Physicochemical Marker for Determination of Value-Adding Component in Over-Ripe Thai Mango Peels
Abstract
1. Introduction
2. Materials and Methods
2.1. Mango Samples
2.2. Proximate Analysis
2.3. Physiolchemical Properties of the Peels
2.3.1. Colour Measurement
2.3.2. pH
2.3.3. Near-Infrared Spectra (NIR)
2.4. Chemical Analysis
2.4.1. Sample Extraction
2.4.2. The Analysis of Total Reducing Sugar
2.4.3. Total Phenolic and Total Flavonoid Content
2.4.4. The Identification of Phenolics and Flavonoids in Mango Peel
2.5. In Vitro Antioxidant Capacity
2.5.1. ABTS Radical Scavenging Assay
2.5.2. DPPH Radical Scavenging Assay
2.5.3. Ferric Reducing Antioxidant Power (FRAP) Assay
2.6. Statistic Analysis
3. Results and Discussion
3.1. Physiochemical Characteristics of Thai Mangoes
3.1.1. Size and Firmness
3.1.2. Colour
3.1.3. pH of the Peel
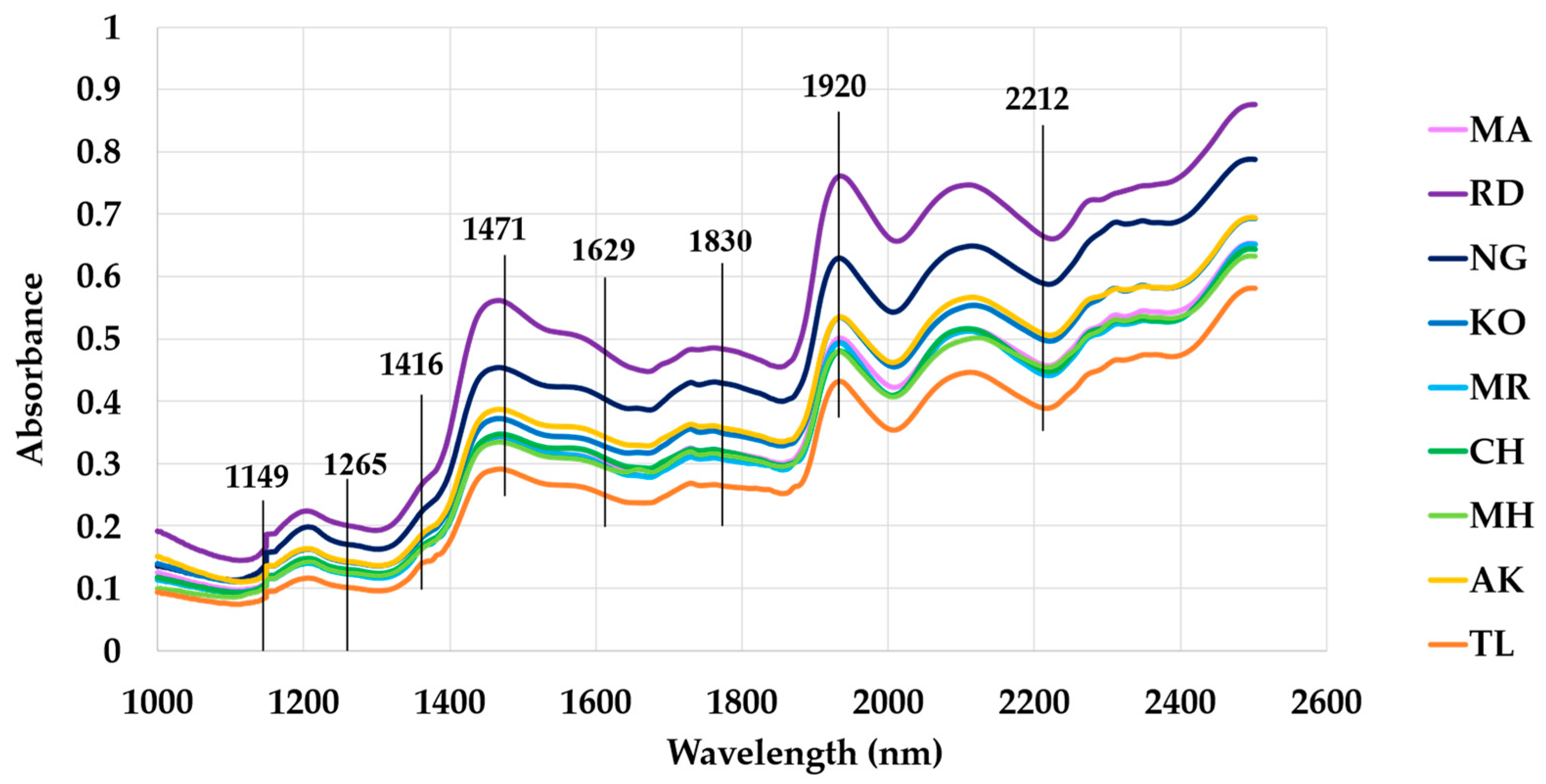
3.2. Near-Infrared (NIR) Spectroscopy Analysis
3.3. Chemical Properties
3.3.1. Proximate Compositions
3.3.2. Total Reducing Sugar
3.3.3. Total Phenolic and Total Flavonoid Content
3.3.4. Phenolics and Flavonoids in Mango Peel
3.3.5. Antioxidant Activity
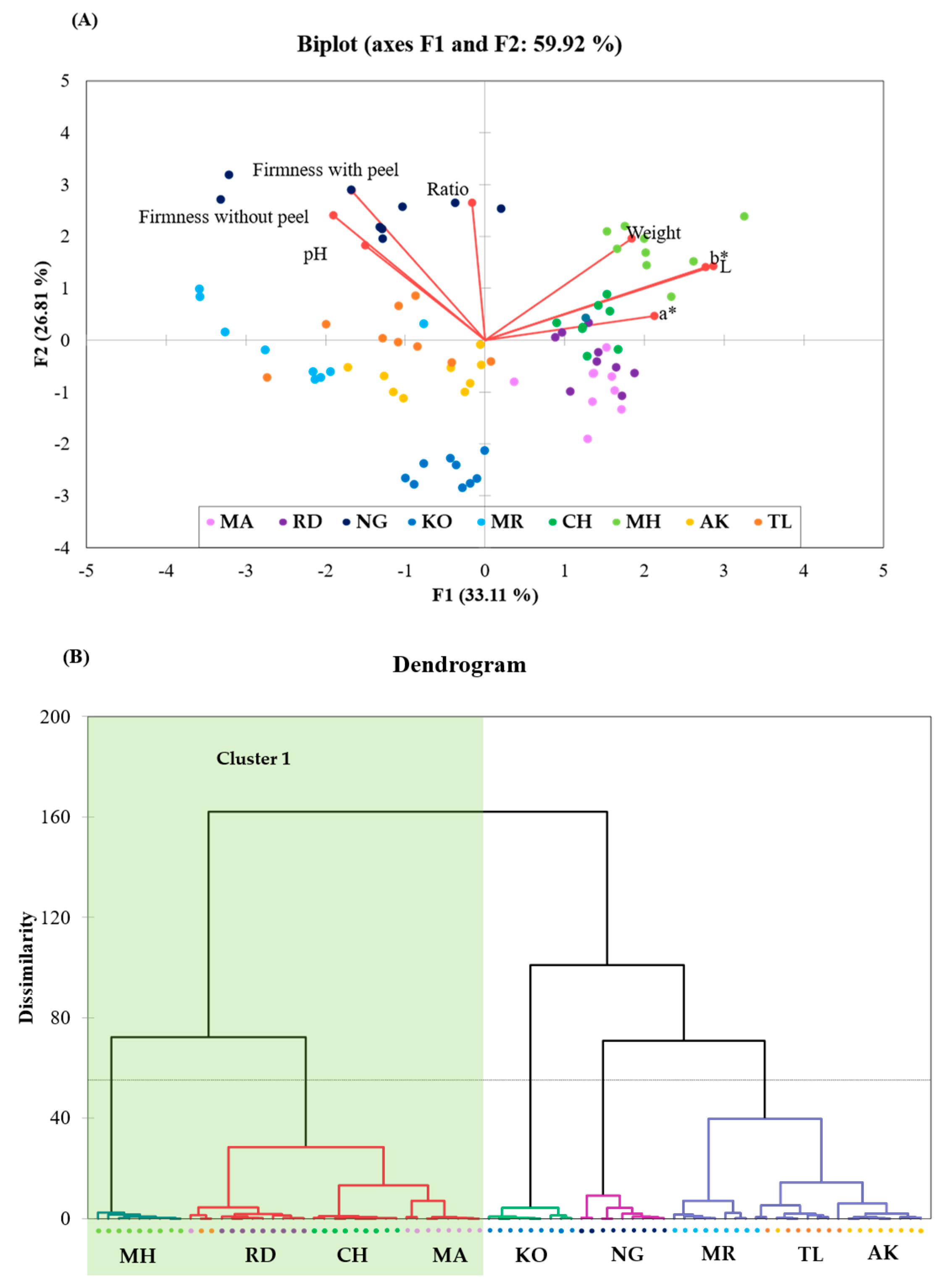
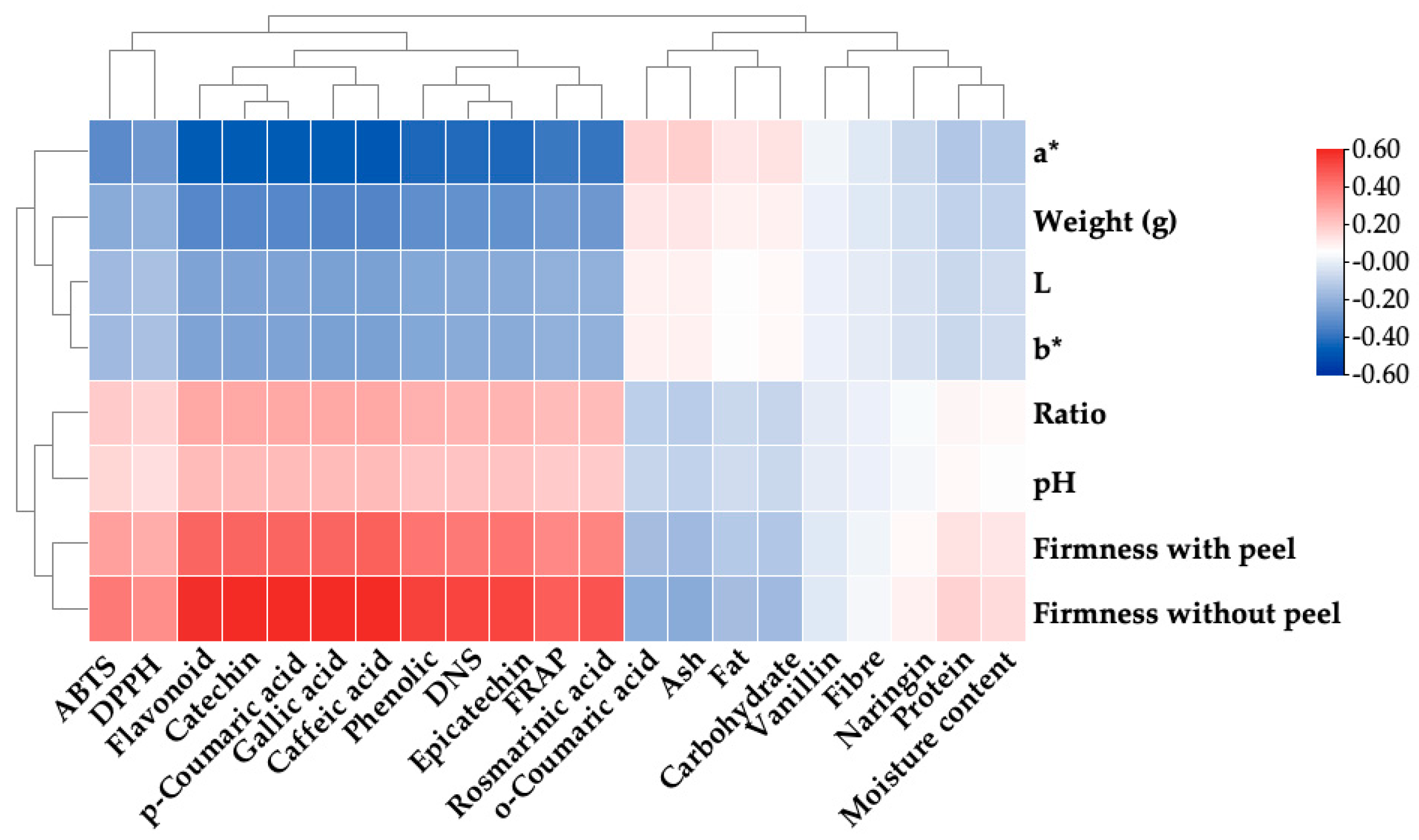
3.4. Physiological and Physiochemical Property Relation among Peel of Thai Mango Cultivars
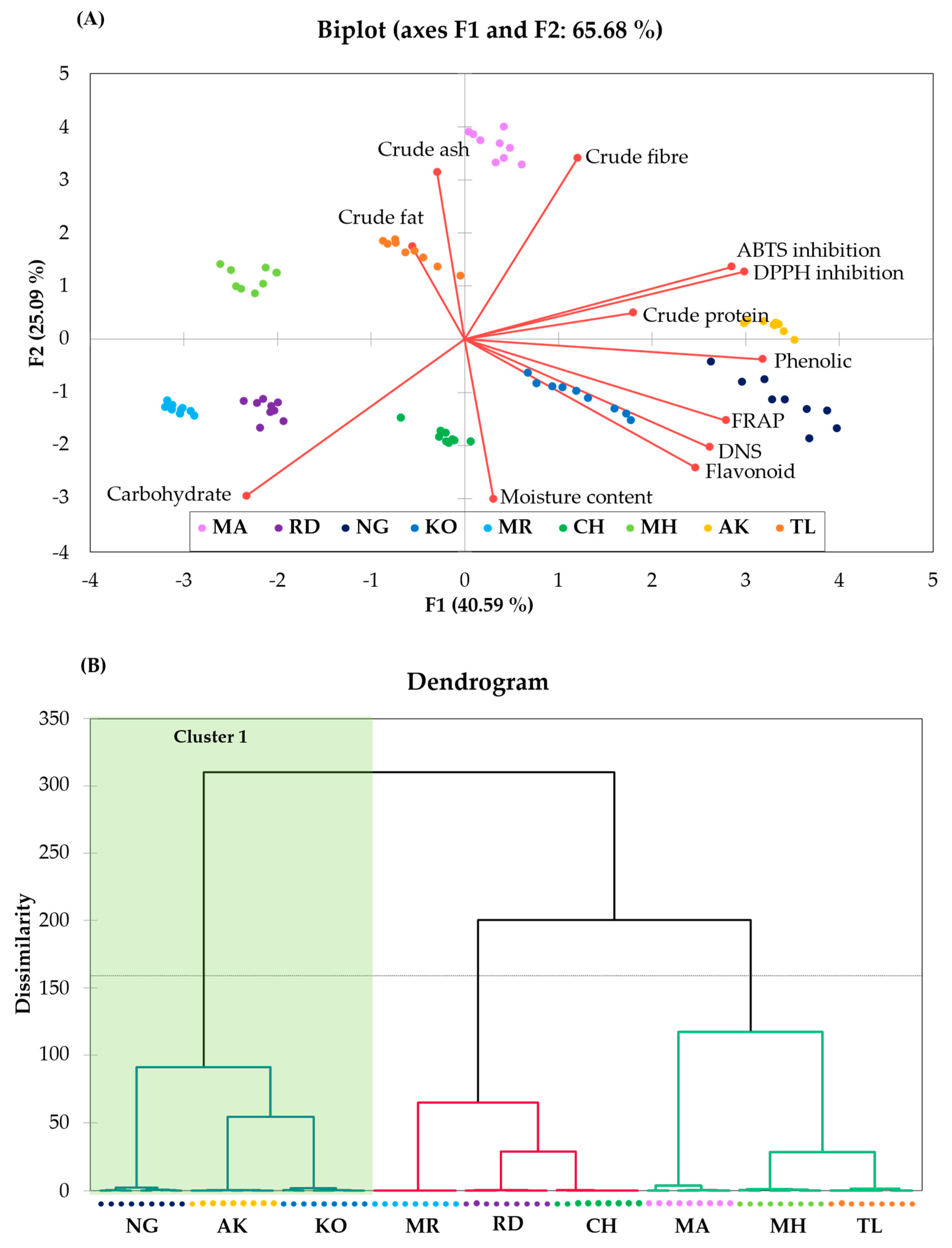
3.5. Chemical Property Relation among Peel of Thai Mango Cultivars
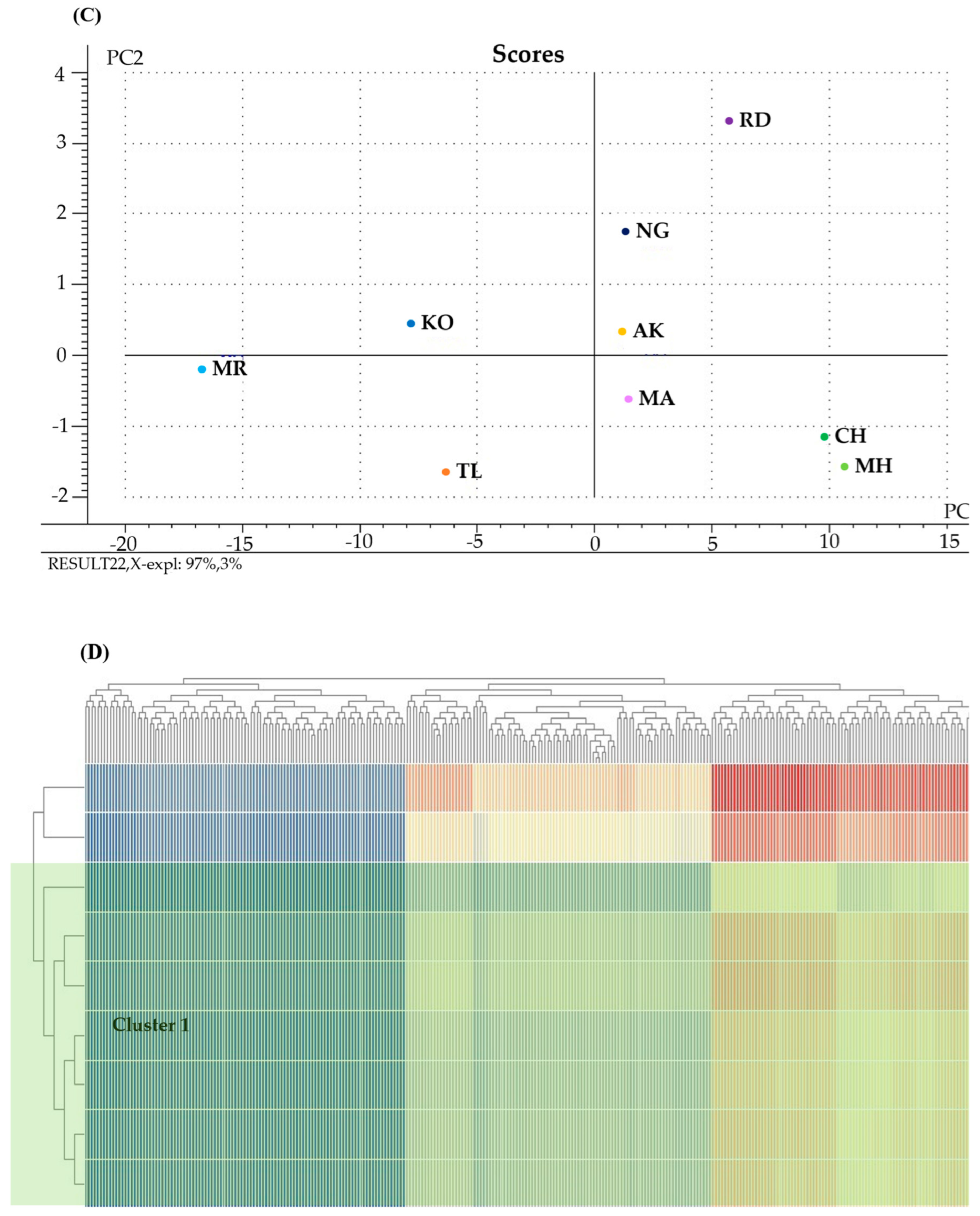
3.6. Partial Least Squares Analysis
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- FAO. Major Tropical Fruits—Preliminary Results 2022; Food and Agriculture Organization of the United Nations: Rome, Italy, 2022. [Google Scholar]
- Madalageri, D.; Bharati, P.; Orsat, V.; Raghavan, V.; Kage, U. Antioxidant activity in pulp and peel of three mango varieties. J. Hortic. Sci. 2015, 10, 199–209. [Google Scholar] [CrossRef]
- Rahman, M.S. Handbook of Food Preservation; CRC Press: Boca Raton, FL, USA, 2020. [Google Scholar]
- Sagar, V.; Khurdiya, D.; Balakrishnan, K. Quality of dehydrated ripe mango slices as affected by packaging material and mode of packaging. J. Food Sci. Technol. 1999, 36, 67–70. [Google Scholar]
- Pott, I.; Konrad, S.; Scherer, R.; Wiriyacharee, P.; Mühlbauer, W. Quality of five Thai mango cultivars (Mangifera indica L.) using a solar drying system. CMU J. 2004, 3, 189–198. [Google Scholar]
- Litz Richard, E. (Ed.) The Mango: Botany, Production and Uses; Cabi: Preston, UK, 2009. [Google Scholar]
- Singh, Z.; Singh, R.K.; Sane, V.A.; Nath, P. Mango-postharvest biology and biotechnology. Crit. Rev. Plant Sci. 2013, 32, 217–236. [Google Scholar] [CrossRef]
- Spreer, W.; Ongprasert, S.; Hegele, M.; Wünsche, J.N.; Müller, J. Yield and fruit development in mango (Mangifera indica L. cv. Chok Anan) under different irrigation regimes. Agric. Water Manag. 2009, 96, 574–584. [Google Scholar] [CrossRef]
- Wongkaew, M.; Tinpovong, B.; Sringarm, K.; Leksawasdi, N.; Jantanasakulwong, K.; Rachtanapun, P.; Hanmoungjai, P.; Sommano, S.R. Crude pectic oligosaccharide recovery from Thai Chok Anan mango peel using pectinolytic enzyme hydrolysis. Foods 2021, 10, 627. [Google Scholar] [CrossRef]
- Kaur, M.; Singh, N.; Sandhu, K.S.; Guraya, H.S. Physicochemical, morphological, thermal and rheological properties of starches separated from kernels of some Indian mango cultivars (Mangifera indica L.). Food Chem. 2004, 85, 131–140. [Google Scholar] [CrossRef]
- Maldonado-Celis, M.E.; Yahia, E.M.; Bedoya, R.; Landázuri, P.; Loango, N.; Aguillón, J.; Restrepo, B.; Guerrero Ospina, J.C. Chemical composition of mango (Mangifera indica L.) fruit: Nutritional and phytochemical compounds. Front. Plant Sci. 2019, 10, 1073. [Google Scholar] [CrossRef]
- Serna-Cock, L.; García-Gonzales, E.; Torres-León, C. Agro-industrial potential of the mango peel based on its nutritional and functional properties. Food Rev. Int. 2016, 32, 364–376. [Google Scholar] [CrossRef]
- Rumainum, I.M.; Worarad, K.; Srilaong, V.; Yamane, K. Fruit quality and antioxidant capacity of six Thai mango cultivars. Agric. Nat. Resour. 2018, 52, 208–214. [Google Scholar] [CrossRef]
- Hussain, S.; Jõudu, I.; Bhat, R. Dietary fiber from underutilized plant resources—A positive approach for valorization of fruit and vegetable wastes. Sustainability 2020, 12, 5401. [Google Scholar] [CrossRef]
- Ajila, C.; Aalami, M.; Leelavathi, K.; Rao, U.P. Mango peel powder: A potential source of antioxidant and dietary fiber in macaroni preparations. Innov. Food Sci. Emerg. Technol. 2010, 11, 219–224. [Google Scholar] [CrossRef]
- Kim, H.; Moon, J.Y.; Kim, H.; Lee, D.-S.; Cho, M.; Choi, H.-K.; Kim, Y.S.; Mosaddik, A.; Cho, S.K. Antioxidant and antiproliferative activities of mango (Mangifera indica L.) flesh and peel. Food Chem. 2010, 121, 429–436. [Google Scholar] [CrossRef]
- Ali, S.; Hossain, M.; Zakaria, M.; Haque, M.; Ahiduzzaman, M. Physio-chemical Characteristics of Seven Cultivars Mango (Mangifera indica L.) in Bangladesh. Int. J. Bus. Soc. Sci. Res 2019, 7, 01–08. [Google Scholar]
- Bora, L.; Singh, A.; Singh, C. Characterization of mango (Mangifera indica L.) genotypes based on physio-chemical quality attributes. J. Appl. Nat. Sci. 2017, 9, 2199–2204. [Google Scholar] [CrossRef]
- Wongkaew, M.; Sangta, J.; Chansakaow, S.; Jantanasakulwong, K.; Rachtanapun, P.; Sommano, S.R. Volatile profiles from over-ripe purée of Thai mango varieties and their physiochemical properties during heat processing. PLoS ONE 2021, 16, e0248657. [Google Scholar] [CrossRef]
- Tangpao, T.; Phuangsaujai, N.; Kittiwachana, S.; George, D.R.; Krutmuang, P.; Chuttong, B.; Sommano, S.R. Evaluation of Markers Associated with Physiological and Biochemical Traits during Storage of ‘Nam Dok Mai Si Thong’ Mango Fruits. Agriculture 2022, 12, 1407. [Google Scholar] [CrossRef]
- Wongkaew, M.; Kittiwachana, S.; Phuangsaijai, N.; Tinpovong, B.; Tiyayon, C.; Pusadee, T.; Chuttong, B.; Sringarm, K.; Bhat, F.M.; Sommano, S.R. Fruit characteristics, peel nutritional compositions, and their relationships with mango peel pectin quality. Plants 2021, 10, 1148. [Google Scholar] [CrossRef]
- Larrauri, J.A.; Rupérez, P.; Saura-Calixto, F. Mango peel fibres with antioxidant activity. Z. Für Leb. Und-Forsch. A 1997, 205, 39–42. [Google Scholar] [CrossRef]
- International, A. Official Methods of Analysis of the Association of Official Analytical Chemists; Association of Official Analytical Chemists: Rockville, MD, USA, 1970. [Google Scholar]
- Ihekoronye, A.I.; Ngoddy, P.O. Integrated Food Science and Technology for the Tropics; Macmillan: London, UK, 1985. [Google Scholar]
- Sunanta, P.; Kontogiorgos, V.; Leksawasdi, N.; Phimolsiripol, Y.; Wangtueai, S.; Wongkaew, M.; Sommano, S.R. Loss Assessment during Postharvest and Handling of Thai Garlic Used for Processing. Horticulturae 2023, 9, 482. [Google Scholar] [CrossRef]
- Sunanta, P.; Pankasemsuk, T.; Jantanasakulwong, K.; Chaiyaso, T.; Leksawasdi, N.; Phimolsiripol, Y.; Rachtanapun, P.; Seesuriyachan, P.; Sommano, S.R. Does Curing Moisture Content Affect Black Garlic Physiochemical Quality? Horticulturae 2021, 7, 535. [Google Scholar] [CrossRef]
- Sunanta, P.; Rachtanapun, P.; Jantanasakulwong, K.; Sommano, S. Antioxidant potential and quality traits of black garlic from microwave heating and hot steam incubation. In Proceedings of the V Asia Symposium on Quality Management in Postharvest Systems 1336, Bangkok, Thailand, 1 December 2021. [Google Scholar]
- Deineka, V.; Oleinits, E.Y.; Kul’chenko, Y.Y.; Blinova, I.; Deineka, L. Control of the Selectivity of Separation and the Determination of Anthocyanins of Fruits of Vaccinium Family Plants Using Acetonitrile–Formic Acid–Water Eluents. J. Anal. Chem. 2020, 75, 1443–1450. [Google Scholar] [CrossRef]
- Ramirez, J.E.; Zambrano, R.; Sepúlveda, B.; Simirgiotis, M.J. Antioxidant properties and hyphenated HPLC-PDA-MS profiling of Chilean Pica mango fruits (Mangifera indica L. Cv. piqueño). Molecules 2013, 19, 438–458. [Google Scholar] [CrossRef] [PubMed]
- Marcillo-Parra, V.; Anaguano, M.; Molina, M.; Tupuna-Yerovi, D.S.; Ruales, J. Characterization and quantification of bioactive compounds and antioxidant activity in three different varieties of mango (Mangifera indica L.) peel from the Ecuadorian region using HPLC-UV/VIS and UPLC-PDA. NFS J. 2021, 23, 1–7. [Google Scholar] [CrossRef]
- Sunanta, P.; Chung, H.H.; Kunasakdakul, K.; Ruksiriwanich, W.; Jantrawut, P.; Hongsibsong, S.; Sommano, S.R. Genomic relationship and physiochemical properties among raw materials used for Thai black garlic processing. Food Sci. Nutr. 2020, 8, 4534–4545. [Google Scholar] [CrossRef]
- Prasad, S.K.; Veeresh, P.M.; Ramesh, P.S.; Natraj, S.M.; Madhunapantula, S.V.; Devegowda, D. Phytochemical fractions from Annona muricata seeds and fruit pulp inhibited the growth of breast cancer cells through cell cycle arrest at G0/G1 phase. J. Cancer Res. Ther. 2020, 16, 1235–1249. [Google Scholar]
- Gillaspy, G.; Ben-David, H.; Gruissem, W. Fruits: A developmental perspective. Plant Cell 1993, 5, 1439. [Google Scholar] [CrossRef]
- Harper, J.L.; Lovell, P.; Moore, K. The shapes and sizes of seeds. Annu. Rev. Ecol. Syst. 1970, 1, 327–356. [Google Scholar] [CrossRef]
- Negi, P.S.; Handa, A.K. Structural deterioration of the produce: The breakdown of cell wall components. Postharvest Biol. Technol. Fruits Veg. Flowers 2008, 978, 0-8138. [Google Scholar]
- Baloch, M.; Bibi, F. Effect of harvesting and storage conditions on the post harvest quality and shelf life of mango (Mangifera indica L.) fruit. S. Afr. J. Bot. 2012, 83, 109–116. [Google Scholar] [CrossRef]
- Mannan, M.; Khan, S.; Islam, M.; Islam, M.S.; Siddiqa, A. A study on the physico-chemical characteristics of some mango varieties in Khulna region. Pak. J. Biol. Sci. 2003, 6, 2034–2039. [Google Scholar] [CrossRef][Green Version]
- Padda, M.S.; do Amarante, C.V.; Garcia, R.M.; Slaughter, D.C.; Mitcham, E.J. Methods to analyze physico-chemical changes during mango ripening: A multivariate approach. Postharvest Biol. Technol. 2011, 62, 267–274. [Google Scholar] [CrossRef]
- Mitcham, E.J.; McDonald, R.E. Cell wall modification during ripening of ‘Keitt’ and ‘Tommy Atkins’ mango fruit. J. Am. Soc. Hortic. Sci. 1992, 117, 919–924. [Google Scholar] [CrossRef]
- Kullaj, E. New insights on postharvest ecophysiology of fresh horticultural crops. In Eco-Friendly Technology for Postharvest Produce Quality; Elsevier: Amsterdam, The Netherlands, 2016; pp. 1–38. [Google Scholar]
- Karanjalker, G.; Ravishankar, K.; Shivashankara, K.; Dinesh, M.; Roy, T.; Sudhakar Rao, D. A study on the expression of genes involved in carotenoids and anthocyanins during ripening in fruit peel of green, yellow, and red colored mango cultivars. Appl. Biochem. Biotechnol. 2018, 184, 140–154. [Google Scholar] [CrossRef]
- Liu, B.; Xin, Q.; Zhang, M.; Chen, J.; Lu, Q.; Zhou, X.; Li, X.; Zhang, W.; Feng, W.; Pei, H. Research progress on mango post-harvest ripening physiology and the regulatory technologies. Foods 2022, 12, 173. [Google Scholar] [CrossRef]
- Geerkens, C.H.; Nagel, A.; Just, K.M.; Miller-Rostek, P.; Kammerer, D.R.; Schweiggert, R.M.; Carle, R. Mango pectin quality as influenced by cultivar, ripeness, peel particle size, blanching, drying, and irradiation. Food Hydrocoll. 2015, 51, 241–251. [Google Scholar] [CrossRef]
- Ali, S.; Zahid, N.; Nawaz, A.; Naz, S.; Ejaz, S.; Ullah, S.; Siddiq, B. Tragacanth gum coating suppresses the disassembly of cell wall polysaccharides and delays softening of harvested mango (Mangifera indica L.) fruit. Int. J. Biol. Macromol. 2022, 222, 521–532. [Google Scholar] [CrossRef] [PubMed]
- Tharanathan, R.; Yashoda, H.; Prabha, T. Mango (Mangifera indica L.), “The king of fruits”—An overview. Food Rev. Int. 2006, 22, 95–123. [Google Scholar] [CrossRef]
- De Girolamo, A.; Cortese, M.; Cervellieri, S.; Lippolis, V.; Pascale, M.; Logrieco, A.F.; Suman, M. Tracing the Geographical Origin of Durum Wheat by FT-NIR Spectroscopy. Foods 2019, 8, 450. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Camargo, A.d.P.; Gutiérrez, L.-F.; Vargas, S.M.; Martinez-Correa, H.A.; Parada-Alfonso, F.; Narváez-Cuenca, C.-E. Valorisation of mango peel: Proximate composition, supercritical fluid extraction of carotenoids, and application as an antioxidant additive for an edible oil. J. Supercrit. Fluids 2019, 152, 104574. [Google Scholar] [CrossRef]
- Onuh, J.O.; Momoh, G.; Egwujeh, S.; Onuh, F. Evaluation of the nutritional, phytochemical and antioxidant properties of the peels of some selected mango varieties. Am. J. Food Sci. Technol. 2017, 5, 176–181. [Google Scholar] [CrossRef]
- Giraldo, L.M.; Correa, H.M.; Gutiérrez, J.B.; Castano, C.C. Aprovechamiento del residuo agroindustrial del mango común (Mangifera indica L.) en la obtención de azúcares fermentables. Ing. Y Cienc. 2007, 3, 41–62. [Google Scholar]
- Dar, M.S.; Oak, P.; Chidley, H.; Deshpande, A.; Giri, A.; Gupta, V. Nutrient and flavor content of mango (Mangifera indica L.) cultivars: An appurtenance to the list of staple foods. In Nutritional Composition of Fruit Cultivars; Elsevier: Amsterdam, The Netherlands, 2016; pp. 445–467. [Google Scholar]
- Yahia, E.M. Postharvest Biology and Technology of Tropical and Subtropical Fruits: Fundamental Issues; Elsevier: Amsterdam, The Netherlands, 2011. [Google Scholar]
- Baddi, J.; Vijayalakshmi, D.; Durgannavar, N.A.; Chandru, R. Mango peel: A potential source of natural bioactive phyto-nutrients in functional food. Asian J. Dairy Food Res. 2015, 34, 75–77. [Google Scholar] [CrossRef]
- de Lourdes García-Magaña, M.; García, H.S.; Bello-Pérez, L.A.; Sáyago-Ayerdi, S.G.; de Oca, M.M.-M. Functional properties and dietary fiber characterization of mango processing by-products (Mangifera indica L., cv Ataulfo and Tommy Atkins). Plant Foods Hum. Nutr. 2013, 68, 254–258. [Google Scholar] [CrossRef]
- Kumar, I.; Yadav, P.; Gautam, M.; Panwar, H. Impact of Heat on Naturally Present Digestive Enzymes in Food. Int. J. Food Nutr. Diet 2022, 10, 57–63. [Google Scholar]
- Singh, R.; Singh, P.; Pathak, N.; Singh, V.; Dwivedi, U.N. Modulation of mango ripening by chemicals: Physiological and biochemical aspects. Plant Growth Regul. 2007, 53, 137–145. [Google Scholar] [CrossRef]
- Grauzdytė, D. Phytochemical Composition and In Vitro Bioactivity of Low Investigated Phyllanthus phillyreifolius and Aphloia theiformis Plant Species Indigenous to Reunion Island. Ph.D. Thesis, Kauno Technologijos Universitetas, Kaunas, Lithuania, 2020. [Google Scholar]
- Berardini, N.; Knödler, M.; Schieber, A.; Carle, R. Utilization of mango peels as a source of pectin and polyphenolics. Innov. Food Sci. Emerg. Technol. 2005, 6, 442–452. [Google Scholar] [CrossRef]
- Ajila, C.; Naidu, K.; Bhat, S.; Rao, U.P. Bioactive compounds and antioxidant potential of mango peel extract. Food Chem. 2007, 105, 982–988. [Google Scholar] [CrossRef]
- Kamtekar, S.; Keer, V.; Patil, V. Estimation of phenolic content, flavonoid content, antioxidant and alpha amylase inhibitory activity of marketed polyherbal formulation. J. Appl. Pharm. Sci. 2014, 4, 061–065. [Google Scholar]
- Kothalawala, S.; Yatiwella, L. Analysis of antioxidant activities in Mango Peel among different Sri Lankan Cultivars. J. Pharmacogn. Phytochem. 2018, 7, 1668–1671. [Google Scholar]
- Vélez-Erazo, E.M.; Pasquel-Reátegui, J.L.; Dorronsoro-Guerrero, O.H.; Martínez-Correa, H.A. Phenolics and carotenoids recovery from agroindustrial mango waste using microwave-assisted extraction: Extraction and modeling. J. Food Process Eng. 2021, 44, e13774. [Google Scholar] [CrossRef]
- Sultana, B.; Hussain, Z.; Asif, M.; Munir, A. Investigation on the antioxidant activity of leaves, peels, stems bark, and kernel of mango (Mangifera indica L.). J. Food Sci. 2012, 77, C849–C852. [Google Scholar] [CrossRef]
- Anal, A.K. Food processing by-products. In Handbook of Plant Food Phytochemicals: Sources, Stability and Extraction; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2013; pp. 180–197. [Google Scholar]
- Pinsirodom, P.; Taprap, R.; Parinyapatthanaboot, T. Antioxidant activity and phenolic acid composition in different parts of selected cultivars of mangoes in Thailand. Int. Food Res. J. 2018, 25, 1435–1443. [Google Scholar]
- Liu, F.-X.; Fu, S.-F.; Bi, X.-F.; Chen, F.; Liao, X.-J.; Hu, X.-S.; Wu, J.-H. Physico-chemical and antioxidant properties of four mango (Mangifera indica L.) cultivars in China. Food Chem. 2013, 138, 396–405. [Google Scholar] [CrossRef] [PubMed]
- Schieber, A.; Ullrich, W.; Carle, R. Characterization of polyphenols in mango puree concentrate by HPLC with diode array and mass spectrometric detection. Innov. Food Sci. Emerg. Technol. 2000, 1, 161–166. [Google Scholar] [CrossRef]
- Lebaka, V.R.; Wee, Y.-J.; Ye, W.; Korivi, M. Nutritional composition and bioactive compounds in three different parts of mango fruit. Int. J. Environ. Res. Public Health 2021, 18, 741. [Google Scholar] [CrossRef] [PubMed]
- Abbasi, A.M.; Guo, X.; Fu, X.; Zhou, L.; Chen, Y.; Zhu, Y.; Yan, H.; Liu, R.H. Comparative assessment of phenolic content and in vitro antioxidant capacity in the pulp and peel of mango cultivars. Int. J. Mol. Sci. 2015, 16, 13507–13527. [Google Scholar] [CrossRef]
- Ma, X.; Wu, H.; Liu, L.; Yao, Q.; Wang, S.; Zhan, R.; Xing, S.; Zhou, Y. Polyphenolic compounds and antioxidant properties in mango fruits. Sci. Hortic. 2011, 129, 102–107. [Google Scholar] [CrossRef]
- Rice-Evans, C.; Miller, N.; Paganga, G. Antioxidant properties of phenolic compounds. Trends Plant Sci. 1997, 2, 152–159. [Google Scholar] [CrossRef]
- Dangles, O. Antioxidant activity of plant phenols: Chemical mechanisms and biological significance. Curr. Org. Chem. 2012, 16, 692–714. [Google Scholar] [CrossRef]
- Rojas, R.; Contreras-Esquivel, J.C.; Orozco-Esquivel, M.T.; Muñoz, C.; Aguirre-Joya, J.A.; Aguilar, C.N. Mango peel as source of antioxidants and pectin: Microwave assisted extraction. Waste Biomass Valorization 2015, 6, 1095–1102. [Google Scholar] [CrossRef]
- Kuganesan, A.; Thiripuranathar, G.; Navaratne, A.; Paranagama, P. Antioxidant and anti-inflammatory activities of peels, pulps and seed kernels of three common mango (Mangifera indical L.) varieties in Sri Lanka. Int. J. Pharm. Sci. Res. 2017, 8, 70. [Google Scholar]
- Xie, J.; Schaich, K. Re-evaluation of the 2, 2-diphenyl-1-picrylhydrazyl free radical (DPPH) assay for antioxidant activity. J. Agric. Food Chem. 2014, 62, 4251–4260. [Google Scholar] [CrossRef] [PubMed]
- Jiménez-Escrig, A.; Jiménez-Jiménez, I.; Sánchez-Moreno, C.; Saura-Calixto, F. Evaluation of free radical scavenging of dietary carotenoids by the stable radical 2, 2-diphenyl-1-picrylhydrazyl. J. Sci. Food Agric. 2000, 80, 1686–1690. [Google Scholar] [CrossRef]
- Parcheta, M.; Świsłocka, R.; Orzechowska, S.; Akimowicz, M.; Choińska, R.; Lewandowski, W. Recent developments in effective antioxidants: The structure and antioxidant properties. Materials 2021, 14, 1984. [Google Scholar] [CrossRef]
- Gulcin, İ.; Alwasel, S.H. DPPH radical scavenging assay. Processes 2023, 11, 2248. [Google Scholar] [CrossRef]
- Foti, M.C. Use and Abuse of the DPPH• Radical. J. Agric. Food Chem. 2015, 63, 8765–8776. [Google Scholar] [CrossRef]
- Charles, D.J. Antioxidant Properties of Spices, Herbs and Other Sources; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2012. [Google Scholar]
- Dudonne, S.; Vitrac, X.; Coutiere, P.; Woillez, M.; Mérillon, J.-M. Comparative study of antioxidant properties and total phenolic content of 30 plant extracts of industrial interest using DPPH, ABTS, FRAP, SOD, and ORAC assays. J. Agric. Food Chem. 2009, 57, 1768–1774. [Google Scholar] [CrossRef]
- Castañeda-Valbuena, D.; Ayora-Talavera, T.; Luján-Hidalgo, C.; Álvarez-Gutiérrez, P.; Martínez-Galero, N.; Meza-Gordillo, R. Ultrasound extraction conditions effect on antioxidant capacity of mango by-product extracts. Food Bioprod. Process. 2021, 127, 212–224. [Google Scholar] [CrossRef]
- Khammuang, S.; Sarnthima, R. Antioxidant and antibacterial activities of selected varieties of Thai mango seed extract. Pak. J. Pharm. Sci. 2011, 24, 37–42. [Google Scholar]
- Singh, A.; Tyagi, M.; Singh, C. Studies on morphology and physical attributes of mango varieties. Int. J. Curr. Microbiol. Appl. Sci 2017, 6, 2324–2330. [Google Scholar] [CrossRef]
- Ahmed, T.H.M.; Mohamed, Z.M.A. Diversity of Mango (Mangifera indica L.) cultivars in Shendi area: Morphological fruit characterization. Int. J. Res. Agric. Sci. 2015, 2, 2348–3997. [Google Scholar]
- Tsao, R. Chemistry and biochemistry of dietary polyphenols. Nutrients 2010, 2, 1231–1246. [Google Scholar] [CrossRef] [PubMed]
- Wong, K.K.-K. Partial least squares structural equation modeling (PLS-SEM) techniques using SmartPLS. Mark. Bull. 2013, 24, 1–32. [Google Scholar]
- Jahurul, M.; Zaidul, I.; Ghafoor, K.; Al-Juhaimi, F.Y.; Nyam, K.-L.; Norulaini, N.; Sahena, F.; Omar, A.M. Mango (Mangifera indica L.) by-products and their valuable components: A review. Food Chem. 2015, 183, 173–180. [Google Scholar] [CrossRef] [PubMed]
- Muralidhara, B.; Veena, G.; Bhattacherjee, A.; Rajan, S. Antioxidants in ripe peel and pulp of twelve mango (Mangifera indica) cultivars. Indian J. Agric. Sci. 2019, 89, 1580–1584. [Google Scholar] [CrossRef]
- Doughty, C.E.; Asner, G.P.; Martin, R.E. Predicting tropical plant physiology from leaf and canopy spectroscopy. Oecologia 2011, 165, 289–299. [Google Scholar] [CrossRef]

| Parameter | MA | RD | NG | KO | MR | CH | MH | AK | TL |
|---|---|---|---|---|---|---|---|---|---|
 |  |  |  |  |  |  |  |  | |
| Physiological characteristics | |||||||||
| Width (cm) | 62.99 ± 1.69 d | 73.54 ± 1.16 e | 53.06 ± 3.14 b | 44.90 ± 0.69 a | 58.02 ± 0.38 c | 65.61 ± 0.88 d | 65.63 ± 0.19 d | 57.43 ± 1.63 c | 61.20 ± 0.91 cd |
| Length (cm) | 79.94 ± 2.38 a | 119.35 ± 1.82 d | 129.31 ± 5.90 e | 80.69 ± 1.64 a | 101.79 ± 2.66 bc | 105.83 ± 2.17 c | 162.58 ± 4.26 f | 96.25 ± 2.23 b | 102.06 ± 1.63 c |
| Length to width ratio | 1.27 ± 0.03 a | 1.63 ± 0.03 b | 2.44 ± 0.07 f | 1.80 ± 0.03 d | 1.76 ± 0.05 cd | 1.61 ± 0.02 b | 2.48 ± 0.04 f | 1.68 ± 0.02 bc | 1.67 ± 0.02 bc |
| Weight (g) | 171.65 ± 9.95 b | 166.22 ± 13.60 b | 301.44 ± 8.74 e | 87.22 ± 4.46 a | 176.11 ± 6.26 bc | 206.78 ± 9.47 c | 379.33 ± 17.38 f | 176.44 ± 10.04 bc | 246.00 ± 13.61 d |
| Fruit firmness (N) | 2.24 ± 0.03 b | 1.90 ± 0.07 a | 3.50 ± 0.11 e | 1.89 ± 0.05 a | 2.93 ± 0.04 d | 2.55 ± 0.07 c | 2.70 ± 0.06 c | 2.10 ± 0.08 b | 3.05 ± 0.09 d |
| Flesh firmness (N) | 0.45C0.04 ab | 0.43 ± 0.03 ab | 1.45 ± 0.08 d | 0.35 ± 0.02 a | 0.90 ± 0.13 c | 0.82 ± 0.03 c | 0.54 ± 0.05 b | 0.81 ± 0.05 c | 0.61 ± 0.06 b |
| Physicochemical characteristics | |||||||||
| L* | 59.69 ± 0.77 c | 63.87 ± 0.62 d | 59.61 ± 0.96 c | 50.54 ± 1.18 b | 40.30 ± 0.88 a | 68.26 ± 0.57 e | 69.07 ± 1.12 e | 59.61 ± 1.71 c | 52.62 ± 0.65 b |
| a* | 14.76 ± 1.25 e | 2.03 ± 0.69 c | −6.00 ± 1.36 b | −5.86 ± 1.44 b | 4.69 ± 1.66 c | 8.71 ± 0.46 d | 10.93 ± 0.57 d | −10.12 ± 1.31 a | −6.67 ± 1.35 b |
| b* | 51.63 ± 1.49 e | 44.77 ± 0.89 d | 46.01 ± 2.53 d | 29.66 ± 1.40 b | 18.65 ± 1.28 a | 51.50 ± 0.67 e | 48.72 ± 1.01 de | 33.40 ± 1.38 bc | 34.94 ± 0.91 c |
| pH | 4.65 ± 0.07 c | 4.66 ± 0.03 c | 4.78 ± 0.01 de | 4.30 ± 0.01 a | 4.84 ± 0.03 e | 4.56 ± 0.02 b | 4.52 ± 0.01 b | 4.67 ± 0.02 c | 4.72 ± 0.01 cd |
| Chemical Characteristics | MA | RD | NG | KO | MR | CH | MH | AK | TL |
|---|---|---|---|---|---|---|---|---|---|
| Proximal contents (%w/w) | |||||||||
| Moisture content | 8.24 ± 0.01 c | 10.68 ± 0.02 g | 8.81 ± 0.02 d | 11.70 ± 0.01 i | 10.27 ± 0.01 f | 10.93 ± 0.11 h | 5.93 ± 0.02 a | 9.22 ± 0.01 e | 7.83 ± 0.01 b |
| Crude fat | 0.29 ± 0.02 f | 0.12 ± 0.00 a | 0.13 ± 0.00 a | 0.24 ± 0.00 d | 0.27 ± 0.00 e | 0.16 ± 0.00 b | 0.18 ± 0.01 c | 0.18 ± 0.00 c | 0.16 ± 0.01 b |
| Crude protein | 4.56 ± 0.01 e | 3.68 ± 0.00 a | 4.13 ± 0.03 c | 6.01 ± 0.02 f | 4.39 ± 0.02 d | 3.98 ± 0.02 b | 4.44 ± 0.04 d | 6.13 ± 0.03 g | 4.43 ± 0.00 d |
| Crude fibre | 13.78 ± 0.00 g | 7.71 ± 0.00 c | 12.17 ± 0.00 ef | 7.06 ± 0.00 a | 7.16 ± 0.00 b | 7.77 ± 0.00 d | 13.75 ± 0.00 f | 13.84 ± 0.00 h | 13.77 ± 0.00 fg |
| Crude ash | 4.07 ± 0.00 g | 3.24 ± 0.02 e | 2.95 ± 0.02 c | 2.92 ± 0.01 c | 2.88 ± 0.00 b | 3.03 ± 0.00 d | 2.89 ± 0.00 b | 2.82 ± 0.00 a | 3.29 ± 0.01 f |
| Carbohydrate | 69.05 ± 0.02 b | 74.58 ± 0.02 h | 71.80 ± 0.01 d | 72.06 ± 0.02 e | 75.04 ± 0.02 i | 74.12 ± 0.12 g | 72.82 ± 0.05 f | 67.81 ± 0.03 a | 70.53 ± 0.01 c |
| Sugar content | |||||||||
| Total reducing sugar (mg/g) | 0.47 ± 0.02 a | 0.53 ± 0.02 ab | 1.14 ± 0.06 e | 0.87 ± 0.06 cd | 0.60 ± 0.03 b | 0.83 ± 0.04 c | 0.61 ± 0.03 b | 0.99 ± 0.04 d | 0.62 ± 0.05 b |
| Bioactive compounds | |||||||||
| TPC (mgGAE/g dry weight) | 113.09 ± 1.74 e | 41.28 ± 0.52 b | 213.36 ± 5.93 h | 149.43 ± 2.51 g | 21.99 ± 0.15 a | 87.61 ± 1.91 d | 19.39 ± 0.44 a | 129.95 ± 2.28 f | 63.25 ± 1.24 c |
| TFC (mgCE/g dry weight) | 2.86 ± 0.01 b | 3.11 ± 0.01 d | 5.43 ± 0.02 h | 3.54 ± 0.00 f | 3.48 ± 0.01 e | 4.33 ± 0.01 g | 2.66 ± 0.01 a | 4.31 ± 0.02 g | 2.97 ± 0.01 c |
| HPLC (mg/g dry bias) | |||||||||
| Gallic acid | 8.45 ± 0.04 e | 6.39 ± 0.00 b | 16.10 ± 0.02 h | 11.05 ± 0.01 g | 6.84 ± 0.03 c | 10.46 ± 0.03 f | 4.94 ± 0.00 a | 10.52 ± 0.02 f | 7.33 ± 0.01 d |
| Cataechin | 6.65 ± 0.14 b | 6.79 ± 0.01 b | 12.22 ± 0.19 f | 8.26 ± 0.18 d | 7.31 ± 0.08 c | 9.64 ± 0.17 e | 5.64 ± 0.01 a | 9.46 ± 0.03 e | 7.33 ± 0.01 c |
| Epicatechin | 11.06 ± 0.01 e | 6.77 ± 0.00 b | 23.49 ± 0.05 h | 13.64 ± 0.01 g | 9.46 ± 0.13 d | 12.59 ± 0.04 f | 5.57 ± 0.01 a | 9.09 ± 0.00 c | 6.75 ± 0.03 b |
| Caffeic acid | 9.12 ± 0.00 e | 6.17 ± 0.05 b | 15.82 ± 0.03 i | 9.99 ± 0.01 f | 7.66 ± 0.01 d | 10.25 ± 0.09 g | 5.02 ± 0.00 a | 11.48 ± 0.02 h | 7.36 ± 0.01 c |
| Naringin | n/d | n/d | n/d | n/d | n/d | 7.88 ± 0.00 a | n/d | n/d | n/d |
| p-Coumeric acid | 5.22 ± 0.00 b | 5.64 ± 0.01 d | 9.83 ± 0.00 i | 6.66 ± 0.00 f | 6.37 ± 0.00 e | 7.96 ± 0.00 h | 5.11 ± 0.00 a | 7.85 ± 0.02 g | 5.41 ± 0.02 c |
| Rosmarinnic acid | 6.47 ± 0.00 c | n/d | 12.16 ± 0.00 i | 8.02 ± 0.00 f | 7.82 ± 0.00 e | 9.80 ± 0.002 h | 5.97 ± 0.01 b | 9.78 ± 0.01 g | 6.71 ± 0.01 d |
| Vanillin acid | 5.85 ± 0.00 b | n/d | n/d | 7.31 ± 0.00 d | n/d | 8.92 ± 0.00 e | n/d | n/d | 6.12 ± 0.00 c |
| o-Coumeric acid | 4.92 ± 0.00 b | n/d | n/d | n/d | n/d | n/d | n/d | n/d | 5.16 ± 0.00 c |
| Quercetin | n/d | n/d | n/d | n/d | n/d | n/d | n/d | n/d | n/d |
| Antioxidant potential | |||||||||
| FRAP (% FRAP value) | 16.71 ± 1.31 b | 29.46 ± 1.34 c | 46.28 ± 3.90 d | 29.32 ± 3.54 c | 10.09 ± 0.34 a | 27.71 ± 0.63 c | 10.05 ± 0.17 a | 53.64 ± 2.46 e | 13.79 ± 0.55 ab |
| ABTS (% scavenging activity ) | 85.67 ± 0.39 f | 24.93 ± 0.61 b | 85.56 ± 1.75 f | 56.40 ± 0.29 c | 24.53 ± 0.38 b | 57.30 ± 0.57 c | 14.44 ± 1.26 a | 71.35 ± 0.40 e | 63.56 ± 1.52 d |
| DPPH (% scavenging activity) | 85.78 ± 0.08 d | 56.22 ± 1.85 b | 87.86 ± 0.08 d | 87.04 ± 0.16 d | 27.30 ± 0.47 a | 62.56 ± 0.31 c | 57.62 ± 2.71 b | 86.42 ± 0.17 d | 64.54 ± 0.88 c |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chaiwan, P.; Rachtanapun, P.; Phimolsiripol, Y.; Ruksiriwanich, W.; Li, C.; Luo, L.; Shen, D.; Chung, H.-H.; George, D.; Tangpao, T.; et al. Physicochemical Marker for Determination of Value-Adding Component in Over-Ripe Thai Mango Peels. Horticulturae 2024, 10, 1036. https://doi.org/10.3390/horticulturae10101036
Chaiwan P, Rachtanapun P, Phimolsiripol Y, Ruksiriwanich W, Li C, Luo L, Shen D, Chung H-H, George D, Tangpao T, et al. Physicochemical Marker for Determination of Value-Adding Component in Over-Ripe Thai Mango Peels. Horticulturae. 2024; 10(10):1036. https://doi.org/10.3390/horticulturae10101036
Chicago/Turabian StyleChaiwan, Pirawan, Pornchai Rachtanapun, Yuthana Phimolsiripol, Warintorn Ruksiriwanich, Chunmei Li, Lu Luo, Dan Shen, Hsiao-Hang Chung, David George, Tibet Tangpao, and et al. 2024. "Physicochemical Marker for Determination of Value-Adding Component in Over-Ripe Thai Mango Peels" Horticulturae 10, no. 10: 1036. https://doi.org/10.3390/horticulturae10101036
APA StyleChaiwan, P., Rachtanapun, P., Phimolsiripol, Y., Ruksiriwanich, W., Li, C., Luo, L., Shen, D., Chung, H.-H., George, D., Tangpao, T., Sommano, S. R., & Sunanta, P. (2024). Physicochemical Marker for Determination of Value-Adding Component in Over-Ripe Thai Mango Peels. Horticulturae, 10(10), 1036. https://doi.org/10.3390/horticulturae10101036












