Abstract
Plant small molecules, such as nitric oxide (NO) and melatonin (MN) as natural and human health-friendly compounds, play important roles in the mitigation of abiotic stresses in plants. Heavy metals such as chromium (Cr) are hazardous for the survival of ornamentals, especially edible flowers. This study evaluated the effects of NO (50 µM; sourced as sodium nitroprusside) and MN (50 µM) applied two times through foliar spraying at 1-week intervals on alleviating Cr (120 µM; K2Cr2O7)-induced oxidative stress in edible flowers of Calendula officinalis cv. Orange King. Cr stress decreased plant dry mass, leaf SPAD values, net photosynthetic rates, and the maximum photochemical quantum yield (Fv/Fm), and increased the oxidative stress markers. The individual application of NO or MN significantly mitigated the adverse effects, and the combined application of NO and MN synergistically enhanced plant tolerance to Cr stress, including increased activities of antioxidant enzymes in plants and concentrations of carbohydrate, ascorbic acid, sugar, total protein, as well as ash contents of edible flowers. The co-application also significantly elevated the concentrations of total phenolics, flavonoids, free reducing power, antioxidant capacity DPPH, and total carotenoids in Cr-treated plants compared with those in Cr-stressed plants. Additionally, the essential oil contents in flowers increased in response to the signaling molecule treatment under Cr stress. Compared with individual applications, the co-application of NO and MN had more significant effects. Our results indicate that the combination of signaling molecules, such as MN and NO, can not only increase the biomass of edible calendula plants but also improve flower quality for use as a novel food.
1. Introduction
Research into novel healthy foods continues to grow due to the increasing demand for nutraceuticals and functional foods, as well as their usefulness in both the prevention and treatment of chronic diseases [1]. In addition to their nutritious value, consumers are constantly searching for products with organoleptic properties. Edible flowers satisfy these requirements by adding vibrant and appealing colors, textures, and enthusiasm to meals [1,2]. Edible flowers have been used for thousands of years, but they account for a small fraction of the market and are now regarded as a culinary curiosity [1,3]. Indeed, no international body has published official lists of edible and nonedible flowers, nor have any legal standards for edible flower marketing been documented, suggesting the need to take necessary precautions for safe production, composition, preservation, and consumption [1]. Several abiotic and biotic factors can influence the qualities of edible flowers; hence, their nutritional value, antioxidant properties, and color are only a few of the significant attributes to consider when evaluating “novel foods” [1,2,3].
The abiotic factors influencing plant growth include soil nutrients, pH, and salinity; heavy metals (HMs); temperatures; and water availability. Plants encountering extreme levels of these factors are commonly referred to as abiotic stresses. It has been estimated that abiotic stresses could lead to 50% yield losses in crop production [4]. One of the abiotic stresses is the increase in harmful HMs and metalloids in soils, which can greatly reduce the productivity of agricultural land and negatively affect plant growth [5,6,7]. These HM levels in the soil are exacerbated because of both natural and anthropogenic activities. Furthermore, with the continuous increase in the human population, there is extreme pressure on agricultural resources, such as the use of sewage water to cater to the water scarcity issue in agriculture. In addition to this, industrialization has posed a threat to agricultural soils due to their direct discharges in irrigation water, which ultimately pollutes the soils [3,4].
Heavy metals are hazardous substances for horticultural plant production systems [7]. Among HMs, chromium (Cr) is a comparatively less explored HM because of its poor impact on the ornamental plant production sector given the growing problem of Cr in agricultural soils. Cr is carcinogenic and may enter the food chain through plants, causing skin eruptions, asthma, coughing, ulcers, gastric disturbances, kidney damage, a weakened immune system, cancers, and genetic mutations [7]. Cr has severe negative effects on plants owing to its redox potential, which makes it highly soluble in plants [8]. Although it can occur under a variety of oxidation conditions in nature, hexavalent Cr(VI) and trivalent Cr(III) are the most prevalent and constant forms. In contrast to Cr(III), Cr(VI) is more mobile in soils, more soluble in water, and more harmful to living things because of its higher oxidation state [9]. Currently, there is an increasing Cr(VI) contamination in soil and water worldwide, which is commonly caused by industrial processes, such as electroplating, steel manufacturing, paint production, leather processing, textile dyeing, metallurgy, wood preservation, and tanning [9]. The exposure of plants to such soils and irrigation water containing Cr can substantially suppress plant growth and development.
Chromium plays no role in plants, and there is no distinct mechanism for its uptake or transport. However, transporters that facilitate the absorption of essential elements can be used for Cr absorption [7]. Owing to their similarity in chemical properties, Cr(IV) can be taken up by phosphate or sulfur transporters [10]. When Cr enters the food chain, it can cause dermatitis, asthma, tumors, and other conditions in living organisms, including humans [11,12]. Cr causes plants to produce more reactive oxygen species (ROS), severely damaging macromolecules, such as proteins, lipids, and membranes, impairing their ability to function [9,13]. The main consequences of Cr in plants include alterations in gene expression, a decrease in enzyme activity and mineral absorption, ultrastructural alterations of the cell membrane and chloroplast, disruption of carbohydrate metabolism, and damage to root cells, which result in leaf chlorosis and necrosis and stunted growth [7,12,13].
Certain naturally occurring vital strategies exist in plants to mitigate the detrimental impacts of Cr on plants. These mechanisms include enzymatic and nonenzymatic antioxidant systems that neutralize ROS; osmoprotectant buildup; compartmentalization; and the sequestration or chelation of toxic metal ions, which work together to protect key metabolic pathways. Studies have shown that the exogenous application of phytohormones, mineral ions, and bioactive molecules can trigger plant defense mechanisms against metal stress and improve crop production [7,13].
Nitric oxide (NO) as a gaseous signaling molecule is not only involved in the regulatory mechanisms of plants but also vitalizes stress tolerance [14]. NO broadly contributes to regulating seed germination, root organogenesis, floral development, senescence, photosynthesis, and environmental stresses in plants [15]. Owing to its synergistic and antagonistic roles with other plant hormones, NO imparts tolerance against abiotic stresses in plants by modulating biochemical pathways and signal transduction [6]. Various studies have revealed that NO promotes abiotic stress tolerance by triggering antioxidant activity and reducing the uptake of metal ions [7,15,16]. NO can either directly scavenge ROS or cause the overexpression of antioxidant genes [7,13,14]. However, the regulatory mechanisms of NO-facilitated mitigation of Cr stress in edible flowers remain unclear.
On the other hand, melatonin (MN) is a regulatory molecule with the ability to enhance plant tolerance to abiotic and biotic stresses. It is a significant gene expression modulator and affects the production and activity of numerous important phytohormones [17]. MN application averts the oxidative effects of HMs by upregulating the antioxidant system and maintaining redox homeostasis, reflecting improved photosynthesis [17]. The upregulation of genes related to vital pathways due to MN application led to an improvement in Cr stress in Brassica juncea (L.) Czern [18]. The generation of anthocyanins and the maintenance of endogenous MN levels in tomatoes are related to MN-mediated Cr tolerance [19].
Both nitric oxide and MN are widely reported to enhance the biosynthesis and accumulation of secondary metabolites and induce tolerance to biotic and abiotic stresses in plants [7,9,19]. However, limited information is available on the role of these signaling molecules in alleviating Cr stress in plants and increasing the levels of secondary metabolites in edible flowers.
Calendula officinalis L., commonly known as calendula, is a vital edible ornamental flowering plant in the family Asteraceae and is grown worldwide because of its high content of phytochemicals and visual appearance [20]. It is consumed in different forms, such as salad, dried, and pharmaceutical products, owing to its antitumor, anti-inflammatory, antioxidant, antiseptic, and antibacterial properties [21]. Flavonoid chemicals, which exhibit antioxidant properties and are vital for human health by fending off harm from oxidizing agents, are among the primary pigment types found in calendula [22]. Moreover, the presence of essential oils and aroma compounds in its flowers makes it a perfect constituent material for the cosmetic and perfume industries [23]. As a result, calendula flowers are considered as a functional food due to their multiple biological activities, taste, and overall appearance. However, there is little information on how heavy metals, such as Cr, affect calendula growth and flower quality.
The effects of signaling molecules on the physicochemical characteristics and antioxidant potential of calendula plants are poorly understood. Furthermore, considering the mobility of Cr(VI) in soils and irrigation water, calendula production could encounter Cr stress. Thus, research into this plant’s responses to signaling molecules, such as NO and MN, under Cr stress and the changes in its pigments, antioxidant activity, and total flavonoid and essential oil contents of flowers is much needed as a biofortification strategy. The primary goal of the present research was to study the impact of Cr on the growth of edible calendula, evaluate the effects of the foliar application of NO and MN on the mitigation of Cr stress in calendula, and analyze physiochemical parameters associated with the mitigating effects. It was anticipated that this effort could lead to the selection of the right mix of signaling molecules for enhancing antioxidant activities and improving the growth and development of calendula plants when grown under Cr stress.
2. Materials and Methods
2.1. Plant Material and Treatments
The experiment was performed at the Islamia University of Bahawalpur, Pakistan (IUB), a semiarid area of Punjab Province. The average annual temperature of the region is 25.5 °C, with 143 mm of annual precipitation. Calendula ‘Orange King’ was selected as a model plant, and its seedlings grown in plug trays filled with peat- and perlite-based growing medium were transplanted to polyethylene plastic pots after twenty days of germination. These pots were filled with sandy loam soil, which was taken from the surface soil (0–30 cm) of a farmland in Bahawalpur and was air dried and sieved (10 mm). It had 12.34 g kg−1 organic matter, a water-holding capacity of 23.22%, 17.69 g kg−1 available phosphorus, 0.81 g kg−1 total nitrogen, and 79.31 g kg−1 available potassium. Each pot contained 4.5 kg of the soil combined with 1.5 kg of compost. Prior to transplanting, each pot received 12 g of composite fertilizer, which was properly mixed with the soil. Plant cultivation and signaling molecule tests for Cr tolerance were carried out under field conditions. These pots were placed on level ground, and potted plants were irrigated as needed and grown under a natural photoperiod, with air temperatures of almost 30 °C and approx. 60–70% relative humidity. During flower bud emergence, each pot received 12 g of 0.46% urea and 15 g of 0.2% potassium dihydrogen phosphate.
Calendula plants were treated with MN (50 µM) and NO (50 µM; sourced as sodium nitroprusside) two times (first at 20 days after transplanting and second at the 1 week interval) during the experiment via a hand sprayer [12,15]. Each pot was sprayed with 20 mL of the corresponding solution, and distilled water was used as a control solution. After 10 days of treatment, the plants were treated with 120 µM Cr (K2Cr2O7) for two times along with irrigation water. There were five treatments: control (without signaling molecules and Cr), Cr only, Cr + NO, Cr + MN, and Cr + NO + MN, and 10 pots per treatment. All the applied compounds were acquired from Sigma-Aldrich (St. Louis, MO, USA).
2.2. Plant Dry Weight
At the end of the experiment, all plants from the five treatments were detached from the soil surface and washed with distilled water to remove the soil from the roots. The plants were cut into shoots, roots, and flowers and dried in an electric oven at 80 °C for two days to measure the dry weights of the roots, shoots, and flowers on a digital balance.
2.3. Leaf Gas Exchange (LGE) and Fv/Fm
An LC4 portable LGE system was used to quantify net photosynthetic rates, stomatal conductance, and internal CO2 concentrations. The gas exchange characteristics of fully expanded leaves were measured between 8:30 and 10:00 a.m. The ratio of fluorescence (Fv) to maximum fluorescence (Fm) denoted as Fv/Fm was quantified with a PAM fluorimeter when the calendula leaves had been cultivated under dark conditions for 20 min (high-performance PAM-2500 chlorophyll fluorometer by WALZ). A soil plant analysis development (SPAD) meter was used to obtain the SPAD values of four leaves from a single plant, and a mean from the four readings of that plant was calculated.
2.4. Oxidative Stress Markers and Antioxidant Enzyme Activities in Leaves
The leaf hydrogen peroxide and malondialdehyde contents of plants subjected to the five treatments were evaluated following the approaches described by Hodges et al. [24] and Heath and Parker [25], respectively. The methodology of Wild et al. [26] was employed to quantify methylglyoxal (MG) contents in calendula leaves. Elstner and Heupel’s [27] methodology was used to evaluate superoxide anion (SA) levels in calendula leaves. Catalase (CAT), superoxide dismutase (SOD), and glutathione reductase (GR) activities were evaluated following Aebi [28], Dhindsa et al. [29], and Foyer and Halliwell [30], respectively.
2.5. Analysis of Key Elements in Leaves
The micro-Kjeldahl and molybdovanado phosphate methods were used to measure the levels of nitrogen (N) and phosphorus (P) in the leaves of plants grown under the five treatments, respectively [31,32]. The amount of potassium (K) in the leaves was determined via the flame photometry method. An atomic absorption spectrometry method was used to detect the Cr contents in the leaves of the calendula.
2.6. Flower Total Carbohydrate (TC)
Fresh calendula flower petals (200 mg) were collected from individual plants grown under the five treatments. The TC level of calendula flowers was assessed via the anthrone method, with some minor alterations [33]. The collected flower petals were heated with 5 mL of HCl (2.5 N) for 3 h, after which sodium carbonate was added. The supernatant was diluted to 100 mL via distilled water, and then, a 2 mL sample was mixed with anthrone reagent (4 mL). Afterwards, the mixture was incubated for 10 min, the resulting mixture was boiled, and the absorbance was spectrophotometrically detected at 630 nm.
2.7. Total Reducing Sugars (TRSs) in Flowers
The TRS was detected following the methodology of Miller [34] with modifications. Fresh calendula flower petals (100 mg) were ground in alcohol solution (80%) and evaporated at 80 °C. Aliquots of approximately 1 mL of 3,5-dinitrosalicylic acid reagent (3 mL) were placed in a glass tube and heated for 5 min. Afterwards, sodium potassium tartrate (1 mL) was added to the glass tube, and the absorbance was spectrophotometrically measured at 510 nm. The level of TRS was calculated via a standard curve of D-glucose and denoted as mg g−1 FW.
2.8. Total Protein in Petals
Freshly collected calendula petals (approximately 1.5 g) from individual plants per treatment were ground with liquid N and combined with 10 mL of phosphate buffer (pH 7.4). After centrifugation (10 min at 8000 rpm), the protein level was assessed following the Lowry methodology [35], and the absorbance was spectrophotometrically measured at 660 nm in contrast to the reagent blank.
2.9. Petal Ascorbic Acid (AsA)
The AsA level was assessed following a titration methodology with small alterations. Freshly collected calendula petals (approximately 5 g) were ground in liquid N, after which 10 mL of oxalic acid (10 mL, 4.0%) was added, after which the mixture was filtered. Then, the filtrate sample (5 mL) was elevated to 15 mL with oxalic acid (4.0%), and titration was performed in contrast to the dye solution. The graph factor was assessed by taking standard AsA (100 µg mL−1 in 4% oxalic acid) following the exact protocol. The burette value was recorded until a pale pink shade formed [36].
2.10. Petal Total Ash Content (TAC)
A porcelain crucible was dried at 100 °C for 1 h, and then it was weighed (W1). Dried calendula petals (1.0 g, denoted W2) were placed in the weighted porcelain crucible, and it was re-weighted (W2). Afterwards, the porcelain crucible containing the calendula petal sample was firstly heated at 250 °C for 1 h and then at 550 °C for a period of 5 h in a muffle furnace heating system. The petal sample was then cooled in a desiccator and weighed again (W3). The TAC was quantified via the formula given below after weighting the ash following Sigel [37].
2.11. Petal Total Phenolics (TPC), Flavonoids (TFC), and Total Carotenoids
Dried calendula petals (1 g) were minced and extracted with methanol (96% v/v). The TPC was assessed spectrophotometrically with the Folin–Ciocalteu reagent [38]. The TFC in calendula petals was detected spectrophotometrically following the methods of Zhishen et al. [39]. The total carotenoid content in flowers was assessed following the methodology of Yang et al. [40].
2.12. Antioxidant Capacity Assays of Petals
Ferric reducing power (FRAP assay) and total antioxidant capacity were detected following the methodology of Benzie and Strain [41], and the absorbance was spectrophotometrically measured at 595 nm. The total antioxidant capacity was detected from the decolorization of the purple methanol solution 2,2-diphenylpyrylhydrazyl, following Tepe et al. [42].
2.13. Essential Oil Contents (EOCs) in Petals
EOC was determined following the methodology of Kapoor et al. [43] via a hydrodistillation process with an attached Clevenger device. For this purpose, 200 g of petals from each treatment was crushed using distilled water in a pestle mortar. The ground petals of each treatment were subjected to hydrodistillation. Hydrodistillation was performed twice against individual treatment and the average values of the extraction output are presented.
2.14. Statistical Analysis
The experiment was set to a completely randomized design with ten pots per treatment and each pot having 1 plant. The normality of the residuals was confirmed by Shapiro–Wilk’s test (p > 0.05), and the means were separated by Tukey’s HSD at the p ≤ 0.05 level. Statistical analysis was performed in Statistix 8.1 and the graphs were prepared in GraphPad Prism 10.
3. Results
3.1. Plant Dry Biomass
Compared with the control, exposure to Cr stress decreased the dry biomass of roots, shoots, and flowers by 55%, 72%, and 60%, respectively (Figure 1A–C). Individual NO and MN applications significantly improved biomass accumulation. The maximum increases occurred in the treatment of Cr + NO + MN, which were 115%, 250%, and 100% for the roots, shoots, and flowers, respectively, when compared with Cr-stressed plants without foliar spraying (Figure 1A–C).
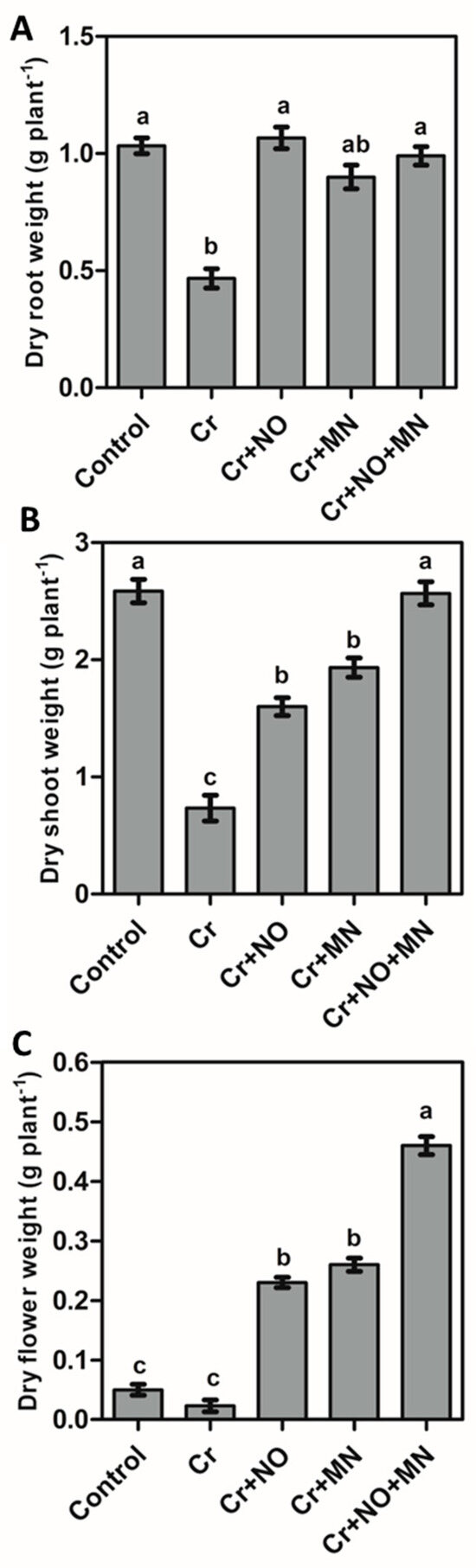
Figure 1.
Dry biomass of calendula roots (A), shoots (B), and flowers (C) in response to Cr (chromium) stress and effects of NO (nitric oxide) and MN (melatonin) applied individually and in combination on biomass accumulation when grown under Cr stress. The values are the means and SEs. Similar small letters within a graph depict no significant differences evaluated by Tukey’s HSD test at p < 0.05 level (n = 10).
3.2. Leaf Gas Exchange and Fv/Fm
The Cr stress decreased net photosynthetic rates (Pn) by 53%, stomatal conductance (Gs) by 57%, intercellular CO2 concentration (Ci) by 47%, the SPAD by 40%, and the Fv/Fm by 43% compared with the control (Table 1). The application of MN and NO individually or in combination to Cr-stressed plants elevated these parameters. The maximum increase was observed in plants treated with Cr + NO + MN, where Pn, Gs, Ci, SPAD, and Fv/Fm were 135%, 123%, 90%, 86%, and 73% greater than those of Cr-stressed plants without foliar spraying (Table 1).

Table 1.
Impact of chromium and signaling molecules on leaf gas exchange parameters, soil plant analysis development (SPAD) index, and the ratio of fluorescence (Fv) to maximum fluorescence (Fm) (Fv/Fm) of calendula.
3.3. Leaf Oxidative Stress Biomarkers
The exposure to Cr increased leaf oxidative stress biomarkers, such as hydrogen peroxide (H2O2), malondialdehyde (MDA), methylglyoxal (MG), and superoxide anion (SA) by 371%, 447%, 374%, and 146%, respectively, compared with the control (Figure 2). Individual applications of NO and MN decreased the levels of these oxidative stress markers. The maximum decrease was observed in the treatment of Cr + NO + MN, where the decreases were 59%, 64%, 67%, and 59% for H2O2, MDA, MG, and SA, respectively, compared with those in Cr-stressed plants (Figure 2).
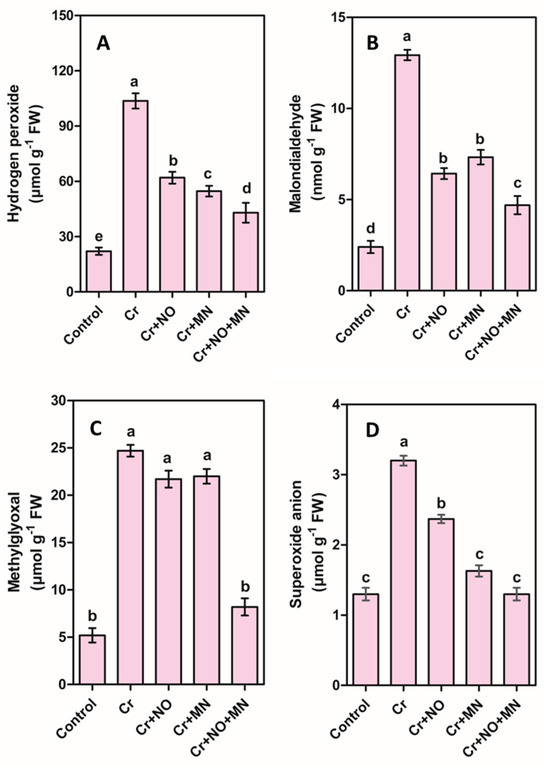
Figure 2.
Changes in oxidative stress markers including hydrogen peroxide (A), malondialdehyde (B), methylglyoxal (C), and superoxide anion (D) in leaves of calendula plants grown under Cr (chromium) stress, and effects of NO (nitric oxide) or MN (melatonin) applied individually or in combination on these stress markers. The values are the means and SEs. Similar small letters within a graph depict no significant differences evaluated by Tukey’s HSD test at p < 0.05 level (n = 10).
3.4. Leaf Antioxidant Enzyme Activities
Compared with the control, exposure to Cr increased the levels of SOD, CAT, APX, and Gr by 180%, 21%, 67%, and 100%, respectively (Figure 3). Individual applications of NO and MN increased the levels of the activities of these antioxidant enzymes. The maximum increase was observed in plants treated with Cr + NO + MN, where the increase in SOD, CAT, APX, and Gr was 24%, 19%, 118%, and 117%, respectively, compared with those in Cr-stressed plants without foliar spraying (Figure 3).
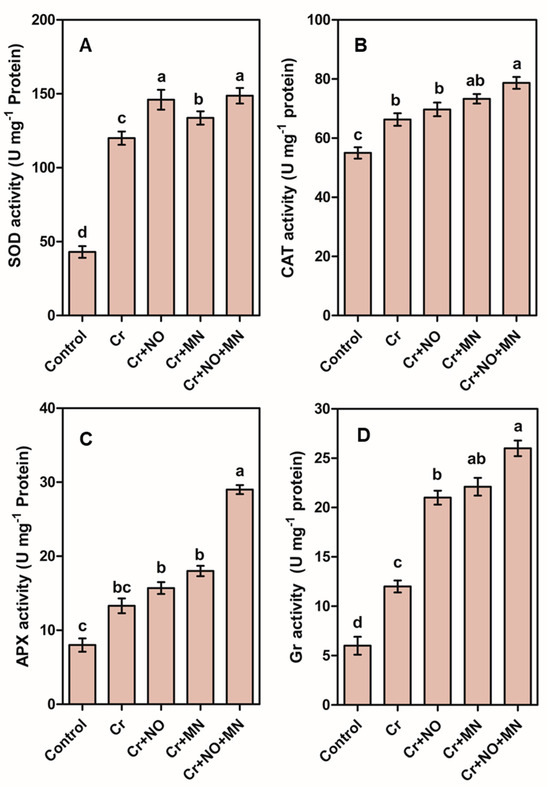
Figure 3.
Enzymatic activities including superoxide dismutase (SOD; A), catalase (CAT; B), ascorbate peroxidase (APX; C), and glutathione reductase (Gr; D) of calendula leaves in response to Cr (chromium) stress and application of NO (nitric oxide) and MN (melatonin) individually or in combination. The values are the means and SEs. Similar small letters within a graph depict no significant differences evaluated by Tukey’s HSD test at p < 0.05 level (n = 10).
3.5. Leaf N, P, K, and Cr Contents
The Cr stress decreased the level of N, P, and K by 38%, 65%, and 54%, respectively, compared with the control (Table 2). Individual applications of NO and MN increased the levels of these macronutrients. The maximum increase was observed in plants treated with Cr + NO + MN, where the increase was 80%, 271%, and 96% for N, P, and K, respectively, compared with that in Cr-stressed plants (Table 2). Additionally, in reference to Cr-stressed plants, the application of NO, MN, and NO + MN decreased Cr contents by 71%, 74%, and 87%, respectively (Table 2).

Table 2.
Impact of chromium and signaling molecules on the leaf macronutrient and chromium contents in calendula.
3.6. Flower Nutritional Composition
The total carbohydrate (TC) contents of calendula flowers ranged from 1.1 g 100 g−1 to 3.0 g 100 g−1 of fresh weight in response to the different treatments. Compared with the control, Cr stress decreased the TC value by 63% (Table 3). Treatment with Cr + NO, Cr + MN, and Cr + NO + MN increased the TC levels by 54%, 63%, and 109%, respectively, compared with Cr-stressed calendula without foliar spraying (Table 3). The total reducing sugars (TRSs) of the calendula petals varied from 22.0 mg g−1 to 56.3 mg g−1. By comparing with the control treatment, the TRS under Cr stress decreased by 61% (Table 3). Cr + NO, Cr + MN, and Cr + NO + MN elevated TRS levels by 57%, 41%, and 108%, respectively, with reference to Cr-stressed calendula without foliar spraying (Table 3).

Table 3.
Impact of chromium and signaling molecules on the nutritional aspects of calendula edible flower.
The total protein (TP) content varied from 1.3 g 100 g−1 to 4.9 g 100 g−1 across the treatments. The TP content decreased by 73% under Cr stress compared with the control conditions (Table 3). Compared with Cr-stressed calendula without foliar spraying, Cr + NO, Cr + MN, and Cr + NO + MN increased TP levels by 100%, 146%, and 238%, respectively (Table 3). The ascorbic acid (AsA) content of the calendula petals differed from 44.5 mg 100g−1 to 65 mg 100g−1 across treatments. The AsA under Cr stress increased by 10% compared with the control treatment (Table 3). The Cr + NO, Cr + MN, and Cr + NO + MN treatments also improved the AsA levels by 11%, 22%, and 33%, respectively, compared with that of Cr-stressed calendula without foliar spraying (Table 3).
The ash content (AC) of the calendula petals ranged from 11.3% to 17.5%. Compared with the control treatment, the AC under Cr stress decreased by 35% (Table 3). Treatment with Cr + NO, Cr + MN, and Cr + NO + MN increased the AC levels by 11%, 13%, and 35%, respectively, compared with that of Cr-stressed calendula flowers derived from the plants without foliar spraying (Table 3).
3.7. Flower Traits
The total phenol concentration (TPC) of the calendula flowers varied in response to the different treatments. Cr stress increased the TPC value by 27% in reference to the control treatment (Figure 4A). The supplementation of Cr + NO, Cr + MN, and Cr + NO + MN further increased the TPC by 10%, 9%, and 27%, respectively, compared with Cr-stressed calendula flowers without foliar spraying (Figure 4A). The total flavonoid concentration (TFC) of calendula flowers increased by 27% due to Cr stress (Figure 4B). The application of Cr + NO, Cr + MN, and Cr + NO + MN further elevated the TFC level by 28%, 7%, and 36%, respectively, compared with that of Cr-stressed calendula without foliar spraying (Figure 4B).
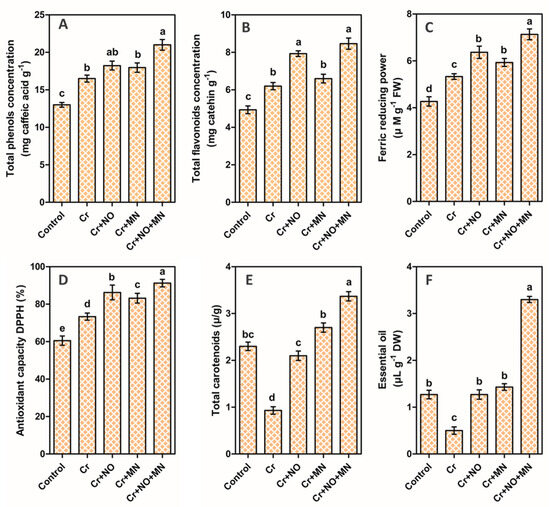
Figure 4.
Changes in total phenols (A), total flavonoids (B), ferric reducing power (C), antioxidant capacity DPPH (D), total carotenoids (E), and essential oil (F) of calendula flowers in response to Cr (chromium) stress and application of NO (nitric oxide) and MN (melatonin) individually or in combination. The values are the means and SEs. Similar small letters within a graph depict no significant differences evaluated by Tukey’s HSD test at p < 0.05 level (n = 10). DW (dry weight), FW (fresh weight), DPPH (2,2-diphenyl-1-picrylhydrazyl).
Cr stress increased the ferric reducing power (FRAP) by 25% in calendula flowers compared with the control (Figure 4C). The application of Cr + NO, Cr + MN, and Cr + NO + MN further improved the antioxidant capacity FRAP level by 19%, 11%, and 34%, respectively, compared with that of Cr-stressed calendula without foliar spraying (Figure 4C). The results related to the DPPH (2,2-diphenyl-1-picrylhydrazyl) antioxidant capacity showed similar responses as FRAP. Cr stress increased the DPPH value by 21% in reference to the control (Figure 4D). Cr + NO, Cr + MN, and Cr + NO + MN further increased the DPPH level by 18%, 13%, and 24%, respectively, compared with that of Cr-stressed calendula without foliar spraying (Figure 4D).
Cr stress decreased the total carotenoids (TCs) by 60% (Figure 4E). Treatment with Cr + NO, Cr + MN, and Cr + NO + MN further improved the TC level by 126%, 190%, and 261%, respectively, compared with that of Cr-stressed calendula without foliar spraying (Figure 4E). Cr stress decreased the EOC value by 58% (Figure 4F). Cr + NO, Cr + MN, and Cr + NO + MN further improved the EOC level by 152%, 186%, and 566%, respectively, compared with that of Cr-stressed calendula without foliar spraying (Figure 4F).
4. Discussion
Chromium is one of the most harmful trace elements found in agricultural soils and irrigation water. Cr(VI) is mobile in soils and water and can be absorbed by plants, resulting in the suppression of plant growth. It can enter the food chain through edible crops, posing a significant risk to human health [44]. The use of signaling compounds and stimulants is being explored as an alternative approach to enhance plant tolerance to Cr stress. Melatonin is known for its stress mitigating effects upon heavy metal exposure via a variety of mechanisms, such as reducing the bioavailability and uptake of heavy metals from the rhizosphere, facilitating the chelation of heavy metal ions, ROS scavenging properties, and the induction of signaling pathways and subsequent gene expression [45]. These properties are all in favor of plants’ adaptation to heavy metal stress including Cr. The gasotransmitter NO, with its short half-life, is also a potent signaling molecule when present in the plasma membrane. The concentration-dependent antioxidant and pro-oxidant properties of NO have been documented earlier in the context of plant growth regulation and oxidative stress response [8,46,47]. The co-occurrence of NO and MN biogenesis or their interplay seems to be essential for their protective role, as was the case in a study on tomatoes subjected to aluminum stress [48], where the presence of an NO scavenger (cPTIO) could reduce the positive effects of MN.
In the present study, the combined application of both nitric oxide (NO) and melatonin (MN) increased the activity of antioxidant enzymes and antioxidant compounds, decreased the Cr uptake, sustained net photosynthetic rates, and increased shoot, flower, and root dry weights. Our results agreed with those of previous reports that individual applications of NO or MN mitigated oxidative stresses and improved plant biomass accumulation and net photosynthetic rates [6,7,14,49,50,51] in Trigonella foenum-graecum L. [12], Oryza sativa L. [14], and Solanum lycopersicum L. [7,8,51]. The synergistic effects of combined NO and MN demonstrated in this study reaffirm the feasibility of the co-application of these stimulants in the alleviation of Cr stress in cultivated plants.
Plants’ antioxidant defense mechanisms play an important role in scavenging excessive ROS [4,5]. This mechanism lessens the oxidative burst that Cr stress can cause in plants [6,7,8]. The results of the present study revealed the negative effects of Cr-associated stress that were reflected by the increase in oxidative stress indicators, such as H2O2, MDA, MG, and SA (Figure 2). The application of NO and MN to calendula plants increased the antioxidant activities and decreased the expression of stress markers, leading to improved biomass accumulation and better stress tolerance (Figure 1 and Figure 3). The role of NO as a signaling molecule that can enhance the antioxidant system and induce plant defense responses under various biotic and abiotic stresses is now well documented [14,15,16]. Similar properties were also reported for melatonin. It is also known that MN can induce endogenous NO biosynthesis with its regulatory effect on activating antioxidant systems, and enhancing the biosynthesis of osmoprotectants [12,15,49]. Recent studies further indicated that the crosstalk between NO and MN exists in plants [15]. The crosstalk of MT and NO with the heavy metals and plant hormones’ signaling networks are believed to be directly involved in plant growth and abiotic stress tolerance [52]. This synergistic effect was also evident in our experiment with calendula plants, where the results indicated the potentiality of the co-application of these signaling molecules as protective agents for enhancing crop tolerance under Cr stress.
Macronutrients are vital for plant growth and development. Cr stress reduces the accumulation of macronutrients in calendula plants, which could be a result of excessive Cr concentrations impeding the biological binding sites of the necessary elements because of their ionic resemblance or disruption of ionic homeostasis [53]. High levels of Cr may influence membrane permeability, altering element transport across the cell membrane and leading to nutrient imbalance [54]. Melatonin proved to be able to immobilize heavy metals in cell walls and sequester them in root cell vacuoles, reducing their translocation from roots to shoots and therefore enhancing the other nutrients’ uptake for a normal cellular function [55]. In this study, NO and MN increased N, P, and K accumulation and decreased Cr in the leaves of Cr-stressed plants (Table 2), indicating that these signaling molecules have the capacity to sustain ionic homeostasis in calendula plants under Cr stress. Our results concurred with several previous reports. For example, the combined application of NO and MN reduced the mobility of cadmium and lead in soil and increased the uptake of calcium (Ca) and K in soybean [56]. In addition, NO has been reported to be essential for MT to enhance root growth and facilitate the accumulation of Ca, magnesium, and iron in cucumber seedlings under nitrate stress [57]. Furthermore, Parwez et al. [12] and Qin et al. [7] reported that NO and MN improved plant growth under Cr stress, promoted macronutrient uptake, and consequently maintained ionic balance.
This study also investigated the quality of the calendula flower, which possesses unique medicinal properties and nutraceutical applicability for its antioxidants and pigments [2,3]. Although the effects of NO and MN have been documented in cut flowers, fruit, and vegetables, their effects on edible flowers such as calendula have not been studied. Edible flower plants are typically valued for the high contents of their active ingredients [58,59]. This has been achieved in our calendula plants by NO and MN treatments, similar to the improved concentration of secondary metabolites in other edible flower plants [60]. Here, we noted that the application of NO or MN, particularly in combination, significantly enhanced flower quality traits such as total carbohydrate, AsA, total protein, EOC, phenolic, flavonoid, and antioxidant compounds under Cr stress conditions (Table 3, Figure 4). Furthermore, the use of MN and NO represents an environmentally benign approach for alleviating Cr toxicity as they increase plant yield and resilience to abiotic stress and reduce the need for mineral fertilizers while maintaining productivity and economic growth [61]. Nevertheless, the synergistic and antagonistic interactions of NO and MN are still the subject of current research to fill the existing gap in the complex interplay of these molecules in plant physiological processes that need to be further investigated.
5. Conclusions
The state of oxidative stress was evident in Calendula officinalis L. plants subjected to 120 µM chromium (Cr) applied in the form of K2Cr2O7 as it was manifested in the reduced growth parameters and the elevated levels of hydrogen peroxide, malondialdehyde, methylglyoxal, and superoxide anions. Based on the outcomes of this study, the application of NO (sourced as sodium nitroprusside) and melatonin (MN), individually or in combination, significantly enhanced antioxidant enzyme activities, the production of antioxidant compounds, secondary metabolite biosynthesis, and the uptake of major macronutrients (N, P, and K). These effects collectively contributed to increased biomass in roots, shoots, and flowers. The enhanced growth and productivity observed were in linear relationship with the reduced uptake and accumulation of Cr in leaves which was significantly decreased due to the applied treatments (NO, MN, NO + MN). The applied treatments substantially alleviated the symptoms of Cr stress with the best outcome being associated with the combined application of MN and NO. Since antioxidant enzymes and compounds are at the forefront of plant responses to abiotic stresses including access Cr, the positive role of MN and NO as inducers of these defensive signaling pathways warrants further investigation. Our findings provide a basis for the further refinement and development of commercial stress-mitigating products or formulations in Cr-contaminated environments.
Author Contributions
Conceptualization, F.Z., A.M., E.F. and J.C.; methodology, F.Z. and A.M.; software, A.D.; validation, Ö.Ş., M.A. and T.İ.; formal analysis, F.Z.; investigation, F.Z. and A.M.; resources, F.Z.; data curation, M.O.A., D.S.A., N.M.A., S.F.A., E.F. and I.M.; writing—original draft preparation, F.Z.; writing—review and editing, F.Z., A.M., A.D., J.C., Ö.Ş., M.A., T.İ., M.O.A., D.S.A., N.M.A., S.F.A., E.F. and I.M.; visualization, F.Z. and A.M.; supervision, J.C.; project administration, F.Z. and A.M.; funding acquisition, A.D. All authors have read and agreed to the published version of the manuscript.
Funding
The author extends her appreciation to the Deanship of Scientific Research at Princess Nourah bint Abdulrahman University Researchers Supporting Project number (PNURSP2024R465), Princess Nourah bint Abdulrahman University, Riyadh, Saudi Arabia, and the authors also extend their appreciation to the Deanship of Scientific Research at King Khalid University for funding this work through a large group Research Project under grant number RGP2/342/45.
Data Availability Statement
Data are contained within the article.
Acknowledgments
The author extends her appreciation to the Deanship of Scientific Research at Princess Nourah bint Abdulrahman University Researchers Supporting Project number (PNURSP2024R465), Princess Nourah bint Abdulrahman University, Riyadh, Saudi Arabia, and the authors also extend their appreciation to the Deanship of Scientific Research at King Khalid University for funding this work through a large group Research Project under grant number RGP2/342/45.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- De Oliveira, I.; Chrysargyris, A.; Finimundy, T.C.; Carocho, M.; Santos-Buelga, C.; Calhelha, R.C.; Heleno, S.A. Magnesium and manganese induced changes on chemical, nutritional, antioxidant and antimicrobial properties of the pansy and viola edible flowers. Food Chem. 2024, 438, 137976. [Google Scholar] [CrossRef] [PubMed]
- Pensamiento-Niño, C.A.; Castañeda-Ovando, A.; Añorve-Morga, J.; Hernández-Fuentes, A.D.; Aguilar-Arteaga, K.; Ojeda-Ramírez, D. Edible flowers and their relationship with human health: Biological activities. Food Rev. Int. 2024, 40, 620–639. [Google Scholar] [CrossRef]
- Sarode, D.K.; Pagariya, M.C.; Jadhav, P.R.; Patil, S.A.; Devarumath, R.M.; Shingote, P.R.; Kawar, P.G. Edible flowers: Biotechnological interventions for improving bioactives of food and health significance. J. Food Compos. Anal. 2024, 134, 106506. [Google Scholar] [CrossRef]
- Kajla, M.; Yadav, V.K.; Khokhar, J.; Singh, S.; Chhokar, R.S.; Meena, R.P.; Sharma, R.K. Increase in wheat production through management of abiotic stresses: A review. J. Appl. Nat. Sci. 2015, 7, 1070–1080. [Google Scholar] [CrossRef]
- Gratão, P.L.; Alves, L.R.; Lima, L.W. Heavy Metal Toxicity and Plant Productivity. In Plant-Metal Interactions: Role of Metal Scavengers; Srivastava, S., Srivastava, A.K., Suprasanna, P., Eds.; Springer Nature: Cham, Switzerland, 2019; pp. 49–60. [Google Scholar]
- Singh, S.; Dubey, N.K.; Tripathi, D.K.; Gupta, R.; Singh, V.P. Nitric oxide and hydrogen peroxide mediated regulation of chromium (VI) toxicity in wheat seedlings involves alterations in antioxidants and high affinity sulfate transporter. Plant Sci. 2023, 332, 111697. [Google Scholar] [CrossRef]
- Qin, C.; Lian, H.; Alqahtani, F.M.; Ahanger, M.A. Chromium mediated damaging effects on growth, nitrogen metabolism and chlorophyll synthesis in tomato can be alleviated by foliar application of melatonin and jasmonic acid priming. Sci. Hort. 2024, 323, 112494. [Google Scholar] [CrossRef]
- Khan, M.N.; Alamri, S.; Al-Amri, A.A.; Alsubaie, Q.D.; Al-Munqedi, B.; Ali, H.M.; Siddiqui, M.H. Effect of nitric oxide on seed germination and seedling development of tomato under chromium toxicity. J. Plant Growth Regul. 2021, 40, 2358–2370. [Google Scholar] [CrossRef]
- Sharma, P.; Singh, S.P.; Tripathi, R.D.; Tong, Y.W. Chromium toxicity and tolerance mechanisms in plants through cross-talk of secondary messengers: An overview of pathways and mechanisms. Environ. Pollut. 2023, 320, 121049. [Google Scholar] [CrossRef]
- de Oliveira, L.M.; Ma, L.Q.; Santos, J.A.; Guilherme, L.R.; Lessl, J.T. Effects of arsenate, chromate, and sulfate on arsenic and chromium uptake and translocation by arsenic hyperaccumulator Pteris vittata L. Environ. Pollut. 2014, 184, 187–192. [Google Scholar] [CrossRef]
- Iyer, M.; Anand, U.; Thiruvenkataswamy, S.; Babu, H.W.S.; Narayanasamy, A.; Prajapati, V.K.; Vellingiri, B. A review of chromium (Cr) epigenetic toxicity and health hazards. Sci. Total Environ. 2023, 882, 163483. [Google Scholar] [CrossRef]
- Parwez, R.; Aqeel, U.; Aftab, T.; Khan, M.M.A.; Naeem, M. Melatonin supplementation combats nickel-induced phytotoxicity in Trigonella foenum-graecum L. plants through metal accumulation reduction, upregulation of NO generation, antioxidant defence machinery and secondary metabolites. Plant Physiol. Biochem. 2023, 202, 107981. [Google Scholar] [CrossRef]
- Kumar, D.; Dhankher, O.P.; Tripathi, R.D.; Seth, C.S. Titanium dioxide nanoparticles potentially regulate the mechanism (s) for photosynthetic attributes, genotoxicity, antioxidants defense machinery, and phytochelatins synthesis in relation to hexavalent chromium toxicity in Helianthus annuus L. J. Hazard. Mater. 2023, 454, 131418. [Google Scholar] [CrossRef] [PubMed]
- Basit, F.; Abbas, S.; Sheteiwy, M.S.; Bhat, J.A.; Alsahli, A.A.; Ahmad, P. Deciphering the alleviation potential of nitric oxide, for low temperature and chromium stress via maintaining photosynthetic capacity, antioxidant defence, and redox homeostasis in rice (Oryza sativa). Plant Physiol. Biochem. 2024, 214, 108957. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Cao, J.; Fan, X.; Jiang, W. Applications of nitric oxide and melatonin in improving postharvest fruit quality and the separate and crosstalk biochemical mechanisms. Trends Food Sci. Technol. 2020, 99, 531–541. [Google Scholar] [CrossRef]
- Kumari, R.; Kapoor, P.; Mir, B.A.; Singh, M.; Parrey, Z.A.; Rakhra, G.; Rakhra, G. Unlocking the versatility of Nitric Oxide in plants and insights into its molecular interplays under biotic and abiotic stress. Nitric Oxide 2024, 150, 1–17. [Google Scholar] [CrossRef]
- Arnao, M.B.; Hernández-Ruiz, J. Melatonin and its relationship to plant hormones. Ann. Bot. 2018, 121, 195–207. [Google Scholar] [CrossRef]
- Kour, J.; Bhardwaj, T.; Chouhan, R.; Singh, A.D.; Gandhi, S.G.; Bhardwaj, R.; Ahmad, P. Phytomelatonin maintained chromium toxicity induced oxidative burst in Brassica juncea L. through improving antioxidant system and gene expression. Environ. Pollut. 2024, 356, 124256. [Google Scholar] [CrossRef]
- Sun, S.; Liu, A.; Li, Z.; Guo, T.; Chen, S.; Ahammed, G.J. Anthocyanin synthesis is critical for melatonin-induced chromium stress tolerance in tomato. J. Hazard. Mater. 2023, 453, 131456. [Google Scholar] [CrossRef]
- Ninama, V.; Shah, H.; Kapadia, C.; Italiya, A.; Datta, R.; Singh, S.; Singh, A. Assessment of phytochemicals, nutritional compositions and metabolite profiling using GCMS–from annual edible flowers. Sci. Hortic. 2024, 323, 112551. [Google Scholar] [CrossRef]
- Peron, G.; Franceschi, C.; Da Dalt, C.; Ferrarese, I.; Sut, S.; Dall’Acqua, S. Biostimulation of Calendula officinalis with a soy protein hydrolysate induces flower and plant biomass and flower count by reversibly altering the floral metabolome. Ind. Crops Prod. 2024, 214, 118508. [Google Scholar] [CrossRef]
- Tavallali, V.; Rahmati, S.; Bahmanzadegan, A.; Lasibi, M.J.M. Phenolic profile and evaluation of antimicrobial and anticancer activities of Calendula officinalis L. using exogenous polyamines application. Ind. Crops Prod. 2024, 214, 118571. [Google Scholar] [CrossRef]
- Varshney, A.; Dahiya, P.; Mohan, S. Antioxidant activity of pot marigold (Calendula officinalis L.) in response to metal (loid) induced oxidative stress from fly ash amended soil. J. Plant Growth Regul. 2023, 42, 5928–5944. [Google Scholar] [CrossRef]
- Hodges, D.M.; DeLong, J.M.; Forney, C.F.; Prange, R.K. Improving the thiobarbituric acid-reactive-substances assay for estimating lipid peroxidation in plant tissues containing anthocyanin and other interfering compounds. Planta 1999, 207, 604–611. [Google Scholar] [CrossRef]
- Heath, R.L.; Packer, L. Photoperoxidation in isolated chloroplasts: I. Kinetics and stoichiometry of fatty acid peroxidation. Arch. Biochem. Biophys. 1968, 125, 189–198. [Google Scholar] [CrossRef] [PubMed]
- Wild, R.; Ooi, L.; Srikanth, V.; Münch, G. A quick, convenient and economical method for the reliable determination of methylglyoxal in millimolar concentrations: The N-acetyl-L-cysteine assay. Anal. Bioanal. Chem. 2012, 403, 2577–2581. [Google Scholar] [CrossRef]
- Elstner, E.F.; Heupel, A. Inhibition of nitrite formation from hydroxylammoniumchloride: A simple assay for superoxide dismutase. Anal. Biochem. 1976, 70, 616–620. [Google Scholar] [CrossRef]
- Aebi, H. Catalase in vitro. Meth. Enzymol. 1948, 105, 121–126. [Google Scholar]
- Dhindsa, R.S.; Plumb-Dhindsa, P.; Thorpe, T.A. Leaf senescence: Correlated with increased levels of membrane permeability and lipid peroxidation and decreased levels of superoxide dismutase and catalase. J. Exp. Bot. 1981, 32, 93–101. [Google Scholar] [CrossRef]
- Foyer, C.H.; Halliwell, B. The presence of glutathione and glutathione reductase in chloroplasts: A proposed role in ascorbic acid metabolism. Planta 1976, 133, 21–25. [Google Scholar] [CrossRef]
- Jackson, M.L. Soil Chemical Analysis, 1st ed.; Prentice Hall of India Pvt. Ltd.: New Delhi, India, 1967; pp. 144–197. [Google Scholar]
- Kitson, R.; Mellon, M. Colorimetric determination of phosphorus as molybdivanado phosphonic acid. Ind. Eng. Chem. Res. 1944, 16, 379–383. [Google Scholar]
- Ludwig, T.G.; Goldberg, H.J.V. The anthrone method for the determination of carbohydrates in foods and in oral rinsing. J. Dent. Res. 1956, 35, 90–94. [Google Scholar] [CrossRef] [PubMed]
- Miller, G.L. Use of dinitrosalicylic acid reagent for determination of reducing sugar. Anal. Chem. 1959, 31, 426–428. [Google Scholar] [CrossRef]
- Lowry, O.H.; Rosebrough, N.J.; Farr, A.L.; Randall, R.J. Protein measurement with the folin phenol reagent. J. Biol. Chem. 1951, 193, 265–275. [Google Scholar] [CrossRef] [PubMed]
- Harris, L.J.; Ray, S.N. Determination of plasma Ascorbic acid by2, 6-dichorphenol indophenols titration. Lancet 1935, 1, 462. [Google Scholar] [CrossRef]
- Sigel, H. Metals in Biological Systems; Marcel Dekker: New York, NY, USA, 1978. [Google Scholar]
- Pfeffer, H.; Dannel, F.; Römheld, V. Are there connection between phenol metabolism, ascorbate metabolism and membrane integrity in leaves of boron-deficient sunflower plants? Physiol. Plant. 1998, 104, 479–485. [Google Scholar] [CrossRef]
- Zhishen, J.; Mengcheng, T.; Jianming, W. The determination of flavonoid contents in mulberry and their scavenging effects on superoxide radicals. Food Chem. 1999, 64, 555–559. [Google Scholar] [CrossRef]
- Yang, C.-M.; Chang, K.-W.; Yin, M.-H.; Huang, H.-M. Method and determination chlorophyll and derivate. Taiwania 1998, 43, 116–122. [Google Scholar]
- Benzie, I.F.; Strain, J.J. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: The FRAP assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef]
- Tepe, B.; Sokmen, M.; Akpulat, H.A.; Sokmen, A. Screening of the antioxidant potentials of six Salvia species from Turkey. Food Chem. 2006, 95, 200–204. [Google Scholar] [CrossRef]
- Kapoor, R.; Giri, B.; Mukerji, K.G. Improved growth and essential oil yield and quality in Foeniculum vulgare mill on mycorrhizal inoculation supplemented with Pfertilizer. J. Bioresour. Technol. 2004, 93, 307–311. [Google Scholar] [CrossRef]
- Alwutayd, K.M.; Alghanem, S.M.S.; Alwutayd, R.; Alghamdi, S.A.; Alabdallah, N.M.; Al-Qthanin, R.N.; Abeed, A.H. Mitigating chromium toxicity in rice (Oryza sativa L.) via ABA and 6-BAP: Unveiling synergistic benefits on morphophysiological traits and ASA-GSH cycle. Sci. Total Environ. 2024, 908, 168208. [Google Scholar] [CrossRef] [PubMed]
- Samal, I.; Bhoi, T.K.; Mahanta, D.K.; Komal, J.; Majhi, P.K.; Murmu, S.; Pradhan, A.K.; Chaurasia, H. Melatonin mediated abiotic stress mitigation in plants: A comprehensive study from biochemical to omics cascades. S. Afr. J. Bot. 2024, 170, 331–347. [Google Scholar] [CrossRef]
- Siddiqui, M.H.; Al-Whaibi, M.H.; Basalah, M.O. Role of nitric oxide in tolerance of plants to abiotic stress. Protoplasma 2011, 248, 447–455. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Lorente, S.E.; Pardo-Hernández, M.; Martí-Guillén, J.M.; López-Delacalle, M.; Rivero, R.M. Interaction between Melatonin and NO: Action mechanisms, main targets, and putative roles of the emerging molecule NOmela. Int. J. Mol. Sci. 2022, 23, 6646. [Google Scholar] [CrossRef] [PubMed]
- Ghorbani, A.; Emamverdian, A.; Pishkar, L.; Chashmi, K.A.; Salavati, J.; Zargar, M.; Chen, M. Melatonin-mediated nitric oxide signaling enhances adaptation of tomato plants to aluminum stress. S. Afr. J. Bot. 2023, 162, 443–450. [Google Scholar] [CrossRef]
- Rizwan, M.; Nawaz, A.; Irshad, S.; Manoharadas, S. Exogenously applied melatonin enhanced chromium tolerance in pepper by up-regulating the photosynthetic apparatus and antioxidant machinery. Sci. Hortic. 2024, 323, 112468. [Google Scholar] [CrossRef]
- Sharma, V.; Garg, N. Nitric oxide and AMF-mediated regulation of soil enzymes activities, cysteine-H2S system and thiol metabolites in mitigating chromium (Cr (VI)) toxicity in pigeonpea genotypes. BioMetals 2024, 37, 185–209. [Google Scholar] [CrossRef]
- Raja, V.; Qadir, S.U.; Kumar, N.; Alsahli, A.A.; Rinklebe, J.; Ahmad, P. Melatonin and strigolactone mitigate chromium toxicity through modulation of ascorbate-glutathione pathway and gene expression in tomato. Plant Physiol. Biochem. 2023, 201, 107872. [Google Scholar] [CrossRef]
- Nabaei, M.; Amooaghaie, R.; Ghorbanpour, M.; Ahadi, A. Crosstalk between melatonin and nitric oxide restrains Cadmium-induced oxidative stress and enhances vinblastine biosynthesis in Catharanthus roseus (L) G Don. Plant Cell Rep. 2024, 43, 139. [Google Scholar] [CrossRef]
- Barzin, G.; Safari, F.; Bishehkolaei, R. Beneficial role of methyl jasmonate on morphological, physiological and phytochemical responses of Calendula officinalis L. under chromium toxicity. Physiol. Mol. Biol. Plants 2022, 28, 1453–1466. [Google Scholar] [CrossRef]
- Kandhol, N.; Srivastava, A.; Rai, P.; Sharma, S.; Pandey, S.; Singh, V.P.; Tripathi, D.K. Cytokinin and indole-3-acetic acid crosstalk is indispensable for silicon mediated chromium stress tolerance in roots of wheat seedlings. J. Hazard. Mater. 2024, 468, 133134. [Google Scholar] [CrossRef] [PubMed]
- Altaf, M.A.; Sharma, N.; Srivastava, D.; Mandal, S.; Adavi, S.; Jena, R.; Bairwa, R.K.; Gopalakrishnan, A.V.; Kumar, A.; Dey, A.; et al. Deciphering the melatonin-mediated response and signalling in the regulation of heavy metal stress in plants. Planta 2023, 257, 115. [Google Scholar] [CrossRef] [PubMed]
- Imran, M.; Khan, A.L.; Mun, B.-G.; Bilal, S.; Shaffique, S.; Kwon, E.-H.; Kang, S.-M.; Yun, B.-W.; Lee, I.-J. Melatonin and nitric oxide: Dual players inhibiting hazardous metal toxicity in soybean plants via molecular and antioxidant signaling cascades. Chemosphere 2022, 308, 136575. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Liu, A.; Hao, Y.; Su, W.; Sun, G.; Song, S.; Liu, H.; Chen, R. Nitric oxide is essential for melatonin to enhance nitrate tolerance of cucumber seedlings. Molecules 2022, 27, 5806. [Google Scholar] [CrossRef] [PubMed]
- Pires, T.C.; Barros, L.; Santos-Buelga, C.; Ferreira, I.C. Edible flowers: Emerging components in the diet. Trends Food Sci. Technol. 2019, 93, 244–258. [Google Scholar] [CrossRef]
- Takahashi, J.A.; Rezende, F.A.G.G.; Moura, M.A.F.; Dominguete, L.C.B.; Sande, D. Edible flowers: Bioactive profile and its potential to be used in food development. Food Res. Int. 2020, 129, 10886. [Google Scholar] [CrossRef]
- Esmaeili, S.; Sharifi, M.; Ghanati, F.; Soltani, B.M.; Samari, E.; Sagharyan, M. Exogenous melatonin induces phenolic compounds production in Linum album cells by altering nitric oxide and salicylic acid. Sci. Rep. 2023, 13, 8. [Google Scholar] [CrossRef]
- Salehi, B.; Sharopov, F.; Fokou, P.V.T.; Kobylinska, A.; Jonge, L.D.; Tadio, K.; Sharifi-Rad, J.; Posmyk, M.M.; Martorell, M.; Martins, N.; et al. Melatonin in medicinal and food plants: Occurrence, bioavailability, and health potential for humans. Cells 2019, 8, 681. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).