SQI was significantly and positively correlated with DW (p < 0.001) and significantly and negatively correlated with AQI (p < 0.001), indicating that soil quality has a negative effect on American ginseng quality but a positive effect on yield. AQI was significantly negatively correlated with DW (p < 0.001) (Figure 4B), indicating a contradiction between yield and quality in the production of American ginseng. Only the nutrient elements in roots, RK, and RMg showed a significant correlation with SQI, AQI, and DW; therefore, RK and RMg are the key factors connecting soil quality and yield and quality of American ginseng.
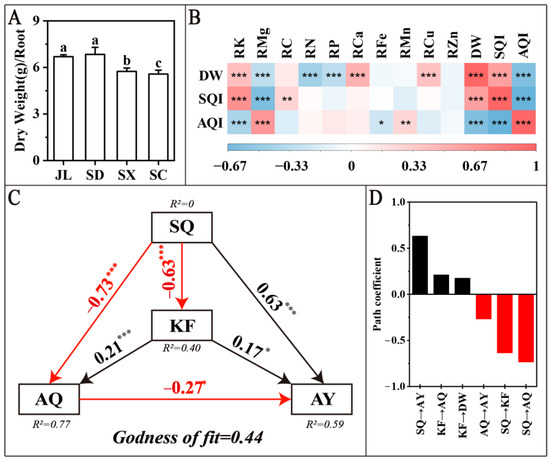
Figure 4.
Interaction of soil quality with nutrient elements, yield and quality of American ginseng. (A) differences in dry weight of American ginseng in the four provinces. (B) Pearson correlation analysis heat map of nutrient elements in roots with DW, SQI, AQI, black is positive, red is negative. (C) PLS-PM analysis among RK, RMg with SQ, AQ and DW, and numbers are path coefficients, SQ is five indicators in MDS (SOM, AK, AMg, TP, ACa), AQ is nine quality indicators (TS, Rb1, Rb1, Rb2, Rb3, Rc, Rd, Re, Rg1,Rg2), AY is yield (DW), KF is two key factors (RK, RMg), black is positive, red is negative. (D) PLS-PM path coefficients among RK, RMg with SQ, AQ and DW, black is positive, red is negative. * p < 0.05, ** p < 0.01, *** p < 0.001.
The PLS-PM analysis showed (Figure 4C,D) the following: The path coefficient of SQ on KF was −0.63 (p < 0.001), with a negative effect. The path coefficients of KF on AQ and AY were 0.21 (p < 0.001) and 0.17 (p < 0.05), respectively, with a positive effect. The path coefficients of SQ on AQ were −0.73 (p < 0.001), and those of SQ on AY were 0.63 (p < 0.001), and AQ on AY, with a path coefficient of −0.27 (p < 0.01). Because SQ had a significant negative effect on KF and KF had a significant positive effect on both AY and AQ, the simultaneous improvement in yield and quality of American ginseng might be achieved by optimising soil quality and regulating the content and ratio of K and Mg in the roots of American ginseng.
3.2. Creation of Predictive Models
K/Mg is an important parameter to evaluate the growth and development of phytomass. Because RK and RMg were negatively correlated (r = 0.54, p < 0.001) (Figure 5A) and RK/RMg was positively correlated (r = 0.89, p < 0.001) with RK (Supplementary Figure S2F) and negatively correlated (r = 0.83, p < 0.001) with RMg (Supplementary Figure S2G), all of which showed a good linear relationship and for the ease of calculation and to reduce repetitive discussion, RK/RMg was used for subsequent experiments.
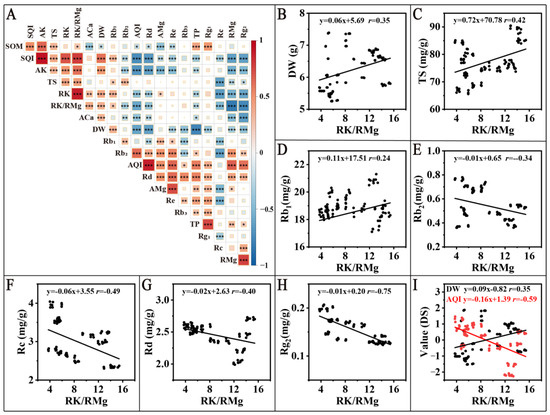
Figure 5.
Creation of predictive models. (A) pearson correlation analysis of MDS, yield, quality, RK, RMg and RK/RMg. (B–I) are the linear fits of the metrics significantly correlated with RK/RMg. * p < 0.05, ** p < 0.01, *** p < 0.001.
To further investigate the effect of RK/RMg on yield and quality and establish a predictive model, the indicators significantly correlated with yield and quality were linearly fitted with RK/RMg in combination with Figure 5A; the results are shown in Figure 6. The prediction model of RK/RMg (x) for yield (DW) (Figure 5B) was y(DW) = 0.06x + 5.69 (r = 0.35, p < 0.001); the prediction model for TG (Figure 5C) was y(TS) = 0.72x + 70.78 (r = 0.42, p < 0.001); the prediction model for Rb1 (Figure 5D): y(Rb1) = 0.11x + 17.51 (r = 0.24, p < 0.05); and the prediction model for Rb2 (Figure 5E): y(Rb2) = −0.01x + 0.65 (r = −0.34, p < 0.001); the prediction model for Rc (Figure 5F): y(Rc) = −0.06x + 3.55 (r = −0.49, p < 0.001); the prediction model for Rd (Figure 5G): y(Rd) = −0.02x + 2.63 (r = −0.40, p < 0.001); and the prediction model for Rg2 (Figure 5H): y(Rg2) = −0.01x + 0.20 (r = 0.75, p < 0.001). For calculating the optimum RK/RMg value to balance the yield and quality of American ginseng, the DW and AQI data were standardised and then linked (Figure 5I), which can be calculated as the RK/RMg of 8.85. The RK of 15.63 g/kg and the RMg of 1.91 g/kg were calculated as the optimum content in American ginseng root, with the data from Figure S2K,L.
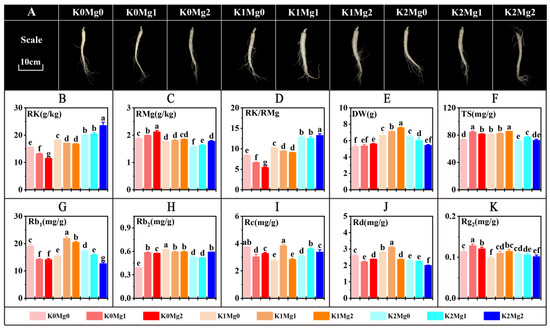
Figure 6.
Effects of exogenous potassium and magnesium on predictive indicators. (A) effects of exogenous potassium and magnesium on root phenotype of American ginseng. (B) potassium content. (C) magnesium content. (D) RK/RMg value. (E) dry weight. (F) total saponin content. (G) Rb1 content. (H) Rb2 content. (I) Rc content. (J) Rd content. (K) Rg2. n = 3; different letters indicate significant differences (least significant difference test, p < 0.05).
3.3. Evaluation of Predictive Models
Effects of Exogenous Potassium and Magnesium on Predictive Indicators
To verify the accuracy of the prediction model, this study regulated the RK and RMg contents in the roots by controlling the exogenous K and Mg doses in a field trial to achieve the effect of controlling the RK/RMg values. The predicted indices were examined after harvest. RK content increased with the increase in potassium application and decreased with the increase in magnesium application at K0 and K1, but increased with the increase in magnesium application at K2; RMg content increased with the increase in magnesium application and decreased with the increase in potassium application; RK/RMg increased with the increase in potassium application and decreased with the increase in magnesium application at K0 and K1; exogenous potassium and magnesium application controlled RK/RMg (Figure 6D); exogenous potassium and magnesium application affected the phenotype of western ginseng roots (Figure 6A).
Among the seven predictors, the DW of K1Mg2 was 7.56 g, which was significantly higher than that of the other treatments (p < 0.05) and K0Mg0 was 5.28 g, which was not significantly different from that of K0Mg1 and K2Mg2 but was lower than that of the other treatments (p < 0.05); the TG content of the K1Mg2 group was 84.47 mg/g, which was significantly higher than that of the other treatments (p < 0.05), and the content of K2Mg0 was 71.02 mg/g, which was not significantly different from K2Mg2 but was lower than that of the other treatments (p < 0.05); K1Mg1 had the highest Rb1 content, 21.83 mg/g, and K2Mg2 had the lowest at 12.6 mg/g, which was significantly different (p < 0.05); K1Mg0 had the highest Rb2 content at 0.62 mg/g, and K0Mg0 had the lowest at 0.39 mg/g, with significant differences (p < 0.05); the Rc content of K1Mg1 was 3.83 mg/g, which was not significantly different from that of K0Mg0 but was significantly higher than that of the other treatments (p < 0.05), and the content of K1Mg0 was 2.74 mg/g, which was significantly lower than that of the other treatments (p < 0.05); the Rd content of K1Mg1 was the highest at 3.10 mg/g, and the lowest content of K2Mg2 was 1.99 mg/g, which was significantly different (p < 0.05); the Rg2 content of K0Mg1 was 0.13 mg/g, which was significantly higher than that of the other treatments (p < 0.05), and the content of K1Mg0 was 0.10 mg/g, which was not significantly different from that of K2Mg2 but significantly lower than that of the other treatments (p < 0.05).
The RK/RMg ratio from the field trial was entered into the prediction model for the calculations. The descriptive statistics of the predicted values of the seven predictors are shown in Table 2. The predicted mean value of DW was 6.27 g, with a range between 5.99 and 6.50 g; the predicted mean value of TG content was 77.83 mg/g, with a range between 74.56 and 80.67 mg/g; the predicted mean value of Rb1 was 18.59 mg/g, with a range between 18.09 and 19.02 mg/g; the predicted mean value of Rb2 was 0.53 mg/g, with a range between 0.49 and 0.59 mg/g; the predicted mean value of Rc was 2.92 mg/g, with a range between 2.67 and 3.21 mg/g; the predicted mean value of Rd was 2.44 mg/g, with a range between 2.36 and 2.52 mg/g; and the predicted mean value of Rg2 was 0.15 mg/g, with a range between 0.13 and 0.18 mg/g. The coefficients of variation for all the predictors were less than 10%, indicating a low level of variability.

Table 2.
Descriptive statistics of forecasting values (n = 27).
The test and predicted values of the predictors were entered into Equation (6) to calculate the prediction accuracy. The descriptive statistics of the calculated results are shown in Table 3. The predictive accuracy of DW ranged from 77.85 to 99.87%; the predictive accuracy of TS ranged from 86.09 to 97.31%; the predictive accuracy of Rb1 ranged from 62.85 to 99.86%; the predictive accuracy of Rb2 ranged from 70.08 to 99.57% and from 64.87 to 99.52%; the predictive accuracy of Rd ranged from 71.56 to 97.76%; and the predictive accuracy of Rg1 ranged from 77.85 to 99.99%; the prediction accuracy of Rg2 ranged from 63.30 to 80.74%. The prediction accuracy rankings of the seven predictors were TS (92.17%) > Rd (89.48%) > Rb2 (89.17%) > DW (88.97%) > Rc (84.51%) > Rb1 (83.17%) > Rg2 (72.75%), of which Rb1, Rb2, and Rc belonged to the medium variance (10% < CV ≤ 30%) and the remaining indicators belonged to the low variance (CV ≤ 10%).

Table 3.
Prediction accuracy (%) (n = 27).
4. Discussion
Soil is the basis for food production, and soil quality is directly related to human survival. Soil quality is an essential property of soil, which is the ability of the soil to provide nutrients to plants. Soil quality is declining owing to the influence of human factors; therefore, an accurate understanding of soil quality and its scientific and objective evaluation would guarantee an accurate understanding of the nature of the soil and improve the use of soil resources [26,27]. In the cultivation process of medicinal plants, soil quality plays a crucial role in their yield and quality [28]. In this study, the quality of American ginseng was used as the basis for evaluating soil quality, and the saponin content varied significantly between provinces (Figure 2). The main reason for this difference was that in addition to natural factors, such as soil formation, climate, and topography, the four provinces provided different nutrient supplies for the growth and development of American ginseng, affecting the accumulation of its active ingredients [29]. This phenomenon may be due to differences in the type and amount of fertiliser applied, which directly affects the efficiency of nutrient uptake and utilisation by the plant.
Many factors affect the quality of American ginseng. Soil quality is one of those crucial factors [30]. According to the literature, using MDS combined with an affiliation function to evaluate soil quality is widely accepted [31]. The indices this study used to determine MDS were common soil indices proposed by Govaerts and Rezaei and others and the full amount of various trace elements [32], such as Cu, Mn, and Zn. Their quick-acting state contents were selected as evaluation indicators, which explains the spatial heterogeneity of certain American ginseng root zone soils. The established MDS comprised five soil factors (SOM, TP, AK, ACa, and AMg). Therefore, these five indicators can be used as key indicators to assess soil quality. Among them, SOM, AK, ACa and AMg can be regulated by fertiliser application, while differences in TP may be related to the composition of the soil-forming parent material in each province. Among these indicators, SOM is widely used as an indicator of soil quality due to its role in a number of soil functions, including stability of soil structure and nutrient availability, cycling, and the main sources of carbon and nitrogen for microorganisms [33,34]. SOM was also examined in this study and was significantly and positively correlated with SQI (Figure S2G). The SQI was obtained from the composite quality index equation, which is a strong soil characteristic because several carefully selected soil indicators in the MDS provide sufficient information for soil quality assessment [35]. In general, spatial heterogeneity in soil quality is caused by multiple environmental factors, each of which does not contribute equally to soil quality [36]. Among the four provinces, JL had the highest SQI with a mean of 0.769, indicating a high level of soil quality, followed by SD, SX, and SC.
There are large differences in the quality of American ginseng among provinces. This phenomenon represents the substantial potential for quality improvement because, for example, the yield and quality of traditional Chinese medicines are closely related to soil nutrients [37]. In our study, the ranking of the AQI was SC (1.64) > SX (1.02) > SD (−0.35) > JL (−2.30) (p < 0.05), which was opposite to the ranking of the SQI, and the two were significantly negatively correlated (p < 0.001), suggesting a contradiction between the quality and soil quality of American ginseng; the AQI was significantly negatively correlated with DW, indicating that the yield and quality of American ginseng are inconsistent. However, there was a significant positive correlation between SQI and DW (p < 0.001), indicating that soil quality has a certain promoting effect on American ginseng yield.
The active ingredients in traditional Chinese medicine are usually secondary metabolites, which are phytoprotectants for plants [38]. Under environmental stress, plants inhibit the growth of other plants by releasing secondary metabolites to improve their competitive abilities. Harsh environments may stimulate the accumulation of secondary metabolites in plants, which is conducive to the authenticity of Chinese herbal medicines [39]. Nutrients absorbed by plants from the soil directly affect primary and secondary metabolism; therefore, nutrients act as mediators, linking soil quality to crop yield and quality [18]. In addition, nutrient elements can, to a certain extent, be used as important indicators for evaluating the pharmacological efficacy of medicinal plants. Thus, screening for key factors that determine the yield and quality of medicinal plants is crucial for the development of Chinese medicinal herbs. For example, Sun et al. reported that SOM and Ca are key factors affecting the yield and quality of Astragalus mongolica [12]. Therefore, soil SOM and Ca were used as important factors in the cultivation and management of Astragalus mongolica. This study observed that RK and RMg, among the nutrient indices, were significantly correlated (p < 0.001) with SQI, AQI, and DW (Figure 4B), indicating that in American ginseng, potassium and magnesium can be used as key factors to measure the quality of soil and to determine American Ginseng yield and quality. PLS-PM analysis (Figure 4C) showed that RK and RMg, as key factors, had positive effects on AQ (p < 0.01) and DW (p < 0.05). Therefore, the positive increase in yield and quality of American ginseng can be achieved by adjusting the content and proportion of RK and RMg in the roots of American ginseng. SQ had a negative effect on KF. Therefore, SQ can be increased by reducing the soil quality of KF. Amg, AK, SOM, and TP in MDS were significantly correlated with SQI (p < 0.001). The reduction in the soil quality and, therefore, the increase in KF could be achieved by increasing P and Mg in fertilisers and decreasing K and organic fertilisers, balancing American Ginseng yield and quality.
Potassium and magnesium are essential elements for plant growth and development. Potassium, the only monovalent metal ion and the most abundant metal element in plants, plays a crucial role in regulating water metabolism, enzyme activation, energy metabolism, substance transport, and other physiological and biochemical processes [40,41]. Magnesium is one of the most abundant cations in plant cells, and optimal plant growth typically requires 1.5–3.5 g/kg [42]. Magnesium is essential for several physiological and biochemical processes in plants, including photosynthesis, carbohydrate partitioning, and protein synthesis, affecting plant growth, development, and quality formation. Mg activates more than 300 enzymes and acts as the central binding ion for chlorophyll molecules [43]. In addition, Mg is involved in the regulation of the cellular anion and cation balance and the co-regulation of cellular osmotic pressure with potassium [44]. The interaction of potassium and magnesium in plants is a research hotspot in the field of plant nutrition [45]. In this study, potassium and magnesium in Panax quinquefolium roots were antagonistic (Figure S2A), which may be because root surface transporter proteins can simultaneously transport K+ and Mg2+ [46]. In addition, the non-selective ion channel is an exclusive osmotic channel for Mg2+ and permeable to K+; thus, competition for the active site or transporter may partially explain the antagonistic effects of K+ and Mg2+ in the root system. Increasing K/Mg increased American ginseng yield but decreased quality (Figure 5I), which may be because potassium ions can activate the activity of starch synthase in many plant species and promote the synthesis of sucrose and starch from monosaccharides. For example, as the potassium content of the plant increases, the activity of the soluble starch synthase starch branching enzyme increases [47]. Magnesium directly mediates the secondary metabolism process of the acetyl coenzyme A pathway and mangiferolic acid pathway [48]. Because acetyl coenzyme A is the precursor of ginsenoside synthesis, the increase in magnesium will increase it, leading to the increase in ginsenoside accumulation to achieve the improvement in the quality of American ginseng. In this study, when RK was 15.63 g/kg, RMg was 1.91 g/kg, and RK/RMg was 8.85 (Figure 5gI), yield and quality could be balanced to optimise productivity. So, potassium and magnesium can be used as key factors to monitor the yield and quality of American ginseng, which would improve the prediction and evaluation of the yield and quality of American ginseng.
Insufficient or unbalanced fertilisation of potassium and magnesium is common in agricultural production, resulting in an unbalanced distribution of potassium and magnesium in plants, limiting plant growth, and nutrient quality of the edible part of the plant; an appropriate ratio of potassium and magnesium promotes plant growth and nutrient uptake and improving crop yield and quality [49]. In this study, yield and quality were balanced when RK/RMg was 8.85, at which time SQI was 0.46 (Figure S2I). Moreover, as shown in Figure S2E–H, American ginseng yield and quality were balanced when the AMg in the soil was 0.21 g/kg, AK was 0.30 g/kg, SOM was 27%, and TP was 1.19 g/kg. Thus, this study provides a reference for nutrient regulation in the production process of American ginseng and is of substantial importance for planting site selection.
Clarifying the key factors that regulate the synthesis and accumulation of active ingredients in T and CM is of substantial importance for the actual production and cultivation of T and CM [50]. With the development of complementary medicine (T and CM), studies on monomeric agents and their medicinal mechanisms are increasing. In this study, linear correlation analysis was performed as the basis for prediction by using the monomer saponins, which is significantly correlated with K/Mg. Using K/Mg for prediction reduces the amount of arithmetic and increases predictive ability. For example, Mg and Rb1 and Rc, and K and Rb2 had no significant correlation, but K/Mg and Rb1 (p < 0.05), Rc (p < 0.001), and Rb2 (p < 0.001) were significantly correlated (Figure 5A). In the field experiment, to regulate K/Mg in roots, we used the prediction model to predict the yield and quality of American ginseng and evaluated the prediction results. The evaluation showed that the average prediction accuracy of the prediction indexes, except Rg2, was more than 80%, indicating that the predicted values were close to the measured values, which further proved the accuracy of our prediction model. The Rg2 content was low, and the variation in Rc and Rb2 was higher than that of Rb1, Rc, and Rb2. The Rg2 content in Panax quinquefolium was low, the range of variation was small, and the fitting effect was good (p < 0.001, r = 0.75). The sources of the seedlings and planting plots used in the validation test were the same, and the climatic conditions in JL may not have been suitable for the accumulation of Rg2 (Figure 2I), resulting in a low prediction accuracy for Rg2. The prediction model was evaluated with the aim of predicting the active ingredient content of American ginseng by detecting the potassium and magnesium content in the roots of American ginseng, which could simplify the tedious step of quality testing.
This study has limitations that highlight topics for further research. Regarding our evaluation method, a large amount of experimental data is necessary to test it in further research because of the small sample size of this study, the differences in field management in different regions, the insufficient baseline data of the literature, and the relatively limited selection of indicators. In addition, we examined a limited set of key factors; thus, other key factors affecting the yield, quality, and soil quality of American ginseng should be investigated. Finally, data from different reproductive periods of American ginseng should be compiled to improve the generalisability of the prediction model.
5. Conclusions
This study showed that the MDS based on PC principal analysis comprised five indicators: SOM, TP, AK, AMg, and ACa. The correlation analysis, PLS-PM analysis, and linear fitting showed that K and Mg can be used as key factors to measure soil quality as key factors in determining the yield and quality of American ginseng. Despite a contradiction between the yield and quality of American ginseng, productivity can be optimised by adjusting the content and ratio of K/Mg in the roots, and the yield and quality of American ginseng can be simultaneously considered when the K content of the roots is 15.63 g/kg and the Mg content is 1.91 g/kg. The prediction and validation of the model showed that the prediction of DW, TG, Rb1, Rb2, Rc, and Rd in American ginseng using K/Mg was reliable, with an average prediction accuracy of more than 80%. This study provides a scientific basis for nutrient regulation, planting site selection, soil quality assessment, quality prediction, and evaluation of American ginseng.
This study aimed to predict the quality of American ginseng by evaluating the content of K and Mg in its roots in order to simplify the cumbersome steps of quality testing and to provide a scientific basis for nutrient regulation, planting site selection, and soil quality evaluation of American ginseng; it is proposed that the soil evaluation method based on yield and quality can also be applied to other medicinal plants. However, as an evaluation method, a large amount of experimental data is needed to be tested in the future, considering the small sample size of the study in this paper and the differences in field management in different regions. In addition, other key factors that more sensitively influence the yield, quality, and soil quality of American ginseng should be investigated to improve the generalisability of the prediction model.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/horticulturae10040344/s1, Figure S1: Determine the type of each indicator in MDS; Figure S2: Linear fitting curve; Table S1: The sampling information; Table S2: Soil background values for validation trial; Table S3: Correlation coefficients between maximum Norm and other indicators in each group.
Author Contributions
J.Q.: Conceptualization, Methodology, Investigation, Formal Analysis, Writing—original draft. H.S.: Investigation, Writing—review and editing. C.S.: Investigation, Writing—review and editing. H.L.: Investigation, Writing—review and editing. W.C.: Investigation, Writing—review and editing. B.L.: Investigation, Writing—review and editing. Y.Z.: Investigation, Writing—review and editing, Project administration, Supervision, Funding acquisition. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the National Key Research and Development Program of China (2021YFD1600902) and the China Agriculture Research System of MOF and MARA (CARS-21) and the Agricultural Science and Technology Innovation Program of the Chinese Academy of Agricultural Sciences (CAAS-XTCX20190025-6).
Data Availability Statement
Data are contained within the article and Supplementary Material.
Conflicts of Interest
The authors declare that they have no competing interests.
References
- Zhang, Z.; Sun, H.; Shao, C.; Lei, H.; Qian, J.; Ruan, Y.; Zhang, Y. Calcium Affects Growth and Physiological Indices of Panax quinquefolium L. HortScience 2022, 57, 112–117. [Google Scholar] [CrossRef]
- Yang, L.; Hou, A.; Zhang, J.; Wang, S.; Man, W.; Yu, H.; Zheng, S.; Wang, X.; Liu, S.; Jiang, H. Panacis Quinquefolii Radix: A Review of the Botany, Phytochemistry, Quality Control, Pharmacology, Toxicology and Industrial Applications Research Progress. Front. Pharmacol. 2020, 11, 602092. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, R.; Bryant, D.L.; Farone, A.L. Panax quinquefolius (North American Ginseng) Polysaccharides as Immunomodulators: Current Research Status and Future Directions. Molecules 2020, 25, 5854. [Google Scholar] [CrossRef]
- Shan, M.; Bai, Y.; Fang, X.; Lan, X.; Zhang, Y.; Cao, Y.; Zhu, D.; Luo, H. American Ginseng for the Treatment of Alzheimer’s Disease: A Review. Molecules 2023, 28, 5716. [Google Scholar] [CrossRef] [PubMed]
- Morshed, M.N.; Akter, R.; Karim, M.R.; Iqbal, S.; Kang, S.C.; Yang, D.C. Bioconversion, Pharmacokinetics, and Therapeutic Mechanisms of Ginsenoside Compound K and Its Analogues for Treating Metabolic Diseases. Curr. Issues Mol. Biol. 2024, 46, 2320–2342. [Google Scholar] [CrossRef] [PubMed]
- Song, M.; Li, J.; Gao, L.; Tian, Y. Comprehensive Evaluation of Effects of Various Carbon-Rich Amendments on Overall Soil Quality and Crop Productivity in Degraded Soils. Geoderma 2023, 436, 116529. [Google Scholar] [CrossRef]
- Biswas, S.; Hazra, G.C.; Purakayastha, T.J.; Saha, N.; Mitran, T.; Singha Roy, S.; Basak, N.; Mandal, B. Establishment of Critical Limits of Indicators and Indices of Soil Quality in Rice-Rice Cropping Systems under Different Soil Orders. Geoderma 2017, 292, 34–48. [Google Scholar] [CrossRef]
- Zhou, Y.; Ma, H.; Xie, Y.; Jia, X.; Su, T.; Li, J.; Shen, Y. Assessment of Soil Quality Indexes for Different Land Use Types in Typical Steppe in the Loess Hilly Area, China. Ecol. Indic. 2020, 118, 106743. [Google Scholar] [CrossRef]
- Juhos, K.; Szabó, S.; Ladányi, M. Explore the Influence of Soil Quality on Crop Yield Using Statistically-Derived Pedological Indicators. Ecol. Indic. 2016, 63, 366–373. [Google Scholar] [CrossRef]
- Yu, P.; Liu, S.; Zhang, L.; Li, Q.; Zhou, D. Selecting the Minimum Data Set and Quantitative Soil Quality Indexing of Alkaline Soils under Different Land Uses in Northeastern China. Sci. Total Environ. 2018, 616–617, 564–571. [Google Scholar] [CrossRef]
- Hamidi Nehrani, S.; Askari, M.S.; Saadat, S.; Delavar, M.A.; Taheri, M.; Holden, N.M. Quantification of Soil Quality under Semi-Arid Agriculture in the Northwest of Iran. Ecol. Indic. 2020, 108, 105770. [Google Scholar] [CrossRef]
- Sun, H.; Jin, Q.; Wang, Q.; Shao, C.; Zhang, L.; Guan, Y.; Tian, H.; Li, M.; Zhang, Y. Effects of Soil Quality on Effective Ingredients of Astragalus Mongholicus from the Main Cultivation Regions in China. Ecol. Indic. 2020, 114, 106296. [Google Scholar] [CrossRef]
- Andrews, S.S.; Karlen, D.L.; Cambardella, C.A. The Soil Management Assessment Framework: A Quantitative Soil Quality Evaluation Method. Soil Sci. Soc. Am. J. 2004, 68, 1945–1962. [Google Scholar] [CrossRef]
- Li, K.; Wang, C. Multiple Soil Quality Assessment Methods for Evaluating Effects of Organic Fertilization in Wheat-Maize Rotation System. Eur. J. Agron. 2023, 150, 126929. [Google Scholar] [CrossRef]
- Liu, N.; Shao, C.; Sun, H.; Liu, Z.; Guan, Y.; Wu, L.; Zhang, L.; Pan, X.; Zhang, Z.; Zhang, Y.; et al. Arbuscular Mycorrhizal Fungi Biofertilizer Improves American Ginseng (Panax quinquefolius L.) Growth under the Continuous Cropping Regime. Geoderma 2020, 363, 114155. [Google Scholar] [CrossRef]
- Falcone Ferreyra, M.L.; Rius, S.P.; Casati, P. Flavonoids: Biosynthesis, Biological Functions, and Biotechnological Applications. Front. Plant Sci. 2012, 3, 34352. [Google Scholar] [CrossRef] [PubMed]
- Kliebenstein, D.J.; Osbourn, A. Making New Molecules—Evolution of Pathways for Novel Metabolites in Plants. Curr. Opin. Plant Biol. 2012, 15, 415–423. [Google Scholar] [CrossRef] [PubMed]
- Francis, B.; Aravindakumar, C.T.; Brewer, P.B.; Simon, S. Plant Nutrient Stress Adaptation: A Prospect for Fertilizer Limited Agriculture. Environ. Exp. Bot. 2023, 213, 105431. [Google Scholar] [CrossRef]
- Ahmed, N.; Zhang, B.; Chachar, Z.; Li, J.; Xiao, G.; Wang, Q.; Hayat, F.; Deng, L.; Narejo, M.-N.; Bozdar, B.; et al. Micronutrients and Their Effects on Horticultural Crop Quality, Productivity and Sustainability. Sci. Hortic. 2024, 323, 112512. [Google Scholar] [CrossRef]
- Rani, M.; Kaushik, P.; Bhayana, S.; Kapoor, S. Impact of Organic Farming on Soil Health and Nutritional Quality of Crops. J. Saudi Soc. Agric. Sci. 2023, 22, 560–569. [Google Scholar] [CrossRef]
- Ng, C.W.W.; So, P.S.; Coo, J.L.; Lau, S.Y.; Wong, J.T.F. Interactions between Nutrient Types and Soil Hydrological Properties on Yield and Quality of Pinellia Ternata, a Medicinal Plant. Ind. Crops Prod. 2023, 195, 116423. [Google Scholar] [CrossRef]
- Vasu, D.; Singh, S.K.; Ray, S.K.; Duraisami, V.P.; Tiwary, P.; Chandran, P.; Nimkar, A.M.; Anantwar, S.G. Soil Quality Index (SQI) as a Tool to Evaluate Crop Productivity in Semi-Arid Deccan Plateau, India. Geoderma 2016, 282, 70–79. [Google Scholar] [CrossRef]
- Zuber, S.M.; Behnke, G.D.; Nafziger, E.D.; Villamil, M.B. Multivariate Assessment of Soil Quality Indicators for Crop Rotation and Tillage in Illinois. Soil Tillage Res. 2017, 174, 147–155. [Google Scholar] [CrossRef]
- Lei, H.; Zhang, H.; Zhang, Z.; Sun, H.; Li, M.; Shao, C.; Liang, H.; Wu, H.; Zhang, Y. Physiological and Transcriptomic Analyses of Roots from Panax Ginseng C. A. Meyer under Drought Stress. Ind. Crops Prod. 2023, 191, 115858. [Google Scholar] [CrossRef]
- Rezaei, S.A.; Gilkes, R.J.; Andrews, S.S. A Minimum Data Set for Assessing Soil Quality in Rangelands. Geoderma 2006, 136, 229–234. [Google Scholar] [CrossRef]
- Karlen, D.L.; Ditzler, C.A.; Andrews, S.S. Soil Quality: Why and How? Geoderma 2003, 114, 145–156. [Google Scholar] [CrossRef]
- Zhang, Z.; Ai, N.; Liu, G.; Liu, C.; Qiang, F. Soil Quality Evaluation of Various Microtopography Types at Different Restoration Modes in the Loess Area of Northern Shaanxi. CATENA 2021, 207, 105633. [Google Scholar] [CrossRef]
- Ražić, S.S.; Đogo, S.M.; Slavković, L.J. Multivariate Characterization of Herbal Drugs and Rhizosphere Soil Samples According to Their Metallic Content. Microchem. J. 2006, 84, 93–101. [Google Scholar] [CrossRef]
- Shuai, M.; Yang, Y.; Bai, F.; Cao, L.; Hou, R.; Peng, C.; Cai, H. Geographical Origin of American Ginseng (Panax quinquefolius L.) Based on Chemical Composition Combined with Chemometric. J. Chromatogr. A 2022, 1676, 463284. [Google Scholar] [CrossRef]
- Goodwin, P.H.; Proctor, E. Review: Molecular Techniques to Assess Genetic Variation within and between Panax Ginseng and Panax quinquefolius. Fitoterapia 2019, 138, 104343. [Google Scholar] [CrossRef] [PubMed]
- Jin, H.; Zhong, Y.; Shi, D.; Li, J.; Lou, Y.; Li, Y.; Li, J. Quantifying the Impact of Tillage Measures on the Cultivated-Layer Soil Quality in the Red Soil Hilly Region: Establishing the Thresholds of the Minimum Data Set. Ecol. Indic. 2021, 130, 108013. [Google Scholar] [CrossRef]
- Govaerts, B.; Sayre, K.D.; Deckers, J. A Minimum Data Set for Soil Quality Assessment of Wheat and Maize Cropping in the Highlands of Mexico. Soil Tillage Res. 2006, 87, 163–174. [Google Scholar] [CrossRef]
- Tenic, E.; Ghogare, R.; Dhingra, A. Biochar—A Panacea for Agriculture or Just Carbon? Horticulturae 2020, 6, 37. [Google Scholar] [CrossRef]
- Abakumov, E.; Petrov, A.; Polyakov, V.; Nizamutdinov, T. Soil Organic Matter in Urban Areas of the Russian Arctic: A Review. Atmosphere 2023, 14, 997. [Google Scholar] [CrossRef]
- Samaei, F.; Emami, H.; Lakzian, A. Assessing Soil Quality of Pasture and Agriculture Land Uses in Shandiz County, Northwestern Iran. Ecol. Indic. 2022, 139, 108974. [Google Scholar] [CrossRef]
- Li, P.; Zhang, T.; Wang, X.; Yu, D. Development of Biological Soil Quality Indicator System for Subtropical China. Soil Tillage Res. 2013, 126, 112–118. [Google Scholar] [CrossRef]
- Thakur, M.; Kumar, R. Mulching: Boosting Crop Productivity and Improving Soil Environment in Herbal Plants. J. Appl. Res. Med. Aromat. Plants 2021, 20, 100287. [Google Scholar] [CrossRef]
- Guo, C.; Wang, X.; Wang, Q.; Zhao, Z.; Xie, B.; Xu, L.; Zhang, R. Plant Defense Mechanisms against Ozone Stress: Insights from Secondary Metabolism. Environ. Exp. Bot. 2024, 217, 105553. [Google Scholar] [CrossRef]
- Kroymann, J. Natural Diversity and Adaptation in Plant Secondary Metabolism. Curr. Opin. Plant Biol. 2011, 14, 246–251. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Jiang, Q.; Zong, J.; Guo, H.; Liu, J.; Chen, J. Effects of Supplemental Potassium on the Growth, Photosynthetic Characteristics, and Ion Content of Zoysia Matrella under Salt Stress. Horticulturae 2023, 10, 31. [Google Scholar] [CrossRef]
- Wang, Y.; Wu, W.-H. Genetic Approaches for Improvement of the Crop Potassium Acquisition and Utilization Efficiency. Curr. Opin. Plant Biol. 2015, 25, 46–52. [Google Scholar] [CrossRef] [PubMed]
- Shaul, O. Magnesium Transport and Function in Plants: The Tip of the Iceberg. Biometals 2002, 15, 307–321. [Google Scholar] [CrossRef] [PubMed]
- Guo, W.; Nazim, H.; Liang, Z.; Yang, D. Magnesium Deficiency in Plants: An Urgent Problem. Crop J. 2016, 4, 83–91. [Google Scholar] [CrossRef]
- Xie, K.; Cakmak, I.; Wang, S.; Zhang, F.; Guo, S. Synergistic and Antagonistic Interactions between Potassium and Magnesium in Higher Plants. Crop J. 2021, 9, 249–256. [Google Scholar] [CrossRef]
- Cakmak, I.; Hengeler, C.; Marschner, H. Changes in Phloem Export of Sucrose in Leaves in Response to Phosphorus, Potassium and Magnesium Deficiency in Bean Plants. J. Exp. Bot. 1994, 45, 1251–1257. [Google Scholar] [CrossRef]
- Tanoi, K.; Kobayashi, N. Leaf Senescence by Magnesium Deficiency. Plants 2015, 4, 756–772. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Liu, X.; Wang, Q.; Zhang, H.; Li, M.; Song, B.; Zhao, Z. Effects of Potassium Fertilization on Potato Starch Physicochemical Properties. Int. J. Biol. Macromol. 2018, 117, 467–472. [Google Scholar] [CrossRef] [PubMed]
- Oliver, D.J.; Nikolau, B.J.; Wurtele, E.S. Acetyl-CoA—Life at the Metabolic Nexus. Plant Sci. 2009, 176, 597–601. [Google Scholar] [CrossRef]
- Gransee, A.; Führs, H. Magnesium Mobility in Soils as a Challenge for Soil and Plant Analysis, Magnesium Fertilization and Root Uptake under Adverse Growth Conditions. Plant Soil 2013, 368, 5–21. [Google Scholar] [CrossRef]
- Han, X.; Yin, M.; Fang, Q.; Tan, X.; Sun, H.; Cheng, M.; Peng, H.; Huang, L. Nutritional Ingredients and Functional Components of Cultivated and Wild-Simulated Astragali Radix Using Widely Targeted Metabolomics. LWT 2023, 185, 115186. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).