Abstract
Efficient genetic transformation approaches play pivotal roles in both gene function research and crop breeding. However, stable transformation in mustard, particularly for different horticultural types, has not been systematically studied and well-established so far. In this study, we optimized the key factors in the genetic transformation of mustard, including the optical density value of Agrobacterium suspension, the age of explants, and the combination of phytohormones at different concentrations. As a result, the optimal conditions for the genetic transformation of leaf and stem mustard included hypocotyl explants derived from 4-day-old seedlings, infection by 0.8 OD600nm Agrobacterium suspension, and then re-differentiation on a medium containing 4 mg/L trans-zeatin (tZ) and 0.1 mg/L indoleacetic acid (IAA) for leaf mustard, and for stem mustard, re-differentiation on a medium containing 2 mg/L tZ and 0.4 mg/L IAA, with positive rates of 4.74% and 5.26%, respectively. Those for root mustard were hypocotyl explants derived from 8-day-old seedlings, infection by 0.2 OD600nm Agrobacterium suspension, and a medium containing 2 mg/L tZ and 0.1 mg/L IAA, with a positive rate of 4.42%. Overall, this work provides an effective tool for both the theoretical study and genetic improvement of Brassica juncea.
1. Introduction
Mustard (Brassica juncea, AABB, 2n = 36) belongs to the Brassica genus in the Cruciferae family. It is a naturally doubled allotetraploid derived from interspecific hybridization between Brassica rape (AA, 2n = 20) and Brassica nigra (BB, 2n = 16) [1]. Mustard is of substantial economic importance and often used as oilseeds, vegetables, and condiments around the world. It is a common fresh and deep-processing vegetable in China, and can be divided into the four categories of juncea (seed mustard), integrifolia (leaf mustard), napiformis (root mustard), and tumida (stem mustard), which together comprise a total of sixteen horticultural types [2]. Selective breeding and heterosis hybridization breeding are common approaches used for the improvement of mustard, which are labor-resource intensive and time-consuming, as well as suffer from certain limitations when used to improve some undesirable characteristics.
Genetic transformation technology is an important means of gene discovery, gene function analysis, and variety improvement. Agrobacterium-mediated transformation is one of the most common methods. Agrobacterium can infect plant wounds and insert the T-DNA from the Ti plasmid into the plant genome, which can achieve the transfer and integration of exogenous genes into plant cells, thereby generating transgenic plants [3,4]. Since a major advance in the genetic transformation of Brassica napus was reported [5,6,7], Agrobacterium transformation has been applied to Brassica transformation with efficiencies ranging from 0.59% to 22.5% [8,9,10,11,12,13], exhibiting significant differences in transformation efficiency among different species.
A relatively limited number of mustard varieties have been successfully transformed with Agrobacterium. For example, Barfield and Pua [14] established an Agrobacterium-mediated transgenic system in Brassica juncea var. India Mustard, which could generate transgenic shoots from hypocotyl explants at the highest transformation efficiency of 9%. Transformation has been tested in a variety of explant types such as hypocotyl, petiole, leaf, and cotyledon, and the transformation efficiency varied with the cultivar, donor plant age, and explant type. Prasad et al. [15] took petiole as the explant and transformed the codA gene into Brassica juncea (L.), which enhanced its tolerance to salt stress. Dutta et al. [16] used leaf pieces as explants for the genetic transformation of five oilseed mustard cultivars, achieving a stable transformation efficiency of approximately 19%. Bhuiyan et al. [17] employed cotyledon as explants, and successfully introduced the yeast cadmium factor 1 (YCF1) gene into Indian mustard, with the transformation efficiency reaching 16.2%. In general, most of these studies of the genetic transformation of mustard have been mainly focused on leaf and seed mustard by employing different tissue culture media and physical treatments to improve the transformation efficiency [11,18,19,20]. Genetic transformation of root mustard has not been reported, which may be due to its poor shoot regeneration capacity and low sensitivity of explants to Agrobacterium. Genotype restriction strictly limits the genetic transformation efficiency of mustard species. The protocols established for model varieties (such as oilseed mustard) are not directly applicable to all horticultural types, and current elite mustard germplasms are often recalcitrant to transformation. Therefore, it is highly necessary to establish an efficient genetic transformation system for different genetic backgrounds of mustard for the breeding of new varieties, which may help realize the full potential of genomics in mustard breeding.
In this study, we developed an efficient and applicable Agrobacterium-mediated genetic transformation system using hypocotyl as explants in Brassica juncea. Moreover, by investigating several factors affecting the infection efficiency of Agrobacterium (Agrobacterium suspension concentration and explants of different seedling ages) and screening the optimal medium formula for indefinite shoot proliferation, we optimized the efficient genetic transformation system of leaf and stem mustard, and established a genetic transformation system for root mustard. This study lays a foundation for the analysis of mustard gene function and provides reference for the genetic transformation of commercial mustard cultivars.
2. Materials and Methods
2.1. Plant Materials
Three commercial mustard cultivars, ‘Lion Head’, ‘Yong An Xiao Ye’, and ‘Hua 501’, were selected (Figure 1), which belong to leaf, stem, and root mustard, respectively, for genetic transformation, which were also their important commodity traits. All the three test materials were self-crossed lines with stable economic characteristics, and provided by the Cruciferous Vegetable Genetic Breeding Research Group of the College of Horticulture and Forestry, Huazhong Agricultural University.

Figure 1.
Different mustard cultivars used in this study. (A) ‘Lion Head’; (B) ‘Yong An Xiao Ye’; (C) ‘Hua 501’. Bar = 5 cm.
2.2. Culture Medium Preparation
Luria–Bertani (LB) liquid medium was used to activate Agrobacterium. Culture medium components for the transformation are provided in Table 1. All media were based on the MS medium [21] with agar (6 g/L), except for the medium for rooting cultivation with agar of 8 g/L. The pH of all media was adjusted to about 5.9. Acetosyringone (AS) and antibiotics including kanamycin (kana), timentin (TMT), and staurosporine (STS) were filtered and sterilized and added to the media, which were cooled to about 55 °C after autoclaving. Additionally, to screen the optimal medium formula for indefinite shoot proliferation (M3), the concentrations of IAA and tZ added were set according to specific experimental requirements.

Table 1.
Culture media and formulas at different stages of cultivation.
2.3. Agrobacterium-Mediated Genetic Transformation of Mustard
Preparation of Agrobacterium suspension: A binary expression vector with a kana selective marker was used in this experiment, which was provided by the Cruciferous Vegetable Genetic and Breeding Laboratory of Huazhong Agricultural University. The plasmid was transformed into Agrobacterium GV3101 by freeze-thaw conversion [22] and cultured in 1 mL of LB medium containing 100 mg/L rifampin and 50 mg/L kana with 200 rpm at 28 °C overnight. The bacterial suspension was transferred to a 50 mL centrifuge tube and added with 25 mL LB for further culture for 24 h, followed by centrifugation at 3900 rpm for 10 min to collect the bacterial precipitate, which was then suspended in DM medium (Table 1).
Agrobacterium suspensions at different optical density (OD) concentrations (OD600nm = 0.2, 0.5, 0.8, 1.2) were diluted with DM medium. The hypocotyls of the three mustard varieties were infected at 7 d of seedling age. Three repeated tests were conducted for each treatment.
Explant preparation: Round and plump seeds were selected, which were then surface-sterilized with 75% ethanol for 1 min, followed by rinsing in sterile water for 1 min, submerging in 10% sodium hypochlorite for 10 min, and washing with sterile water for 1 min 5 times; the seeds were stirred with tweezers throughout the entire disinfection process. Finally, the seeds were neatly discharged into M0 medium (Table 1) with about 64 seeds (8 × 8) being planted in each box and dark cultured. All subsequent cultivation temperatures were set at 22 °C (Figure 2A).
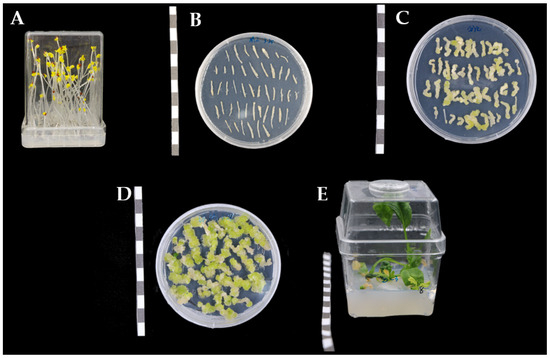
Figure 2.
Diagram of the genetic transformation process in root mustard. (A) Sterile seedling culture stage; (B) co-culture stage; (C) callus induction stage; (D) adventitious bud differentiation stage; (E) regeneration stage. Bar = 1 cm.
Agrobacterium infection and co-cultivation: The elongated hypocotyl after dark culture was cut into small explants (about 7~10 mm) in DM liquid medium, and put into the above-mentioned bacterial suspension for 10 min of infection, and then put on the filter paper to be dried for 20 min. The dried explant was put onto a co-cultivation medium M1 (Table 1) and cultivated in a dark environment for 36 h (Figure 2B).
The seedlings of the three mustard varieties were cultured in the dark for 4, 6, and 8 d, using the optimal concentration of Agrobacterium infection solution from experiments for infection. Three repeated tests were conducted for each treatment.
Dedifferentiation and re-differentiation of explants: Explants were transferred from the co-cultivation medium to a callus induction medium M2 (Table 1) and cultivated for 12 d, and then transferred to a differentiation medium (Table 1) for adventitious shoot-inducing cultivation for 13 d (Figure 2C).
Elongation and rooting of adventitious shoots: Calli with adventitious shoots were transferred to an adventitious shoot proliferation medium M3 (Table 1) for growth culture of adventitious shoots at 14 d intervals, until the development of resistant shoots (Figure 2D). When the adventitious shoots grew to 2–3 cm in length, they were cut and transferred to a root-induction medium (M4) for rooting cultivation (Figure 2E).
The optimum hormone concentration for adventitious shoot proliferation was determined by regulating the concentration ratio of tZ and IAA in M3. By using the minimum 2 mg/L tZ concentration as the standard, five treatments with tZ/IAA multiples of 40, 20, 10, 5, and 1 were set: 0.1 mg/L IAA + 4 mg/L tZ; 0.1 mg/L IAA + 2 mg/L tZ; 0.4 mg/L IAA + 4 mg/L tZ; 0.4 mg/L IAA + 2 mg/L tZ; and 2 mg/L IAA + 2 mg/L tZ. Three repeated tests were conducted for each treatment.
2.4. Molecular Detection of Transgenic Plants
Total genomic DNA was isolated from young leaves of transgenic and non-transformed mustard following the cetyltrimethylammonium bromide (CATB) method as described previously [23]. The plasmid was used as the positive control and the DNA of non-transformed mustard was extracted as the negative control. The PCR amplification detection of regenerated mustard plants was performed by specific primers of the kanamycin resistance gene (Kana-F: 5′-CTCCCAATCAGGCTTGATCC-3′, Kana-R: 5′-CTGATCGAAAAATACCGCTGC-3′). The size of the PCR product was 720 bp. The PCR reaction mixture comprised 5 μL of Ex Taq (TaKaRa, Shiga, Japan), 3 μL of distilled sterile water, 100 ng of genomic DNA, and 0.5 μL of each primer, and the PCR reaction procedure was as follows: an initial denaturation step of 94 °C for 5 min; followed by 28 cycles of 94 °C for 30 s, 60 °C for 30 s, and 72 °C for 1 min; and a final extension of 72 °C for 5 min. The PCR amplification products were added into gel with 1% agarose for electrophoresis under 150 V for 20 min. Finally, the gel was observed under a UV transilluminator (Bio-Rad, Hercules, CA, USA).
2.5. Statistical Analysis
The software IBM SPSS Statistics 25 was used for statistical analysis, and Duncan’s method (SSR method) was employed to perform multiple comparison analysis on the test data at a p < 0.05 level. Each treatment was repeated three times. The callus induction rate = the amounts of explants with callus/total number of infected explants × 100%. The rate of differentiation = amounts of explants with adventitious shoots/total number of infected explants × 100%; the regeneration rate = total number of regeneration plants/total number of infected explants × 100%; and the transformation efficiency = total number of positive transformants/total number of infected explants × 100%.
3. Results
3.1. Factors Influencing the Infection Efficiency of Agrobacterium
3.1.1. Effects of Agrobacteria Suspension Concentration in Three Types of Mustard
An appropriate Agrobacterium suspension concentration might promote the growth of positively transformed plants. Our results showed that a higher OD600nm value with increasing concentration of Agrobacterium suspension promoted the hypocotyl explant callus induction rate and differentiation rate of ‘Lion Head’. The highest callus induction rate (94.78%) and differentiation rate (24.03%) were observed when the OD600nm value was 1.2, which were significantly different from those of the groups with an OD600nm of 0.5 and 0.2 (Table 2). However, when the OD600nm value was 0.8, ‘Lion Head’ showed a higher regeneration rate (8.26%) and positive rate (3.56%).

Table 2.
Factors influencing the infection efficiency of Agrobacterium in three types of mustard (%).
The callus induction rate of ‘Yong An Xiao Ye’ showed no significant differences among the groups with an OD600nm of 0.5, 0.8, and 1.2, all of which reached around 100% and significantly differed from that of the groups with an OD600nm of 0.2. Moreover, it was observed that an increase in the OD600nm value could improve the differentiation rate, and a rate of 32.06% was observed when the OD600nm value was 1.2. However, an OD600nm value of 0.8 resulted in the highest regeneration rate (7.06%) and positive rate (4.48%) (Table 2).
The Agrobacterium suspension concentration had no significant effect on the callus induction rate of ‘Hua 501’, while the differentiation rate showed a downtrend with an increasing OD600nm value. As a result, an OD600nm value of 0.2 resulted in the highest differentiation rate (11.99%), as well as the highest plant regeneration rate (3.57%) and positive rate (1.61%) among OD600nm values (Table 2).
The callus tissue of the root mustard variety ‘Hua 501’ had a compact texture and a pale green color (Figure 3C), while that of the leaf mustard variety ‘Lion Head’ and stem mustard variety ‘Yong An Xiao Ye’ was thin with a delicate texture and faint yellow color, and that of the leaf mustard variety ‘Lion Head’ was easier to brown (Figure 3A,B).
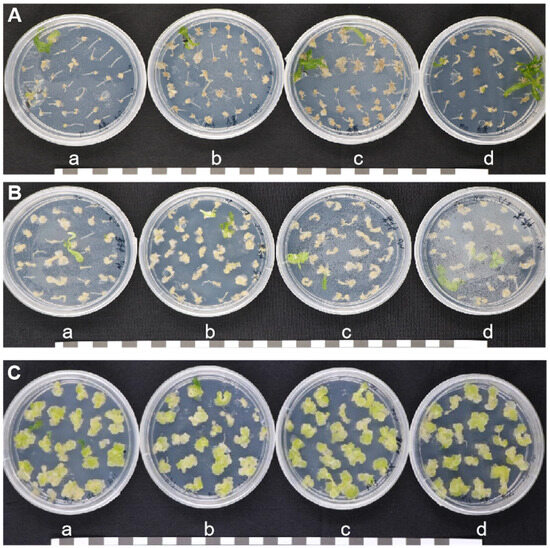
Figure 3.
Callus induction of three types of mustard. (A) Leaf mustard variety ‘Lion Head’; (B) stem mustard variety ‘Yong An Xiao Ye’; (C) root mustard variety ‘Hua 501’; a. OD600nm = 0.2; b. OD600nm = 0.5; c. OD600nm = 0.8; d. OD600nm = 1.2. Bar = 2 cm.
3.1.2. Optimization of Seedling Age
Table 2 shows that the highest callus induction rate (98.36%) and differentiation rate (53.42%) of ‘Lion Head’ were observed in the group with the seedling age of 4 d, both of which were significantly different from the values of the groups with seedling ages of 6 d and 8 d. However, seedling age exhibited no significant effect on the plant regeneration rate and positive rate. The callus induction rate was 100% for ‘Yong An Xiao Ye’ when the seedling age was 4 d and 6 d, and the differentiation rate, regeneration rate, and positive rate reached 22.33%, 7.45%, and 1.93% at 4 d, respectively, which were significantly higher than those of the groups with seedling ages of 6 d and 8 d. Seedling age showed no significant influence on the callus induction rate of ‘Hua 501’, which reached 100% in all three groups. However, an increase in seedling age significantly increased the differentiation rate of ‘Hua 501’, and the highest differentiation rate (21.67%) was observed when the seedling age was 8 d, when the regeneration rate and positive rate were 5.64% and 2.81%, respectively.
3.2. Effects of Different Hormone Combinations
To study the effect of different combinations of tZ and IAA concentrations on the callus induction and differentiation rates of three types of mustard, we set five combinations, and selected the optimal Agrobacterium suspension concentration and seedling age (Table 3). Significantly higher differentiation rates of ‘Lion Head’ were observed on M3 supplemented with 0.1 mg/L IAA + 4 mg/L tZ and 0.4 mg/L IAA + 2 mg/L tZ, which reached 29.83% and 31.67%, respectively. The hormone combination of 0.1 mg/L IAA + 4 mg/L tZ resulted in the highest regeneration rate (7.07%) and positive rate (4.74%). The callus induction rate in ‘Yong An Xiao Ye’ was not significantly different under different hormone combinations. The differentiation rate (40.83%) under the hormone combination of 0.4 mg/L IAA + 2 mg/L tZ was significantly higher than that under other four combinations, and the plant regeneration rate (7.83%) and positive rate (5.26%) were the highest as well. ‘Hua 501’ showed a strong ability to induce callus. The differentiation rate was the lowest under the combination of 2 mg/L IAA + 2 mg/L tZ, while it showed no significant difference among the other four hormone combinations. Moreover, the hormone combination of 0.1 mg/L IAA + 2 mg/L tZ brought about a slightly higher regeneration rate (7.51%) and positive rate (4.42%).

Table 3.
Effect of hormone levels on the differentiation rate of three types of mustard plants (%).
3.3. Validation of Transgenic Plants
As shown in Figure 4, with nine randomly selected regeneration plants, positive regeneration plants were identified based on the same size of the target band and the positive control band (approximately 720 bp). A total of 108, 97, and 49 positive plants were observed in the leaf mustard variety ‘Lion Head’, stem mustard variety ‘Yong An Xiao Ye’, and root mustard variety ‘Hua 501’, respectively. It took about three months to obtain transgenic positive plants using this protocol.
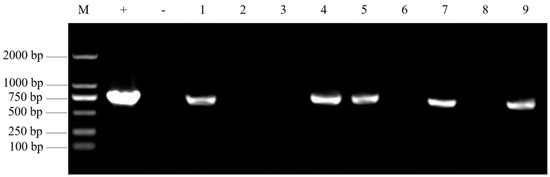
Figure 4.
PCR detection of transgenic plants in Brassica juncea. M. DL2000 marker; +. Positive control (recombinant plasmid); −. Negative control (untransformed mustard plant); 1–9. In the transgenic regenerated plants of mustard, the size of kana resistance gene products was 720 bp.
4. Discussion
Agrobacterium-mediated transformation has allowed the production of feasible transgenic plants from many Brassica species [8,24,25]. Previous studies have demonstrated that oilseed mustard is receptive to Agrobacterium transformation [17,26]. Genetic transformation primarily relies on the efficiency of gene delivery into plant cells and the ability to regenerate transgenic plants. PLETHORA (PLT5) can significantly improve shoot regeneration and transformation in two Brassica cabbage varieties (Brassica rapa) [27]. However, the different horticultural varieties chosen in this study are of unknown transformability. An efficient transformation protocol suitable for various genetic backgrounds is a necessity for the genetic transformation of mustard. This study identified various factors influencing the Agrobacterium-mediated transformation of mustard. The density of bacterial cells, as measured by the optical density of bacterial suspensions, is directly related to the cell mass or cell number. Selection of a suitable OD is critical for the genetic transformation with Agrobacterium. Numerous studies have shown that the OD600nm of Agrobacterium suspension plays a vital role in the genetic transformation of brassica vegetable, and an OD600nm value between 0.2 and 1.0 was found to promote the differentiation rate. The differentiation rate of the cabbage inbred line YL-1 reached the highest with an OD600nm of 0.3 [28], and Chinese cabbage performed well in genetic transformation with an OD600nm between 0.6 and 0.8 [29,30]. This study found that the optimum OD600nm value for the regeneration and positive plants of the leaf mustard variety ‘Lion Head’ and stem mustard variety ‘Yong An Xiao Ye’ was 0.8, which is similar to the values reported for other brassica plants in previous studies. Although a higher Agrobacterium suspension concentration could contribute to higher callus induction and differentiation rates, an extremely high Agrobacterium suspension concentration (1.2) would decrease the infection efficiency. The results were just opposite in the root mustard variety ‘Hua 501’, for which the optimum OD600nm value for regeneration and positive plants was 0.2. The three varieties of mustard showed significant differences in hypocotyl thickness; we observed that root mustard had the largest hypocotyl, with a relatively uniform distribution of vascular bundles and plant hormones, which is conducive to the formation of callus. In contrast, stem and leaf mustard had a relatively uneven distribution of vascular bundles in the hypocotyl, which may affect the formation of callus. Therefore, the optimal Agrobacterium infection concentration for the genetic transformation of mustard needs to be selected according to the variety.
Seedling age also has a great impact on the regeneration of adventitious shoots. A decrease in seedling age can generally enhance the differentiation ability of adventitious shoots. Seedlings aged at 3 to 8 d are usually selected for the genetic transformation of Brassica Crucifer plants [23,28,29,31]. In this study, the optimum seedling age of the leaf mustard variety ‘Lion Head’ and stem mustard variety ‘Yong An Xiao Ye’ was 4 d, when they were in a young and tender state with strong morphogenetic ability, while the hypocotyl was gradually aged when the seedling age was 6 d and 8 d. This result is consistent with the previous study of genetic transformation of Indian mustard [32]. The optimal seedling age for root mustard is 8 d, and the differentiation rate, regeneration rate, and positivity rate are significantly better than those of 4 and 6 d, when the hypocotyl was still in a tender state and the morphogenetic ability was stronger. In addition, due to the influence of seed vitality or disinfectants, the seedling age cannot accurately reflect the growth of sterile seedlings. The hypocotyl length will be considered as a measurement index in the following experiments.
Many factors involved in plant regeneration efficiency have been identified. Preparing media with the appropriate ratio of auxin to cytokinin is significantly important for enhancing regeneration efficiency [33]. Numerous studies have reported that the hormones 6-BA and NAA can be added in the differentiation medium. A maximum regeneration rate of 92% was observed when cotyledon was used as an explant to be incubated on a medium supplemented with 4 µM BA and 1 µM NAA in mustard [11]. Similarly, a differentiation medium with NAA (0.1–0.2 mg/L) can improve the differentiation of adventitious shoots in Brassica [34,35,36]. In this study, we selected the combination of IAA and tZ hormones to induce adventitious shoots. The highest differentiation rate of the leaf mustard variety ‘Lion Head’ and stem mustard variety ‘Yong An Xiao Ye’ was observed when 2 mg/L tZ was combined with 0.4 mg/L IAA. However, the differentiation rate of the root mustard variety ‘Hua 501’ showed no significant differences under four different combinations of tZ and IAA concentrations. It is possible that the leaf mustard variety ‘Lion Head’ and stem mustard variety ‘Yong An Xiao Ye’ have higher sensitivity to hormones in vitro than the root mustard variety ‘Hua 501’. This difference may be caused by different horticultural types, and some genes related to hormone signal transmission are absent in ‘Hua 501’.
5. Conclusions
These results demonstrate that the Agrobacterium transformation procedure is applicable to a diverse range of commercial mustard varieties. Unsurprisingly, there were clear differences in the transformation efficiency between different mustard varieties, given that plant transformation is known to be dependent on genotype. In this study, by evaluating the effects of Agrobacterium suspension concentration, seedling age, and hormone combination, we optimized the genetic transformation system of leaf mustard and stem mustard, and established a genetic transformation system for root mustard. In the transformation system for root mustard, Agrobacterium suspension with an OD600nm value of 0.2 is used to infect the hypocotyl of 8-day seedlings, and callus is induced from the infected hypocotyl, which is then cultured on M3 medium containing 0.1 mg/L IAA + 2 mg/L tZ to generate adventitious shoots, resulting in the highest positive rate of 4.42%.
Author Contributions
Conceptualization, Z.W.; methodology, W.F., C.Y. and Z.W.; software, X.Z.; formal analysis, X.Z.; investigation, W.F.; resources, Z.W.; data curation, W.F.; writing—original draft preparation, W.F.; writing—review and editing, X.Z., C.Y. and Z.W.; visualization, W.F.; supervision, Z.W.; project administration, Z.W.; funding acquisition, W.F. and Z.W. All authors have read and agreed to the published version of the manuscript.
Funding
This research is supported by the National Natural Science Foundation of China (NSFC 32072573, NSFC 31872096, NSFC 32460766), the China Agriculture Research System (CARS-24-A-06), the National Key Research and Development Program of China (2023YFD1200102-03), and the Guizhou Science and Technology Support Plan (No. 2022-086).
Data Availability Statement
The data that support the findings of this study are available from the corresponding author, Zhengjie Wan, upon reasonable request.
Conflicts of Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Yang, J.H.; Liu, D.Y.; Wang, X.W.; Ji, C.M.; Cheng, F.; Liu, B.N.; Hu, Z.Y.; Chen, S.; Pental, D.; Ju, Y.H.; et al. The genome sequence of allopolyploid Brassica juncea and analysis of differential homoeolog gene expression influencing selection. Nat. Genet. 2016, 48, 1225–1232. [Google Scholar] [CrossRef] [PubMed]
- Kang, L.; Qian, L.W.; Zheng, M.; Chen, L.Y.; Chen, H.; Yang, L.; You, L.; Yang, B.; Yan, M.L.; Gu, Y.G.; et al. Genomic insights into the origin, domestication and diversification of Brassica juncea. Nat. Genet. 2021, 53, 1392–1402. [Google Scholar] [CrossRef] [PubMed]
- Zambryski, P.; Joos, H.; Genetello, C.; Leemans, J.; Vanmontagu, M.; Schell, J. Ti plasmid vector for the introduction of DNA into plant cells without alteration of their normal regeneration capacity. EMBO J. 1983, 2, 2143–2150. [Google Scholar] [CrossRef] [PubMed]
- Fraley, R.T.; Rogers, S.G.; Horsch, R.B.; Eichholtz, D.A.; Flick, J.S.; Fink, C.L.; Hoffmann, N.L.; Sanders, P.R. The SEV system: A new disarmed Ti plasmid vector system for plant transformation. Nat. Biotechnol. 1985, 3, 629–635. [Google Scholar] [CrossRef]
- Pua, E.C.; Mehrapalta, A.; Nagy, F.; Chua, N.H. Transgenic plants of Brassica napus L. Nat. Biotechnol. 1987, 5, 815–817. [Google Scholar] [CrossRef]
- Moloney, M.M.; Walker, J.M.; Sharma, K.K. High efficiency transformation of Brassica napus using Agrobacterium vectors. Plant Cell Rep. 1989, 8, 238–242. [Google Scholar] [CrossRef]
- Deblock, M.; Debrouwer, D.; Tenning, P. Transformation of Brassica napus and Brassica oleracea using Agrobacterium tumefaciens and the expression of the bar and neo genes in the transgenic plants. Plant Physiol. 1989, 91, 694–701. [Google Scholar] [CrossRef] [PubMed]
- Li, X.N.; Li, H.Y.; Zhao, Y.Z.; Zong, P.X.; Zhan, Z.X.; Piao, Z.Y. Establishment of a simple and efficient Agrobacterium-mediated genetic transformation system to Chinese Cabbage (Brassica rapa L. ssp. pekinensis). Hortic. Plant J. 2021, 7, 117–128. [Google Scholar] [CrossRef]
- Dai, C.; Li, Y.Q.; Li, L.; Du, Z.L.; Lin, S.L.; Tian, X.; Li, S.J.; Yang, B.; Yao, W.; Wang, J.; et al. An efficient Agrobacterium-mediated transformation method using hypocotyl as explants for Brassica napus. Mol. Breed. 2020, 40, 96. [Google Scholar] [CrossRef]
- Chen, G.; Zeng, F.; Wang, J.; Ye, X.; Zhu, S.; Yuan, L.; Hou, J.; Wang, C. Transgenic Wucai (Brassica campestris L.) produced via Agrobacterium-mediated anther transformation in planta. Plant Cell Rep. 2019, 38, 577–586. [Google Scholar] [CrossRef]
- Naeem, I.; Munir, I.; Durrett, T.P.; Iqbal, A.; Aulakh, K.S.; Ahmad, M.A.; Khan, H.; Khan, I.A.; Hussain, F.; Shuaib, M.; et al. Feasible regeneration and agrobacterium-mediated transformation of Brassica juncea with Euonymus alatus diacylglycerol acetyltransferase (EaDAcT) gene. Saudi J. Biol. Sci. 2020, 27, 1324–1332. [Google Scholar] [CrossRef] [PubMed]
- Pavlović, S.; Savić, J.; Milojević, J.; Vinterhalter, B.; Girek, Z.; Adžić, S.; Zečević, B.; Banjac, N. Introduction of the Nicotiana protein kinase (NPK1) gene by combining Agrobacterium-mediated transformation and recurrent somatic embryogenesis to enhance salt tolerance in cauliflower. Plant Cell Tissue Organ Cult. 2020, 143, 635–651. [Google Scholar] [CrossRef]
- Sheng, X.G.; Gu, H.H.; Yu, H.F.; Wang, J.S.; Zhao, Z.Q.; Qi, Z.R. An efficient shoot regeneration system and Agrobacterium-mediated transformation with codA gene in a doubled haploid line of broccoli. Can. J. Plant Sci. 2016, 96, 1014–1020. [Google Scholar] [CrossRef]
- Barfield, D.G.; Pua, E.C. Gene transfer in plants of Brassica juncea using Agrobacterium tumefaciens-mediated transformation. Plant Cell Rep. 1991, 10, 308–314. [Google Scholar] [CrossRef]
- Prasad KV, S.K.; Sharmila, P.; Kumar, P.A.; Saradhi, P.P. Transformation of Brassica juncea (L.) Czern with bacterial codA gene enhances its tolerance to salt stress. Mol. Breed. 2000, 6, 489–499. [Google Scholar] [CrossRef]
- Dutta, I.; Saha, P.; Das, S. Efficient Agrobacterium-mediated genetic transformation of oilseed mustard [Brassica juncea (L.) Czern.] using leaf piece explants. Vitr. Cell. Dev. Biol.-Plant 2008, 44, 401–411. [Google Scholar] [CrossRef]
- Bhuiyan, M.S.U.; Min, S.R.; Jeong, W.J.; Sultana, S.; Choi, K.S.; Lim, Y.P.; Song, W.Y.; Lee, Y.; Liu, J.R. An improved method for Agrobacterium-mediated genetic transformation from cotyledon explants of Brassica juncea. Plant Biotechnol. 2011, 28, 17–23. [Google Scholar] [CrossRef][Green Version]
- Assou, J.; Zhang, D.B.; Roth KD, R.; Steinke, S.; Hust, M.; Reinard, T.; Winkelmann, T.; Boch, J. Removing the major allergen Bra j I from brown mustard (Brassica juncea) by CRISPR/Cas9. Plant J. 2022, 109, 649–663. [Google Scholar] [CrossRef]
- Nambiar, D.M.; Kumari, J.; Augustine, R.; Kumar, P.; Bajpai, P.K.; Bisht, N.C. GTR1 and GTR2 transporters differentially regulate tissue-specific glucosinolate contents and defence responses in the oilseed crop Brassica juncea. Plant Cell Environ. 2021, 44, 2729–2743. [Google Scholar] [CrossRef]
- Shekhar, S.; Panwar, R.; Prasad, S.C.; Kumar, D.; Rustagi, A. Overexpression of flowering locus D (FLD) in Indian mustard (Brassica juncea) enhances tolerance to Alternaria brassicae and Sclerotinia sclerotiorum. Plant Cell Rep. 2023, 42, 1233–1250. [Google Scholar] [CrossRef]
- Murashige, T.; Skoog, F. A revised medium for rapid growth and bio assays with tobacco tissue cultures. Physiol. Plant. 1962, 15, 473–497. [Google Scholar] [CrossRef]
- Chilton, M.D.; Currier, T.C.; Farrand, S.K.; Bendich, A.J.; Gordon, M.P.; Nester, E.W. Agrobacterium tumefaciens DNA and PS8 bacteriophage DNA not detected in crown gall tumors. Proc. Natl. Acad. Sci. USA 1974, 71, 3672–3676. [Google Scholar] [CrossRef] [PubMed]
- Murray, M.G.; Thompson, W.F. Rapid isolation of high molecular weight plant DNA. Nucleic Acids Res. 1980, 8, 4321–4326. [Google Scholar] [CrossRef] [PubMed]
- Bhalla, P.L.; Singh, M.B. Agrobacterium-mediated transformation of Brassica napus and Brassica oleracea. Nat. Protoc. 2008, 3, 181–189. [Google Scholar] [CrossRef]
- Hu, D.; Bent, A.F.; Hou, X.; Li, Y. Agrobacterium-mediated vacuum infiltration and floral dip transformation of rapid-cycling Brassica rapa. BMC Plant Biol. 2019, 19, 1–9. [Google Scholar] [CrossRef]
- Aminedi, R.; Dhatwalia, D.; Jain, V.; Bhattacharya, R. High efficiency in planta transformation of Indian mustard (Brassica juncea) based on spraying of floral buds. Plant Cell Tissue Organ Cult. 2019, 138, 229–237. [Google Scholar] [CrossRef]
- Lian, Z.; Nguyen, C.D.; Liu, L.; Wang, G.; Chen, J.; Wang, S.; Yi, G.; Wilson, S.; Ozias-Akins, P.; Gong, H.; et al. Application of developmental regulators to improve in planta or in vitro transformation in plants. Plant Biotechnol. J. 2022, 20, 1622–1635. [Google Scholar] [CrossRef]
- Cui, H.L.; Li, Z.Y.; Fang, Z.Y.; Yang, L.M.; Zhuang, M.; Lu, H.H.; Liu, Y.M.; Song, J.H.; Zhang, Y.Y. Establishment and application of YL-1 high-efficiency genetic transformation system in cabbage (Brassica oleracea L. var. capitata). Acta Hortic. Sin. 2019, 46, 345–355. [Google Scholar] [CrossRef]
- Vanjildorj, E.; Song, S.Y.; Yang, Z.H.; Choi, J.E.; Noh, Y.S.; Park, S.; Lim, W.J.; Cho, K.M.; Yun, H.D.; Lim, Y.P. Enhancement of tolerance to soft rot disease in the transgenic Chinese cabbage (Brassica rapa L. ssp. pekinensis) inbred line, Kenshin. Plant Cell Rep. 2009, 28, 1581–1591. [Google Scholar] [CrossRef]
- Baskar, V.; Gangadhar, B.H.; Park, S.W.; Nile, S.H. A simple and efficient Agrobacterium tumefaciens-mediated plant transformation of Brassica rapa ssp. Pekinensis. 3 Biotech 2016, 6, 88. [Google Scholar] [CrossRef]
- Liu, X.X.; Lang, S.R.; Su, L.Q.; Liu, X.; Wang, X.F. Improved Agrobacterium-mediated transformation and high efficiency of root formation from hypocotyl meristem of spring Brassica napus ‘Precocity’cultivar. Genet. Mol. Res. 2015, 14, 16840–16855. [Google Scholar] [CrossRef] [PubMed]
- Gasic, K.; Korban, S.S. Indian mustard [Brassica juncea (L.) Czern.]. In Agrobacterium Protocols; Methods in Molecular Biology; Wang, K., Ed.; Humana Press: Totowa, NJ, USA, 2006; Volume 343, pp. 281–289. [Google Scholar] [CrossRef]
- Lee, S.; Park, Y.S.; Rhee, J.H.; Chu, H.J.; Frost, J.M.; Choi, Y.H. Insights into plant regeneration: Cellular pathways and DNA methylation dynamics. Plant Cell Rep. 2024, 43, 120. [Google Scholar] [CrossRef] [PubMed]
- Kong, F.M.; Li, J.; Tan, X.L.; Zhang, L.L.; Zhang, Z.Y.; Qi, C.K.; Ma, X.K. New time-saving transformation system for Brassica napus. Afr. J. Biotechnol. 2009, 8, 2497–2502. [Google Scholar]
- Kamal, G.B.; Illich, K.G.; Asadollah, A. Effects of genotype, explant type and nutrient medium components on canola (Brassica napus L.) shoot in vitro organogenesis. Afr. J. Biotechnol. 2007, 6, 861–867. [Google Scholar]
- Sharma, M.; Sahni, R.; Kansal, R.; Koundal, K.R. Transformation of oilseed mustard Brassica juncea (L.) Czern & Coss cv. Pusajaikisan with snowdrop lectin gene. Indian J. Biotechnol. 2004, 1, 97–102. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).