Evaluation of Drought Tolerance and Trehalose Response in Auricularia heimuer
Abstract
1. Introduction
2. Materials and Methods
2.1. Auricularia heimuer Test Strains
2.2. Experimental Design
2.3. Physiological Index Determination
2.4. Evaluation of Drought Tolerance in A. heimuer
2.5. Total RNA Extraction and Transcriptome Sequencing
2.6. Real-Time Quantitative PCR Analysis
2.7. DEG Identification and Enrichment Analysis
2.8. Measurement of Trehalose Content
2.9. Data Processing and Analysis
3. Results
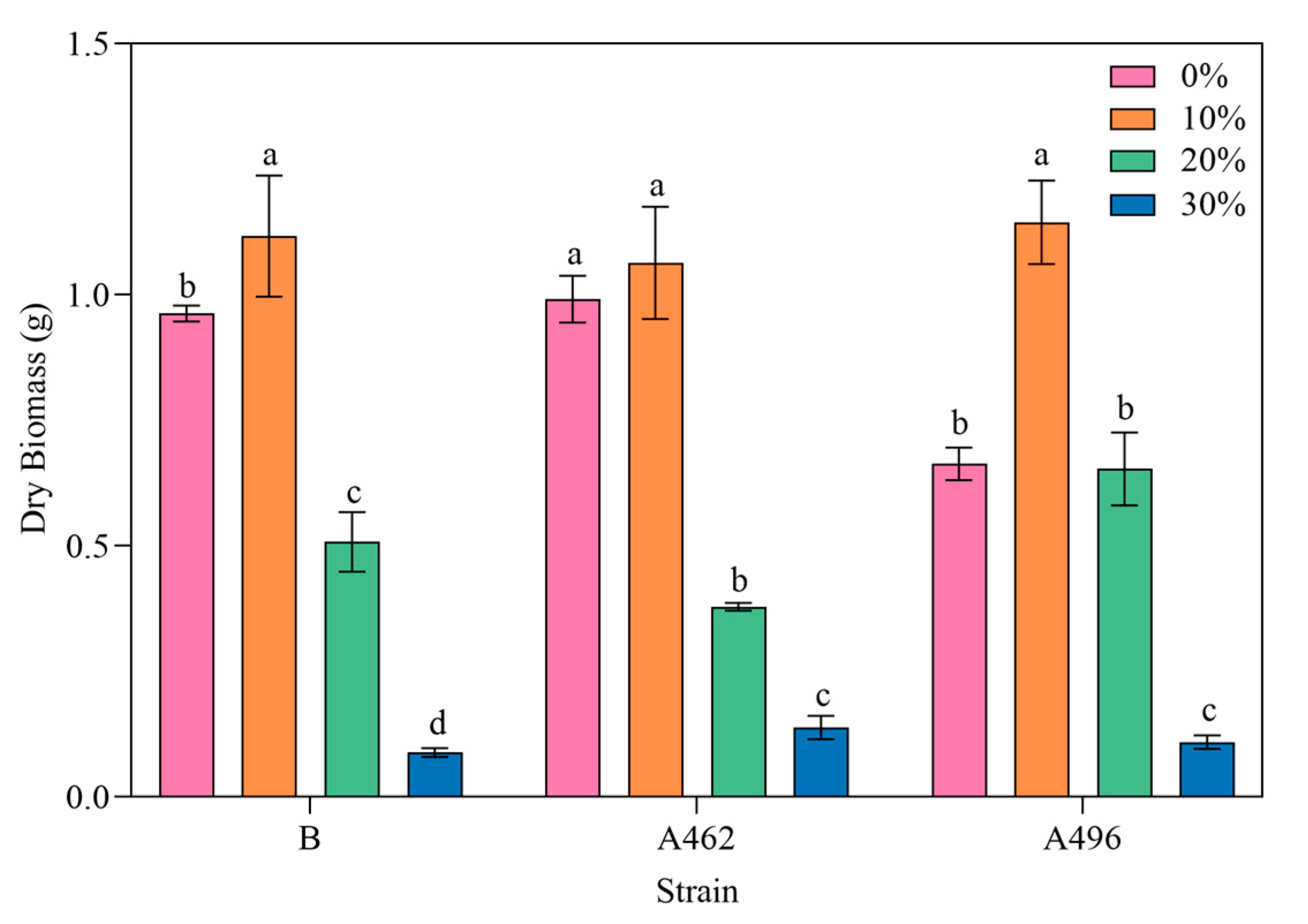
3.1. Study on Drought Tolerance of A. heimuer
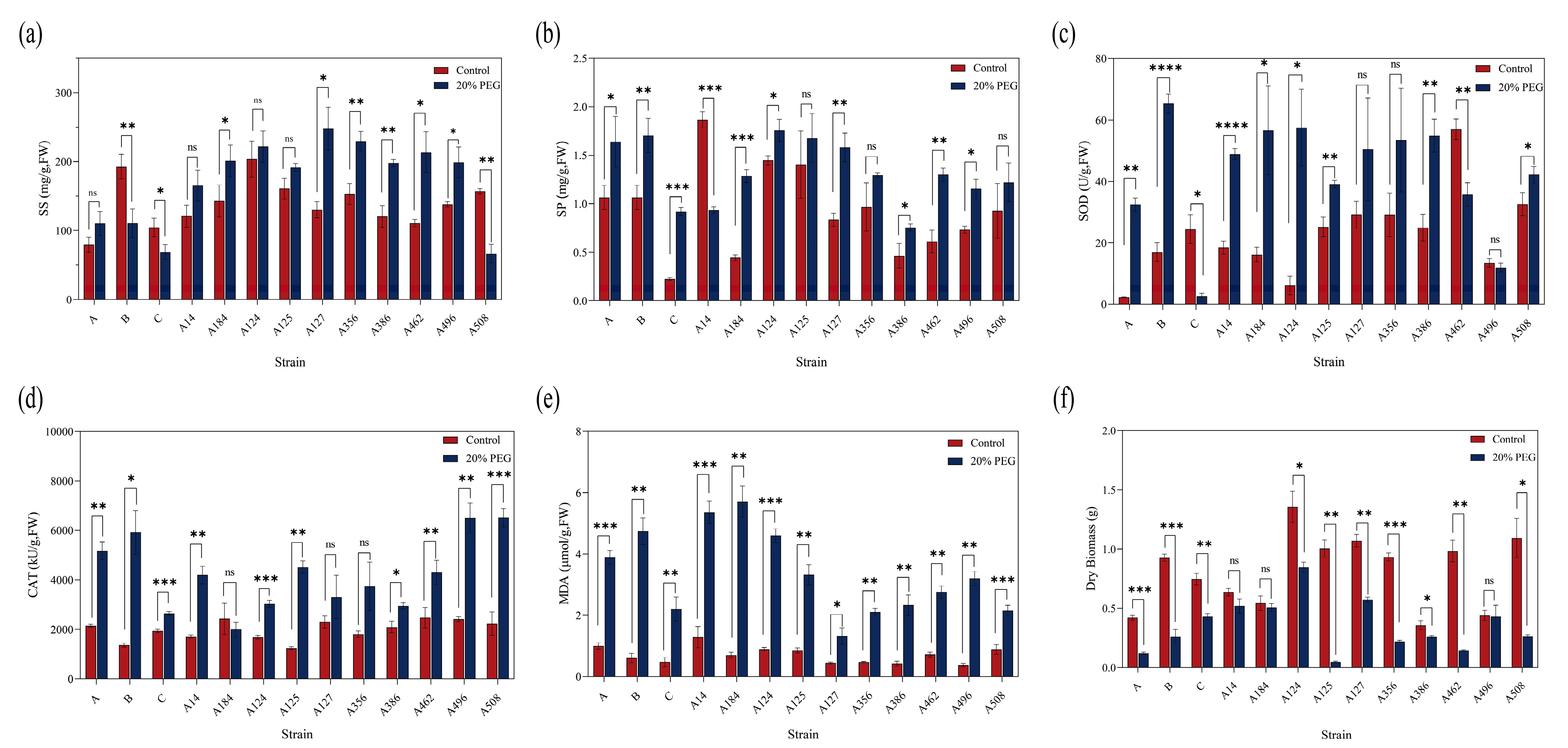
3.2. Physiological Response of A. heimuer to Drought Stress
3.3. Identification and Evaluation of Drought Tolerance in A. heimuer
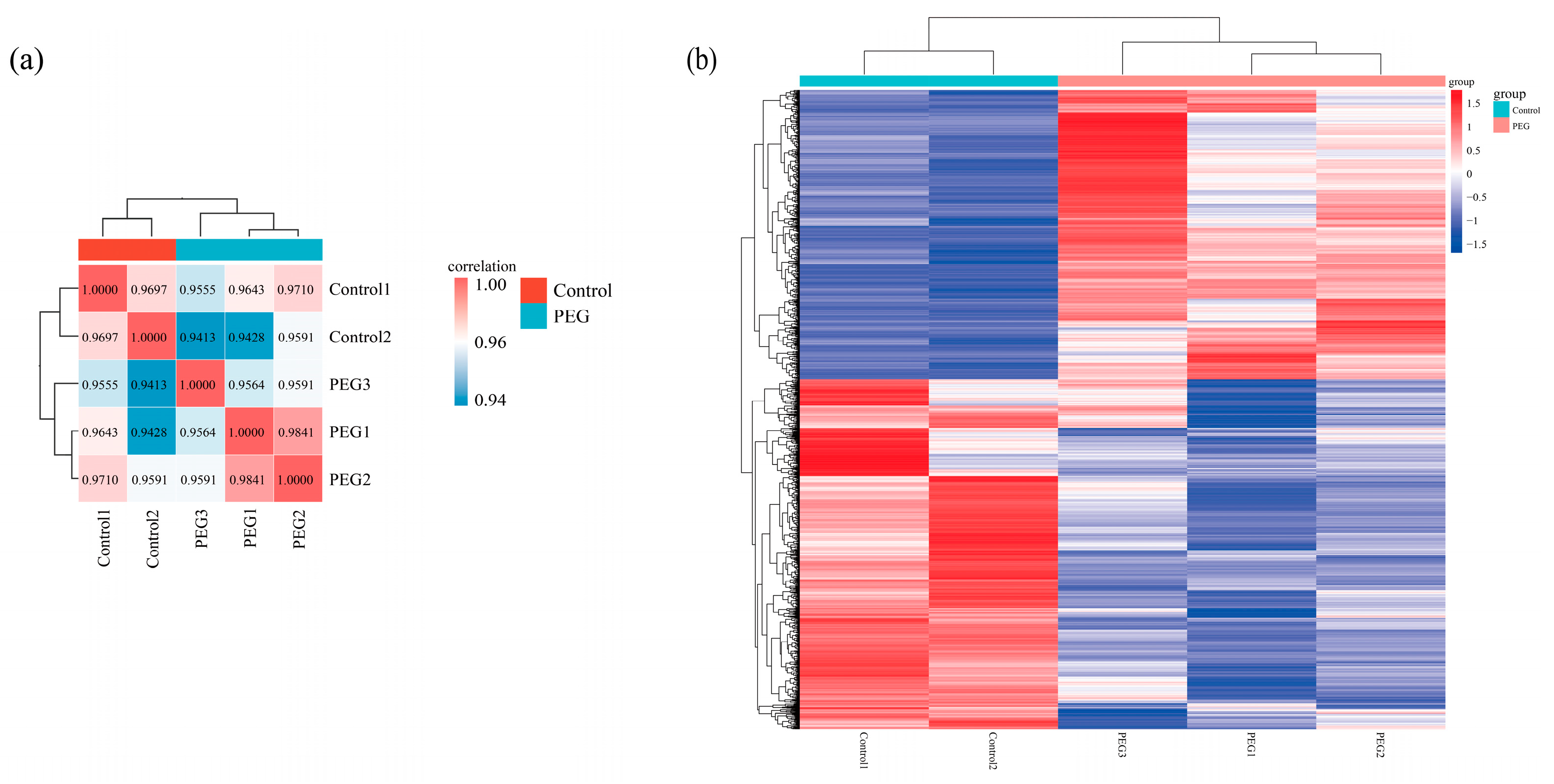
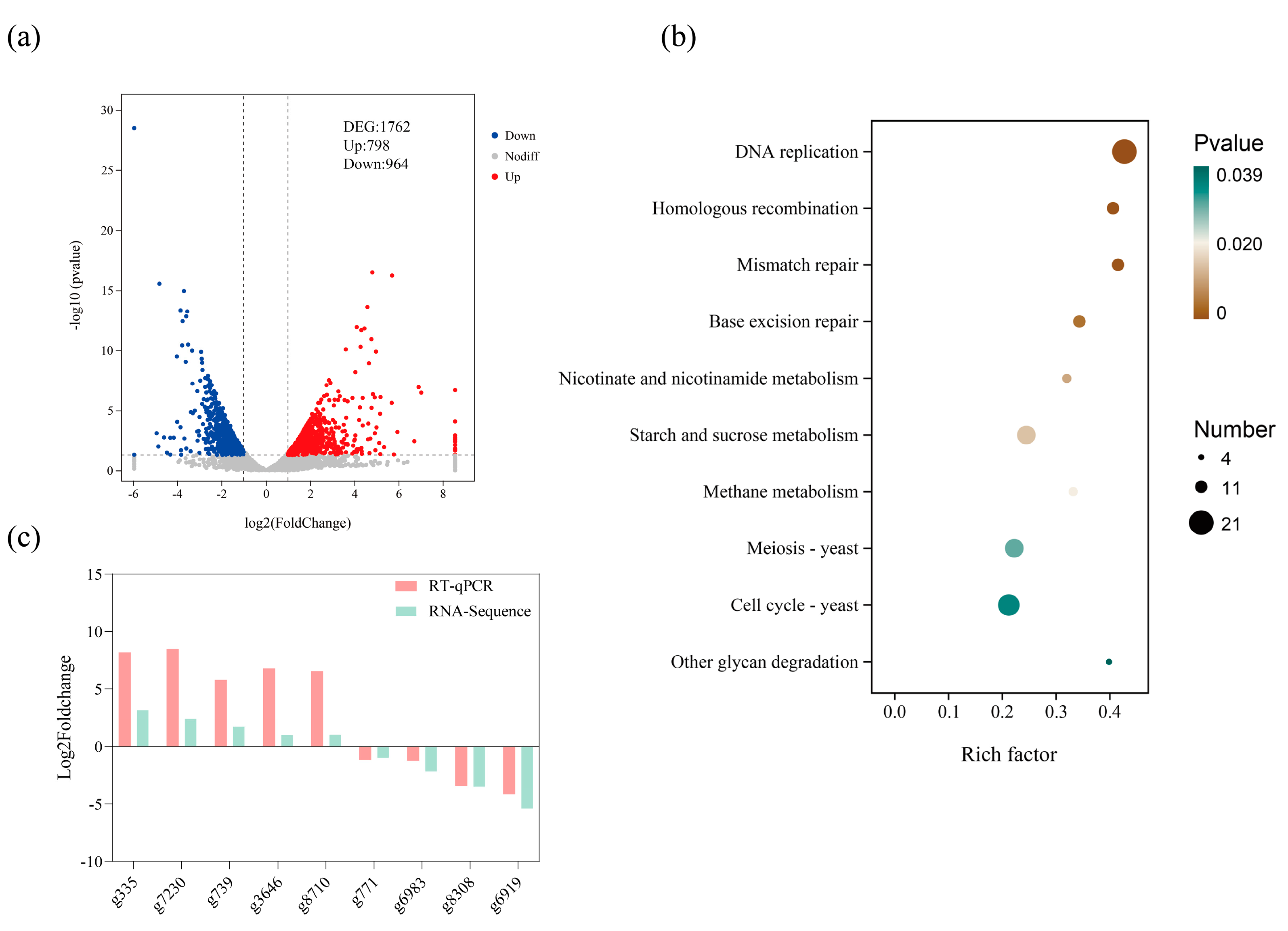
3.4. High-Throughput RNA Sequencing of Strains Under Drought Stress
3.5. KEGG Enrichment Analysis and Quantitative Real-Time PCR Verification
3.6. Involvement of Trehalose in the Drought Stress Response of A. heimuer
4. Discussion
4.1. Physiological Responses to Abiotic Stress
4.2. Drought Tolerance Assessment
4.3. Study on the Drought Tolerance of A. heimuer
4.4. Trehalose Synthesis Pathway
4.5. Trehalose Involvement in Stress Response
4.6. Potential Mechanisms of Drought Stress Response in A. heimuer
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lu, L.X.; Yao, F.J.; Wang, P.; Fang, M.; Zhang, Y.M.; Zhang, W.T.; Kong, X.H.; Lu, J. Construction of a genetic linkage map and QTL mapping of agronomic traits in Auricularia auricula-judae. J. Microbiol. 2017, 55, 792–799. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.; Lu, L.; Yao, F.; Fang, M.; Ma, X.; Meng, J.; Shao, K. Dicarboxylic Amino Acid Permease 7219 Regulates Fruiting Body Type of Auricularia heimuer. J. Fungi 2023, 9, 876. [Google Scholar] [CrossRef]
- Wu, F.; Yuan, Y.; Malysheva, V.F.; Du, P.; Dai, Y.-C. Species clarification of the most important and cultivated Auricularia mushroom “Heimuer”: Evidence from morphological and molecular data. Phytotaxa 2014, 186, 241–253. [Google Scholar] [CrossRef]
- Yao, F.J. The Cultivation Spread of Auricularia auricula from North to South in China. In Edible Fungi of China; Chinese Edible Fungi Editorial Department: Kunming, China, 2012. [Google Scholar]
- Ma, H.; Xu, X.; Feng, L. Responses of antioxidant defenses and membrane damage to drought stress in fruit bodies of Auricularia auricula-judae. World J. Microbiol. Biotechnol. 2013, 30, 119–124. [Google Scholar] [CrossRef]
- Yao, F.; Zhang, Y.; Lu, L.; Fang, M. Research Progress on Genetics and Breeding of Auricularia auricula-judae. J. Fungal Res. 2015, 19, 125–128. [Google Scholar]
- Dubois, M.; Inzé, D. Plant growth under suboptimal water conditions: Early responses and methods to study them. J. Exp. Bot. 2020, 71, 1706–1722. [Google Scholar] [CrossRef]
- Osmolovskaya, N.; Shumilina, J.; Kim, A.; Didio, A.; Grishina, T.; Bilova, T.; Keltsieva, O.A.; Zhukov, V.; Tikhonovich, I.; Tarakhovskaya, E.; et al. Methodology of Drought Stress Research: Experimental Setup and Physiological Characterization. Int. J. Mol. Sci. 2018, 19, 4089. [Google Scholar] [CrossRef] [PubMed]
- Sobahan, M.A.; Akter, N.; Rana, M.M. Polyethylene glycol mediated drought stress impacts on germination, growth and accumulation of proline in rice (Oryza sativa L.): Drought stress impacts in rice. SAARC J. Agric. 2022, 20, 107–119. [Google Scholar] [CrossRef]
- Lei, C.; Bagavathiannan, M.; Wang, H.; Sharpe, S.M.; Meng, W.; Yu, J. Osmopriming with Polyethylene Glycol (PEG) for Abiotic Stress Tolerance in Germinating Crop Seeds: A Review. Agronomy 2021, 11, 2194. [Google Scholar] [CrossRef]
- Zhao, Y.; Cheng, X.; Liu, X.; Wu, H.; Bi, H.; Xu, H. The Wheat MYB Transcription Factor TaMYB (31) Is Involved in Drought Stress Responses in Arabidopsis. Front. Plant Sci. 2018, 9, 1426. [Google Scholar] [CrossRef]
- Rong, W.; Qi, L.; Wang, A.; Ye, X.; Du, L.; Liang, H.; Xin, Z.; Zhang, Z. The ERF transcription factor TaERF3 promotes tolerance to salt and drought stresses in wheat. Plant Biotechnol. J. 2014, 12, 468–479. [Google Scholar] [CrossRef] [PubMed]
- Mishra, S.S.; Panda, D. Leaf Traits and Antioxidant Defense for Drought Tolerance During Early Growth Stage in Some Popular Traditional Rice Landraces from Koraput, India. Rice Sci. 2017, 24, 207–217. [Google Scholar] [CrossRef]
- Limapichat, W. Genetically encoded voltage indicator proteins revealed differential effects of hyperosmotic stress on yeast plasma membrane potential imposed by different stress conditions. FEMS Microbiol. Lett. 2022, 368, 21–24. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, X.; Zhou, Q.; Zhang, X.; Wei, J. Comparative transcriptome analysis of the lichen-forming fungus Endocarpon pusillum elucidates its drought adaptation mechanisms. Sci. China Life Sci. 2015, 58, 89–100. [Google Scholar] [CrossRef] [PubMed]
- Duran, R.; Cary, J.W.; Calvo, A.M. Role of the osmotic stress regulatory pathway in morphogenesis and secondary metabolism in filamentous fungi. Toxins 2010, 2, 367–381. [Google Scholar] [CrossRef]
- Zadrąg-Tęcza, R.; Maślanka, R.; Bednarska, S.; Kwolek-Mirek, M. Response Mechanisms to Oxidative Stress in Yeast and Filamentous Fungi. In Stress Response Mechanisms in Fungi: Theoretical and Practical Aspects; Skoneczny, M., Ed.; Springer International Publishing: Cham, Switzerland, 2018; pp. 1–34. [Google Scholar]
- Yaakoub, H.; Mina, S.; Calenda, A.; Bouchara, J.P.; Papon, N. Oxidative stress response pathways in fungi. Cell. Mol. Life Sci. 2022, 79, 333. [Google Scholar] [CrossRef]
- Crowe, J.H.; Crowe, L.M.; Chapman, D. Preservation of Membranes in Anhydrobiotic Organisms: The Role of Trehalose. Science 1984, 223, 701–703. [Google Scholar] [CrossRef]
- Ribeiro, G.D.; de Holanda Paranhos, L.; Eleutherio, E.C.A. Trehalose promotes biological fitness of fungi. Fungal Biol. 2024. [Google Scholar] [CrossRef]
- Fernandez, O.; Béthencourt, L.; Quero, A.; Sangwan, R.S.; Clément, C. Trehalose and plant stress responses: Friend or foe? Trends Plant Sci. 2010, 15, 409–417. [Google Scholar] [CrossRef]
- Iturriaga, G.; Suárez, R.; Nova-Franco, B. Trehalose metabolism: From osmoprotection to signaling. Int. J. Mol. Sci. 2009, 10, 3793–3810. [Google Scholar] [CrossRef]
- Morabito, C.; Secchi, F.; Schubert, A. Grapevine TPS (trehalose-6-phosphate synthase) family genes are differentially regulated during development, upon sugar treatment and drought stress. Plant Physiol. Biochem. 2021, 164, 54–62. [Google Scholar] [CrossRef] [PubMed]
- El-Bashiti, T.; Hamamcı, H.; Öktem, H.A.; Yücel, M. Biochemical analysis of trehalose and its metabolizing enzymes in wheat under abiotic stress conditions. Plant Sci. 2005, 169, 47–54. [Google Scholar] [CrossRef]
- Han, B.; Fu, L.; Zhang, D.; He, X.; Chen, Q.; Peng, M.; Zhang, J. Interspecies and Intraspecies Analysis of Trehalose Contents and the Biosynthesis Pathway Gene Family Reveals Crucial Roles of Trehalose in Osmotic-Stress Tolerance in Cassava. Int. J. Mol. Sci. 2016, 17, 1077. [Google Scholar] [CrossRef] [PubMed]
- Jiang, D.; Chen, W.; Gao, J.; Yang, F.; Zhuang, C. Overexpression of the trehalose-6-phosphate phosphatase OsTPP3 increases drought tolerance in rice. Plant Biotechnol. Rep. 2019, 13, 285–292. [Google Scholar] [CrossRef]
- Ren, J.; Wang, Q.; Zuo, J.; Jiang, S. Study of thermotolerant mechanism of Stropharia rugosoannulata under high temperature stress based on the transcriptome sequencing. Mycoscience 2021, 62, 95–105. [Google Scholar] [CrossRef]
- Yan, Z.Y.; Zhao, M.R.; Huang, C.Y.; Zhang, L.J.; Zhang, J.X. Trehalose alleviates high-temperature stress in Pleurotus ostreatus by affecting central carbon metabolism. Microb. Cell Fact. 2021, 20, 82. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.; Yan, P.; Leng, D.; Shang, L.; Zhang, C.; Wu, Z.; Wang, Z. Salicylic Acid Treatment Alleviates the Heat Stress Response by Reducing the Intracellular ROS Level and Increasing the Cytosolic Trehalose Content in Pleurotus ostreatus. Microbiol. Spectr. 2023, 11, e03113-22. [Google Scholar] [CrossRef]
- Zhang, Q.; Feng, R.; Miao, R.; Lin, J.; Cao, L.; Ni, Y.; Li, W.; Zhao, X. Combined transcriptomics and metabolomics analysis reveals the molecular mechanism of heat tolerance of Le023M, a mutant in Lentinula edodes. Heliyon 2023, 9, e18360. [Google Scholar] [CrossRef]
- Fang, M.; Wang, X.; Chen, Y.; Wang, P.; Lu, L.X.; Lu, J.; Yao, F.J.; Zhang, Y.M. Genome Sequence Analysis of Auricularia heimuer Combined with Genetic Linkage Map. J. Fungi 2020, 6, 37. [Google Scholar] [CrossRef]
- Islam, T.; Yao, F.; Kang, W.; Lu, L.; Xu, B. A systematic study on mycochemical profiles, antioxidant, and anti-inflammatory activities of 30 varieties of Jew’s ear (Auricularia auricula-judae). Food Sci. Hum. Well. 2022, 11, 781–794. [Google Scholar] [CrossRef]
- Ying, C.; Fang-Jie, Y.; You-Min, Z.; Ming, F. Numerical classification of cultivated germplasm of Auricularia auricula-judae. Mycosystema 2014, 33, 984–996. [Google Scholar] [CrossRef]
- Gao, S.; Kong, Y.; Lv, Y.; Cao, B.; Chen, Z.; Xu, K. Effect of different LED light quality combination on the content of vitamin C, soluble sugar, organic acids, amino acids, antioxidant capacity and mineral elements in green onion (Allium fistulosum L.). Food Res. Int. 2022, 156, 111329. [Google Scholar] [CrossRef] [PubMed]
- Lin, K.-H.; Huang, M.-Y.; Huang, W.-D.; Hsu, M.-H.; Yang, Z.-W.; Yang, C.-M. The effects of red, blue, and white light-emitting diodes on the growth, development, and edible quality of hydroponically grown lettuce (Lactuca sativa L. var. capitata). Sci. Hortic. 2013, 150, 86–91. [Google Scholar] [CrossRef]
- Sarker, U.; Oba, S. Catalase, superoxide dismutase and ascorbate-glutathione cycle enzymes confer drought tolerance of Amaranthus tricolor. Sci. Rep. 2018, 8, 16496. [Google Scholar] [CrossRef]
- Hadwan, M.H.; Abed, H.N. Data supporting the spectrophotometric method for the estimation of catalase activity. Data Brief 2016, 6, 194–199. [Google Scholar] [CrossRef] [PubMed]
- Quagliata, G.; Abdirad, S.; Celletti, S.; Sestili, F.; Astolfi, S. Screening of Triticum turgidum genotypes for tolerance to drought stress. Plant Physiol. Biochem. 2023, 194, 271–280. [Google Scholar] [CrossRef]
- Scheid, S.S.; Faria, M.G.I.; Velasquez, L.G.; do Valle, J.S.; Gonçalves, A.C., Jr.; Dragunski, D.C.; Colauto, N.B.; Linde, G.A. Iron biofortification and availability in the mycelial biomass of edible and medicinal basidiomycetes cultivated in sugarcane molasses. Sci. Rep. 2020, 10, 12875. [Google Scholar] [CrossRef]
- Chen, X.; Min, D.; Yasir, T.A.; Hu, Y.-G. Evaluation of 14 morphological, yield-related and physiological traits as indicators of drought tolerance in Chinese winter bread wheat revealed by analysis of the membership function value of drought tolerance (MFVD). Field Crops Res. 2012, 137, 195–201. [Google Scholar] [CrossRef]
- Sekova, V.Y.; Dergacheva, D.I.; Isakova, E.P.; Gessler, N.N.; Tereshina, V.M.; Deryabina, Y.I. Soluble Sugar and Lipid Readjustments in the Yarrowia lipolytica Yeast at Various Temperatures and pH. Metabolites 2019, 9, 307. [Google Scholar] [CrossRef]
- Pouris, J.; Kapnopoulou, K.; Papadopoulou, S.; Pyrri, I. Studying free Proline and Soluble Sugars Accumulation in the Fungus Aspergillus creber Exposed to Salt Stress. J. Biomed. Res. Environ. Sci. 2023, 4, 440–445. [Google Scholar] [CrossRef]
- Bhandari, U.; Gajurel, A.; Khadka, B.; Thapa, I.; Chand, I.; Bhatta, D.; Poudel, A.; Pandey, M.; Shrestha, S.; Shrestha, J. Morpho-physiological and biochemical response of rice (Oryza sativa L.) to drought stress: A review. Heliyon 2023, 9, e13744. [Google Scholar] [CrossRef] [PubMed]
- Dong, Q.; Chen, M.; Zhang, Y.; Song, P.; Yang, H.; Zhao, Y.; Yu, C.; Zha, L. Integrated physiologic and proteomic analysis of Stropharia rugosoannulata mycelia in response to Cd stress. J. Hazard. Mater. 2023, 441, 129877. [Google Scholar] [CrossRef]
- Li, P.; Hu, C.; Li, Y.; Ge, L.; Wu, G.; Lv, B.; Jiang, W.; Xi, D. The cold—Resistance mechanism of a mutagenic Volvariella volvacea strain VH3 with outstanding traits revealed by transcriptome profiling. BMC Microbiol. 2021, 21, 336. [Google Scholar] [CrossRef] [PubMed]
- Ma, H.L.; Ma, S.Y.; Fu, C.X.; Yang, J.Q.; Li, D.L. Antioxidant Defenses Against Air Humidity Stress in Fruit Bodies of Auricularia heimuer (Agaricomycetes). Int. J. Med. Mushrooms 2024, 26, 53–61. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Prasad, A.; Sedlářová, M.; Pospíšil, P. Malondialdehyde enhances PsbP protein release during heat stress in Arabidopsis. Plant Physiol. Biochem. 2023, 202, 107984. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Huang, D.; Zhao, X.; Zhang, M. Evaluation of drought resistance and transcriptome analysis for the identification of drought-responsive genes in Iris germanica. Sci. Rep. 2021, 11, 16308. [Google Scholar] [CrossRef] [PubMed]
- Ma, H.-l.; Xu, X.-h.; Zhao, X.-y.; Liu, H.-j.; Chen, H. Impacts of drought stress on soluble carbohydrates and respiratory enzymes in fruit body of Auricularia auricula. Biotechnol. Biotechnol. Equip. 2014, 29, 10–14. [Google Scholar] [CrossRef] [PubMed]
- Argüelles, J.C.; Guirao-Abad, J.P.; Sánchez-Fresneda, R. Trehalose: A Crucial Molecule in the Physiology of Fungi. In Encyclopedia of Microbiology, 4th ed.; Schmidt, T.M., Ed.; Academic Press: Oxford, UK, 2017; pp. 486–494. [Google Scholar]
- Saito, K.; Kase, T.; Takahashi, E.; Takahashi, E.; Horinouchi, S. Purification and Characterization of a Trehalose Synthase from the Basidiomycete Grifola frondosa. Appl. Environ. Microbiol. 1998, 64, 4340–4345. [Google Scholar] [CrossRef]
- Kitamoto, Y.; Akashi, H.; Tanaka, H.; Mori, N. α-Glucose-1-phosphate formation by a novel trehalose phosphorylase from Flammulina velutipes. FEMS Microbiol. Lett. 1988, 55, 147–149. [Google Scholar] [CrossRef][Green Version]
- Han, S.-E.; Kwon, H.-B.; Lee, S.-B.; Yi, B.-Y.; Murayama, I.; Kitamoto, Y.; Byun, M.-O. Cloning and characterization of a gene encoding trehalose phosphorylase (TP) from Pleurotus sajor-caju. Protein Expr. Purif. 2003, 30, 194–202. [Google Scholar] [CrossRef]
- Lei, M.; Wu, X.; Huang, C.; Qiu, Z.; Wang, L.; Zhang, R.; Zhang, J. Trehalose induced by reactive oxygen species relieved the radial growth defects of Pleurotus ostreatus under heat stress. Appl. Microbiol. Biotechnol. 2019, 103, 5379–5390. [Google Scholar] [CrossRef]
- Zhao, X.; Song, X.; Li, Y.; Yu, C.; Zhao, Y.; Gong, M.; Shen, X.; Chen, M. Gene expression related to trehalose metabolism and its effect on Volvariella volvacea under low temperature stress. Sci. Rep. 2018, 8, 11011. [Google Scholar] [CrossRef] [PubMed]
- Eleutherio, E.; Panek, A.; De Mesquita, J.F.; Trevisol, E.; Magalhães, R. Revisiting yeast trehalose metabolism. Curr. Genet. 2015, 61, 263–274. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Yang, Z.; Shi, L.; Yang, R.; Guo, H.; Zhang, S.; Geng, G. Transcriptome analysis of Auricularia fibrillifera fruit-body responses to drought stress and rehydration. BMC Genom. 2022, 23, 58. [Google Scholar] [CrossRef] [PubMed]
- Bagri, D.S.; Upadhyaya, D.C.; Kumar, A.; Upadhyaya, C.P. Overexpression of PDX-II gene in potato (Solanum tuberosum L.) leads to the enhanced accumulation of vitamin B6 in tuber tissues and tolerance to abiotic stresses. Plant Sci. 2018, 272, 267–275. [Google Scholar] [CrossRef]
- Guhr, A.; Horn, M.A.; Weig, A.R. Vitamin B2 (riboflavin) increases drought tolerance of Agaricus bisporus. Mycologia 2017, 109, 860–873. [Google Scholar] [CrossRef]

| Experiment ID | Preservation ID | Strain Type | Source |
|---|---|---|---|
| A | JAUAH590 | Cultivated | Jilin Province |
| B | JAUAH591 | Cultivated | Jilin Province |
| C | JAUAH592 | Cultivated | Jilin Province |
| A14 | JAUAH14 | Cultivated | Heilongjiang Province |
| A184 | JAUAH184 | Cultivated | Shandong Province |
| A124 | JAUAH124 | Wild | Heilongjiang Province |
| A125 | JAUAH125 | Wild | Heilongjiang Province |
| A127 | JAUAH127 | Wild | Jilin Province |
| A356 | JAUAH356 | Wild | Yunnan Province |
| A386 | JAUAH386 | Wild | North Korea |
| A462 | JAUAH462 | Wild | Shandong Province |
| A496 | JAUAH496 | Wild | Jilin Province |
| A508 | JAUAH508 | Wild | Yunnan Province |
| Strain | D | Rank | Drought Tolerance |
|---|---|---|---|
| A | 0.565 | 1 | HDT |
| A127 | 0.515 | 2 | DT |
| C | 0.508 | 3 | DT |
| A124 | 0.470 | 4 | MDT |
| A14 | 0.465 | 5 | MDT |
| A386 | 0.451 | 6 | MDT |
| A462 | 0.441 | 7 | MDT |
| A184 | 0.430 | 8 | MDT |
| A496 | 0.426 | 9 | MDT |
| A125 | 0.397 | 10 | MDT |
| B | 0.391 | 11 | MDT |
| A356 | 0.385 | 12 | S |
| A508 | 0.357 | 13 | S |
| Sample | Raw Read Number | Clean Read Number | Q30 Score | Gene Map Rate (%) | Expressed Gene |
|---|---|---|---|---|---|
| Control 1 | 38,785,758 | 37,001,576 | 94.22 | 88.14 | 9683 |
| Control 2 | 46,536,944 | 44,469,130 | 94.79 | 90.89 | 9204 |
| 20% PEG1 | 49,262,512 | 46,986,024 | 94.57 | 91.15 | 9797 |
| 20% PEG2 | 46,805,826 | 44,663,836 | 94.45 | 91.65 | 10,054 |
| 20% PEG3 | 51,027,092 | 48,367,260 | 94.78 | 91.04 | 10,183 |
| Gene | Fold Change (20% PEG/Control) | Pval | Regulation |
|---|---|---|---|
| trehalose 6-phosphate synthase (TPS) | 2.23 | 0.019 | Up |
| trehalose 6-phosphate phosphatase (TPP) | 2.12 | 0.025 | Up |
| trehalase (TREH) | / | / | / |
| trehalose phosphorylase (TreP) | 2.37 | 0.010 | Up |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, J.; Yao, F.; Lu, L.; Zhang, Y.; Fang, M.; Ma, X.; Shao, K.; Sun, X. Evaluation of Drought Tolerance and Trehalose Response in Auricularia heimuer. Horticulturae 2024, 10, 1312. https://doi.org/10.3390/horticulturae10121312
Sun J, Yao F, Lu L, Zhang Y, Fang M, Ma X, Shao K, Sun X. Evaluation of Drought Tolerance and Trehalose Response in Auricularia heimuer. Horticulturae. 2024; 10(12):1312. https://doi.org/10.3390/horticulturae10121312
Chicago/Turabian StyleSun, Jian, Fangjie Yao, Lixin Lu, Youmin Zhang, Ming Fang, Xiaoxu Ma, Kaisheng Shao, and Xu Sun. 2024. "Evaluation of Drought Tolerance and Trehalose Response in Auricularia heimuer" Horticulturae 10, no. 12: 1312. https://doi.org/10.3390/horticulturae10121312
APA StyleSun, J., Yao, F., Lu, L., Zhang, Y., Fang, M., Ma, X., Shao, K., & Sun, X. (2024). Evaluation of Drought Tolerance and Trehalose Response in Auricularia heimuer. Horticulturae, 10(12), 1312. https://doi.org/10.3390/horticulturae10121312





