Gibberellin Treatment Accelerates Starch Decomposition and Seed Germination in Sticky Nightshade (Solanum sisymbriifolium Lam.)
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Materials and Growth Conditions
2.2. Characterization of Seed Germination
2.3. Quantification of GA3 Content in Seeds
2.4. Paraffin Section of Seeds
2.5. Measurement of Malondialdehyde (MDA) Content in Seeds
2.6. Statistical Analyses
3. Results
3.1. Gibberellic Acid Alleviates the Seed Germination Obstacles in Sticky Nightshade
3.2. Differences in Starch Granules of Seeds Between Sticky Nightshade and Cultivated Eggplant
3.3. GA3 Treatment Accelerates the Decomposition of Starch Granules in Sticky Nightshade Seeds
3.4. MDA Content of Seeds After GA3 Treatment
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yin, P.; Ding, W.Y.; Zhang, H.P.; Liu, X.; Zhang, H.Y.; Zeng, J.W.; Xu, J. Morphological, physiological and molecular characteristics of the seedless ‘Hongjiangcheng’ sweet orange. Hortic. Plant J. 2023, 9, 437–449. [Google Scholar] [CrossRef]
- Zhang, T.Y.; Wang, Y.R.; Munir, S.; Wang, T.T.; Ye, Z.B.; Zhang, J.H.; Zhang, Y.Y. Cyclin gene SlCycB1 alters plant architecture in association with histone H3.2 in tomato. Hortic. Plant J. 2022, 8, 341–350. [Google Scholar] [CrossRef]
- Liu, Y.; Fang, J.; Xu, F.; Chu, J.F.; Yan, C.Y.; Schläppi, M.R.; Wang, Y.P.; Chu, C.C. Expression patterns of ABA and GA metabolism genes and hormone levels during rice seed development and imbibition: A comparison of dormant and non-dormant rice cultivars. J. Genet. Genom. 2014, 41, 327–338. [Google Scholar] [CrossRef] [PubMed]
- Yan, A.; Chen, Z. The pivotal role of abscisic acid signaling during transition from seed maturation to germination. Plant Cell Rep. 2017, 36, 689–703. [Google Scholar] [CrossRef]
- Gallardo, K.; Job, C.; Groot, S.P.C.; Puype, M.; Demol, H.; Vandekerckhove, J.; Job, D. Proteomics of Arabidopsis seed germination. A comparative study of wild-type and gibberellin-deficient seeds. Plant Physiol. 2002, 129, 823–837. [Google Scholar] [CrossRef]
- Nan, Y.; Ya, C.C.; Bing, Q.W.; Li, H.W. Research progress on seed germination and dormancy of monocot and dicot plants. Plant Genet. Resour. 2022, 23, 1249–1257. [Google Scholar]
- Pendield, S. Seed dormancy and germination. Curr. Biol. 2017, 27, R874–R878. [Google Scholar] [CrossRef] [PubMed]
- Matilla, A.J.; Matilla-Vázquez, M.A. Involvement of ethylene in seed physiology. Plant Sci. 2008, 175, 87–97. [Google Scholar] [CrossRef]
- Steber, C.M.; McCourt, P. A role for brassinosteroids in germination in Arabidopsis. Plant Physiol. 2001, 125, 763–769. [Google Scholar] [CrossRef] [PubMed]
- Gómez-Cadenas, A.; Zentella, R.; Walker-Simmons, M.K.; Ho, T.H.D. Gibberellin/abscisic acid antagonism in barley aleurone cells:: Site of action of the protein kinase PKABA1 in relation to gibberellin signaling molecules. Plant Cell 2001, 13, 667–679. [Google Scholar] [CrossRef] [PubMed]
- Kato-Noguchi, H.; Macías, F.A. Effects of 6-methoxy-2 benzoxazolinone on the germination and α-amylase activity in lettuce seeds. J. Plant Physiol. 2005, 162, 1304–1307. [Google Scholar] [CrossRef] [PubMed]
- Mishra, P.; Dubey, R.S. Effect of aluminium on metabolism of starch and sugars in growing rice seedlings. Acta Physiol. Plant 2008, 30, 265–275. [Google Scholar] [CrossRef]
- Borek, S.; Ratajczak, W.; Ratajczak, L. Ultrastructural and enzymatic research on the role of sucrose in mobilization of storage lipids in germinating yellow lupine seeds. Plant Sci. 2006, 170, 441–452. [Google Scholar] [CrossRef]
- Moller, I.M.; Jensen, P.E.; Hansson, A. Oxidative modifications to cellular components in plants. Annu. Rev. Plant Biol. 2007, 58, 459–481. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.K.; Jia, H.; Wang, P.; Zhou, T.; Wu, Y.; Liu, Z.M. Exogenous gibberellin weakens lipid breakdown by increasing soluble sugars levels in early germination of zanthoxylum seeds. Plant Sci. 2019, 280, 155–163. [Google Scholar] [CrossRef]
- Wu, Y.; Bao, W.Q.; Hu, H.; Shen, Y.B. Mechanical constraints in the endosperm and endocarp are major causes of dormancy in Hu (Styracaceae) seeds. J. Plant Growth Regul. 2023, 42, 644–657. [Google Scholar] [CrossRef]
- Smykal, P.; Vernoud, V.; Blair, M.W.; Soukup, A.; Thompson, R.D. The role of the testa during development and in establishment of dormancy of the legume seed. Front. Plant Sci. 2014, 5, 351. [Google Scholar]
- Liao, D.Q.; Chen, Y.L.; Qi, J.J.; Zhang, H.L.; Sun, P.; Chen, C.X.; Li, X.E. Temporal transcriptomics reveal the molecular mechanism of dormancy and germination regulated by temperature in seed. Hortic. Plant J. 2023, 9, 848–866. [Google Scholar] [CrossRef]
- Biswas, D.; Santra, I.; Ghosh, B. EMA-based chromosome characterization and karyotype analysis in two wild spiny medicinally and nutritionally important Solanaceaous species -Solanum sisymbriifolium Lam. and Solanum virginianum L. Cytologia 2024, 89, 65–70. [Google Scholar] [CrossRef]
- Rosa-Martínez, E.; Villanueva, G.; Sahin, A.; Gramazio, P.; García-Martínez, M.D.; Raigón, M.D.; Vilanova, S.; Prohens, J.; Plazas, M. Characterization and QTL identification in eggplant introgression lines under two N fertilization levels. Hortic. Plant J. 2023, 9, 971–985. [Google Scholar] [CrossRef]
- Song, L.J.; Tan, Z.; Zhang, W.W.; Li, Q.; Jiang, Z.X.; Shen, S.X.; Luo, S.X.; Chen, X.P. Exogenous melatonin improves the chilling tolerance and preharvest fruit shelf life in eggplant by affecting ROS- and senescence-related processes. Hortic. Plant J. 2023, 9, 523–540. [Google Scholar] [CrossRef]
- Xiong, Z.; Yong, C.; Meng, J.L.; Ya, H.Y.; Wei, L.; Zong, J.L.; Qing, Q.O.; Peng, C. Research on seed germination technology of Solanum sisymbriifolium. China Veg. 2019, 6, 58–63. [Google Scholar]
- Li, N.G.; Chen, S.; Yan, S. Germination characteristics and drought resistance of Indigofera bungeana Walpers under PEG simulated drought stress. SJAS 2023, 69(02), 13–18. [Google Scholar]
- Wen, J.J.; Qing, S.Y.; Guo, L.L.; Fei, Y.; Zhan, H.S.; Wen, L. Effects of storage substances and endogenous hormones on seed germination and seedling formation of Quercus variabilis. J. Beijing For. Univ. 2024, 46, 19–26. [Google Scholar]
- Zhou, Y.H.; Yu, H.Y.; Tang, Y.P.; Chen, R.; Luo, J.Y.; Shi, C.M.; Tang, S.; Li, X.; Shen, X.Y.; Chen, R.F.; et al. Critical roles of mitochondrial fatty acid synthesis in tomato development and environmental response. Plant Physiol. 2022, 190, 576–591. [Google Scholar] [CrossRef]
- Agüero-Martínez, P.F.; Cardozo, L.; Gómez, C.A.; López-Spahr, D.; Baskin, C.C.; Bertero, D.; Galíndez, G.; Curti, R. Variation in thickness of embryo covering structures and their role in the regulation of seed physiological dormancy of Amaranthaceae. Plants-Basel 2024, 13, 2832. [Google Scholar] [CrossRef]
- Mazer, S.J. Seeds, ecology, biogeography, and evolution of dormancy and germination. Science 1999, 283, 334. [Google Scholar] [CrossRef]
- Groot, S.P.C.; Kieliszewskarokicka, B.; Vermeer, E.; Karssen, C.M. Gibberellin-induced hydrolysis of endosperm cell-walls in gibberellin-deficient tomato seeds prior to radicle protrusion. Planta 1988, 174, 500–504. [Google Scholar] [CrossRef] [PubMed]
- Nonogaki, H. Seed germination and dormancy: The classic story, new puzzles, and evolution. J. Integr. Plant Biol. 2019, 61, 541–563. [Google Scholar] [CrossRef] [PubMed]
- Xing, M.Q.; Chen, S.H.; Zhang, X.F.; Xue, H.W. Rice OsGA2ox9 regulates seed GA metabolism and dormancy. Plant Biotechnol. J. 2023, 21, 2411–2413. [Google Scholar] [CrossRef]
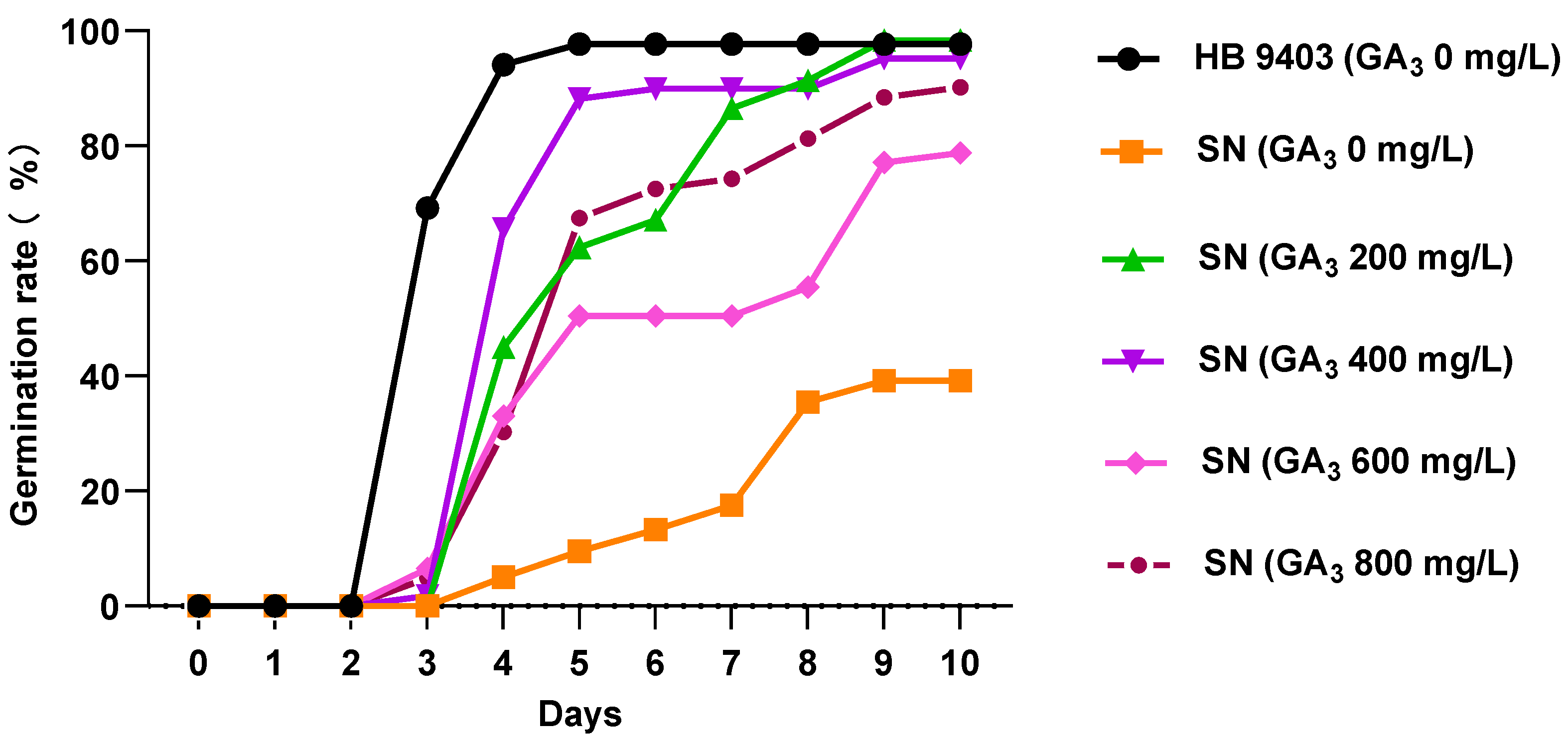
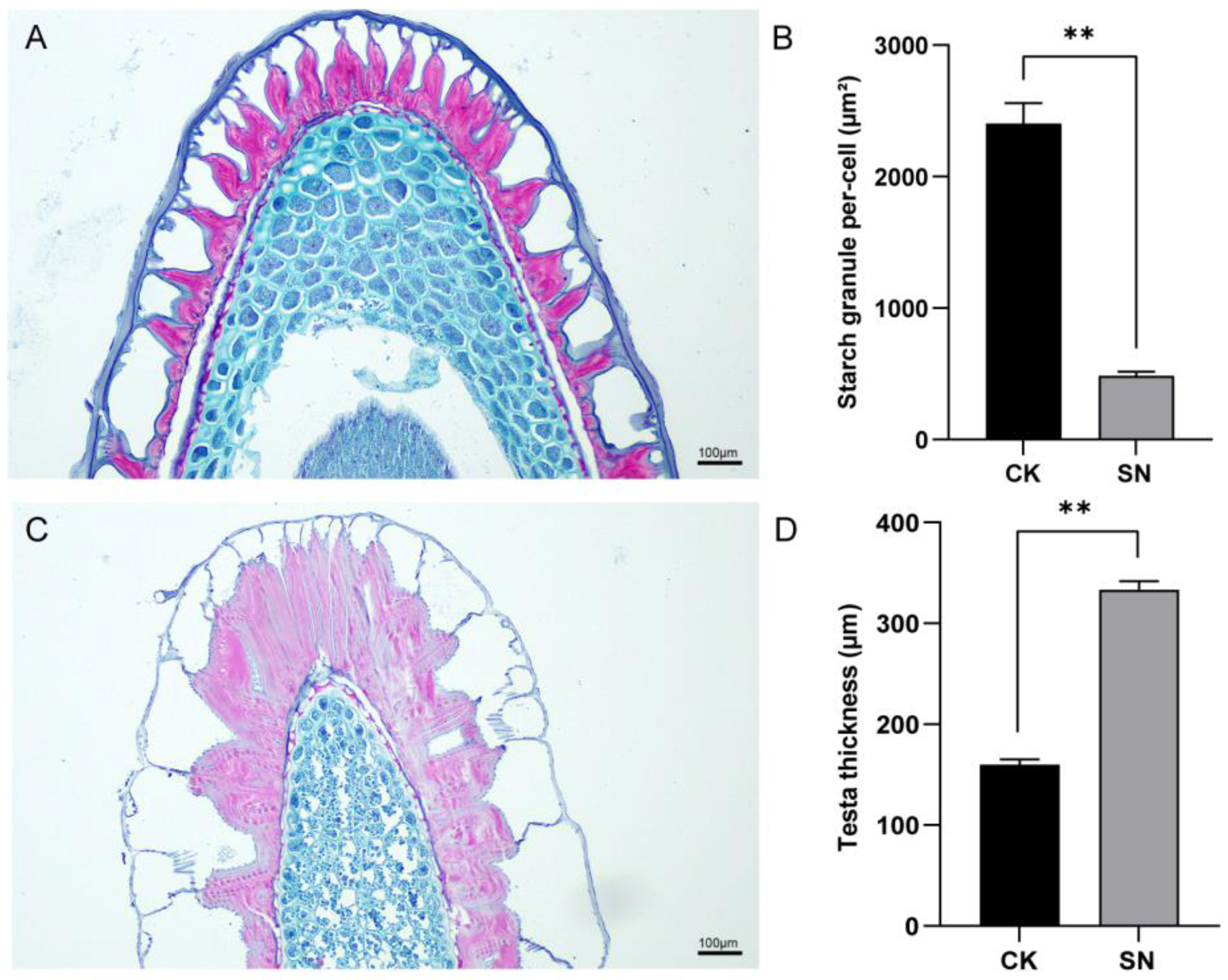
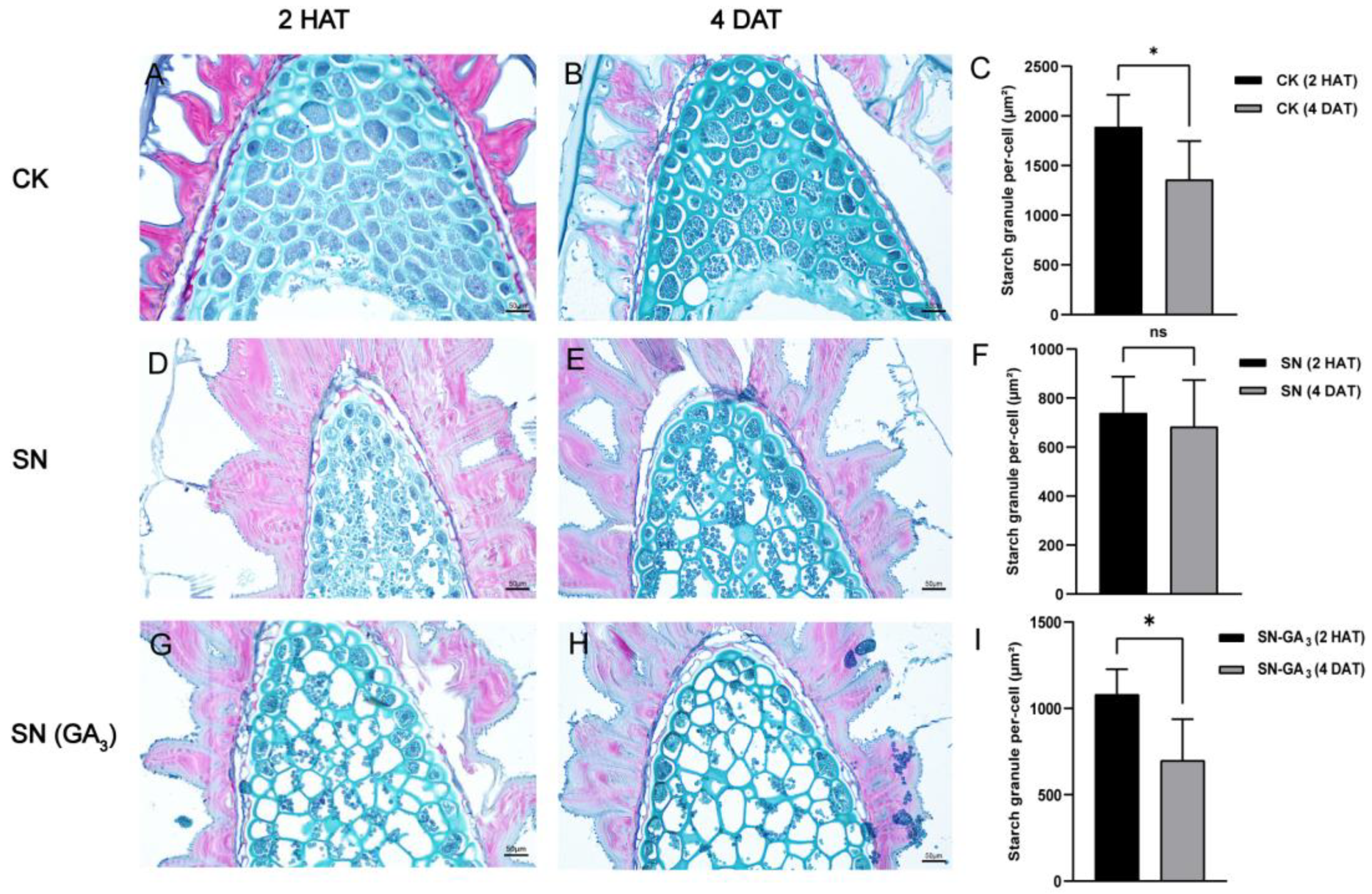
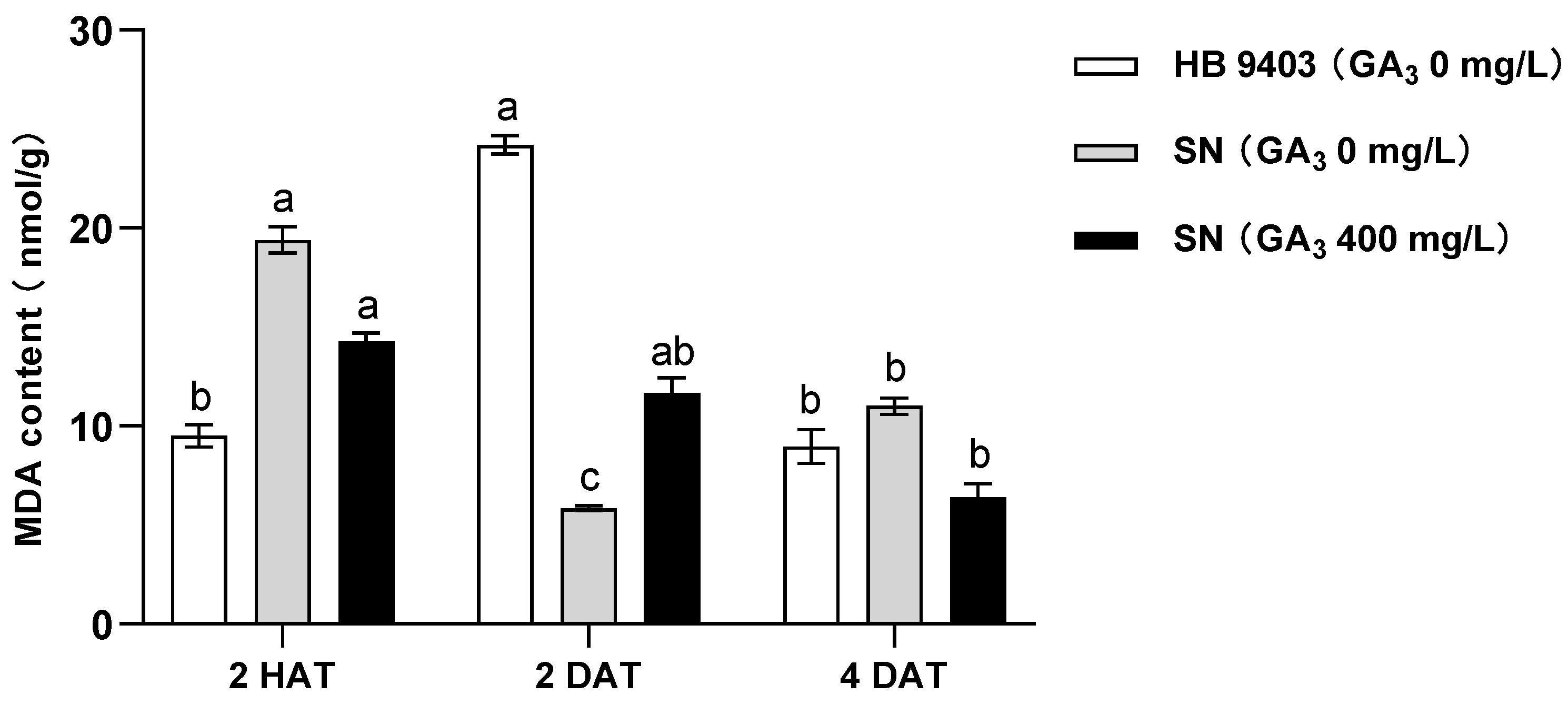
| Treatment | Germination Period (d) | Germination Potential (%) | Germination Index |
|---|---|---|---|
| HB-9403 0 mg/L | 5 | 69.21 ± 0.32 aA | 8.34 ± 1.03 aA |
| SN-GA3 0 mg/L | 9 | 0.00 ± 0.00 bB | 1.04 ± 1.07 cC |
| SN-GA3 200 mg/ | 9 | 0.00 ± 0.00 bB | 3.77 ± 0.70 bB |
| SN-GA3 400 mg/L | 5 | 1.75 ± 0.03 bB | 4.50 ± 0.22 bB |
| SN-GA3 600 mg/L | 9 | 6.52 ± 0.08 bB | 3.23 ± 1.29 bBC |
| SN-GA3 800 mg/L | 9 | 4.90 ± 0.05 bB | 3.23 ± 1.29 bBC |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, H.; Mo, D.; Zhang, X.; Li, F.; Tao, J.; Ge, P.; Yang, Y.; Wang, Z.; Zhang, Y. Gibberellin Treatment Accelerates Starch Decomposition and Seed Germination in Sticky Nightshade (Solanum sisymbriifolium Lam.). Horticulturae 2024, 10, 1342. https://doi.org/10.3390/horticulturae10121342
Xu H, Mo D, Zhang X, Li F, Tao J, Ge P, Yang Y, Wang Z, Zhang Y. Gibberellin Treatment Accelerates Starch Decomposition and Seed Germination in Sticky Nightshade (Solanum sisymbriifolium Lam.). Horticulturae. 2024; 10(12):1342. https://doi.org/10.3390/horticulturae10121342
Chicago/Turabian StyleXu, Haobo, Danni Mo, Xingyu Zhang, Fangman Li, Jinbao Tao, Pingfei Ge, Yang Yang, Ziyuan Wang, and Yuyang Zhang. 2024. "Gibberellin Treatment Accelerates Starch Decomposition and Seed Germination in Sticky Nightshade (Solanum sisymbriifolium Lam.)" Horticulturae 10, no. 12: 1342. https://doi.org/10.3390/horticulturae10121342
APA StyleXu, H., Mo, D., Zhang, X., Li, F., Tao, J., Ge, P., Yang, Y., Wang, Z., & Zhang, Y. (2024). Gibberellin Treatment Accelerates Starch Decomposition and Seed Germination in Sticky Nightshade (Solanum sisymbriifolium Lam.). Horticulturae, 10(12), 1342. https://doi.org/10.3390/horticulturae10121342







