Morphology and Allometry of Juvenile Açaí Palms Under Cultivation Conditions in Central Amazonia
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Site and Climate Conditions
2.2. Soil Preparation and Planting Procedures
2.3. Nutrient Management and Pruning Practices
2.4. Phenotypic Assessments
2.4.1. Nutritional Deficiencies and the Reproductive Stage of Plants
2.4.2. Stipe Growth and Number of Leaves on Plants
2.4.3. Leaf Morphology
2.4.4. Leaf Area Measurement
2.4.5. Statistical Analyses
2.4.6. Allometric Models for Estimating Leaf Área
3. Results and Discussion
3.1. Impact of Nutritional Deficiencies on Plant Reproductive Development
3.2. Plants’ Morphology and Development
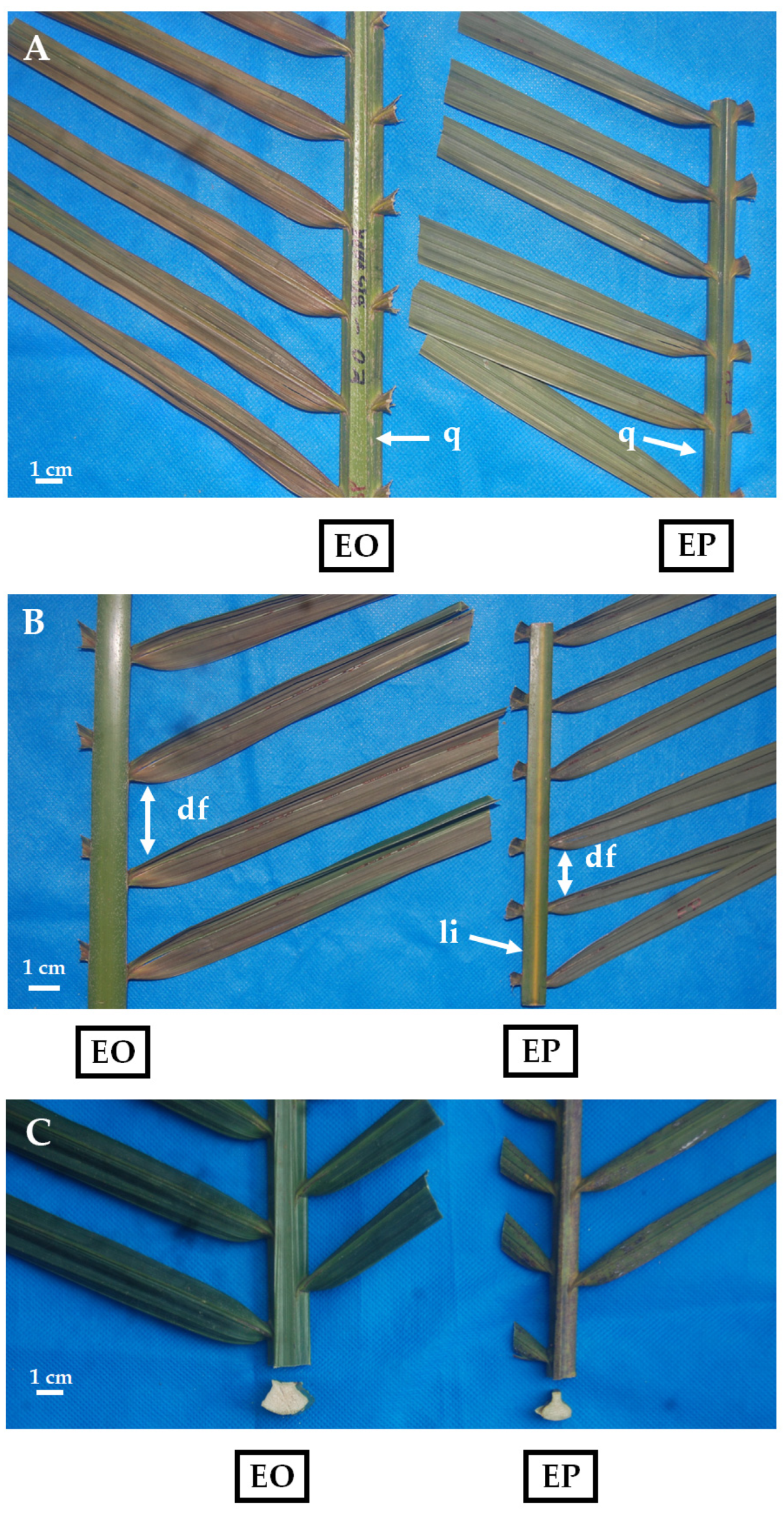
3.3. Morphology and Leaf Area
3.4. Leaf Allometry
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- IBGE (Instituto Brasileiro de Geografia e Estatística-IBGE). Sistema IBGE de Recuperação Automática. Banco de Dados Agregados. Tabela 1613: Área Destinada à Colheita, Área Colhida, Quantidade Produzida, Rendimento Médio e Valor da Produção das Lavouras Permanentes—Açaí. Rio de Janeiro. 2022. Available online: https://sidra.ibge.gov.br/tabela/1613#resultado (accessed on 6 July 2024).
- Vianna, S.A. Euterpe in Flora e Funga do Brasil. Jardim Botânico do Rio de Janeiro. Available online: https://floradobrasil.jbrj.gov.br/FB22142 (accessed on 30 July 2024).
- Castro, A.F.; Santos, A.C.; Silva, L.J.S.; Meneghetti, G.A.; Lopes, R. Adoção da cultivar BRS Pará no Amazonas: Um estudo exploratório da expansão de açaizais com tecnologia agropecuária disponibilizada pela Embrapa. In O Despertar para a Ciência: Contribuições dos Alunos de Iniciação Científica para a Pesquisa Socioeconômica na Amazônia; Silva, L.J.S., Meneghetti, G.A., Pinheiro, J.O.C., Eds.; Embrapa: Brasília, Brazil, 2022; pp. 17–41. Available online: https://ainfo.cnptia.embrapa.br/digital/bitstream/item/237885/1/Livro-bolsista-final-atual-p17.pdf (accessed on 8 August 2024).
- Kang, J.; Thakali, K.M.; Xie, C.; Kondo, M.; Tong, Y.; Ou, B.; Jensen, G.; Medina, M.B.; Schauss, A.L.; Wu, X. Bioactivities of açaí (Euterpe precatoria Mart.) fruit pulp, superior antioxidant and anti-inflammatory properties to Euterpe oleracea Mart. Food Chem. 2012, 133, 671–677. [Google Scholar] [CrossRef]
- Matos, C.B.; Sampaio, P.; Rivas, A.A.A.; Matos, J.C.S.; Hodges, D.G. Economic profile of two species of Genus Euterpe, producers of açaí fruits, from the Pará and Amazonas States—Brazil. Int. J.Environ. Agric. Biotechnol. 2017, 2, 1822–1828. [Google Scholar] [CrossRef]
- da Silva Melo, G.; da Costa, F.S.; da Silva, L.C. Productivity of Açai Euterpe Precatoria and Production System Management Recommendations in the South Of The Amazonas. Rev. Gest. Soc. Ambient. 2024, 18, e06972. [Google Scholar]
- Lopes, R.; Cunha, R.N.V.; Tavares, M.S.; Raizer, M.D.M.; Santos, C.A.; Silva, E.J.D.; Lopes, M.T.G. Seasonality of fruit production of Euterpe oleracea and E. precatoria açaí palm trees cultivated in the metropolitan region of Manaus (AM). Agro@mbiente On-Line 2022, 16, e7282. [Google Scholar] [CrossRef]
- Henderson, A.; Galeano, G. Euterpe, Prestoea, and Neonicholsonia (Palmae: Euterpeinae). Flora Neotrop. 1996, 72, 98–103. [Google Scholar]
- Henderson, A. The Palms of the Amazon, 1st. ed.; Oxford University Press: New York, NY, USA, 1995; p. 101. [Google Scholar]
- Clement, C.R.; de Cristo-Araujo, M.; d’Eeckenbrugge, G.C.; Alves Pereira, A.; Picanço-Rodrigues, D. Origin and domestication of native Amazonian crops. Diversity 2010, 2, 72–106. [Google Scholar] [CrossRef]
- Oliveira, M.S.P.; Mochiutti, S.; Farias-Neto, J.T. Domestication and Breeding for Assai Palm. In Domestication and Breendig: Amazonian Species; Borém, A., Lopes, M.T.G., Clement, C.R., Noda, H., Eds.; Suprema: Viçosa, Brazil, 2012; pp. 209–236. [Google Scholar]
- Nogueira, A.K.M.; Santana, A.C. Benefícios socioeconômicos da adoção de novas tecnologias no cultivo do açaí no Estado do Pará. Rev. Ceres 2016, 63, 1–7. [Google Scholar] [CrossRef]
- Chaves, S.F.S.; Alves, R.M.; Dias, L.A.S. Contribution of breeding to agriculture in the Brazilian Amazon. I. Açaí palm and oil palm. Crop Breed. Appl. Biotechnol. 2021, 21, e386221S8. [Google Scholar] [CrossRef]
- Ayres, M.I.; De Souza, E.I.; Uguen, K.; Alfaia, S.S.; Pereira, H. Dos Santo. Caracterização dos agroecossistemas de açaí-do-amazonas em Codajás, Amazonas—Brasil. RBA 2024, 19, 167–190. [Google Scholar] [CrossRef]
- Fritz, M.A.; Rosa, S.; Sicard, A. Mechanisms underlying the environmentally induced plasticity of leaf morphology. Front. Genet. 2018, 9, 478. [Google Scholar] [CrossRef]
- Moreira, A.; Fagueria, N.K. Potential of Brazilian Amazon Soils for Food and Fiber Production. Dyn. Soil Dyn. Plant 2008, 2, 82–88. [Google Scholar]
- Fisch, G.; Marenco, J.A.; Nobre, C.A. The climate of Amazonia–A review. Acta Amaz. 1999, 28, 101–126. [Google Scholar] [CrossRef]
- Oliveira, M.S.P.; Carvalho, J.E.U.; Nascimento, W.M.O.; Müller, C.H. Açaí Tree Cultivation for Fruit Production, 1st ed.; Embrapa: Belém, Brazil, 2002; pp. 6–7. [Google Scholar]
- Field, A. Discovering Statistics Using IBM SPSS Statistics, 4th ed.; SAGE: London, UK, 2013; pp. 156–215. [Google Scholar]
- IBM Corp. IBM SPSS Statistics for Windows; Version 20.0.; IBM Corp: Armonk, NY, USA, 2011. [Google Scholar]
- Viégas, I.J.M.; Frazão, D.A.C.; Thomaz, M.A.A.; Conceição, H.E.O.; Pinheiro, E. Limitações nutricionais para o cultivo de açaizeiros em Latossolo Amarelo textura media, Estado do Pará. Rev. Bras. De Frutic. 2004, 26, 382–384. [Google Scholar] [CrossRef]
- Viégas, I.J.M.; Gonçalves, A.A.S.; Frazão, D.A.C.; Conceição, H.E.O. Efeito das omissões de macronutrientes e Boro na sintomatologia e crescimento de plantas de açaizeiros (Euterpe oleracea Mart.). Rev. Ciências Agrárias 2008, 50, 129–142. [Google Scholar]
- Viégas, I.J.M.; Meireles, R.O.; Frazão, D.A.C.; Conceição, H.E.O. Avaliação da fertilidade de um Latossolo Amarelo textura média para o cultivo do açaizeiro no estado do Pará. Rev. Ciências Agrárias 2009, 52, 23–36. [Google Scholar]
- Cordeiro, R.A.M. Crescimento e Nutrição Mineral do Açaízeiro (Euterpe oleracea Mart.), em Função da Idade em Sistemas Agroflorestais no Município de Tomé-Açu, Pará. Ph.D. Thesis, Universidade Federal Rural da Amazônia, Belém, Brazil, 2011. [Google Scholar]
- Araujo, F.R.R.; Viegas, I.D.J.M.; Da Cunha, R.L.M.; De Vasconcelos, W.L.F. Efeito da omissão de nutrientes no crescimento e estado nutricional de mudas de açaizeiro. Pes. Agropec. Trop. 2016, 46, 374–382. [Google Scholar]
- Ribeiro, F.O. Estado Nutricional e Produtividade de Açaizeiro Fertirrigado em Função Variabilidade Espacial. Master’s Thesis, Universidade Federal Rural da Amazônia, Curió Utinga, Brazil, 2017. [Google Scholar]
- Cajueiro, L.R.; Costa, L.G.B.; Feitosa, I.d.L. Adubação orgânica na produção de açaí (Euterpe oleracea e E. precatoria), visando à geração de emprego e renda, em pequenas propriedades do estado de Rondônia. Cad. Agroecol. 2018, 13, 1–6. [Google Scholar]
- Lindolfo, M.M.; Matos, G.S.B.; Pereira, W.V.; Fernandes, A.R. Productivity and nutrition of fertigated açaí palms according to boron fertilization. Rev. Bras. Frutic. 2020, 42, e-601. [Google Scholar] [CrossRef]
- Almeida, U.O.D.; Andrade Neto, R.D.C.; Lunz, A.M.P.; Nogueira, S.R.; Costa, D.A.D.; Araújo, J.M.D. Environment and slow-release fertilizer in the production of Euterpe precatoria seedlings. Pesqui. Agropecu. Trop. 2018, 48, 382–389. [Google Scholar] [CrossRef]
- Araújo, C.S.; Lunz, A.M.P.; dos Santos, V.B.; Neto, R.D.C.A.; Nogueira, S.R.; dos Santos, R.S. Uso de resíduos agroindustriais como substrato para a produção de mudas de Euterpe precatoria. Pes. Agropec. Trop. 2020, 50, e58709. [Google Scholar] [CrossRef]
- Araújo, J.C. Adubação Nitrogenada e Fosfatada para Produção de Mudas de Açaizeiro Solteiro (Euterpe Precatoria Mart.). Ph.D. Thesis, Universidade Federal do Acre, Rio Branco, Brazil, 2021. [Google Scholar]
- Butzke, A.G.; de Brito, R.S.; Neto, R.D.C.A.; Lunz, A.M.P.; da Silva Fiuza, S.; Andrade, R.A. Produção de mudas de Euterpe precatoria Mart. em resposta a diferentes dosagens de nitrogênio e potássio. Comun. Sci. 2023, 14, e4000. [Google Scholar] [CrossRef]
- Pimentel-Gomes, F. Curso de Estatística Experimental, 15th ed.; FEALQ: Piracicaba, Brazil, 2009; p. 451. [Google Scholar]
- Avalos, G.; Fernández, O.M. Allometry and stilt root structure of the neotropical palm Euterpe precatoria (Arecaceae) across sites and successional stages. Am. J. Bot. 2010, 97, 388–394. [Google Scholar] [CrossRef] [PubMed]
- Avalos, G.; Sylvester, O. Allometric estimation of total leaf area in the neotropical palm Euterpe oleracea at La Selva, Costa Rica. Trees 2010, 24, 969–974. [Google Scholar] [CrossRef]
- Brum, H.D.; Souza, A.F. Flood disturbance and shade stress shape the population structure of açaí palm Euterpe precatoria, the most abundant Amazon species. Botany 2020, 8, 147–160. [Google Scholar] [CrossRef]
- Clement, C. Growth and Genetic Analysis of Pejibaye (bactris gasipaes Kunth, Palmae) in Hawaii. Ph.D. Thesis, University of Hawaii, Honolulu, HI, USA, 1995. [Google Scholar]
- Ramos, A.; Bovi, M.L.A.; Folegatti, M.V.; Diotto, A.V. Leaf area and aboveground biomass estimates in peach palm using allometric relationships. Hortic. Bras. 2008, 26, 138–143. [Google Scholar] [CrossRef][Green Version]
- Gotelli, N.J.; Ellison, A.M. A Primer of Ecological Statistic, 1st ed.; Sinauer Associates: Sunderland, MA, USA, 2011; pp. 277–282. [Google Scholar]
| Character Evaluated | F | Coefficient of Variation (%) |
|---|---|---|
| Petiole Length (cm) | 0.74 | 47 |
| Petiole Height (cm) | 31.16 ** | 22 |
| Petiole Width (cm) | 13.55 ** | 11 |
| Rachis Length (cm) | 1.39 | 9 |
| Leaf Width (cm) | 0.05 | 13 |
| Number of Leaflets | 5.73 ** | 11 |
| Leaflet Length (cm) | 1.86 | 10 |
| Leaflet Width (cm) | 51.72 ** | 17 |
| Distance Between Leaflets (cm) | 8.18 ** | 12 |
| Variables * | E. oleracea cv. BRS Pará | E. oleracea cv. Chumbinho | E. precatoria |
|---|---|---|---|
| Stem | |||
| N | 27 | 17 | 121 |
| Stipe height | 576 ± 93 a | 599 ± 86 a | 357 ± 101 b |
| Internode distance | 63 ± 8 a | 86 ± 3 a | 44 ± 13 b |
| Stem diameter | 23 ± 3 a | 24 ± 2 a | 19 ± 5 b |
| Number of leaves | 26 ± 10 a | 26 ± 12 a | 10 ± 2 b |
| Leaf | |||
| N | 3 | 3 | 3 |
| Petiole length (cm) | 13.7 ± 6.8 a | 17.3 ± 6.1 a | 17.1 ± 9.2 a |
| Rachis length (cm) | 251.7 ± 20.2 a | 268.3 ± 23.0 a | 255.1 ± 26.7 a |
| Leaf width (cm) | 167.9 ± 26.5 a | 171.0 ± 21.5 a | 169.5 ± 17.7 a |
| Number of leaflets (count) | 115.4 ± 15.8 b | 116.8 ± 7.1 b | 130.2 ± 9.9 a |
| Rachis width (cm) | 3.5 ± 0.2 a | 3.6 ± 0.3 a | 3.0 ± 0.3 b |
| Rachis height (cm) | 2.1 ± 0.2 a | 2.1 ± 0.3 a | 1.4 ± 0.1 b |
| Leaflet length (cm) | 93.5 ± 6.9 a | 93.4 ± 8.3 a | 87.0 ± 11.4 a |
| Leaflet width (cm) | 4.1 ± 0.2 a | 3.9 ± 0.3 a | 2.9 ± 0.3 b |
| Distance between leaflets (cm) | 4.4 ± 0.5 a | 4.6 ± 0.4 a | 3.9 ± 0.4 b |
| Yellow stripe on the rachis | Absent | Absent | Present |
| Rachis shape | Trapezoidal | Trapezoidal | Triangular |
| Leaf Area | |||
| N | 3 | 3 | |
| Plant leaf area (m2) | 25.21 ± 9.23 a | - | 16.72 ± 2.76 b |
| N | 34 | 32 | |
| Leaf area (m2) | 5.82 ± 1.47 a | - | 4.18 ± 1.19 b |
| Af | Cfa | Lfa | Cfm | Lfm | Cr | Nf | |
|---|---|---|---|---|---|---|---|
| Af | - | 0.922 ** | 0.662 ** | 0.956 ** | 0.415 * | 0.848 ** | 0.725 ** |
| Cfa | 0.934 ** | - | 0.712 ** | 0.940 ** | 0.339 | 0.813 ** | 0.618 ** |
| Lfa | 0.622 ** | 0.538 ** | - | 0.644 ** | 0.550 ** | 0.572 ** | 0.303 |
| Cfm | 0.915 ** | 0.962 ** | 0.517 ** | - | 0.293 | 0.886 ** | 0.720 ** |
| Lfm | 0.805 ** | 0.711 ** | 0.533 ** | 0.614 ** | - | 0.025 | 0.039 |
| Cr | 0.911 ** | 0.925 ** | 0.544 ** | 0.912 ** | 0.752 ** | - | 0.717 ** |
| Nf | 0.745 ** | 0.729 ** | 0.380 * | 0.699 ** | 0.575 ** | 0.716 ** | - |
| Model | t-Test for Model Parameters | S | CV (%) | R2 | ||||
|---|---|---|---|---|---|---|---|---|
| ta | tb1 | tb2 | tb3 | tb4 | ||||
| AFo = –3.32 + 3.52 Cf + 43.80 Lf − 0.034 Cr + 0.01 Nf | –7.8 ** | 5.3 ** | 5.1 ** | –0.1 ns | 1.5 ns | 0.15 | 7 | 94 |
| AFo = –2.85 + 3.71 Cf + 45.48 Lf + 0.02 Cr | –9.6 ** | 5.6 ** | 5.2 ** | 0.1 ns | 0.15 | 7 | 93 | |
| AFo = –2.86 + 3.75 Cf + 45.88 Lf | –11.5 ** | 11.5 ** | 6.6 ** | 0.15 | 7 | 93 | ||
| AFo = –2.10 + 5.09 Cf | –6.2 ** | 12.9 ** | 0.23 | 10 | 84 | |||
| AFp = –1.77 + 1.90 Cf + 32.61 Lf + 0.17 Cr + 0.004 Nf | –4.2 ** | 6.7 ** | 1.3 ** | –0.1 ns | 0.79 ns | 0.08 | 5 | 94 |
| AFp = –1.43 + 2.11 Cf + 30.26 Lf + 0.19 Cr | –6.3 ** | 5.8 ** | 3.7 ** | 2.0 ns | 0.09 | 6 | 94 | |
| AFp = –1.21 + 2.78 Cf + 21.67 Lf | –5.8 ** | 18.4 ** | 3.0 ** | 0.09 | 6 | 93 | ||
| AFp = –0.70 + 2.91 | –5.5 ** | 17.9 ** | 0.10 | 7 | 92 | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Delgado, J.P.M.; da Cunha, R.N.V.; de Morais, R.R.; Lopes, M.T.G.; Ramos, S.L.F.; Rodrigues, M.d.R.L.; de Medeiros, N.M.C.; Meneses, C.H.S.G.; Barcelos, E.; Lopes, R. Morphology and Allometry of Juvenile Açaí Palms Under Cultivation Conditions in Central Amazonia. Horticulturae 2024, 10, 1119. https://doi.org/10.3390/horticulturae10101119
Delgado JPM, da Cunha RNV, de Morais RR, Lopes MTG, Ramos SLF, Rodrigues MdRL, de Medeiros NMC, Meneses CHSG, Barcelos E, Lopes R. Morphology and Allometry of Juvenile Açaí Palms Under Cultivation Conditions in Central Amazonia. Horticulturae. 2024; 10(10):1119. https://doi.org/10.3390/horticulturae10101119
Chicago/Turabian StyleDelgado, Jhon Paul Mathews, Raimundo Nonato Vieira da Cunha, Ronaldo Ribeiro de Morais, Maria Teresa Gomes Lopes, Santiago Linorio Ferreyra Ramos, Maria do Rosário Lobato Rodrigues, Nathalia Maíra Cabral de Medeiros, Carlos Henrique Salvino Gadelha Meneses, Edson Barcelos, and Ricardo Lopes. 2024. "Morphology and Allometry of Juvenile Açaí Palms Under Cultivation Conditions in Central Amazonia" Horticulturae 10, no. 10: 1119. https://doi.org/10.3390/horticulturae10101119
APA StyleDelgado, J. P. M., da Cunha, R. N. V., de Morais, R. R., Lopes, M. T. G., Ramos, S. L. F., Rodrigues, M. d. R. L., de Medeiros, N. M. C., Meneses, C. H. S. G., Barcelos, E., & Lopes, R. (2024). Morphology and Allometry of Juvenile Açaí Palms Under Cultivation Conditions in Central Amazonia. Horticulturae, 10(10), 1119. https://doi.org/10.3390/horticulturae10101119








