Selenium Seed Priming and Biostimulation Influence the Seed Germination and Seedling Morphology of Jalapeño (Capsicum annuum L.)
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material and Experimental Site
2.2. Treatments and Experimental Design
2.3. Seed Priming
2.4. Germination
2.5. Measurements
2.6. Statistical Analysis
3. Results
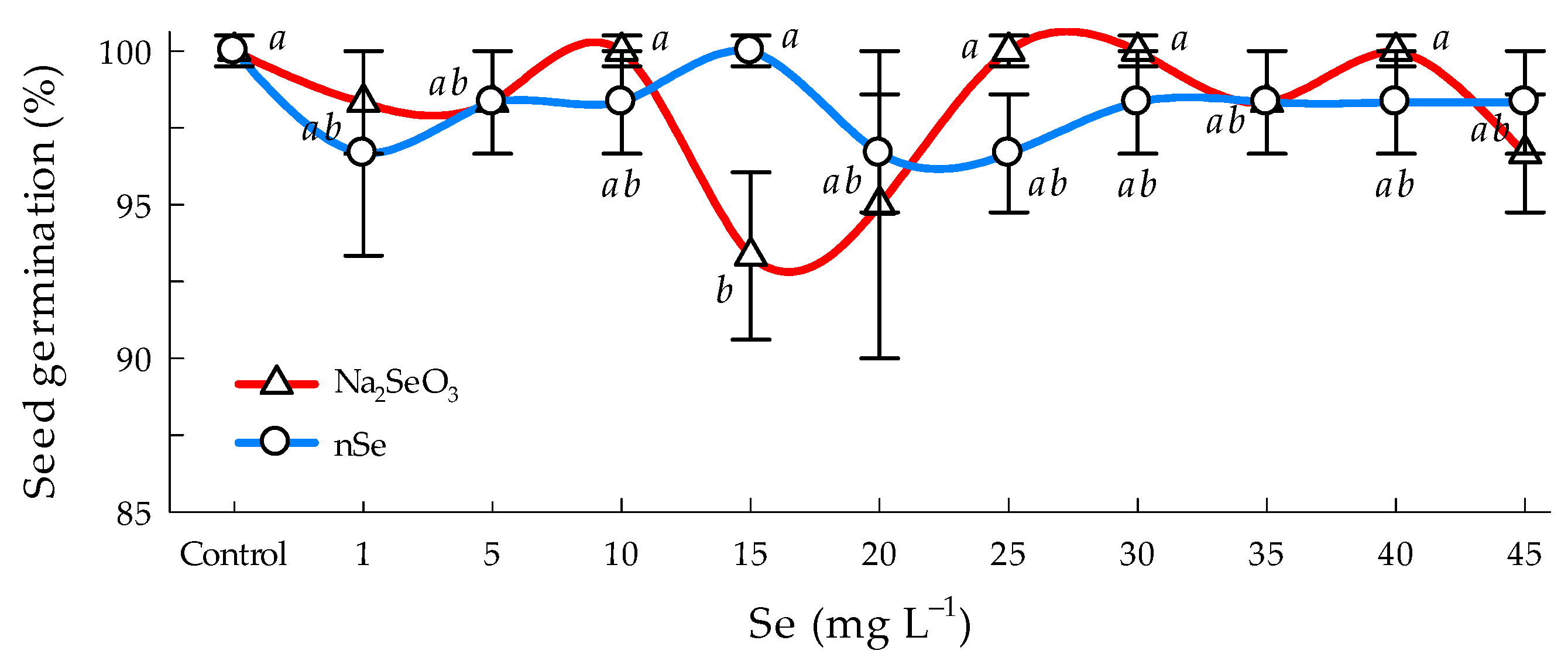
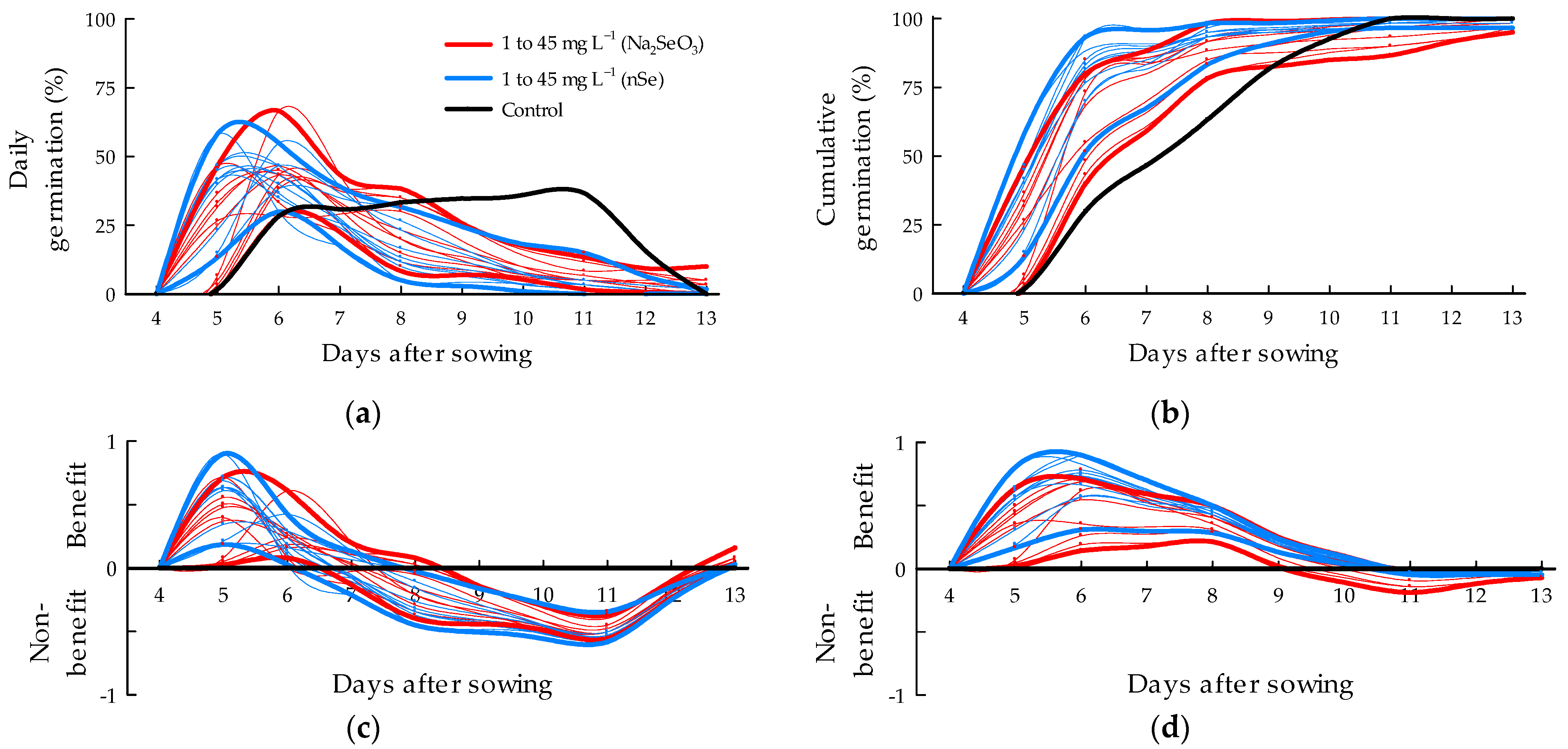
3.1. Germination Percent
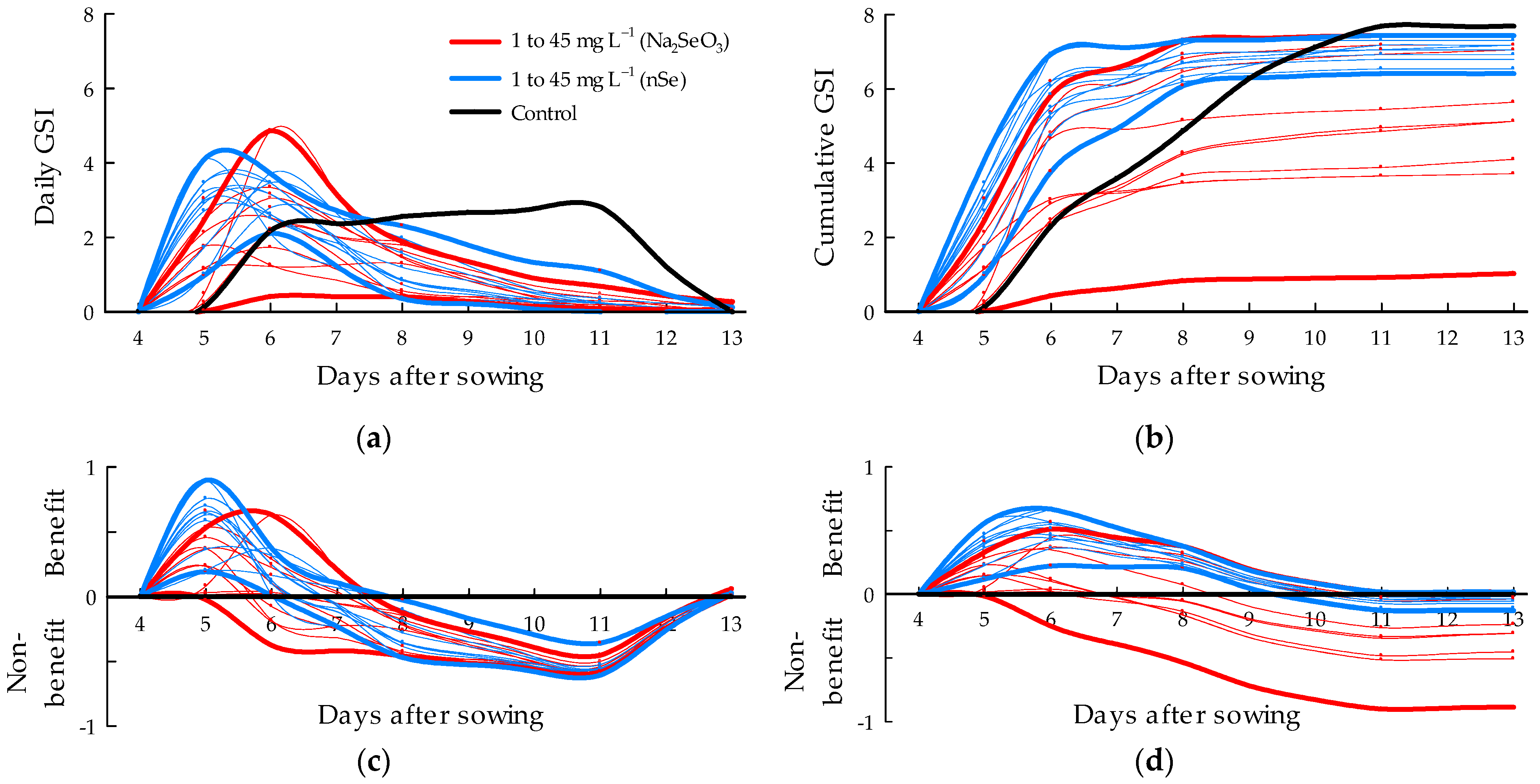
3.2. Germination Speed Index
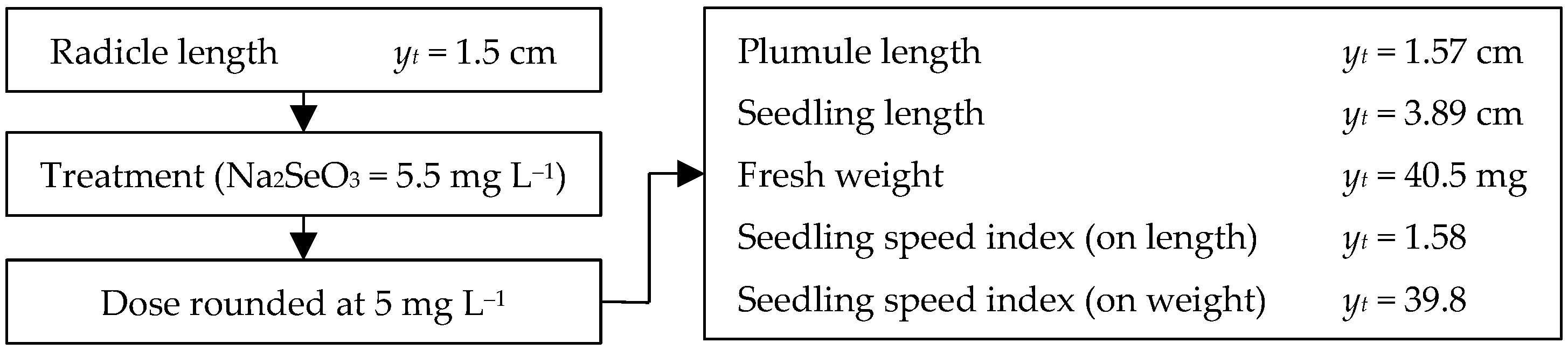
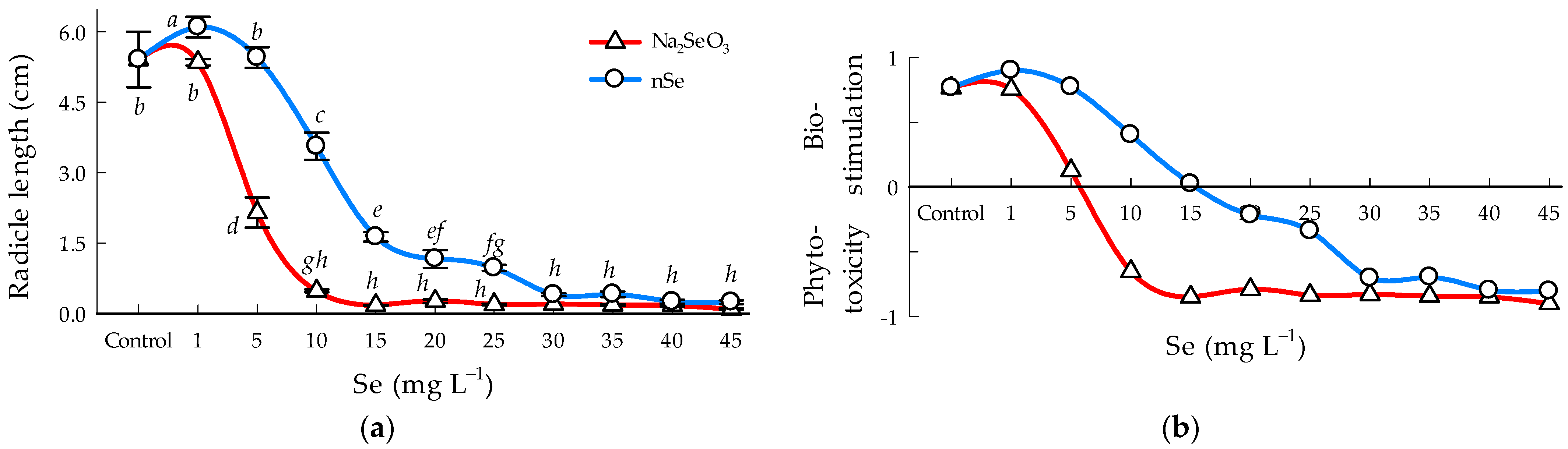
3.3. Radicle Length
3.4. Plumule Length
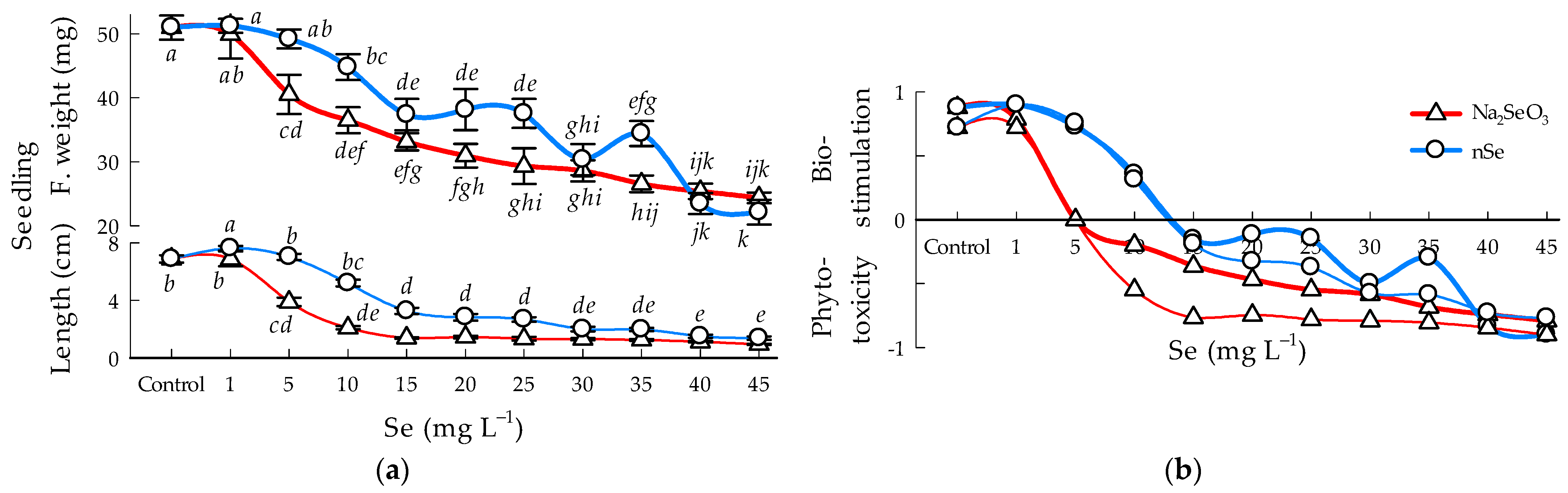
3.5. Fresh Weight and Seedling Length
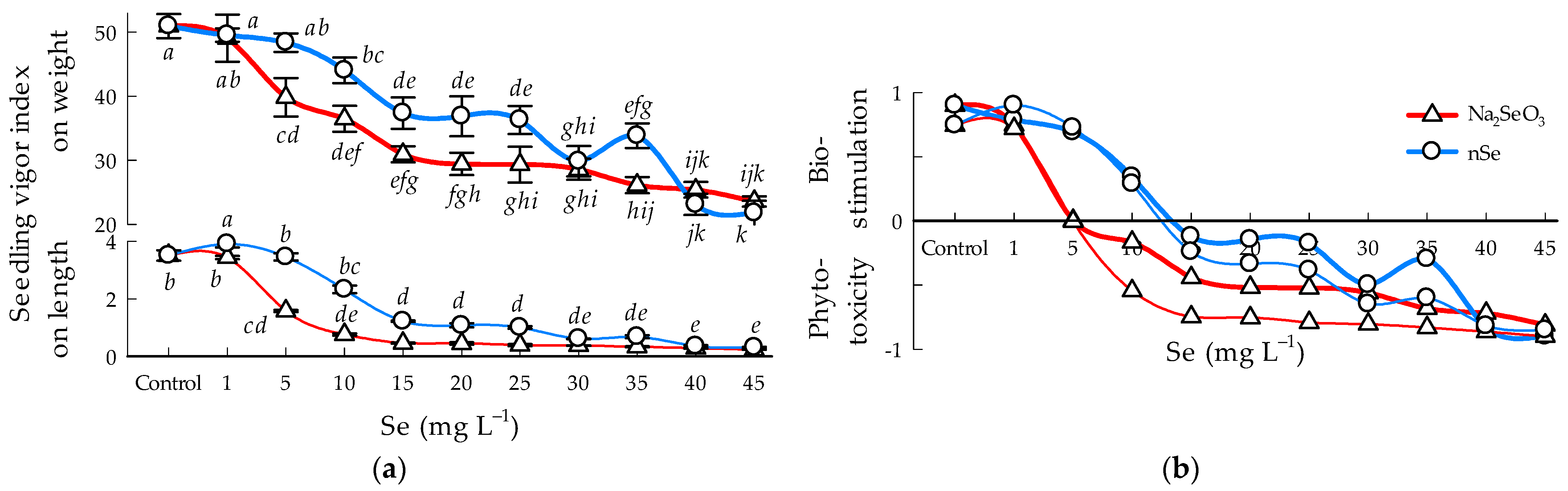
3.6. Seedling Vigor Index
4. Discussion
4.1. Germination Percent
4.2. Response of Seedlings to Seed Priming with Minerals—Salts and Nanomaterials
4.3. Bioestimulation and Phitotoxicity
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Parera, C.A.; Cantliffe, D.J. Presowing seed priming. Hort. Rev. 2010, 16, 109–141. [Google Scholar] [CrossRef]
- Nawaz, J.; Hussain, M.; Jabbar, A.; Nadeem, G.A.; Sajid, M.; Subtain, M.; Shabbir, I. Seed priming a technique. Int. J. Agric. Crop Sci. 2013, 6, 1373. [Google Scholar]
- Abbasi Khalaki, M.; Moameri, M.; Asgari Lajayer, B.; Astatkie, T. Influence of nano-priming on seed germination and plant growth of forage and medicinal plants. Plant Growth Regul. 2020, 93, 13–28. [Google Scholar] [CrossRef]
- Gomes, D.G.; Pelegrino, M.T.; Ferreira, A.S.; Bazzo, J.H.; Zucareli, C.; Seabra, A.B.; Oliveira, H.C. Seed priming with copper-loaded chitosan nanoparticles promotes early growth and enzymatic antioxidant defense of maize (Zea mays L.) seedlings. J. Chem. Technol. Biotechnol. 2021, 96, 2176–2184. [Google Scholar] [CrossRef]
- Salehzade, H.; Izadkhah-Shishvan, M.; Chiyasi, M. Effect of seed priming on germination and seedling growth of wheat (Triticum aestivum L.). J. Biol. Sci. 2009, 4, 629–631. [Google Scholar]
- Li, Y.; Liang, L.; Li, W.; Ashraf, U.; Ma, L.; Tang, X.; Pan, S.; Tian, H.; Mo, Z. ZnO nanoparticle-based seed priming modulates early growth and enhances physio-biochemical and metabolic profiles of fragrant rice against cadmium toxicity. J. Nanobiotechnol. 2021, 19, 75. [Google Scholar] [CrossRef]
- López-Vargas, E.R.; González-García, Y.; Pérez-Álvarez, M.; Cadenas-Pliego, G.; González-Morales, S.; Benavides-Mendoza, A.; Cabrera, R.I.; Juárez-Maldonado, A. Seed priming with carbon nanomaterials to modify the germination, growth, and antioxidant status of tomato seedlings. Agronomy 2020, 10, 639. [Google Scholar] [CrossRef]
- Moaaz Ali, M.; Javed, T.; Mauro, R.P.; Shabbir, R.; Afzal, I.; Yousef, A.F. Effect of seed priming with potassium nitrate on the performance of tomato. Agriculture 2020, 10, 498. [Google Scholar] [CrossRef]
- Ghoohestani, A.; Gheisary, H.; Zahedi, S.M.; Dolatkhahi, A. Effect of seed priming of tomato with salicylic acid, ascorbic acid and hydrogen peroxideon germination and plantlet growth in saline conditions. Int. J. Agron. Plant Prod. 2012, 3, 700–704. [Google Scholar]
- Acharya, P.; Jayaprakasha, G.K.; Crosby, K.M.; Jifon, J.L.; Patil, B.S. Nanoparticle-mediated seed priming improves germination, growth, yield, and quality of watermelons (Citrullus lanatus) at multi-locations in Texas. Sci. Rep. 2020, 10, 5037. [Google Scholar] [CrossRef] [PubMed]
- Yildirim, K.C.; Canik Orel, D.; Okyay, H.; Gursan, M.M.; Demir, I. Quality of immature and mature pepper (Capsicum annuum L.) seeds in relation to bio-priming with endophytic pseudomonas and Bacillus spp. Horticulturae 2021, 7, 75. [Google Scholar] [CrossRef]
- da Silva, M.B.P.; Silva, V.N.; Vieira, L.C. Biopriming of sweet pepper and tomato seeds with Ascophyllum nodosum. Rev. Fac. Nac. Agron. Medellín 2021, 74, 9423–9430. [Google Scholar] [CrossRef]
- Khan, H.; Ayub, C.; Pervez, M.; Bilal, R.; Shahid, M.; Ziaf, K. Effect of seed priming with NaCl on salinity tolerance of hot pepper (Capsicum annuum L.) at seedling stage. Soil Environ. 2009, 2, 81–87. [Google Scholar]
- Puccinelli, M.; Malorgio, F.; Pezzarossa, B. Selenium enrichment of horticultural crops. Molecules 2017, 22, 933. [Google Scholar] [CrossRef] [PubMed]
- Moulick, D.; Ghosh, D.; Chandra Santra, S. Evaluation of effectiveness of seed priming with selenium in rice during germination under arsenic stress. Plant Physiol. Biochem. 2016, 109, 571–578. [Google Scholar] [CrossRef] [PubMed]
- Adhikary, S.; Biswas, B.; Chakraborty, D.; Timsina, J.; Pal, S.; Tarafdar, J.C.; Banerjee, S.; Hossain, A.; Roy, S. Seed priming with selenium and zinc nanoparticles modifies germination, growth, and yield of direct-seeded rice (Oryza sativa L.). Sci. Rep. 2022, 12, 7103. [Google Scholar] [CrossRef] [PubMed]
- Hu, F.Q.; Jiang, S.C.; Wang, Z.; Hu, K.; Xie, Y.M.; Zhou, L.; Zhu, J.Q.; Xing, D.Y.; Du, B. Seed priming with selenium: Effects on germination, seedling growth, biochemical attributes, and grain yield in rice growing under flooding conditions. Plant Direct 2022, 6, e378. [Google Scholar] [CrossRef]
- Gholami, S.; Dehaghi, M.A.; Rezazadeh, A.; Naji, A.M. Seed germination and physiological responses of quinoa to selenium priming under drought stress. Bragantia 2022, 81, e0722. [Google Scholar] [CrossRef]
- Khaliq, A.; Aslam, F.; Matloob, A.; Hussain, S.; Geng, M.; Wahid, A.; ur Rehman, H. Seed priming with selenium: Consequences for emergence, seedling growth, and biochemical attributes of rice. Biol. Trace Elem. Res. 2015, 166, 236–244. [Google Scholar] [CrossRef] [PubMed]
- Abedi, S.; Iranbakhsh, A.; Oraghi Ardebili, Z.; Ebadi, M. Seed priming with cold plasma improved early growth, flowering, and protection of Cichorium intybus against selenium nanoparticle. J. Theor. Appl. Phys. 2020, 14, 113–119. [Google Scholar] [CrossRef]
- Al-Omairi, A.A.; Al-Hilfy, I.H. Effect of soaking maize seeds with selenium and chitosan on improving germination, vigour and viability of seed and seedling. IOP Conf. Ser. Earth Environ. Sci. 2021, 904, 012075. [Google Scholar] [CrossRef]
- Nie, M.; Ning, N.; Liang, D.; Zhang, H.; Li, S.; Li, S.; Fan, X.; Zhang, Y. Seed priming with selenite enhances germination and seedling growth of Sorghum [Sorghum bicolor (L.) Moench] under salt stress. Acta Agric. Scand. Sect. B Soil Plant Sci. 2023, 73, 42–53. [Google Scholar] [CrossRef]
- Xia, F.S.; Wang, C.C.; Li, Y.Y.; Yang, Y.Y.; Zheng, C.; Fan, H.F.; Zhang, Y.D. Influence of priming with exogenous selenium on seed vigour of alfalfa (Medicago sativa L.). Legume Res. 2021, 44, 1124–1127. [Google Scholar] [CrossRef]
- Xia, F.S.; Wang, C.C.; Li, H.Y.; Liu, M.; Zheng, C.; Zhang, Y.D.; Fan, H.F. Effect of selenium priming on the antioxidation of alfalfa seeds. Acta Agrestia Sin. 2021, 29, 472–477. [Google Scholar] [CrossRef]
- Raza, M.A.S.; Aslam, M.U.; Valipour, M.; Iqbal, R.; Haider, I.; Mustafa, A.E.M.A.; Elshikh, M.S.; Ali, I.; Roy, R.; Elshamly, A.M.S. Seed priming with selenium improves growth and yield of quinoa plants suffering drought. Sci. Rep. 2024, 14, 886. [Google Scholar] [CrossRef]
- Ashraf Ganjouii, F.; Nasibi, F.; Manouchehri Kalantari, K.; Ahmadi Mousavi, E. Effect of seed priming with selenium nanoparticles and plant growth promoting rhizobacteria on improving Quinoa seedling growth under salinity stress. J. Plant Process Funct. 2023, 11, 65–73. [Google Scholar]
- Bayat, H.; Aminifard, M.H. Seed priming with selenium improves growth, water relation and antioxidant activity of pot marigold (Calendula officinalis L.) under drought conditions. Acta Sci. Pol. Hortorum Cultus 2021, 20, 27–36. [Google Scholar] [CrossRef]
- Hussain, S.; Ahmed, S.; Akram, W.; Li, G.; Yasin, N.A. Selenium seed priming enhanced the growth of salt-stressed Brassica rapa L. through improving plant nutrition and the antioxidant system. Front. Plant Sci. 2023, 13, 1050359. [Google Scholar] [CrossRef] [PubMed]
- Nasibi, F.; Aminian, F.; Mohammadinejad, G.; Hassanshahian, M. Seed priming with selenium nanoparticle and plant growth promoting rhizobacteria improve seedling development of foxtail millet (Setaria italica) under salinity stress. Res. Square 2022, 1–26. [Google Scholar] [CrossRef]
- Mehrkish, M.; Ghobadi, M.; Jalali Honarmand, S. Evaluation the ability of seed priming with selenium to improving deteriorated seeds in lentil (Lens culinaris Medic.). Iran. J. Seed Sci. Res. 2021, 8, 13–28. [Google Scholar] [CrossRef]
- Mejía-Ramírez, F.; Benavides-Mendoza, A.; González-Morales, S.; Juárez-Maldonado, A.; Lara-Viveros, F.M.; Morales-Díaz, A.B.; Morelos-Moreno, Á. Seed priming based on iodine and selenium influences the nutraceutical compounds in tomato (Solanum lycopersicum L.) crop. Antioxidants 2023, 12, 1265. [Google Scholar] [CrossRef]
- Ishtiaq, M.; Mazhar, M.W.; Maqbool, M.; Hussain, T.; Hussain, S.A.; Casini, R.; Abd-ElGawad, A.M.; Elansary, H.O. Seed priming with the selenium nanoparticles maintains the redox status in the water stressed tomato plants by modulating the antioxidant defense enzymes. Plants 2023, 12, 1556. [Google Scholar] [CrossRef]
- Feizi, H.; Kamali, M.; Jafari, L.; Rezvani Moghaddam, P. Phytotoxicity and stimulatory impacts of nanosized and bulk titanium dioxide on fennel (Foeniculum vulgare Mill). Chemosphere 2013, 91, 506–511. [Google Scholar] [CrossRef]
- Forigo Beloti, I.; Alves de Sousa, L.; Oliveira, R.C.; Miranda Lemes, E. Bell pepper germination and seedling’s parameters under different osmotic potentials. Comun. Sci. 2022, 13, e3843. [Google Scholar] [CrossRef]
- García-López, J.I.; Zavala-García, F.; Olivares-Sáenz, E.; Lira-Saldívar, R.H.; Díaz Barriga-Castro, E.; Ruiz-Torres, N.A.; Ramos-Cortez, E.; Vázquez-Alvarado, R.; Niño-Medina, G. Zinc oxide nanoparticles boosts phenolic compounds and antioxidant activity of Capsicum annuum L. during germination. Agronomy 2018, 8, 215. [Google Scholar] [CrossRef]
- Iosob, G.A.; Nedeff, V.; Sandu, I.; Cristea, T.O.; Prisecaru, M.; Sandu, I.G. The effect of heavy metals (copper and cadmium) on the germination of bell pepper seeds (Capsicum annuum L. var. Dariana Bac). Rev. Chim. 2019, 70, 3262–3266. [Google Scholar] [CrossRef]
- León-Morales, J.M.; Panamá-Raymundo, W.; Langarica-Velázquez, E.C.; García-Morales, S. Selenium and vanadium on seed germination and seedling growth in pepper (Capsicum annuum L.) and radish (Raphanus sativus L.). Rev. Bio Cienc. 2019, 6, e425. [Google Scholar] [CrossRef]
- Hassan, M.S.M.F.; Belal, H.E.E.; Abou-Sreea, A.I.B.; Rady, M.M. Exogenous application of selenium or iodine improves the growth, yield and antioxidant status of Capsicum annuum L. Labyrinth Fayoum J. Sci. Interdiscipl. Stud. 2023, 1, 76–83. [Google Scholar] [CrossRef]
- Aloui, H.; Souguir, M.; Latique, S.; Hannachi, C. Germination and growth in control and primed seeds of pepper as affected by salt stress. Cercet. Agron. Mold. 2014, 47, 83–95. [Google Scholar] [CrossRef]
- Aloui, H.; Mohamed Aymen, E.; Chérif, H. Seed priming to improve seedling growth of pepper cultivars exposed to salt concentrations. Int. J. Vegetable Sci. 2017, 23, 489–507. [Google Scholar] [CrossRef]
- Smith, P.T.; Cobb, B.G. Accelerated germination of pepper seed by priming with salt solutions and water. HortScience 1991, 26, 417–419. [Google Scholar] [CrossRef]
- Sotoodehnia-Korani, S.; Iranbakhsh, A.; Ebadi, M.; Majd, A.; Ardebili, Z.O. Selenium nanoparticles induced variations in growth, morphology, anatomy, biochemistry, gene expression, and epigenetic DNA methylation in Capsicum annuum; an in vitro study. Environ. Poll. 2020, 265, 114727. [Google Scholar] [CrossRef]
- Quiterio-Gutiérrez, T.; Ortega-Ortiz, H.; Cadenas-Pliego, G.; Hernández-Fuentes, A.D.; Sandoval-Rangel, A.; Benavides-Mendoza, A.; Cabrera-de la Fuente, M.; Juárez-Maldonado, A. The application of selenium and copper nanoparticles modifies the biochemical responses of tomato plants under stress by Alternaria solani. Int. J. Mol. Sci. 2019, 20, 1950. [Google Scholar] [CrossRef]
- Kong, H.; Yang, J.; Zhang, Y.; Fang, Y.; Nishinari, K.; Phillips, G.O. Synthesis and antioxidant properties of gum arabic-stabilized selenium nanoparticles. Int. J. Biol. Macromol. 2014, 65, 155–162. [Google Scholar] [CrossRef]
- Portuguez-Garcia, M.P.; Rodriguez-Ruiz, A.M.; Porras-Martinez, C.; Gonzalez-Lutz, M.I. Imbibition and temperature to rupture latency of Ischaemum rugosum Salisb. Agron. Mesoam. 2020, 31, 780–789. [Google Scholar] [CrossRef]
- Carballo-Méndez, F.J.; Olivares-Sáenz, E.; Bolivar-Duarte, M.; Antonio-Bautista, A.; Vazquez-Badillo, M.E.; Niño-Medina, G. Effect of silicon on germination of Moringa oleifera Lam. in different types of salts. Fresen. Environ. Bull. 2019, 28, 8823–8830. [Google Scholar]
- ISTA. International Rules for Seed Testing; International Seed Testing Association: Bassersdorf, Switzerland, 2019; 298p. [Google Scholar]
- MAPA. Regras Para Análise de Sementes. In Ministério da Agricultura, Pecuária e Abastecimento; Secretaria de Defesa Agropecuária: Brasília, Brazil, 2009; 395p. [Google Scholar]
- Qi, Y.; Yan, B.; Fu, G.; Guan, X.; Du, L.; Li, J. Germination of seeds and seedling growth of Amaranthus retroflexus L. following sublethal exposure of parent plants to herbicides. Sci. Rep. 2017, 7, 157. [Google Scholar] [CrossRef]
- Zaghdoud, C.; Ollio, I.; Solano, C.J.; Ochoa, J.; Suardiaz, J.; Fernández, J.A.; Martínez Ballesta, M.C. Red LED light improves pepper (Capsicum annuum L.) seed radicle emergence and growth through the modulation of aquaporins, hormone homeostasis, and metabolite remobilization. Int. J. Mol. Sci. 2023, 24, 4779. [Google Scholar] [CrossRef]
- Romano, A.; Stevanato, P. Germination data analysis by time-to-event approaches. Plants 2020, 9, 617. [Google Scholar] [CrossRef]
- McNair, J.N.; Sunkara, A.; Frobish, D. How to analyse seed germination data using statistical time-to-event analysis: Non-parametric and semi-parametric methods. Seed Sci. Res. 2012, 22, 77–95. [Google Scholar] [CrossRef]
- Ranal, M.A.; de Santana, D.G. How and why to measure the germination process? Braz. J. Bot. 2006, 29, 1–11. [Google Scholar] [CrossRef]
- de Oliveira, G.R.F.; de Salles, K.F.L.; Batista, T.B.; da Silva, M.S.; Cicero, S.M.; Gomes-Junior, F.G. Morphological parameters of image processing to characterize primary root emergence in evaluation of tomato seed vigor. J. Seed Sci. 2021, 43, e202143005. [Google Scholar] [CrossRef]
- Martínez-Solís, J.; Romero, A.S.; Pena, G.; Virgen, J. Índice de velocidad de emergencia en líneas de maíz. Rev. Mex. Cienc. Agríc. 2010, 1, 288–303. [Google Scholar]
- Koukounaras, A.; Boursianis, A.; Kostas, S.; Theopoulos, A.; Bantis, F.; Samaras, T. Pre-sowing static magnetic field treatment of vegetable seeds and its effect on germination and young seedlings development. Seeds 2023, 2, 394–405. [Google Scholar] [CrossRef]
- Pradeep, P. Seed quality parameters (germination percentage and seedling vigor index) of rabi sorghum seeds influenced by rice weevil infestation. MOJ Toxicol. 2018, 4, 391–396. [Google Scholar] [CrossRef][Green Version]
- Abdul-Baki, A.A.; Anderson, J.D. Vigor determination in soybean seed by multiple criteria. Crop Sci. 1973, 13, 630–633. [Google Scholar] [CrossRef]
- González-Moscoso, M.; Juárez-Maldonado, A.; Cadenas-Pliego, G.; Meza-Figueroa, D.; SenGupta, B.; Martínez-Villegas, N. Silicon nanoparticles decrease arsenic translocation and mitigate phytotoxicity in tomato plants. Environ. Sci. Pollut. Res. 2022, 29, 34147–34163. [Google Scholar] [CrossRef]
- Parera, V.; Parera, C.A.; Feresin, G.E. Germination and early seedling growth of high Andean native plants under heavy metal stress. Diversity 2023, 15, 824. [Google Scholar] [CrossRef]
- Sinkkonen, A. Modeling the effect of density-dependent chemical interference upon seed germination. Nonlinearity Biol. Toxicol. Med. 2005, 3, 225–233. [Google Scholar] [CrossRef]
- Belz, R.G.; Duke, S.O. Stepping beyond hormesis modeling and sub-NOAEL predictions in plant biology. Curr. Opin. Environ. Sci. Health 2022, 28, 100366. [Google Scholar] [CrossRef]
- Di Rienzo, J.A.; Casanoves, F.; Balzarini, M.G.; Gonzalez, L.; Tablada, M.; Robledo, C.W. InfoStat Versión 2016. Grupo InfoStat, FCA, Universidad Nacional de Córdoba, Argentina. Available online: http://www.infostat.com.ar (accessed on 12 December 2020).
- Rajjou, L.; Duval, M.; Gallardo, K.; Catusse, J.; Bally, J.; Job, C.; Job, D. Seed germination and vigor. Ann. Rev. Plant Biol. 2012, 63, 507–533. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Zeng, X.; Yang, W.; Xie, H.; Ashraf, U.; Mo, Z.; Liu, J.; Li, G.; Li, W. Seed priming with multiwall carbon nanotubes (MWCNTs) modulates seed germination and early growth of maize under cadmium (Cd) toxicity. J. Soil. Sci. Plant Nutr. 2021, 21, 1793–1805. [Google Scholar] [CrossRef]
- El-Badri, A.M.; Batool, M.; Wang, C.; Hashem, A.M.; Tabl, K.M.; Nishawy, E.; Kuai, J.; Zhou, G.; Wang, B. Selenium and zinc oxide nanoparticles modulate the molecular and morpho-physiological processes during seed germination of Brassica napus under salt stress. Ecotox. Environ. Saf. 2021, 225, 112695. [Google Scholar] [CrossRef] [PubMed]
- Nawaz, F.; Zulfiqar, B.; Ahmad, K.S.; Majeed, S.; Shehzad, M.A.; Javeed, H.M.R.; Tahir, M.N.; Ahsan, M. Pretreatment with selenium and zinc modulates physiological indices and antioxidant machinery to improve drought tolerance in maize (Zea mays L.). S. Afr. J. Bot. 2021, 138, 209–216. [Google Scholar] [CrossRef]
- Hussain, S.; Khan, F.; Hussain, H.A.; Nie, L. Physiological and biochemical mechanisms of seed priming-induced chilling tolerance in rice cultivars. Front. Plant Sci. 2016, 7, 116. [Google Scholar] [CrossRef]
- do Espirito Santo Pereira, A.; Caixeta Oliveira, H.; Fernandes Fraceto, L.; Santaella, C. Nanotechnology potential in seed priming for sustainable agriculture. Nanomaterials 2021, 11, 267. [Google Scholar] [CrossRef] [PubMed]
- Calabrese, E.J. The maturing of hormesis as a credible dose-response model. Nonlinearity Biol. Toxicol. Med. 2003, 1, 319–343. [Google Scholar] [CrossRef] [PubMed]
- Calabrese, E.J. Hormesis: Principles and applications. Homeopathy 2015, 104, 69–82. [Google Scholar] [CrossRef]
- Su, Y.; Zhou, X.; Meng, H.; Xia, T.; Liu, H.; Rolshausen, P.; Roper, C.; McLean, J.E.; Zhang, Y.; Keller, A.A.; et al. Cost-benefit analysis of nanofertilizers and nanopesticides emphasizes the need to improve the efficiency of nanoformulations for widescale adoption. Nat. Food 2022, 3, 1020–1030. [Google Scholar] [CrossRef]
- Coppola, G.; Costantini, M.; Orsi, L.; Facchinetti, D.; Santoro, F.; Pessina, D.; Bacenetti, J. A comparative cost-benefit analysis of conventional and organic hazelnuts production systems in center Italy. Agriculture 2020, 10, 409. [Google Scholar] [CrossRef]
- Tröster, F.; Sauer, J. Characteristics of cost-efficient fertilization plans at the farm level. NJAS Impact Agric. Life Sci. 2022, 94, 184–216. [Google Scholar] [CrossRef]
- García Márquez, V.; Morelos Moreno, Á.; Benavides Mendoza, A.; Medrano Macías, J. Ionic selenium and nanoselenium as biofortifiers and stimulators of plant metabolism. Agronomy 2020, 10, 1399. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
de los Ángeles Sariñana-Navarrete, M.; Benavides-Mendoza, A.; González-Morales, S.; Juárez-Maldonado, A.; Preciado-Rangel, P.; Sánchez-Chávez, E.; Cadenas-Pliego, G.; Antonio-Bautista, A.; Morelos-Moreno, Á. Selenium Seed Priming and Biostimulation Influence the Seed Germination and Seedling Morphology of Jalapeño (Capsicum annuum L.). Horticulturae 2024, 10, 119. https://doi.org/10.3390/horticulturae10020119
de los Ángeles Sariñana-Navarrete M, Benavides-Mendoza A, González-Morales S, Juárez-Maldonado A, Preciado-Rangel P, Sánchez-Chávez E, Cadenas-Pliego G, Antonio-Bautista A, Morelos-Moreno Á. Selenium Seed Priming and Biostimulation Influence the Seed Germination and Seedling Morphology of Jalapeño (Capsicum annuum L.). Horticulturae. 2024; 10(2):119. https://doi.org/10.3390/horticulturae10020119
Chicago/Turabian Stylede los Ángeles Sariñana-Navarrete, María, Adalberto Benavides-Mendoza, Susana González-Morales, Antonio Juárez-Maldonado, Pablo Preciado-Rangel, Esteban Sánchez-Chávez, Gregorio Cadenas-Pliego, Adriana Antonio-Bautista, and Álvaro Morelos-Moreno. 2024. "Selenium Seed Priming and Biostimulation Influence the Seed Germination and Seedling Morphology of Jalapeño (Capsicum annuum L.)" Horticulturae 10, no. 2: 119. https://doi.org/10.3390/horticulturae10020119
APA Stylede los Ángeles Sariñana-Navarrete, M., Benavides-Mendoza, A., González-Morales, S., Juárez-Maldonado, A., Preciado-Rangel, P., Sánchez-Chávez, E., Cadenas-Pliego, G., Antonio-Bautista, A., & Morelos-Moreno, Á. (2024). Selenium Seed Priming and Biostimulation Influence the Seed Germination and Seedling Morphology of Jalapeño (Capsicum annuum L.). Horticulturae, 10(2), 119. https://doi.org/10.3390/horticulturae10020119










