Functional Analysis of Cytochrome b5 in Regulating Anthocyanin Biosynthesis in Malus domestica
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Materials and Treatments
2.2. Gene Identification, Cloning, and Biological Informational Analysis
2.3. Subcellular Localization of MdCYB5
2.4. Genetic Transformation of Apple Calli and Apple Tissue Culture Seedlings
2.5. DNA, RNA Extraction and qRT-PCR Analysis
2.6. Determinationthe Accumulation of Measurement of Anthocyanin
2.7. Statistical Analysis
3. Results
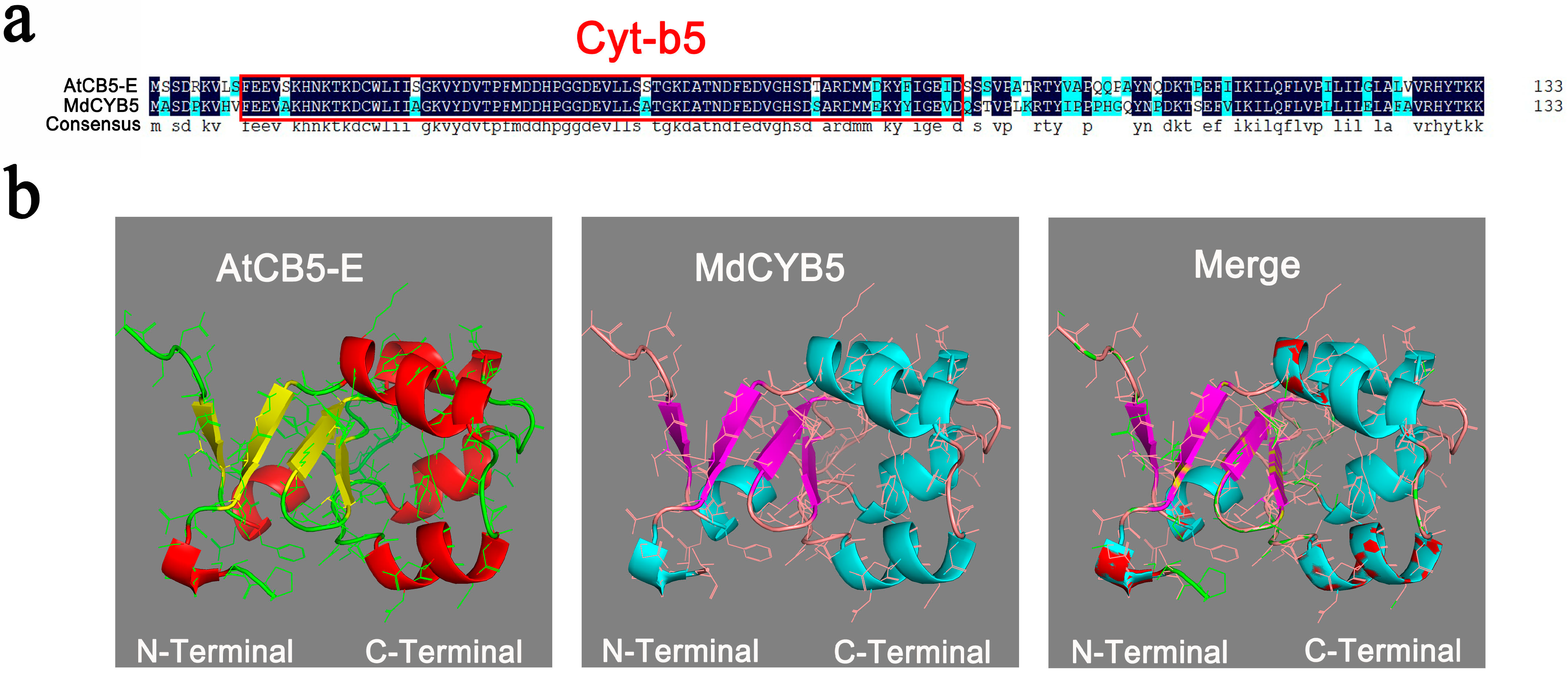
3.1. Cloning of MdCYB5 Gene and Protein Structure Analysis of MdCYB5
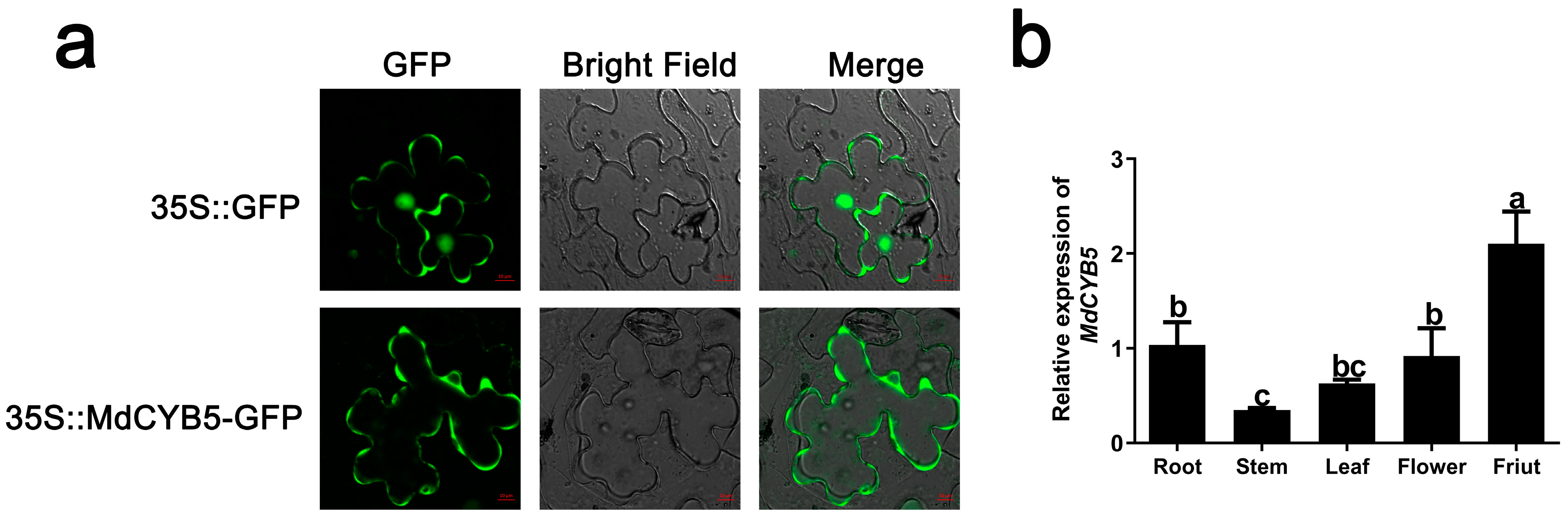
3.2. Subcellular Localization and Tissue Specificity Analysis of MdCYB5
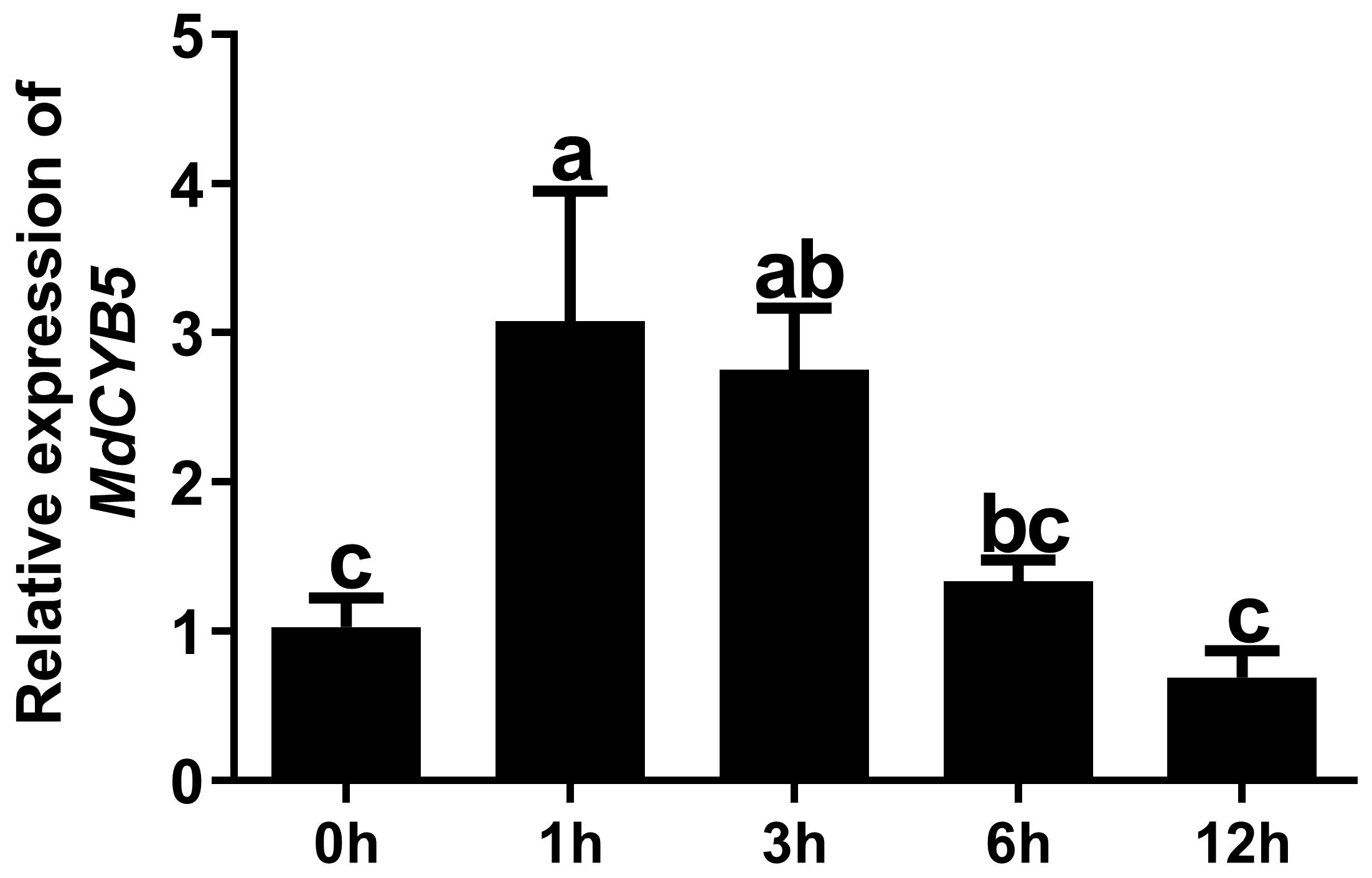
3.3. MdCYB5 Responds to Light Signals
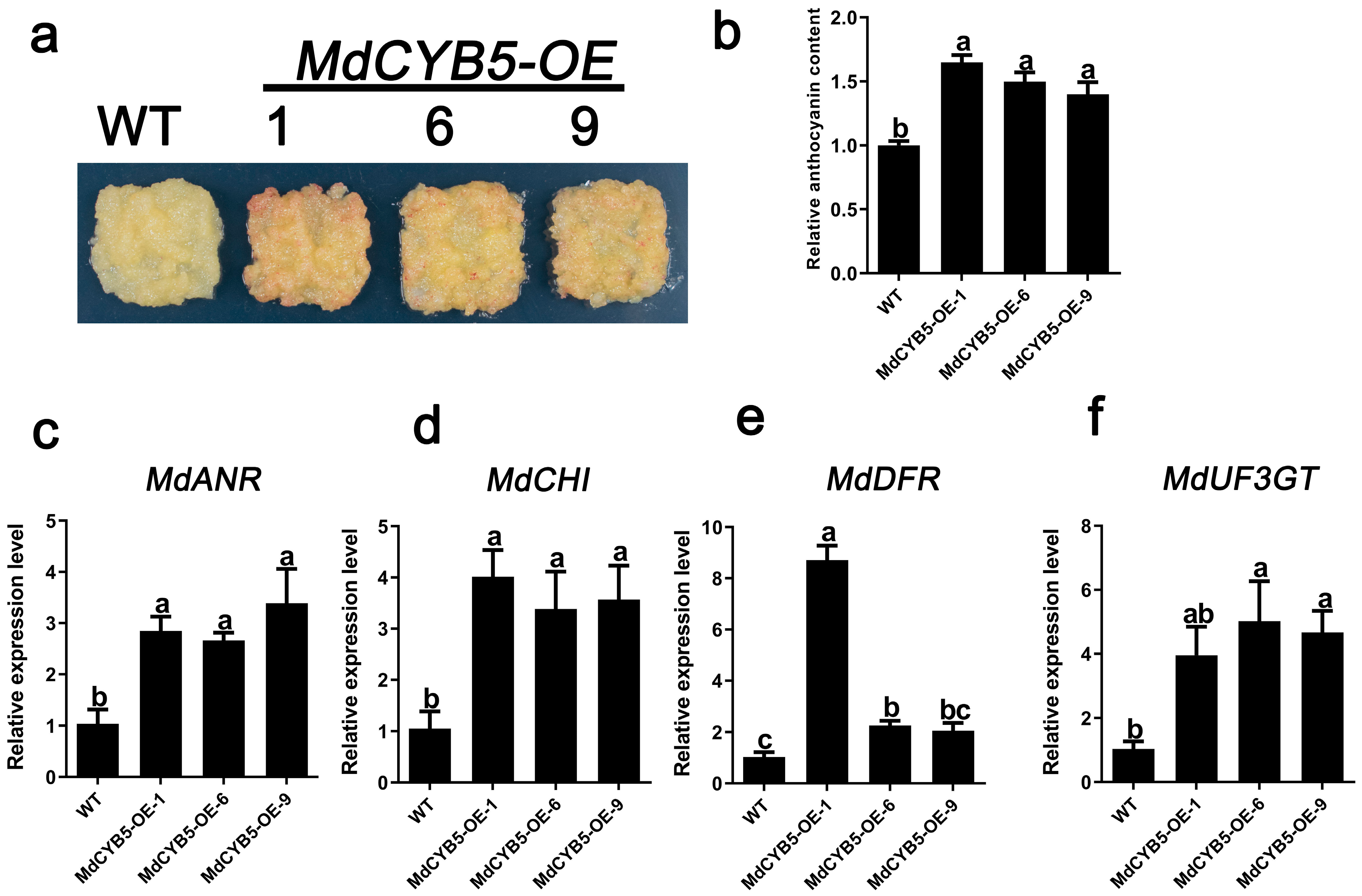
3.4. MdCYB5 Is a Positive Regulator of Anthocyanin Accumulation in Apple Calli
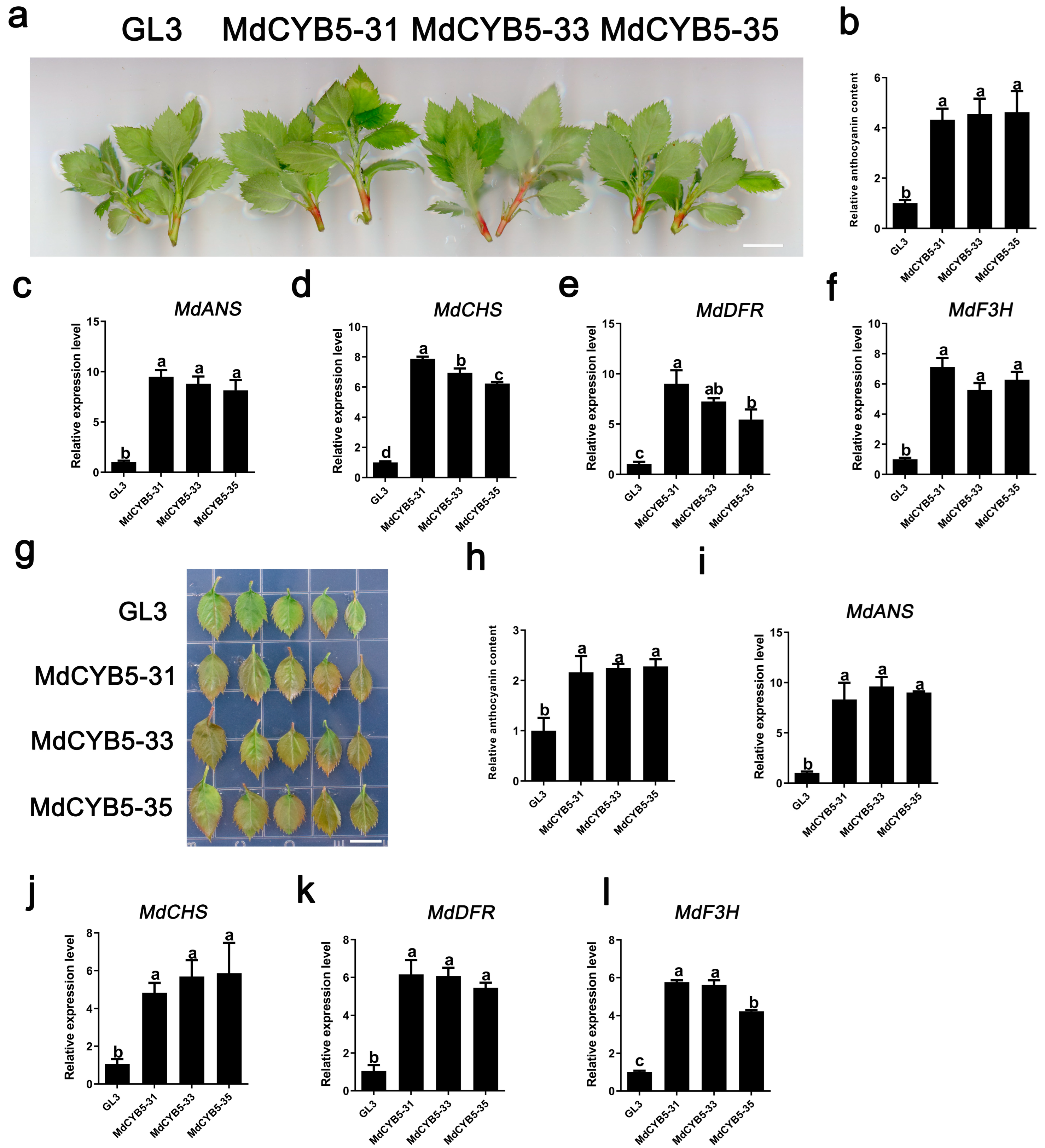
3.5. Overexpression of MdCYB5 Increases Anthocyanin Accumulation in Leaves and Stems of Apple Tissue Culture Seedlings
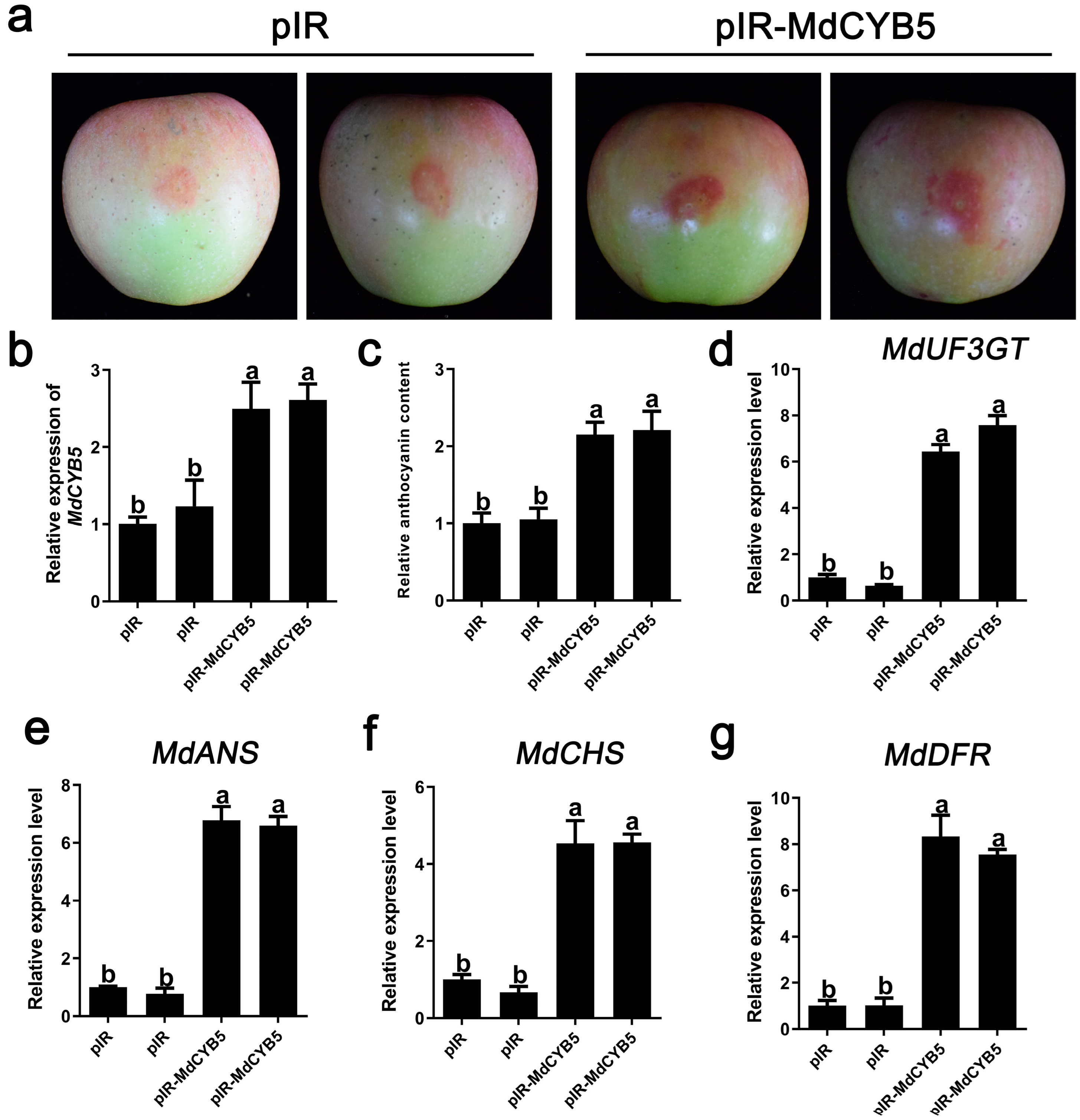
3.6. Transient Overexpression of MdCYB5 Increased Anthocyanin Accumulation in Apple Fruits
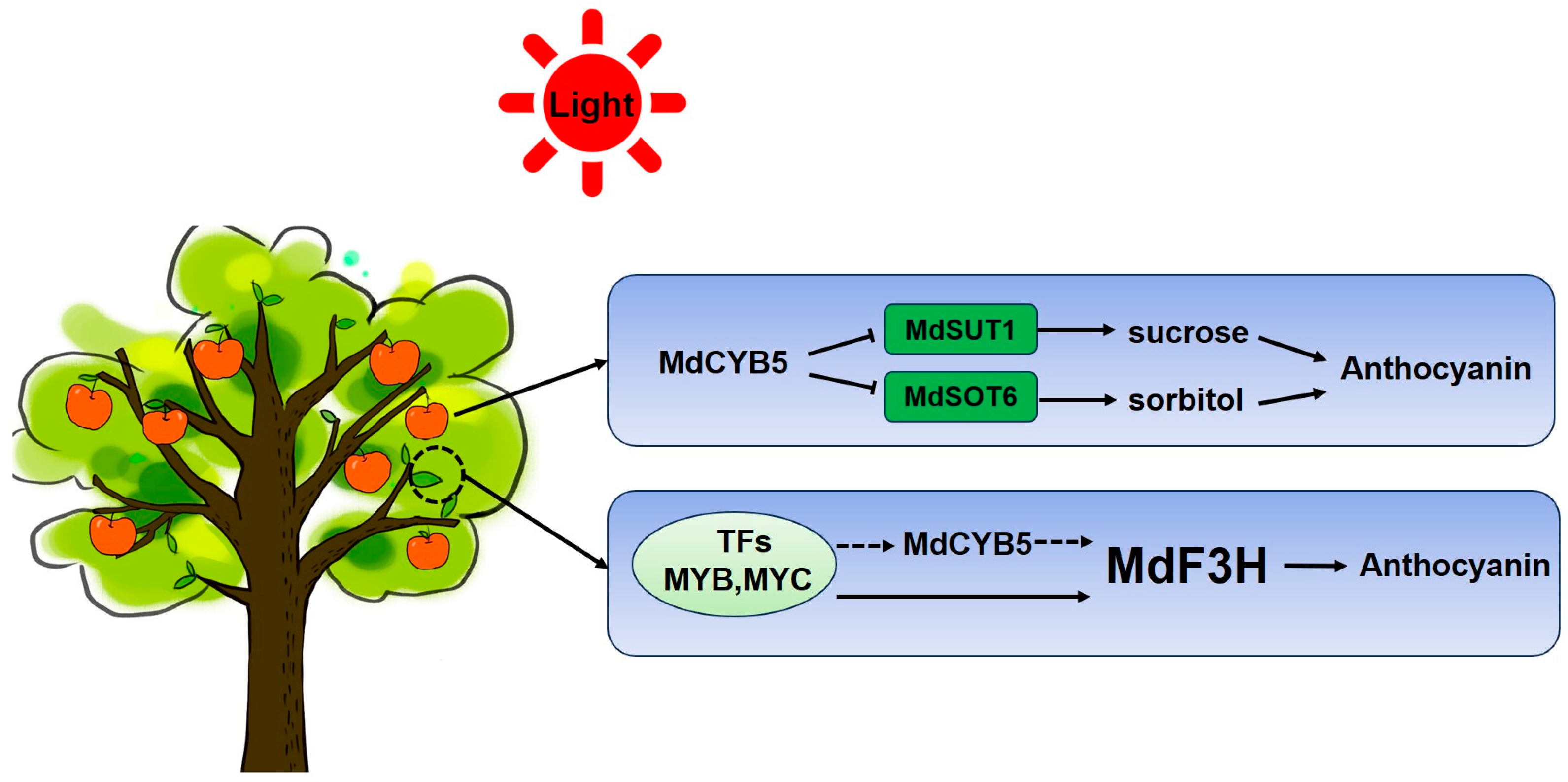
4. Discussion
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Nakabayashi, R.; Yonekura-Sakakibara, K.; Urano, K.; Suzuki, M.; Yamada, Y.; Nishizawa, T.; Matsuda, F.; Kojima, M.; Sakakibara, H.; Shinozaki, K.; et al. Enhancement of oxidative and drought tolerance in Arabidopsis by overaccumulation of antioxidant flavonoids. Plant J. 2014, 77, 367–379. [Google Scholar] [CrossRef]
- Butelli, E.; Titta, L.; Giorgio, M.; Mock, H.P.; Matros, A.; Peterek, S.; Schijlen, E.G.; Hall, R.D.; Bovy, A.G.; Luo, J.; et al. Enrichment of tomato fruit with health-promoting anthocyanins by expression of select transcription factors. Nat. Biotechnol. 2008, 26, 1301–1308. [Google Scholar] [CrossRef] [PubMed]
- Gould, K.S. Nature’s Swiss Army Knife: The Diverse Protective Roles of Anthocyanins in Leaves. J. Biomed. Biotechnol. 2004, 2004, 314–320. [Google Scholar] [CrossRef] [PubMed]
- Koes, R.; Verweij, W.; Quattrocchio, F. Flavonoids: A colorful model for the regulation and evolution of biochemical pathways. Trends Plant Sci. 2005, 10, 236–242. [Google Scholar] [CrossRef] [PubMed]
- Hichri, I.; Barrieu, F.; Bogs, J.; Kappel, C.; Delrot, S.; Lauvergeat, V. Recent advances in the transcriptional regulation of the flavonoid biosynthetic pathway. J. Exp. Bot. 2011, 62, 2465–2483. [Google Scholar] [CrossRef] [PubMed]
- Shan, X.; Zhang, Y.; Peng, W.; Wang, Z.; Xie, D. Molecular mechanism for jasmonate-induction of anthocyanin accumulation in Arabidopsis. J. Exp. Bot. 2009, 60, 3849–3860. [Google Scholar] [CrossRef]
- Rubin, G.; Tohge, T.; Matsuda, F.; Saito, K.; Scheible, W.R. Members of the LBD family of transcription factors repress anthocyanin synthesis and affect additional nitrogen responses in Arabidopsis. Plant Cell 2009, 21, 3567–3584. [Google Scholar] [CrossRef]
- Liu, Y.; Lin-Wang, K.; Espley, R.V.; Wang, L.; Li, Y.; Liu, Z.; Zhou, P.; Zeng, L.; Zhang, X.; Zhang, J.; et al. StMYB44 negatively regulates anthocyanin biosynthesis at high temperatures in tuber flesh of potato. J. Exp. Bot. 2019, 70, 3809–3824. [Google Scholar] [CrossRef]
- Zhang, Y.; Butelli, E.; De Stefano, R.; Schoonbeek, H.J.; Magusin, A.; Pagliarani, C.; Wellner, N.; Hill, L.; Orzaez, D.; Granell, A.; et al. Anthocyanins double the shelf life of tomatoes by delaying overripening and reducing susceptibility to gray mold. Curr. Biol. 2013, 23, 1094–1100. [Google Scholar] [CrossRef]
- An, J.P.; Zhang, X.W.; Bi, S.Q.; You, C.X.; Wang, X.F.; Hao, Y.J. The ERF transcription factor MdERF38 promotes drought stress-induced anthocyanin biosynthesis in apple. Plant J. 2020, 101, 573–589. [Google Scholar] [CrossRef]
- Xie, X.B.; Li, S.; Zhang, R.F.; Zhao, J.; Chen, Y.C.; Zhao, Q.; Yao, Y.X.; You, C.X.; Zhang, X.S.; Hao, Y.J. The bHLH transcription factor MdbHLH3 promotes anthocyanin accumulation and fruit colouration in response to low temperature in apples. Plant Cell Environ. 2012, 35, 1884–1897. [Google Scholar] [CrossRef]
- Gan, Y.; Li, H.; Xie, Y.; Wu, W.; Li, M.; Wang, X.; Huang, J. THF1 mutations lead to increased basal and wound-induced levels of oxylipins that stimulate anthocyanin biosynthesis via COI1 signaling in Arabidopsis. J. Integr. Plant Biol. 2014, 56, 916–927. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.Y.; Mao, K.; Zhao, C.; Zhao, X.Y.; Zhang, H.L.; Shu, H.R.; Hao, Y.J. MdCOP1 ubiquitin E3 ligases interact with MdMYB1 to regulate light-induced anthocyanin biosynthesis and red fruit coloration in apple. Plant Physiol. 2012, 160, 1011–1022. [Google Scholar] [CrossRef]
- Steyn, W.J.; Wand, S.J.E.; Holcroft, D.M.; Jacobs, G. Anthocyanins in vegetative tissues: A proposed unified function in photoprotection. New Phytol. 2002, 155, 349–361. [Google Scholar] [CrossRef]
- Gangappa, S.N.; Botto, J.F. The Multifaceted Roles of HY5 in Plant Growth and Development. Mol. Plant 2016, 9, 1353–1365. [Google Scholar] [CrossRef] [PubMed]
- Fang, H.; Dong, Y.; Yue, X.; Hu, J.; Jiang, S.; Xu, H.; Wang, Y.; Su, M.; Zhang, J.; Zhang, Z.; et al. The B-box zinc finger protein MdBBX20 integrates anthocyanin accumulation in response to ultraviolet radiation and low temperature. Plant Cell Environ. 2019, 42, 2090–2104. [Google Scholar] [CrossRef]
- An, J.P.; Xu, R.R.; Wang, X.N.; Zhang, X.W.; You, C.X.; Han, Y. MdbHLH162 connects the gibberellin and jasmonic acid signals to regulate anthocyanin biosynthesis in apple. J. Integr. Plant Biol. 2024, 66, 265–284. [Google Scholar] [CrossRef]
- An, J.P.; Zhang, X.W.; Liu, Y.J.; Wang, X.F.; You, C.X.; Hao, Y.J. ABI5 regulates ABA-induced anthocyanin biosynthesis by modulating the MYB1-bHLH3 complex in apple. J. Exp. Bot. 2021, 72, 1460–1472. [Google Scholar] [CrossRef]
- Maggio, C.; Barbante, A.; Ferro, F.; Frigerio, L.; Pedrazzini, E. Intracellular sorting of the tail-anchored protein cytochrome b5 in plants: A comparative study using different isoforms from rabbit and Arabidopsis. J. Exp. Bot. 2007, 58, 1365–1379. [Google Scholar] [CrossRef] [PubMed]
- Hwang, Y.T.; Pelitire, S.M.; Henderson, M.P.; Andrews, D.W.; Dyer, J.M.; Mullen, R.T. Novel targeting signals mediate the sorting of different isoforms of the tail-anchored membrane protein cytochrome b5 to either endoplasmic reticulum or mitochondria. Plant Cell 2004, 16, 3002–3019. [Google Scholar] [CrossRef]
- Vergères, G.; Ramsden, J.; Waskell, L. The carboxyl terminus of the membrane-binding domain of cytochrome b5 spans the bilayer of the endoplasmic reticulum. J. Biol. Chem. 1995, 270, 3414–3422. [Google Scholar] [CrossRef] [PubMed]
- Porter, T.D. The roles of cytochrome b5 in cytochrome P450 reactions. J. Biochem. Mol. Toxicol. 2002, 16, 311–316. [Google Scholar] [CrossRef] [PubMed]
- Schenkman, J.B.; Jansson, I. The many roles of cytochrome b5. Pharmacol. Ther. 2003, 97, 139–152. [Google Scholar] [CrossRef] [PubMed]
- Pascal, S.; Bernard, A.; Deslous, P.; Gronnier, J.; Fournier-Goss, A.; Domergue, F.; Rowland, O.; Joubès, J. Arabidopsis CER1-LIKE1 Functions in a Cuticular Very-Long-Chain Alkane-Forming Complex. Plant Physiol. 2019, 179, 415–432. [Google Scholar] [CrossRef] [PubMed]
- Bernard, A.; Domergue, F.; Pascal, S.; Jetter, R.; Renne, C.; Faure, J.D.; Haslam, R.P.; Napier, J.A.; Lessire, R.; Joubès, J. Reconstitution of plant alkane biosynthesis in yeast demonstrates that Arabidopsis ECERIFERUM1 and ECERIFERUM3 are core components of a very-long-chain alkane synthesis complex. Plant Cell 2012, 24, 3106–3118. [Google Scholar] [CrossRef] [PubMed]
- Ruan, H.; Gao, L.; Fang, Z.; Lei, T.; Xing, D.; Ding, Y.; Rashid, A.; Zhuang, J.; Zhang, Q.; Gu, C.; et al. A flavonoid metabolon: Cytochrome b(5) enhances B-ring trihydroxylated flavan-3-ols synthesis in tea plants. Plant J. 2024, 118, 1793–1814. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Zhao, Y.; Gou, M.; Liu, C.J. Tissue-preferential recruitment of electron transfer chains for cytochrome P450-catalyzed phenolic biosynthesis. Sci. Adv. 2023, 9, eade4389. [Google Scholar] [CrossRef]
- Gou, M.; Yang, X.; Zhao, Y.; Ran, X.; Song, Y.; Liu, C.J. Cytochrome b 5 Is an Obligate Electron Shuttle Protein for Syringyl Lignin Biosynthesis in Arabidopsis. Plant Cell 2019, 31, 1344–1366. [Google Scholar] [CrossRef]
- Zhao, X.; Zhao, Y.; Zeng, Q.Y.; Liu, C.J. Cytochrome b5 diversity in green lineages preceded the evolution of syringyl lignin biosynthesis. Plant Cell 2024, 36, 2709–2728. [Google Scholar] [CrossRef]
- Resnick, J.S.; Rivarola, M.; Chang, C. Involvement of RTE1 in conformational changes promoting ETR1 ethylene receptor signaling in Arabidopsis. Plant J. 2008, 56, 423–431. [Google Scholar] [CrossRef]
- Chang, J.; Clay, J.M.; Chang, C. Association of cytochrome b5 with ETR1 ethylene receptor signaling through RTE1 in Arabidopsis. Plant J. 2014, 77, 558–567. [Google Scholar] [CrossRef] [PubMed]
- Fan, R.C.; Peng, C.C.; Xu, Y.H.; Wang, X.F.; Li, Y.; Shang, Y.; Du, S.Y.; Zhao, R.; Zhang, X.Y.; Zhang, L.Y.; et al. Apple sucrose transporter SUT1 and sorbitol transporter SOT6 interact with cytochrome b5 to regulate their affinity for substrate sugars. Plant Physiol. 2009, 150, 1880–1901. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Li, L.L.; Fan, R.C.; Peng, C.C.; Sun, H.L.; Zhu, S.Y.; Wang, X.F.; Zhang, L.Y.; Zhang, D.P. Arabidopsis sucrose transporter SUT4 interacts with cytochrome b5-2 to regulate seed germination in response to sucrose and glucose. Mol. Plant 2012, 5, 1029–1041. [Google Scholar] [CrossRef] [PubMed]
- Daccord, N.; Celton, J.M.; Linsmith, G.; Becker, C.; Choisne, N.; Schijlen, E.; van de Geest, H.; Bianco, L.; Micheletti, D.; Velasco, R.; et al. High-quality de novo assembly of the apple genome and methylome dynamics of early fruit development. Nat. Genet. 2017, 49, 1099–1106. [Google Scholar] [CrossRef]
- Rombauts, S.; Déhais, P.; Van Montagu, M.; Rouzé, P. PlantCARE, a plant cis-acting regulatory element database. Nucleic Acids Res. 1999, 27, 295–296. [Google Scholar] [CrossRef]
- Kelley, L.A.; Mezulis, S.; Yates, C.M.; Wass, M.N.; Sternberg, M.J. The Phyre2 web portal for protein modeling, prediction and analysis. Nat. Protoc. 2015, 10, 845–858. [Google Scholar] [CrossRef]
- Zhang, Z.L.; Zhang, F.J.; Zheng, P.F.; Xie, Y.H.; You, C.X.; Hao, Y.J. Determination of Protein Interactions among Replication Components of Apple Necrotic Mosaic Virus. Viruses 2020, 12, 474. [Google Scholar] [CrossRef]
- Zhang, F.J.; Xie, Y.H.; Jiang, H.; Wang, X.; Hao, Y.J.; Zhang, Z.; You, C.X. The ankyrin repeat-containing protein MdANK2B regulates salt tolerance and ABA sensitivity in Malus domestica. Plant Cell Rep. 2020, 40, 405–419. [Google Scholar] [CrossRef]
- Zhang, F.J.; Li, Z.Y.; Zhang, D.E.; Ma, N.; Wang, Y.X.; Zhang, T.T.; Zhao, Q.; Zhang, Z.; You, C.X.; Lu, X.Y. Identification of Hsp20 gene family in Malus domestica and functional characterization of Hsp20 class I gene MdHsp18.2b. Physiol. Plant 2024, 176, e14288. [Google Scholar] [CrossRef]
- Bai, S.; Tao, R.; Tang, Y.; Yin, L.; Ma, Y.; Ni, J.; Yan, X.; Yang, Q.; Wu, Z.; Zeng, Y.; et al. BBX16, a B-box protein, positively regulates light-induced anthocyanin accumulation by activating MYB10 in red pear. Plant Biotechnol. J. 2019, 17, 1985–1997. [Google Scholar] [CrossRef]
- An, J.P.; Liu, Y.J.; Zhang, X.W.; Bi, S.Q.; Wang, X.F.; You, C.X.; Hao, Y.J. Dynamic regulation of anthocyanin biosynthesis at different light intensities by the BT2-TCP46-MYB1 module in apple. J. Exp. Bot. 2020, 71, 3094–3109. [Google Scholar] [CrossRef]
- Zhao, L.; Zhang, Y.; Sun, J.; Yang, Q.; Cai, Y.; Zhao, C.; Wang, F.; He, H.; Han, Y. PpHY5 is involved in anthocyanin coloration in the peach flesh surrounding the stone. Plant J. 2023, 114, 951–964. [Google Scholar] [CrossRef]
- Ren, Y.R.; Zhao, Q.; Yang, Y.Y.; Zhang, R.; Wang, X.F.; Zhang, T.E.; You, C.X.; Huo, H.Q.; Hao, Y.J. Interaction of BTB-TAZ protein MdBT2 and DELLA protein MdRGL3a regulates nitrate-mediated plant growth. Plant Physiol. 2021, 186, 750–766. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, Z.; Liu, J.; Lin, S.; Wang, J.; Lin, W.; Xu, W. GA-DELLA pathway is involved in regulation of nitrogen deficiency-induced anthocyanin accumulation. Plant Cell Rep. 2017, 36, 557–569. [Google Scholar] [CrossRef]
- Smith, M.A.; Jonsson, L.; Stymne, S.; Stobart, K. Evidence for cytochrome b5 as an electron donor in ricinoleic acid biosynthesis in microsomal preparations from developing castor bean (Ricinus communis L.). Biochem. J. 1992, 287 Pt 1, 141–144. [Google Scholar] [CrossRef]
- Napier, J.A.; Smith, M.A.; Stobart, A.K.; Shewry, P.R. Isolation of a cDNA encoding a cytochrome b5 specifically expressed in developing tobacco seeds. Planta 1995, 197, 200–202. [Google Scholar] [CrossRef]
- Fukuchi-Mizutani, M.; Mizutani, M.; Tanaka, Y.; Kusumi, T.; Ohta, D. Microsomal electron transfer in higher plants: Cloning and heterologous expression of NADH-cytochrome b5 reductase from Arabidopsis. Plant Physiol. 1999, 119, 353–362. [Google Scholar] [CrossRef]
- Kumar, R.; Tran, L.S.; Neelakandan, A.K.; Nguyen, H.T. Higher plant cytochrome b5 polypeptides modulate fatty acid desaturation. PLoS ONE 2012, 7, e31370. [Google Scholar] [CrossRef]
- Vogt, T. Phenylpropanoid biosynthesis. Mol. Plant 2010, 3, 2–20. [Google Scholar] [CrossRef]
- de Vetten, N.; ter Horst, J.; van Schaik, H.P.; de Boer, A.; Mol, J.; Koes, R. A cytochrome b5 is required for full activity of flavonoid 3′, 5′-hydroxylase, a cytochrome P450 involved in the formation of blue flower colors. Proc. Natl. Acad. Sci. USA 1999, 96, 778–783. [Google Scholar] [CrossRef]
- Yoon, J.; Cho, L.H.; Tun, W.; Jeon, J.S.; An, G. Sucrose signaling in higher plants. Plant Sci. 2021, 302, 110703. [Google Scholar] [CrossRef]
- Xu, D. COP1 and BBXs-HY5-mediated light signal transduction in plants. New Phytol. 2020, 228, 1748–1753. [Google Scholar] [CrossRef]
- An, J.P.; Wang, X.F.; Zhang, X.W.; Bi, S.Q.; You, C.X.; Hao, Y.J. MdBBX22 regulates UV-B-induced anthocyanin biosynthesis through regulating the function of MdHY5 and is targeted by MdBT2 for 26S proteasome-mediated degradation. Plant Biotechnol. J. 2019, 17, 2231–2233. [Google Scholar] [CrossRef]
- Tao, R.; Yu, W.; Gao, Y.; Ni, J.; Yin, L.; Zhang, X.; Li, H.; Wang, D.; Bai, S.; Teng, Y. Light-Induced Basic/Helix-Loop-Helix64 Enhances Anthocyanin Biosynthesis and Undergoes Constitutively Photomorphogenic1-Mediated Degradation in Pear. Plant Physiol. 2020, 184, 1684–1701. [Google Scholar] [CrossRef]
- Zhang, Z.; Li, Z.Y.; Zhang, F.J.; Zheng, P.F.; Ma, N.; Li, L.; Li, H.; Sun, P.; Zhang, S.; Wang, X.F.; et al. A viroid-derived small interfering RNA targets bHLH transcription factor MdPIF1 to regulate anthocyanin biosynthesis in Malus domestica. Plant Cell Environ. 2024, 1–19. [Google Scholar] [CrossRef]
- Strygina, K.V.; Khlestkina, E.K. Myc-like transcriptional factors in wheat: Structural and functional organization of the subfamily I members. BMC Plant Biol. 2019, 19 (Suppl. S1), 50. [Google Scholar] [CrossRef]
- Liu, S.; Wang, Y.; Shi, M.; Maoz, I.; Gao, X.; Sun, M.; Yuan, T.; Li, K.; Zhou, W.; Guo, X.; et al. SmbHLH60 and SmMYC2 antagonistically regulate phenolic acids and anthocyanins biosynthesis in Salvia miltiorrhiza. J. Adv. Res. 2022, 42, 205–219. [Google Scholar] [CrossRef]
- Yan, H.; Pei, X.; Zhang, H.; Li, X.; Zhang, X.; Zhao, M.; Chiang, V.L.; Sederoff, R.R.; Zhao, X. MYB-Mediated Regulation of Anthocyanin Biosynthesis. Int. J. Mol. Sci. 2021, 22, 3103. [Google Scholar] [CrossRef]
- Jiang, L.; Yue, M.; Liu, Y.; Zhang, N.; Lin, Y.; Zhang, Y.; Wang, Y.; Li, M.; Luo, Y.; Zhang, Y.; et al. A novel R2R3-MYB transcription factor FaMYB5 positively regulates anthocyanin and proanthocyanidin biosynthesis in cultivated strawberries (Fragaria × ananassa). Plant Biotechnol. J. 2023, 21, 1140–1158. [Google Scholar] [CrossRef]
- Wang, P.; Ge, M.; Yu, A.; Song, W.; Fang, J.; Leng, X. Effects of ethylene on berry ripening and anthocyanin accumulation of ‘Fujiminori’ grape in protected cultivation. J. Sci. Food Agric. 2022, 102, 1124–1136. [Google Scholar] [CrossRef]
- Zhang, J.; Xu, H.; Wang, N.; Jiang, S.; Fang, H.; Zhang, Z.; Yang, G.; Wang, Y.; Su, M.; Xu, L.; et al. The ethylene response factor MdERF1B regulates anthocyanin and proanthocyanidin biosynthesis in apple. Plant Mol. Biol. 2018, 98, 205–218. [Google Scholar] [CrossRef] [PubMed]
- Ni, J.; Premathilake, A.T.; Gao, Y.; Yu, W.; Tao, R.; Teng, Y.; Bai, S. Ethylene-activated PpERF105 induces the expression of the repressor-type R2R3-MYB gene PpMYB140 to inhibit anthocyanin biosynthesis in red pear fruit. Plant J. 2021, 105, 167–181. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Yu, L.; Zhang, M.; Wu, T.; Song, T.; Yao, Y.; Zhang, J.; Tian, J. MdWER interacts with MdERF109 and MdJAZ2 to mediate methyl jasmonate- and light-induced anthocyanin biosynthesis in apple fruit. Plant J. 2024, 118, 1327–1342. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Li, M.; Li, T.; Chen, Y.; Zhang, L.; Zhao, G.; Zhuang, J.; Zhao, W.; Gao, L.; Xia, T. Airborne fungus-induced biosynthesis of anthocyanins in Arabidopsis thaliana via jasmonic acid and salicylic acid signaling. Plant Sci. 2020, 300, 110635. [Google Scholar] [CrossRef]
| Regulatory Sequence | Sequence | Function of Site | Location |
|---|---|---|---|
| G-Box | CACGTT GACGAC GACGTC | cis-acting regulatory element involved in light responsiveness | +1962 +995 +1024 |
| GT1-motif | GGTTAA | light responsive element | −360 −1742 |
| Box 4 | ATTAAT | part of a conserved DNA module involved in light responsiveness | −1641 |
| TCCC-motif | TCTCCCT | part of a light responsive element | −270 +1483 |
| TCT-motif | TCTTAC | part of a light responsive element | +370 +1090 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, F.-J.; Ma, N.; Li, H.-J.; Li, L.-Z.; Zhang, D.-E.; Zhang, Z.-L.; You, C.-X.; Lu, X.-Y. Functional Analysis of Cytochrome b5 in Regulating Anthocyanin Biosynthesis in Malus domestica. Horticulturae 2024, 10, 1075. https://doi.org/10.3390/horticulturae10101075
Zhang F-J, Ma N, Li H-J, Li L-Z, Zhang D-E, Zhang Z-L, You C-X, Lu X-Y. Functional Analysis of Cytochrome b5 in Regulating Anthocyanin Biosynthesis in Malus domestica. Horticulturae. 2024; 10(10):1075. https://doi.org/10.3390/horticulturae10101075
Chicago/Turabian StyleZhang, Fu-Jun, Ning Ma, Hao-Jian Li, Lian-Zhen Li, De-En Zhang, Zhen-Lu Zhang, Chun-Xiang You, and Xiao-Yan Lu. 2024. "Functional Analysis of Cytochrome b5 in Regulating Anthocyanin Biosynthesis in Malus domestica" Horticulturae 10, no. 10: 1075. https://doi.org/10.3390/horticulturae10101075
APA StyleZhang, F.-J., Ma, N., Li, H.-J., Li, L.-Z., Zhang, D.-E., Zhang, Z.-L., You, C.-X., & Lu, X.-Y. (2024). Functional Analysis of Cytochrome b5 in Regulating Anthocyanin Biosynthesis in Malus domestica. Horticulturae, 10(10), 1075. https://doi.org/10.3390/horticulturae10101075





